Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity
Abstract
:1. Introduction
2. Homophase TiO2
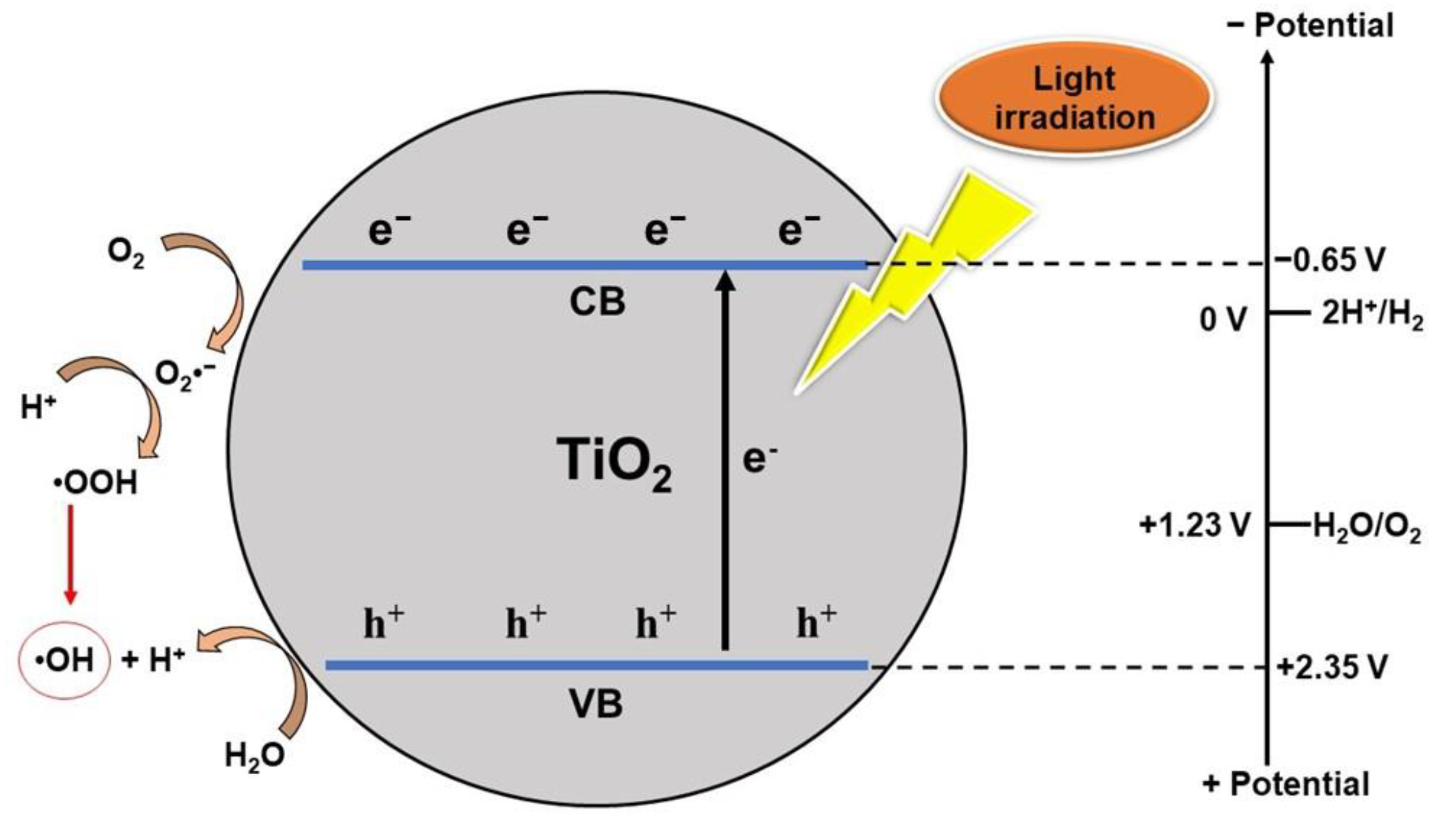
2.1. Photocatalysis Mechanism of TiO2
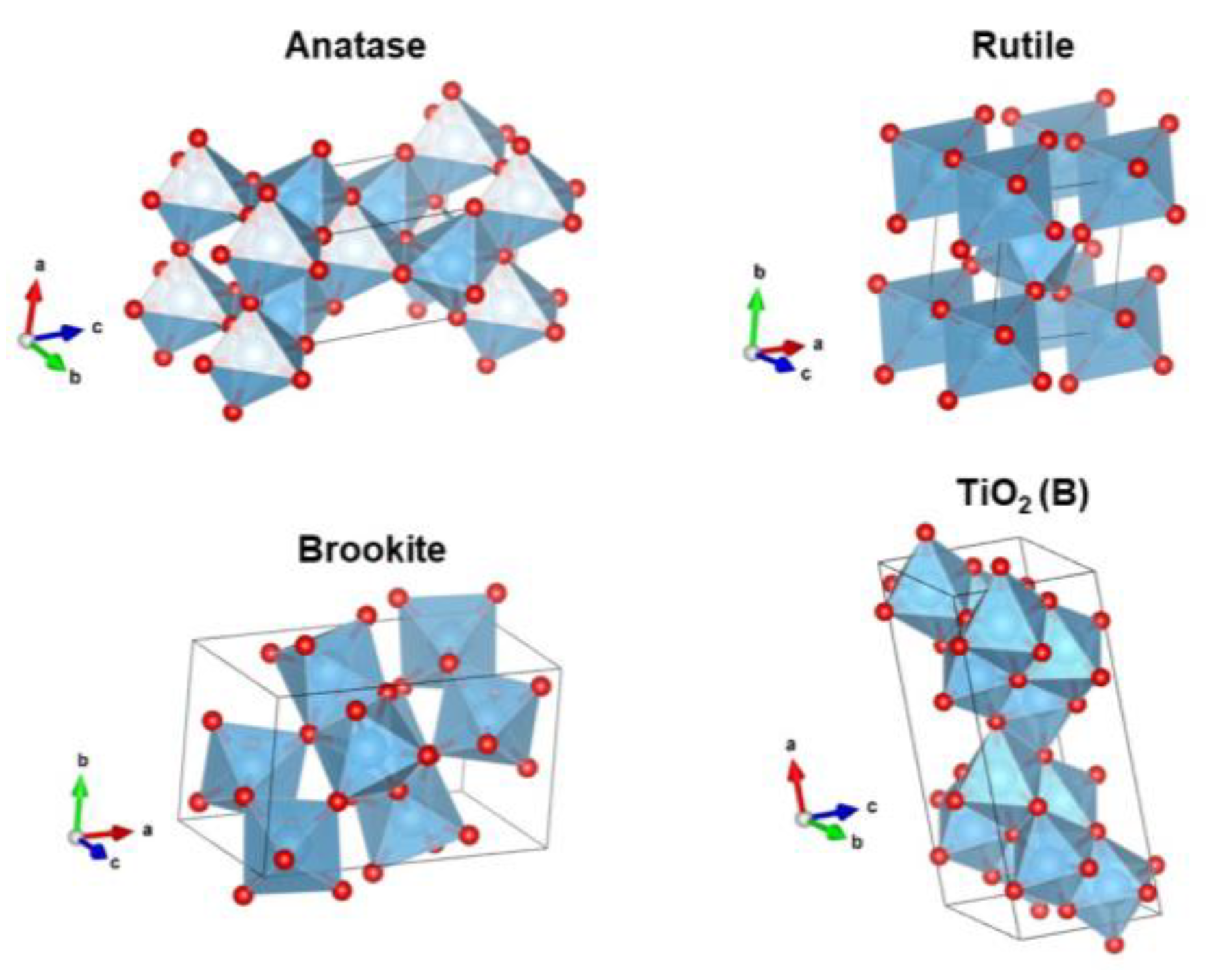
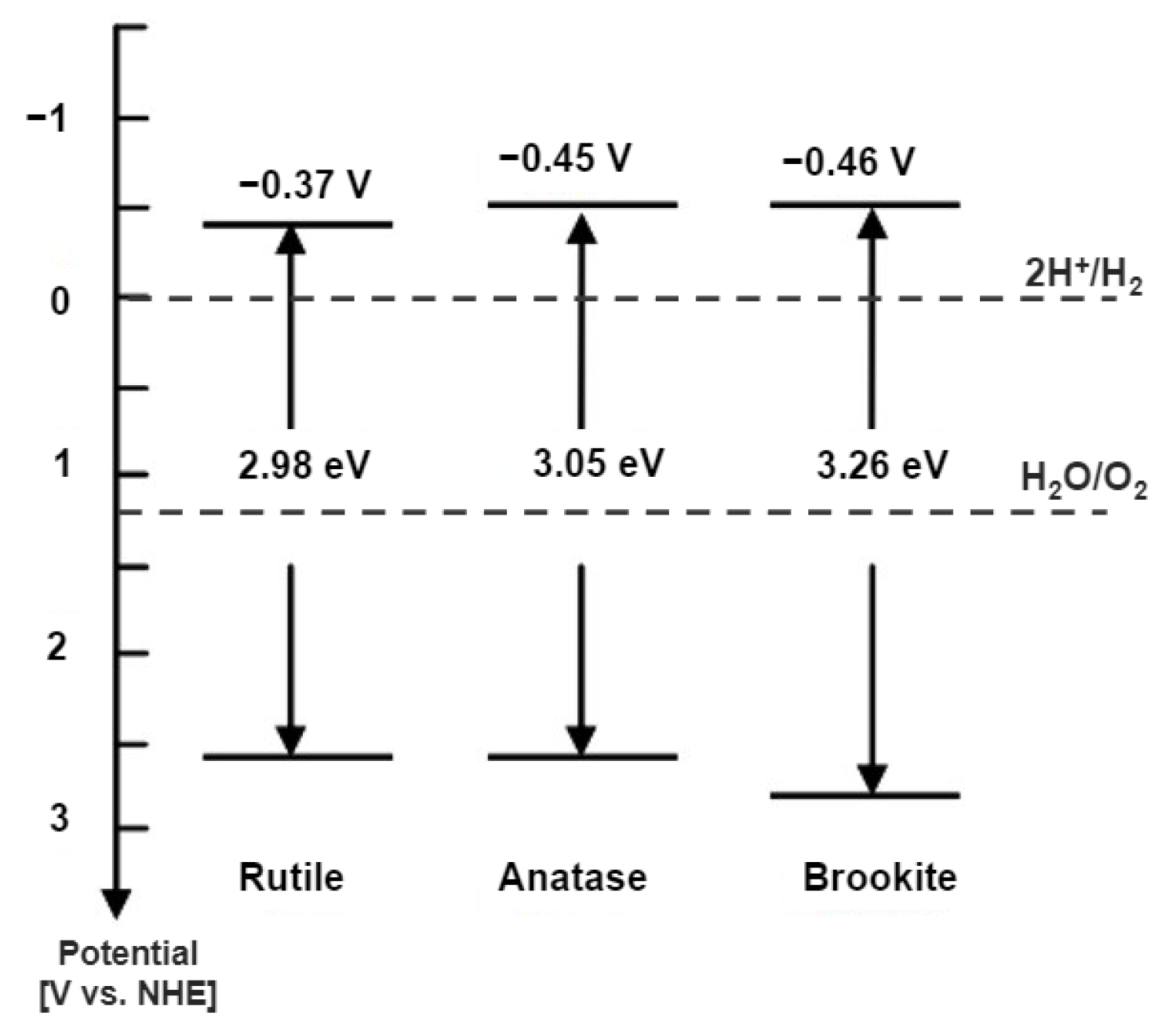
2.2. Phase of TiO2
2.2.1. Anatase
2.2.2. Rutile
2.2.3. Brookite
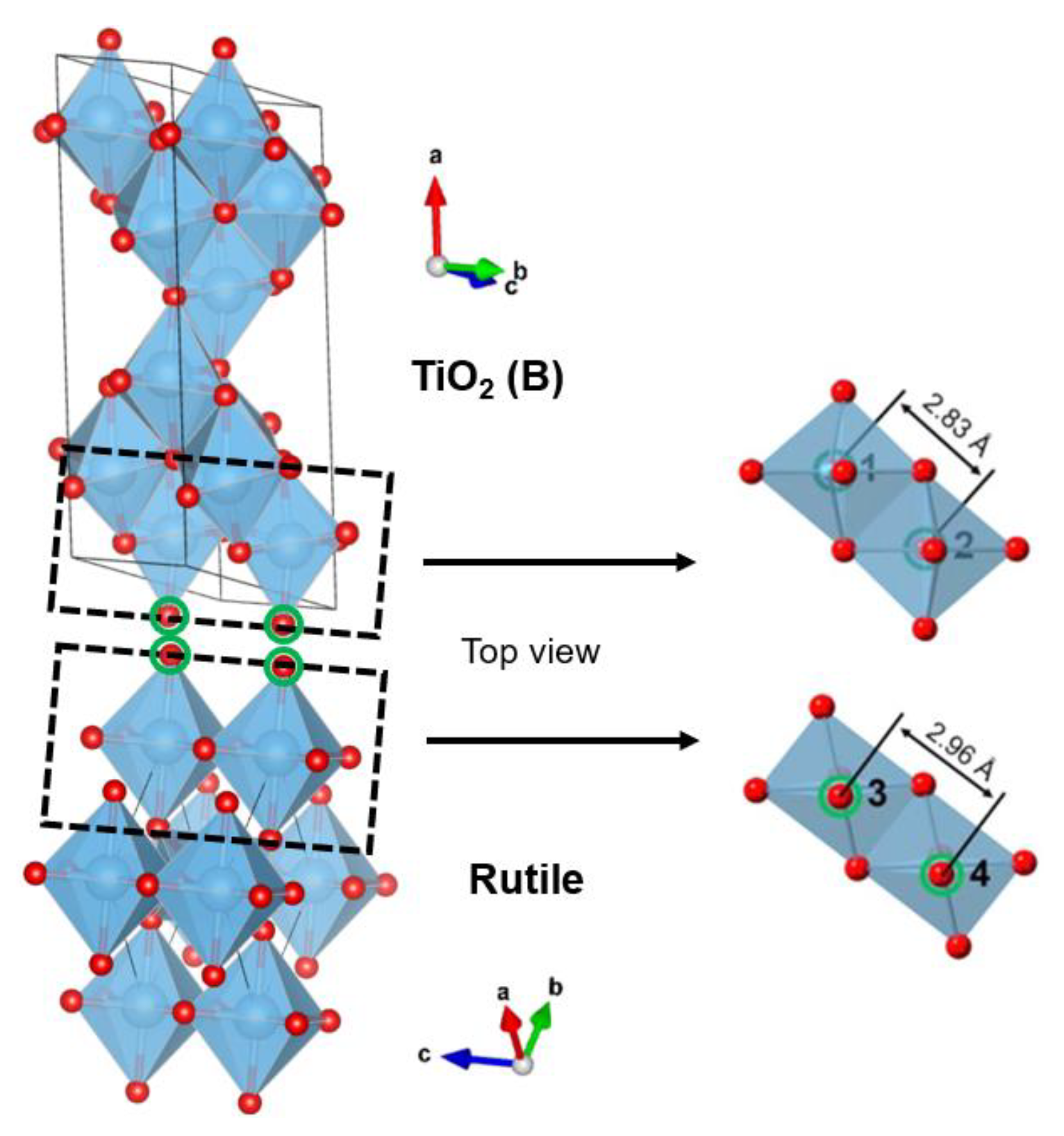
2.2.4. TiO2 (B)
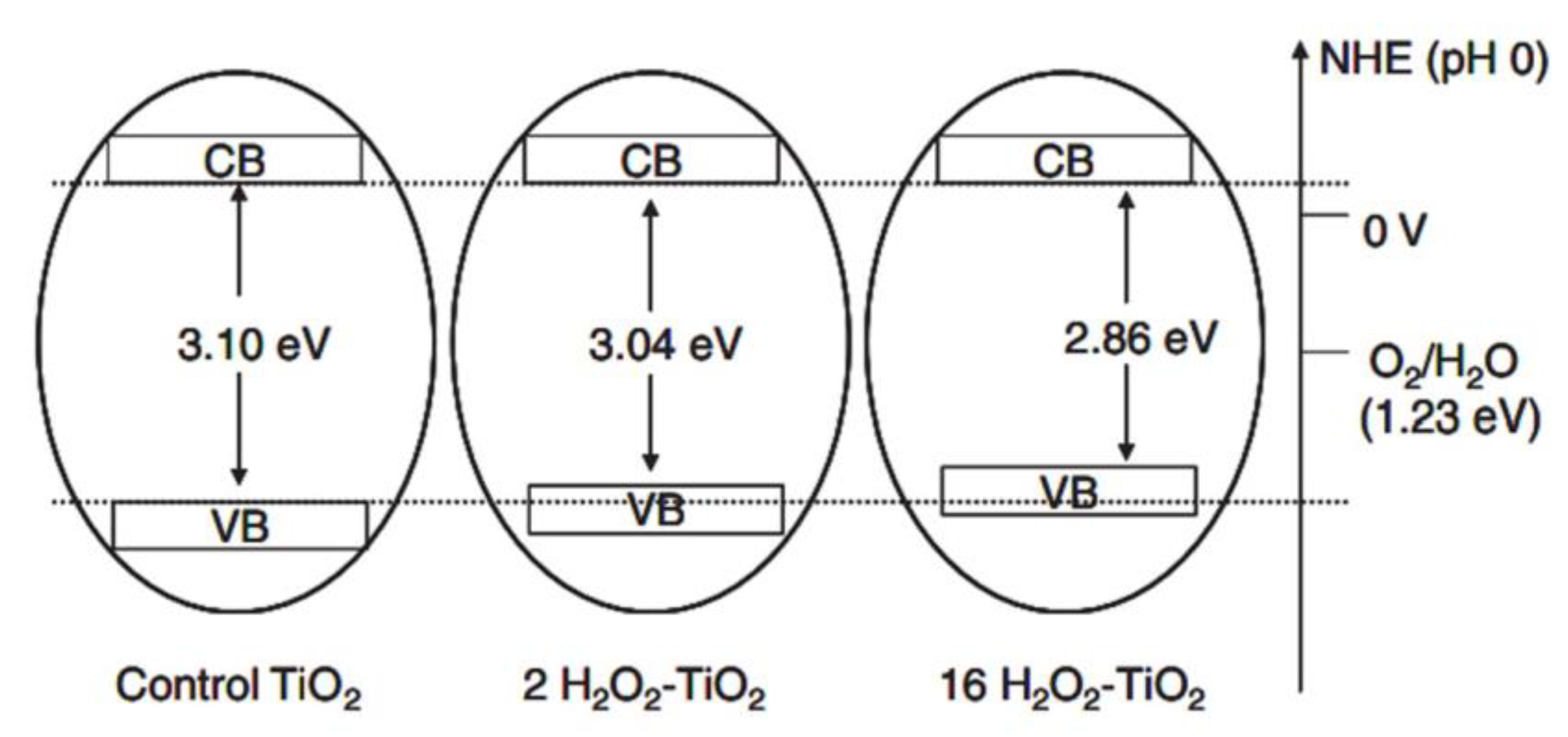
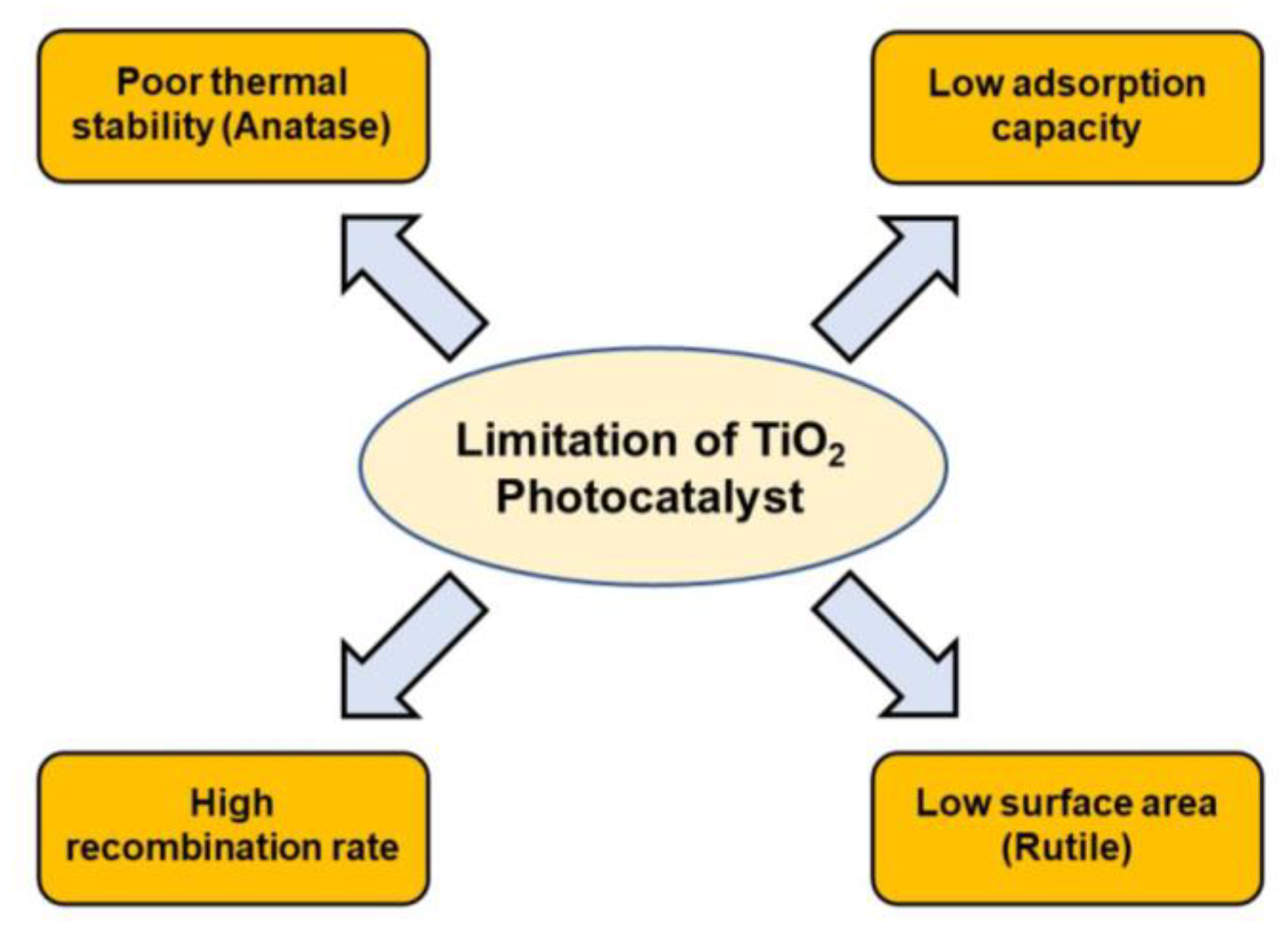
2.3. Disadvantages of TiO2 Homophase Photocatalysis
3. Heterophase TiO2 in Photocatalysts
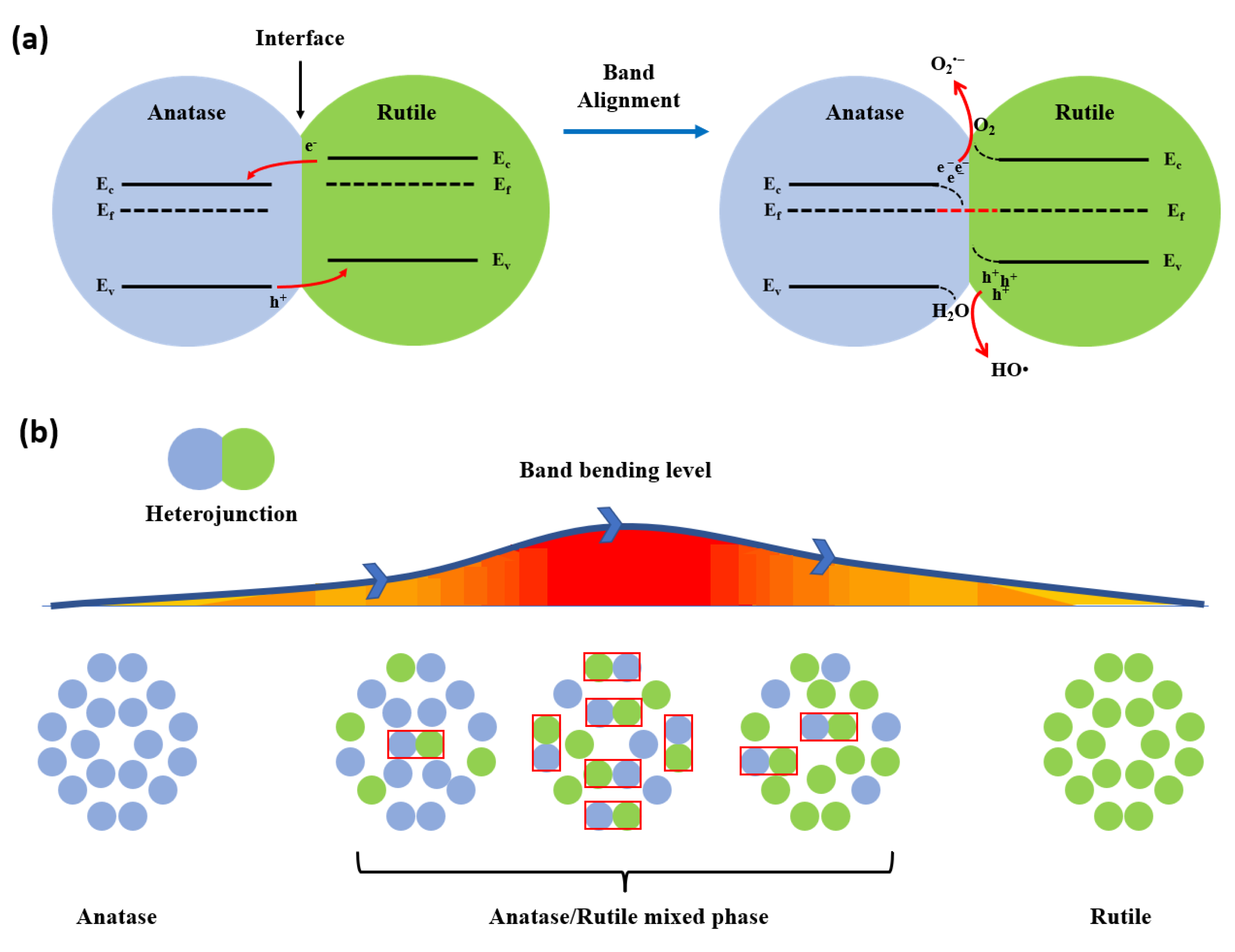
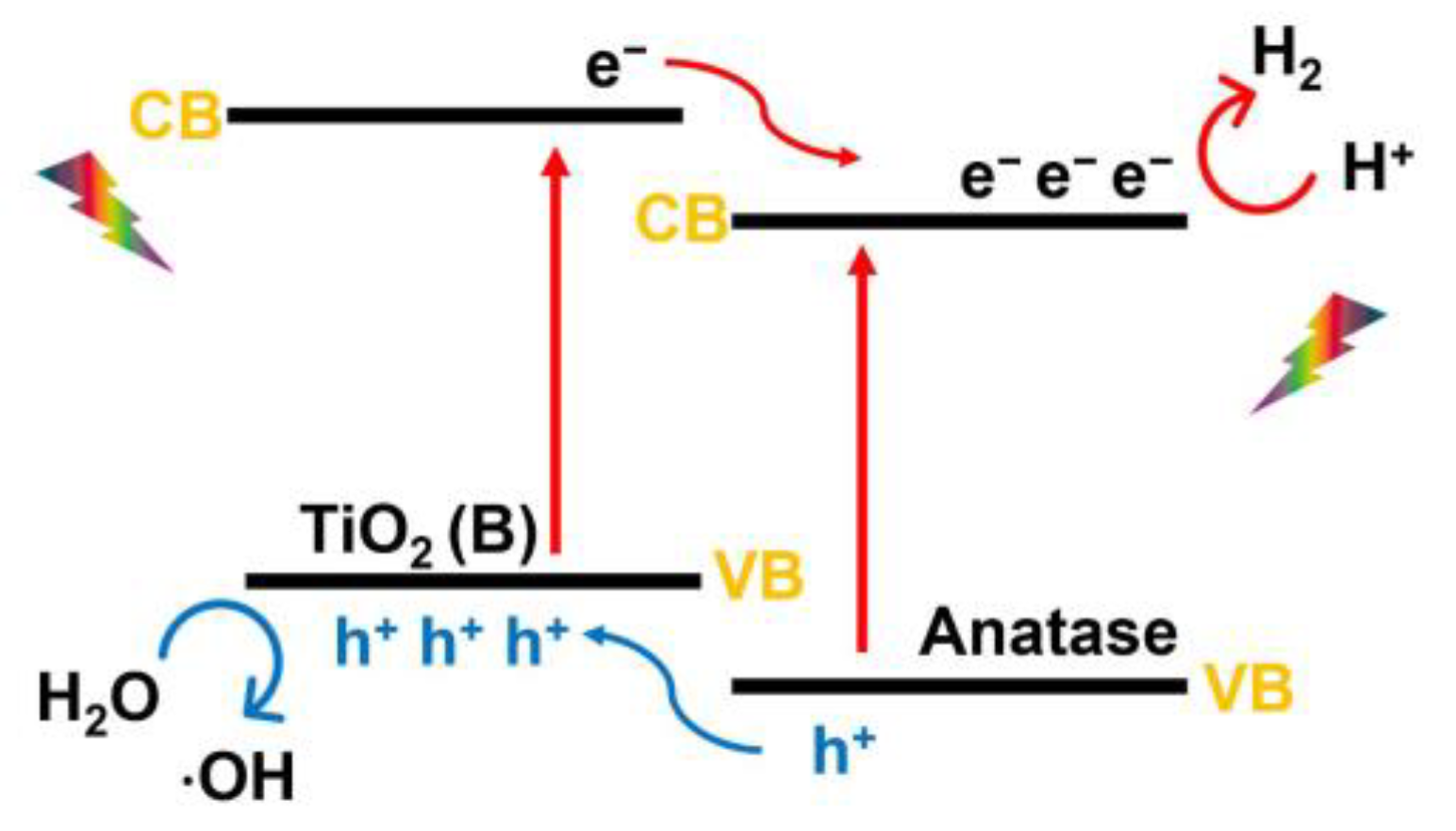
3.1. Photocatalysis Mechanism of TiO2 Heterophase
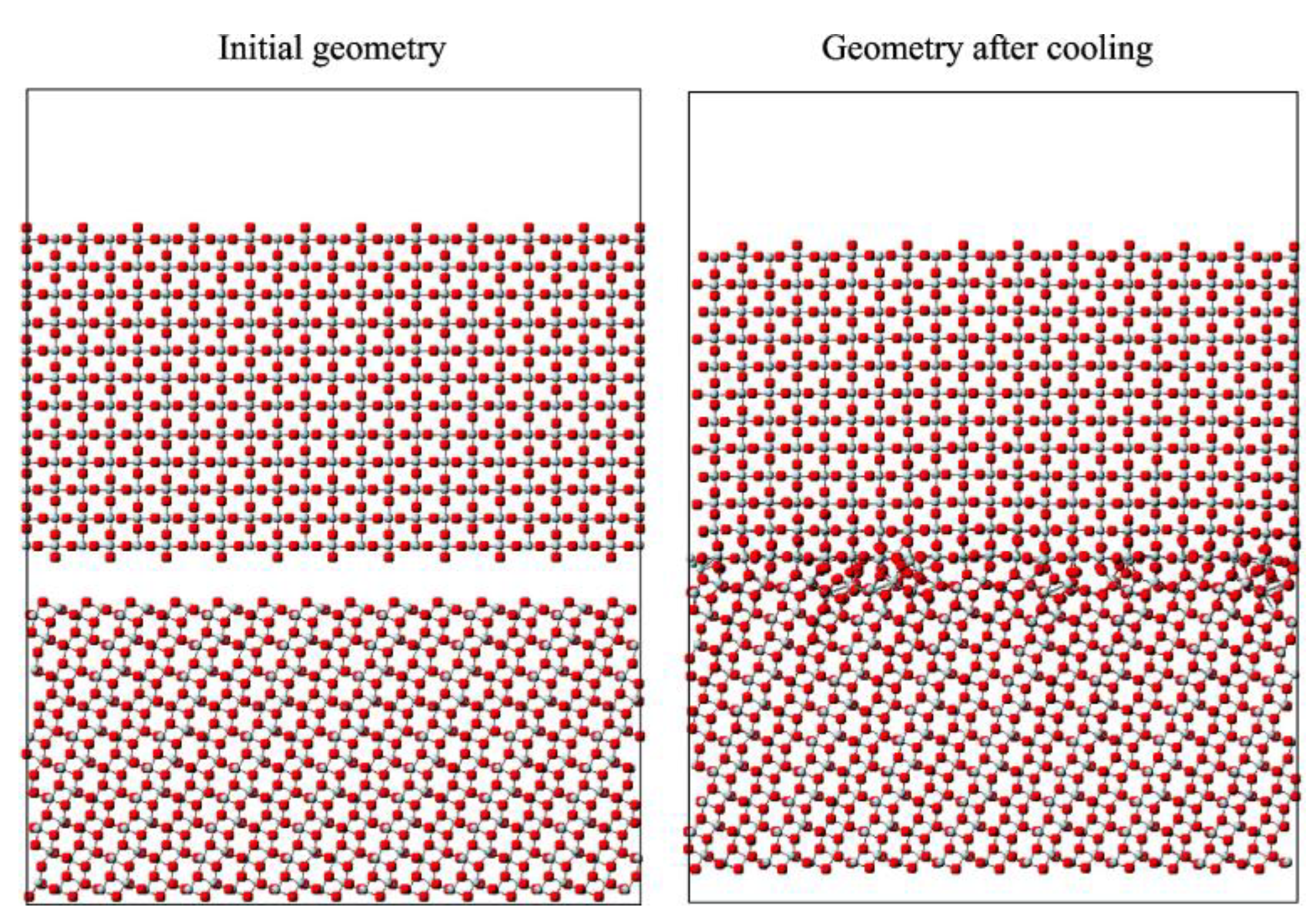
3.2. Computational Study of Heterophase TiO2
3.3. Fabrication Methods of Heterophase TiO2
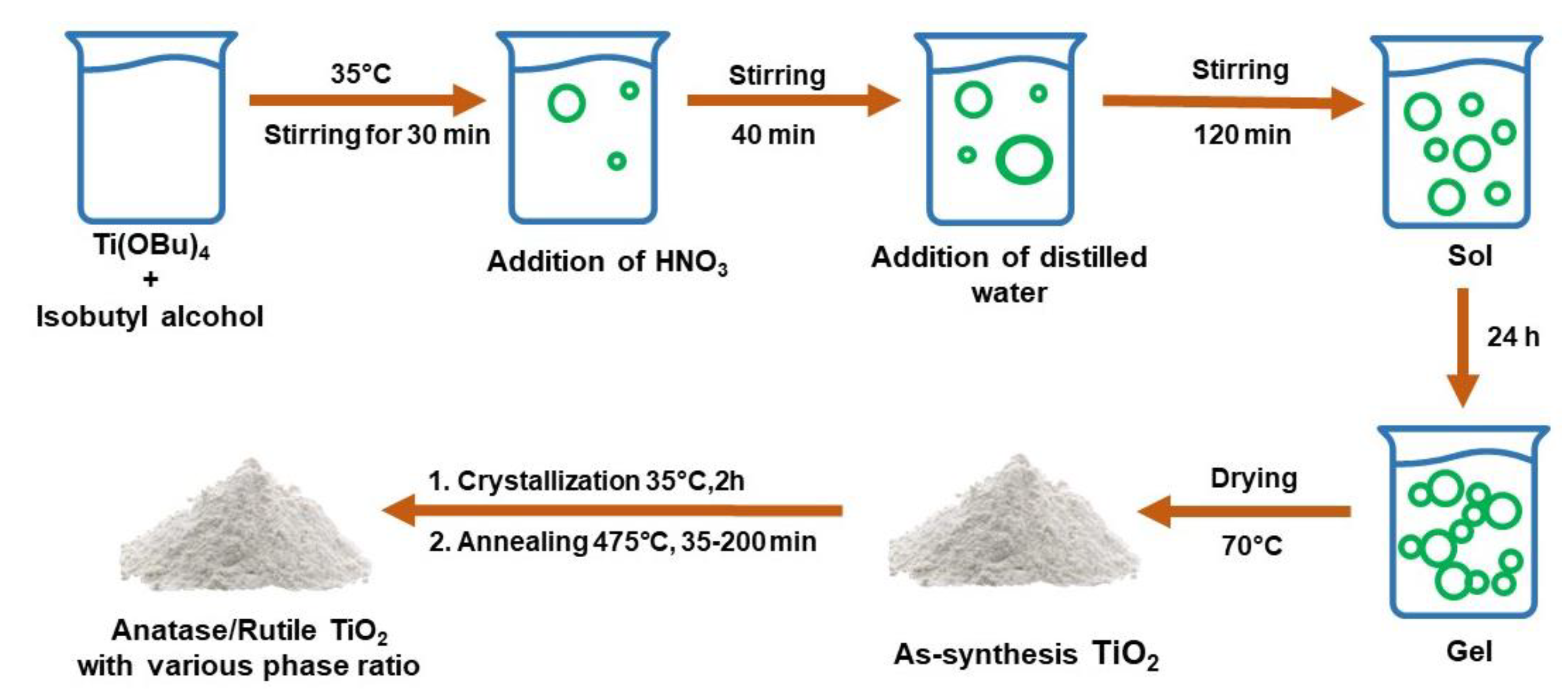
3.3.1. Sol–Gel Method
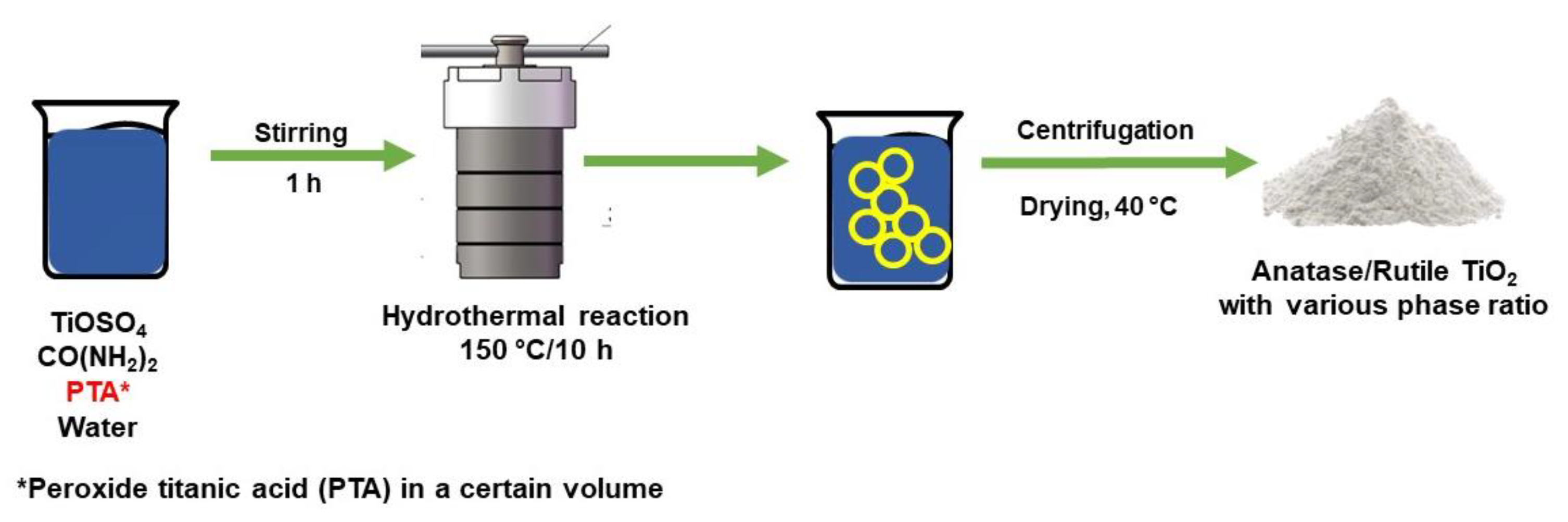
3.3.2. Hydrothermal Method
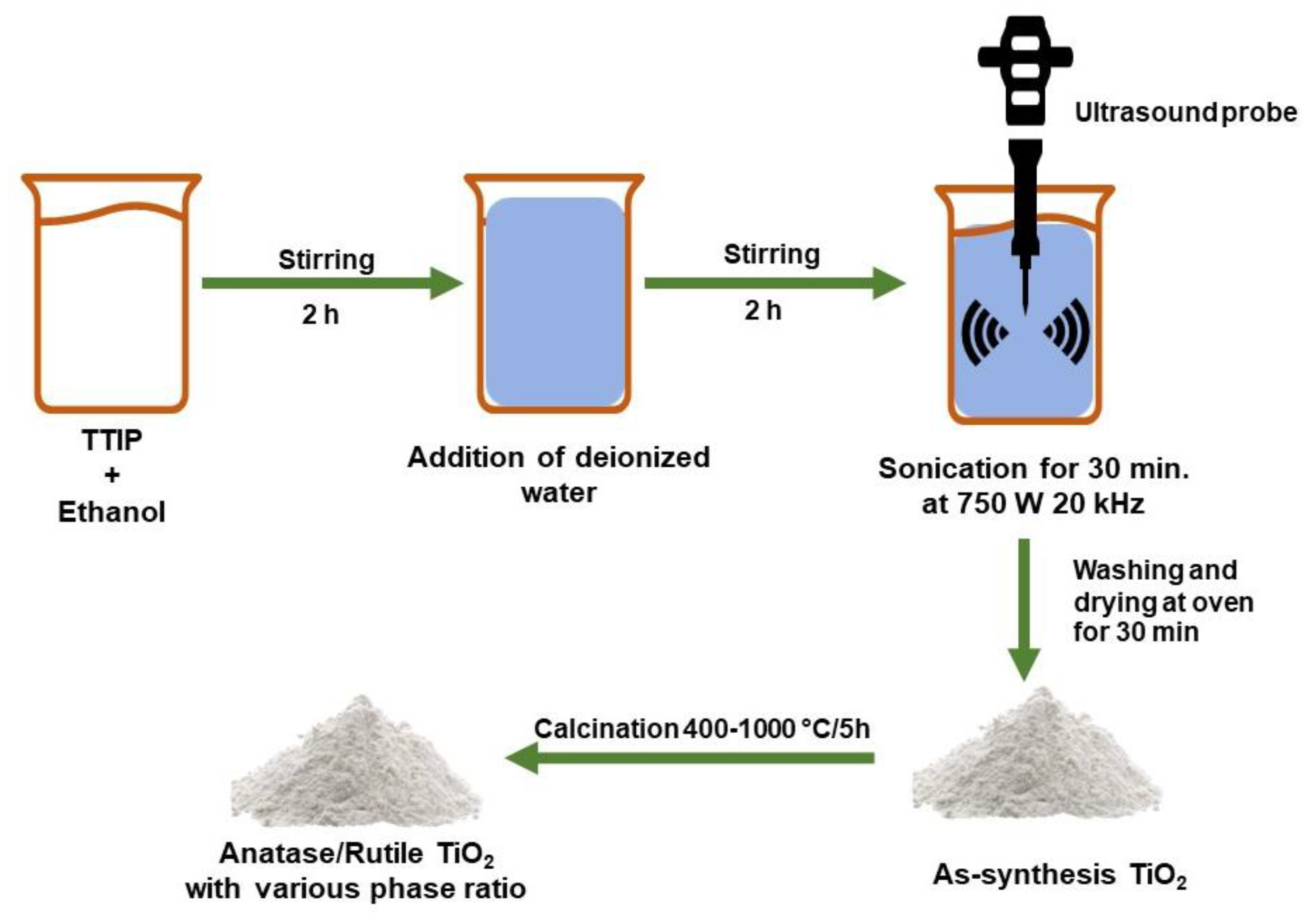
3.3.3. Sonochemical Method
3.4. Transformation of Polymorph TiO2
4. Photocatalytic Activity of Heterophase TiO2
4.1. Biphase of TiO2
4.1.1. Anatase/Rutile
4.1.2. Anatase/Brookite
4.1.3. Rutile/Brookite
4.1.4. Biphase of TiO2 (B)
4.2. Triphase of TiO2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium dioxide applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L. Synthesis of titanium dioxide/silicon dioxide from beach sand as photocatalyst for Cr and Pb remediation. Catalysts 2020, 10, 1248. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Glassford, K.M.; Chelikowsky, J.R. Structural and electronic properties of titanium dioxide. Phys. Rev. B 1992, 46, 1284–1298. [Google Scholar] [CrossRef]
- Grant, F.A. Properties of rutile (titanium dioxide). Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- Breckenridge, R.G.; Hosler, W.R. Electrical properties of titanium dioxide semiconductors. Phys. Rev. 1953, 91, 793–802. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Neto, D.M.A.; Freire, R.M.; Rocha, J.S.; Fechine, L.M.U.D.; Denardin, J.C.; Valentini, A.; de Araújo, T.G.; Mazzetto, S.E.; Fechine, P.B.A. Ultrafast sonochemistry-based approach to coat TiO2 commercial particles for sunscreen formulation. Ultrason. Sonochem. 2018, 48, 340–348. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Bolívar, J.P.; García-Tenorio, R.; Vaca, F. A review of the production cycle of titanium dioxide pigment. Mater. Sci. Appl. 2014, 2, 441–458. [Google Scholar] [CrossRef]
- Day, R.E. The role of titanium dioxide pigments in the degradation and tabilization of polymers in the plastics industry. Polym. Degrad. Stab. 1990, 29, 73–92. [Google Scholar] [CrossRef]
- Middlemas, S.; Fang, Z.Z.; Fan, P. A new method for production of titanium dioxide pigment. Hydrometallurgy 2013, 131, 107–113. [Google Scholar] [CrossRef]
- Gesenhues, U. Calcination of metatitanic acid to titanium dioxide white pigments. Chem. Eng. Technol. 2001, 24, 685–694. [Google Scholar] [CrossRef]
- Yang, B.; Wang, M.; Hu, X.; Zhou, T.; Zang, Z. Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low-temperature processed In2S3 as electron-transport-layer. Nano Energy 2019, 57, 718–727. [Google Scholar] [CrossRef]
- Tan, X.; Liu, X.; Liu, Z.; Sun, B.; Li, J.; Xi, S.; Shi, T.; Tang, Z.; Liao, G. Enhancing the optical, morphological and electronic properties of the solution-processed CsPbIBr2 films by Li doping for efficient carbon-based perovskite solar cells. Appl. Surf. Sci. 2020, 499, 143990. [Google Scholar] [CrossRef]
- Islam Molla, M.A.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of Reaction Mechanism for Photocatalytic Degradation of Dye with Self-Sensitized TiO2 under Visible Light Irradiation. Open J. Inorg. Non met. Mater. 2017, 7, 1–7. [Google Scholar]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dziechciarz, A. Self-cleaning coatings and surfaces of modern building materials for the removal of some air pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production. Mater. Sci. Semicond. Process. 2013, 16, 561–575. [Google Scholar] [CrossRef]
- Teets, T.S.; Nocera, D.G. Photocatalytic hydrogen production. ChemComm. 2011, 47, 9268–9274. [Google Scholar] [CrossRef]
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 4, 260–269. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.N.; Shen, C.C.; Xu, A.W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chinese J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. In Organic Pollutants-Monitoring, Risk, and Treatment; Rashed, M.N., Ed.; IntechOpen: London, UK, 2013; Volume 8, pp. 196–197. [Google Scholar]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Ameta, R.; Benjamin, S.; Ameta, A.; Ameta, S.C. Photocatalytic degradation of organic pollutants: A review. Mater. Sci. Forum 2013, 734, 247–272. [Google Scholar] [CrossRef]
- Wang, C.C.; Du, X.D.; Li, J.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr (VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Lin, Z.; Zheng, Y.; Deng, F.; Luo, X.; Zou, J.; Shao, P.; Zhang, S.; Tang, H. Target-directed design of dual-functional Z-scheme AgIn5S8/SnS2 heterojunction for Pb (II) capture and photocatalytic reduction of Cr (VI): Performance and mechanism insight. Sep. Purif. Technol. 2021, 277, 119430. [Google Scholar] [CrossRef]
- Hubbard, J.S.; Hardy, J.P.; Voecks, G.E.; Golub, E.E. Photocatalytic synthesis of organic compounds from CO and water: Involvement of surfaces in the formation and stabilization of products. J. Mol. Evol. 1973, 2, 149–166. [Google Scholar] [CrossRef]
- Lima, M.J.; Silva, A.M.; Silva, C.G.; Faria, J.L.; Reis, N.M. Selective photocatalytic synthesis of benzaldehyde in microcapillaries with immobilized carbon nitride. Chem. Eng. J. 2022, 430, 132643. [Google Scholar] [CrossRef]
- Luo, J.; Wang, M.; Chen, L.; Shi, J. Efficient benzaldehyde photosynthesis coupling photocatalytic hydrogen evolution. J. Energy Chem. 2022, 66, 52–60. [Google Scholar] [CrossRef]
- Lima, M.J.; Tavares, P.B.; Silva, A.M.; Silva, C.G.; Faria, J.L. Selective photocatalytic oxidation of benzyl alcohol to benzaldehyde by using metal-loaded g-C3N4 photocatalysts. Catal. Today 2017, 287, 70–77. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.; Zhang, R.; Wei, W.; Wang, H.; Lü, X.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Morphology-controlled self-assembly and synthesis of photocatalytic nanocrystals. Nano Lett. 2014, 14, 7175–7179. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; He, G.; Zhao, R.; Liu, D. Morphology control of TiO2 nanoparticle in microemulsion and its photocatalytic property. ACS Sustain. Chem. Eng. 2014, 2, 288–295. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 nanotubes: Synthesis and applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene modified TiO2 composite photocatalysts: Mechanism, progress and perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef]
- He, J.; Kumar, A.; Khan, M.; Lo, I.M. Critical review of photocatalytic disinfection of bacteria: From noble metals-and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef]
