Inorganic Nanoparticles as Radiosensitizers for Cancer Treatment
Abstract
1. Introduction
2. Tumor Physiology
3. Current Approaches to Cancer Treatment
3.1. Implementation of Hirudin in Cancer Treatment
3.2. Progresses in Detection and Therapy
3.3. Chemotherapy
3.4. Surgery
3.5. Hormonal Therapy
3.6. Bone Marrow Transplant and Stem-Cell Therapy
3.7. Immunotherapy
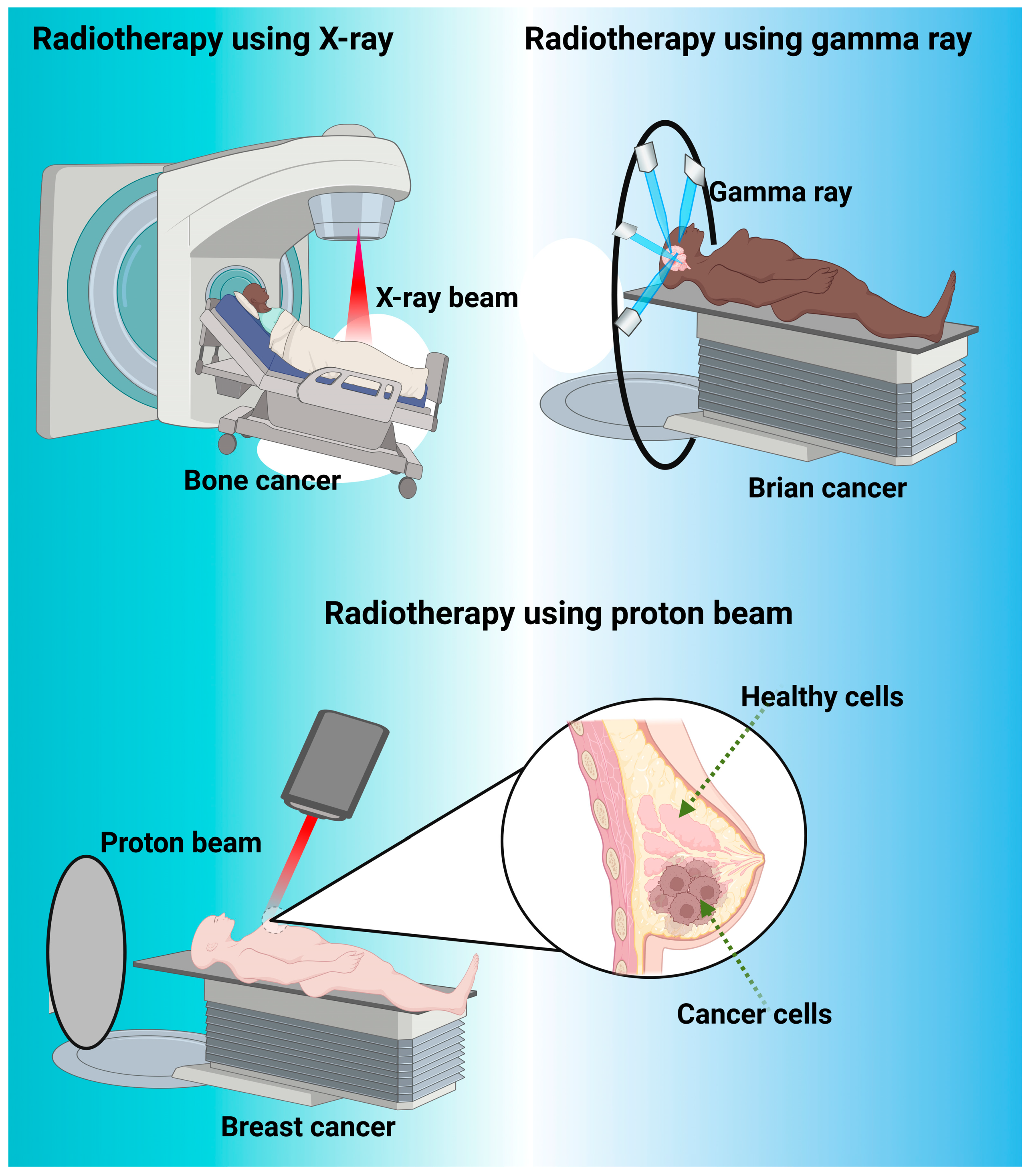
4. Radiotherapy
4.1. Radiotherapy Success
4.2. Modern Radiotherapy
4.3. Proton Therapy
5. Radio sensitization Using Organic Molecular Compounds
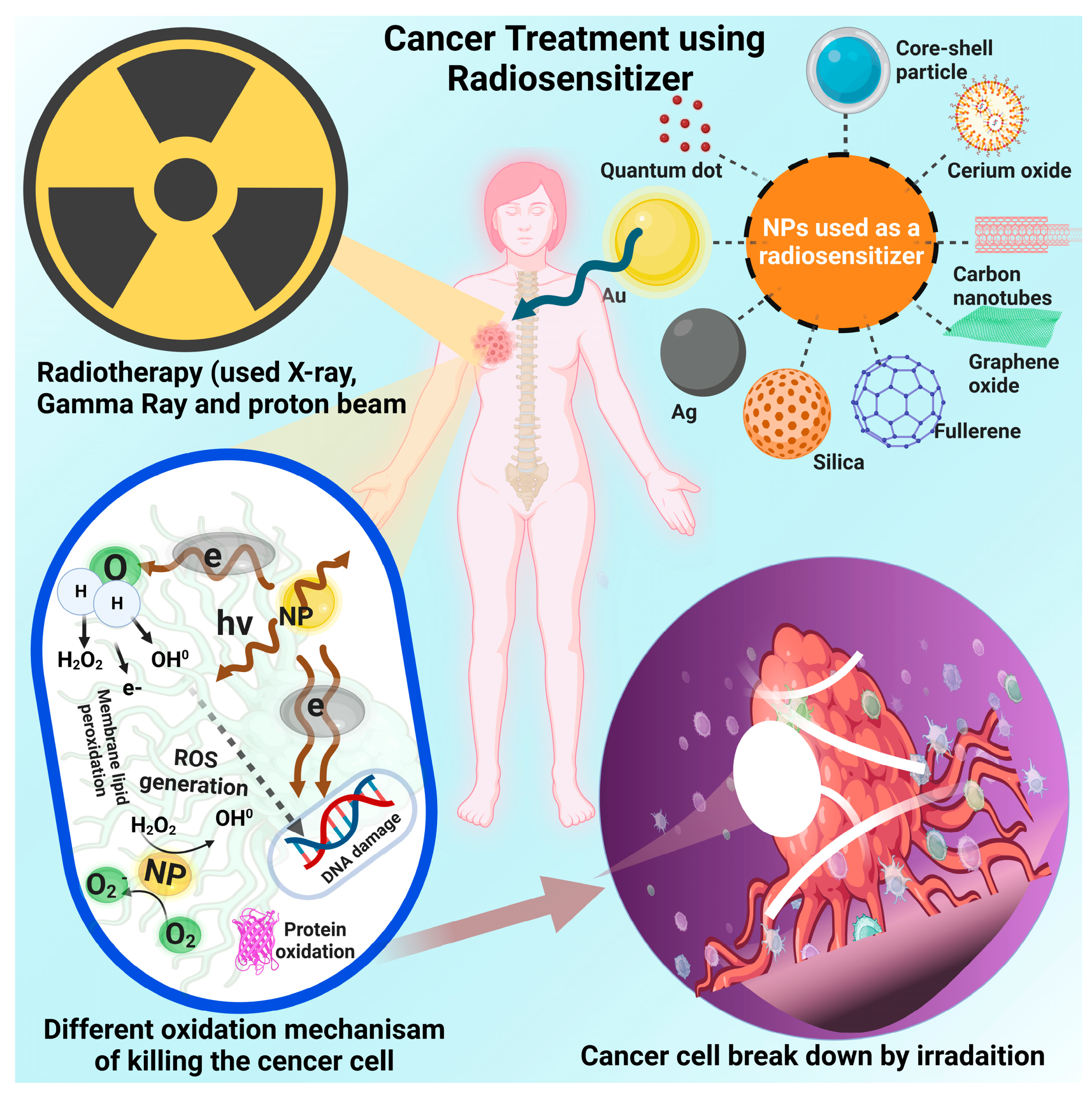
6. Inorganic Nanomaterials
6.1. Gold Nanoparticles
| Nanoparticle and Functionalization | Synthesis Method and Size | Experimental System | Radiation Utilized and Dose | Mechanism | Notable Results | Ref. |
|---|---|---|---|---|---|---|
| Spherical AuNPs in the center of a water cube | Simulated model, 10 nm to 150 nm in diameter | Tumor cell in the head in a spherical shape with a radius of 0.8 cm | X-ray radiation, 20 keV and 50 keV | Simulated tumor cells absorbed high amounts of energy in the presence of gold. During irradiation, gold increased the production of secondary electrons as well as photons in cancerous cells. | The absorbed energy for the 20 keV was higher than any other dosage. Irradiation in the presence of AuNPs was significantly better than irradiation alone. | [155,156] |
| AuNPs | Spherical, 0.18 nm | Tumor Cells | X-rays, 81 keV | There is a significant dose enhancement near the Au/Tissue interface due to the enhancement at a low Z/high Z interface. | Results showed an increase of about 550% in dose enhancement for the AuNPs. | [157] |
| Transferrin peptide targeted AuNPs | Modified Brust-Schifrin, 8.2 nm on average diameter | Human glioma cancer lines | Photosensitizers | Utilizing Tfpep improves AuNPs specificity. PDT-killing efficacy is effectively reached. | Experimental system shows a successful delivery system to brain tumors utilizing AuNPs. | [161] |
| AuNPs | 55 nm on average | dsDNA | Femtosecond laser excitation, 750 nm | The AuNPs can modulate the local rate of denaturation by forming a structure with single-stranded DNA. | The results showed that modulation increased nearly threefold during the use of gold nanoparticle intermediates. | [165] |
| 2-d gold nanoribbons | Pre-synthesized by mammalian cells, 20 nm | MCF7 Breast Cancer cells | NIR irradiation, 0.6 W | The nanoribbons aided in many different areas, including SERS imaging as well as NIR hypothermia and potential for a new nanomedicine. | Nanoribbons using the seed-mediated method could potentially produce a state-of-the-art cancer therapeutic treatment. | [173] |
| Chitosan-coated AuNPs | Chemical methods, 3.7 nm on average | Chronic myeloid leukemia cell line (K562) | Not stated | Cell death was dependent on ROS production. | TheNPs induced selective cell death in leukemia cells. | [178] |
6.2. Silver Nanoparticles
6.3. Silica Nanoparticles
6.4. Carbonaceous Nanomaterials
6.5. Cerium Oxide
7. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M. Funding of cardiovascular research in the USA: Robert Califf and Peter Libby—Speak about cardiovascular research funding in the United States and what the latest trends are with Mark Nicholls. Eur. Heart J. 2018, 39, 3629–3631. [Google Scholar] [CrossRef]
- ReFaey, K.; Tripathi, S.; Grewal, S.S.; Bhargav, A.G.; Quinones, D.J.; Chaichana, K.L.; Antwi, S.O.; Cooper, L.T.; Meyer, F.B.; Dronca, R.S.; et al. Cancer Mortality Rates Increasing vs. Cardiovascular Disease Mortality Decreasing in the World: Future Implications. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Abudu, R.; Bouche, G.; Bourougaa, K.; Davies, L.; Duncan, K.; Estaquio, C.; Font, A.D.; Hurlbert, M.S.; Jackson, P.; Kroeskop-Bossenbroek, L.; et al. Trends in International Cancer Research Investment 2006–2018. JCO Glob. Oncol. 2021, 7, 602–610. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef]
- Idikio, H.A. Human cancer classification: A systems biology- based model integrating morphology, cancer stem cells, proteomics, and genomics. J. Cancer 2011, 2, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget 2023, 14, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zeman, E.M.; Schreiber, E.C.; Tepper, J.E. 27—Basics of RadiationTherapy. In Abeloff’s Clinical Oncology, 5th ed.; Niederhuber, J.E., Armitage, J.O., Doroshow, J.H., Kastan, M.B., Tepper, J.E., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2014; pp. 393–422.e393. [Google Scholar]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Dabiri, S.M.H.; Walsh, T.; Behrooz, A.B.; Yathindranath, V.; Clark, C.; Aghajani, S.; Ahmadi, M.; Madrakian, T.; Miller, D.W.; Ghavami, S. Chapter 27—Potential theranostic targets in glioblastoma. In New Insights Into Glioblastoma; Vitorino, C., Balaña, C., Cabral, C., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 631–665. [Google Scholar]
- Wang, H.; Mu, X.; He, H.; Zhang, X.-D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Price, J.M.; Prabhakaran, A.; West, C.M.L. Predicting tumour radiosensitivity to deliver precision radiotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Kolanthai, E.; Fu, Y.; Kumar, U.; Babu, B.; Venkatesan, A.K.; Liechty, K.W.; Seal, S. Nanoparticle mediated RNA delivery for wound healing. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1741. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Shakil, M.S.; Mahmud, K.M. The Promise of Nanoparticles-Based Radiotherapy in Cancer Treatment. Cancers 2023, 15, 1892. [Google Scholar] [CrossRef]
- Loo, J.F.-C.; Chien, Y.-H.; Yin, F.; Kong, S.-K.; Ho, H.-P.; Yong, K.-T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord. Chem. Rev. 2019, 400, 213042. [Google Scholar] [CrossRef]
- Babu, B.; Pawar, S.; Mittal, A.; Kolanthai, E.; Neal, C.J.; Coathup, M.; Seal, S. Nanotechnology enabled radioprotectants to reduce space radiation-induced reactive oxidative species. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1896. [Google Scholar] [CrossRef]
- Yao, W.; Wu, B.; Liu, Y. Growth and Grain Boundaries in 2D Materials. ACS Nano 2020, 14, 9320–9346. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, I.J.; Caro, A.; Demkowicz, M.J.; Mara, N.A.; Misra, A.; Uberuaga, B.P. Radiation damage tolerant nanomaterials. Mater. Today 2013, 16, 443–449. [Google Scholar] [CrossRef]
- Verger, A.; Brandhonneur, N.; Molard, Y.; Cordier, S.; Kowouvi, K.; Amela-Cortes, M.; Dollo, G. From molecules to nanovectors: Current state of the art and applications of photosensitizers in photodynamic therapy. Int. J. Pharm. 2021, 604, 120763. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.; Sangeetha, K.; Magesan, P.; Johnson, J.; Zhang, X.; Jayamoorthy, K. Photodynamic cancer therapy: Role of Ag-and Au-based hybrid nano-photosensitizers. J. Biomol. Struct. Dyn. 2022, 40, 4766–4773. [Google Scholar] [CrossRef]
- Liu, W.; Chen, B.; Zheng, H.; Xing, Y.; Chen, G.; Zhou, P.; Qian, L.; Min, Y. Advances of nanomedicine in radiotherapy. Pharmaceutics 2021, 13, 1757. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, D.; Lu, J.; Qin, D.; Zhou, J.; Liu, H.-J. Cancer nanomedicine in preoperative therapeutics: Nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy. Bioact. Mater. 2023, 24, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.C.; Sanderson, D.; Caspani, S.; Magalhães, R.; Cortés-Llanos, B.; Granja, A.; Reis, S.; Belo, J.H.; Azevedo, J.; Gómez-Gaviro, M.V. New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo-and Radiotherapy. Cancers 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Timashev, P.; Zhang, Y.; Xue, X.; Liang, X.-J. Nanotechnology-based combinatorial phototherapy for enhanced cancer treatment. RSC Adv. 2022, 12, 9725–9737. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Z.; Hu, R.; Dong, M.; Zhou, X.; Ren, S.; Zhang, Y.; Chen, C.; Huang, R.; Zhu, M. Metabolic Intervention Liposome Boosted Lung Cancer Radio-Immunotherapy via Hypoxia Amelioration and PD-L1 Restraint. Adv. Sci. 2023, 10, 2207608. [Google Scholar] [CrossRef]
- Zhang, L.; Forgham, H.; Huang, X.; Shen, A.; Davis, T.; Qiao, R.; Guo, B. All-in-one inorganic nanoagents for near-infrared-II photothermal-based cancer theranostics. Mater. Today Adv. 2022, 14, 100226. [Google Scholar] [CrossRef]
- Zhou, L.-L.; Guan, Q.; Zhou, W.; Kan, J.-L.; Dong, Y.-B. An iodide-containing covalent organic framework for enhanced radiotherapy. Chem. Sci. 2023, 14, 3642–3651. [Google Scholar] [CrossRef]
- Roy, A.; Patra, C.R. Inorganic nanosystems for cancer theranostics. In Inorganic Nanosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 509–547. [Google Scholar]
- Zhang, C.; Yan, L.; Gu, Z.; Zhao, Y. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy. Chem. Sci. 2019, 10, 6932–6943. [Google Scholar] [CrossRef] [PubMed]
- Thanekar, A.M.; Sankaranarayanan, S.A.; Rengan, A.K. Role of nano-sensitizers in radiation therapy of metastatic tumors. Cancer Treat. Res. Commun. 2021, 26, 100303. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Wen, L.-F.; Xu, G.; Lin, H.-H.; Wang, S.; Liu, J.-Y. Multifunctional organic nanomaterials with ultra-high photothermal conversion efficiency for photothermal therapy and inhibition of cancer metastasis. Bio Org. Chem. 2023, 130, 106220. [Google Scholar] [CrossRef]
