Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes
Abstract
:1. Introduction
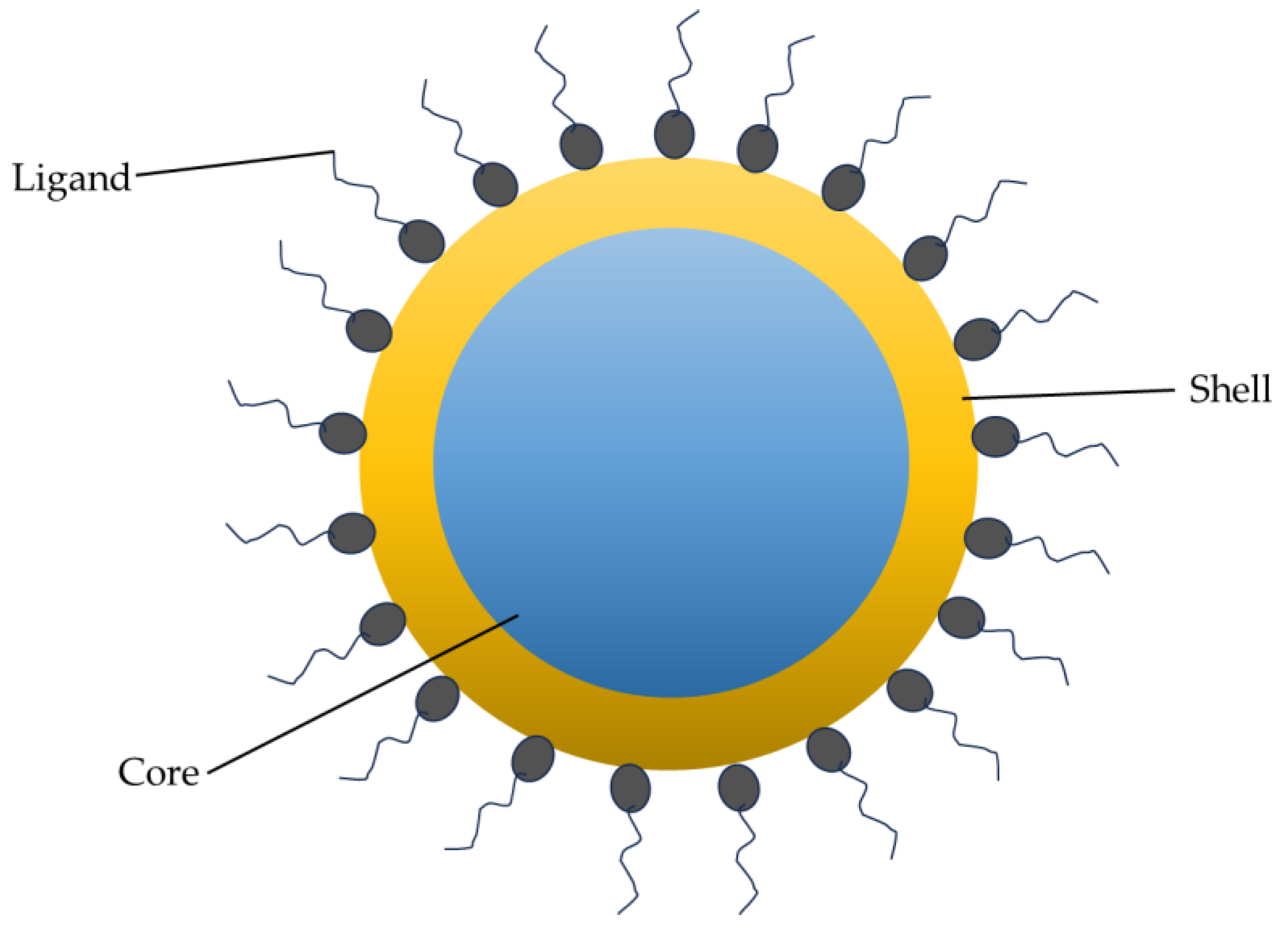
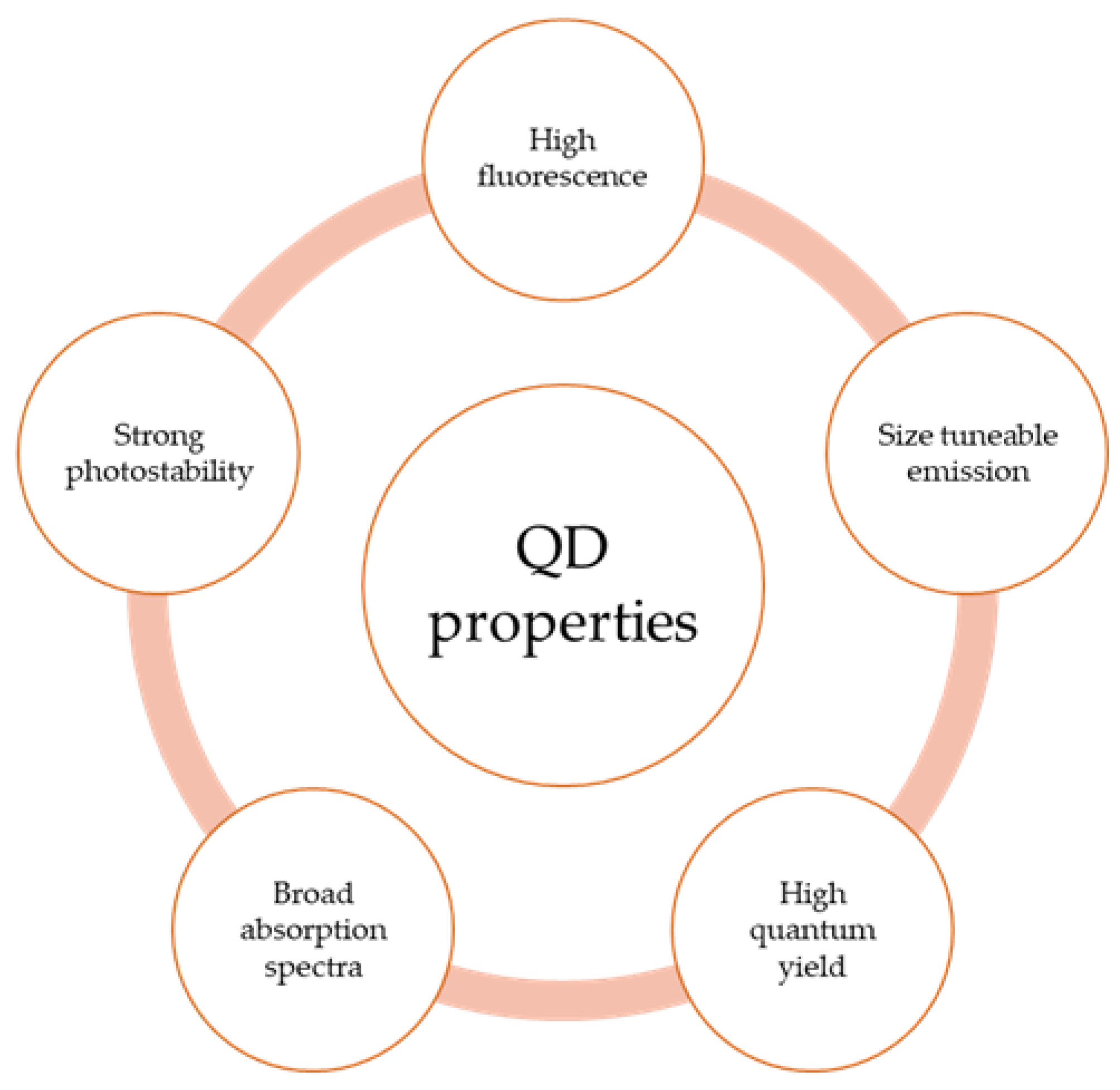
2. Structural and Optical Properties of QDs
3. Synthesis of QDs
3.1. Top-Down Approach
3.1.1. Electron Beam Lithography (EBL)
3.1.2. Reactive Ion Etching
3.1.3. Focused Ion Beam (FIB)
3.2. Bottom-Up Approach
3.2.1. Wet Chemical Methods
Sol–Gel
Microemulsion Process
3.2.2. Vapor-Phase Method
Molecular Beam Epitaxy (MBE)
Physical Vapor Deposition (PVD)
Sputtering
3.3. Other Syntheses
4. Surface Functionalization of QDs
4.1. Ligand Exchange
4.2. Surface Silanization
4.3. Amphiphilic Ligands
4.4. Microsphere Coating
5. Application of QDs
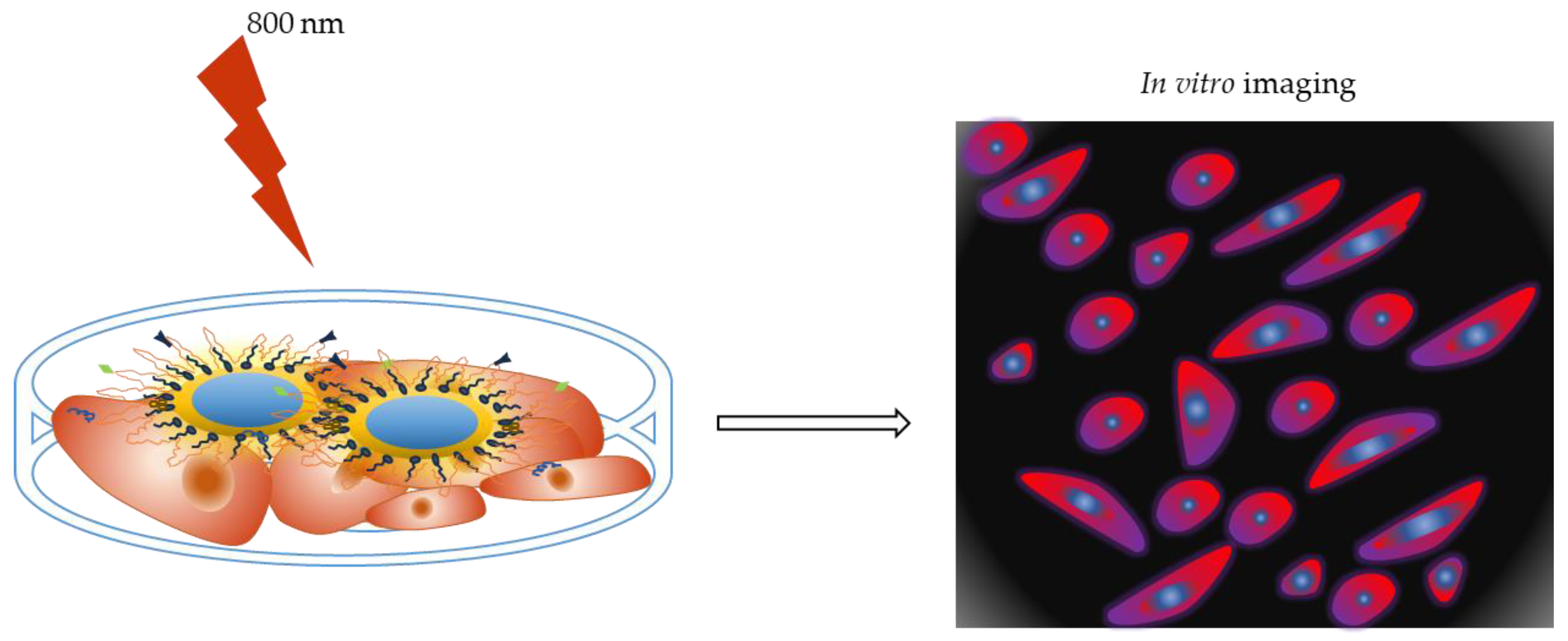
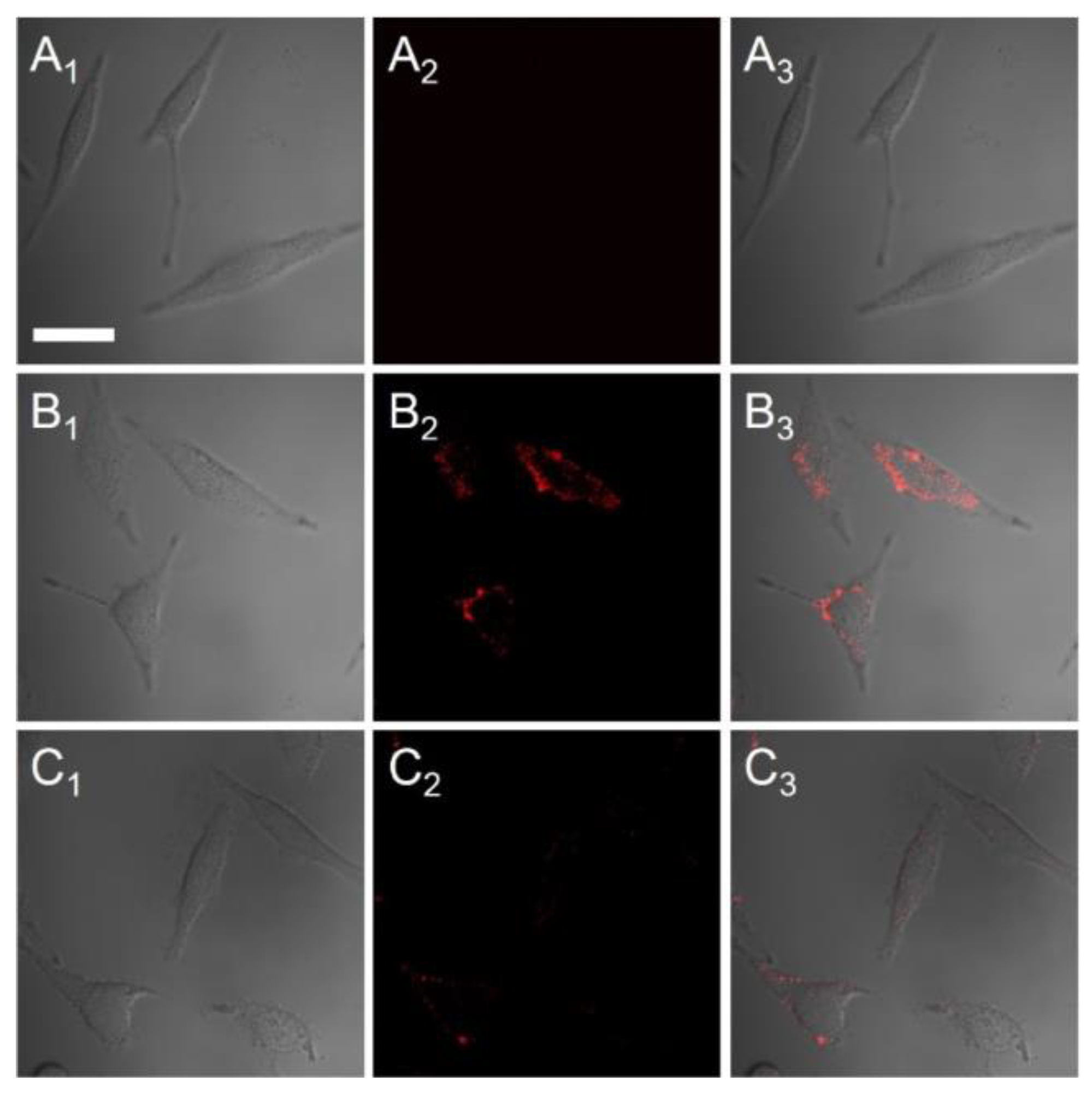
5.1. QDs for In Vitro Tumor Imaging
5.2. QDs for In Vivo Tumor Imaging
5.3. QDs for Drug Delivery
6. Cytotoxicity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute, N.C. What-Is-Cancer. 2015. Available online: http://www.cancer.gov/cancertopics/what-is-cancer (accessed on 18 June 2023).
