Abstract
This article discusses the features of the synthesis and application of porous two-dimensional nanomaterials in developing conductometric gas sensors based on metal oxides. It is concluded that using porous 2D nanomaterials and 3D structures based on them is a promising approach to improving the parameters of gas sensors, such as sensitivity and the rate of response. The limitations that may arise when using 2D structures in gas sensors intended for the sensor market are considered.
1. Introduction
Metal oxide nanomaterials with 1D (nanotubes, nanobelts, nanowires) and 2D structures (nanoflakes (NFs), nanoplates, and nanosheets (NSs)) are increasingly used in the development of conductometric gas sensors. Studies have shown that the unique properties of such structures make it possible to optimize the main parameters of gas sensors, such as sensitivity and rate of response [1,2,3,4,5]. In [5,6,7], a detailed consideration of the manufacturing features of gas sensors based on 1D and 2D structures and nanofibers was carried out, and the advantages and disadvantages of using such structures in this area were shown. In this article, we will continue the analysis of nanomaterials suitable for the development of gas sensors and focus on the consideration of porous 2D structures. At that, by porosity, we will understand not the traditionally considered porosity of the gas-sensitive layer formed by 2D structures but the porosity of nanoflakes and nanosheets, i.e., the presence of nanoholes in them. Some reviews about porous 2D materials and their applications have been published [8,9,10,11]. However, it should be noted that there is still a lack of a comprehensive review of the use of porous two-dimensional metal oxides for gas sensors, which is critical for the further design and manufacture of a new generation of solid-state gas sensors.
This review is organized as follows: the advantages of 2D nanomaterials for the design of gas sensors are commented on in Section 2. Then, in Section 3, we consider the limitations of developing gas sensors based on 2D nanomaterials. In Section 4, we analyze the advantages of porous 2D nanostructures for applications in gas sensors, while Section 5 discusses approaches that can be used to fabricate porous 2D structures. As regards the gas-sensitive characteristics of conductometric gas sensors based on two-dimensional structures, Section 6 and Section 7 are devoted to these issues. In Section 6, attention is focused on gas sensors using 2D structures, and in Section 7, on sensors developed on the basis of 3D nanostructures.
It should be noted that in this review, we will not consider the mechanism of gas sensitivity of metal oxide with 2D and 3D structures since this mechanism is no different from the sensitivity mechanism of conventional metal oxide gas sensors and gas sensors based on 1D nanomaterials. It has been described in numerous published articles, reviews, and books [11,12,13,14]. There is no point in repeating them. It is only important to recall that the sensory response of conductometric gas sensors is controlled by such processes as adsorption or chemisorption, desorption, diffusion, catalysis, and charge transfer, in which sensing material acts as a charge acceptor or donor with respect to the adsorbed gas molecule. As a result of these processes, an increase or decrease in the conductivity of the metal oxides occurs. The basic requirements for metal oxides to achieve maximum sensory response are described in sufficient detail in [15].
2. Advantages of 2D Nanomaterials for Gas Sensor Design
Korotcenkov [5] highlighted the following advantages of 2D nanostructures, such as nanosheets, nanoplates, and nanoflakes, for developing gas sensors:
- First, 2D-layered nanomaterials, such as 1D nanomaterials, possess a large surface area and high surface-to-volume ratio, allowing more atoms of these materials to interact with the atmosphere. This is especially important for gas sensors, as such structure of the gas-sensitive material facilitates surface reactions such as adsorption, chemisorption, and catalysis-controlling the gas-sensing effects [15,16].
- Second, the unique structure of 2D nanomaterials can provide these materials with properties that cannot be achieved with conventional bulk materials.
- The possibility of assembly into three-dimensional (3D) architectures can also be attributed to the advantages of 2D nanomaterials [17].
- In addition, it should be taken into account that only one or two crystallographic planes are involved in gas-sensitive effects in 2D nanomaterials. This, as in the case of 1D structures, greatly simplifies the modeling of processes occurring on the surface of these materials and contributes to a better understanding of the nature of gas-sensitive effects [5,18]. The same factor can contribute to better sensor selectivity.
- The advantages of 2D structures also include their relatively large lateral size, which, in contrast to 1D nanostructures, greatly facilitates the formation of Ohmic contacts, but, unfortunately, does not completely solve this problem.
At present, based on various 2D nanomaterials, a large number of prototypes of gas sensors with increased sensitivity to various gases have been manufactured. An analysis of these results, as well as methods used to synthesize 2D structures, can be found in numerous reviews devoted to the consideration of these materials [16,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Therefore, these topics will not be considered in this article.
3. Limitations Arising from the Development of Gas Sensors Based on 2D Nanomaterials
The results of testing gas sensors based on 2D nanomaterials showed that, along with the undeniable advantages of these materials, the following difficulties might arise when using 2D nanomaterials to develop high-sensitivity gas sensors [33,34,35,36]:
First, it is very difficult to grow 2D nanomaterials that are large but thin using traditional approaches to metal oxide synthesis. When materials take the form of a real 2D nanomaterial, their thickness turns out to be more than 30–50 nm, which does not contribute to achieving the high sensitivity of sensors. To achieve a high-sensor response, the thickness of the 2D nanomaterial should be comparable with the Debye length (2–5 nm) [37]. Only in this case, modulation of the surface space charge region due to a change in the surface charge caused by adsorption–desorption and catalytic processes can have a noticeable effect on the conductivity of the gas-sensitive layer. However, if we try to synthesize nanosheets with a smaller thickness using traditional approaches, then, in the end, we will get a material that differs little from conventional nanocrystals used in conventional gas sensors [37].
If 2D nanomaterials are in the form of large plates, then, in this case, when using thick-film technology for forming a gas-sensitive layer, there is also a threat of forming a gas-sensitive material with a denser arrangement of nanosheets (see Figure 1). This makes it difficult for gas to penetrate the inter-sheet space, further reducing sensitivity and significantly increasing the response and recovery time. This statement has been experimentally confirmed by Miao et al. [38], who established that an increase in the number of nanosheets in gas-sensitive layers, as previously predicted, is accompanied by a decrease in sensitivity (see Figure 2) and an increase in the response time.
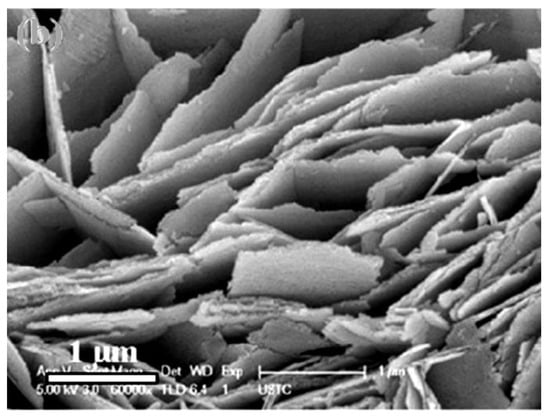
Figure 1.
Field-emission-scanning electron microscopy (FE-SEM) image of the ZnO nanosheets. Reprinted with permission from [39]. Copyright 2012: Elsevier.
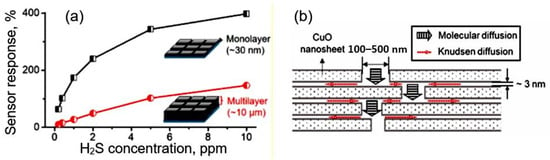
Figure 2.
(a) Sensor response of monolayer- and multilayer-based sensors to H2S; (b) Model of a multilayer film with lamellar structure. Reproduced from [38]. Published 2019 by ACS as open access.
Of course, it is possible to fabricate gas sensors based on individual nanosheets (Figure 3). In such structures, the sensor response is determined either by the resistance of metal–metal oxide contacts or by the conductivity of the nanosheet itself, which is modulated by a change in the width of the space-charge region upon interaction with the gas environment. In this case, the sensor response kinetics is not controlled by diffusion processes. In this case, technological difficulties arise in the manufacture of sensors, especially when organizing mass production, described in [5]. These include separating, manipulating, and fixing nanosheets in the right place on the sensor platform, as well as manufacturing low-resistance ohmic contacts.
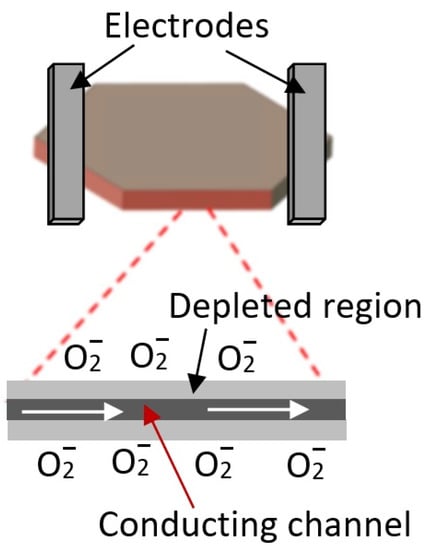
Figure 3.
Schematic diagram of gas sensor based on individual 2D nanosheets.
Some of the problems associated with the excessive thickness of nanosheets have been solved on the basis of new technologies [22,40] using hydro/solvothermal synthesis [41,42], the interface-mediated synthesis method [43], 2D-oriented attachment method [44], 2D-templated synthesis [45], self-assembly of nanocrystals [46], and the on-surface synthesis method [47].
Using this strategy, 2D structures of TiO2, ZnO, Co3O4, WO3, Fe3O4, V2O5, and MnO2 nanosheets with planar sizes from 0.2 μm to 10 μm and thicknesses ranging from 1.6 nm to 5.2 nm (corresponding to 2−7 stacking monolayers) have been prepared [48,49]. It is important to note that metal oxides of this thickness must have unique properties in terms of gas-sensing effects. For example, five-atomic-layer-thick 0.66 nm SnO2 nanosheets have 40% surface atom occupancy by highly-reactive surface Sn and O atoms with low coordination numbers [49]. These surface atoms could serve as adsorption centers to efficiently adsorb gaseous molecules and/or catalytically active sites, favoring the surface reactions of adsorbed species.
However, it turned out that the smaller the thickness of the nanosheets, the worse their mechanical strength and stability. Wang et al. [50] drew attention to this in their research. By studying the synthesis process of ~10 nm thick WO3 nanosheets and analyzing the parameters of gas sensors, they found that the stability of much thinner WO3 nanosheets is worse, and the removal of P123 templates was accompanied by the formation of large agglomerates, which led to a decrease in sensor response. Zhang et al. [51] also pointed out the importance of this process. They noted that sensors with highly agglomerated ZnO nanosheets have significantly poorer gas-detection performance. This is natural since the presence of free channels for the penetration of gases and an open space between the nanosheets is a prerequisite for good gas permeability of the sensitive layer, which is necessary to achieve high sensitivity and fast response [37].
Studies have shown that several approaches can be used to increase the gas permeability of the sensitive layer based on two-dimensional structures. For example, Cao et al. [52] used the surface decoration of In2O3 nanosheets with WO3 nanoparticles for this purpose. As a result, both an increase in sensitivity and an improvement in the kinetics of the sensor response were achieved. It can be assumed that the WO3 nanoparticles provided the required gap between the In2O3 nanosheets. The same approach was taken by Yin et al. [53], who showed that the response of sensors with WO3 nanosheets coated with SnO2 nanoparticles to 50 ppm acetone vapor was 10 times higher than that of sensors based on pristine WO3 nanosheets. However, it seems that a more promising approach is the use of porous 2D structures, the synthesis technology of which was considered in the previous sections.
4. Advantages of Porous 2D Nanostructures for Application in Gas Sensors
An analysis of the results obtained allows us to highlight the following advantages of porous 2D nanostructures for the development of gas sensors. At first, porous 2D materials can combine the structural advantages of both 2D and porous materials and thus exhibit outstanding chemical and physical properties [10]. Due to the porosity of the 2D materials, more boundaries and edges can be introduced, and nearly all the framework of porous 2D materials can be exposed to the surrounding gas. This means that more accessible surface area and active sites, such as steps, edges, kinks, terraces, and corner atoms with low-coordinated binding states, will be available for interaction with gas surroundings [37]. For example, it is reported that in porous Co3O4 nanosheets, the presence of pores decreased the coordination number of Co3+ atoms to unsaturated four or even three, which served as active sites for many oxidation reactions [54]. In addition, these numerous high-energy sites on the surfaces of metal oxide NFs and NSs can serve as centers for nucleation and adsorption of noble metals and other materials used to functionalize the surface and increase its catalytic activity [55,56]. Similar conclusions were made in [57] based on an analysis of the difference in gas-sensing performance between hole-rich WO3 and non-porous WO3 nanosheets. They explained the improved response of the sensor based on porous WO3 nanosheets to C2H6S3 by the participation of active-edge centers on hole-rich WO3 nanosheets in the gas-sensing effect. DFT calculations confirmed that the edge regions show higher adsorption energy and greater charge transfer for gas, and the W–S bond is formed at the edge regions due to strong chemical adsorption.