- Weng, X.-L.; Liu, J.-Y. Strategies for maximizing photothermal conversion efficiency based on organic dyes. Drug Discov. Today 2021, 26, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ge, J.; Miao, Q.; Zhu, R.; Wen, L.; Zeng, J.; Gao, M. Biodegradable inorganic nanoparticles for cancer theranostics: Insights into the degradation behavior. Bioconjugate. Chem. 2019, 31, 315–331. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, P.R.; Gobalakrishnan, S.; Rabender, C.; Vijayaragavan, V.; Zweit, J. Molecular Imaging Investigations of Polymer-Coated Cerium Oxide Nanoparticles as a Radioprotective Therapeutic Candidate. Pharmaceutics 2023, 15, 2144. [Google Scholar] [CrossRef] [PubMed]
- Varzandeh, M.; Sabouri, L.; Mansouri, V.; Gharibshahian, M.; Beheshtizadeh, N.; Hamblin, M.R.; Rezaei, N. Application of nano-radiosensitizers in combination cancer therapy. Bioeng. Transl. Med. 2023, 8, e10498. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef]
- Babaei, M.; Ganjalikhani, M. The potential effectiveness of nanoparticles as radio sensitizers for radiotherapy. Bioimpacts 2014, 4, 15–20. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Jia, P.; Dai, C.; Cao, P.; Sun, D.; Ouyang, R.; Miao, Y. The role of reactive oxygen species in tumor treatment. RSC Adv. 2020, 10, 7740–7750. [Google Scholar] [CrossRef]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008, 266, 53–59. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xing, C.; Ding, C.; Zhang, H.; Chen, L.; You, L.; Dai, M.; Zhao, Y. Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer. Am. J. Cancer Res. 2020, 10, 1937. [Google Scholar]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, oxidative stress, and metastasis. In Proceedings of the Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2016; pp. 163–175. [Google Scholar]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Nechifor, M.T.; Neagu, T.-M. Reactive oxygen species, cancer and anti-cancer therapies. Curr. Chem. Biol. 2009, 3, 22–46. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.A.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bio Conjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Zhao, J.; Li, D.; Ma, J.; Yang, H.; Chen, W.; Cao, Y.; Liu, P. Increasing the accumulation of aptamer AS1411 and verapamil conjugated silver nanoparticles in tumor cells to enhance the radiosensitivity of glioma. Nanotechnology 2021, 32, 145102. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019, 31, 1802244. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Zhao, L. Hirudin inhibits cell growth via ERK/MAPK signaling in human glioma. Int. J. Clin. Exp. Med. 2015, 8, 20983–20987. [Google Scholar] [PubMed]
- Junren, C.; Xiaofang, X.; Huiqiong, Z.; Gangmin, L.; Yanpeng, Y.; Xiaoyu, C.; Yuqing, G.; Yanan, L.; Yue, Z.; Fu, P.; et al. Pharmacological Activities and Mechanisms of Hirudin and Its Derivatives—A Review. Front. Pharmacol. 2021, 12, 660757. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Z.; Li, S.; Zhang, W.; Foda, M.F.; Zhao, Y.; Han, H. Platelet-Covered Nanocarriers for Targeted Delivery of Hirudin to Eliminate Thrombotic Complication in Tumor Therapy. ACS Nano 2022, 16, 18483–18496. [Google Scholar] [CrossRef]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Klein, J.S.; Sun, C.; Pratx, G. Radioluminescence in biomedicine: Physics, applications, and models. Phys. Med. Biol. 2019, 64, 04TR01. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.S.; Mitchell, G.S.; Cherry, S.R. Quantitative assessment of Cerenkov luminescence for radioguided brain tumor resection surgery. Phys. Med. Biol. 2017, 62, 4183–4201. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Riedl, C.C.; Grimm, J. Clinical Cerenkov Luminescence Imaging of 18F-FDG. J. Nucl. Med. 2014, 55, 95–98. [Google Scholar] [CrossRef]
- Hu, H.; Cao, X.; Kang, F.; Wang, M.; Lin, Y.; Liu, M.; Li, S.; Yao, L.; Liang, J.; Liang, J.; et al. Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: First human results. Eur. Radiol. 2015, 25, 1814–1822. [Google Scholar] [CrossRef]
- Grootendorst, M.R.; Cariati, M.; Pinder, S.E.; Kothari, A.; Douek, M.; Kovacs, T.; Hamed, H.; Pawa, A.; Nimmo, F.; Owen, J.; et al. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery Using (18)F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J. Nucl. Med. 2017, 58, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Mroz, P.; Zhiyentayev, T.; Sharma, S.K.; Balasubramanian, T.; Ruzié, C.; Krayer, M.; Fan, D.; Borbas, K.E.; Yang, E.; et al. In Vitro Photodynamic Therapy and Quantitative Structure−Activity Relationship Studies with Stable Synthetic Near-Infrared-Absorbing Bacteriochlorin Photosensitizers. J. Med. Chem. 2010, 53, 4018–4027. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.; Clasie, B.; Partridge, M. Review on the characteristics of radiation detectors for dosimetry and imaging. Phys. Med. Biol. 2014, 59, R303–R347. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, T. Inorganic scintillating materials and scintillation detectors. Proc. Jpn. Acad Ser. B Phys. Biol. Sci. 2018, 94, 75–97. [Google Scholar] [CrossRef]
- Ahmad, M.; Pratx, G.; Bazalova, M.; Xing, L. X-Ray Luminescence and X-Ray Fluorescence Computed Tomography: New Molecular Imaging Modalities. IEEE Access 2014, 2, 1051–1061. [Google Scholar] [CrossRef]
- Beddar, A.S. Plastic scintillation dosimetry and its application to radiotherapy. Radiat. Meas. 2006, 41, S124–S133. [Google Scholar] [CrossRef]
- Sengupta, D.; Pratx, G. Single-Cell Characterization of 18F-FLT Uptake with Radioluminescence Microscopy. J. Nucl. Med. 2016, 57, 1136–1140. [Google Scholar] [CrossRef]
- Pratx, G.; Chen, K.; Sun, C.; Axente, M.; Sasportas, L.; Carpenter, C.; Xing, L. High-resolution radioluminescence microscopy of 18F-FDG uptake by reconstructing the β-ionization track. J. Nucl. Med. 2013, 54, 1841–1846. [Google Scholar] [CrossRef]
- Tringale, K.R.; Pang, J.; Nguyen, Q.T. Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1412. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Audisio, R.A.; Poston, G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Miyagishima, D.F.; Moliterno, J.; Claus, E.; Günel, M. Hormone therapies in meningioma-where are we? J. Neuro-Oncol. 2023, 161, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2016, 44, 30–33. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef]
- Sagar, J.; Chaib, B.; Sales, K.; Winslet, M.; Seifalian, A. Role of stem cells in cancer therapy and cancer stem cells: A review. Cancer Cell Int. 2007, 7, 9. [Google Scholar] [CrossRef]
- Chu, D.-T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.-K.; Jeong, J.-H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent progress of stem cell therapy in cancer treatment: Molecular mechanisms and potential applications. Cells 2020, 9, 563. [Google Scholar] [CrossRef]
- Samadani, A.A.; Keymoradzdeh, A.; Shams, S.; Soleymanpour, A.; Norollahi, S.E.; Vahidi, S.; Rashidy-Pour, A.; Ashraf, A.; Mirzajani, E.; Khanaki, K. Mechanisms of cancer stem cell therapy. Clin. Chim. Acta 2020, 510, 581–592. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal stem cells and cancer: Clinical challenges and opportunities. BioMed Res. Int. 2019, 2019, 2820853. [Google Scholar] [CrossRef]
- Albarenque, S.M.; Zwacka, R.M.; Mohr, A. Both human and mouse mesenchymal stem cells promote breast cancer metastasis. Stem Cell Res. 2011, 7, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Tong, Y.; Li, Y.; Yuan, J.; Hu, S.; Hu, T.; Song, G. Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget 2017, 8, 73693. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, K.; Kitadai, Y.; Tanaka, M.; Sumida, T.; Kodama, M.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int. J. Cancer 2010, 127, 2323–2333. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Gilkes, D.M.; Wong, C.C.L.; Luo, W.; Zhang, H.; Wei, H.; Takano, N.; Schito, L.; Levchenko, A.; Semenza, G.L. Hypoxia-inducible factor–dependent breast cancer–mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Investig. 2012, 123. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Zhu, Y.; Smith, D.J.; Zhou, Y.; Li, Y.-R.; Yu, J.; Lee, D.; Wang, Y.-C.; Di Biase, S.; Wang, X.; Hardoy, C. Development of hematopoietic stem cell-engineered invariant natural killer T cell therapy for cancer. Cell Stem Cell 2019, 25, 542–557.e549. [Google Scholar] [CrossRef]
- Kanojia, D.; Balyasnikova, I.V.; Morshed, R.A.; Frank, R.T.; Yu, D.; Zhang, L.; Spencer, D.A.; Kim, J.W.; Han, Y.; Yu, D. Neural stem cells secreting anti-HER2 antibody improve survival in a preclinical model of HER2 overexpressing breast cancer brain metastases. Stem Cells 2015, 33, 2985–2994. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Doo, S.W.; Kim, D.H.; Cha, Y.J.; Kim, J.H.; Song, Y.S.; Kim, S.U. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Lett. 2013, 335, 58–65. [Google Scholar] [CrossRef]