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer. 2022, p. 1. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 18 June 2023).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Chen, Z.; Shin, D.M. Application of Nanotechnology in Cancer Therapy and Imaging. CA A Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Zareyi, H.; Sheervalilou, R.; Laurent, S.; Ghaznavi, H.; Samadian, H. Gold nanoparticle-mediated bubbles in cancer nanotechnology. J. Control. Release 2020, 330, 49–60. [Google Scholar] [CrossRef]
- Sharma, A.; Saini, A.K.; Kumar, N.; Tejwan, N.; Singh, T.A.; Thakur, V.K.; Das, J. Methods of preparation of metal-doped and hybrid tungsten oxide nanoparticles for anticancer, antibacterial, and biosensing applications. Surf. Interfaces 2021, 28, 101641. [Google Scholar] [CrossRef]
- Almanghadim, H.G.; Nourollahzadeh, Z.; Khademi, N.S.; Tezerjani, M.D.; Sehrig, F.Z.; Estelami, N.; Shirvaliloo, M.; Sheervalilou, R.; Sargazi, S. Application of nanoparticles in cancer therapy with an emphasis on cell cycle. Cell Biol. Int. 2021, 45, 1989–1998. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Shirvaliloo, M.; Sargazi, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Recent advances in iron oxide nanoparticles for brain cancer theranostics: From in vitro to clinical applications. Expert Opin. Drug Deliv. 2021, 18, 949–977. [Google Scholar] [CrossRef] [PubMed]
- Irajirad, R.; Ahmadi, A.; Najafabad, B.K.; Abed, Z.; Sheervalilou, R.; Khoei, S.; Shiran, M.B.; Ghaznavi, H.; Shakeri-Zadeh, A. Combined thermo-chemotherapy of cancer using 1 MHz ultrasound waves and a cisplatin-loaded sonosensitizing nanoplatform: An in vivo study. Cancer Chemother. Pharmacol. 2019, 84, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Sargazi, S.; Zaboli, M.; Barani, M.; Baino, F.; Bilal, M.; Sanchooli, E. Biochemical, Ameliorative and Cytotoxic Effects of Newly Synthesized Curcumin Microemulsions: Evidence from In Vitro and In Vivo Studies. Nanomaterials 2021, 11, 817. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Sargazi, S.; Amiri, M.S.; Sharma, P.K.; Bhalla, N. Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sens. Bio-Sens. Res. 2021, 32, 100417. [Google Scholar] [CrossRef]
- Arshad, R.; Barani, M.; Rahdar, A.; Sargazi, S.; Cucchiarini, M.; Pandey, S.; Kang, M. Multi-Functionalized Nanomaterials and Nanoparticles for Diagnosis and Treatment of Retinoblastoma. Biosensors 2021, 11, 97. [Google Scholar] [CrossRef]
- Zarrabi, A.; Zarepour, A.; Khosravi, A.; Alimohammadi, Z.; Thakur, V.K. Synthesis of Curcumin Loaded Smart pH-Responsive Stealth Liposome as a Novel Nanocarrier for Cancer Treatment. Fibers 2021, 9, 19. [Google Scholar] [CrossRef]
- Burz, C.; Pop, V.-V.; Buiga, R.; Daniel, S.; Samasca, G.; Aldea, C.; Lupan, I. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget 2018, 9, 24561–24571. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ren, L.; Liu, X.; Zhou, M.; Li, L.; Xu, J.; Zhu, X. DNA Nanotechnology for Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2018, 19, 1671. [Google Scholar] [CrossRef] [PubMed]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Kagan, C.R.; Murray, C.B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 2015, 10, 1013–1026. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I. Nanomaterials and their classification. In EMR/ESR/EPR Spectroscopy for Characterization of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–45. [Google Scholar]
- Rizwan, M.; Shoukat, A.; Ayub, A.; Razzaq, B.; Tahir, M.B. Chapter 3-Types and classification of nanomaterials. In Nanomaterials: Synthesis, Characterization, Hazards and Safety; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–54. [Google Scholar]
- Ali, A.A.; Abuwatfa, W.H.; Al-Sayah, M.H.; Husseini, G.A. Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy. Nanomaterials 2022, 12, 3706. [Google Scholar] [CrossRef]
- Chen, W.; Goldys, E.M.; Deng, W. Light-induced liposomes for cancer therapeutics. Prog. Lipid Res. 2020, 79, 101052. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 1–28. [Google Scholar] [CrossRef]
- Ekimov, A.; Efros, A.; Onushchenko, A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Remya, V.R.; Prajitha, V.; George, J.S.; Jibin, K.P.; Thomas, S. Chapter 7-Quantum dots: A brief introduction. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 181–196. [Google Scholar]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2003, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, L.A.; Ebenstein, Y.; Weiss, S. Quantum Dots for In Vivo Small-Animal Imaging. J. Nucl. Med. 2009, 50, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xiao, F.-X.; Phan, H.; Chen, S.; Yu, Z.; Wang, R.; Nguyen, T.-Q.; Yang Tan, T.T. Unraveling the cooperative synergy of zero-dimensional graphene quantum dots and metal nanocrystals enabled by layer-by-layer assembly. J. Mater. Chem. A 2018, 6, 1700–1713. [Google Scholar] [CrossRef]
- Ji, X.; Peng, F.; Zhong, Y.; Su, Y.; He, Y. Fluorescent quantum dots: Synthesis, biomedical optical imaging, and biosafety assessment. Colloids Surf. B:Biointerfaces 2014, 124, 132–139. [Google Scholar] [CrossRef]
- Shamsi, J.; Dang, Z.; Bianchini, P.; Canale, C.; Di Stasio, F.; Brescia, R.; Prato, M.; Manna, L. Colloidal Synthesis of Quantum Confined Single Crystal CsPbBr3 Nanosheets with Lateral Size Control up to the Micrometer Range. J. Am. Chem. Soc. 2016, 138, 7240–7243. [Google Scholar] [CrossRef] [PubMed]
- Segets, D. Analysis of Particle Size Distributions of Quantum Dots: From Theory to Application. KONA Powder Part. J. 2016, 33, 48–62. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef]
- Reimann, S.M.; Manninen, M. Electronic structure of quantum dots. Rev. Mod. Phys. 2002, 74, 1283–1342. [Google Scholar] [CrossRef]
- Maxwell, T.; Nogueira Campos, M.G.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Chapter 15-Quantum Dots. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–265. [Google Scholar]
- Fomenko, V.; Nesbitt, D.J. Solution Control of Radiative and Nonradiative Lifetimes: A Novel Contribution to Quantum Dot Blinking Suppression. Nano Lett. 2007, 8, 287–293. [Google Scholar] [CrossRef]
- Ornes, S. Quantum dots. Proc. Natl. Acad. Sci. USA 2016, 113, 2796–2797. [Google Scholar] [CrossRef]
- Sumanth Kumar, D.; Jai Kumar, B.; Mahesh, H.M. Chapter 3-Quantum Nanostructures (QDs): An Overview. In Micro and Nano Technologies; Woodhead Publishing: Sawston, UK, 2018; pp. 59–88. [Google Scholar]
- Yoffe, A.D. Semiconductor quantum dots and related systems: Electronic, optical, luminescence and related properties of low dimensional systems. Adv. Phys. 2001, 50, 1–208. [Google Scholar] [CrossRef]
- Hong, N.H. Chapter 1-Introduction to Nanomaterials: Basic Properties, Synthesis, and Characterization. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Jin, T.; Tiwari, D.K.; Tanaka, S.-I.; Inouye, Y.; Yoshizawa, K.; Watanabe, T.M. Antibody–ProteinA conjugated quantum dots for multiplexed imaging of surface receptors in living cells. Mol. Biosyst. 2010, 6, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Khawar, M.B.; Liang, J.; Sun, H. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front. Oncol. 2021, 11, 749970. [Google Scholar] [CrossRef] [PubMed]
- Misra, K.P.; Misra, R.D.K. ZnO-Based Quantum Dots for Biosensing, Cancer Imaging and Therapy: An Overview. Biomed. Mater. Devices 2022, 11, 749970. [Google Scholar] [CrossRef]
- Brunetti, J.; Riolo, G.; Gentile, M.; Bernini, A.; Paccagnini, E.; Falciani, C.; Lozzi, L.; Scali, S.; Depau, L.; Pini, A.; et al. Near-infrared quantum dots labelled with a tumor selective tetrabranched peptide for in vivo imaging. J. Nanobiotechnol. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Ranjbar-Navazi, Z.; Eskandani, M.; Johari-Ahar, M.; Nemati, A.; Akbari, H.; Davaran, S.; Omidi, Y. Doxorubicin-conjugated D-glucosamine- and folate- bi-functionalised InP/ZnS quantum dots for cancer cells imaging and therapy. J. Drug Target. 2017, 26, 267–277. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Mohamed, W.A.A.; El-Gawad, H.A.; Mekkey, S.; Galal, H.; Handal, H.; Mousa, H.; Labib, A. Quantum dots synthetization and future prospect applications. Nanotechnol. Rev. 2021, 10, 1926–1940. [Google Scholar] [CrossRef]
- Kara, H.E.Ş. Quantum Dots for Pharmaceutical and Biomedical Analysis; Zafar, N.E.E.-E.S.E.-F., Ed.; IntechOpen: Rijeka, Croatia, 2017; p. 8. [Google Scholar]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef]
- Bailey, R.E.; Smith, A.M.; Nie, S. Quantum dots in biology and medicine. Phys. E:Low-Dimens. Syst. Nanostructures 2004, 25, 1–12. [Google Scholar] [CrossRef]
- Sutherland, A.J. Quantum dots as luminescent probes in biological systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 365–370. [Google Scholar] [CrossRef]