- Tekin, D.; Birhan, D.; Kiziltas, H. Thermal, photocatalytic, and antibacterial properties of calcinated nano-TiO2/polymer composites. Mat. Chem. Phys. 2020, 251, 123067. [Google Scholar] [CrossRef]
- Murad, E. Identification of minor amounts of anatase in kaolins by Raman spectroscopy. Am. Mineral. 1997, 82, 203–206. [Google Scholar] [CrossRef]
- Lu, Y.; Jaeckel, B.; Parkinson, B.A. Preparation and characterization of terraced surfaces of low-index faces of anatase, rutile, and brookite. Langmuir 2006, 22, 4472–4475. [Google Scholar] [CrossRef]
- Haggerty, J.E.; Schelhas, L.T.; Kitchaev, D.A.; Mangum, J.S.; Garten, L.M.; Sun, W. High-fraction brookite films from amorphous precursors. Sci. Rep. 2017, 7, 15232. [Google Scholar] [CrossRef]
- Permana, M.D.; Noviyanti, A.R.; Lestari, P.R.; Kumada, N.; Eddy, D.R.; Rahayu, I. The Effect of Organometallic Precursors on TiO2 Synthesis and Their Photocatalytic Activity on Phenol Degradation. Kuwait J. Sci. 2022, 49, 1–13. [Google Scholar]
- Deo, G.; Turek, A.M.; Wachs, I.E.; Machej, T.; Haber, J.; Das, N.; Eckert, H.; Hirt, A.M. Physical and chemical characterization of surface vanadium oxide supported on titania: Influence of the titania phase (anatase, rutile, brookite and B). Appl. Catal. A Gen. 1992, 91, 27–42. [Google Scholar] [CrossRef]
- Dachille, F.; Simons, P.Y.; Roy, R. Pressure-temperature studies of anatase, brookite, rutile and TiO2-II. Am. Mineral. 1968, 53, 1929–1939. [Google Scholar]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Zhang, Q. Synthesizing and comparing the photocatalytic properties of high surface area rutile and anatase titania nanoparticles. J. Am. Ceram. Soc. 2003, 86, 1677–1682. [Google Scholar] [CrossRef]
- Maver, K.; Arčon, I.; Fanetti, M.; Emin, S.; Valant, M.; Štangar, U.L. Improved photocatalytic activity of anatase-rutile nanocomposites induced by low-temperature sol-gel Sn-modification of TiO2. Catal. Today 2021, 361, 124–129. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Lee, M.S.; Hong, S.-S.; Mohseni, M. Synthesis of photocatalytic nanosized TiO2–Ag particles with sol–gel method using reduction agent. J. Mol. Catal. A: Chem. 2005, 242, 135–140. [Google Scholar] [CrossRef]
- Roy, P.; Ho, L.; Periasamy, A.P.; Lin, Y.; Huang, M.; Chang, H. Graphene-ZnO-Au nanocomposites based photocatalytic oxidation of benzoic acid. ScienceJet 2015, 4, 120. [Google Scholar]
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly active non-metals doped mixed-phase TiO2 for photocatalytic oxidation of ibuprofen under visible light. J. Photochem. Photobiol. A Chem. 2017, 346, 530–540. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.C.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2 (B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef]
- Kang, X.; Dong, G.; Dong, T. Oxygen Vacancy Defect Engineering of Heterophase Junction TiO2: Interfacial/Surface Oxygen Vacancies Coadjust the Photocatalytic ROS Production. ACS Appl. Energy Mater. 2022, 6, 1025–1036. [Google Scholar] [CrossRef]
- Ding, L.; Yang, S.; Liang, Z.; Qian, X.; Chen, X.; Cui, H.; Tian, J. TiO2 nanobelts with anatase/rutile heterophase junctions for highly efficient photocatalytic overall water splitting. J. Colloid Interface Sci. 2020, 567, 181–189. [Google Scholar] [CrossRef]
- Berger, T.; Sterrer, M.; Diwald, O.; Knözinger, E.; Panayotov, D.; Thompson, T.L.; Yates, J.T. Light-induced charge separation in anatase TiO2 particles. J. Phys. Chem. B 2005, 109, 6061–6068. [Google Scholar] [CrossRef]
- Cao, F.; Xiong, J.; Wu, F.; Liu, Q.; Shi, Z.; Yu, Y.; Wang, X.; Li, L. Enhanced photoelectrochemical performance from rationally designed anatase/rutile TiO2 heterostructures. ACS Appl. Mater. Interfaces 2016, 8, 12239–12245. [Google Scholar] [CrossRef]
- Tian, G.; Fu, H.; Jing, L.; Xin, B.; Pan, K. Preparation and characterization of stable biphase TiO2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity. J. Phys. Chem. C 2008, 112, 3083–3089. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Zhou, Y.; Bai, Y.; Feng, X.; Yang, Z.; Lu, L.; Lu, X.; Chan, K.Y. Enhanced photocatalytic activity in anatase/TiO2 (B) core−shell nanofiber. J. Phys. Chem. C 2008, 112, 20539–20545. [Google Scholar] [CrossRef]
- Mu, R.; Ao, Y.; Wu, T.; Wang, C.; Wang, P. Synthesis of novel ternary heterogeneous anatase-TiO2 (B) biphase nanowires/Bi4O5I2 composite photocatalysts for the highly efficient degradation of acetaminophen under visible light irradiation. J. Hazard. Mater. 2020, 382, 121083. [Google Scholar] [CrossRef]
- Chen, J.; Guan, M.; Zhang, X.; Gong, X. Insights into a rutile/brookite homojunction of titanium dioxide: Separated reactive sites and boosted photocatalytic activity. RSC Adv. 2019, 9, 36615–36620. [Google Scholar] [CrossRef]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Abdul Latif, A.; Griebel, J.; Prager, A.; Schulze, A. Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Hierarchical nanotubular anatase/rutile/TiO2 (B) heterophase junction with oxygen vacancies for enhanced photocatalytic H2 production. Langmuir 2018, 34, 1883–1889. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Y. Coexistence of an anatase/TiO2 (B) heterojunction and an exposed (001) facet in TiO2 nanoribbon photocatalysts synthesized via a fluorine-free route and topotactic transformation. Nanoscale 2014, 6, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Zhang, W.; Wang, Z.; Ma, Y.; Zhou, X. Fabrication of TiO2 (B)/anatase heterophase junctions at high temperature via stabilizing the surface of TiO2 (B) for enhanced photocatalytic activity. J. Phys. Chem. C 2019, 123, 1779–1789. [Google Scholar] [CrossRef]
- Tanemura, S.; Miao, L.; Wunderlich, W.; Tanemura, M.; Mori, Y.; Toh, S.; Kaneko, K. Fabrication and characterization of anatase/rutile–TiO2 thin films by magnetron sputtering: A review. Sci. Technol. Adv. Mater. 2005, 6, 11–17. [Google Scholar] [CrossRef]
- Yamakata, A.; Vequizo, J.J.M. Curious behaviors of photogenerated electrons and holes at the defects on anatase, rutile, and brookite TiO2 powders: A review. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 234–243. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, S. Challenges in band alignment between semiconducting materials: A case of rutile and anatase TiO2. Prog. Nat. Sci. Mater. Int. 2019, 29, 277–284. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/co-doped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Connelly, K.; Wahab, A.K.; Idriss, H. Photoreaction of Au/TiO2 for hydrogen production from renewables: A review on the synergistic effect between anatase and rutile phases of TiO2. Mater. Renew. Sustain. Energy 2012, 1, 1–12. [Google Scholar]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Adachi, S. Physical Properties of III–V Semiconductor Compounds; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Sitt, A.; Hadar, I.; Banin, U. Band-gap engineering, optoelectronic properties and applications of colloidal heterostructured semiconductor nanorods. Nano Today 2013, 8, 494–513. [Google Scholar] [CrossRef]