Second, pores improve the gas permeability of the formed gas-sensitive layer [37]. This means that both the access of gas molecules to the inner surface of the gas sensing layer and the mass transfer of reaction products are facilitated, which increases the efficiency of reactions occurring on the surface of metal oxides [12,37,58,59]. As is known, the mass transfer process is one of the decisive processes determining the performance of any chemical reaction.
Third, the appearance of pores in nanoflakes and nanosheets makes it possible to form a conductive network in them, which enhances the influence of the atmosphere on the resistance of 2D nanoflakes and nanosheets. This effect is associated with the formation of space charge regions around the pores, which, when the gas atmosphere changes, can also participate in the modulation of the conductivity of nanoflakes and nanosheets (see Figure 4). In this case, the maximum effect should be achieved at a distance between the pores comparable to the Debye length. This makes it possible to block the conduction channels between the pores even at nanosheet thicknesses significantly exceeding the Debye length. A similar effect is often used to explain the increased sensitivity of gas sensors based on porous metal oxide [12,37,58,60,61]. This means that high sensitivity of the sensors can be achieved even with a large thickness of nanoflakes and nanosheets, which cannot be obtained using monolithic (nonporous) nanoflakes and nanosheets. For example, Duy et al. [41], Chen et al. [62], and Choi et al. [63] have shown that gas sensors based on porous 2D nanostructures can indeed have high sensitivity even at nanosheet thicknesses of 80–100 nm.
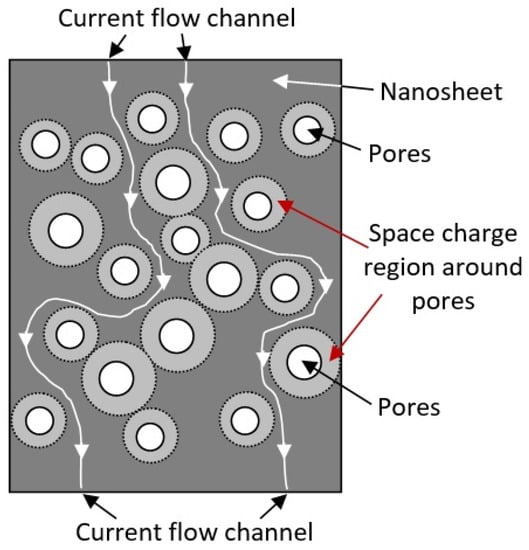
Figure 4.
Diagram illustrating the mechanism of current flow channel formation in porous two-dimensional nanostructures. When oxidizing gases are detected, the space charge regions expand, and current flow channels are blocked.
At the same time, it can be assumed that with an equal volume of pores in nanosheets, a large number of small pores is preferable for gas-sensor development, compared to a small number of large pores. The large thickness of the nanoflakes, in turn, should also improve the stability of the parameters of sensors based on them. This approach has been intensively developed in recent years. The parameters of gas sensors using porous 2D structures are given in Table S1 (Supplementary Materials). Based on the foregoing, it can be argued that the use of porous 2D materials in developing solid-state gas sensors is indeed a promising direction that can significantly increase the sensitivity and improve the rate of response of gas sensors. Of course, to achieve such a result, a corresponding optimization of the electrophysical properties and structure of 2D nanomaterials is necessary.
5. Features of Manufacturing Porous 2D Structures
5.1. Creation of Nanoholes in 2D Structures
Currently, there are two main approaches to fabricating porous 2D nanostructures: nanoflakes and nanosheets. The first approach combines methods based on creating nanoholes in already-formed 2D structures. Obtaining nanoholes in 2D structures, in this case, is carried out as a result of various post-processing treatments:
5.1.1. Reorganization of the Structure or Phase Transition
The most common is the group of methods that result in the reorganization of the structure of 2D nanomaterials or phase transition. The experiment has shown that the reorganization of the structure of a 2D material can be observed when the oxidation states of atoms that form 2D structures change or when some cations or anions from the composition of 2D nanomaterials are replaced by other cations and anions [64,65,66,67]. The first can occur during the oxidation or reduction of atoms forming 2D structures, and the second as a result of ion exchange reactions, which lead to structural-chemical deformation in the grid of chemical bonds and the formation of nanoholes. Studies have shown that changing oxidation states can be implemented for nanoflakes containing transition metals, such as V, Co, Cu, Cr, Fe, etc., with multiple stable oxidation states. For example, such a reaction was used by Hong et al. [67] in the synthesis of CoOOH NSs. In this work, for the synthesis of CoOOH NSs with a large number of holes about 1 nm in size, a simple coordination-oxidative-hydrolysis method was used. The formation of porous CoOOH NSs was facilitated by the Co(II) → Co(III) oxidation reaction occurring in a solution of an oxidation reagent H2O2.
An example of a reverse reaction is the reduction of HCoOx NSs with a solution of SnCl2 at 90 °C, proposed by Jang et al. [68]. As was established, this reaction leads to the reduction of Co(III) cations to the oxidation state of Co(II) and the oxidation of Sn(II) cations to Sn(IV). As a result, nanoholes with a size of 2–50 nm are formed in HCoOx NSs. During heat treatment of metal oxides in an atmosphere of reducing gases, such a mechanism can also be realized. In particular, Li et al. [69] observed the formation of nanoholes with a size of 30–40 nm in CoMnO4 nanosheets after thermal treatment in a mixture of hydrogen and argon at 400 °C. Li et al. [69] explain the appearance of nanoholes by the removal of some oxygen atoms from the composition of metal oxide.
As for the preparation of porous NSs using a cation exchange reaction, a good example of such a method is the process of forming porous TiO2 nanosheets based on the treatment of K-titanate films in an HCl solution [70]. As a result of this treatment, the reaction of ion exchange of K+ cations to protons and the formation of H-titanate occurs (see Figure 5). After heating the obtained samples in the air at 400 °C, the transformation of its structure and the formation of porous TiO2 NSs with the anatase crystal structure and nanoholes of 2–4 nm in size are observed. In some works, the appearance of pores during phase transition is associated with rapid kinetic and deformation stresses caused by a large mismatch between the lattices of two phases present in metal oxides [71,72].
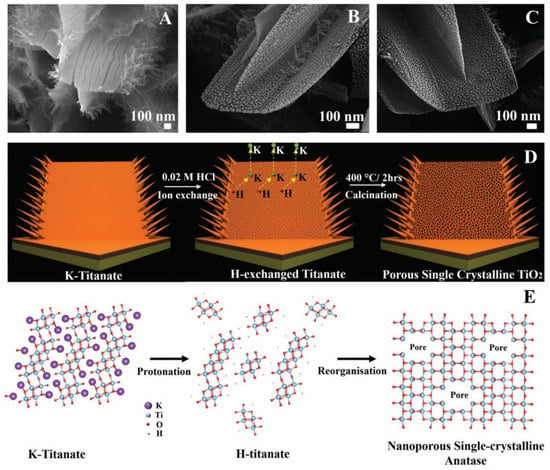
Figure 5.
Nanostructure evolution mechanism. SEM images of (A) K-titanate, (B) H-titanate, and (C) porous single-crystalline TiO2, respectively. (D) Schematic illustration of converting K-titanate to porous single-crystalline TiO2 via ion exchange followed by calcination. (E) Atomic crystal model of crystal structure changes during ion exchange and calcination processes. Reprinted with permission from Butburee et al. [70]. Copyright 2018: Wiley.
The experiment has shown that phase transition with pore formation can also occur in the process of simple thermal annealing of metal oxides. For example, Adpakpang et al. [73] have found that calcinating the layered MnO2 nanosheets in the ambient atmosphere can result in the formation of holey Mn2O3 nanosheets with mesopores (see Figure 6). The holes originate from the removal of oxygen ions during the reductive phase transition, and the pore dimensions were directly related to the heating temperature.
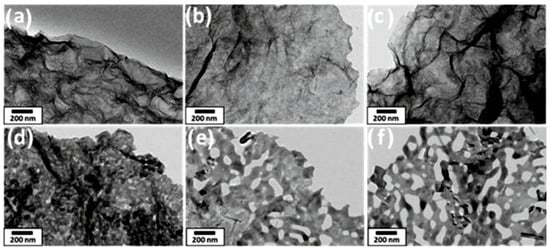
Figure 6.
TEM images of MnO2 nanosheets after annealing in air at different temperatures: (a) as-synthesized, (b) Tan = 300 °C, (c) Tan = 400 °C, (d) Tan = Tan = 500 °C, (e) Tan = 600 °C, (f) Tan = 700 °C. Reprinted with permission from Adpakpang et al. [73]. Copyright 2018: Wiley.
5.1.2. Etching of Synthesized 2D Structures
This method works most successfully when processing two-dimensional structures in a non-thermal gas plasma. An example of such treatment is the preparation of porous heterostructural Co3O4-CoO nanosheets after their treatment in cold Ar-H2 plasma [74]. With an increase in the treatment time in the Ar-H2 plasma, gradual etching of the Co3O4-CoO NSs is observed, which leads to the formation of numerous nanoholes. At first glance, the formation of holes occurs in the areas with increased defectiveness, where the etching rate is higher. However, Ar-H2 plasma treatment is also accompanied by partial oxide reduction [75], i.e., loss of lattice oxygen and defect generation. This is naturally accompanied by a violation of the symmetry and arrangement of atoms in the NSs and leads to structural changes, which should contribute to the formation of pores.
5.1.3. Chemical Dissolution of Individual Components of 2D Materials
This method is based on chemical etching, which makes it possible to remove some of the atoms of a certain type from the compound and form nanoholes due to ongoing structural changes (see Figure 7). The main problem of this method is the selection of appropriate easily-soluble components, their inclusion in metal oxides or oxyhydroxides in which pore formation is necessary, and the creation of conditions for their selective etching. At present, this approach is usually implemented by introducing Zn(II), Al(III), Ga(III), Cr(III), or Na cations into the composition of NSs at the stage of their synthesis, followed by their dissolution in an alkaline medium. An example of the implementation of this method is the process of nanopore formation in Ni(OH)2 NSs proposed by Kong et al. [76]. For chemical dissolution of Zn in Ni(OH)2:Zn, NSs were treated in 5 M NaOH solution heated to 85 °C for 30 min. As a result, nanoholes up to 5 nm in diameter were formed in such NSs. The same approach was taken by Lu et al. [77] for the formation of holey LiCoO2 nanosheets. The formation of pores occurred due to the extraction of lithium from LiCoO2 during acid etching.
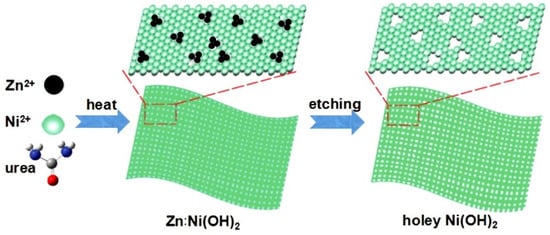
Figure 7.
Schematic illustration for the formation of clean and freestanding tiny holey 2D Ni(OH)2 nanosheets. Reprinted with permission from Kong et al. [76]. Copyright 2017: Wiley.
5.1.4. Removal of Volatile Components
Removal of volatile components from the composition of 2D structures by thermal heating in a controlled gaseous environment or vacuum is perhaps the simplest and most commonly used method [78,79]. This treatment removes water molecules and other highly-volatile components from NSs, resulting in the formation of nanoholes due to the reformatting of metal–oxygen bonds and phase transition. This means that this method, in principle, can be attributed to one of the varieties of phase transition methods. One example of this approach is shown in Figure 8. As a result of heat treatment of Cu2(OH)2CO3 synthesized by the low-temperature hydrothermal method, a dramatic change in the structure is observed, associated with the decomposition of Cu2(OH)2CO3 (reaction (1)) and accompanied by the formation of highly porous CuO nanosheets with the pore sizes around 15–30 nm [78].
Cu2(OH)2CO3 → 2CuO + H2O↑ +CO2↑
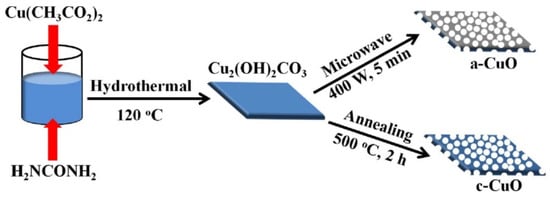
Figure 8.
The designed synthetic route of the c-CuO and a-CuO porous nanosheets. Reprinted from Tian et al. [78]. Published 2020 by Wiley as open access.
The same process occurs during the heat treatment of zinc nitrate hydroxide hydrate (ZNH) polygonal nanoflakes at 300–600 °C [62]. ZNH polygonal nanoflakes were synthesized using a microwave-assisted approach. As a result of heat treatment, uniform porous ZnO polygonal nanoflakes are formed with a thickness of about 50–90 nm, an average size of less than 900 nm, and pore size in the range of 30–60 nm. Cobalt-oxalate coordination complex (CoC2O4·2H2O), thin sheets synthesized by the hydrothermal method after thermal treatment at 400–600 °C, also transforms into porous 2D Co3O4 thin nanosheets with a pore size of 5–100 nm [80].
