Three-Dimensional Evaluation of the Cytotoxicity and Antibacterial Properties of Alpha Lipoic Acid-Capped Silver Nanoparticle Constructs for Oral Applications
Abstract
:1. Introduction
2. Methods
2.1. Alpha-Lipoic-Acid-Capped Silver Nanoparticles Preparation and Characterisation
2.2. Optimisation and Characterisation of the Hydrogel Constructs
2.2.1. HyStem®-C Reconstitution and Sample Preparation
2.2.2. GelMA Synthesis
2.2.3. GelMA Reconstitution and Sample Preparation
2.2.4. Cell Culture of Primary Human Gingival Fibroblasts
2.2.5. Cell Encapsulation in HyStem®-C and GelMA Constructs
2.2.6. Metabolic Activity of HGFs within the Hydrogel Constructs
2.2.7. HGF Cell Viability by Live/Dead Staining
2.3. Silver Nanoparticles and their Interaction with Hydrogels
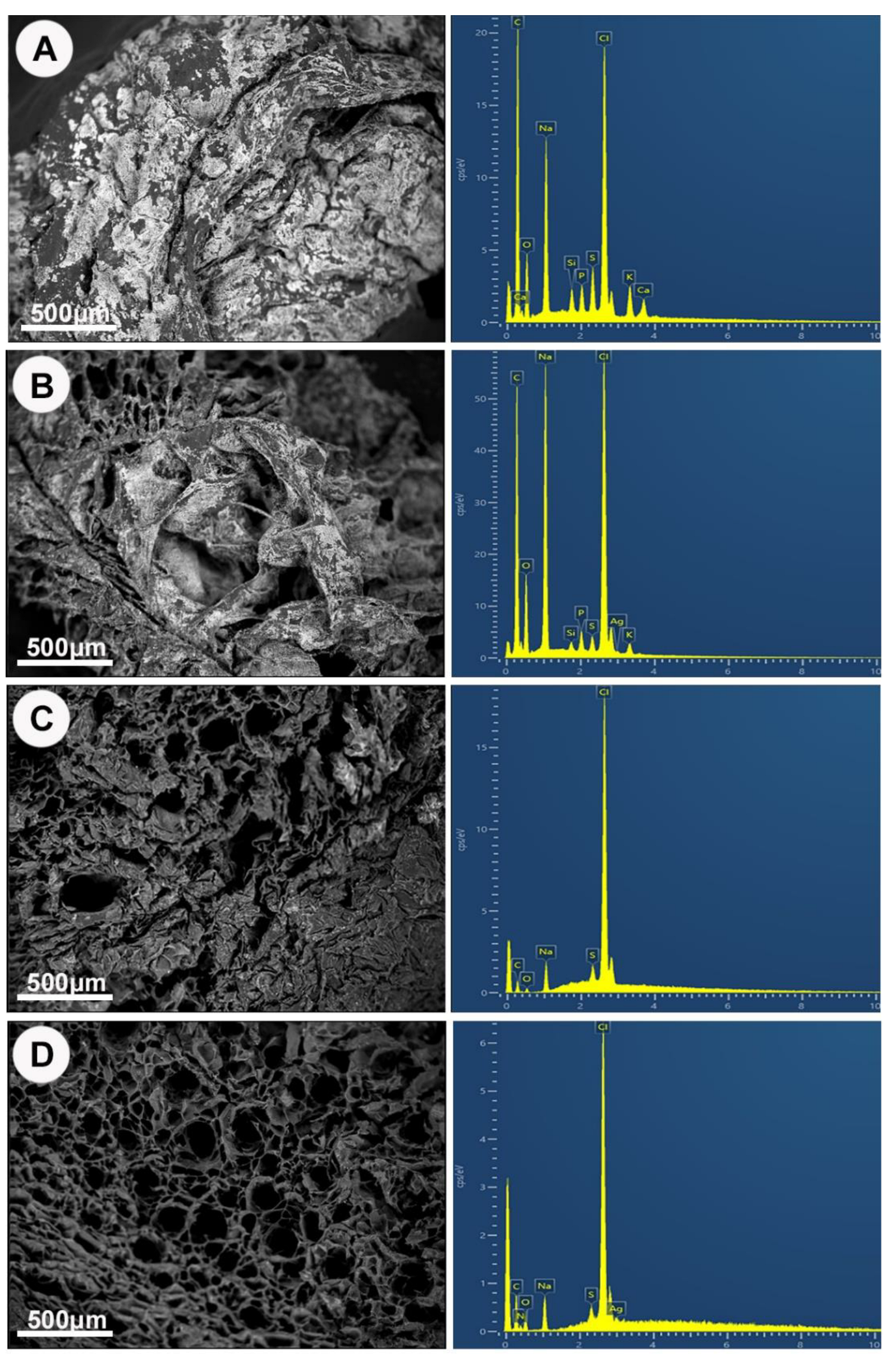
2.3.1. Morphology and Elemental Analysis
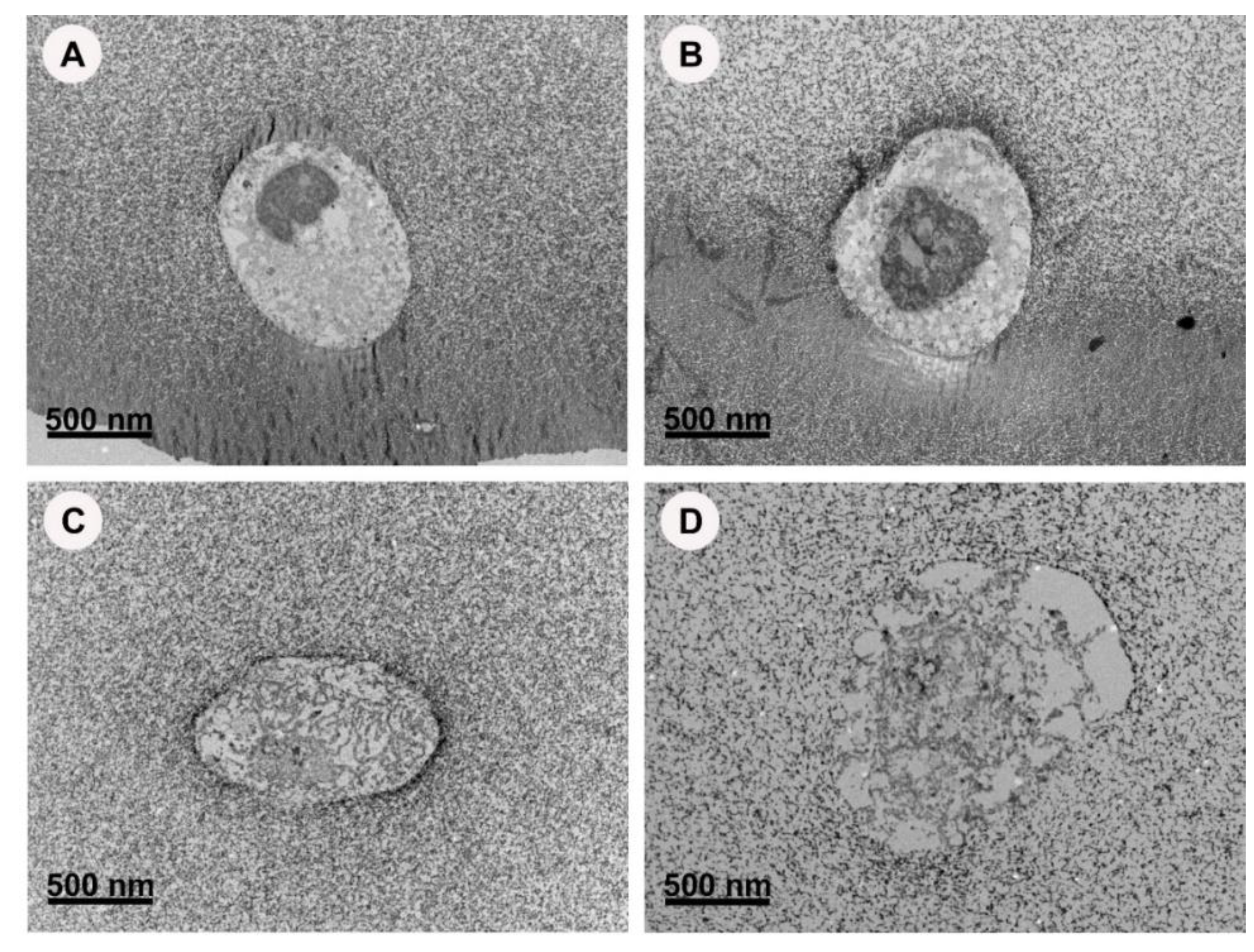
2.3.2. TEM Imaging of Hydrogels Constructs with and without AgNPs
2.3.3. Fourier Transform Infrared-Attenuated Total Reflection (FTIR-ATR)
2.4. GelMA as a 3D Model for Cytotoxicity
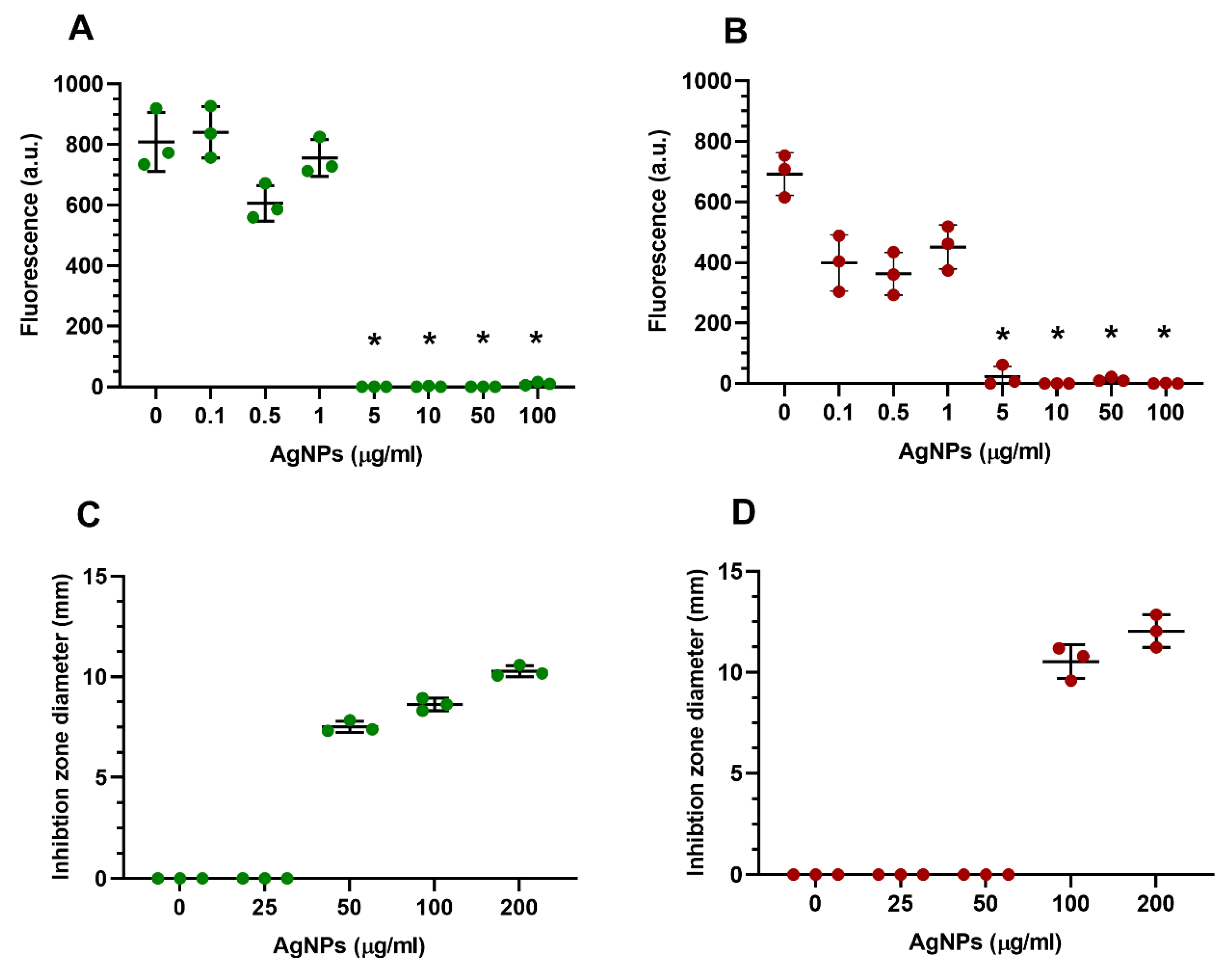
2.4.1. AgNP Cytotoxicity on Encapsulated HGFs in Standard GelMA Constructs
2.4.2. Silver Nanoparticles Release from GelMA Constructs
2.4.3. TEM Imaging of GelMA Constructs
2.5. Antibacterial Properties of AgNPs Encapsulated in GelMA
2.5.1. Bacterial Culture