- Yi, B.-R.; Kim, S.U.; Choi, K.-C. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget 2014, 5, 12835. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, J.S.; Lee, D.-S.; Hong, S.H.; Lee, H.J. Anti-Cancer Effect of Neural Stem Cells Transfected with Carboxylesterase and sTRAIL Genes in Animals with Brain Lesions of Lung Cancer. Pharmaceuticals 2023, 16, 1156. [Google Scholar] [CrossRef] [PubMed]
- Mercer-Smith, A.R.; Jiang, W.; Bago, J.R.; Valdivia, A.; Thang, M.; Woodell, A.S.; Montgomery, S.A.; Sheets, K.T.; Anders, C.K.; Hingtgen, S.D. Cytotoxic Engineered Induced Neural Stem Cells as an Intravenous Therapy for Primary Non–Small Cell Lung Cancer and Triple-Negative Breast Cancer. Mol. Cancer Ther. 2021, 20, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Nianias, A.; Themeli, M. Induced pluripotent stem cell (iPSC)–derived lymphocytes for adoptive cell immunotherapy: Recent advances and challenges. Curr. Hematol. Malig. Rep. 2019, 14, 261–268. [Google Scholar] [CrossRef]
- Chen, X.; Lei, F.; Wang, L.; Xiong, X.; Song, J. Generation of tumor antigen-specific cytotoxic T lymphocytes from pluripotent stem cells. Cancer Immunosurveill. Methods Protoc. 2019, 1884, 43–55. [Google Scholar] [CrossRef]
- Ueda, T.; Kaneko, S. Induced pluripotent stem cell-derived natural killer cells gene-modified to express chimeric antigen receptor-targeting solid tumors. Int. J. Hematol. 2021, 114, 572–579. [Google Scholar] [CrossRef]
- Walk, E.E.; Yohe, S.L.; Beckman, A.; Schade, A.; Zutter, M.M.; Pfeifer, J.; Berry, A.B.; on behalf of the College of American Pathologists Personalized Health Care Committee. The cancer immunotherapy biomarker testing landscape. Arch. Pathol. Lab. Med. 2020, 144, 706–724. [Google Scholar] [CrossRef]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker testing for patients with advanced non–small cell lung cancer: Real-world issues and tough choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Finck, A.; Gill, S.I.; June, C.H. Cancer immunotherapy comes of age and looks for maturity. Nat. Commun. 2020, 11, 3325. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, J.; McEntee, M.C. Principles and applications of radiation therapy. Clin. Tech. Small Anim. Pract. 2003, 18, 82–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Lan, G.; Tang, H.; Pelizzari, C.; Fu, Y.-X.; Spiotto, M.T.; et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018, 2, 600–610. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, I.; Uchino, Y.; Kobayashi, E.; Yamaura, A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: A systematic review of outcome and risk of three therapeutic options. Neurol. Res. 2003, 25, 682–690. [Google Scholar] [CrossRef]
- Waksman, R.; Ajani, A.E.; White, R.L.; Chan, R.; Bass, B.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Torguson, R.; Deible, R.; et al. Five-Year Follow-Up After Intracoronary Gamma Radiation Therapy for In-Stent Restenosis. Circulation 2004, 109, 340–344. [Google Scholar] [CrossRef]
- Gieschen, H.L.; Spiro, I.J.; Suit, H.D.; Ott, M.J.; Rattner, D.W.; Ancukiewicz, M.; Willett, C.G. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 127–131. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sole, C.V.; Rutten, H.J.; Poortmans, P.; Asencio, J.M.; Serrano, J.; Aristu, J.; Roeder, F.; Dries, W.J. ESTRO/ACROP IORT recommendations for intraoperative radiation therapy in primary locally advanced rectal cancer. Clin. Transl. Radiat Oncol. 2020, 25, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Esperança-Martins, M.; Melo-Alvim, C.; Dâmaso, S.; Lopes-Brás, R.; Peniche, T.; Nogueira-Costa, G.; Abreu, C.; Luna Pais, H.; de Sousa, R.T.; Torres, S.; et al. Breast Sarcomas, Phyllodes Tumors, and Desmoid Tumors: Turning the Magnifying Glass on Rare and Aggressive Entities. Cancers 2023, 15, 3993. [Google Scholar] [CrossRef]
- Loeffler, J.S.; Shih, H.A. Radiation Therapy in the Management of Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2011, 96, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Cannon, G.M.; Tomé, W.A.; Robins, H.I.; Howard, S.P. Pulsed reduced dose-rate radiotherapy: Case report. J. Neuro-Oncol. 2007, 83, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.C. Pulsed low dose-rate radiotherapy: Radiobiology and dosimetry. Phys. Med. Biol. 2022, 67, 03TR01. [Google Scholar] [CrossRef]
- Bhatia, K.; Bhumika; Das, A. Combinatorial drug therapy in cancer—New insights. Life Sci. 2020, 258, 118134. [Google Scholar] [CrossRef]
- Yeo, E.L.; Li, Y.Q.; Soo, K.-C.; Wee, J.T.; Chua, M.L. Combinatorial strategies of radiotherapy and immunotherapy in nasopharyngeal carcinoma. Chin. Clin. Oncol. 2018, 7, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Hou, J.; Xiong, W.; Kim, H.; Chen, J.; Zheng, C.; Jiang, X.; Yoon, J.; Shen, J. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv. Mater. 2022, 34, 2206121. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Zhu, Y.; Pei, M.; Wang, K.; Qu, X.; Zhang, Y.; Gao, J.; Qin, H. Targeting inorganic nanoparticles to tumors using biological membrane-coated technology. MedComm 2022, 3, e192. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, Z.Q.; Tang, H.X.; Shi, Z.E.; Kang, J.; Liu, Q.; Qi, J. Efficacy-shaping nanomedicine by loading Calcium Peroxide into Tumor Microenvironment-responsive Nanoparticles for the Antitumor Therapy of Prostate Cancer. Theranostics 2020, 10, 9808–9829. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar] [CrossRef]
- Peng, T.; Xu, T.; Liu, X. Research progress of the engagement of inorganic nanomaterials in cancer immunotherapy. Drug Deliv. 2022, 29, 1914–1932. [Google Scholar] [CrossRef]
- Debele, T.A.; Yeh, C.F.; Su, W.P. Cancer Immunotherapy and Application of Nanoparticles in Cancers Immunotherapy as the Delivery of Immunotherapeutic Agents and as the Immunomodulators. Cancers 2020, 12, 3773. [Google Scholar] [CrossRef]
- Vyfhuis, M.A.L.; Onyeuku, N.; Diwanji, T.; Mossahebi, S.; Amin, N.P.; Badiyan, S.N.; Mohindra, P.; Simone, C.B. Advances in proton therapy in lung cancer. Ther. Adv. Respir. Dis. 2018, 12, 1753466618783878. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, J.; Ekman, M.; Ericsson, S.R.; Jönsson, B.; Glimelius, B. Proton therapy of cancer: Potential clinical advantages and cost-effectiveness. Acta Oncol. 2005, 44, 850–861. [Google Scholar] [CrossRef]
- Paganetti, H.; Bortfeld, T. Proton Therapy. In New Technologies in Radiation Oncology; Schlegel, W., Bortfeld, T., Grosu, A.-L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 345–363. [Google Scholar]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Valais, I.; Michail, C.; Bakas, A.; Fountzoula, C.; Cantzos, D.; Bhattacharyya, D.; Sianoudis, I.; Fountos, G.; Yannakopoulos, P.; et al. Radioluminescence properties of the CdSe/ZnS Quantum Dot nanocrystals with analysis of long-memory trends. Radiat. Meas. 2016, 92, 19–31. [Google Scholar] [CrossRef]
- Osakada, Y.; Pratx, G.; Hanson, L.; Solomon, P.E.; Xing, L.; Cui, B. X-ray excitable luminescent polymer dots doped with an iridium(iii) complex. Chem. Commun. 2013, 49, 4319–4321. [Google Scholar] [CrossRef] [PubMed]
- Osakada, Y.; Pratx, G.; Sun, C.; Sakamoto, M.; Ahmad, M.; Volotskova, O.; Ong, Q.; Teranishi, T.; Harada, Y.; Xing, L.; et al. Hard X-ray-induced optical luminescence via biomolecule-directed metal clusters. Chem. Commun. 2014, 50, 3549–3551. [Google Scholar] [CrossRef]
- Wang, C.; Volotskova, O.; Lu, K.; Ahmad, M.; Sun, C.; Xing, L.; Lin, W. Synergistic Assembly of Heavy Metal Clusters and Luminescent Organic Bridging Ligands in Metal–Organic Frameworks for Highly Efficient X-ray Scintillation. J. Am. Chem. Soc. 2014, 136, 6171–6174. [Google Scholar] [CrossRef]
- Morgan, N.Y.; Kramer-Marek, G.; Smith, P.D.; Camphausen, K.; Capala, J. Nanoscintillator Conjugates as Photodynamic Therapy-Based Radiosensitizers: Calculation of Required Physical Parameters. Radiat. Res. 2009, 171, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.P.; Gregas, M.K.; Lauly, B.; Zhang, Y.; Vo-Dinh, T. Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation. ACS Nano 2011, 5, 4679–4687. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Kusuzaki, K.; Murata, H.; Takeshita, H.; Hashiba, M.; Nishimura, T.; Ashihara, T.; Hirasawa, Y. Acridine Orange Excited by Low-Dose Radiation Has a Strong Cytocidal Effect on Mouse Osteosarcoma. Oncology 2002, 62, 85–93. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Kim, B.; Auguste, D.T. Incorporating gold nanoclusters and target-directed liposomes as a synergistic amplified colorimetric sensor for HER2-positive breast cancer cell detection. Theranostics 2017, 7, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Liu, J. Protection and Promotion of UV Radiation-Induced Liposome Leakage via DNA-Directed Assembly with Gold Nanoparticles. Adv. Mater. 2011, 23, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, H.; Wang, X. Thiols-Induced Rapid Photoluminescent Enhancement of Glutathione-Capped Gold Nanoparticles for Intracellular Thiols Imaging Applications. Anal. Chem. 2015, 87, 10230–10236. [Google Scholar] [CrossRef]
- Townley, H.E.; Kim, J.; Dobson, P.J. In vivo demonstration of enhanced radiotherapy using rare earth doped titania nanoparticles. Nanoscale 2012, 4, 5043–5050. [Google Scholar] [CrossRef]