5.1.5. Physical Methods
There are also proposals to form pores in 2D materials by physical methods, such as high-energy light, ion, or electron-beam exposure [10]. For example, a focused ion beam (FIB) can provide high-energy heat to ‘‘drill’’ pores of a controllable size. However, it must be admitted that this method is unlikely to find application in the development of sensors for the market.
5.2. Direct Synthesis of Porous 2D Nanostructures
The second approach to the creation of porous two-dimensional structures is based on the direct synthesis of such structures. Undoubtedly, this is the most optimal approach to fabricating porous 2D structures since it excludes the multi-stage fabrication process. The most common method for the direct synthesis of porous two-dimensional structures is considered to be the hydrothermal/solvothermal method due to its high versatility, high yield, and high efficiency. The hydrothermal/solvothermal method can carefully control the crystal structure and morphology of synthesized materials, which cannot be achieved at atmospheric pressure. For example, porous ZnO [79] and Ni-Co bimetal oxide nanosheets [81] have been synthesized using this method. The one-pot solvothermal method was used by Yan et al. [82] for the synthesis of segregated ZnO-In2O3 porous nanosheets. SEM images of these nanosheets are shown in Figure 9. However, large aggregation is observed in ZnO nanosheets (Figure 9a,b). With an increase in the In2O3 content, aggregation decreases (Figure 9c,d), and in samples with the highest In2O3 content, aggregation is not observed on SEM images (Figure 9e,f). This suggests that the addition of indium ions suppresses the stacking of the nanosheets, which results in high dispersion and efficient gas diffusion inside the ZnO-In2O3 nanosheets. According to Yan et al. [82], the porous structure of the nanosheets could be attributed to the loss of volatile gas, such as H2O and CO2, during the synthesis procedure. Zhu et al. [83] reported the synthesis of a 3D hierarchical Co3O4 structure assembled by ultrathin nanosheets with a pore size of 8–10 nm via direct hydrothermal decomposition of an aqueous solution of cobalt nitrate in the presence of benzoic acid (BA). In addition to binary metal oxides, ternary materials with porous 2D structures, such as porous ultrathin BiWO6 nanosheets [84], have also been synthesized by the hydrothermal method. Although the hydrothermal method is an effective strategy for the fabrication of porous 2D materials, continuous efforts should still be made to investigate the corresponding working mechanism of hydrothermal treatment as well as the influence of processing conditions on the microstructure and morphology of porous 2D materials for precise control. For example, Li et al. [85] have found that during the hydrothermal synthesis of SnO2 flowerlike nanostructures, the semi-blooming nanoflowers assembled by high-yield and uniform-ordered mesoporous nanosheets are formed after calcination only when the reaction time is extended to 12 h. When the hydrothermal time is less than 12 h, the product shows a prototype of flowerlike architectures comprised of relatively uniform nanosheets. If the hydrothermal time increases to 16 h, flowerlike hierarchical nanostructures are destroyed into irregular nanosheets.

Figure 9.
SEM images of ZnO-In2O3 porous nanosheets of various compositions synthesized by solvothermal method at different magnifications: (a,b) –Zn2+/In3+ = 10:0 M ratio; (c,d) Zn2+/In3+ = 10:2 M ratio, and (e,f) Zn2+/In3+ = 10:6 M ratio. Reprinted with permission from Yan et al. [82]. Copyright 2021: Elsevier.
In addition, it should be kept in mind that, in most cases, the formation of porous metal oxide 2D nanostructures requires annealing at temperatures of 300–500 °C at the final stage to transfer hydroxyls to the metal oxide phase [79,81,85,86]. For example, Ryu et al. [87] synthesized ZnO nanosheets by the hydrothermal method and found that the nanosheets became highly porous only after calcination at 350 °C for 5 h. This means that the formation of pores in such structures often occurs according to the mechanism corresponding to the phase-transition method. For example, Sharma et al. [86] showed that the formation of porous NiMoO4 nanosheets requires annealing at 400 °C in the presence of air, during which there is a transition from NiMoO4·xH2O, which has a 3D honeycomb structure, to NiMoO4 porous nanosheets, which have 3D rectangular structures. When using other synthetic methods, such as sol-gel [88], electrodeposition [89], chemical bath deposition [90], and wet chemical method [91], designed for the synthesis of ZnO, WO3, SnO2, Co3O4, In2O3, NiMoO4, CeO2, and other metal oxide-based porous 2D structures, post-processing high-temperature annealing is also generally required.
Of course, there are other methods for the direct synthesis of porous 2D materials [92,93,94]. For example, Wang et al. [93] synthesized Co0.75Ni0.25(OH)2 nanosheets with numerous nanopores by pulsed laser ablation in liquid (see Figure 10). Pulsed laser ablation can also be used to form nanocomposites, such as ZnO NSs-CNT [87]. Such hybrids can be promising for the formation of NSs-based gas-sensitive layers with increased gas permeability. CNTs prevent nanosheets from agglomerating.
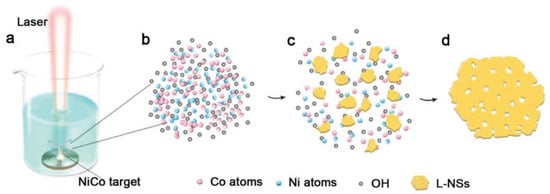
Figure 10.
The formation of L-NSs. (a) Laser ablation of CoNi alloy target in 1 m KOH solution, (b) mixed vapor of cobalt, nickel, and OH−, (c) formation of hydroxide debris in the vapor, (d) formation of L-NSs via orientation attachment. Reprinted with permission from Wang et al. [93]. Copyright 2019: Wiley.
Zhu et al. [94] suggested using direct calcination of an Sn-based metal-organic framework that was prepared via a facile wet chemistry method with deionized water as solvent at 80 °C to form porous SnO2 nanosheets intended for fabricating highly-sensitive formaldehyde sensors. The porous SnO2 nanosheets are found to be constructed by the interconnection of ultrafine nanoparticles with sizes of 4–9 nm, comparable to the Debye length of SnO2. The synthesized SnO2 nanosheets demonstrated a relatively narrow pore-size distribution of 2–12 nm, with a peak occurring at 7 nm.
There are also attempts to form porous 2D structures using template methods [72,95,96]. For example, porous 2D metal oxides can be obtained by depositing metal oxides on a porous hard template and then removing it. As a hard-templating material, porous silicon or Al2O3 can be used, the porosity of which will also determine the porosity of the formed 2D metal oxide. However, this method is laboratory in nature and is unacceptable for large-scale application. Huang et al. [97] have taken a different approach to preparing porous 2D nanostructures. Cross-linked porous tin dioxide nanosheets were obtained using three-dimensional reduced-graphene oxide as a hard template followed by an annealing process at 550 °C in the air for 4 h (Figure 11). During this annealing, SnS2@rGO precursor is transformed in semiconductor metal oxide nanostructures with large specific surface areas (77.4 m2/g), high porosity, and, as a result, improved gas-sensing characteristics. The pore size was in the range of 2–28 nm.
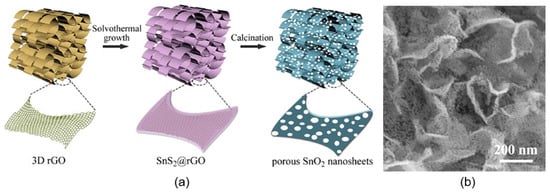
Figure 11.
(a) Schematic diagram of the synthesis procedure and (b) SEM image of porous SnO2 nanosheets. Reprinted with permission from Huang et al. [97]. Copyright 2022: Elsevier.
As for the soft-template method, it is more suitable for practical use. The soft-template method has distinct advantages in preparing porous-structured 2D nanomaterials because it is flexible for controlling the morphology of prepared materials. In particular, ZnO mesoporous single-crystal nanosheets (ZnO-MSN) with exposed {0001} polar facets and pore size ~35 nm were synthesized using this method [98]. Soft templates were 250 nm monodispersed colloids of poly(styrene-methyl methacrylate-sulfo propyl methacrylate potassium) (P(St-MMA-SPMAP)) (see Figure 12).
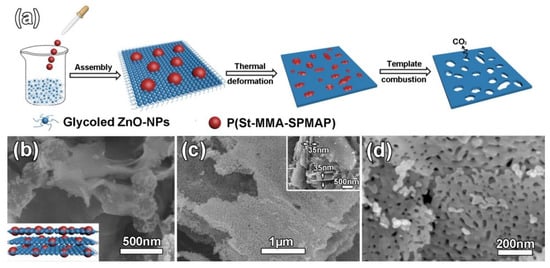
Figure 12.
(a) Schematic illustration of the ZnO-MSN synthesis, (b) SEM images of the layer assembled ZnO/P(St-MMA-SPMAP) composites with the inset showing the perspective view of schematic image, (c) low magnification SEM image with the inset showing the thickness of ZnO-MSN and (d) high magnification SEM image of ZnO-MSN. Reprinted with permission from Liu et al. [98]. Copyright 2016: Elsevier.
6. Performances of Gas Sensors Based on Porous 2D Nanostructures
As shown in Table S1, porous nanosheets and nanoflakes of various materials, from binary metal oxides to complex compounds, such as LaFe1-xPxO3-δ [99], have been tested for the manufacture of gas sensors. However, the main 2D materials used to manufacture gas sensors are still metal oxides such as ZnO [39,100,101], WO3 [90], In2O3 [102,103], SnO2 [88,104], CuO [105], Co3O4 [106], NiO [107], CeO2 [108], LaFeO3 [55], and TiO2 [56] which have physical—chemical properties most suitable for work in aggressive environments [15]. As a rule, gas sensors were fabricated using conventional ceramic and thick-film technology [13]. The maximum sensitivity of the developed sensors, depending on the material used, its structural parameters, and the nature of the detected gas, is observed in the temperature range from RT [102] to 400 °C [41,109]. The operation temperature depends on the semiconductor used and its structural condition. However, as a rule for most sensors, the maximum sensory response to reducing gases is observed at temperatures of 200–340 °C [88,97,110], and to oxidizing gases, at a temperature of 100–200 °C [62,90]. This difference is associated with a different mechanism of sensory response to these gases and is typical of all metal oxide sensors [15,111,112].
The best examples of gas sensors developed on the basis of porous 2D structures also have a fast response and an increased sensitivity to various gases that exhibit both reducing (CO, H2S, NH3) [113,114] and oxidizing properties (O3, NO2) [115,116]. High sensitivity is also shown to the vapors of organic solvents [51,101,117]. Although metal oxide sensors are not selective-gas sensors (see Figure 13a), under certain conditions, sensors based on 2D materials, as well as conventional metal oxide gas sensors, can exhibit good selectivity to gases (Figure 13b,c), such as O3, NO2, H2S, which have specific properties [15,118,119]. Zhang et al. [39] reported that their ZnO-based sensors developed using porous 2D nanostructures had high selectivity to ethanol (Figure 13d). However, the mechanism of such selectivity is unclear since similar ZnO-based sensors developed in other laboratories did not have such a pronounced selectivity to ethanol [19,62,120]. This suggests that the technology for the synthesis of nanosheets and nanoflakes plays an important role in the formation of their gas-sensitive properties, and this role needs to be studied.
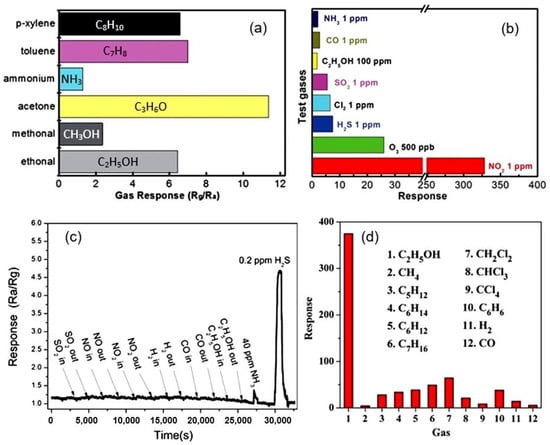
Figure 13.
(a) The conductometric responses of sensors based on porous 2D Co3O4 nanosheets to several reducing gases with concentrations of 100 ppm at 150 °C. Reprinted with permission from [106]. Copyright 2015: RSC; (b) Cross-responses of the sensor based on 2D porous In2O3 nanoflakes to various test gases at 140 °C. Reprinted with permission from [116]. Copyright 2015: RSC; (c) Response and recovery curves of the porous CuO nanosheet-based gas sensors to difference gases (SO2, NO, NO2, H2, CO, C2H5OH, NH3) of 40 ppm and 0.2 ppm H2S at room temperature. Reprinted with permission from [105]. Copyright 2016: ACS; (d) The cross-response of the ZnO-based sensor to ethanol and other 11 interfering gases at 400 °C. Reprinted with permission from [39]. Copyright 2012: Elsevier.