- Edgar, J.H. Prospects for device implementation of wide band gap semiconductors. J. Mater. Res. 1992, 7, 235–252. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates Jr, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Pawar, R.R.; Kim, M.; Kim, J.G.; Hong, S.M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Mechanism of photocatalysis. In Photocatalysis: Fundamentals, Materials and Applications; Zhang, J., Tian, B., Wang, L., Xing, M., Lei, J., Eds.; Springer: Singapore, 2018; pp. 1–15. [Google Scholar]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding mechanism of photocatalytic microbial decontamination of environmental wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ye, X.; Zhang, Q.; Hui, Z.; Wang, X.; Chen, S. Efficient utilization of photogenerated electrons and holes for photocatalytic redox reactions using visible light-driven Au/ZnIn2S4 hybrid. J. Hazard. Mater. 2019, 367, 277–285. [Google Scholar] [CrossRef]
- Gerischer, H. Electron-transfer kinetics of redox reactions at the semiconductor/electrolyte contact. A new approach. J. Phys. Chem. 1991, 95, 1356–1359. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Graetzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Feng, X.; Guo, H.; Patel, K.; Zhou, H.; Lou, X. High performance, recoverable Fe3O4ZnO nanoparticles for enhanced photocatalytic degradation of phenol. Chem. Eng. J. 2014, 244, 327–334. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Sobczyński, A.; Duczmal, Ł.; Zmudziński, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Mol. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, S.; Yu, X.; Dong, C.; Ji, J.; Zhang, D.; Xing, M. Research progress of photocatalytic sterilization over semiconductors. RSC Adv. 2019, 9, 19278–19284. [Google Scholar] [CrossRef] [PubMed]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored preparation methods of TiO2 anatase, rutile, brookite: Mechanism of formation and electrochemical properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (PBCA, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Beltran, A.; Gracia, L.; Andres, J. Density functional theory study of the brookite surfaces and phase transitions between natural titania polymorphs. J. Phys. Chem. B 2006, 110, 23417–23423. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Fornasiero, P. Brookite: Nothing new under the sun? Catalysts 2017, 7, 304. [Google Scholar] [CrossRef]
- Li, Z.; Cong, S.; Xu, Y. Brookite vs anatase TiO2 in the photocatalytic activity for organic degradation in water. ACS Catal. 2014, 4, 3273–3280. [Google Scholar] [CrossRef]
- Banfield, J.F.; Veblen, D.R.; Smith, D.J. The identification of naturally occurring TiO2 (B) by structure determination using high-resolution electron microscopy, image simulation, and distance-least-squares refinement. Am. Mineral. 1991, 76, 343–353. [Google Scholar]
- Xiang, G.; Wang, Y.G.; Li, J.; Zhuang, J.; Wang, X. Surface-specific interaction by structure-match confined pure high-energy facet of unstable TiO2 (B) polymorph. Sci. Rep. 2013, 3, 1411. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; Bruce, P.G. TiO2-B nanowires. Angew. Chem. Int. Ed. 2004, 43, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Nosheen, S.; Galasso, F.S.; Suib, S.L. Role of Ti-O bonds in phase transitions of TiO2. Langmuir 2009, 25, 7623–7630. [Google Scholar] [CrossRef] [PubMed]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L. Recent efforts in design of TiO2 (B) anodes for high-rate lithium-ion batteries: A review. J. Power Sources 2019, 442, 227225. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Moon, G.H.; Majima, T.; Choi, W. Molecular-level understanding of the photocatalytic activity difference between anatase and rutile Nanoparticles. Angew. Chem. 2014, 126, 14260–14265. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Wu, X.; Ng, Y.H.; Wang, L.; Du, Y.; Dou, S.X.; Amal, R.; Scott, J. Improving the photo-oxidative capability of BiOBr via crystal facet engineering. J. Mater. Chem. A 2017, 5, 8117–8124. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Porkodi, K.; Arokiamary, S.D. Synthesis and spectroscopic characterization of nanostructured anatase titania: A photocatalyst. Mater. Charact. 2007, 58, 495–503. [Google Scholar] [CrossRef]
- Sivalingam, G.; Nagaveni, K.; Hegde, M.S.; Madras, G. Photocatalytic degradation of various dyes by combustion synthesized nano anatase TiO2. Appl. Catal. B 2003, 45, 23–38. [Google Scholar] [CrossRef]
- Periyat, P.; Naufal, B.; Ullattil, S.G. A review on high temperature stable anatase TiO2 photocatalysts. Mater. Sci. Forum 2016, 855, 78–93. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of intrinsic band alignments within anatase and rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef]
- Yuan, L.D.; Deng, H.X.; Li, S.S.; Wei, S.H.; Luo, J.W. Unified theory of direct or indirect band-gap nature of conventional semiconductors. Phys. Rev. B 2018, 98, 245203. [Google Scholar] [CrossRef]
- Gu, L.; Srot, V.; Sigle, W.; Koch, C.; van Aken, P.; Scholz, F.; Thapa, S.B.; Kirchner, C.; Jetter, M.; Rühle, M. Band-gap measurements of direct and indirect semiconductors using monochromated electrons. Phys. Rev. B 2007, 75, 195214. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Zhou, W.; Umezawa, N.; Ma, R.; Sakai, N.; Ebina, Y.; Sano, K.; Liu, M.; Ishida, Y.; Aida, T.; Sasaki, T. Spontaneous direct band gap, high hole mobility, and huge exciton energy in atomic-thin TiO2 nanosheet. Chem. Mater. 2018, 30, 6449–6457. [Google Scholar] [CrossRef]
- Wetchakun, N.; Incessungvorn, B.; Wetchakun, K.; Phanichphant, S. Influence of calcination temperature on anatase to rutile phase transformation in TiO2 nanoparticles synthesized by the modified sol–gel method. Mater. Lett. 2012, 82, 195–198. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M. Mixed-phase TiO2 photocatalysis: Correlation between phase composition and photodecomposition of water pollutants. Rev. Inorg. Chem. 2017, 37, 11–28. [Google Scholar] [CrossRef]
- Ou, X.; Liu, X.; Liu, W.; Rong, W.; Li, J.; Lin, Z. Surface defects enhance the adsorption affinity and selectivity of Mg(OH)2 towards As (V) and Cr (VI) oxyanions: A combined theoretical and experimental study. Environ. Sci. Nano 2018, 5, 2570–2578. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.; Synnott, D.W.; McCormack, D.E.; Pillai, S.C. An effective method for the preparation of high temperature stable anatase TiO2 photocatalysts. Appl. Surf. Sci. 2016, 371, 447–452. [Google Scholar] [CrossRef]
- Bubacz, K.; Choina, J.; Dolat, D.; Morawski, A.W. Methylene Blue and Phenol Photocatalytic Degradation on Nanoparticles of Anatase TiO2. Pol. J. Environ. Stud. 2010, 19, 685–691. [Google Scholar]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen rich titania: A dopant free, high temperature stable, and visible-light active anatase photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef]
- Lv, K.; Cheng, B.; Yu, J.; Liu, G. Fluorine ions-mediated morphology control of anatase TiO2 with enhanced photocatalytic activity. Phys. Chem. Chem. Phys. 2012, 14, 5349–5362. [Google Scholar] [CrossRef]