- Klein, S.; Sommer, A.; Distel, L.V.; Neuhuber, W.; Kryschi, C. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem. Biophys. Res. Commun. 2012, 425, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bai, L.; Wu, H.; Tian, F.; Guo, G. Radiosensitization of paclitaxel, etanidazole and paclitaxel+etanidazole nanoparticles on hypoxic human tumor cells in vitro. Biomaterials 2007, 28, 3724–3730. [Google Scholar] [CrossRef] [PubMed]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Paquot, H.; Daouk, J.; Chateau, A.; Retif, P.; Barberi-Heyob, M.; Pinela, S. Radiation-Induced Mitotic Catastrophe Enhanced by Gold Nanoparticles: Assessment with a Specific Automated Image Processing Workflow. Radiat Res. 2019, 192, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci. Rep. 2020, 10, 12096. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA A Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Khan, M.K.; Nasti, T.H.; Buchwald, Z.S.; Weichselbaum, R.R.; Kron, S.J. Repurposing Drugs for Cancer Radiotherapy: Early Successes and Emerging Opportunities. Cancer J. 2019, 25, 106–115. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Berrezoug, A.; Dib, A.; Belbachir, A. Enhanced X-ray absorption by using gold nanoparticles in a biological tissue. Radioprotection 2015, 50, 281–285. [Google Scholar] [CrossRef]
- Liu, Y.; Murray-Stewart, T.; Casero, R.A.; Kagiampakis, I.; Jin, L.; Zhang, J.; Wang, H.; Che, Q.; Tong, H.; Ke, J. Targeting hexokinase 2 inhibition promotes radiosensitization in HPV16 E7-induced cervical cancer and suppresses tumor growth. Int. J. Oncol. 2017, 50, 2011–2023. [Google Scholar] [CrossRef]
- Roeske, J.C.; Nunez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold nanoparticles in cancer treatment. Mol. Pharm. 2018, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Et. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Hastman, D.A.; Melinger, J.S.; Aragones, G.L.; Cunningham, P.D.; Chiriboga, M.; Salvato, Z.J.; Salvato, T.M.; Brown III, C.W.; Mathur, D.; Medintz, I.L. Femtosecond laser pulse excitation of DNA-labeled gold nanoparticles: Establishing a quantitative local nanothermometer for biological applications. ACS Nano 2020, 14, 8570–8583. [Google Scholar] [CrossRef]
- Akouibaa, A.; Masrour, R.; Jabar, A.; Benhamou, M.; Ouarch, M.; Derouiche, A. Study of the optical and thermoplasmonics properties of gold nanoparticle embedded in Al2O3 matrix. Plasmonics 2022, 17, 1157–1169. [Google Scholar] [CrossRef]
- Moustaoui, H.; Saber, J.; Djeddi, I.; Liu, Q.; Diallo, A.T.; Spadavecchia, J.; Lamy de la Chapelle, M.; Djaker, N. Shape and size effect on photothermal heat elevation of gold nanoparticles: Absorption coefficient experimental measurement of spherical and urchin-shaped gold nanoparticles. J. Phys. Chem. C 2019, 123, 17548–17554. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Beik, J.; Mirrahimi, M.; Alamzadeh, Z.; Teymouri, S.; Mahabadi, V.P.; Eslahi, N.; Tazehmahalleh, F.E.; Ghaznavi, H.; Shakeri-Zadeh, A. Triple combination of heat, drug and radiation using alginate hydrogel co-loaded with gold nanoparticles and cisplatin for locally synergistic cancer therapy. Int. J. Biol. Macromol. 2020, 158, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.K.; Sun, I.-C.; Ryu, J.H.; Koo, H.; Park, C.W.; Youn, I.-C.; Choi, K.; Kwon, I.C.; Kim, K.; Ahn, C.-H. Matrix metalloproteinase sensitive gold nanorod for simultaneous bioimaging and photothermal therapy of cancer. Bio Conjug. Chem. 2010, 21, 2173–2177. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Yang, P. Pd-Tipped Au Nanorods for Plasmon-Enhanced Electrocatalytic Hydrogen Evolution with Photoelectric and Photothermal Effects. ChemElectroChem 2018, 5, 778–784. [Google Scholar] [CrossRef]
- Zhu, X.; Yip, H.K.; Zhuo, X.; Jiang, R.; Chen, J.; Zhu, X.-M.; Yang, Z.; Wang, J. Realization of red plasmon shifts up to∼ 900 nm by AgPd-tipping elongated Au nanocrystals. J. Am. Chem. Soc. 2017, 139, 13837–13846. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Hao, H.-L.; Zhao, W.; Zhao, X.; Chen, H.-Y.; Xu, J.-J. A plasmon-enhanced theranostic nanoplatform for synergistic chemo-phototherapy of hypoxic tumors in the NIR-II window. Chem. Sci. 2021, 12, 10848–10854. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Alapan, Y.; Jahnke, T.; Laux, P.; Luch, A.; Aghakhani, A.; Kharratian, S.; Onbasli, M.C.; Bill, J.; Sitti, M. Seed-mediated synthesis of plasmonic gold nanoribbons using cancer cells for hyperthermia applications. J. Mater. Chem. B 2018, 6, 7573–7581. [Google Scholar] [CrossRef]
- Kumar, S.; Mongia, A.; Gulati, S.; Singh, P.; Diwan, A.; Shukla, S. Emerging theranostic gold nanostructures to combat cancer: Novel probes for combinatorial immunotherapy and photothermal therapy. Cancer Treat. Res. Commun. 2020, 25, 100258. [Google Scholar] [CrossRef]
- Piktel, E.; Ościłowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Wollny, T.; Janion, M.; Parlinska-Wojtan, M. ROS-mediated apoptosis and autophagy in ovarian cancer cells treated with peanut-shaped gold nanoparticles. Int. J. Nanomed. 2021, 16, 1993. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Yao, X.; Chen, D.; Fan, M.; Jin, Z.; He, Q. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat. Commun. 2021, 12, 1345. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, S.; Zhao, X.; Chang, L.; Ren, X.; Mei, X.; Chen, Z. Preparation of photothermal responsive and ROS generative gold nanocages for cancer therapy. Chem. Eng. J. 2021, 421, 129744. [Google Scholar] [CrossRef]
- Martínez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zarate-Triviño, D.G.; Rodríguez-Padilla, C. Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells. Int. J. Nanomed. 2019, 14, 7173–7190. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, Y.; Li, Z.; Li, B.; Zhang, H.; Li, L.; Majeed, I.; Zou, P.; Tan, B. Biolabeling hematopoietic system cells using near-infrared fluorescent gold nanoclusters. J. Phys. Chem. C 2011, 115, 16753–16763. [Google Scholar] [CrossRef]
- Shenoi, M.M.; Shah, N.B.; Griffin, R.J.; Vercellotti, G.M.; Bischof, J.C. Nanoparticle preconditioning for enhanced thermal therapies in cancer. Nanomedicine 2011, 6, 545–563. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef] [PubMed]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 17120. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, Y.; Lu, H.; Zhao, D. Silver nanoparticles coupled to anti-EGFR antibodies sensitize nasopharyngeal carcinoma cells to irradiation. Mol. Med. Rep. 2017, 16, 9005–9010. [Google Scholar] [CrossRef]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J. Nanomater. 2017, 2017, 5107485. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, H.; Zhang, X.; Chen, F.; Zhou, Z.; Hu, X.; Chang, S.; Liu, P.; Zhang, H. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 922–930. [Google Scholar] [CrossRef]
- Alidadi, S.; Mohammadian, M.; Zohdiaghdam, R.; Esgandari, K.; Behrouzkia, Z. Evaluation of the Silver Graphene Quantum Dot Effect when Combined with Resveratrol and Radiation in Colorectal Cancer Cells. Iran. Red Crescent Med. J. 2022, 24. [Google Scholar] [CrossRef]
- Elshawy, O.E.; Helmy, E.A.; Rashed, L.A. Preparation, characterization and in vitro evaluation of the antitumor activity of the biologically synthesized silver nanoparticles. Adv. Nanoparticles 2016, 5, 149–166. [Google Scholar] [CrossRef]
- Sabbaghizadeh, R.; Shamsudin, R.; Deyhimihaghighi, N.; Sedghi, A. Enhancement of dose response and nuclear magnetic resonance image of PAGAT polymer gel dosimeter by adding silver nanoparticles. PLoS ONE 2017, 12, e0168737. [Google Scholar] [CrossRef]
- Kleinauskas, A.; Rocha, S.; Sahu, S.; Sun, Y.-P.; Juzenas, P. Carbon-core silver-shell nanodots as sensitizers for phototherapy and radiotherapy. Nanotechnology 2013, 24, 325103. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, W.; Gordon, A.; Gates, V.; Lewandowski, R.; Salem, R.; Larson, A.; Kim, D. Bimetal nano-radiosensitizers for 90Y radioembolization. J. Vasc. Interv. Radiol. 2017, 28, S10. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hayama, K.; Sasagawa, I.; Okada, Y.; Kawase, T.; Tsubokawa, N.; Tsuchimochi, M. HER2-Targeted Multifunctional Silica Nanoparticles Specifically Enhance the Radiosensitivity of HER2-Overexpressing Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 908. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Zhou, Y.; Jiang, Y.; Li, S. Dual Functional Mesoporous Silicon Nanoparticles Enhance the Radiosensitivity of VPA in Glioblastoma. Transl. Oncol. 2017, 10, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Dell’Arciprete, M.L.; Wegmann, M.; Distel, L.V.R.; Neuhuber, W.; Gonzalez, M.C.; Kryschi, C. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem. Biophys. Res. Commun. 2013, 434, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Chen, M.H.; Li, C.Y.; Tung, F.I.; Chen, S.Y.; Liu, T.Y. Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials 2021, 11, 2235. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Zhang, M.; Li, G.; Hao, L. Gadolinium-Coated Mesoporous Silica Nanoparticle for Magnetic Resonance Imaging. Front. Chem. 2022, 10, 837032. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Zhang, C.; Zhu, S.; Li, L.; Gu, Z.; Zhao, Y. Ultrasmall BiOI Quantum Dots with Efficient Renal Clearance for Enhanced Radiotherapy of Cancer. Adv. Sci. 2020, 7, 1902561. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, Y.; Li, F.; Jia, R.; Zhou, G.; Shao, C.; Zhu, L.; Cui, M.; Yang, D.-P.; Ge, S. Graphene Quantum Dots for Radiotherapy. ACS Appl. Mater. Interfaces 2018, 10, 14342–14355. [Google Scholar] [CrossRef]