2.5.2. Bacterial Encapsulation in GelMA
2.5.3. Minimal Inhibitory Concentration (MICs) and Minimal Bactericidal Concentration (MBCs) in 3D Culture
2.5.4. Antibacterial Properties of GelMA Incorporated AgNPs by Disc Diffusion Assay
2.6. Statistical Analysis
3. Results
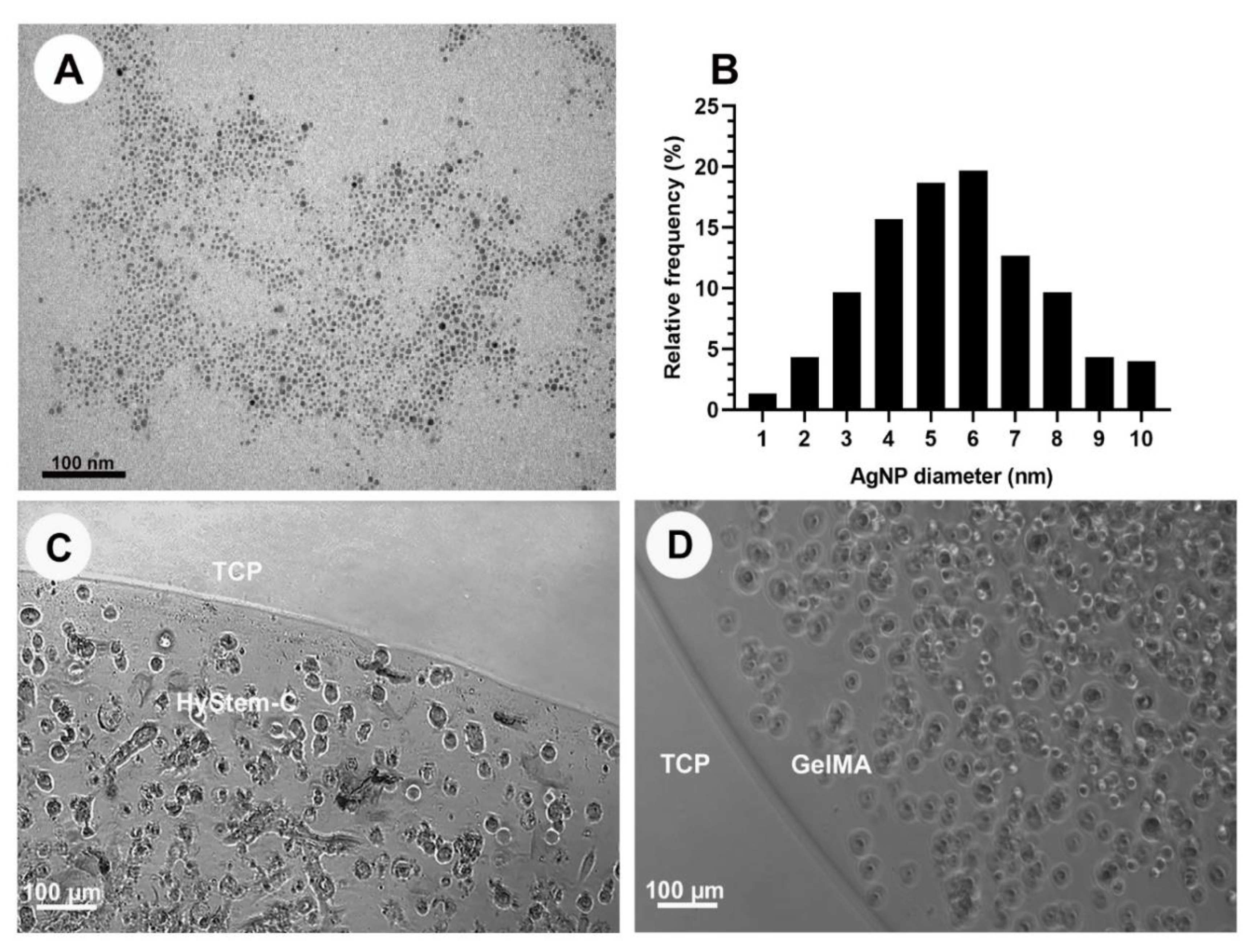
3.1. Synthesis of Alpha-Lipoic-Acid-Capped Silver Nanoparticles
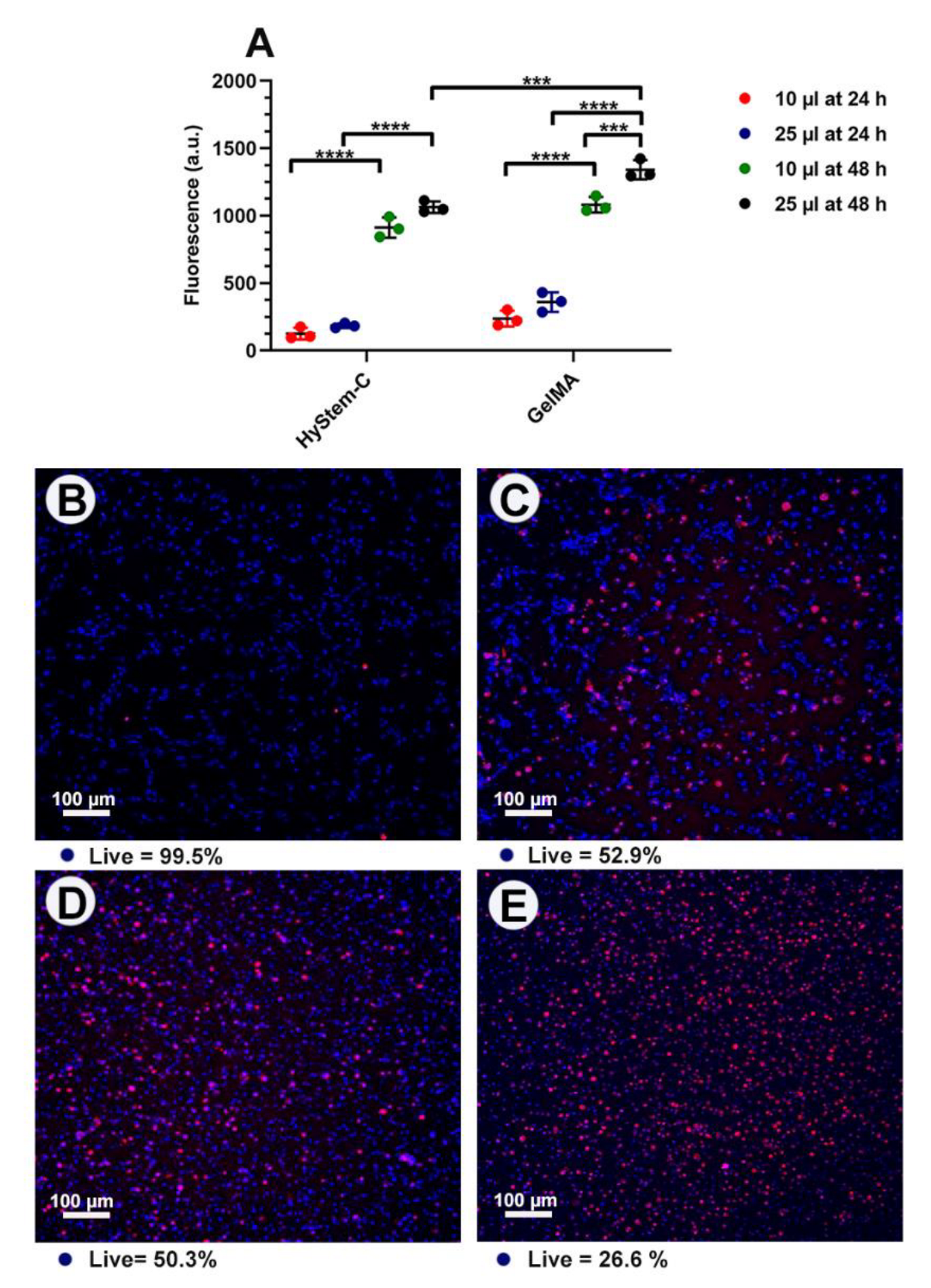
3.2. Optimisation and Characterisation
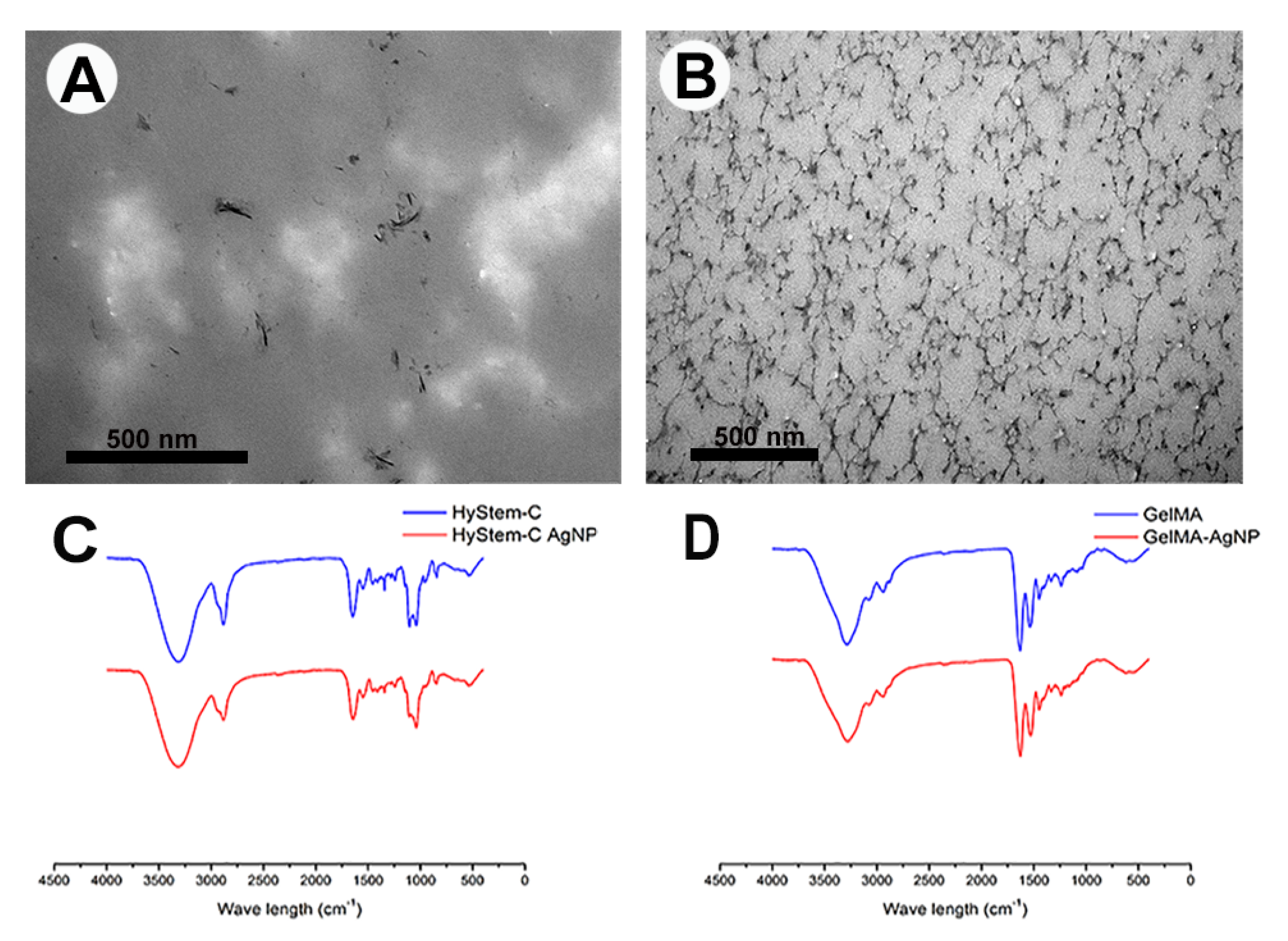
3.3. AgNPs-Hydrogel Matrix Interaction
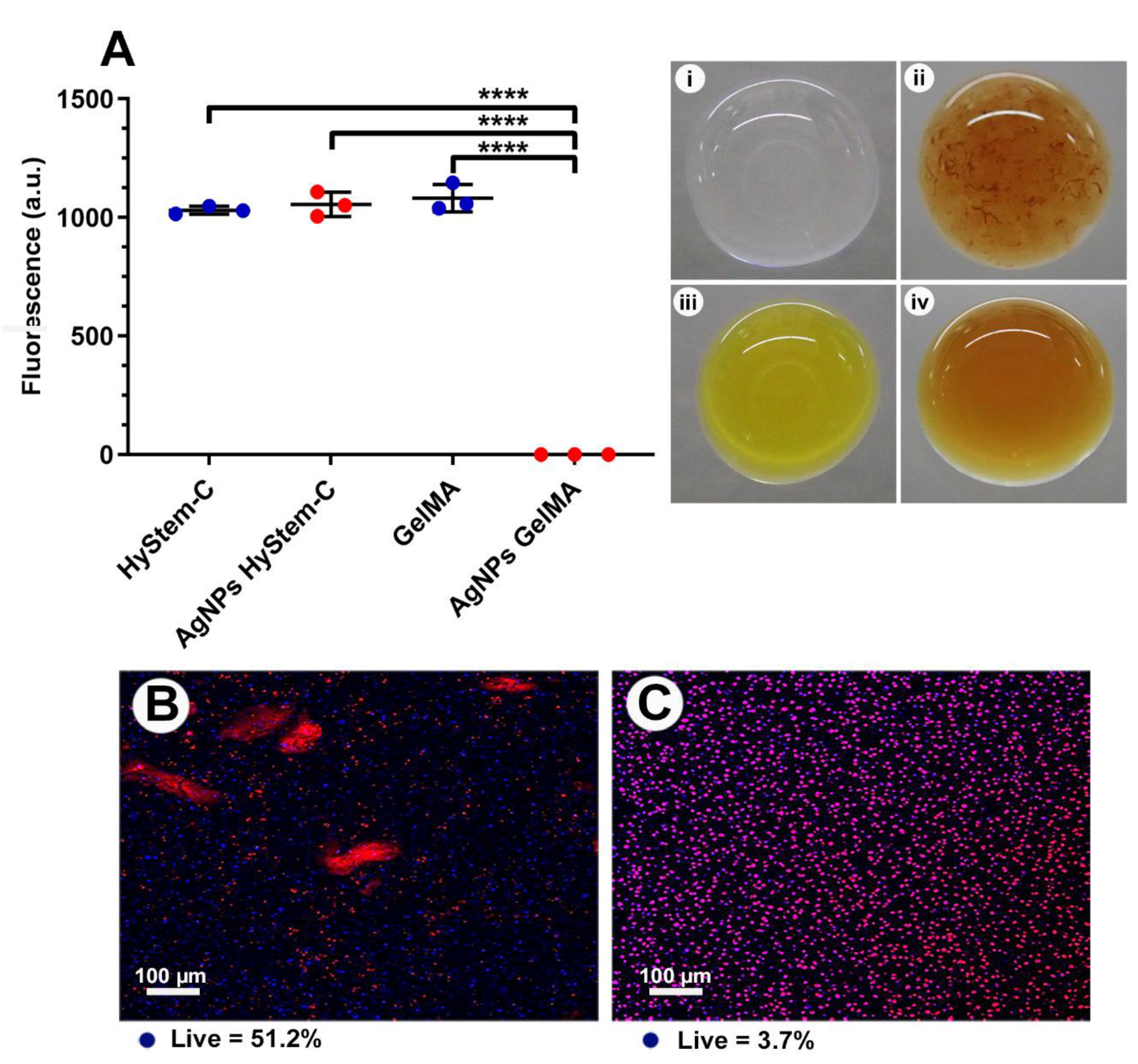
3.4. GelMA as a 3D Model
3.5. Antibacterial Properties of GelMA Incorporated with AgNPs
4. Discussion
4.1. Significance of the Photo-Cross-Linked GelMA-AgNPs as Bifunctional Non-Antibiotic Scaffold
4.2. Construct Design
4.3. Polymeric Matrices and Interactions with AgNPs
4.4. Cytotoxicity
4.5. Mechanism of Action
4.6. Antimicrobial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campoccia, D.; Montanaro, L.; Arciola, C. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [PubMed]
- Actis, L.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Ong, J.L. Effect of silver nanoparticle geometry on methicillin susceptible and resistant Staphylococcus aureus, and osteoblast viability. J. Mater. Sci. Mater. Med. 2015, 26, 215. [Google Scholar] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.; Roh, J.; Kim, S.; Choi, K.; Yi, J.; Kim, Y.; Yoon, J. Biofilm-inactivating activity of silver nanoparticles: A comparison with silver ions. J. Ind. Eng. Chem. 2013, 19, 614–619. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araujo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vargas, J.M.; Echeverry-Cardona, L.M.; Moreno-Montoya, L.E.; Restrepo-Parra, E. Evaluation of Antifungal Activity of Ag Nanoparticles Synthetized by Green Chemistry against Fusarium solani and Rhizopus stolonifera. Nanomaterials 2023, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Singh, S.K.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009, 3, 1357–1364. [Google Scholar] [CrossRef]
- Asharani, P.V.; Hande, M.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008, 4, 241–251. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [PubMed]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [PubMed]
- Alarcon, R.; Walter, M.; Paez, M.; Azócar, M.I. Ostwald Ripening and Antibacterial Activity of Silver Nanoparticles Capped by Anti-Inflammatory Ligands. Nanomaterials 2023, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Cotton, G.C.; Gee, C.; Jude, A.; Duncan, W.J.; Abdelmoneim, D.; Coates, D.E. Efficacy and safety of alpha lipoic acid-capped silver nanoparticles for oral applications. RSC Adv. 2019, 9, 6973–6985. [Google Scholar] [CrossRef]
- Mette, M.; Connolly, L.; Vishwanath, N.; Allu, S.; Whitaker, C.; Stone, B.K.; Garcia, D. Silver Carboxylate as an Antibiotic-Independent Antimicrobial: A Review of Current Formulations, in vitro Efficacy, and Clinical Relevance. Med. Res. Arch. 2022, 10, 3388. [Google Scholar] [CrossRef]
- Kapalczynska, M. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Beningo, K.A.; Dembo, M.; Wang, Y. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 18024–18029. [Google Scholar] [CrossRef]
- Friedl, P.; Brocker, E. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. 2000, 57, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef]
- Liu, Y.; Chan-Park, M. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [PubMed]
- Lim, K.S.; Klotz, B.J.; Lindberg, G.C.J.; Melchels, F.P.W.; Hooper, G.J.; Malda, J.; Gawlitta, D.; Woodfield, T.B.F. Visible Light Cross-Linking of Gelatin Hydrogels Offers an Enhanced Cell Microenvironment with Improved Light Penetration Depth. Macromol. Biosci. 2019, 19, e1900098. [Google Scholar] [PubMed]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [PubMed]
- Kim, H.; Shin, M.; Han, S.; Kwon, W.; Hahn, S.K. Hyaluronic Acid Derivatives for Translational Medicines. Biomacromolecules 2019, 20, 2889–2903. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Kuo, J. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 2008, 9, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.A.; Scott, A.; Prestwich, G. Use of hyaluronan-derived hydrogels for three-dimensional cell culture and tumor xenografts. Curr. Protoc. Cell Biol. 2008, 40, 10–14. [Google Scholar] [CrossRef]