- Wang, X.; Kafizas, A.; Li, X.; Moniz, S.J.; Reardon, P.J.; Tang, J.; Parkin, I.P.; Durrant, J.R. Transient absorption spectroscopy of anatase and rutile: The impact of morphology and phase on photocatalytic activity. J. Phys. Chem. C 2015, 119, 10439–10447. [Google Scholar] [CrossRef]
- Bickley, R.I.; Stone, F.S. Photoadsorption and photocatalysis at rutile surfaces: I. Photoadsorption of oxygen. J. Catal. 1973, 31, 389–397. [Google Scholar] [CrossRef]
- Bickley, R.I.; Munuera, G.; Stone, F.S. Photoadsorption and photocatalysis at rutile surfaces: II. Photocatalytic oxidation of isopropanol. J. Catal. 1973, 31, 398–407. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Rutile titanium dioxide prepared by hydrogen reduction of Degussa P25 for highly efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2016, 6, 5693–5699. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Lian, Y.; Wang, X.; Li, C. Photoluminescence characteristics of TiO2 and their relationship to the photoassisted reaction of water/methanol mixture. J. Phys. Chem. C 2007, 111, 693–699. [Google Scholar] [CrossRef]
- Park, N.G.; Van de Lagemaat, J.; Frank, A.A. Comparison of dye-sensitized rutile-and anatase-based TiO2 solar cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.; Zhang, X.; Xu, Y. Different effects of fluoride and phosphate anions on TiO2 photocatalysis (rutile). Catal. Sci. Technol. 2020, 10, 6552–6561. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low temperature synthesis and photocatalytic activity of rutile TiO2 nanorod superstructures. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Miyoshi, A.; Nishioka, S.; Maeda, K. Water splitting on rutile TiO2-based photocatalysts. Chem. Eur. J. 2018, 24, 18204–18219. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, H.D.; Tang, R.; Cheng, Q.; Yuan, Y.J. Rutile TiO2 nanoparticles with oxygen vacancy for photocatalytic nitrogen fixation. ACS Appl. Nano Mater. 2021, 4, 8674–8679. [Google Scholar] [CrossRef]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, H. Origin of low photocatalytic activity of rutile TiO2. Electron. Mater. Lett. 2009, 5, 73–76. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Zhang, G.; Liu, Z.; Qu, D.; Miao, X.; Feng, P.; Sun, Z. Effect of defects on photocatalytic activity of rutile TiO2 nanorods. Nano Res. 2015, 8, 4061–4071. [Google Scholar] [CrossRef]
- Djokić, V.R.; Marinković, A.D.; Petrović, R.D.; Ersen, O.; Zafeiratos, S.; Mitrić, M.; Ophus, C.; Radmilović, V.R.; Janaćković, D.T. Highly active rutile TiO2 nanocrystalline photocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 33058–33068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Y.; Lu, F.; Fei, W.; Mengqiong, Y.; Genxiang, L.; Qian, X.; Xiang, W.; Can, L. Photocatalytic degradation of rhodamine B on anatase, rutile, and brookite TiO2. Chinese J. Catal. 2011, 32, 983–991. [Google Scholar] [CrossRef]
- Pauling, L.; Sturdivant, J.H., XV. The crystal structure of brookite. Z. Kristallogr. Cryst. Mater. 1928, 68, 239–256. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Xie, J.; Lü, X.; Liu, J.; Shu, H. Brookite titania photocatalytic nanomaterials: Synthesis, properties, and applications. Pure Appl. Chem. 2009, 81, 2407–2415. [Google Scholar] [CrossRef]
- Thuong, H.T.T.; Kim, C.T.T.; Quang, L.N.; Kosslick, H. Highly active brookite TiO2-assisted photocatalytic degradation of dyes under the simulated solar−UVA radiation. Prog. Nat. Sci. Mater. Int. 2019, 29, 641–647. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaima, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Khan, S.; Je, M.; Kim, D.; Lee, S.; Cho, S.H.; Song, T.; Choi, H. Mapping point defects of brookite TiO2 for photocatalytic activity beyond anatase and P25. J. Phys. Chem. C 2020, 124, 10376–10384. [Google Scholar] [CrossRef]
- Zhuang, B.; Shi, H.; Zhang, H.; Zhang, Z. Sodium doping in brookite TiO2 enhances its photocatalytic activity. Beilstein J. Nanotechnol. 2022, 13, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: Whats behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Absolute crystallinity and photocatalytic activity of brookite TiO2 samples. Appl. Catal. B 2017, 201, 150–158. [Google Scholar] [CrossRef]
- Choi, M.; Lim, J.; Baek, M.; Choi, W.; Kim, W.; Yong, K. Investigating the unrevealed photocatalytic activity and stability of nanostructured brookite TiO2 film as an environmental photocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 16252–16260. [Google Scholar] [CrossRef]
- Do, H.H.; Tran, T.K.C.; Ung, T.D.T.; Dao, N.T.; Nguyen, D.D.; Trinh, T.H.; Hoang, T.D.; Le, T.L.; Tran, T.T.H. Controllable fabrication of photocatalytic TiO2 brookite thin film by 3D-printing approach for dyes decomposition. J. Water Process. Eng. 2021, 43, 102319. [Google Scholar] [CrossRef]
- Marchand, R.; Brohan, L.; Tournoux, M. TiO2 (B) a new form of titanium dioxide and the potassium octatitanate K2Ti8O17. Mater. Res. Bull. 1980, 15, 1129–1133. [Google Scholar] [CrossRef]
- Dylla, A.G.; Henkelman, G.; Stevenson, K.J. Lithium insertion in nanostructured TiO2 (B) architectures. Acc. Chem. Res. 2013, 46, 1104–1112. [Google Scholar] [CrossRef]
- Fehse, M.; Ventosa, E. Is TiO2 (B) the future of titanium-based battery materials? ChemPlusChem 2015, 80, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Liu, Z.; Bruce, P.G.; Grey, C.P. The morphology of TiO2 (B) nanoparticles. J. Am. Chem. Soc. 2015, 137, 13612–13623. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, H.; Han, L.; Wang, J.; Gao, P.; Xu, D.; Yang, J.; Che, S. Nanosheet-constructed porous TiO2–B for advanced lithium-ion batteries. Adv. Mater. 2012, 24, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A.R.; Grey, C.P.; Bruce, P.G. Nanoparticulate TiO2 (B): An anode for lithium-ion batteries. Angew. Chem. 2012, 124, 2206–2209. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Qi, Z.; Chai, S.Y.; Lee, C.; Park, S.Y.; Jang, D.J.; Lee, W.I. Formation of highly crystallized TiO2 (B) and its photocatalytic behavior. Appl. Catal. B 2010, 93, 368–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ self-doped blue TiO2 (B) single-crystalline nanorods for efficient solar-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; You, K.H.; Park, C.B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 2012, 24, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, N.S.; Khameneh Asl, S.; Mohammadpour, R.; Asl, S.K. Band-gap narrowing and electrochemical properties in N-doped and reduced anodic TiO2 nanotube arrays. Electrochim. Acta 2018, 270, 245–255. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Han, Y.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2017, 321–322, 9–17. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M. Photocatalysis; Springer: Singapore, 2018. [Google Scholar]
- Huang, Y.; Cao, J.J.; Kang, F.; You, S.J.; Chang, C.W.; Wang, Y.F. High selectivity of visible-light-driven La-doped TiO2 photocatalysts for NO removal. Aerosol Air Qual. Res. 2017, 17, 2555–2565. [Google Scholar] [CrossRef]
- Marques, J.; Gomes, T.D.; Forte, M.A.; Silva, R.F.; Tavares, C.J. A new route for the synthesis of highly-active N-doped TiO2 nanoparticles for visible light photocatalysis using urea as nitrogen precursor. Catal. Today 2019, 326, 36–45. [Google Scholar] [CrossRef]