The important role of technology in the synthesis of 2D structures used to develop gas sensors is evidenced by the data presented in Table S1. They show that even when using the same synthesis method, depending on the used precursors, solvents, temperature, and time regimes of synthesis, the parameters of sensors can differ radically. Studies performed by Chen et al. [62] and Fan et al. [120] showed that in addition to the synthesis conditions, in order to achieve the best parameters of gas sensors, it is also necessary to control the temperature regimes of annealing of already-synthesized 2D structures (see Figure 14a). This is quite understandable since such heat treatment is accompanied by a change in the morphology and size of the crystallites that form nanoflakes or nanosheets (Figure 14a) and the size of the pores formed in them (Figure 14b).
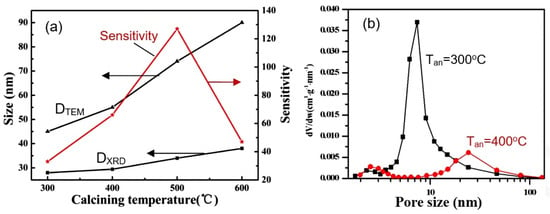
Figure 14.
(a) Effect of annealing on crystallite size (DTEM and DXRD) and sensitivity of sensors based on porous ZnO nanoflakes. Reprinted with permission from [62]. Copyright 2011: RSC; (b) Pore−size-distribution curves of 3D hierarchical ZnO porous structure after annealing at 300 °C and 400 °C. Reprinted with permission from [120]. Copyright 2015: Elsevier.
However, as follows from the results presented in [121], it is not always clear what determines the change in sensor parameters—a change in the size and density of pores or a change in the electrical and surface properties of metal oxides under the influence of heat treatment. In particular, Feng et al. [121] found that the maximum response of sensors based on porous NiO nanosheets to N2H4 at 92 °C was observed after annealing at 700 °C. At the same time, if at a temperature of 500 °C the nanosheets had a mesoporous structure with a large number of pores, then, as the calcination temperature increased to 600 °C, the pore size on the surface of the NiO nanosheets became larger. When the calcination temperature reached 700 °C, the pore size became much larger, and only a few large pores remained on the surface of the nanosheets. Upon reaching the calcination temperature of 800 °C, the NiO nanosheets melted and were destroyed. The described behavior of the nanosheet morphology indicates that the observed change in the parameters of sensors based on NiO NSs during annealing is more consistent with the effect of heat treatment on the bulk and surface properties of NiO.
Measurements performed in [40,94,100] showed that sensors based on porous 2D nanostructures, as well as conventional metal oxide-based gas sensors, with appropriate fabrication optimization [122,123], are characterized by good temporal stability of parameters (see Figure 15). All this indicates that porous 2D nanostructures are indeed promising materials for the development of gas sensors for various applications.
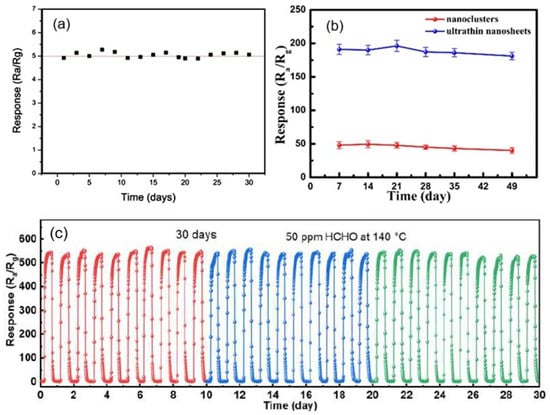
Figure 15.
(a) Long-term stability of the porous CuO nanosheet-based gas sensor exposed to 200 ppb H2S gas at room temperature. Reprinted with permission from [40]. Copyright 2016: ACS; (b) Long-term stability of the response of ZnO-based sensor toward acetylacetone at 340 °C. Reprinted with permission from [100]. Copyright 2019: ACS. (c) Durability of SnO2 porous nanosheet-based sensor of formaldehyde (HCHO). Reprinted with permission from Zhu et al. [94]. Copyright 2021: Elsevier.
As for the effect of air humidity on the parameters of sensors based on porous two-dimensional nanomaterials, the results presented in various articles [92,94,124,125,126] show that, similar to conventional metal oxide gas sensors, they are affected by humidity. This effect depends on the metal oxide used, the type of gas detected, the operating temperature, and the side effects. In particular, the mesoporous structure contributes to the condensation of water vapor in nanopores even at a sufficiently low humidity level. Production technology is also of great importance. For example, Spagnoli et al. [124] showed that WO3 NSs have less effect on film conductivity, while according to Liu et al. [125], WO3 NS-based sensors showed a noticeable effect of humidity on sensor performance. The influence of humidity on the properties of metal oxides and sensors based on them is considered in sufficient detail in [127,128].
The typical effect of humidity on the conductometric response of various 2D-based sensors is shown in Figure 16a. It can be seen that the influence of humidity can be significant, and this influence cannot be neglected if the sensor is designed to measure gas concentration. However, the experiment showed that with appropriate calibration and independent measurement of the air humidity, the effect of humidity could be compensated by the electronic method [123]. If the structure and composition of the gas-sensitive material are optimized to reduce the influence of humidity (Figure 16b), and the sensor is used in alarm systems, then, with sufficient sensitivity of the sensors to the detected gas, the influence of humidity can be neglected. This is exactly the situation we have with the CeO2/SnO2 sensors developed by Zhu et al. [94]. It is believed [94] that the humidity-independent property of CeO2/SnO2 sensors is mainly attributed to the presence of cerium oxide. CeO2 nanoparticles can be considered an absorber of hydroxyl groups due to a facile redox reaction (Ce3+/Ce4+) [129,130].
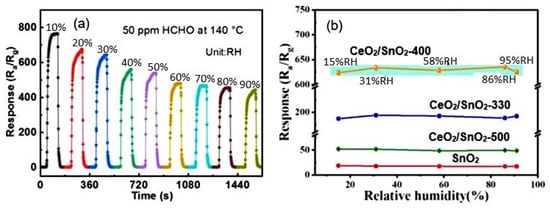
Figure 16.
(a) Response-recovery curves of SnO2 porous nanosheet-based sensor to HCHO under different RH. Zhu et al. [94]. Copyright 2021. Elsevier. (b) Relationship between relative humidity and response of porous CeO2-SnO2 nanosheet-based sensor towards 3-hydroxy-2-butanone, a biomarker for pathogenic bacterium Listeria monocytogenes: 330, 400, and 500 correspond to the annealing temperature. Reprinted with permission from Yang et al. [92]. Copyright 2022: Elsevier.
It is important to note that, in the fabrication of sensors based on porous 2D nanomaterials, all approaches developed earlier for optimizing the parameters of conventional metal-oxide gas sensors can be used: namely, doping of metal oxides or surface modification with catalytically-active noble metals [56,91,131], metal oxides [52,53,132], II-VI compounds [126], and even phosphorus [133]. Just as in the case of conventional metal oxides [119,134], these approaches provide a significant improvement in sensor performances, such as sensitivity, selectivity, and response and recovery times. For example, Figure 17 shows the influence of surface modification with Pd on the conductometric response of sensors based on 2D porous ZnO nanosheets. It is seen that surface modification strongly increases response and improves selectivity to CO. At the same time, it should be borne in mind that the achievement of the desired result is possible only at a certain concentration of dopants, specific for each metal oxide and doping element, and usually does not exceed a few percent [126,135,136]. Deviation from this value is accompanied by a drop in sensor response (see Figure 18). Moreover, such behavior is observed both during surface modification and bulk doping with noble metals [55,56] and transition metals [133,136]. For example, according to Wang et al. [56], the maximum response of a porous TiO2 nanosheet-based sensor to H2 was observed when the amount of Pd loaded during impregnation was 1.5 wt%. Even with phosphorus doping of the LaFe1-xPxO3-δ compound, to achieve the maximum sensory response, the phosphorus concentration should not exceed 1% [99]. The mechanism of such influence is described in [119,134,137,138,139]. If, however, we are applying the second gas-sensitive oxide to the surface of the already formed porous metal-oxide nanosheets, as was done in [140], then completely different patterns can be observed here. For example, in gas sensors based on the SnO2 nanorods/2D NiO porous nanosheet composite, the maximum sensory response was observed at a Ni:Sn ratio of 1:0.5 [140]. When developing sensors based on a composite of mesoporous ZnO/Co3O4 nanosheets, the optimal Zn:Co atomic ratio is 1:0.15 [141].
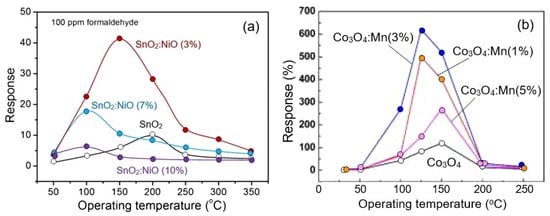
Figure 17.
(a) Response versus operating temperature of gas sensors based on Pd-modified porous ZnO nanosheets to different gases; (b) Response of Pd/ZnO and ZnO-based sensors to different gases at 220 °C. Reprinted with permission from [143]. Copyright 2012: Elsevier.
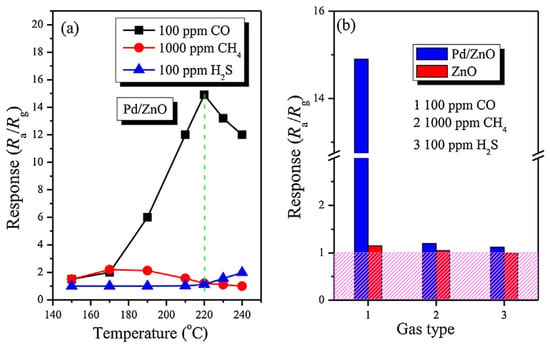
Figure 18.
(a) Response of sensors based on SnO2 and SnO2 doped with NiO nanoflowers to 100 ppm formaldehyde. Adapted with permission from [136]. Copyright 2018: Elsevier. (b) The responses of bare Co3O4 and Co3O4 nanosheets doped with Mn to 100 ppm CO at different temperatures. Reprinted with permission from Qin et al. [133]. Copyright 2022: Elsevier.
Porous nanoflakes can also be used to make hybrid nanocomposites. For example, Bai et al. [142] suggested using CuO nanoflakes/rGO nanosheet composites for the detection of NO2. The composite was synthesized via an ultra-low-cost hydrothermal process with a thermal treatment. The gas sensor based on the as-synthesized CuO/rGO was fabricated by the spin-coated method and applied to detect NO2 gas at room temperature (23 °C). The sensor exhibited a conductometric response of approximately 40 towards 5 ppm NO2, and the limit of detection was down to 50 ppb.
7. 3D nanostructures Based on Porous 2D Nanosheets and Nanoflakes
The next step in optimizing the parameters of gas sensors based on 2D porous nanoflakes and nanosheets is the development of 3D structures based on them. 3D structures can have a wide variety of shapes: nanoflowers and various hierarchical structures (see Figure 19). It should be noted that the structure of the gas-sensitive layer formed in this way favorably differs from the structure of the gas-sensitive layer formed using traditional thick-film technology [37,144]. If mesoporous or microporous structures are formed as a result of using traditional gas-sensor technology, then 3D structures formed by aggregation of 2D nanosheets and nanoflakes are macroporous. Such structures are optimal for gas sensors since a nanoscale network system formed provides maximum porosity and free gas access to almost all elements of the gas-sensing layer, which facilitates fast and effective gas adsorption onto the entire sensing surface, thereby providing a significant improvement in sensitivity and a reduction in response time.
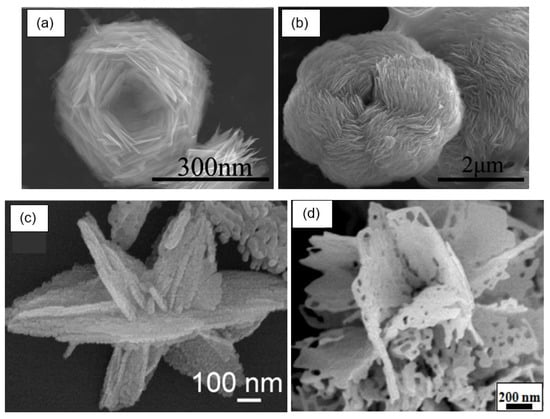
Figure 19.
Varieties of 3D structures: (a,b) SEM images of nestlike 3D ZnO porous structures synthesized at different temperatures. Reprinted with permission from [145]. Copyright 2012: ACS; (c) FE-SEM images of In2O3 hierarchical nanostructure. Reprinted with permission from [102]. Copyright 2012: Elsevier; (d) SEM image of porous flowerlike SnO2 nanostructures. Reprinted with permission from [146]. Copyright 2015: Elsevier.