- Chan, L.; Gao, P.; Zhou, W.; Mei, C.; Huang, Y.; Yu, X.F.; Chu, P.K.; Chen, T. Sequentially Triggered Delivery System of Black Phosphorus Quantum Dots with Surface Charge-Switching Ability for Precise Tumor Radiosensitization. ACS Nano 2018, 12, 12401–12415. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Tian, X.; Dong, H.Y.; Zhang, X.X.; Yu, L.; Li, M.; Gu, M.M.; Gao, D.; Shang, Z.F. Black Phosphorus Quantum Dots Enhance the Radiosensitivity of Human Renal Cell Carcinoma Cells through Inhibition of DNA-PKcs Kinase. Cells 2022, 11, 1651. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Yang, Y.; Zhao, X.; Wang, Q.; Li, B.; Dong, L.; Tian, R.; Bao, Z. Iron-Palladium Decorated Carbon Nanotubes Achieve Radiosensitization via Reactive Oxygen Species Burst. Front. Bioeng. Biotechnol. 2021, 9, 683363. [Google Scholar] [CrossRef]
- Fox, C.R.; Kedarinath, K.; Neal, C.J.; Sheiber, J.; Kolanthai, E.; Kumar, U.; Drake, C.; Seal, S.; Parks, G.D. Broad-Spectrum, Potent, and Durable Ceria Nanoparticles Inactivate RNA Virus Infectivity by Targeting Virion Surfaces and Disrupting Virus–Receptor Interactions. Molecules 2023, 28, 5190. [Google Scholar] [CrossRef] [PubMed]
- Wallbank, A.M.; Vaughn, A.E.; Niemiec, S.; Bilodeaux, J.; Lehmann, T.; Knudsen, L.; Kolanthai, E.; Seal, S.; Zgheib, C.; Nozik, E. CNP-miR146a improves outcomes in a two-hit acute-and ventilator-induced lung injury model. Nanomed. Nanotechnol. Biol. Med. 2023, 50, 102679. [Google Scholar] [CrossRef]
- Daré, R.G.; Kolanthai, E.; Neal, C.J.; Fu, Y.; Seal, S.; Nakamura, C.V.; Lautenschlager, S.O. Cerium Oxide Nanoparticles Conjugated with Tannic Acid Prevent UVB-Induced Oxidative Stress in Fibroblasts: Evidence of a Promising Anti-Photodamage Agent. Antioxidants 2023, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kolanthai, E.; Neal, C.J.; Kumar, U.; Zgheib, C.; Liechty, K.W.; Seal, S. Engineered faceted cerium oxide nanoparticles for therapeutic miRNA delivery. Nanomaterials 2022, 12, 4389. [Google Scholar] [CrossRef]
- Shin, C.S.; Veettil, R.A.; Sakthivel, T.S.; Adumbumkulath, A.; Lee, R.; Zaheer, M.; Kolanthai, E.; Seal, S.; Acharya, G. Noninvasive Delivery of Self-Regenerating Cerium Oxide Nanoparticles to Modulate Oxidative Stress in the Retina. ACS Appl. Bio Mater. 2022, 5, 5816–5825. [Google Scholar] [CrossRef]
- Kolanthai, E.; Neal, C.J.; Kumar, U.; Fu, Y.; Seal, S. Antiviral nanopharmaceuticals: Engineered surface interactions and virus-selective activity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1823. [Google Scholar] [CrossRef]
- El Ghzaoui, C.; Neal, C.J.; Kolanthai, E.; Fu, Y.; Kumar, U.; Hu, J.; Zgheib, C.; Liechty, K.W.; Seal, S. Assessing the bio-stability of microRNA-146a conjugated nanoparticles via electroanalysis. Nanoscale Adv. 2023, 5, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Zhang, H.; Cheng, M.-j.; Lu, Q. Application of Ceria in CO2 Conversion Catalysis. ACS Catal. 2020, 10, 613–631. [Google Scholar] [CrossRef]
- Ziemba, M.; Schilling, C.; Ganduglia-Pirovano, M.V.; Hess, C. Toward an Atomic-Level Understanding of Ceria-Based Catalysts: When Experiment and Theory Go Hand in Hand. Acc. Chem. Res. 2021, 54, 2884–2893. [Google Scholar] [CrossRef]
- Tang, J.L.Y.; Moonshi, S.S.; Ta, H.T. Nanoceria: An innovative strategy for cancer treatment. Cell Mol. Life Sci. 2023, 80, 46. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Fu, Y.; Omer, M.; Adhikary, A.; Ward, S.; Ta, K.M.; Moxon, S.; Molinari, M.; et al. A novel approach for the prevention of ionizing radiation-induced bone loss using a designer multifunctional cerium oxide nanozyme. Bioact. Mater. 2023, 21, 547–565. [Google Scholar] [CrossRef]
- Das, S.; Neal, C.J.; Ortiz, J.; Seal, S. Engineered nanoceria cytoprotection in vivo: Mitigation of reactive oxygen species and double-stranded DNA breakage due to radiation exposure. Nanoscale 2018, 10, 21069–21075. [Google Scholar] [CrossRef]
- Popov, A.L.; Zaichkina, S.I.; Popova, N.R.; Rozanova, O.M.; Romanchenko, S.P.; Ivanova, O.S.; Smirnov, A.A.; Mironova, E.V.; Selezneva, I.I.; Ivanov, V.K. Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv. 2016, 6, 106141–106149. [Google Scholar] [CrossRef]
- Liu, S.; Fang, L.; Ding, H.; Zhang, Y.; Li, W.; Liu, B.; Dong, S.; Tian, B.; Feng, L.; Yang, P. Alternative Strategy to Optimize Cerium Oxide for Enhanced X-ray-Induced Photodynamic Therapy. ACS Nano 2022, 16, 20805–20819. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Gomez-Cerezo, N.; Kubacka, A.; Fernandez-Garcia, M. Oxide-based composites: Applications in thermo-photocatalysis. Catal. Sci. Technol. 2021, 11, 6904–6930. [Google Scholar] [CrossRef]
- Yang, C.-J.; Nguyen, D.D.; Lai, J.-Y. Poly(l-Histidine)-Mediated On-Demand Therapeutic Delivery of Roughened Ceria Nanocages for Treatment of Chemical Eye Injury. Adv. Sci. 2023, 10, 2302174. [Google Scholar] [CrossRef]
- Zhou, X.; You, M.; Wang, F.; Wang, Z.; Gao, X.; Jing, C.; Liu, J.; Guo, M.; Li, J.; Luo, A.; et al. Multifunctional Graphdiyne–Cerium Oxide Nanozymes Facilitate MicroRNA Delivery and Attenuate Tumor Hypoxia for Highly Efficient Radiotherapy of Esophageal Cancer. Adv. Mater. 2021, 33, 2100556. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, J.; Hu, J.; Akakuru, O.U.; Sun, S.; Chen, T.; Wu, A. Navigating nMOF-mediated enzymatic reactions for catalytic tumor-specific therapy. Mater. Horiz. 2020, 7, 3176–3186. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.-G.; Kim, D.; Park, J.Y.; Oh, Y.-K. Nanoformulation-based sequential combination cancer therapy. Adv. Drug Deliv. Rev. 2017, 115, 57–81. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Woodford, K.; Panettieri, V.; Ruben, J.D.; Senthi, S. Limiting the risk of cardiac toxicity with esophageal-sparing intensity modulated radiotherapy for locally advanced lung cancers. J. Thorac. Dis. 2016, 8, 942–949. [Google Scholar] [CrossRef]
- Li, X.-R.; Cheng, X.-H.; Zhang, G.-N.; Wang, X.-X.; Huang, J.-M. Cardiac safety analysis of first-line chemotherapy drug pegylated liposomal doxorubicin in ovarian cancer. J. Ovarian Res. 2022, 15, 96. [Google Scholar] [CrossRef]
- Shreffler, J.W.; Pullan, J.E.; Dailey, K.M.; Mallik, S.; Brooks, A.E. Overcoming Hurdles in Nanoparticle Clinical Translation: The Influence of Experimental Design and Surface Modification. Int. J. Mol. Sci. 2019, 20, 6056. [Google Scholar] [CrossRef]
- Schmidt, J.; Marques, M.R.G.; Botti, S.; Marques, M.A.L. Recent advances and applications of machine learning in solid-state materials science. npj Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]


| Mechanism of Action | Common Drugs |
|---|---|
| Alkylating Agents: This class of drugs reacts with nucleophilic sites on nucleic acids and proteins to stimulate the formation of unstable alkyl groups. This reaction then inhibits DNA replication and transcription. | Bendamustine, cyclophosphamide, ifosfamide, carmustine, lomustine, temozolomide, carboplatin, thiotepa, cisplatin, oxaliplatin, busulfan, dacarbazine, procarbazine. |
| Antimetabolites: Interfere with DNA methyltransferase and/or DNA polymerase to inhibit DNA replication. | Cytidine analogs: cytarabine; azacitidine; gemcitabine; decitabine. Folate antagonists: methotrexate; pemetrexed. Purine analogs: cladribine; clofarabine; nelarabine. Pyrimidine analogs: fluorouracil; capecitabine. |
| Antimicrotubular Agents: Topoisomerase Inhibitors: Inhibit topoisomerase 1 or topoisomerase 2 to inhibit DNA repair and block DNA and RNA synthesis. Taxanes: Disruption of microtubule assembly, thereby inhibiting cell cycle progression in the M phase. Vinca alkaloids: Bind to tubulin to inhibit microtubule development. This complex then causes cell cycle arrest in metaphase. | Topoisomerase 1 Inhibitors: irinotecan; topotecan. Topoisomerase 2 Inhibitors: doxorubicin; Daunorubicin; idarubicin; mitoxantrone. Taxanes: paclitaxel; docetaxel; cabazitaxel. Vinca alkaloids: vinblastine; vincristine; vinorelbine. |
| Antibiotics: Inhibit the synthesis of RNA and DNA Binds to DNA to produce single and double-stranded breaks in DNA. | Actinomycin D, bleomycin, daunomycin. |
| Inhibits ribonucleoside diphosphate reductase, thus cell cycle progression in the S phase. | Hydroxyurea. |
| Targets RAR-alpha pathway, thereby promoting cell differentiation. | Tretinoin. |
| Stimulates cell differentiation. | Arsenic trioxide. |
| Inhibits the functions of proteasomes. | Bortezomib. |
| Radiation Type | Methodology | Subjects | Results | Why | Source |
|---|---|---|---|---|---|