- Liu, D.; Tian, R.; Wang, J.; Nie, E.; Piao, X.; Li, X.; Sun, Z. Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 2017, 185, 574–581. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of sulfur doped-TiO2 by flame spray pyrolysis for visible light photocatalytic degradation of acetaldehyde. Chem. Eng. J. 2018, 339, 249–258. [Google Scholar] [CrossRef]
- Payormhorm, J.; Idem, R. Synthesis of C-doped TiO2 by sol-microwave method for photocatalytic conversion of glycerol to value-added chemicals under visible light. Appl. Catal. A-Gen. 2020, 590, 117362. [Google Scholar] [CrossRef]
- Nagaraj, G.; Raj, A.D.; Irudayaraj, A.A.; Josephine, R.L. Tuning the optical band Gap of pure TiO2 via photon induced method. Optik 2018, 179, 889–894. [Google Scholar]
- Hu, J.; Zhang, S.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Novel highly active anatase/rutile TiO2 photocatalyst with hydrogenated heterophase interface structures for photoelectrochemical water splitting into hydrogen. ACS Sustain. Chem. Eng. 2018, 6, 10823–10832. [Google Scholar] [CrossRef]
- Lu, S.; Yang, S.; Hu, X.; Liang, Z.; Guo, Y.; Xue, Y.; Cui, H.; Tian, J. Fabrication of TiO2 nanoflowers with bronze (TiO2 (B))/anatase heterophase junctions for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 24398–24406. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Hydroxyl radical and free and shallowly trapped electron generation and electron/hole recombination rates in TiO2 photocatalysis using different combinations of anatase and rutile. Appl. Catal. A-Gen. 2018, 565, 20–25. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Remarkable charge separation and photocatalytic efficiency enhancement through TiO2 (B)/anatase heterophase junctions of TiO2 nanobelts. Int. J. Hydrogen Energy 2019, 44, 27311–27318. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, X.; Cui, Y.; Fan, X.; Li, Z.; Xie, T.; Ba, K.; Jia, G.; Zhang, H.; Zhang, L.; et al. Favorable energy band alignment of TiO2 anatase/rutile heterophase homojunctions yields photocatalytic hydrogen evolution with quantum efficiency exceeding 45.6%. Adv. Energy Mater. 2022, 12, 2200298. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Wang, D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps. Chem. Eng. J. 2019, 355, 399–415. [Google Scholar] [CrossRef]
- Luo, Z.; Poyraz, A.S.; Kuo, C.H.; Miao, R.; Meng, Y.; Chen, S.Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline mixed phase (anatase/rutile) mesoporous titanium dioxides for visible light photocatalytic activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Franciosi, A.; Van de Walle, C.G. Heterojunction band offset engineering. Surf. Sci. Rep. 1996, 25, 1–140. [Google Scholar] [CrossRef]
- Tung, R.T.; Kronik, L. Charge density and band offsets at heterovalent semiconductor interfaces. Adv. Theory Simul. 2018, 1, 1700001. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Mehata, M.S. Phase-dependent optical and photocatalytic performance of synthesized titanium dioxide (TiO2) nanoparticles. Optik 2019, 193, 163011. [Google Scholar] [CrossRef]
- Sato, T.; Taya, M. Enhancement of phage inactivation using photocatalytic titanium dioxide particles with different crystalline structures. Biochem. Eng. J. 2006, 28, 303–308. [Google Scholar] [CrossRef]
- Zhu, W.; Kong, L.; Long, W.; Shi, X.; Zhou, D.; Sun, L.; Zhou, L.; Yang, G.; Liu, X.; Liu, H.; et al. Band Structure Engineering of Black Phosphorus/Graphene/MoS2 van der Waals Heterojunctions for Photovoltaic and Op-toelectronic Device Application. J. Phys. Conf. Ser. 2021, 1865, 022021. [Google Scholar] [CrossRef]
- Sun, X.; Chang, Y.; Cheng, Y.; Feng, Y.; Zhang, H. Band alignment-driven oxidative injury to the skin by anatase/rutile mixed-phase titanium dioxide nanoparticles under sunlight exposure. Toxicol. Sci. 2018, 164, 300–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Vittadini, A.; Casarin, M.; Selloni, A. Chemistry of and on TiO2-anatase surfaces by DFT calculations: A partial review. Theor. Chem. Acc. 2007, 117, 663–671. [Google Scholar] [CrossRef]
- Ju, M.G.; Sun, G.; Wang, J.; Meng, Q.; Liang, W. Origin of high photocatalytic properties in the mixed-phase TiO2: A first-principles theoretical study. ACS Appl. Mater. Interfaces 2014, 6, 12885–12892. [Google Scholar] [CrossRef] [PubMed]
- Deskins, N.A.; Kerisit, S.; Rosso, K.M.; Dupuis, M. Molecular dynamics characterization of rutile-anatase interfaces. J. Phys. Chem. C 2007, 111, 9290–9298. [Google Scholar] [CrossRef]
- Davlatshoevich, N.D.; Ashur, K.M.; Saidali, B.A.; Kholmirzotagoykulovich, K.; Lyubchyk, A.; Ibrahim, M. Investigation of structural and optoelectronic properties of N-doped hexagonal phases of TiO2 (TiO2-xNx) nanoparticles with DFT realization: Optimization of the band gap and optical properties for visible-light absorption and photovoltaic applications. Biointerface Res. Appl. Chem. 2022, 12, 3836–3848. [Google Scholar]
- Liang, B.; Mianxin, S.; Tianliang, Z.; Xiaoyong, Z.; Qingqing, D. Band gap calculation and photo catalytic activity of rare earths doped rutile TiO2. J. Rare Earths 2009, 27, 461–468. [Google Scholar]
- Quesada-Cabrera, R.; Sotelo-Vazquez, C.; Bear, J.C.; Darr, J.A.; Parkin, I.P. Photocatalytic Evidence of the Rutile-to-Anatase Electron Transfer in Titania. Adv. Mater. Interfaces 2014, 1, 1400069. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. Sol-gel synthesis of titanium dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Pillai, S.C., Hehir, S., Eds.; Springer Cham: New York, NY, USA, 2017; pp. 271–283. [Google Scholar]
- Wang, C.C.; Ying, J.Y. Sol− gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem. Mater. 1999, 11, 3113–3120. [Google Scholar] [CrossRef]
- Permana, M.D.; Noviyanti, A.R.; Lestari, P.R.; Kumada, N.; Eddy, D.R.; Rahayu, I. Enhancing the photocatalytic activity of TiO2/Na2Ti6O13 composites by gold for the photodegradation of phenol. ChemEngineering 2022, 6, 69. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol–gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Castrejón-Sánchez, V.H.; López, R.; Ramón-González, M.; Enríquez-Pérez, Á.; Camacho-López, M.; Villa-Sánchez, G. Annealing control on the anatase/rutile ratio of nanostructured titanium dioxide obtained by sol-gel. Crystals 2018, 9, 22. [Google Scholar] [CrossRef]
- Noviyanti, A.R.; Asyiah, E.N.; Permana, M.D.; Dwiyanti, D.; Eddy, D.R. Preparation of Hydroxyapatite-Titanium Dioxide Composite from Eggshell by Hydrothermal Method: Characterization and Antibacterial Activity. Crystals 2022, 12, 1599. [Google Scholar] [CrossRef]
- Aruna, S.T.; Tirosh, S.; Zaban, A. Nanosize rutile titania particle synthesis via hydrothermal method without mineralizers. J. Mater. Chem. 2000, 10, 2388–2391. [Google Scholar] [CrossRef]
- Li, G.; Ciston, S.; Saponjic, Z.V.; Chen, L.; Dimitrijevic, N.M.; Rajh, T.; Gray, K.A. Synthesizing mixed-phase TiO2 nanocomposites using a hydrothermal method for photo-oxidation and photoreduction applications. J. Catal. 2008, 253, 105–110. [Google Scholar] [CrossRef]