However, despite the extremely large macroporosity of such structures, which is preferable for achieving the maximum efficiency of gas sensors, the formation of pores in nanosheets and nanoflakes, even in this case, gives a positive result, which manifests itself in an increase in the sensory response (see Figure 20). Apparently, this growth occurs due to an increase in the number of active centers formed during the formation of pores [10,54]. For example, density-functional theory (DFT) calculations for porous MoO3 nanosheets designed to detect H2S show that Mo atoms at the pore edges exhibit higher adsorption activity for H2S and O2 molecules [147]. Xie et al. [148] and Zhang et al. [39] also believe that mesopores in 2D ZnO nanoflakes contributed to the increased sensitivity of sensors based on 2D ZnO nanoflowers to ethanol. Due to the porosity of the nanoflakes, successful detection of ethanol has been demonstrated down to 0.1–1 ppm. The same conclusion was reached by Cao et al. [149]. Moreover, Cao et al. [149] showed that such sensors could have a very fast response and recovery (see Figure 20d). The increased selectivity of sensors based on In2O3 nanoflakes to methanol is also explained by the presence of mesopores in [150] (see Figure 20c).
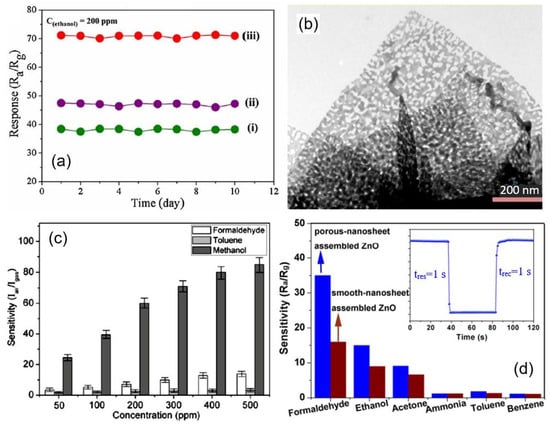
Figure 20.
(a) Repeatability test of the (i) blooming, (ii) semi-blooming, and (iii) mesoporous semi-blooming SnO2 nanoflowers-based sensors towards 200 ppm ethanol at optimal operating temperature. (b) TEM images of mesoporous semi-blooming SnO2 nanoflowers with an average pore size of 31 nm. Reprinted with permission from Li et al. [85]. Copyright 2016: Elsevier; (c) Sensor responses of the gas sensor based on porous In2O3 nanoflowers toward formaldehyde, toluene, and methanol. Reprinted with permission from Liu et al. [150]. Copyright 2010: ACS; (d) responses of porous-nanosheet assembled ZnO and smooth-nanosheet-assembled ZnO-based sensors to 100 ppm different testing gases at 260 °C. The inset is the response transient of porous-nanosheet-assembled ZnO nanoflowers to 100 ppm HCHO at 260 °C. Reprinted with permission from Cao et al. [149]. Copyright 2017: Elsevier.
However, it should be kept in mind that not every 3D flower-based aggregation of nanosheets is optimal for gas sensors. For example, Juang et al. [151] designed resistor-type ammonia gas sensors with two architectures of porous NiO nanosheets—spherically assembled and dispersed nanosheets (Figure 21a,c,d)— and established the devices with dispersed porous NiO nanosheets exhibited higher and faster sensing response (Figure 21b). Apparently, the spherically-assembled architecture was too dense.
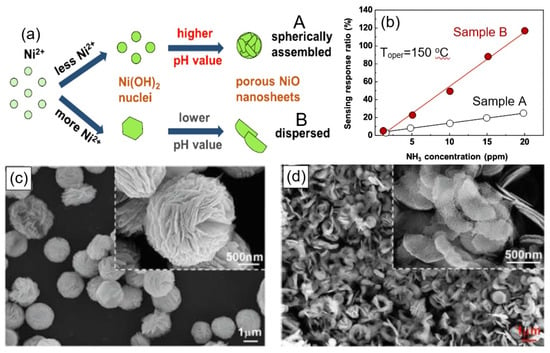
Figure 21.
(a) Growing mechanism of spherically-assembled (A) and dispersed porous NiO nanosheets (B). (b) Sensing-response ratio to different ammonia gas concentrations for samples A and B. (c,d) SEM images of (c) spherically-assembled porous NiO nanosheets and (d) dispersed porous NiO nanosheets. Reprinted with permission from Juang et al. [151]. Copyright 2022: Elsevier.
It should be noted that the porosification of NFs and NSs-based 3D structures can be organized using the same methods that were developed for the porosification of NFs and NSs and described earlier in Section 5 [150,152]. For example, Liu et al. [150] prepared a porous In2O3 nanoflower-based structure using the phase-transformation method, namely oxidation at 350–550 °C of In2S3 nanoflower synthesized via a hydrothermal route (Figure 22a). Since the radius of S atoms (0.109 nm) is larger than that of O atoms (0.065 nm), structural parameters, such as bond lengths and angles, change during the oxidation of In2S3, which manifests itself in the substitution of S by O atoms. In this condition, internal tensile stress in the crystal is formed because of the distortion of the crystal lattice. It will be released through the breakup of the crystal structure, leading to the formation of the porous structure (Figure 22c). The average pore size in such nanoflakes was around 20 nm. The same approach was used by Li et al. [153] for preparing MoO3 microporous nanoflowers: MoSe2 nanoflowers were calcined under an oxygen atmosphere at 400 °C for 3 h to be fully oxidized. Jing and Zhan [154] used annealing at 400 °C for 2 h of Zn5(CO3)2(OH)6 monoclinic hydrozincite nanoplates with an edge thickness of about 19 nm to fabricate porous ZnO nanoplates with a pore size of 20–80 nm. According to Jing and Zhan [154], during the thermal decomposition of Zn5(CO3)2(OH)6, a phase transition occurs to ZnO, in which zinc octahedra are destroyed and reorganized into tetrahedra. Therefore, they believe that the reorganization of the crystal structure and the release of water and carbon dioxide from the Zn5(CO3)2(OH)6 nanoplates are the reasons leading to the formation of holes in ZnO nanoplates and a decrease in their thickness.

Figure 22.
(a) FESEM image of the as-synthesized In2S3 precursors, (b) FESEM image of the products (In2O3) obtained by oxidizing In2S3 precursors. (c) HRTEM images of In2O3 nanoflakes. Reprinted with permission from Liu et al. [150]. Copyright 2010: ACS.
Optimization of the parameters of gas sensors based on porous 3D flowerlike metal oxides is also carried out using the approaches that were described earlier in relation to nanoflakes and nanosheets. For example, Li et al. [153] used atomic layer deposition (ALD) of Rh onto ZnO flowerlike nanostructures to optimize sensory response to trimethylamine (TMA). The ZnO nanostructures composed of porous nanosheets were prepared by a hydrothermal method. The maximum sensory response was observed after 10 Rh deposition cycles. It was suggested that the surface modification with Rh promotes an increase in the concentration of chemisorbed oxygen on the surface of ZnO nanosheets, which is extremely important in the detection of reducing gases. The surface modification with Rh has also been found to reduce the effect of humidity on the sensors parameters (see Figure 23), which is of paramount importance for their operation in a typical atmosphere with varying humidity levels.

Figure 23.
Response of (a) unmodified and (b) modified with Rh ZnO flower-based sensor to 10 ppm TMA at 220 °C under different RH. Reprinted with permission from Li et al. [153]. Copyright 2022: Elsevier.
Chen et al. [155] used bulk doping of ZnO with Fe (0%, 0.5%, 1%, and 3%), carried out during hydrothermal synthesis, to optimize the gas-sensitive properties of ZnO flowerlike nanostructures. They found that in order to achieve the maximum sensory response to acetone (~105 at 100 ppm) in the temperature range of 250–450 °C, the concentration of Fe should not exceed 0.5%. Otherwise, the sensory response was significantly reduced (Figure 24a). Under these conditions, the pores in the nanosheets had a maximum diameter (~20 nm), and ZnO was characterized by the maximum concentration of oxygen vacancies and the narrower band gap. As for the BET surface area, it increased with increasing Fe concentration from 21.2 m2/g (0%Fe) to 65.7 m2/g (3%Fe) and was not a factor determining the magnitude of the sensory response. Zhang et al. [156], to optimize the conductometric response of 3D In2O3 microflower-based sensors to NO2, proposed to modify the surface of porous In2O3 nanosheets with MoS2. The results showed that the In2O3/MoS2 composite-based sensor at room temperature exhibits a response as high as 343 at 5 ppm NO2, which is 309 and 73 times higher than the response of the sensors based on the pristine MoS2 and In2O3. In addition, the sensors had better selectivity to NO2. Improving the sensory properties of the In2O3/MoS2 composite-based sensor, Zhang et al. [156] explained by (i) the presence of an electron depletion layer at the interface of In2O3 and MoS2, (ii) the highly exposed edges of MoS2 to provide more active sites, and (iii) 2D/3D hybrid structure to improve the adsorption-desorption process of NO2. However, one should always keep in mind that the improvement of some properties may be accompanied by the deterioration of others [37,157]. In particular, it turned out that the In2O3/MoS2 composite-based sensor exhibited increased sensitivity to air humidity (Figure 24b). This naturally complicates the use of such sensors since in order to avoid the effect of humidity on the sensor, it is necessary either to first remove moisture from the gases or to use a complex electronic system to compensate for the effect of humidity.
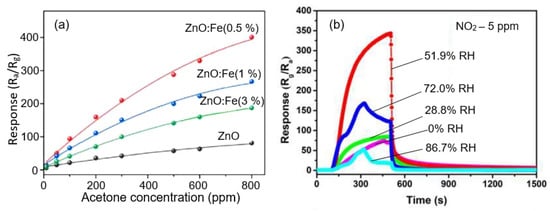
Figure 24.
(a) The response of sensors based on pure ZnO and 0.5%, 1%, and 3% Fe–ZnO to 50 ppm acetone at 365 °C. Reprinted with permission from Chen et al. [155]. Copyright 2022: Elsevier; (b) Dynamic response curves of the In2O3/MoS2 sensor to 5 ppm NO2 in various humidity at RT. Reprinted with permission from Zhang et al. [156]. Copyright 2022: Elsevier.
Despite the macroporosity of flowerlike structures, it is believed that the most optimal 3D nanoflakes-based structure for developing gas sensors is still the structure with vertical walls shown in Figure 25b. In such structures, unlike nanoflower-based structures, there is no nonporous core, which may not participate in gas-sensing effects. Studies have shown that the structures can show the highest sensitivity and response rate when interacting with a gas environment [158,159]. At nanoflakes thicknesses comparable with the Debye length, the change in the resistance of a gas-sensitive layer having such a structure will be controlled not only by the resistance of inter-nanoflakes contacts but also by the resistance of the nanoflakes themselves. In particular, if the thickness of the nanosheets of n-type conductivity is less than twice the Debye length, then the whole nanosheet body will be almost electron-depleted in air, which will result in significantly enhanced sensor response based on the grain-control model [60] during interaction with reducing gases. As known, reducing gases reduce surface band bending due to interaction with chemisorbed oxygen [111]. When detecting oxidizing gases, this condition is unacceptable since the sensory response will be insignificant in this case. Under these conditions, an increase in the surface potential caused by interaction with oxidizing gases [112] will not be accompanied by a noticeable increase in the resistance of NSs and NFs. To achieve a significant effect in this case, the thickness of NFs and NSs must be greater than twice the Debye length, and complete blocking of the current-flow path through NFs and NSs must be observed only when an oxidizing gas appears (see Figure 3). Experimental confirmation of the influence of the thickness of porous nanosheets and nanoplates on the sensor response can be found in [160] in relation to ZnO-based gas sensors.
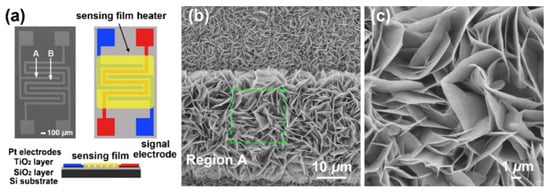
Figure 25.
(a) Schematic diagrams of the different views for the sensor platform; (b,c) FE-SEM images of ZnO nanosheets (NSs) having a 3D structure taken from different regions in the sensor platform. Reprinted with permission from [161]. Copyright 2012: Elsevier.
Gas-sensitive layers with the structure shown in Figure 25b are usually grown directly on the surface of the platform used to fabricate the gas sensor [161,162,163,164]. For example, in [162,163,164], NiO, Co3O4, and CuO nanosheets were synthesized directly on an Al2O3 tube, inside which a heater was subsequently mounted, and in [161,164], ZnO and NiO nanosheets were synthesized directly on a substrate with already-formed contacts (see Figure 25a). However, the technology proposed in these works is poorly consistent with large-scale sensor production. If such structures are synthesized separately and then attempted to be transferred to a sensor platform, then the probability of preserving such structures in the gas-sensitive layer is minimal. In this regard, 3D structures in the form of nanospheres and nanoflowers are more likely to retain their structure in manufacturing sensors based on the principles of thick-film technology. Therefore, it must be recognized that, despite the progress made, the efficient integration of 2D nanoflakes and nanosheets, especially in the form of 3D structures, with a sensor platform remains a serious problem that limits the use of such nanostructures in the development of gas sensors [165].