- Garside, P.; Lahlil, S.; Wyeth, P. Characterization of historic silk by polarized attenuated total reflectance Fourier transform infrared spectroscopy for informed conservation. Appl. Spectrosc. 2005, 59, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Wang, Y.; Li, Z.; Wang, W.X.; Sun, H.H.; Liu, M.X. Synthesis and Characterization of Hyaluronic Acid Modified Colloidal Mesoporous Silica Nanoparticles. In 5th Annual International Conference on Material Science and Engineering (Icmse2017); IOP Publishing: Bristol, UK, 2018; Volume 275. [Google Scholar]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [PubMed]
- Bryant, S.J.; Nuttelman, C.; Anseth, K. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef]
- Marambio-Jones., C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.; Goulart, M.O. Lipoic Acid: Its antioxidant and anti-inflammatory role and clinical applications. Curr. Top Med. Chem. 2015, 15, 458–483. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Hobgood, C.D.; Webb, L.X.; Myrvik, Q.N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 1987, 8, 423–426. [Google Scholar] [CrossRef]
- Martins, N.; Rodrigues, C. Biomaterial-Related Infections. J. Clin. Med. 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Pan, Z.; Ghosh, K.; Liu, Y.; Clark, R.A.; Rafailovich, M.H. Traction stresses and translational distortion of the nucleus during fibroblast migration on a physiologically relevant ECM mimic. Biophys. J. 2009, 96, 4286–4298. [Google Scholar] [CrossRef] [PubMed]
- Zarembinski, T.I.; Skardal, A. HyStem®: A Unique Clinical Grade Hydrogel for Present and Future Medical Applications, in Hydrogels—Smart Materials for Biomedical Applications. In Hydrogels-Smart Materials for Biomedical Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed. Mater. Res. A 2018, 106, 201–209. [Google Scholar] [CrossRef]
- Heo, D.N. Enhanced bone regeneration with a gold nanoparticle-hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T. Silver nanoparticles improve structural stability and biocompatibility of decellularized porcine liver. Artif. Cells Nanomed. Biotechnol. 2018, 46, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Yang, X.; Zhang, W.; Jiang, M.; Wen, T.; Wang, J.; Guo, R.; Liu, H. Preparation and Application of Quaternized Chitosan- and AgNPs-Base Synergistic Antibacterial Hydrogel for Burn Wound Healing. Molecules 2021, 26, 4037. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zu, D.; Kong, L.; Chen, S.; Yao, J.; Lin, J.; Lu, L.; Wu, B.; Fang, B. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater. Res. 2022, 26, 36. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Walker, M.; Parsons, D. The biological fate of silver ions following the use of silver-containing wound care products—A review. Int. Wound J. 2014, 11, 496–504. [Google Scholar] [CrossRef]
- Martinez-Higuera, A. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci. Rep. 2021, 11, 11312. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynski, C.J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Development of Silver Nanoparticles/Gelatin Thermoresponsive Nanocomposites: Characterization and Antimicrobial Activity. Curr. Pharm. Des. 2019, 25, 4121–4129. [Google Scholar]
- Sumitha, M.S.; Shalumon, K.T.; Sreeja, V.N.; Jayakumar, R.; Nair, S.V.; Menon, D. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar]
- Porter, G.C.; Duncan, W.J.; Jude, A.; Abdelmoneim, D.; Easingwood, R.A.; Coates, D.E. Endocytosed silver nanoparticles degrade in lysosomes to form secondary nanoparticle structures during expression of autophagy genes in osteogenic cells. Nanomedicine 2021, 33, 102355. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [PubMed] [Green Version]
- Panacek, A.; Kilianova, M.; Prucek, R.; Husickova, V.; Vecerova, R.; Kolar, M.; Kvitek, L.; Zboril, R. Preparation and in vitro Bactericidal and Fungicidal Efficiency of NanoSilver / Methylcellulose Hydrogel. Int. J. Chem. Mol. Eng. 2014, 8, 493–498. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmoneim, D.; Porter, G.; Duncan, W.; Lim, K.; Easingwood, R.; Woodfield, T.; Coates, D. Three-Dimensional Evaluation of the Cytotoxicity and Antibacterial Properties of Alpha Lipoic Acid-Capped Silver Nanoparticle Constructs for Oral Applications. Nanomaterials 2023, 13, 705. https://doi.org/10.3390/nano13040705
Abdelmoneim D, Porter G, Duncan W, Lim K, Easingwood R, Woodfield T, Coates D. Three-Dimensional Evaluation of the Cytotoxicity and Antibacterial Properties of Alpha Lipoic Acid-Capped Silver Nanoparticle Constructs for Oral Applications. Nanomaterials. 2023; 13(4):705. https://doi.org/10.3390/nano13040705
Chicago/Turabian StyleAbdelmoneim, Dina, Gemma Porter, Warwick Duncan, Khoon Lim, Richard Easingwood, Tim Woodfield, and Dawn Coates. 2023. "Three-Dimensional Evaluation of the Cytotoxicity and Antibacterial Properties of Alpha Lipoic Acid-Capped Silver Nanoparticle Constructs for Oral Applications" Nanomaterials 13, no. 4: 705. https://doi.org/10.3390/nano13040705
APA StyleAbdelmoneim, D., Porter, G., Duncan, W., Lim, K., Easingwood, R., Woodfield, T., & Coates, D. (2023). Three-Dimensional Evaluation of the Cytotoxicity and Antibacterial Properties of Alpha Lipoic Acid-Capped Silver Nanoparticle Constructs for Oral Applications. Nanomaterials, 13(4), 705. https://doi.org/10.3390/nano13040705