- Sun, C.; Wang, N.; Zhou, S.; Hu, X.; Zhou, S.; Chen, P. Preparation of self-supporting hierarchical nanostructured anatase/rutile composite TiO2 film. Chem. Comm. 2008, 28, 3293–3295. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wei, P.; Xia, X.; Huang, Z.; Homewood, K.; Gao, Y. Remarkably enhanced H2 response and detection range in Nb doped rutile/anatase heterophase junction TiO2 thin film hydrogen sensors. Sens. Actuators B Chem. 2019, 301, 127143. [Google Scholar] [CrossRef]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, M.; Gao, B.; Ding, W.; Zhang, Z.; Anandan, S.; Umar, A. Hydrothermally regulating phase composition of TiO2 nanocrystals toward high photocatalytic activity. J. Alloys Compd. 2021, 850, 156653. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Jaafar, J.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Salehmin, M.N.I.; Rosmi, M.S.; Salleh, W.N.; Othman, M.H.D. Concurrent growth, structural and photocatalytic properties of hybridized C, N co-doped TiO2 mixed phase over g-C3N4 nanostructured. Scr. Mater. 2018, 142, 143–147. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Liu, X.; Qin, W.; Wang, M.; Pan, L. Metal-organic frameworks derived cake-like anatase/rutile mixed phase TiO2 for highly efficient photocatalysis. J. Alloys Compd. 2017, 690, 640–646. [Google Scholar] [CrossRef]
- Peng, F.; Gao, H.; Zhang, G.; Zhu, Z.; Zhang, J.; Liu, Q. Synergistic effects of Sm and C co-doped mixed phase crystalline TiO2 for visible light photocatalytic activity. Materials 2017, 10, 209. [Google Scholar] [CrossRef]
- Jacob, K.A.; Peter, P.M.; Jose, P.E.; Balakrishnan, C.J.; Thomas, V.J. A simple method for the synthesis of anatase-rutile mixed phase TiO2 using a convenient precursor and higher visible-light photocatalytic activity of Co–doped TiO2. Mater. Today Proc. 2021, 49, 1408–1417. [Google Scholar] [CrossRef]
- Li, H.; Shen, X.; Liu, Y.; Wang, L.; Lei, J.; Zhang, J. Facile phase control for hydrothermal synthesis of anatase-rutile TiO2 with enhanced photocatalytic activity. J. Alloys Compd. 2015, 646, 380–386. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, Z.; Jiang, P.; Kuang, J.; Liu, W.; Cao, W. Hydrothermal synthesis and enhanced photocatalytic activity of mixed-phase TiO2 powders with controllable anatase/rutile ratio. Solid State Sci. 2018, 77, 14–19. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Cui, H.; An, H.; Pan, Y.; Zhai, J. Synthesis of crystal-controlled TiO2 nanorods by a hydrothermal method: Rutile and brookite as highly active photocatalysts. J. Phys. Chem. C 2015, 119, 16905–16912. [Google Scholar] [CrossRef]
- Luthfiah, A.; Permana, M.D.; Deawati, Y.; Firdaus, M.L.; Rahayu, I.; Eddy, D.R. Photocatalysis of nanocomposite titania–natural silica as antibacterial against Staphylococcus aureus and Pseudomonas aeruginosa. RSC Adv. 2021, 11, 38528–38536. [Google Scholar] [CrossRef] [PubMed]
- Duvarci, Ö.Ç.; Çiftçioğlu, M. Preparation and characterization of nanocrystalline titania powders by sonochemical synthesis. Powder Technol. 2012, 228, 231–240. [Google Scholar] [CrossRef]
- Garibay-Febles, V.; Hernández-Pérez, I.; Arceo, L.D.B.; Meza-Espinoza, J.S.; Espinoza-Tapia, J.C.; González-Reyes, L. Microstructural study by electron microscopy of sonochemical synthesized TiO2 nanoparticles. Acta Microsc. 2017, 26, 56–64. [Google Scholar]
- Hernández-Perez, I.; Maubert, A.M.; Rendón, L.; Santiago, P.; Herrera-Hernández, H.; Díaz-Barriga Arceo, L.; Garibay Febles, V.; Palacios Gonzalez, E.; González-Reyes, L. Ultrasonic synthesis: Structural, optical and electrical correlation of TiO2 nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 8832–8847. [Google Scholar]
- Noman, M.T.; Militky, J.; Wiener, J.; Saskova, J.; Ashraf, M.A.; Jamshaid, H.; Azeem, M. Sonochemical synthesis of highly crystalline photocatalyst for industrial applications. Ultrasonics 2018, 83, 203–213. [Google Scholar] [CrossRef]
- Arami, H.; Mazloumi, M.; Khalifehzadeh, R.; Sadrnezhaad, S.K. Sonochemical preparation of TiO2 nanoparticles. Mater. Lett. 2007, 61, 4559–4561. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mekprasart, W.; Pecharapa, W. Anatase/Rutile TiO2 composite prepared via sonochemical process and their photocatalytic activity. Mater. Today: Proc. 2017, 4, 6159–6165. [Google Scholar]
- Yu, J.C.; Zhang, L.; Yu, J. Direct sonochemical preparation and characterization of highly active mesoporous TiO2 with a bicrystalline framework. Chem. Mater. 2002, 14, 4647–4653. [Google Scholar] [CrossRef]
- Ozawa, T.; Iwasaki, M.; Tada, H.; Akita, T.; Tanaka, K.; Ito, S. Low-temperature synthesis of anatase–brookite composite nanocrystals: The junction effect on photocatalytic activity. J. Colloid Interface Sci. 2005, 281, 510–513. [Google Scholar] [CrossRef]
- Matthews, A. The crystallization of anatase and rutile from amorphous titanium dioxide under hydrothermal conditions. Am. Mineral. 1976, 61, 419–424. [Google Scholar]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Sikong, L.; Damchan, J.; Kooptarnond, K.; Niyomwas, S. Effect of doped SiO2 and calcinations temperature on phase transformation of TiO2 photocatalyst prepared by sol-gel method. Songklanakarin J. Sci. Technol. 2008, 30, 385–391. [Google Scholar]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of High-Temperature Stable Anatase TiO2 Photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef]
- Yuangpho, N.; Le, S.T.T.; Treerujiraphapong, T.; Khanitchaidecha, W.; Nakaruk, A. Enhanced photocatalytic performance of TiO2 particles via effect of anatase–rutile ratio. Physica E Low Dimens. Syst. Nanostruct. 2015, 67, 18–22. [Google Scholar] [CrossRef]
- Scarpelli, F.; Mastropietro, T.F.; Poerio, T.; Godbert, N. Mesoporous TiO2 thin films: State of the art. In Titanium Dioxide-Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen: London, UK, 2018; Volume 508, pp. 135–142. [Google Scholar]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Mancinelli, R.; Botti, A.; Bruni, F.; Ricci, M.A.; Soper, A.K. Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J. Phys. Chem. B 2007, 111, 13570–13577. [Google Scholar] [CrossRef]
- Zaw, Y.Y.; Channei, D.A.D.; Threrujirapapong, T.; Khanitchaidecha, W.; Nakaruk, A. Effect of anatase/rutile phase ratio on the photodegradation of methylene blue under uv irradiation. Mater. Sci. Forum 2020, 998, 78–83. [Google Scholar] [CrossRef]
- Wetchakun, N.; Phanichphant, S. Effect of temperature on the degree of anatase–rutile transformation in titanium dioxide nanoparticles synthesized by the modified sol–gel method. Curr. Appl. Phys. 2008, 8, 343–346. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Li, L.; Shen, Z.; Cao, Y.; Wang, Y.; Cui, L.; Cheng, J.; Wang, Y.; Li, X. Engineering of anatase/rutile TiO2 heterophase junction via in-situ phase transformation for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 599, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Bakardjieva, S.; Šubrt, J.; Štengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of anatase–rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase. Appl. Catal. B 2005, 58, 193–202. [Google Scholar] [CrossRef]