| X-rays | Metal–organic frameworks were injected into local tumors, then treated with X-ray radiation. | Mouse models of breast and colorectal cancer. | Low-level X-ray radiation was able to remove the local tumors and subsequently prevent tumors from reoccurrence. | The frameworks coupled with X-ray radiation helped to overcome the limitations of cytotoxic T-cell response, and X-ray radiation served to perform an “in situ vaccination”. | [110] |
| X-rays | X-rays are used to induce a photodynamic therapy process, which is coupled with radiation therapy to produce significantly better tumor-killing abilities. | In vitro: H1299-Luc cells In vivo: 5–6 week athymic nude mice | The development of a treatment known as X-PDT demonstrated increased efficiency both in vivo and in vitro against tumorous cells, especially more thermodynamically resistant cells. | X-PDT was shown to increase the penetrance of light, allowing for a PDT process to occur while combining it with radiotherapy. | [111] |
| Gamma rays | Gamma Knife surgery allows for precise treatments to occur, most commonly found in the brain. | A total of 1475 patients afflicted with acoustic neurinomas. | The study showed that among the 1475 patients who underwent Gamma Knife Surgery, only 8% had enlargement afterward over a 3-year period. Compared with microsurgery, Gamma Knife also allows for a lower morbidity rate and fewer complications. | Gamma Knife suppresses tumor growth and provides tumor control by breaking the ability for the tumor to reform and grow through its DNA damage. | [112] |
| Gamma Rays | Gamma rays used through prompt exposure were found to leave nearly the same amount of radiation in soft tissue as compared to the lower energy waves that were used. | Results obtained from statistical analysis | The study found that gamma rays differed by a factor of two in their dosing when used in a therapeutic setting and allowed for the necessary increase in energy when dealing with thermoresistant cells in cancer treatment. | Gamma rays produce significantly more energy than X-ray radiation and can be modified to provide a therapeutic treatment. | [113] |
| Electron Beam | Patients underwent an external beam treatment with dosages of up to 45 gy. | 37 patients with soft tissue sarcoma | The study found that among those who underwent electron beam treatment, nearly 83% remained sarcoma-free, whereas that percentage dropped to 59% for those who did not. Additionally, patients provided excellent local control and a disease-free state at acceptable mortality. | The treatment allowed for the removal of the sarcoma from the soft tissue and allowed for the area to remain cancer-free. | [114] |
| Electron Beam | Intraoperative electron beam radiation therapy (IORT). | Sixty-five patients with recurrent areas or high-risk areas of cancer. | The study showed that the 5-year rate of survivability and disease-free rate increased from 60 and 32%, respectively, to almost 88% and 53% with the undergoing of IORT. | IORT functions to help the complete recitation of dangerous or recurrent tumors. | [115] |
| Type | Application | Radiation Used | Mechanism | Synthesis | Size | Ref. |
|---|---|---|---|---|---|---|
| Liposomes functionalized with gold nanoclusters | Imaging and Diagnostic | Wide variety | Allows for colorimetric detection of HER-2-positive breast cancer cells | Extrusion Method | 175.04 +/− 2.45 nm | [142] |
| AuNPs and Liposomes | Therapeutic | Ultraviolet (UV) radiation | DNA-directed assembly of biomolecules for alleviation of pathogenic symptoms of various diseases | Extrusion Method | 103 nm | [143] |
| AuNPs | Imaging | Photoluminescence | Allows for detection and imaging of intracellular thiols that can be significant biomarkers for chronic diseases and their progression | Not Specified | 1.8–3.0 nm | [144] |
| Titanium Dioxide | Therapeutic | UV radiation, X-ray radiation (when titanium dioxide NPs were functionalized with gadolinium) | Amplify the formation of reactive oxygen species (ROS) in corresponding tumor tissue to facilitate apoptosis for cancer therapy (i.e., glioblastoma) | Not Specified | Not Specified | [145] |
| Quantum Dots synthesized from CaF, LaF, ZnS, or ZnO | Therapeutic | Light waves (most biocompatible), X-rays, gamma rays | Generation of radicals upon light radiation for cancer therapy | Not Specified | Not Specified | |
| Superparamagnetic Iron Oxide | Therapeutic | X-rays | Exhibit cytotoxic effects on cancer cell lines through the facilitation of radicals’ production | Not Specified | Not Specified | [146] |
| Polymer | Therapeutic | 60 Co source emitting gamma rays | Function as an Amifostine carrier and radiosensitizer to allow cancer cells to amplify the effects of radiation therapy (part of synergistic therapy) | Not Specified | Not Specified | [147] |
| Solid Lipid NPs | Therapeutic | N/A | Function as a radiosensitizer and deliver small interfering RNAs (siRNAs) antagonists for programmed cell death ligand-1 (PD-L1) and epidermal growth factor receptor (EGFR) | Melt–Emulsification | 51.3 nm | [148] |
| AuNPs | Therapeutic | Photon Beam | Function as a synergistic radiation therapy to amplify mitosis perturbation pathways | Not Specified | Not Specified | [149] |
| AuNPs | Therapeutic | Photon Beam | Control the release and dosimetry of radiotherapy in cancer cells (synergy therapy) | Citrate-Reduction Technique | 20.90 +/− 0.14 nm | [150] |
| Nanoparticle and Functionalization | Synthesis Method and Size | Experimental System | Radiation Utilized and Dose | Mechanism | Notable Results | Ref. |
|---|---|---|---|---|---|---|
| AgNPs Functionalized with Polyethylene Glycol and Aptamer As1411 | Electrochemical Synthesis, 18 nm. | C6 glioma cells, human microvascular endothelial cells. | X-Rays, 6 MV, 200 cGy/min. | Dark-field microscopy and confocal laser scanning microscopy were utilized to evaluate the targeting properties of the synthesized NPs MTT and Annexin V-FITC/PI assays and C6 glioma spheroid models were utilized to assess tumor spheroid penetration and apoptotic effects. | PEG and As1411 surface modifications significantly enhanced Ag nanoparticle uptake in glioma tumor cells. The synthesized NPs successfully penetrated the core of tumor spheroids. The AgNPs modified with PEG and As1411 facilitated amplified apoptosis rates. Synergistic therapy of PEG-As1411 AgNPs and X-ray irradiation prolonged survival time of C6 glioma mice. | [180] |
| PEGylated graphene quantum dot-decorated Silver Nanoprisms | Synthesized by exploiting the non-covalent electrostatic interactions created between the AgNPs and the PEG-graphene quantum dots Size: 18–45 nm. | In vitro: HCT 116 and HT29 (relatively radiation-resistant) colorectal cancer cells; both cell lines are derived from humans. In vivo: Male Swiss nu/nu mice (age: 5–8 weeks). | X-rays, 2–10 Gy. | The PEGylated AgNPs did not significantly amplify ROS production in irradiated HCT 116 but increased ROS production by 18% in irradiated HT29 cells. The NPs were able to pose radiosensitive effects on colorectal cancer cells by amplifying ROS production pathways. | The combination therapy of conventional radiation and the administration of the synthesized NPs stunted colorectal tumor growth and prolonged the survival time when compared to groups treated with only radiotherapy. | [181] |
| AgNPs functionalized with anti-EGFR antibodies | Thermal Reduction Method Size: 20 nm. | In vitro: Human Nasopharyngeal carcinoma epithelial cells. In vivo: Mouse model with nasopharyngeal carcinoma. | X-rays 0, 2, 4, 6, or 8 Gy | The AgNPs functionalized with anti-EGFR antibodies posed radiosensitive effects by downregulating the expression of the mitosis arrest proteins, Rad51, Ku-80, and Ku-70, in human nasopharyngeal carcinoma epithelial cells. This downregulation facilitated apoptosis in the cancer cells. | The Ag-anti EGFR NPs inhibited the proliferation of nasopharyngeal carcinoma epithelial cells and stimulated their apoptosis via G2 cell cycle arrest. | [182] |
| AgNPs in Conjunction with Cisplatin | AgNPs were capped with polyvinyl pyrrolidone. Size: 23.1 +/− 6.9 nm. | In vitro: Ovarian cancer cell lines (SKOV3, A2780, and OVCAR3). | Not Specified. | AgNPs facilitated radio sensitization by amplifying ROS production pathways and inducing DNA damage. A2780 cells treated with Ag NPs (100 µg/mL) became rounded and enhanced the loss of adherent ovarian cancer cells. A secondary mechanism in which the Ag NPs facilitated apoptosis was by decreasing the glutathione/homodimer disulfide ratio. | The AgNPs inflicted cytotoxic effects via DNA damage and ROS amplification on the A2780 and SKOV3 cell lines. The OVCAR3 cell lines were not as responsive to the AgNPs. Overall, the combination therapy of cisplatin and AgNPs displayed synergistic effects and resulted in a favorable cisplatin dose reduction in the treatment of ovarian cancer cells. | [183] |