- Ha, Y.; Jung, H.S.; Jeong, S.; Kim, H.-M.; Kim, T.H.; Cha, M.G.; Kang, E.J.; Pham, X.-H.; Jeong, D.H.; Jun, B.-H. Fabrication of Remarkably Bright QD Densely-Embedded Silica Nanoparticle. Bull. Korean Chem. Soc. 2019, 40, 9–13. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.; Mohanan, P. Quantum dots: Applications and safety consequences. J. Lumin 2018, 205, 287–298. [Google Scholar] [CrossRef]
- Tennant, D.; Bleier, A. Electron Beam Lithography of Nanostructures. Handb. Nanofabrication 2010, 4, 121–148. [Google Scholar] [CrossRef]
- Nagpal, R.; Gusain, M. Chapter 25-Synthesis methods of quantum dots. In Woodhead Publishing Series in Electronic and Optical Materials; Al-Douri, Y.B.T.-G., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 599–630. [Google Scholar]
- Nandwana, V.; Subramani, C.; Yeh, Y.-C.; Yang, B.; Dickert, S.; Barnes, M.D.; Tuominen, M.T.; Rotello, V.M. Direct patterning of quantum dot nanostructures via electron beam lithography. J. Mater. Chem. 2011, 21, 16859–16862. [Google Scholar] [CrossRef]
- Palankar, R.; Medvedev, N.; Rong, A.; Delcea, M. Fabrication of Quantum Dot Microarrays Using Electron Beam Lithography for Applications in Analyte Sensing and Cellular Dynamics. ACS Nano 2013, 7, 4617–4628. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Lee, L.K.; Ku, P. Fabrication of site-controlled InGaN quantum dots using reactive-ion etching. Phys. Status Solidi C 2011, 9, 609–612. [Google Scholar] [CrossRef]
- Choi, M.; Jun, S.; Woo, K.Y.; Song, H.G.; Yeo, H.-S.; Choi, S.; Park, D.; Park, C.-H.; Cho, Y.-H. Nanoscale Focus Pinspot for High-Purity Quantum Emitters via Focused-Ion-Beam-Induced Luminescence Quenching. ACS Nano 2021, 15, 11317–11325. [Google Scholar] [CrossRef]
- Zhang, H.; Ross, I.M.; Walther, T. Study of site controlled quantum dot formation on focused ion beam patterned GaAs substrate. J. Physics: Conf. Ser. 2013, 471, 012047. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Aftab, S.; Shah, A.; Erkmen, C.; Kurbanoglu, S.; Uslu, B. Chapter 1-Quantum dots: Synthesis and characterizations. In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–35. [Google Scholar]
- Bang, J.; Yang, H.; Holloway, P.H. Enhanced and stable green emission of ZnO nanoparticles by surface segregation of Mg. Nanotechnology 2006, 17, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Qian, L.; Sabui, S.; Santra, S.; Holloway, P.H. Photoluminescence of ZnO quantum dots produced by a sol–gel process. Opt. Mater. 2008, 30, 1233–1239. [Google Scholar] [CrossRef]
- Rai, A.K.; Jat, K.K. Chapter 3-Sol–gel synthesis of quantum dots. In Quantum Dots; Elsevier: Amsterdam, The Netherlands, 2023; pp. 35–52. [Google Scholar]
- Sashchiuk, A.; Lifshitz, E.; Reisfeld, R.; Saraidarov, T.; Zelner, M.; Willenz, A. Optical and Conductivity Properties of PbS Nanocrystals in Amorphous Zirconia Sol-Gel Films. J. Sol-Gel Sci. Technol. 2002, 24, 31–38. [Google Scholar] [CrossRef]
- Javed, S.; Islam, M.; Mujahid, M. Synthesis and characterization of TiO2 quantum dots by sol gel reflux condensation method. Ceram. Int. 2018, 45, 2676–2679. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, X.; Che, J.; Wang, M.; Kong, F. Preparation of ZnSe quantum dots embedded in SiO2 thin films by sol–gel process. Ceram. Int. 2004, 30, 1685–1689. [Google Scholar] [CrossRef]
- Moghaddam, E.; Youzbashi, A.; Kazemzadeh, A.; Eshraghi, M. Preparation of surface-modified ZnO quantum dots through an ultrasound assisted sol–gel process. Appl. Surf. Sci. 2015, 346, 111–114. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. Arab. J. Chem. 2010, 5, 397–417. [Google Scholar] [CrossRef]
- Shakur, H.R. A detailed study of physical properties of ZnS quantum dots synthesized by reverse micelle method. Phys. E Low-dimens. Syst. Nanostructures 2011, 44, 641–646. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Alexandridis, P.; Itskos, G.; Petrou, A.; Mountziaris, T.J. Synthesis and Size Control of Luminescent ZnSe Nanocrystals by a Microemulsion−Gas Contacting Technique. Langmuir 2004, 20, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Lien, V.T.K.; Ha, C.V.; Ha, L.T.; Dat, N.N. Optical properties of CdS and CdS/ZnS quantum dots synthesized by reverse micelle method. J. Phys. Conf. Ser. 2009, 187, 012028. [Google Scholar] [CrossRef]
- Mohagheghpour, E.; Rabiee, M.; Moztarzadeh, F.; Tahriri, M.; Jafarbeglou, M.; Bizari, D.; Eslami, H. Controllable synthesis, characterization and optical properties of ZnS:Mn nanoparticles as a novel biosensor. Mater. Sci. Eng. C 2009, 29, 1842–1848. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Kamali, M. Synthesis and characterization of aspartic acid-capped CdS/ZnS quantum dots in reverse micelles and its application to Hg(II) determination. J. Lumin 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Darbandi, M.; Thomann, R.; Nann, T. Single Quantum Dots in Silica Spheres by Microemulsion Synthesis. Chem. Mater. 2005, 17, 5720–5725. [Google Scholar] [CrossRef]
- Saran, A.D.; Bellare, J.R. Green engineering for large-scale synthesis of water-soluble and bio-taggable CdSe and CdSe–CdS quantum dots from microemulsion by double-capping. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 165–175. [Google Scholar] [CrossRef]
- Arthur, J.R. Molecular beam epitaxy. Surf. Sci. 2002, 500, 189–217. [Google Scholar] [CrossRef]
- Brault, J.; Matta, S.; Ngo, T.-H.; Al Khalfioui, M.; Valvin, P.; Leroux, M.; Damilano, B.; Korytov, M.; Brändli, V.; Vennéguès, P.; et al. Internal quantum efficiencies of AlGaN quantum dots grown by molecular beam epitaxy and emitting in the UVA to UVC ranges. J. Appl. Phys. 2019, 126, 205701. [Google Scholar] [CrossRef]
- Dhawan, S.; Dhawan, T.; Vedeshwar, A.G. Growth of Nb2O5 quantum dots by physical vapor deposition. Mater. Lett. 2014, 126, 32–35. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Yap, Y.K.; Zhang, D. Physical Vapor Deposition BT-Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–8. [Google Scholar]
- Son, H.H.; Seo, G.H.; Jeong, U.; Shin, D.Y.; Kim, S.J. Capillary wicking effect of a Cr-sputtered superhydrophilic surface on enhancement of pool boiling critical heat flux. Int. J. Heat Mass Transf. 2017, 113, 115–128. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering deposition of nanoparticles onto liquid substrates: Recent advances and future trends. Co-Ord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Balu, S.K.; Andra, S.; Danquah, M.K.; Vidyavathi, M.; Muthalagu, M. Quantum Dots Synthesis and Application BT-Contemporary Nanomaterials in Material Engineering Applications; Mubarak, N.M., Khalid, M., Walvekar, R., Numan, A., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 229–265. [Google Scholar]
- Tiwari, P.K.; Sahu, M.; Kumar, G.; Ashourian, M. Pivotal Role of Quantum Dots in the Advancement of Healthcare Research. Comput. Intell. Neurosci. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dahi, A.; Colson, P.; Jamin, C.; Cloots, R.; Lismont, M.; Dreesen, L. Radio-frequency magnetron sputtering: A versatile tool for CdSe quantum dots depositions with controlled properties. J. Mater. Environ. Sci. 2016, 7, 2277–2287. [Google Scholar]
- Bhatt, J.P.; Godha, N. Chapter 2-Hydrothermal synthesis of quantum dots. In Quantum Dots; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–34. [Google Scholar]
- Shen, T.-Y.; Jia, P.-Y.; Chen, D.-S.; Wang, L.-N. Hydrothermal synthesis of N-doped carbon quantum dots and their application in ion-detection and cell-imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 248, 119282. [Google Scholar] [CrossRef]
- Dalvand, P.; Mohammadi, M.R. Controlling morphology and structure of nanocrystalline cadmium sulfide (CdS) by tailoring solvothermal processing parameters. J. Nanopart. Res. 2011, 13, 3011–3018. [Google Scholar] [CrossRef]
- Tian, R.; Zhong, S.; Wu, J.; Jiang, W.; Shen, Y.; Wang, T. Solvothermal method to prepare graphene quantum dots by hydrogen peroxide. Opt. Mater. 2016, 60, 204–208. [Google Scholar] [CrossRef]
- Luo, K.; Chen, H.; Zhou, Q.; Yan, Z.; Su, Z.; Li, K. A facile one step solvothermal controllable synthesis of FeS2 quantum dots with multiple color emission for the visual detection of aconitine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118563. [Google Scholar] [CrossRef]
- Bharti, D.; Bharati, A.V.; Wankhade, A.V. Synthesis, characterization and optical property investigation of CdS nanoparticles. Luminescence 2018, 33, 1445–1449. [Google Scholar] [CrossRef]
- Khan, A.; Shkir, M.; Manthrammel, M.; Ganesh, V.; Yahia, I.; Ahmed, M.; El-Toni, A.M.; Aldalbahi, A.; Ghaithan, H.; AlFaify, S. Effect of Gd doping on structural, optical properties, photoluminescence and electrical characteristics of CdS nanoparticles for optoelectronics. Ceram. Int. 2019, 45, 10133–10141. [Google Scholar] [CrossRef]