In addition, it must be borne in mind that excessive porosity can cause a decrease in the mechanical strength of the gas-sensitive layer. Therefore, the process of nanoflakes and nanosheet porosification must be optimized to achieve a porosity that provides maximum sensor response while maintaining the mechanical strength and stability of the formed gas-sensitive layer. This primarily applies to 3D structures intended to manufacture gas sensors using thick-film technology. Unfortunately, research in this direction has not yet been carried out.
8. Summary
Our analysis shows that the porosification of 2D materials, such as nanoflakes and nanosheets, is an effective way to improve the performance of gas sensors. However, the use of porous structures, in addition to complicating the process of manufacturing gas-sensitive materials, introduces additional uncertainty in achieving the required sensor parameters. Sensor parameters become dependent not only on the structural and electrophysical parameters of 2D nanoflakes and nanosheets but also on the density of nanopores in NSs and NFs and their diameter, which will certainly be accompanied by a decrease in the reproducibility of the parameters of sensors based on such materials. However, we did not find any studies aimed at establishing the influence of pore parameters in nanoflakes and nanosheets on gas-sensitive effects and sensor parameters. This can become a significant obstacle to using porous 2D structures in developing gas sensors intended for the market. The lack of systematic studies aimed at a more detailed and deep understanding of the relationship between synthesis conditions, the structure of 2D nanoflakes, and the performances of gas sensors based on such materials also hinder progress in this direction. Unfortunately, research in this area is mostly demonstrative—what is obtained as a result of synthesis is then tested without proper optimization of the process and attempts to understand why these results were obtained and what needs to be done to improve them.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13020237/s1, Table S1: Parameters of gas sensors based on porous 2D metal oxide nanostructures.
Author Contributions
G.K.—conceptualization, methodology, data analysis, visualization, writing—original draft preparation and editing. V.P.T.—writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Program of the Republic of Moldova (project 20.80009.5007.02).
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declare no conflict of interest.
References
- Hernandez-Ramírez, F.; Tarancón, A.; Casals, O.; Arbiol, A.J.; Rodríguez, R.; Morante, J.R. High response and stability in CO and humidity measures using a single SnO2 nanowire. Sens. Actuators B 2007, 121, 3–17. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Kolmakov, A. Some recent trends in the fabrication, functionalisation and characterisation of metal oxide nanowire gas sensors. Int. J. Nanotechnol. 2008, 5, 450–474. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Mathur, S.; Kolmakov, A. Metal Oxide Nanomaterials for Chemical Sensors; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Electrospun metal oxide nanofibers and their conductometric gas sensor application. Part 2: Gas sensors and their advantages and limitations. Nanomaterials 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Electrospun metal oxide nanofibers and their conductometric gas sensor application. Part 1: Nanofibers and features of their forming. Nanomaterials 2021, 11, 1544. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhao, X.; Wang, H.; Xu, H.; Xie, L.; Zhao, C.; Chen, L. Novel two-dimensional porous materials for electrochemical energy storage: A mini review. Chem. Rec. 2020, 20, 922–935. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Gowda, C.C.; Mahapatra, P.L.; Mukherjee, M.; Malviya, K.D.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.M.; Jariwala, D.; et al. Emerging 2D metal oxides and their applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: A review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Sysoev, V. Chapter 3: Conductometric metal oxide gas sensors. In Chemical Sensors: Comprehensive Sensor Technologies. Solid State Devices; Korotcenkov, G., Ed.; Momentum Press: New York, NY, USA, 2011; Volume 4, pp. 53–186. [Google Scholar]
- Barsan, N.; Schierbaum, K. (Eds.) Gas Sensors Based on Conducting Metal Oxides; Elsevier Metal Oxide Series edited by G. Korotcenkov; Elsevier: Cambridge, MA, USA, 2018. [Google Scholar]
- Korotcenkov, G. Metal oxides for solid state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Rajkumar, K.; Rajendra Kumar, R.T. Gas sensors based on two-dimensional materials and its mechanisms. In Fundamentals and Sensing Applications of 2D Materials; Rout, C.S., Late, D., Morgan, H., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 205–258. [Google Scholar]
- Chen, X.; Liu, J.; Jing, X. Self-assembly of ZnO nanosheets into flowerlike architectures and their gas sensing properties. Mater. Lett. 2013, 112, 23–25. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-dimensional nanomaterials for gas sensing applications: The role of theoretical calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.G. Two-dimensional zinc oxide nanostructures for gas sensor applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar] [CrossRef]
- Neri, G. Thin 2D: The new dimensionality in gas sensing. Chemosensors 2017, 5, 21. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-L.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D. Recent developments in 2D nanomaterials for chemiresistive-type gas sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Ge, L.; Mu, X.; Tian, G.; Huang, Q.; Ahmed, J.; Hu, Z. Current applications of gas sensor based on 2-D nanomaterial: A mini review. Front. Chem. 2019, 7, 839. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Bhat, A.; Rout, C.S. Fundamentals and properties of 2D materials in general and sensing applications. In Fundamentals and Sensing Applications of 2D Materials; Rout, C.S., Late, D., Morgan, H., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 5–24. [Google Scholar]
- Shinde, P.V.; Saxena, M.; Singh, M.K. Recent developments in graphene-based two-dimensional heterostructures for sensing applications. In Fundamentals and Sensing Applications of 2D Materials; Rout, C.S., Late, D., Morgan, H., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 407–436. [Google Scholar]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent advances in emerging 2D material-based gas sensors: Potential in disease diagnosis. Adv. Mater. Interfaces 2019, 2019, 1901329. [Google Scholar] [CrossRef]
- Dral, A.P.; ten Elshof, J.E. 2D metal oxide nanoflakes for sensing applications: Review and perspective. Sens. Actuators B 2018, 272, 369–392. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zeng, W. Preparation and application of 2D MXene-based gas sensors: A review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent progress of nanostructured sensing materials from 0D to 3D: Overview of structure–property-application relationship for gas sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Ji, F.; Ren, X.; Zheng, X.; Liu, Y.; Pang, L.; Jiang, J.; Liu, S. 2D-MoO3 nanosheets for superior gas sensors. Nanoscale 2016, 8, 8696–8703. [Google Scholar] [CrossRef]
- Yu, H.; Yang, T.; Zhao, R.; Xiao, B.; Li, Z.; Zhang, M. Fast formaldehyde gas sensing response properties of ultrathin SnO2 nanosheets. RSC Adv. 2015, 5, 104574–104581. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Yu, M.; Yin, Y.; Du, B.; Tang, W.; Jiang, T.; Yang, B.; Cao, W.; Ashfold, M.N.R. Enhanced ethanol sensing properties of ultrathin ZnO nanosheets decorated with CuO nanoparticles. Sens. Actuators B 2018, 255, 3384–3390. [Google Scholar] [CrossRef]
- Hoa, N.D.; El-Safty, S.A. Synthesis of mesoporous NiO nanosheets for the detection of toxic NO2 gas. Chem. Eur. J. 2011, 17, 12896–12901. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Miao, J.; Chen, C.; Meng, L.; Lin, Y.S. Self-assembled monolayer of metal oxide nanosheet and structure and gas-sensing property relationship. ACS Sens. 2019, 4, 1279–1290. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. Facile synthesis and ultrahigh ethanol response of hierarchically porous ZnO nanosheets. Sens. Actuators B 2012, 161, 209–215. [Google Scholar] [CrossRef]
- Li, D.J.; Huang, Z.; Hwang, T.H.; Narayan, R.; Choi, J.W.; Kim, S.O. Atomic thin titania nanosheet-coupled reduced graphene oxide 2D heterostructures for enhanced photocatalytic activity and fast lithium storage. Electron. Mater. Lett. 2016, 12, 211–218. [Google Scholar] [CrossRef]
- Duy, L.V.; Hanh, N.H.; Son, D.N.; Hung, P.T.; Hung, C.M.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. Facile hydrothermal synthesis of two-dimensional porous ZnO nanosheets for highly sensitive ethanol sensor. J. Nanomater. 2019, 4867909. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Gao, H.; Meng, F. Ethanol sensors based on porous In2O3 nanosheet-assembled micro-flowers. Sensors 2020, 20, 3353. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Seo, J.-H.; Luo, G.; Starr, M.B.; Li, Z.; Geng, D.; Yin, X.; Wang, S.; Fraser, D.G.; Morgan, D.; et al. Nanometre-thick single-crystalline nanosheets grown at the water−air interface. Nat. Commun. 2016, 7, 10444. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Zhang, H. Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat. Commun. 2015, 6, 7873. [Google Scholar] [CrossRef]
- Cheng, W.R.; He, J.F.; Yao, T.; Sun, Z.H.; Jiang, Y.; Liu, Q.H.; Jiang, S.; Hu, F.; Xie, Z.; He, B.; et al. Half-unit-cell alpha Fe2O3 semiconductor nanosheets with intrinsic and robust ferromagnetism. J. Am. Chem. Soc. 2014, 136, 10393–10398. [Google Scholar] [CrossRef]
- Zhong, Y.T.; Yang, Y.J.; Ma, Y.; Yao, J.N. Controlled synthesis of ultrathin lamellar Eu2O3 nanocrystals: Self-assembly of 1D nanowires to 2D nanosheets. Chem. Commun. 2013, 49, 10355–10357. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, X.; Yu, Y.; Tian, W.Q.; Ma, J.; Lei, S. Surface confined crystalline two-dimensional covalent organic frameworks via on-surface schiff-base coupling. ACS Nano 2013, 7, 8066–8073. [Google Scholar] [CrossRef]
- Rui, X.; Lu, Z.; Yin, Z.; Sim, D.H.; Xiao, N.; Lim, T.M.; Hng, H.H.; Zhang, H.; Yan, Q. Oriented molecular attachments through sol-gel chemistry for synthesis of ultrathin hydrated vanadium pentoxide nanosheets and their applications. Small 2013, 9, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.-S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Sun, J. Controlled synthesis of defect-rich ultrathin two-dimensional WO3 nanosheets for NO2 gas detection. Sens. Actuators B 2017, 245, 828–834. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Lim, J.-O.; Huh, J.-S.; Noh, J.-S.; Lee, W. Two-step fabrication of ZnO nanosheets for high-performance VOCs gas sensor. Curr. Appl. Phys. 2013, 13, S156–S161. [Google Scholar] [CrossRef]
- Cao, Y.; He, Y.; Zou, X.; Li, G.-D. Tungsten oxide clusters decorated ultrathin In2O3 nanosheets for selective detecting formaldehyde. Sens. Actuators B 2017, 252, 232–238. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 nanosheet composites: Hydrothermal synthesis and gas sensing mechanism. J. Alloys Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Liu, J.; Liang, L.; Xie, Y. Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci. 2014, 5, 3976–3982. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, Q.; Yu, X.; Wang, D.; Chen, Y. Ag-modified porous perovskite-type LaFeO3 for efficient ethanol detection. Nanomaterials 2022, 12, 1768. [Google Scholar] [CrossRef]