| AgNPs | Electrochemical Synthesis Method Size: 26.87 nm. | U251 and C6 glioma cell lines. | X-rays, 6 MeV Dose Rate: 200 cGy/min. | AgNPs facilitate therapeutic effects in cancer by stimulating apoptosis and facilitating destructive autophagy through manipulation of 3-methyladenine Hypoxic U251 glioma cell lines treated with X-rays and Ag NPs exhibited significantly lower mitochondrial membrane potential when compared to hypoxic U251 glioma cell lines treated with X-ray irradiation alone. A decrease in mitochondrial membrane potential is an early step in the apoptosis signaling cascade. | The combination therapy of AgNPs coupled with radiotherapy elicited significant anti-glioma effects in hypoxic C6 and U251 glioma cell lines. | [184] |
| Resveratrol-loaded AgNPs Conjugated with Graphene Quantum Dots | Not Specified. | HCT-116 colorectal cancer cells. | X-rays 2 Gy. | The resveratrol-loaded AgNPs conjugated with graphene quantum dots promoted radio sensitization in HCT-116 colorectal cancer cells by reducing superoxide dismutase (SOD) and glutathione peroxidase (GPX) enzyme activities while increasing malondialdehyde (MDA) levels. Additionally, the NPs facilitated apoptosis by upregulating caspase-3 mRNA expression and decreasing cyclooxygenase (COX-2) protein expression. | Colorectal cancer cell viability was significantly hindered, and apoptosis was amplified when treated with X-ray irradiation and silver quantum dot-Resveratrol NPs The synergistic treatment upregulated caspase-3 mRNA and decreased COX-2 protein expression in colorectal cancer cells. | [185] |
| AgNPs | Rapid Extracellular Biosynthesis by using the fungus Penicillium aurantiogresium. Size: 12.7 nm. | MCF-7 and MCT breast cancer cell lines. | Gamma rays 6 Gy Dose Rate: 0.675 Gy/s. | The Ag NPs complemented gamma-ray irradiation treatment by promoting apoptosis through activation of lactate dehydrogenase, downregulation of Bcl-2 genes upregulation of caspase-3. | AgNPs displayed dose-dependent cytotoxicity and confirmed their capability to function as a potent radiosensitizer for gamma irradiation. Additionally, administration of silver NPs altered cell morphology, inhibited cell proliferation, activated lactate dehydrogenase, and caspase-3. The activation of lactate dehydrogenase and caspase-3 and the downregulation of Bcl-2 genes induced apoptosis. | [186] |
| AgNPs Synthesized with normoxic polyacrylamide gelatin and tetrakis hydroxy methyl phosphonium chloride polymer gel | The AgNPs were synthesized by utilizing laser ablation. Silver NPs were embedded in the final step of the polymer gel preparation process. Size: 20 nm. | Not Applicable. | Gamma rays 6–25 Gy. | Different volumes of AgNPs were experimented with to find the ideal configuration for applications in dose enhancement. At an ideal dose, the AgNPs facilitated polymerization of the polymer gel, which thereby increased the received dose of anti-cancer drugs. | The dose response of anti-cancer drug accumulated in tumor tissue increased by 11.82% when AgNPs were implemented at a concentration of 2 mL. In the presence of AgNPs, the maximum penetration dose of drugs observed was 0.5 cm. Overall, the integration of silver NPs in the system increased the optical density of the drug delivery fabrication. | [187] |
| AgNPs Functionalized with Carbon Nanodots and Polyethylene Glycol | Synthesis Method is not specified. Size: 5–100 nm. | In vitro: Du145 prostate cancer cells. | X-rays Not Specified | PEG was utilized to improve the NPs’ affinity to accumulate within prostate cancer cell tissue. Carbon nanodots were incorporated within the Ag nanostructure to improve stability and radio-sensitizing effects. The fabricated NPs were thought to induce radiosensitization by modulating DNA damage and inducing hyperthermia. | The combination therapy of radiation and Ag NPs provided a synergistic effect and reduced prostate cancer cell survival by 50%. | [188] |
| Bimetallic Au/AgNPs | Bile acid molecules were used to synthesize the branched bimetallic Au/AgNPs. The branched structure of the nanoparticulate system optimized surface area and led to an enhancement in catalytic activity. Size: Not Specified. | In vitro: N1-S1 rodent hepatocellular carcinoma cells | External Beam Radiation, 90Y Thera Sphere exposure. 0–10 Gy. | The bimetallic gold/silver nanoparticulate system functioned as radiosensitizer by facilitating the generation of reactive oxygen species. This process occurred by gold/silver-mediated transfer of electrons to intracellular oxygen. | Exposure to external beam radiation or 90Y Thera Sphere and administration of the bimetallic Au/AgNPs caused significant increases in ROS production. The ROS generation induced oxidative stress and facilitated apoptosis in hepatocellular carcinoma cells. | [189] |
| Nanoparticle and Functionalization and Composition | Synthesis Method and Size | Experimental System | Radiation Utilized and Dose | Mechanism | Notable Results | Ref. |
|---|---|---|---|---|---|---|
| Hyperbranched polyamidoamine grafted onto the surface of amorphous SiNPs | Hyper-branched PAMAM was grafted onto the surface of synthetic amorphous NPs; 40 mg was dissolved in a water/ethanol mixture; the particles were conjugated in fluorescent dye; 20–50 nm sized particles were yielded. | In vitro SK-BR3 Breast cancer cells. | X-ray; 8 Gy. | SiNPs were internalized by the breast cancer cells, allowing for stronger local control. Internalized SiNPs helped to disturb the permeability of the lysosomal membrane, leading to disruptions that can cause apoptosis or cellular necrosis on a larger scale. | Radiation reduced the presence of breast cancer cells when it was applied with the SiNPs, showing that silica could be used to potentially help normal cells during cancer treatment. | [190] |
| Mesoporous SiNPs | MSN-benzimidazole was prepared using a previous method. The particles were dissolved in a valproic acid solution and then centrifuged. No specified particle size. | In vitro Rat glioma C6 and human glioma U87. | X-ray and IR; 4 and 8 Gy | Recognizes the over-producing folic acid in cancerous cells. The NPs then, in turn, release VPA into the environment. Silica is used to do targeted drug delivery and controlled release. | Targeted cancer cells reduced the effects of radiation and also showed a higher rate of cell death and enhanced inhibition. A lower radiation dose may be required to kill cancer cells. | [191] |
| Uncapped and aminosilanized SiNPs | Reverse-micelle wet-chemistry procedure. No specified size. | MCF tumor cells and 3T3 tissue cells. | X-rays; 3 Gy | The synthesized NPs punctured into the cellular mitochondria, creating ROS in the cellular body and provoking oxidative stress. | Increased ROS concentrations in the tumorous cells by almost 180%, whereas only changing the tissue by 120%. Results show that cytotoxicity favors the tumor cells. | [192] |
| Gold-Nanorod-Filled Mesoporous Silica Nanobeads | Formulate a synergistic therapy by combining conventional radiotherapy with gold-nanorod-filled mesoporous silica nanobeads to treat oral squamous carcinoma. | Organic Template Method 120–160 nm CAL-27 and L929 cells. | X-ray 2 Gy | Synthesis procedures included the inclusion of cetyltrimethylammonium bromide (CTAB) in the nanostructure to avoid aggregation and agglomeration. | Effective cellular uptake of the synthesized NPs was achieved in CAL-27 cells. Compared to groups treated only with irradiation, groups treated with the synergistic therapy of radiotherapy and Mesoporous Silica Nanobeads had significantly higher incidences of ROS formation. | [193] |
| Gadolinium Mesoporous Silica Nanoparticles (Gd-MSNPs) | Develop a novel and more effective radiotherapeutic for the eradication of tumor masses. | Sol–Gel method 139 nm OVCAR8 human ovarian cancer cells; HEK293 human embryonic kidney cells. | X-rays 50.25 keV. | The surface of the Gd-MSNPs was functionalized with amine groups to ensure the loading of gadopentetic acid onto the Gd-MSNs | In vitro studies indicated that incubation of the cancer cells with 50 ng of the synthesized NPs completely disintegrated the tumor spheroids. | [194] |
| Nanoparticle and Functionalization | Objective | Radiation Utilized and Dose | Experimental System | Synthesis Method | Size | Mechanism | Notable Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ultrasmall BiOI QDs Surface-Coated with Tween 20 | Develop a nanomaterial-based intratumoral injection radiosensitizer with optimal tumor permeability and renal clearance for biocompatible and practical use in cancer treatment. | X-ray 6 Gy | HUVECs, 4T1 cells, and HeLa cells | KI was dissolved in a solution mixture of DI water and ethanol. Ultrasonication was performed, and Bi(NO3)3·5H2O was added to the solution. After this, centrifugation was performed, and the supernatant was injected into Tween 20 solution. Centrifugation was performed again, and ultrafiltration was used to process the supernatant. The resulting supernatant solution was the BiOI QDs. | 3 nm | The ultrasmall BiOI QDs function as a radiosensitizer by facilitating the catalysis of the abundant hydrogen peroxide present in the tumor microenvironment into hydroxyl free radicals. | The ultrasmall BiOI QDs surface-coated with Tween 20 are biocompatible and mitigate toxicity attributed to long-term retention through systemic elimination via renal metabolic clearance pathways. The synthesized ultrasmall BiOI QDs surface-coated with Tween 20 showed great promise to complement radiotherapy by facilitating the formation of hydroxyl free radicals in the tested experimental systems. | [196] |