- Abolghasemi, R.; Rasuli, R.; Alizadeh, M. Microwave-assisted growth of high-quality CdSe quantum dots and its application as a sensitizer in photovoltaic cells. Mater. Today Commun. 2020, 22, 100827. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.-A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, G.; Jiang, H.; Chen, L.; Zhang, X. One-step ultrasonic synthesis of graphene quantum dots with high quantum yield and their application in sensing alkaline phosphatase. Chem. Commun. 2014, 51, 948–951. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tseng, Z.-L.; Chen, S.-Y.; Yang, S. An ultrasonic synthesis method for high-luminance perovskite quantum dots. Ceram. Int. 2017, 43, 16032–16035. [Google Scholar] [CrossRef]
- Mahdi, H.S.; Parveen, A.; Azam, A. Microstructural and Optical Properties of Ni doped CdS Nanoparticles Synthesized by Sol Gel route. Mater. Today Proc. 2018, 5, 20636–20640. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Xu, Y.; Ren, Q.-G.; Xia, Y.-Y. Stable Aqueous ZnO@Polymer Core−Shell Nanoparticles with Tunable Photoluminescence and Their Application in Cell Imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef]
- Ye, Y. Photoluminescence property adjustment of ZnO quantum dots synthesized via sol–gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 4967–4974. [Google Scholar] [CrossRef]
- Entezari, M.H.; Ghows, N. Micro-emulsion under ultrasound facilitates the fast synthesis of quantum dots of CdS at low temperature. Ultrason. Sonochem. 2011, 18, 127–134. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Handal, H.T.; Ibrahem, I.A.; Galal, H.R.; Mousa, H.A.; Labib, A.A. Recycling for solar photocatalytic activity of Dianix blue dye and real industrial wastewater treatment process by zinc oxide quantum dots synthesized by solvothermal method. J. Hazard. Mater. 2020, 404, 123962. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Aladesuyi, O.A.; Oluwafemi, O.S. Synthesis of N, S co-doped carbon quantum dots (N,S-CQDs) for sensitive and selective determination of mercury (Hg2+) in Oreochromis niloctus (Tilapia fish). Inorg. Chem. Commun. 2023, 153, 110843. [Google Scholar] [CrossRef]
- Liao, B.; Wang, W.; Deng, X.; He, B.; Zeng, W.; Tang, Z.; Liu, Q. A facile one-step synthesis of fluorescent silicon quantum dots and their application for detecting Cu2+. RSC Adv. 2016, 6, 14465–14467. [Google Scholar] [CrossRef]
- Nathiya, D.; Gurunathan, K.; Wilson, J. Size controllable, pH triggered reduction of bovine serum albumin and its adsorption behavior with SnO2/SnS2 quantum dots for biosensing application. Talanta 2019, 210, 120671. [Google Scholar] [CrossRef]
- Safardoust-Hojaghan, H.; Salavati-Niasari, M.; Amiri, O.; Hassanpour, M. Preparation of highly luminescent nitrogen doped graphene quantum dots and their application as a probe for detection of Staphylococcus aureus and E. coli. J. Mol. Liq. 2017, 241, 1114–1119. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Tong, X.; Wang, X.; Yang, P.; Yao, F.; Guo, R.; Yuan, C. Preparation of graphene quantum dots with high quantum yield by a facile one-step method and applications for cell imaging. Mater. Lett. 2020, 271, 127806. [Google Scholar] [CrossRef]
- Aouassa, M.; Franzò, G.; Assaf, E.; Sfaxi, L.; M’ghaieth, R.; Maaref, H. MBE growth of InAs/GaAs quantum dots on sintered porous silicon substrates with high optical quality in the 1.3 μm band. J. Mater. Sci. Mater. Electron. 2020, 31, 4605–4610. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Boopathyraja, A. Structural and optical properties of Mg doped ZnS quantum dots and biological applications. Superlattices Microstruct. 2018, 113, 236–243. [Google Scholar] [CrossRef]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, Y.; Park, J.C.; Oh, M.H.; Jeon, D.Y.; Nam, Y.S. Highly luminescent, off-stoichiometric CuxInyS2/ZnS quantum dots for near-infrared fluorescence bio-imaging. RSC Adv. 2015, 5, 43449–43455. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, H.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Huang, Y.; Deng, Q.; Zhou, J.; Du, S.; et al. Photoluminescent Ti3C2MXene Quantum Dots for Multicolor Cellular Imaging. Adv. Mater. 2017, 29, 1604847. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Wei, N.; Li, L.; Zhang, H.; Wang, W.; Pan, C.; Qi, S.; Zhang, H.; Chen, H.; Chen, X. Characterization of the Ligand Exchange Reactions on CdSe/ZnS QDs by Capillary Electrophoresis. Langmuir 2019, 35, 4806–4812. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Zhang, F.; Lees, E.; Amin, F.; RiveraGil, P.; Yang, F.; Mulvaney, P.; Parak, W.J. Polymer-Coated Nanoparticles: A Universal Tool for Biolabelling Experiments. Small 2011, 7, 3113–3127. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Tang, J.; Tang, W. Surface ligands engineering of semiconductor quantum dots for chemosensory and biological applications. Mater. Today 2017, 20, 360–376. [Google Scholar] [CrossRef]
- Lees, E.E.; Nguyen, T.-L.; Clayton, A.H.A.; Mulvaney, P. The Preparation of Colloidally Stable, Water-Soluble, Biocompatible, Semiconductor Nanocrystals with a Small Hydrodynamic Diameter. ACS Nano 2009, 3, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Heyne, B.; Arlt, K.; Geßner, A.; Richter, A.F.; Döblinger, M.; Feldmann, J.; Taubert, A.; Wedel, A. Mixed Mercaptocarboxylic Acid Shells Provide Stable Dispersions of InPZnS/ZnSe/ZnS Multishell Quantum Dots in Aqueous Media. Nanomaterials 2020, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Clapp, A. Overview of Stabilizing Ligands for Biocompatible Quantum Dot Nanocrystals. Sensors 2011, 11, 11036–11055. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, H.; Zhang, M.; Zhang, Z. Quantum Dots (QDs) for Tumor Targeting Theranostics. In Nanomaterials for Tumor Targeting Theranostics: A Proactive Clinical Perspective; World Scientific: Singapore, 2016; pp. 85–141. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid Interface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef]
- Wang, J.; Han, S.; Ke, D.; Wang, R. Semiconductor Quantum Dots Surface Modification for Potential Cancer Diagnostic and Therapeutic Applications. J. Nanomater. 2012, 2012, 129041. [Google Scholar] [CrossRef]
- He, X.; Gao, L.; Ma, N. One-Step Instant Synthesis of Protein-Conjugated Quantum Dots at Room Temperature. Sci. Rep. 2013, 3, 2825. [Google Scholar] [CrossRef]
- Sanjayan, C.; Jyothi, M.; Sakar, M.; Balakrishna, R.G. Multidentate ligand approach for conjugation of perovskite quantum dots to biomolecules. J. Colloid Interface Sci. 2021, 603, 758–770. [Google Scholar] [CrossRef]
- Chen, O.; Yang, Y.; Wang, T.; Wu, H.; Niu, C.; Yang, J.; Cao, Y.C. Surface-Functionalization-Dependent Optical Properties of II–VI Semiconductor Nanocrystals. J. Am. Chem. Soc. 2011, 133, 17504–17512. [Google Scholar] [CrossRef]
- Shi, X.-H.; Dai, Y.-Y.; Wang, L.; Wang, Z.-G.; Liu, S.-L. Water-Soluble High-Quality Ag2Te Quantum Dots Prepared by Mutual Adaptation of Synthesis and Surface Modification for In Vivo Imaging. ACS Appl. Bio Mater. 2021, 4, 7692–7700. [Google Scholar] [CrossRef]
- Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles. Nat. Commun. 2013, 4, 2326. [Google Scholar] [CrossRef]
- Serrano, I.C.; Vazquez-Vazquez, C.; Adams, A.M.; Stoica, G.; Correa-Duarte, M.A.; Palomares, E.; Alvarez-Puebla, R.A. The effect of the silica thickness on the enhanced emission in single particle quantum dots coated with gold nanoparticles. RSC Adv. 2013, 3, 10691–10695. [Google Scholar] [CrossRef]
- Thanh, N.T.; Green, L.A. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Wang, X.; Lv, W.; Zhou, H.; Zhao, G.; Li, L.; Sun, Y.; Wu, Y.; Zhao, M. A novel strategy to create bifunctional silica-protected quantum dot nanoprobe for fluorescence imaging. Sens. Actuators B Chem. 2019, 282, 27–35. [Google Scholar] [CrossRef]
- Kambayashi, M.; Yamauchi, N.; Nakashima, K.; Hasegawa, M.; Hirayama, Y.; Suzuki, T.; Kobayashi, Y. Silica coating of indium phosphide nanoparticles by a sol–gel method and their photobleaching properties. SN Appl. Sci. 2019, 1, 1576. [Google Scholar] [CrossRef]
- Knopp, D.; Tang, D.; Niessner, R. Review: Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 2009, 647, 14–30. [Google Scholar] [CrossRef]
- Jun, B.H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H.; et al. Ultrasensitive, Biocompatible, Quantum-Dot-Embedded Silica Nanoparticles for Bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Giersig, M.; Liz-Marzán, L.M. Stabilization of CdS semiconductor nanoparticles against photodegradation by a silica coating procedure. Chem. Phys. Lett. 1998, 286, 497–501. [Google Scholar] [CrossRef]
- Du, Y.; Yang, P.; Matras-Postolek, K.; Wang, J.; Che, Q.; Cao, Y.; Ma, Q. Low toxic and highly luminescent CdSe/CdxZn1−x S quantum dots with thin organic SiO2 coating for application in cell imaging. J. Nanopart. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Ham, K.-M.; Kim, M.; Bock, S.; Kim, J.; Kim, W.; Jung, H.S.; An, J.; Song, H.; Kim, J.-W.; Kim, H.-M.; et al. Highly Bright Silica-Coated InP/ZnS Quantum Dot-Embedded Silica Nanoparticles as Biocompatible Nanoprobes. Int. J. Mol. Sci. 2022, 23, 10977. [Google Scholar] [CrossRef]