- Yaemsunthorn, K.; Kobielusz, M.; Macyk, W. TiO2 with tunable anatase-to-rutile nanoparticles ratios: How does the photoactivity depend on the phase composition and the nature of photocatalytic reaction? ACS Appl. Nano Mater. 2021, 4, 633–643. [Google Scholar] [CrossRef]
- Xia, X.; Peng, S.; Bao, Y.; Wang, Y.; Lei, B.; Wang, Z.; Huang, Z.; Gao, Y. Control of interface between anatase TiO2 nanoparticles and rutile TiO2 nanorods for efficient photocatalytic H2 generation. J. Power Sources 2018, 376, 11–17. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, Y.; Zhang, P.; Zhou, J.; Wu, J.; Li, K.; Wang, W.; Chen, L. Controllable one-step synthesis of mixed-phase TiO2 nanocrystals with equivalent anatase/rutile ratio for enhanced photocatalytic performance. Nanomaterials 2021, 11, 1347. [Google Scholar] [CrossRef]
- Bernardini, C.; Cappelletti, G.; Dozzi, M.V.; Selli, E. Photocatalytic degradation of organic molecules in water: Photoactivity and reaction paths in relation to TiO2 particles features. J. Photochem. Photobiol. A: Chem. 2010, 211, 185–192. [Google Scholar] [CrossRef]
- Bojinova, A.; Kralchevska, R.; Poulios, I.; Dushkin, C. Anatase/rutile TiO2 composites: Influence of the mixing ratio on the photocatalytic degradation of Malachite Green and Orange II in slurry. Mater. Chem. Phys. 2007, 106, 187–192. [Google Scholar] [CrossRef]
- Cong, S.; Xu, Y. Explaining the high photocatalytic activity of a mixed phase TiO2: A combined effect of O2 and crystallinity. J. Phys. Chem. C 2011, 115, 21161–21168. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Li, X. Preparation and photocatalytic performance of anatase/rutile mixed-phase TiO2 nanotubes. Catal. Lett. 2010, 139, 129–133. [Google Scholar] [CrossRef]
- Almashhori, K.; Ali, T.T.; Saeed, A.; Alwafi, R.; Aly, M.; Al-Hazmi, F.E. Antibacterial and photocatalytic activities of controllable (anatase/rutile) mixed phase TiO2 nanophotocatalysts synthesized via a microwave-assisted sol–gel method. New J. Chem. 2020, 44, 562–570. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, M.; Cheng, G. Facile polyol-triggered anatase–rutile heterophase TiO2-x nanoparticles for enhancing photocatalytic CO2 reduction. J. Colloid Interface Sci. 2020, 579, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, L.; Zhao, M.; Huang, X.; Chen, X.; Li, G.; Yu, R. Synthesis of high-quality brookite TiO2 single-crystalline nanosheets with specific facets exposed: Tuning catalysts from inert to highly reactive. J. Am. Chem. Soc. 2012, 134, 8328–8331. [Google Scholar] [CrossRef] [PubMed]
- Khedr, T.M.; El-Sheikh, S.M.; Kowalska, E.; Abdeldayem, H.M. The synergistic effect of anatase and brookite for photocatalytic generation of hydrogen and diclofenac degradation. J. Environ. Chem. Eng. 2021, 9, 106566. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored titanium dioxide nanomaterials: Anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Tian, B.; Anpo, M. Tartaric acid-assisted preparation and photocatalytic performance of titania nanoparticles with controllable phases of anatase and brookite. J. Mater. Sci. 2012, 47, 5743–5751. [Google Scholar] [CrossRef]
- Shen, X.; Tian, B.; Zhang, J. Tailored preparation of titania with controllable phases of anatase and brookite by an alkalescent hydrothermal route. Catal. Today 2013, 201, 151–158. [Google Scholar] [CrossRef]
- Tay, Q.; Liu, X.; Tang, Y.; Jiang, Z.; Sum, T.C.; Chen, Z. Enhanced photocatalytic hydrogen production with synergistic two-phase anatase/brookite TiO2 nanostructures. J. Phys. Chem. C 2013, 117, 14973–14982. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guel, M.; Díaz-Jiménez, L.; Cortés-Hernández, D.; Cabello-Alvarado, C.; Ávila-Orta, C.; Bartolo-Pérez, P.; Gamero-Melo, P. Microwave assisted sol–gel synthesis of titanium dioxide using hydrochloric and acetic acid as catalysts. Bol. Soc. Esp. Ceram. Vidr. 2019, 58, 171–177. [Google Scholar] [CrossRef]
- Quintero, Y.; Mosquera, E.; Diosa, J.; García, A. Ultrasonic-assisted sol–gel synthesis of TiO2 nanostructures: Influence of synthesis parameters on morphology, crystallinity, and photocatalytic performance. J. Sol-Gel Sci. Technol. 2020, 94, 477–485. [Google Scholar] [CrossRef]
- Cihlar, J.; Kasparek, V.; Kralova, M.; Castkova, K. Biphasic anatase-brookite nanoparticles prepared by sol–gel complex synthesis and their photocatalytic activity in hydrogen production. Int. J. Hydrogen Energy 2015, 40, 2950–2962. [Google Scholar] [CrossRef]
- Cihlar, J.; Navarro, L.K.T.; Kasparek, V.; Michalicka, J.; Cihlar Jr, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti molar ratio on the complex synthesis of anatase/brookite nanoparticles and their hydrogen production. Int. J. Hydrogen Energy 2021, 46, 8578–8593. [Google Scholar] [CrossRef]
- Di Paola, A.; Cufalo, G.; Addamo, M.; Bellardita, M.; Campostrini, R.; Ischia, M.; Ceccato, R.; Palmisano, L. Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 366–376. [Google Scholar] [CrossRef]
- Luo, B.; Li, Z.; Xu, Y. The positive effect of anatase and rutile on the brookite-photocatalyzed degradation of phenol. RSC Adv. 2015, 5, 105999–106004. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L. Controllable one-pot synthesis and enhanced photocatalytic activity of mixed-phase TiO2 nanocrystals with tunable brookite/rutile ratios. J. Phys. Chem. C 2009, 113, 1785–1790. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Bian, Z.; Fuhr, A.; Zhang, D.; Zhu, J. Highly photocatalytic activity of brookite/rutile TiO2 nanocrystals with semi-embedded structure. Appl. Catal. B 2016, 180, 551–558. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, J.E.; Kim, K.S.; Kang, J.M.; Lee, J.H.; Kim, K.H.; Cho, M.; Lee, S.G. Photocatalytic degradation of methylene blue under UV and visible light by brookite–rutile bi-crystalline phase of TiO2. New J. Chem. 2021, 45, 3485–3497. [Google Scholar] [CrossRef]
- Qi, L.; Liu, Y.; Li, C. Controlled synthesis of TiO2-B nanowires and nanoparticles for dye-sensitized solar cells. Appl. Surf. Sci. 2010, 257, 1660–1665. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.; Lu, W.; Li, J. Photoelectrochemical study on charge transfer properties of TiO2−B nanowires with an application as humidity sensors. J. Phys. Chem. B 2006, 110, 22029–22034. [Google Scholar] [CrossRef] [PubMed]
- Feist, T.P.; Davies, P.K. The soft chemical synthesis of TiO2 (B) from layered titanates. J. Solid State Chem. 1992, 101, 275–295. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tomita, K.; Fujita, K.; Kobayashi, M.; Petrykin, V.; Kakihana, M. Synthesis of TiO2 (B) using glycolato titanium complex and post-synthetic hydrothermal crystal growth of TiO2 (B). J. Cryst. Growth 2009, 311, 619–622. [Google Scholar] [CrossRef]
- Kobayashi, M.; Petrykin, V.V.; Kakihana, M.; Tomita, K.; Yoshimura, M. One-step synthesis of TiO2 (B) nanoparticles from a water-soluble titanium complex. Chem. Mater. 2007, 19, 5373–5376. [Google Scholar] [CrossRef]