| GQDs | Synthesize a nano-radiosensitizer with enhanced uptake in tumor tissue to complement conventional tumor radiotherapy for more effective treatment of colorectal carcinoma. | Gamma rays 3–6 Gy | SW620 and HCT116 cells | Hummer’s method. The graphene quantum dots were synthesized through the photo-Fenton reaction of graphene oxide NPs. | 18 nm | The synergy between the radiosensitization of the GQDs and IR radiotherapy have the potential to upregulate G2/M cell cycle arrest, enhance apoptosis, decrease cell proliferation, and facilitate ROS production in colorectal carcinoma cells. | The GQDs showed great promise in enhancing the sensitivity of colorectal carcinoma cells to IR therapy. The GQDs and IR synergy therapy were able to facilitate cell membrane blebbing, enhance the agglutination of chromatin, induce mitochondrial damage, condense cytoplasmic contents, and increase double-stranded DNA breaks in colorectal carcinoma cells. | [197] |
| Poly(lactic-co-glycolic acid) (PLGA) Ultrasmall Black PQDs | Design a nano-system for precise tumor radiosensitization to minimize the dose required for radiotherapy to be effective. | X-rays 2 Gy | A375 cancer cells, HeLa cancer cells, L02 normal cells. | Emulsion Evaporation Method. PLGA NPs are loaded with black PQDs to create the PLGA-ultrasmall black PQDs. | 150 nm | The PLGA NPs function as carriers of the ultrasmall black PQDs to achieve controlled radiosensitization of tumors by mitigating off-target release and prolonging systemic circulation. The PLGA-ultrasmall black phosphorous quantum dot nanoparticulate system targets the Arg-Gly-Asp-Gys sequence abundant in tumor tissue. In the acidic tumor microenvironment, the 2,3-dimethylmaleic acid anhydride shell decomposes, and the NPs acquire a positive charge, stimulating tumor cell uptake. Additionally, glutathione deoxidizes the disulfide bond of cysteine and enhances the release of the synthesized NP to increase tumor cell sensitivity to radiotherapy. | The synthesized nanosystem facilitated apoptosis of cancer cells by promoting the formation of free radicals and exhibited optimal systemic biocompatibility. | [198] |
| Black PQDs | Synthesize black PQDs to complement the efficacy of conventional radiotherapy for applications in treatment of aggressive renal cell carcinoma. | X-rays 5 or 10 Gy. | 786-O Renal Carcinoma Cells, A498. | 1 g of black PQDs was immersed within N-methyl-2-pyrrolidinone for 12 h at 140 degrees Celsius. | 10 nm | The black PQDs amplify ionizing radiotherapy, inducing double-stranded DNA breaks through their interactions with DNA-protein kinase catalytic subunit and the enhancement of its kinase activity. Additionally, the black PQDs inhibit the autophosphorylation of the DNA-protein kinase catalytic subunit at S2056, which is an essential site for DNA double-strand break repair. | Overall, the black PQDs amplify ionizing radiation-induced apoptosis of renal carcinoma cells, as confirmed by the upregulation of DNA damage biomarkers γH2AX and 53 BP1. Animal experiments confirmed that the synergistic therapy of radiosensitizing black PQDs and ionizing radiotherapy have enhanced efficacy for treating renal cell carcinoma. | [199] |
| GQDs doped with rare-earth upconversion NPs | Design a novel radiosensitization nanosystem to amplify the organelle-specific photodynamic effects of cancer therapy for clinical applications. | UV rays not Specified. | 4T1 cells derived from the mouse mammary tumor cell line. | Not Specified. | 100 nm | When excited with UV rays, the GQDs doped with rare-earth NPs promote the formation of radicals that facilitate the decrease in mitochondrial membrane potential. This process then stimulates irreversible tumor cell apoptosis. | The conjugated nanosystem addresses the limitations of conventional cancer therapy and offers a feasible approach to organelle-specific precision to amplify tumor cell apoptosis. | [200] |
| AgGQDs | Assess the combined therapeutic effects of silver graphene quantum dots, Resveratrol, and radiotherapy on HCT-116 colorectal cancer cells. | X-rays 2 Gy | HCT-116 colorectal cancer cells. | Not Specified. | Not Specified | The AgGQDs stimulated colorectal cancer cell apoptosis by inhibiting superoxide dismutase and glutathione peroxidase activities. Additionally, the AgGQDs increased malondialdehyde concentrations, upregulated caspase-3 mRNA levels, and decreased cyclooxygenase 2 protein expression levels. | The combination therapy of AgGQDs, Resveratrol, and neoadjuvant radiotherapy significantly increased apoptosis of colorectal cancer cells. | [186] |
| Iron–Palladium-Decorated Single-Walled Carbon Nanotubes | Analyze the potential for iron–palladium-decorated single-walled carbon nanotubes to amplify free radical formation to supplement radiotherapy for the treatment of cancer. | X-rays 0, 2, 4, 6, 8 Gy. | MCF-7 cells. | Chemical Reduction Method. | 3–4 nm | The Fe-Pd carbon nanotubes enhanced the frequency of DNA double-stranded breaks by facilitating the generation of reactive oxygen species (ROS). ROS was generated by converting the abundant hydrogen peroxide levels in the tumor microenvironment to hydroxyl radicals. | The Fe-Pd carbon nanotubes at 200 µg/mL had optimal biocompatibility and demonstrated the potential to function as radiosensitizers for improved cancer therapy. | [201] |
| Nanoparticle and Functionalization and Composition | Synthesis Method and Size | Experimental System | Radiation Utilized and Dose | Mechanism | Notable Results | Ref. |
|---|---|---|---|---|---|---|
| CNPs | Core–Shell structure. | In vitro Methyl Orange. | UV-Vis radiation. | Light absorption by the shell structure allows for the formation of defects and photo-excited electrons. These electrons, once excited, are trapped between the CeO2 and the Ce2O3. These electrons are captured by oxygen at the surface, forming O2− radicals. | Degradation was measured to be around 1.5–2.7 times better when the structure was exposed to UV-VIS radiation | [215] |
| Cerium Oxide SCNPs@DMSN@CeOx-PEG | Multifunctional core–shell radiosensitizer. | In vitro (in situ experiment). Measurement as a blue-shift scale. | X-rays. | Core-radiosensitizer absorbs high-energy X-rays, resulting in the formation of a photoinduced electron hole to generate reactive oxygen species. | Use of the radiosensitizer is about 3.79 times better than the use of iohexol (a clinical contrast agent). | [214] |
| GDY-Cerium Oxide nanocomposites | Novel 2D graphdiyene can anchor and disperse CNPs. | In vitro use of radioresistant cell models to determine capabilities of a nanostructure GDY. In vivo study of the distribution of nanostructure in mice with subcutaneous tumors. | Multistage radiation therapy. | GDY is a 2D carbon material that enables unique drug loading as well as photothermal ability. | Nanozymes with the GDY-CeO2 complex are found to provide increased CAT-mimetic behavior as well as effective radio sensitization for ESCC. Also found to be nontoxic for cells and well dispersed throughout live animal models. | [217] |
| CNPs Metal–Organic Frameworks | Synthesized through the one-step method in which ultrasmall homogenous cerium oxide particles are isolated. | In vitro blocked energy supply effects in cells. | None listed. | CNPs have catalytic hydrolysis on ATP and can be utilized to treat synergistic tumors with little to no side effects. | OXD-like and ATP deprivation allowed for high oxidative damage and blockage of energy supply effects. | [218] |
| Amine-modified CNPs | Conjugated with neogambogic acid. | In vitro MCF-7 breast cancer cells in G2/M phase. | X-rays 5-Gy radiation therapy (30 Gy total). | Extreme anticancer behavior was found using different mechanisms. Autophagy was induced by killing the cancer cells. | Surface modification of CNPs additionally decreased radiation-induced ROS formation. | [219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, B.; Stoltz, S.A.; Mittal, A.; Pawar, S.; Kolanthai, E.; Coathup, M.; Seal, S. Inorganic Nanoparticles as Radiosensitizers for Cancer Treatment. Nanomaterials 2023, 13, 2873. https://doi.org/10.3390/nano13212873
Babu B, Stoltz SA, Mittal A, Pawar S, Kolanthai E, Coathup M, Seal S. Inorganic Nanoparticles as Radiosensitizers for Cancer Treatment. Nanomaterials. 2023; 13(21):2873. https://doi.org/10.3390/nano13212873
Chicago/Turabian StyleBabu, Balaashwin, Samantha Archer Stoltz, Agastya Mittal, Shreya Pawar, Elayaraja Kolanthai, Melanie Coathup, and Sudipta Seal. 2023. "Inorganic Nanoparticles as Radiosensitizers for Cancer Treatment" Nanomaterials 13, no. 21: 2873. https://doi.org/10.3390/nano13212873
APA StyleBabu, B., Stoltz, S. A., Mittal, A., Pawar, S., Kolanthai, E., Coathup, M., & Seal, S. (2023). Inorganic Nanoparticles as Radiosensitizers for Cancer Treatment. Nanomaterials, 13(21), 2873. https://doi.org/10.3390/nano13212873