- Goftman, V.V.; Aubert, T.; Ginste, D.V.; Van Deun, R.; Beloglazova, N.V.; Hens, Z.; De Saeger, S.; Goryacheva, I.Y. Synthesis, modification, bioconjugation of silica coated fluorescent quantum dots and their application for mycotoxin detection. Biosens. Bioelectron. 2016, 79, 476–481. [Google Scholar] [CrossRef]
- Anderson, R.E.; Chan, W.C.W. Systematic Investigation of Preparing Biocompatible, Single, and Small ZnS-Capped CdSe Quantum Dots with Amphiphilic Polymers. ACS Nano 2008, 2, 1341–1352. [Google Scholar] [CrossRef]
- Yoon, C.; Yang, K.P.; Kim, J.; Shin, K.; Lee, K. Fabrication of highly transparent and luminescent quantum dot/polymer nanocomposite for light emitting diode using amphiphilic polymer-modified quantum dots. Chem. Eng. J. 2019, 382, 122792. [Google Scholar] [CrossRef]
- Abdolahi, G.; Dargahi, M.; Ghasemzadeh, H. Synthesis of starch-g-poly (acrylic acid)/ZnSe quantum dot nanocomposite hydrogel, for effective dye adsorption and photocatalytic degradation: Thermodynamic and kinetic studies. Cellulose 2020, 27, 6467–6483. [Google Scholar] [CrossRef]
- Speranskaya, E.S.; Beloglazova, N.V.; Lenain, P.; De Saeger, S.; Wang, Z.; Zhang, S.; Hens, Z.; Knopp, D.; Niessner, R.; Potapkin, D.V.; et al. Polymer-coated fluorescent CdSe-based quantum dots for application in immunoassay. Biosens. Bioelectron. 2014, 53, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrion, C.; Parak, W.J. Design of pyridyl-modified amphiphilic polymeric ligands: Towards better passivation of water-soluble colloidal quantum dots for improved optical performance. J. Colloid Interface Sci. 2016, 478, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Kong, X.; Sun, L. Application of nanodiagnostics in point-of-care tests for infectious diseases. Int. J. Nanomed. 2017, 12, 4789–4803. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, P.; Dou, H.; Li, W.; Sun, K.; He, X.; Han, J.; Xiao, H.; Li, Y. Efficient Incorporation of Quantum Dots into Porous Microspheres through a Solvent-Evaporation Approach. Langmuir 2012, 28, 6141–6150. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Osipova, V.; Tkach, A.; Miropoltsev, M.; Kurshanov, D.; Sokolova, A.; Cherevkov, S.; Zakharov, V.; Fedorov, A.; Baranov, A.; et al. Lab-on-Microsphere—FRET-Based Multiplex Sensor Platform. Nanomaterials 2021, 11, 109. [Google Scholar] [CrossRef]
- Li, Z.; Ma, H.; Guo, Y.; Fang, H.; Zhu, C.; Xue, J.; Wang, W.; Luo, G.; Sun, Y. Synthesis of uniform Pickering microspheres doped with quantum dot by microfluidic technology and its application in tumor marker. Talanta 2023, 262, 124495. [Google Scholar] [CrossRef]
- Vaidya, S.V.; Couzis, A.; Maldarelli, C. Reduction in Aggregation and Energy Transfer of Quantum Dots Incorporated in Polystyrene Beads by Kinetic Entrapment due to Cross-Linking during Polymerization. Langmuir 2015, 31, 3167–3179. [Google Scholar] [CrossRef]
- Zhao, C.; Li, W.; Liang, Y.; Tian, Y.; Zhang, Q. Synthesis of BiOBr/carbon quantum dots microspheres with enhanced photoactivity and photostability under visible light irradiation. Appl. Catal. A Gen. 2016, 527, 127–136. [Google Scholar] [CrossRef]
- Khan, M.R.; Mitra, T.; Sahoo, D. Metal oxide QD based ultrasensitive microsphere fluorescent sensor for copper, chromium and iron ions in water. RSC Adv. 2020, 10, 9512–9524. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Niu, Y.; Meng, R.; Huang, L.; Cao, H.; Zhang, Z.; Qin, H.; Peng, X. Shell-thickness dependent optical properties of CdSe/CdS core/shell nanocrystals coated with thiol ligands. Nano Res. 2016, 9, 260–271. [Google Scholar] [CrossRef]
- Ko, J.; Jeong, B.G.; Chang, J.H.; Joung, J.F.; Yoon, S.-Y.; Lee, D.C.; Park, S.; Huh, J.; Yang, H.; Bae, W.K.; et al. Chemically resistant and thermally stable quantum dots prepared by shell encapsulation with cross-linkable block copolymer ligands. NPG Asia Mater. 2020, 12, 19. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, P.; Liz-Marzán, L.M.; Giersig, M.; Ung, T. Silica encapsulation of quantum dots and metal clusters. J. Mater. Chem. 2000, 10, 1259–1270. [Google Scholar] [CrossRef]
- Aubert, T.; Soenen, S.J.; Wassmuth, D.; Cirillo, M.; Van Deun, R.; Braeckmans, K.; Hens, Z. Bright and Stable CdSe/CdS@SiO2 Nanoparticles Suitable for Long-Term Cell Labeling. ACS Appl. Mater. Interfaces 2014, 6, 11714–11723. [Google Scholar] [CrossRef]
- Pham, X.-H.; Park, S.-M.; Ham, K.-M.; Kyeong, S.; Son, B.S.; Kim, J.; Hahm, E.; Kim, Y.-H.; Bock, S.; Kim, W.; et al. Synthesis and Application of Silica-Coated Quantum Dots in Biomedicine. Int. J. Mol. Sci. 2021, 22, 10116. [Google Scholar] [CrossRef]
- Cheng, R.; Li, F.; Zhang, J.; She, X.; Zhang, Y.; Shao, K.; Lin, Y.; Wang, C.-F.; Chen, S. Fabrication of amphiphilic quantum dots towards high-colour-quality light-emitting devices. J. Mater. Chem. C 2019, 7, 4244–4249. [Google Scholar] [CrossRef]
- Li, C.; Ji, Y.; Wang, C.; Liang, S.; Pan, F.; Zhang, C.; Chen, F.; Fu, H.; Wang, K.; Cui, D. BRCAA1 antibody- and Her2 antibody-conjugated amphiphilic polymer engineered CdSe/ZnS quantum dots for targeted imaging of gastric cancer. Nanoscale Res. Lett. 2014, 9, 244. [Google Scholar] [CrossRef]
- Nie, Q.; Tan, W.B.; Zhang, Y. Synthesis and characterization of monodisperse chitosan nanoparticles with embedded quantum dots. Nanotechnology 2005, 17, 140–144. [Google Scholar] [CrossRef]
- Sheng, W.; Kim, S.; Lee, J.; Kim, S.-W.; Jensen, K.; Bawendi, M.G. In-Situ Encapsulation of Quantum Dots into Polymer Microspheres. Langmuir 2006, 22, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yuan, H.; Shen, H.; Guo, Y.; Li, X.; Liu, D.; Xu, L.; Ma, L.; Li, L.S. Synthesis of size-tunable photoluminescent aqueous CdSe/ZnS microspheres via a phase transfer method with amphiphilic oligomer and their application for detection of HCG antigen. J. Mater. Chem. 2011, 21, 7393–7400. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Wang, K.; Wong, W.-K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, P.; Liu, H.; Zhan, H.; Zhang, Q.; Zhao, Y.; Chen, Y. Design of bright near-infrared-emitting quantum dots capped with different stabilizing ligands for tumor targeting. RSC Adv. 2018, 8, 4221–4229. [Google Scholar] [CrossRef]
- Tao, J.; Zeng, Q.; Wang, L. Near-infrared quantum dots based fluorescent assay of Cu2+ and in vitro cellular and in vivo imaging. Sens. Actuators B Chem. 2016, 234, 641–647. [Google Scholar] [CrossRef]
- Shi, M.; Dong, L.; Zheng, S.; Hou, P.; Cai, L.; Zhao, M.; Zhang, X.; Wang, Q.; Li, J.; Xu, K. “Bottom-up” preparation of MoS2 quantum dots for tumor imaging and their in vivo behavior study. Biochem. Biophys. Res. Commun. 2019, 516, 1090–1096. [Google Scholar] [CrossRef]
- Tao, J.; Feng, S.; Liu, B.; Pan, J.; Li, C.; Zheng, Y. Hyaluronic acid conjugated nitrogen-doped graphene quantum dots for identification of human breast cancer cells. Biomed. Mater. 2021, 16, 055001. [Google Scholar] [CrossRef]
- Zhu, C.-N.; Chen, G.; Tian, Z.-Q.; Wang, W.; Zhong, W.-Q.; Li, Z.; Zhang, Z.-L.; Pang, D.-W. Near-Infrared Fluorescent Ag2Se-Cetuximab Nanoprobes for Targeted Imaging and Therapy of Cancer. Small 2016, 13, 1602309. [Google Scholar] [CrossRef]
- Yao, C.; Tu, Y.; Ding, L.; Li, C.; Wang, J.; Fang, H.; Huang, Y.; Zhang, K.; Lu, Q.; Wu, M.; et al. Tumor Cell-Specific Nuclear Targeting of Functionalized Graphene Quantum Dots In Vivo. Bioconjug. Chem. 2017, 28, 2608–2619. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Sun, J.; Zhang, Y.; Pu, M.; Zhang, G.; He, N.; Zeng, X. Effective Integration of Targeted Tumor Imaging and Therapy Using Functionalized InP QDs with VEGFR2 Monoclonal Antibody and miR-92a Inhibitor. ACS Appl. Mater. Interfaces 2017, 9, 13068–13078. [Google Scholar] [CrossRef]
- Sun, X.; Shi, M.; Zhang, C.; Yuan, J.; Yin, M.; Du, S.; Yu, S.; Ouyang, B.; Xue, F.; Yang, S.-T. Fluorescent Ag–In–S/ZnS Quantum Dots for Tumor Drainage Lymph Node Imaging In Vivo. ACS Appl. Nano Mater. 2021, 4, 1029–1037. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Ji, W.; Zhou, S.; Li, L.; Qiu, L.; Qian, Z.; Wang, X.; Zhang, H. Biomimetic black phosphorus quantum dots-based photothermal therapy combined with anti-PD-L1 treatment inhibits recurrence and metastasis in triple-negative breast cancer. J. Nanobiotechnol. 2021, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dong, H.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and In Vivo Bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Yaghini, E.; Turner, H.D.; Le Marois, A.M.; Suhling, K.; Naasani, I.; MacRobert, A.J. In vivo biodistribution studies and ex vivo lymph node imaging using heavy metal-free quantum dots. Biomaterials 2016, 104, 182–191. [Google Scholar] [CrossRef]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2015, 98, 19–34. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef]