- Wang, D.; Yang, J.; Bao, L.; Cheng, Y.; Tian, L.; Ma, Q.; Xu, J.; Li, H.-J.; Wang, X. Pd nanocrystal sensitization two-dimension porous TiO2 for instantaneous and high efficient H2 detection. J. Colloid Interface Sci. 2021, 597, 29–38. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.; Yang, H.; Liu, S.; Qin, Z.; Xie, C.; Zeng, D. Active edge effect of pothole-rich WO3 nanosheets for enhancing dimethyl trisulfide gas sensing performance. Sens. Actuators B 2022, 352, 131103. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Belberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Matko, I.; Gaidi, M.; Chenevier, B.; Charai, A.; Saikaly, W.; Labeau, M. Pt doping of SnO2 thin films. A transmission electron microscopy analysis of the porosity evolution. J. Electrochem. Soc. 2002, 149, H153–H158. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Dräger, J.; Russ, S.; Sauerwald, T.; Kohl, C.-D.; Bunde, A. Percolation transition in the gas-induced conductance of nanograin metal oxide films with defects. J. Appl. Phys. 2013, 113, 223701. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO polygonal nanoflakes: Synthesis, use in high-sensitivity NO2 gas sensor, and proposed mechanism of gas sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.-S.; Kim, S.-I.; Baek, S.-H.; Chun, D.W.; Jin, C.; Lee, K.H.; Lee, K.Y. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Tian, R.; Huang, S.; Chen, R.; Li, M.; Wan, T.; Han, Z.; Wang, D.; Chu, D. Oxygen vacancies and band gap engineering of vertically aligned MnO2 porous nanosheets for efficient oxygen evolution reaction. Surf. Interfaces 2021, 26, 101398. [Google Scholar] [CrossRef]
- He, X.; Liu, B.; Zhang, S.; Li, H.; Liu, J.; Sun, Z.; Chang, H. Nickel nitrate hydroxide holey nanosheets for efficient oxygen evolution electrocatalysis in alkaline condition. Electrocatalysis 2022, 13, 37–46. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Hu, X.; Zhang, J.; Wu, J.; Yang, H.; Xie, C.; Zeng, D. A universal strategy for the synthesis of porous two-dimensional transition metal oxide nanosheets based on chemical topology transformation. Sci. China Mater. 2021, 64, 2477–2485. [Google Scholar] [CrossRef]
- Hong, Q.-L.; Zhai, Q.-G.; Liang, X.-L.; Yang, Y.; Li, F.-M.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Chen, Y. Holey cobalt oxyhydroxide nanosheets for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 3297. [Google Scholar] [CrossRef]
- Jang, J.-S.; Lee, S.-E.; Choi, S.-J.; Koo, W.-T.; Kim, D.-H.; Shin, H.; Park, H.J.; Kim, I.-D. Heterogeneous, porous 2D oxide sheets via rapid galvanic replacement: Toward superior HCHO sensing application. Adv. Funct. Mater. 2019, 29, 1903012. [Google Scholar] [CrossRef]
- Li, P.; Ruan, C.; Xu, J.; Xie, Y. Supercapacitive performance of CoMoO4 with oxygen vacancy porous nanosheets. Electrochim. Acta 2020, 330, 135334. [Google Scholar] [CrossRef]
- Butburee, T.; Bai, Y.; Wang, H.; Chen, H.; Wang, Z.; Liu, G.; Zou, J.; Khemthong, P.; Lu, G.Q.M.; Wang, L. 2D porous TiO2 single-crystalline nanostructure demonstrating high photo-electrochemical water splitting performance. Adv. Mater. 2018, 30, 1705666. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wu, X.; Zhao, W.; Zhang, B. Nanoporous single-crystal-like CdxZn1-xS nanosheets fabricated by the cation-exchange reaction of inorganic-organic hybrid ZnS-amine with cadmium ions. Angew. Chem. Int. Ed. 2012, 51, 897–900. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous two-dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Adpakpang, K.; Oh, S.M.; Agyeman, D.A.; Jin, X.; Jarulertwathana, N.; Kim, I.Y.; Sarakonsri, T.; Kang, Y.-M.; Hwang, S.-J. Holey 2D nanosheets of low-valent manganese oxides with an excellent oxygen catalytic activity and a high functionality as a catalyst for Li-O2 batteries. Adv. Funct. Mater. 2018, 28, 1707106. [Google Scholar] [CrossRef]
- Qi, J.; Yan, Y.; Liu, T.; Zhou, X.; Cao, J.; Feng., J. Plasma-induced surface reorganization of porous Co3O4-CoO heterostructured nanosheets for electrocatalytic water oxidation. J. Colloid Interface Sci. 2020, 565, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, T.; Thiebaut, J.M.; Michel, H.; Cardoso, R.P.; Maliska, A. Reduction of metallic oxides by late Ar–H2–N2 postdischarges. I. Application to copper oxides. J. Vac. Sci. Technol. A 2002, 20, 1347–1352. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, C.; Hwang, S.Y.; Chen, Q.; Peng, Z. Free-standing holey Ni(OH)2 nanosheets with enhanced activity for water oxidation. Small 2017, 13, 1700334. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Li, Y.; Wang, H.; Xie, J.; Liao, L.; Liu, C.; Liu, Y.; Wu, T.; Li, Y.; et al. Identifying the active surfaces of electrochemically tuned LiCoO2 for oxygen evolution reaction. J. Am. Chem. Soc. 2017, 139, 6270–6276. [Google Scholar] [CrossRef]
- Tian, Z.; Bai, H.; Li, Y.; Liu, W.; Li, J.; Kong, Q.; Xi, G. Gas-sensing activity of amorphous copper oxide porous nanosheets. ChemistryOpen 2020, 9, 80–86. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatt, V.; Kim, J.; Abhyankar, A.C.; Chung, H.-J.; Singh, K.; Cho, Y.B.; Yun, Y.J.; Lim, K.S.; Yun, J.-H. Holey engineered 2D ZnO-nanosheets architecture for supersensitive ppm level H2 gas detection at room temperature. Sens. Actuators B 2021, 326, 128839. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, L.; Zhang, H.; Zhang, Q.; Chen, W.; Zhu, J.; Song, D. Two-dimensional Co3O4 thin sheets assembled by 3D interconnected nanoflake array framework structures with enhanced supercapacitor performance derived from coordination complexes. Chem. Eng. J. 2016, 292, 1–12. [Google Scholar] [CrossRef]
- Gou, Y.; Liang, X.; Chen, B. Porous Ni-Co bimetal oxides nanosheets and catalytic properties for CO oxidation. J. Alloys Compd. 2013, 574, 181–187. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Y.; Zeng, X.; Wu, G.; Jiang, W.; Wei, D.; Ling, M.; Ng, K.W.; Qin, Y. Ultrasensitive ethanol sensor based on segregated ZnO-In2O3 porous nanosheets. Appl. Surf. Sci. 2021, 535, 147697. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, B.; Zhao, C.; Xu, H.; Wang, S.; Chen, Y.; Xie, L.; Chen, L. Benzoic acid-assisted substrate-free synthesis of ultrathin nanosheets assembled two-dimensional porous Co3O4 thin sheets with 3D hierarchical micro-/nano-structures and enhanced performance as battery-type materials for supercapacitors. Electrochim. Acta 2019, 313, 194–204. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.; Xu, C.; Wu, D.; Gao, Z.; Zhang, Q.; Jiang, K. Ultra-thin Bi2WO6 porous nanosheets with high lattice coherence for enhanced performance for photocatalytic reduction of Cr(VI). J. Colloid Interf. Sci. 2018, 525, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, W.; Long, H.; Wang, Z. Nanosheet-assembled hierarchical SnO2 nanostructures for efficient gas-sensing applications. Sens. Actuators B 2016, 231, 120–128. [Google Scholar] [CrossRef]
- Sharma, B.; Karuppasamy, K.; Vikraman, D.; Jo, E.-B.; Sivakumar, P.; Kim, H.-S. Porous, 3D-hierarchical α-NiMoO4 rectangular nanosheets for selective conductometric ethanol gas sensors. Sens. Actuators B 2021, 347, 130615. [Google Scholar] [CrossRef]
- Ryu, Y.Y.; Kim, T.; Han, H.S. Synthesis of porous ZnO nanosheets and carbon nanotube hybrids as efficient photocatalysts via pulsed laser ablation. Catalysts 2019, 9, 787. [Google Scholar] [CrossRef]
- Yan, S.; Liang, X.; Song, H.; Ma, S.; Lu, Y. Synthesis of porous CeO2-SnO2 nanosheets gas sensors with enhanced sensitivity. Ceram Intern. 2018, 44, 358–363. [Google Scholar] [CrossRef]
- Zheng, X.; Mofarah, S.S.; Cazorla, C.; Daiyan, R.; Esmailpour, A.; Scott, J.; Yao, Y.; Lim, S.; Wong, V.; Chen, E.Y.; et al. Decoupling the impacts of engineering defects and band gap alignment mechanism on the catalytic performance of holey 2D CeO2−x-based heterojunctions. Adv. Funct. Mater. 2021, 31, 2103171. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, X.; Ge, C.; Hussain, S.; Liu, G.; Qiao, G. WO3 porous nanosheet arrays with enhanced low temperature NO2 gas sensing performance. Sens. Actuators B 2020, 316, 128050. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Zhang, C.; Hou, P.; Xu, X. Improvement toluene detection of gas sensors based on flower-like porous indium oxide nanosheets. J. Alloys Compd. 2022, 897, 163222. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Shi, Y.-T.; Gong, F.-L.; Chen, J.-L.; Jin, G.-X.; Guo, Q.; Zhang, H.-L.; Zhang, Y.-H. Asymmetric interfacial oxygen sites of porous CeO2-SnO2 nanosheets enabling highly sensitive and selective detection of 3-hydroxy-2-butanone biomarkers. Sens. Actuators B 2022, 371, 132500. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Wu, D.-Y.; Shen, G.-R.; Zou, C.; Feng, Y.; Liu, H.; Dong, C.-K.; Du, X.-W. Porous cobalt–nickel hydroxide nanosheets with active cobalt ions for overall water splitting. Small 2019, 15, 1804832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X.; Chang, X.; Li, J.; Pan, L.; Jiang, Y.; Gao, W.; Gao, C.; Sun, S. Metal-organic framework-derived porous SnO2 nanosheets with grain sizes comparable to Debye length for formaldehyde detection with high response and low detection limit. Sens. Actuators B 2021, 347, 130599. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, X.; Zheng, C.; Liu, Z. Two-dimensional porous micro/nano metal oxides templated by graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 11984–11990. [Google Scholar] [CrossRef]
- Huang, J.; Meng, C.; Wang, H.; Ren, H.; Lu, X.; Joo, S.W. Preparation of cross-linked porous SnO2 nanosheets using three- dimensional reduced graphene oxide as a template and their gas sensing property. J. Alloys Compd. 2022, 910, 164763. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.-Y.; Peng, Y.; Huang, H.-W.; Li, Y.; Wu, M.; Ke, X.-X.; Tendeloo, G.V.; Su, B.-L. 2D ZnO mesoporous single crystal nanosheets with exposed {0001} polar facets for the depollution of cationic dye molecules by highly selective adsorption and photocatalytic decomposition. Appl. Catal. B 2016, 181, 138–145. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Shen, Y.; Zhang, R.; Meng, F. Phosphorus-doped porous perovskite LaFe1-xPxO3-δ nanosheets with rich surface oxygen vacancies for ppb level acetone sensing at low temperature. Chem. Eng. J. 2022, 431, 134280. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Chen, X.; Song, X.; Tian, J.; Cui, H. Porous ZnO ultrathin nanosheets with high specific surface areas and abundant oxygen vacancies for acetylacetone gas sensing. ACS Appl. Mater. Interfaces 2019, 11, 24757–24763. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Li, L.; Zheng, J.; Zhang, J.; Li, H.; Zhu, Z. Highly sensitive and selective dimethylamine sensors based on hierarchical ZnO architectures composed of nanorods and nanosheet-assembled microspheres. Sens. Actuators B 2012, 171–172, 1101–1109. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Wang, W.; Sun, P.; Ma, J.; Liang, X.; Sun, Y.; Ma, Y.; Lu, G. Porous hierarchical In2O3 nanostructures: Hydrothermal preparation and gas sensing properties. Sens. Actuators B 2012, 171–172, 1066–1072. [Google Scholar] [CrossRef]
- Zhang, W.H.; Ding, S.-J.; Zhang, Q.-S.; Yi, H.; Liu, Z.-X.; Shi, M.-L.; Guan, R.-F.; Yue, L. Rare earth element-doped porous In2O3 nanosheets for enhanced gas-sensing performance. Rare Met. 2021, 40, 1662–1668. [Google Scholar] [CrossRef]
- Song, L.; Lukianov, A.; Butenko, D.; Li, H.; Zhang, J.; Feng, M.; Liu, L.; Chen, D.; Klyui, N.I. Facile synthesis of hierarchical tin oxide nanoflowers with ultra-high methanol gas sensing at low working temperature. Nanoscale Res. Lett. 2019, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Gas sensors based on ultrathin porous Co3O4 nanosheets to detect acetone at low temperature. RSC Adv. 2015, 5, 59976–59982. [Google Scholar] [CrossRef]
- Dong, C.; Xiao, X.; Chen, G.; Guan, H.; Wang, Y.; Djerdj, I. Porous NiO nanosheets self-grown on alumina tube using a novel flash synthesis and their gas sensing properties. RSC Adv. 2015, 5, 4880–4885. [Google Scholar] [CrossRef]
- Li, P.; Wang, D.; Wang, Y. Ultrafast CO sensor based on flame-annealed porous CeO2 nanosheets for environmental application. J. Inorg. Mater. 2021, 36, 1223–1230. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Duan, G.; Zhang, H.; Li, Y.; Liu, G.; Xu, L.; Cai, W. In situ growth of porous ZnO nanosheet-built network film as high-performance gas sensor. Sens. Actuators B 2015, 221, 350–356. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Z.; Meng, F.; Luo, T.; Li, M.; Liu, J. Novel porous single-crystalline ZnO nanosheets fabricated by annealing ZnS(en)0.5 (en=ethylenediamine) precursor. Application in a gas sensor for indoor air contaminant detection. Nanotechnology 2009, 20, 125501. [Google Scholar] [CrossRef] [PubMed]