- Giannuzzi, R.; Manca, M.; De Marco, L.; Belviso, M.R.; Cannavale, A.; Sibillano, T.; Giannini, C.; Cozzoli, P.D.; Gigli, G. Ultrathin TiO2 (B) nanorods with superior lithium-ion storage performance. ACS Appl. Mater. Interfaces 2014, 6, 1933–1943. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Zhu, Y.; Wu, M.; Zhang, H.; Li, X.; Jiang, Z.; Meng, M. In situ formation of disorder-engineered TiO2 (B)-anatase heterophase junction for enhanced photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2015, 7, 24987–24992. [Google Scholar] [CrossRef]
- Mikrut, P.; Kobielusz, M.; Indyka, P.; Macyk, W. Photocatalytic activity of TiO2 polymorph B revisited: Physical, redox, spectroscopic, and photochemical properties of TiO2 (B)/anatase series of titanium dioxide materials. Mater. Today Sustain. 2020, 10, 100052. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Luo, S.; Chang, C.; Cui, Z. Rutile-coated B-phase TiO2 heterojunction nanobelts for photocatalytic H2 evolution. ACS Appl. Nano Mater. 2020, 3, 10349–10359. [Google Scholar] [CrossRef]
- Chalastara, K.; Guo, F.; Elouatik, S.; Demopoulos, G.P. Tunable composition aqueous-synthesized mixed-phase TiO2 nanocrystals for photo-assisted water decontamination: Comparison of anatase, brookite and rutile photocatalysts. Catalysts 2020, 10, 407. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Kaplan, R.; Erjavec, B.; Dražić, G.; Grdadolnik, J.; Pintar, A. Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants. Appl. Catal. B 2016, 181, 465–474. [Google Scholar] [CrossRef]


| Phase | Crystal System, Space Group | Lattice Parameter | Density (g/cm3) | Band Gap Energy (eV) | Ref. |
|---|---|---|---|---|---|
| Brookite | Ortorombik, Pbca | a = 9.148 Å | 4.12 | 3.14–3.31 | [117] |
| b = 5.447 Å | |||||
| c = 5.145 Å | |||||
| V = 257.38 Å3 | |||||
| Rutile | Tetragonal, P42/mnm | a = b = 4.594 Å | 4.25 | 3.02–3.04 | [117] |
| c = 2.959 Å | |||||
| V = 62.45 Å3 | |||||
| Anatase | Tetragonal, I41/amd | a = b = 3.784 Å | 3.89 | 3.20–3.23 | [117] |
| c = 9.515 Å | |||||
| V = 136.24 Å3 | |||||
| TiO2 (B) | Monoclinic, C2/m | a = 12.179 Å | 3.73 | 3.09–3.22 | [113] |
| b = 3.741 Å | |||||
| c = 6.525 Å | |||||
| β = 107.054 | |||||
| V = 284.22 Å3 |
| Precursor | Method | Phase Controller | Application | Ref. |
|---|---|---|---|---|
| TiO2 P25 | Hydrothermal | Calcination temperature | Water splitting | [63] |
| TTIP | Hydrothermal | Calcination temperature | Methylene blue degradation | [247] |
| TTIP | Sol–gel | Calcination temperature | Oxalic acid degradation | [248] |
| TBOT | Hydrothermal | pH | Hydrogen generation | [252] |
| TiOSO4 | Urea precipitation method | Calcination temperature | 4-chlorophenol degradation | [250] |
| TiCl4 | One step condensing reflux | Solvent | Rhodamine B degradation | [253] |
| TiCl3 | Hydrothermal | Calcination temperature | Hydrogen generation | [251] |
| Pollutant | Optimum Composition (%) * | Dye Concentration | Light Source | Irradiation Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Methylene blue | A 78.5 R 21.5 | 1 × 10−5 M | UV lamp | 20 | ~94% | [248] |
| Rhodamine B | A 48.0 R 52.0 | 4.8 mg/L | 300 W Xenon lamp (365 nm) | 60 | 99% | [253] |
| Methyl orange | A 70.0 R 30.0 | 20 mg/L | 125 W Mercury lamp (365 nm) | 40 | 90.6% | [258] |
| Methylene blue | A 30.8 R 69.2 | 2.92 × 10−5 M | 175 W UV lamp | 90 | 85.8% | [220] |
| Crystal violet | A 81.6 R 18.4 | 100 ppm | UV lamp (365 nm) | 300 | 92.65% | [259] |
| Methylene blue | A 81.6 R 18.4 | 100 ppm | UV lamp (365 nm) | 300 | 94.77% | [259] |
| Precursor | Method | Additive | Application | Ref. |
|---|---|---|---|---|
| TALH | Hydrothermal | Urea | Hydrogen production and dichloroacetic acid (DCA) degradation | [263] |
| TiCl3 | Hydrothermal | Tartaric acid | Rhodamine B degradation | [264] |
| TiCl3 | Hydrothermal | NaCl and NH4OH | Rhodamine B degradation | [265] |
| TiS2 | Hydrothermal | NaOH | Hydrogen generation | [266] |
| Ti2(SO4)3 | Hydrothermal | Glycine with NH4OH or NaOH | Cylindrospermopsin degradation | [267] |
| TTIP | Sol–gel | HNO3 | Methylene blue degradation | [268] |
| TTIP | Microwave assisted sol–gel | HCl and CH3COOH | - | [269] |
| TTIP | Ultrasonic-assisted sol–gel | HNO3 and CH3COOH | Methylene blue degradation | [270] |
| TTIP | Sol–gel complex | hydroxycarboxylic acids | Hydrogen generation | [271] |
| Ti(Opr)4 | Sol–gel complex | Lactic acid | Hydrogen generation | [272] |
| Ti2(SO4)3 | Hydrothermal | Glycine with NH4OH or NaOH | Diclofenac degradation | [273] |
| Pollutant | Optimum Composition (%) * | Photocatalyst Loading | Dye Concentration | Light Source | Irradiation Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Rhodamine B | A 78.7 B 21.3 | - | 20 mg/L | 300 W Hg lamp (365 nm) | 120 | 98 | [264] |
| Rhodamine B | A 46.6 B 53.4 | - | 20 mg/L | 300 W Hg lamp (365 nm) | 100 | 95 | [265] |
| Methylene blue | A 80.0 B 20.0 | 0.6 g/L | 32 mg/L | 100 W mercury lamp | 70 | 98 | [268] |
| Methylene blue | A 79.0 B 21.0 | 1 g/L | 5 mg/L | 100 W UV lamp (365 nm) | 240 | 60 | [270] |
| Cylindrospermopsin (CYN) | A 61.8 B 38.2 | 0.25 g/L | 1 × 10−6 M | 15 W florescence lamp (310–720 nm) | 15 | 100 | [267] |
| Diclofenac (DCF) | A 61.8 B 38.2 | 1 g/L | 10 mg/L | UV-A irradiation | 120 | 100 | [262] |
| Optimum Composition (%) * | Amount of Catalyst | Light Source | H2 Evolution Rate | Ref. |
|---|---|---|---|---|
| A 73.5 R 26.5 | 20 mg/100 mL | 300 W Xe lamp | 584 μmol g−1 h−1 | [63] |
| A 12.0 R 88.0 | 20 mg/80 mL | 350 W Xe lamp | 74,400 μmol g−1 h−1 | [252] |
| A 72.0 B 28.0 | 37.5 mg/75 mL | 1000 W Xe lamp | 4267 μmol g−1 h−1 | [263] |
| A 88.4 B 11.6 | 45 mg/100 mL | 800 W Xe−Hg lamp | ~3500 μmol g−1 h−1 | [266] |
| A 74.0 B 36.0 | 20 mg/100 mL | 450 W Xe lamp | 101.4 μmol g−1 min−1 | [271] |
| A 54.0 B 46.0 | 20 mg/100 mL | 450 W Xe lamp | 101.4 μmol g−1 min−1 | [272] |
| Precursor | Method | Additive | Application | Ref. |
|---|---|---|---|---|
| Titanium metal powder | Post-synthetic Hydrothermal crystal growth | H2SO4 | - | [281] |
| Titanium metal powder | Hydrothermal | H2SO4 | - | [282] |
| TiCl4 | Solvothermal | - | Lithium-ion batteries | [173] |
| TiO2 P25 | Hydrothermal | - | DSSC | [278] |
| TiO2 P25 | Hydrothermal | - | Humidity sensors | [279] |
| TTIP | Surfactant-assisted nonaqueous sol–gel route | Oleic acid | Lithium ion-batteries | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. https://doi.org/10.3390/nano13040704
Eddy DR, Permana MD, Sakti LK, Sheha GAN, Solihudin, Hidayat S, Takei T, Kumada N, Rahayu I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials. 2023; 13(4):704. https://doi.org/10.3390/nano13040704
Chicago/Turabian StyleEddy, Diana Rakhmawaty, Muhamad Diki Permana, Lintang Kumoro Sakti, Geometry Amal Nur Sheha, Solihudin, Sahrul Hidayat, Takahiro Takei, Nobuhiro Kumada, and Iman Rahayu. 2023. "Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity" Nanomaterials 13, no. 4: 704. https://doi.org/10.3390/nano13040704
APA StyleEddy, D. R., Permana, M. D., Sakti, L. K., Sheha, G. A. N., Solihudin, Hidayat, S., Takei, T., Kumada, N., & Rahayu, I. (2023). Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials, 13(4), 704. https://doi.org/10.3390/nano13040704