- Grigoletto, A.; Maso, K.; Mero, A.; Rosato, A.; Schiavon, O.; Pasut, G. Drug and protein delivery by polymer conjugation. J. Drug Deliv. Sci. Technol. 2016, 32, 132–141. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Gopinath, P. Self-Assembled Hybrids of Fluorescent Carbon Dots and PAMAM Dendrimers for Epirubicin Delivery and Intracellular Imaging. ACS Appl. Mater. Interfaces 2015, 7, 11423–11435. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Lin, G.; He, Z.; Wang, Y. Metal complex-based liposomes: Applications and prospects in cancer diagnostics and therapeutics. J. Control. Release Off. J. Control. Release 2022, 348, 1066–1088. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Gottsacker, C.; He, X.; Waterkotte, T.; Park, Y.C. Repetitive drug delivery using Light-Activated liposomes for potential antimicrobial therapies. Adv. Drug Deliv. Rev. 2022, 187, 114395. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Kurawattimath, V.; Wilson, B.; Geetha, K.M. Nanoparticle-based drug delivery across the blood-brain barrier for treating malignant brain glioma. OpenNano 2023, 10, 100128. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf. B Biointerfaces 2023, 228, 113419. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Qiu, J.; Kong, L.; Cao, X.; Li, A.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with β-Cyclodextrin. Nanomaterials 2018, 8, 131. [Google Scholar] [CrossRef]
- Zou, J.; Zhu, B.; Li, Y. Functionalization of Silver Nanoparticles Loaded with Paclitaxel-induced A549 Cells Apoptosis Through ROS-Mediated Signaling Pathways. Curr. Top. Med. Chem. 2020, 20, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, S.; Yang, L.; Ling, X.; Liao, J.; Zhou, X.; Li, M.; Zhong, W. Functionalized Silver Nanoparticles Enhance Therapeutic Effect of Paclitaxel for Prostate Cancer Therapy by Arresting the Cellular Cycle and Producing ROS. Nano 2021, 16, 2150126. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Omar, S.M.; Teiama, M.S.; Khairy, A.; Magdy, M.; Anis, B. Fabrication of Gold Nanoparticles in Absence of Surfactant as In Vitro Carrier of Plasmid DNA. Int. J. Nanomed. 2019, 14, 8399–8408. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, J.; Aghanejad, A.; Safari, B.; Barar, J.; Rasta, S.H.; Davaran, S. Aptamer-conjugated gold nanoparticles for targeted paclitaxel delivery and photothermal therapy in breast cancer. J. Drug Deliv. Sci. Technol. 2021, 67, 102954. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Zhang, W.; Huang, Q.; Hu, M.; Peng, D.; Peng, C.; Wang, L.; Chen, W. Acidity and Glutathione Dual-Responsive Polydopamine-Coated Organic-Inorganic Hybrid Hollow Mesoporous Silica Nanoparticles for Controlled Drug Delivery. ChemMedChem 2020, 15, 1940–1946. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Yan, K.; Li, J.; Xu, X.; Yuan, T.; Wang, T.; Zheng, J. Magnetic Janus particles as a multifunctional drug delivery system for paclitaxel in efficient cancer treatment. Mater. Sci. Eng. C 2019, 104, 110001. [Google Scholar] [CrossRef]
- Kong, X.; Qi, Y.; Wang, X.; Jiang, R.; Wang, J.; Fang, Y.; Gao, J.; Hwang, K.C. Nanoparticle drug delivery systems and their applications as targeted therapies for triple negative breast cancer. Prog. Mater. Sci. 2023, 134, 101070. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Zhu, B.-J. The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Chapter 13-Quantum dots hybrid systems for drug delivery. In Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 323–338. [Google Scholar]
- Garcia-Cortes, M.; González-Iglesias, H.; Ruiz Encinar, J.; Costa-Fernández, J.M.; Coca-Prados, M.; Sanz-Medel, A. Sensitive targeted multiple protein quantification based on elemental detection of Quantum Dots. Anal. Chim. Acta 2015, 879, 77–84. [Google Scholar]
- Banerjee, A.; Pons, T.; Lequeux, N.; Dubertret, B. Quantum dots–DNA bioconjugates: Synthesis to applications. Interface Focus 2016, 6, 20160064. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Wang, Y.; Roy, I.; Rui, H.; Swihart, M.T.; Law, W.-C.; Kwak, S.K.; Ye, L.; Liu, J.; Mahajan, S.D.; et al. Preparation of Quantum Dot/Drug Nanoparticle Formulations for Traceable Targeted Delivery and Therapy. Theranostics 2012, 2, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dang, G.; Younis, M.R.; Cao, Y.; Wang, K.; Sun, X.; Zhang, W.; Zou, X.; Shen, H.; An, R.; et al. Peptide functionalized actively targeted MoS2 nanospheres for fluorescence imaging-guided controllable pH-responsive drug delivery and collaborative chemo/photodynamic therapy. J. Colloid Interface Sci. 2023, 639, 302–313. [Google Scholar] [CrossRef]
- Zoghi, M.; Pourmadadi, M.; Yazdian, F.; Nigjeh, M.N.; Rashedi, H.; Sahraeian, R. Synthesis and characterization of chitosan/carbon quantum dots/Fe2O3 nanocomposite comprising curcumin for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2023, 249, 125788. [Google Scholar] [CrossRef]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin delivery to breast cancer cells with transferrin-targeted carbon quantum dots: An in vitro and in silico study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Mohammed-Ahmed, H.K.; Nakipoglu, M.; Tezcaner, A.; Keskin, D.; Evis, Z. Functionalization of graphene oxide quantum dots for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104199. [Google Scholar] [CrossRef]
- Ziaee, N.; Farhadian, N.; Abnous, K.; Matin, M.M.; Khoshnood, A.; Yaghoobi, E. Dual targeting of Mg/N doped-carbon quantum dots with folic and hyaluronic acid for targeted drug delivery and cell imaging. BioMedicine 2023, 164, 114971. [Google Scholar] [CrossRef]
- Khodadadei, F.; Safarian, S.; Ghanbari, N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater. Sci. Eng. C 2017, 79, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, H.; Dong, J.; Zhang, W.; Dang, G.; Yang, M.; Li, Y.; Chen, H.; Ji, H.; Dong, L. PEGylated MoS2 quantum dots for traceable and pH-responsive chemotherapeutic drug delivery. Colloids Surf. B Biointerfaces 2020, 185, 110590. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhan, Y.; Wang, P.; Zhang, B.; Zhang, Y. Novel Surface Modification of ZnO QDs for Paclitaxel-Targeted Drug Delivery for Lung Cancer Treatment. Dose-Response 2020, 18, 1559325820926739. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Habiba, K.; Encarnacion-Rosado, J.; Garcia-Pabon, K.; Villalobos-Santos, J.C.; Makarov, V.I.; Avalos, J.A.; Weiner, B.R.; Morell, G. Improving cytotoxicity against cancer cells by chemo-photodynamic combined modalities using silver-graphene quantum dots nanocomposites. Int. J. Nanomed. 2015, 11, 107–119. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin. Int. J. Biol. Macromol. 2020, 156, 648–659. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoum, A.; Gaber, S.A.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalil, A.S. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef]
- Tan, L.; Huang, R.; Li, X.; Liu, S.; Shen, Y.-M.; Shao, Z. Chitosan-based core-shell nanomaterials for pH-triggered release of anticancer drug and near-infrared bioimaging. Carbohydr. Polym. 2017, 157, 325–334. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Alitheen, N.B.; Hussein, M.Z.; Abu, N.; Mohammed, N.E.; Nordin, N.; Zamberi, N.R.; et al. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interface Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef]
- Hu, F.; Li, C.; Zhang, Y.; Wang, M.; Wu, D.; Wang, Q. Real-time in vivo visualization of tumor therapy by a near-infrared-II Ag2S quantum dot-based theranostic nanoplatform. Nano Res. 2015, 8, 1637–1647. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Duan, S.; Li, Y.; Wang, J.; Zhu, J. Graphene quantum dots mediated magnetic chitosan drug delivery nanosystems for targeting synergistic photothermal-chemotherapy of hepatocellular carcinoma. Cancer Biol. Ther. 2022, 23, 281–293. [Google Scholar] [CrossRef]
- Su, W.; Guo, R.; Yuan, F.; Li, Y.; Li, X.; Zhang, Y.; Zhou, S.; Fan, L. Red-Emissive Carbon Quantum Dots for Nuclear Drug Delivery in Cancer Stem Cells. J. Phys. Chem. Lett. 2020, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, L.; Han, B.; Wang, Y.; Fei, J.; Xia, K.; Zhai, Y.; Yu, Z. Black phosphorus quantum dots camouflaged with platelet-osteosarcoma hybrid membrane and doxorubicin for combined therapy of osteosarcoma. J. Nanobiotechnol. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, A.; Farhadian, N.; Abnous, K.; Matin, M.M.; Ziaee, N.; Yaghoobi, E. N doped-carbon quantum dots with ultra-high quantum yield photoluminescent property conjugated with folic acid for targeted drug delivery and bioimaging applications. J. Photochem. Photobiol. A Chem. 2023, 444, 114972. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Lo, P.-Y.; Cho, E.-C.; Zheng, J.-H.; Li, M.; Huang, J.-H.; Lee, K.-C. Integration of PEG and PEI with graphene quantum dots to fabricate pH-responsive nanostars for colon cancer suppression in vitro and in vivo. FlatChem 2021, 31, 100320. [Google Scholar] [CrossRef]