- Brynzari, V.; Korotchenkov, G.; Dmitriev, S. Theoretical study of semiconductor thin film gas sensitivity: Attempt to consistent approach. J. Electron. Technol. 2000, 33, 225–235. [Google Scholar]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3 and SnO2 based ozone sensors: Fundamentals. J. Sens. 2016, 2016, 3816094. [Google Scholar] [CrossRef]
- Dun, M.; Tan, J.; Tan, W.; Tang, M.; Huang, X. CdS quantum dots supported by ultrathin porous nanosheets assembled into hollowed-out Co3O4 microspheres: A room-temperature H2S gas sensor with ultra-fast response and recovery. Sens. Actuators B 2019, 298, 126839. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, B.; Zhang, D.; Xu, J. Enhanced CO sensing properties of Pd modified ZnO porous nanosheets. Chin. Chem. Lett. 2020, 31, 2033–2036. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.-D.; Shi, Z.; Zou, H.; Zou, X. Ultrathin In2O3 nanosheets with uniform mesopores for highly sensitive nitric oxide detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Zhang, H.; Feng, C.; Wang, C.; Liu, F. Synthesis, characterization and gas sensing properties of porous flower-like indium oxide nanostructures. RSC Adv. 2015, 5, 30297–30302. [Google Scholar] [CrossRef]
- Tan, W.; Tan, J.; Fan, L.; Yu, Z.; Qian, J.; Huang, X. Fe2O3-loaded NiO nanosheets for fast response/recovery and high response gas sensor. Sens. Actuators B 2018, 256, 282–293. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches to improvement operating characteristics of conductometric gas sesors. Part 1: Improvement of sensor sensitivity and selectivity. Sens. Actuators B 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxides: State of the art and approaches. Sens. Actuators B 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Fan, F.; Tang, P.; Wang, Y.; Feng, Y.; Chen, A.; Luo, R.; Li, D. Facile synthesis and gas sensing properties of tubular hierarchical ZnO self-assembled by porous nanosheets. Sens. Actuators B 2015, 215, 231–240. [Google Scholar] [CrossRef]
- Feng, S.; Yu, H.; Zhang, X.; Huo, L.; Gao, R.; Wang, P.; Cheng, X.; Major, Z.; Gao, S.; Xu, Y. Ionic liquid-assisted synthesis of 2D porous lotus root slice-shaped NiO nanomaterials for selective and highly sensitive detection of N2H4. Sens. Actuators B 2022, 359, 131529. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement. Sens. Actuators B 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches to improvement operating characteristics of conductometric gas sensors. Part 2: Decrease of dissipated (consumable) power and improvement stability and reliability. Sens. Actuators B 2014, 198, 316–341. [Google Scholar] [CrossRef]
- Spagnoli, E.; Krik, S.; Fabbri, B.; Valt, M.; Ardit, M.; Gaiardo, A.; Vanzetti, L.; Ciana, M.D.; Cristino, V.; Vola, G.; et al. Development and characterization of WO3 nanoflakes for selective ethanol sensing. Sens. Actuators B 2021, 347, 130593. [Google Scholar] [CrossRef]
- Liu, H.; Duan, L.; Xia, K.; Chen, Y.; Li, Y.; Deng, S.; Xu, J.; Hou, Z. Microwave synthesized 2D WO3 nanosheets for VOCs gas sensors. Nanomaterials 2022, 12, 3211. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z.; Xu, M.-D.; Zhang, Y.; Huang, J.; Cheng, H.; Wang, X.-F.; Zheng, Z.-L.; Ding, Y. Enhanced isopropanol sensing performance of the CdS nanoparticle decorated ZnO porous nanosheets based gas sensors. IEEE Sens. J. 2021, 21, 13041–13047. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Humidity Measurement: Methods, Materials and Technologies; Sensing Materials and Technologies; CRC Press: Boca Raton, FL, USA, 2020; Volume 3. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Humidity Measurement: Methods, Materials and Technologies; Electronic and Electrical Humidity Sensors; CRC Press: Boca Raton, FL, USA, 2019; Volume 2. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.S.; Kim, T.H.; Hong, Y.J.; Kang, Y.C.; Lee, J.H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef]
- Kim, J.S.; Na, C.W.; Kwak, C.H.; Li, H.Y.; Yoon, J.W.; Kim, J.H.; Jeong, S.-Y.; Lee, J.-H. Humidity-independent gas sensors using Pr-doped In2O3 macroporous spheres: Role of cyclic Pr3+/Pr4+ redox reactions in suppression of water-poisoning effect. ACS Appl. Mater. Interfaces 2019, 11, 25322–25329. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, S.J.; Jang, J.S.; Cho, H.J.; Koo, W.T.; Tuller, H.L.; Kim, I.D. Exceptional high-performance of Pt-based bimetallic catalysts for exclusive detection of exhaled biomarkers. Adv. Mater. 2017, 29, 1700737. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Z.; Xu, M.; Hong, Y.; Li, N.; Fu, P.; Chen, Z. Effect of gas sensing properties by Sn–Rh codoped ZnO nanosheets. Electron. Mater. Lett. 2016, 12, 343–349. [Google Scholar] [CrossRef]
- Qin, C.; Wang, B.; Wang, Y. Metal-organic frameworks-derived Mn-doped Co3O4 porous nanosheets and enhanced CO sensing performance. Sens. Actuators B 2022, 351, 130943. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide-based composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Li, H.-Y.; Ding, J.-C.; Guo, X. Hierarchical flowerlike WO3 nanostructures assembled by porous nanoflakes for enhanced NO gas sensing. Sens. Actuators B 2017, 246, 225–234. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.; Wang, G.; Shen, Y.; San, X.; Li, M.; Meng, F. Low-temperature formaldehyde gas sensors based on NiO-SnO2 heterojunction microflowers assembled by thin porous nanosheets. Sens. Actuators B 2018, 273, 418–428. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline metal oxides: Effect of the real structure and surface chemistry on the sensor properties. Russ. Chem. Bull. Int. Ed. 2008, 57, 1106–1125. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxide modified with gold nanoparticles: A review. MicroChim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Nehasil, V. Ozone sensing by In2O3 films modified with Rh: Dimension effect. Sensors 2021, 21, 1886. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Tian, J.; Cui, H.; Wang, X. Synthesis of 1D SnO2 nanorods / 2D NiO porous nanosheets p-n heterostructures for enhanced ethanol gas sensing performance. Vacuum 2022, 205, 111399. [Google Scholar] [CrossRef]
- Gong, F.-L.; Peng, M.-X.; Yue, L.-J.; Chen, J.-L.; Xie, K.-F.; Zhang, Y.-H. Design of p-n heterojunction on mesoporous ZnO/Co3O4 nanosheets for triethylamine sensor. Chem. Phys. Lett. 2021, 779, 138891. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B 2021, 337, 129783. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. Synthesis and ethanol sensing properties of SnO2 nanosheets via a simple hydrothermal route. Solid-State Electron. 2012, 76, 91–94. [Google Scholar] [CrossRef]
- Qiao, P.-Y.; Zhang, L.-X.; Zhu, M.-Y.; Yin, Y.-Y.; Zhao, Z.-W.; Sun, H.-N.; Dong, J.-Y.; Bie, L.-J. Acetylene sensing enhancement of mesoporous ZnO nanosheets with morphology and defect induced structural sensitization. Sens. Actuators B 2017, 250, 189–197. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Liu, J.; Wang, F.; Kong, J.; Qiu, S.; He, C.; Luan, L. Synthesis of nestlike ZnO hierarchically porous structures and analysis of their gas sensing properties. ACS Appl. Mater. Interfaces 2012, 4, 817–882. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Xu, X.L.; Luo, J.; Jiang, X.H.; Li, W.Q.; Jin, W.X.; Sun, X.X. Performance of 3D SnO2 microstructure with porous nanosheets for acetic acid sensing. Mater. Lett. 2015, 142, 141–144. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Hu, X.; Wu, Q.; Xiong, W.; Qin, Z.; Xie, C.; Zeng, D. Exposed Mo atoms induced by micropores enhanced H2S sensing of MoO3 nanoflowers. J. Hazard. Mater. 2022, 429, 127270. [Google Scholar] [CrossRef]
- Xie, X.; Wang, X.; Tian, J.; Song, X.; Wei, N.; Cui, H. Growth of porous ZnO single crystal hierarchical architectures with ultrahigh sensing performances to ethanol and acetone gases. Ceram. Int. 2017, 43, 1121–1128. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Zhang, H. Controllable synthesis of zinc oxide hierarchical architectures and their excellent formaldehyde gas sensing performances. Mater. Lett. 2017, 202, 44–47. [Google Scholar] [CrossRef]
- Liu, J.; Luo, T.; Meng, F.; Qian, K.; Wan, Y.; Liu, J. Porous hierarchical In2O3 micro-/nanostructures: Preparation, formation mechanism, and their application in gas sensors for noxious volatile organic compound detection. J. Phys. Chem. C 2010, 114, 4887–4894. [Google Scholar] [CrossRef]
- Juang, F.-R.; Hsieh, C.-H.; Huang, L.-Y.; Wang, W.-Y.; Lin, W.-B.; Yen, L. Dispersed and spherically assembled porous NiO nanosheets for low concentration ammonia gas sensing applications. Solid State Electron. 2022, 189, 108224. [Google Scholar] [CrossRef]
- Cui, J.; Sun, J.; Liu, X.; Li, J.; Ma, X.; Chen, T. Fabrication of hierarchical flower-like porous ZnO nanostructures from layered ZnC2O4·3Zn(OH)2 and gas sensing properties. Appl. Surf. Sci. 2014, 308, 17–23. [Google Scholar] [CrossRef]
- Li, Z.; Lou, C.; Lei, G.; Lu, G.; Pan, H.; Liu, X.; Zhang, J. Atomic layer deposition of Rh/ZnO nanostructures for anti-humidity detection of trimethylamine. Sens. Actuators B 2022, 355, 131347. [Google Scholar] [CrossRef]
- Jing, Z.; Zhan, J. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Huang, D.; Wang, X.; Wang, Y.; Wang, W.; Yi, M.; Cheng, Q.; Song, Y.; Han, G. Highly sensitive and selective acetone gas sensors based on modified ZnO nanomaterials. Mater. Sci. Semicond. Proc. 2022, 148, 106807. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Duan, Z.; Wu, Y.; Zhao, Q.; Liu, B.; Huang, Q.; Yuan, Z.; Li, X.; Tai, H. Edge-enriched MoS2 nanosheets modified porous nanosheet-assembled hierarchical In2O3 microflowers for room temperature detection of NO2 with ultrahigh sensitivity and selectivity. J. Hazard. Mater. 2022, 434, 128836. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Cho, B.K.; Brinzari, V. Grain size effects in sensor response of nanostructured SnO2- and In2O3-based conductometric gas sensor. Crit. Rev. Sol. St. Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Guo, W.; Liu, T.; Wang, J.; Yu, W.; Sun, R.; Chen, Y.; Hussain, S.; Peng, X.; Wang, Z. Hierarchical ZnO porous microspheres and their gas-sensing properties. Ceram. Intern. 2013, 39, 5919–5924. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Luo, Q.; Ge, C.; Liu, G.; Qiao, G.; Kim, E.J. Below-room-temperature solution-grown ZnO porous nanosheet arrays with ppb-level NO2 sensitivity under intermittent UV irradiation. Appl. Surf. Sci. 2021, 566, 150750. [Google Scholar] [CrossRef]
- Duy, L.V.; Nguyet, T.T.; Hung, C.M.; Le, D.T.T.; Duy, N.V.; Hoa, N.D.; Biasioli, F.; Tonezzer, M.; Natale, C.D. Ultrasensitive NO2 gas sensing performance of two dimensional ZnO nanomaterials: Nanosheets and nanoplates. Ceram. Intern. 2021, 47, 28811–28820. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiao, L.; Bing, Y.; Wen, M.; Zou, B.; Zheng, W.; Zhang, T.; Zou, G. Development of microstructure CO sensor based on hierarchically porous ZnO nanosheet thin films. Sens. Actuators B 2012, 173, 897–902. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, X.; Zhang, X.; Sui, L.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Highly sensitive H2S detection sensors at low temperature based on hierarchically structured NiO porous nanowall arrays. J. Mater. Chem. A 2015, 3, 11991–11999. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Wang, N.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. High precision NH3 sensing using network nano-sheet Co3O4 arrays based sensor at room temperature. Sens. Actuators B 2016, 235, 222–231. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jang, J.-S.; Park, H.J.; Kim, I.-D. Optically sintered 2D RuO2 nanosheets: Temperature-controlled NO2 reaction. Adv. Funct. Mater. 2017, 27, 1606026. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, C.; Du, S.; Yang, J.; Yu, B.; Luo, J.; Yin, W.; Li, E.; Dong, S.; Ye, P.; et al. Contacts between two- and three-dimensional materials: Ohmic, Schottky, and p–n heterojunctions. ACS Nano 2016, 10, 4895–4919. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).