- Hao, R.; Luo, S.; Wang, F.; Pan, X.; Yao, J.; Wu, J.; Fang, H.; Li, W. Enhancement of fluorescence and anti-tumor effect of ZnO QDs by La doping. Front. Chem. 2022, 10, 1042038. [Google Scholar] [CrossRef]
- Gautam, A.; Pal, K. Gefitinib conjugated PEG passivated graphene quantum dots incorporated PLA microspheres for targeted anticancer drug delivery. Heliyon 2022, 8, e12512. [Google Scholar] [CrossRef]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, X.; Li, Y.; Zhang, M.; He, J.; Zhang, X.; Liu, H.; Wang, X.; Gu, H. Fluorescence and drug loading properties of ZnSe:Mn/ZnS-Paclitaxel/SiO2 nanocapsules templated by F127 micelles. J. Colloid Interface Sci. 2017, 490, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.D.; Akkoc, Y.; Demirci, G.; Bavili, N.; Kiraz, A.; Gozuacik, D.; Acar, H.Y. Bypassing pro-survival and resistance mechanisms of autophagy in EGFR-positive lung cancer cells by targeted delivery of 5FU using theranostic Ag2S quantum dots. J. Mater. Chem. B 2019, 7, 7363–7376. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yao, X.; Dong, J.; Wang, N.; Du, Y.; Sun, S.; Gao, L.; Zhong, Y.; Qian, C.; Hong, H. Design and Investigation of Core/Shell GQDs/hMSN Nanoparticles as an Enhanced Drug Delivery Platform in Triple-Negative Breast Cancer. Bioconjug. Chem. 2018, 29, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 61, 102287. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Li, Z.; Cui, H.; Zhou, Y.; Li, W.; Tao, W.; Zhang, H.; Wang, H.; Chu, P.K.; et al. As an Efficient Contrast Agent for In Vivo Photoacoustic Imaging of Cancer. Small 2017, 13, 1602896. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Bahadur, R.; Kaku, T.; Prabhuraj, R.S.; Suchitta, A.; Srivastava, R. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Mater. Sci. Eng. C 2019, 103, 109774. [Google Scholar] [CrossRef] [PubMed]
- Saljoughi, H.; Khakbaz, F.; Mahani, M. Synthesis of folic acid conjugated photoluminescent carbon quantum dots with ultrahigh quantum yield for targeted cancer cell fluorescence imaging. Photodiagnosis Photodyn. Ther. 2020, 30, 101687. [Google Scholar] [CrossRef]
- Zhang, F.; He, X.; Ma, P.; Sun, Y.; Wang, X.; Song, D. Rapid aqueous synthesis of CuInS/ZnS quantum dots as sensor probe for alkaline phosphatase detection and targeted imaging in cancer cells. Talanta 2018, 189, 411–417. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Jiang, C.; Lin, J.; Huang, P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020, 400, 126009. [Google Scholar] [CrossRef]
- Xu, N.; Piao, M.; Arkin, K.; Ren, L.; Zhang, J.; Hao, J.; Zheng, Y.; Shang, Q. Imaging of water soluble CdTe/CdS core-shell quantum dots in inhibiting multidrug resistance of cancer cells. Talanta 2019, 201, 309–316. [Google Scholar] [CrossRef]
- Sobhani, Z.; Khalifeh, R.; Banizamani, M.; Rajabzadeh, M. Water-soluble ZnO quantum dots modified by polyglycerol: The pH-sensitive and targeted fluorescent probe for delivery of an anticancer drug. J. Drug Deliv. Sci. Technol. 2022, 76, 103452. [Google Scholar] [CrossRef]
- Wang, J.; Su, X.; Zhao, P.; Gao, D.; Chen, R.; Wang, L. Cancer photothermal therapy based on near infrared fluorescent CdSeTe/ZnS quantum dots. Anal. Methods 2021, 13, 5509–5515. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-L.; Huang, B.; Zhang, Z.-L.; Liu, X.; He, M.; Yu, Z.; Hu, B.; Cui, R.; Liang, X.-J.; Pang, D.-W. Glucose-functionalized near-infrared Ag2Se quantum dots with renal excretion ability for long-term in vivo tumor imaging. J. Mater. Chem. B 2019, 7, 5782–5788. [Google Scholar] [CrossRef]
- Li, X.; Vinothini, K.; Ramesh, T.; Rajan, M.; Ramu, A. Combined photodynamic-chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Deliv. 2020, 27, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, K.; Sun, L.; Sun, B.; Yang, M.; Chen, H.; Wang, Y.; Sun, J.; Dong, L. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens. Actuators B Chem. 2018, 256, 616–623. [Google Scholar] [CrossRef]
- Kim, E.-M.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. Facile synthesis of near-infrared CuInS2/ZnS quantum dots and glycol-chitosan coating for in vivo imaging. J. Nanopart. Res. 2017, 19, 251. [Google Scholar] [CrossRef]
- Michalska, M.; Florczak, A.; Dams-Kozlowska, H.; Gapinski, J.; Jurga, S.; Schneider, R. Peptide-functionalized ZCIS QDs as fluorescent nanoprobe for targeted HER2-positive breast cancer cells imaging. Acta Biomater. 2016, 35, 293–304. [Google Scholar] [CrossRef]
- Guo, Y.; Nie, Y.; Wang, P.; Li, Z.; Ma, Q. MoS2 QDs-MXene heterostructure-based ECL sensor for the detection of miRNA-135b in gastric cancer exosomes. Talanta 2023, 259, 124559. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Yu, B.; Chen, Y.; Shen, Y.; Cong, H. ZnO Quantum Dots Modified by pH-Activated Charge-Reversal Polymer for Tumor Targeted Drug Delivery. Polymers 2018, 10, 1272. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, K.; Zhu, P.; Zou, X.; Ma, G.; Zhang, W.; Wang, D.; Wan, J.; Ma, Y.; Sun, X.; et al. A near-infrared triggered upconversion/MoS2 nanoplatform for tumour-targeted chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2022, 213, 112393. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, M.; Bai, H.; He, M.; Dong, L.; Cai, L.; Zhao, M.; Wang, Q.; Xu, K.; Li, J. Preparation of AS1411 Aptamer Modified Mn-MoS2 QDs for Targeted MR Imaging and Fluorescence Labelling of Renal Cell Carcinoma. Int. J. Nanomed. 2019, 14, 9513–9524. [Google Scholar] [CrossRef] [PubMed]
- Badıllı, U.; Mollarasouli, F.; Bakirhan, N.K.; Ozkan, Y.; Ozkan, S.A. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Anal. Chem. 2020, 131, 116013. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mortensen, L.; Ravichandran, S.; Bentley, K.; Delouise, L. Effect of Nanoparticle Surface Coating on Cell Toxicity and Mitochondria Uptake. J. Biomed. Nanotechnol. 2017, 13, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Manshian, B.B.; Martens, T.F.; Kantner, K.; Braeckmans, K.; De Smedt, S.C.; Demeester, J.; Jenkins, G.J.S.; Parak, W.J.; Pelaz, B.; Doak, S.H.; et al. The role of intracellular trafficking of CdSe/ZnS QDs on their consequent toxicity profile. J. Nanobiotechnol. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Tian, J.; Wang, X.; Xu, G.; Jiang, W.; Yang, Z.; Liu, D.; Lin, G. Cardiotoxicity of Intravenously Administered CdSe/ZnS Quantum Dots in BALB/c Mice. Front. Pharmacol. 2019, 10, 1179. [Google Scholar] [CrossRef]
- Zou, W.; Li, L.; Chen, Y.; Chen, T.; Yang, Z.; Wang, J.; Liu, D.; Lin, G.; Wang, X. In Vivo Toxicity Evaluation of PEGylated CuInS2/ZnS Quantum Dots in BALB/c Mice. Front. Pharmacol. 2019, 10, 437. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef]
- Zhang, M.; Yue, J.; Cui, R.; Ma, Z.; Wan, H.; Wang, F.; Zhu, S.; Zhou, Y.; Kuang, Y.; Zhong, Y.; et al. Bright quantum dots emitting at ∼1600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595. [Google Scholar] [CrossRef]
- Hu, S.-H.; Gao, X. Stable Encapsulation of Quantum Dot Barcodes with Silica Shells. Adv. Funct. Mater. 2010, 20, 3721–3726. [Google Scholar] [CrossRef]
- Murase, N.; Horie, M.; Sawai, T.; Kawasaki, K. Silica layer-dependent leakage of cadmium from CdSe/ZnS quantum dots and comparison of cytotoxicity with polymer-coated analogues. J. Nanopart. Res. 2019, 21, 10. [Google Scholar] [CrossRef]
- Ko, N.R.; Nafiujjaman, M.; Lee, J.S.; Lim, H.-N.; Lee, Y.-K.; Kwon, I.K. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef]

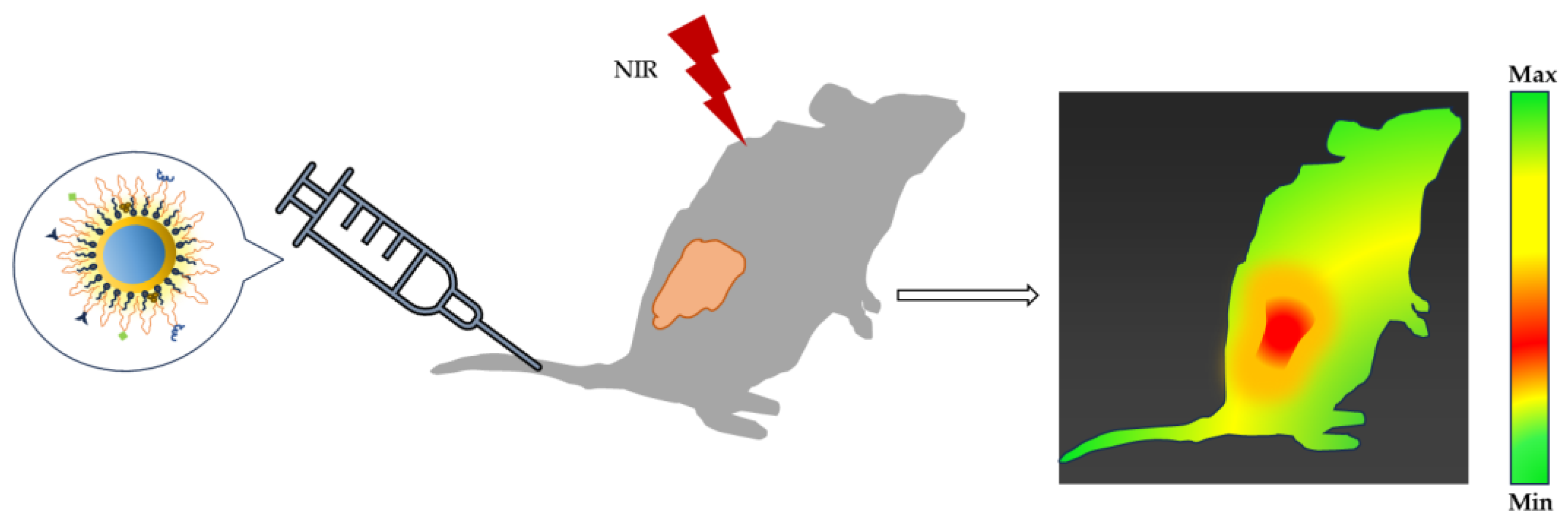
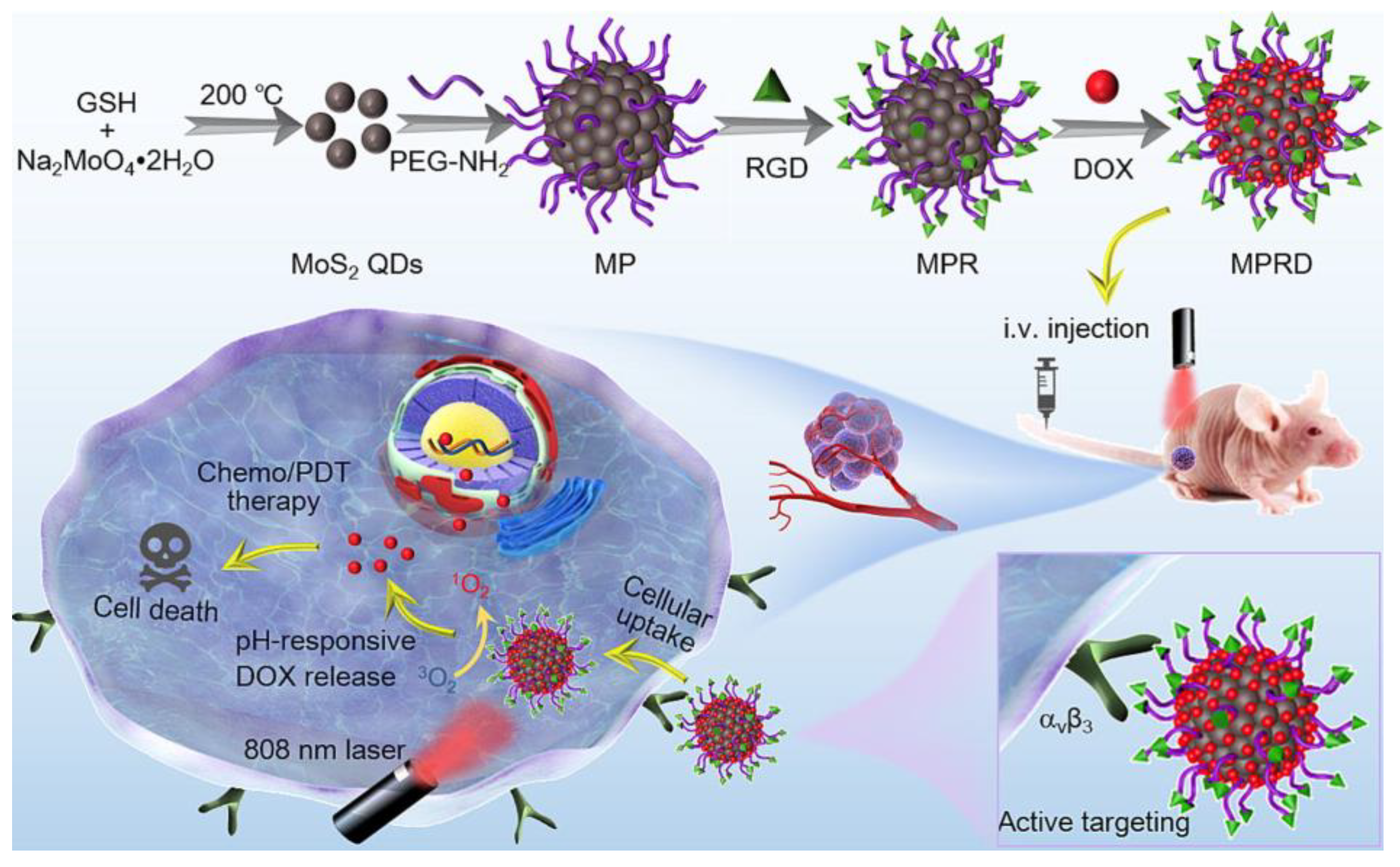
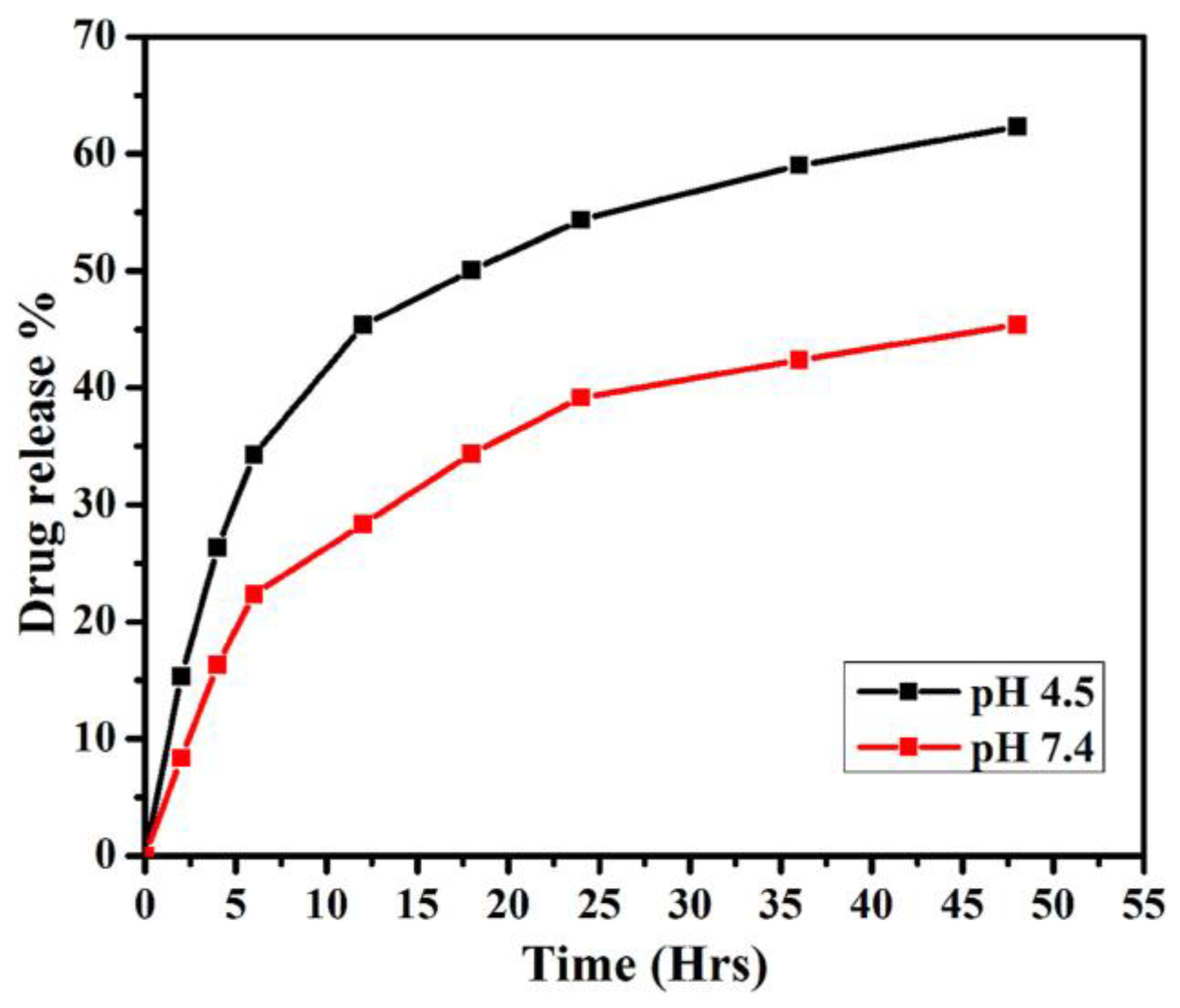
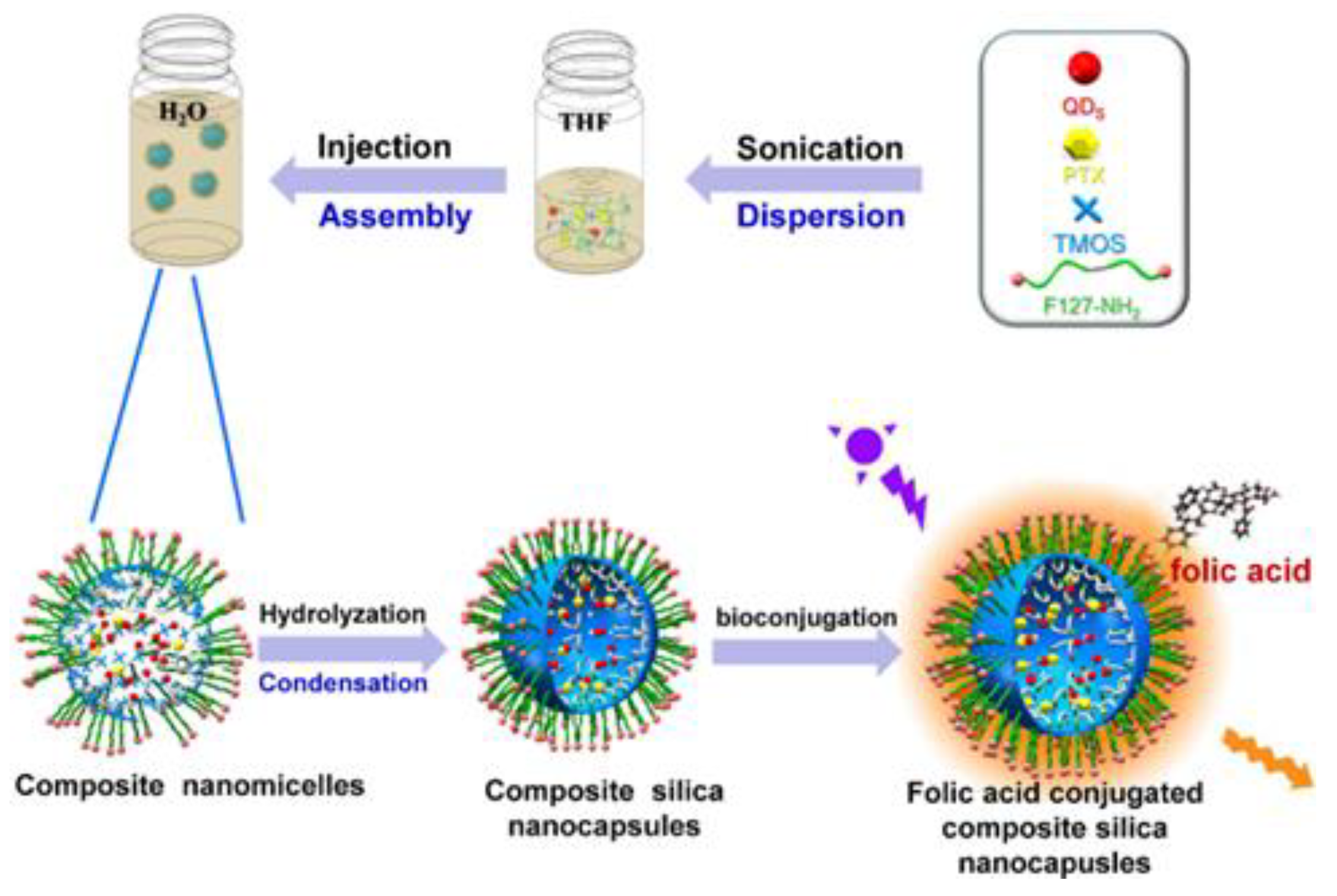
| Quantum Dots | Size Range (Diameter nm) | Emission Range (nm) |
|---|---|---|
| Cadmium sulfide (CdS) | 2.8–5.4 | 410–460 |
| Cadmium telluride (CdTe) | 3.1–9.1 | 520–750 |
| Cadmium selenide (CdSe) | 2–8 | 480–680 |
| CdTe/CdSe | 4–9.2 | 650–840 |
| Indium phosphide (InP) | 2.5–4.5 | 610–710 |
| Indium arsenide (InAs) | 3.2–6 | 860–1270 |
| Lead selenide (PbSe) | 3.2–4.1 | 1110–1310 |
| 1-Dodecanethiol silver sulfide ((Dt)-Ag2S) | 5.4–10 | 1000–1300 |
| Synthesis Methods | QDs Fabricated | Properties | Refs. |
|---|---|---|---|
| Electron beam lithography | QD nanostructures | Optical properties retained after cross-linking | [67] |
| QD microarrays | Fluorescence Bioaffinity | [68] | |
| Reactive ion etching | Indium gallium nitride (InGaN) QDs | Strong and distinct photoluminescence signal | [71] |
| Sol-gel | Titanium dioxide (TiO2) QDs | large surface area photocatalytic properties | [80] |
| Zinc selenide (ZnSe) QDs embedded in Silicon dioxide (SiO2) | - | [81] | |
| Cadmium sulfide (CdS) and Ni-doped CdS | Highly crystalline | [113] | |
| Zinc oxide (ZnO)@polymer core/shell | Quantum yield above 50% | [114] | |
| Zinc oxide (ZnO) QD | High photoluminescence quantum yield | [115] | |
| Microemulsion (reverse micelle) | Zinc sulfide (ZnS) QDs | Pure nanocrystal Quantum confinement effect Photoluminescence peak at 365 nm | [84] |
| Cadmium sulfide/Zinc sulfide (CdS/ZnS) semiconductor QDs | Excellent luminescence and photostability | [86] | |
| Cadmium selenide@Zinc sulfide (CdSe@ZnS) within monodisperse silica | Good monodispersity High luminescence | [89] | |
| Microemulsion (gas contacting technique) | Zinc selenide (ZnSe) QDs | Excellent photostability and size-dependent luminescence | [85] |
| Microemulsion method + ultrasonic waves (sono-microemulsion method) | Cadmium sulfide (CdS) | Narrow size distribution High crystallinity and purity | [116] |
| Physical vapor deposition | Niobium pentoxide (Nb2O5) QDs | Quantum confinement effect | [93] |
| RF magnetron sputtering | Cadmium selenide (CdSe) QDs | Optical properties | [100] |
| Solvothermal | Zinc Oxide (ZO) QDs | Small size Pure, high crystallinity and surface area | [117] |
| Graphene QDs (GQDs) | 11.4% photoluminescence quantum yield High stability Biocompatibility Low toxicity | [118] | |
| Hydrothermal | Nitrogen- and sulfur-doped carbon QDs (N, S-doped CQDs) | Small Spherical Green emission | [119] |
| Fluorescence quantum yield (10.35%) | |||
| Nitrogen-doped carbon QDs (N-CQDs) | Low toxicity Good photostability | [102] | |
| Silicon QDs | Good water dispersibility Strong photoluminescence High pH stability | [120] | |
| Tin oxide/Tin sulfide in reduced bovine serum albumin (SnO2/SnS2 @r-BSA2) | Specific selectivity Long term stability Enhanced reproducibility | [121] | |
| Nitrogen-doped Graphene QDs (N-GQDs) | High quantum yield Long-term fluorescence stability High sensitivity and specificity | [122,123] | |
| Molecular beam epitaxy | Indium arsenide gallium arsenide core/shell (InAs/GaAs) QDs | Strong photoluminescence intensity High structural properties | [124] |
| Surface Modification Techniques | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Ligand exchange | Ease of processing Small QD size | Degradation of QD photophysical properties in an aqueous environment (i.e., reduced PLQY) QD core is susceptible to oxidation | [51,168,169,170] |
| Surface silanization | Improves biocompatibility Highly cross-linked ligand molecules End terminal groups allow further coating through the exposure of the terminal ends (e.g., thiol). Control of silica shell thickness encourages fine-tuning of QD response to light. Improves PLQY of QDs Improves photochemical stability | Large hydrodynamic size Aggregation of QDs in aqueous solution | [171,172,173] |
| Amphiphilic ligands | More chemically stable Increased colloidal stability Good biocompatibility and strong, stable fluorescence signals | Size enlargementSurface defects | [138,174,175] |
| Microsphere coating | Improve QD stability High fluorescence Can mask QD toxicity effectively | The formation of a uniform microsphere is hindered. Reduced PLQY Encapsulation of high concentrations of QDs results in QD aggregation | [167,176,177] |
| Organic Nanoparticles | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Liposomes | Enhances drug solubility | Decreased stability | [199,200] |
| Reduces drug toxicity | |||
| Micelles | Improves circulation time | Lack of targeting moieties | [201,202] |
| Protects aqueous drug cargo | |||
| Polymer NP (Chitosan) | Increase drug residence time in the bloodstream | Initial burst release results in loss of drug efficiency | [203,204] |
| Dendrimers | The hydrophobic core allows insoluble anti-tumor drugs to be absorbed and provides smooth delivery. | Rapid clearance of reticuloendothelial system | [205,206] |
| The hydrophilic part increases stability and limits the particles' interaction with serum proteins. | |||
| Inorganic Nanoparticles | |||
| Silver NPs | Enhances PTX distribution in tumor microenvironment | Release of silver ions in cytosol | [207,208] |
| Gold NPs | Enhances photothermal therapy | Low tissue clearance | [209,210,211] |
| Easily functionalized | |||
| Mesoporous silica NPs | Controlled drug release | Slow biodegradation | [204,212] |
| Magnetic NPs (iron oxide) | Precise targeting of cancer cells Release of PTX under external magnetic field | Removal by macrophages | [213,214,215] |
| Quantum Dots | Improves the bioavailability of the drug | Leaching of heavy metals | [216,217] |
| QDs Used In Vitro | Drug | Cell Line | Ref. |
|---|---|---|---|
| Iron oxide carbon QDs encapsulated in chitosan (Fe2O3/CQDs/Chitosan) | Curcumin | MCF-7 cells | [224] |
| Transferrin (TF)-conjugated Carbon QDs | Doxorubicin | MCF-7 cells | [225] |
| Graphene oxide QDs conjugated with glucosamine and boric acid (GOQDs-GlcN-BA) | Doxorubicin | MCF-7 cells | [226] |
| Magnesium nitride (Mg/N) doped carbon QDs (CQDs) | Epirubicin (EPI) | 4T1 and MCF-7 cells | [227] |
| Nitrogen-doped Graphene QDs (N-GQDs) | Methotrexate (MTX) | MCF-7 human breast cancer cells | [228] |
| PEGylated molybdenum disulfide QDs (PEG-MoS2 QDs) | Doxorubicin | U251 cells | [229] |
| Zinc oxide adipic dihydrazide heparin (ZnO-ADH-Hep) | Paclitaxel | A549 cells | [230] |
| Cadmium-sulfide-modified chitosan (CdS@CTS) | Sesamol | MCF-7 cell | [231] |
| PEGylated Silver graphene QDs (Ag-GQDs) | Doxorubicin | HeLa and DU145 cells | [232] |
| Magnetic carbon triazine dendrimer reacted with graphene QDs (Fe3O4@C@TD GQDs) microsphere | Doxorubicin | A549 cell | [233] |
| QDs used in vivo | |||
| Graphene QDs | Doxorubicin | MCF-7 cells | [234] |
| Silver sulfide (Ag2S) QDs conjugated with chitosan | Doxorubicin | HeLa cells | [235] |
| Manganese doped zinc sulfide (Mn-ZnS) QDs conjugated with folic acid (FA) | 5-fluorouracil (5-FU) | 4T1 breast cancer cells | [236] |
| PEGylated silver sulfide Ag2S QDs | Doxorubicin | MDA-MB-231 human breast tumor cells | [237] |
| Graphene QD (GQD)-modified magnetic chitosan Fe3O4@CS | Doxorubicin | Hepatocellular carcinoma | [238] |
| Red-emissive carbon QDs (CQDs) | Doxorubicin | HeLa cells | [239] |
| Black phosphorus QDs (BPQDs) encapsulated in platelet-osteosarcoma hybrid membrane (OPM) | Doxorubicin | Osteosarcoma | [240] |
| Nitrogen-doped carbon QDs conjugated with folic acid (FA) | Doxorubicin | 4T1 and MCF-7 cells | [241] |
| PEGylated molybdenum disulfide (MoS2) QDs conjugated with arginylglycylaspartic acid (RGD) peptide | Doxorubicin | HepG2 cells | [223] |
| Polyethyleneimine (PEI)-conjugated graphene QDs (GQDs) | Doxorubicin | HCT116 cells | [242] |
| QDs Utilized | Application | Target Cells |
|---|---|---|
| Carbon QDs (CQDs) | Drug delivery | Breast cancer cell line |
| Carbon QDs (CQDs) | Drug delivery | Breast MCF-7 cancer cells |
| Graphene QDs (GQDs) | Drug delivery | U251 glioma cells |
| Near-infrared (NIR) copper indium sulfide zinc sulfide core/shell (CuInS2/ZnS) QDs | In vivo | RR1022 Cancer cell |
| Alloyed Zinc copper indium sulfide (ZCIS) QDs | In vitro | HER2-positive SKBR3 cancer cells |
| Molybdenum disulfide (MoS2) QDs-MXene | Electrochemiluminescence (ECL) sensor for detection | Gastric cancer cell exosome |
| Zinc oxide (ZnO) QDs | Drug delivery | HepG2 cells |
| Molybdenum disulfide (MoS2) QDs | Photodynamic therapy Drug delivery | HeLa and HepG2 cells |
| Manganese-doped molybdenum disulfide (Mn-MoS2) QDs | In vivo MR imaging Fluorescence labeling | 786-O Renal carcinoma cells |
| Titanium-ligand-coordinated black phosphorus QDs (TiL4@BPQDs) | In vivo Photoacoustic Imaging | MCF-7 cancer cells |
| Graphene QDs (GQDs) | Photothermal therapy | MDA-MB-231 |
| Folic-acid-conjugated carbon QDs (FA-CQDs) | Fluorescence imaging | MCF-7 cells and ovarian cancer (HeLa) |
| Copper indium sulfide zinc sulfide core/shell (CuInS/ZnS) QDs | Sensor probe for targeted imaging | BEL-7402 cancer cells |
| Titanium nitride (Ti2N) QDs | Photoacoustic (PA) imaging-guided photothermal therapy (PTT) in near-infrared (NIR-I/II) biowindows | 293T, 4T1 and U87 cancer cells |
| Cadmium telluride cadmium sulfide (CdTe/CdS) core–shell QDs | Fluorescence imaging | MDA-MB-231/MDR |
| Zinc oxide (ZnO) QDs | Drug delivery | MCF-7 |
| Cadmium selenide telluride zinc sulfide (CdSeTe/ZnS) QDs | Photothermal therapy | Hepatoma cells Huh7 |
| Graphene QDs (GQDs) | Drug delivery | MCF-7 cells |
| Near-infrared (NIR) silver selenide (Ag2Se) QDs | In vivo tumor imaging | MCF-7 human breast cancer cells and SW1990 pancreatic cancer cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials 2023, 13, 2566. https://doi.org/10.3390/nano13182566
Hamidu A, Pitt WG, Husseini GA. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials. 2023; 13(18):2566. https://doi.org/10.3390/nano13182566
Chicago/Turabian StyleHamidu, Aisha, William G. Pitt, and Ghaleb A. Husseini. 2023. "Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes" Nanomaterials 13, no. 18: 2566. https://doi.org/10.3390/nano13182566
APA StyleHamidu, A., Pitt, W. G., & Husseini, G. A. (2023). Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials, 13(18), 2566. https://doi.org/10.3390/nano13182566






