Ordered Hierarchical Porous Structure of PtSn/3DOMM-Al2O3 Catalyst for Promoting Propane Non-Oxidative Dehydrogenation
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Characterization
2.3. Evaluation of Catalytic Performance
3. Results and Discussion
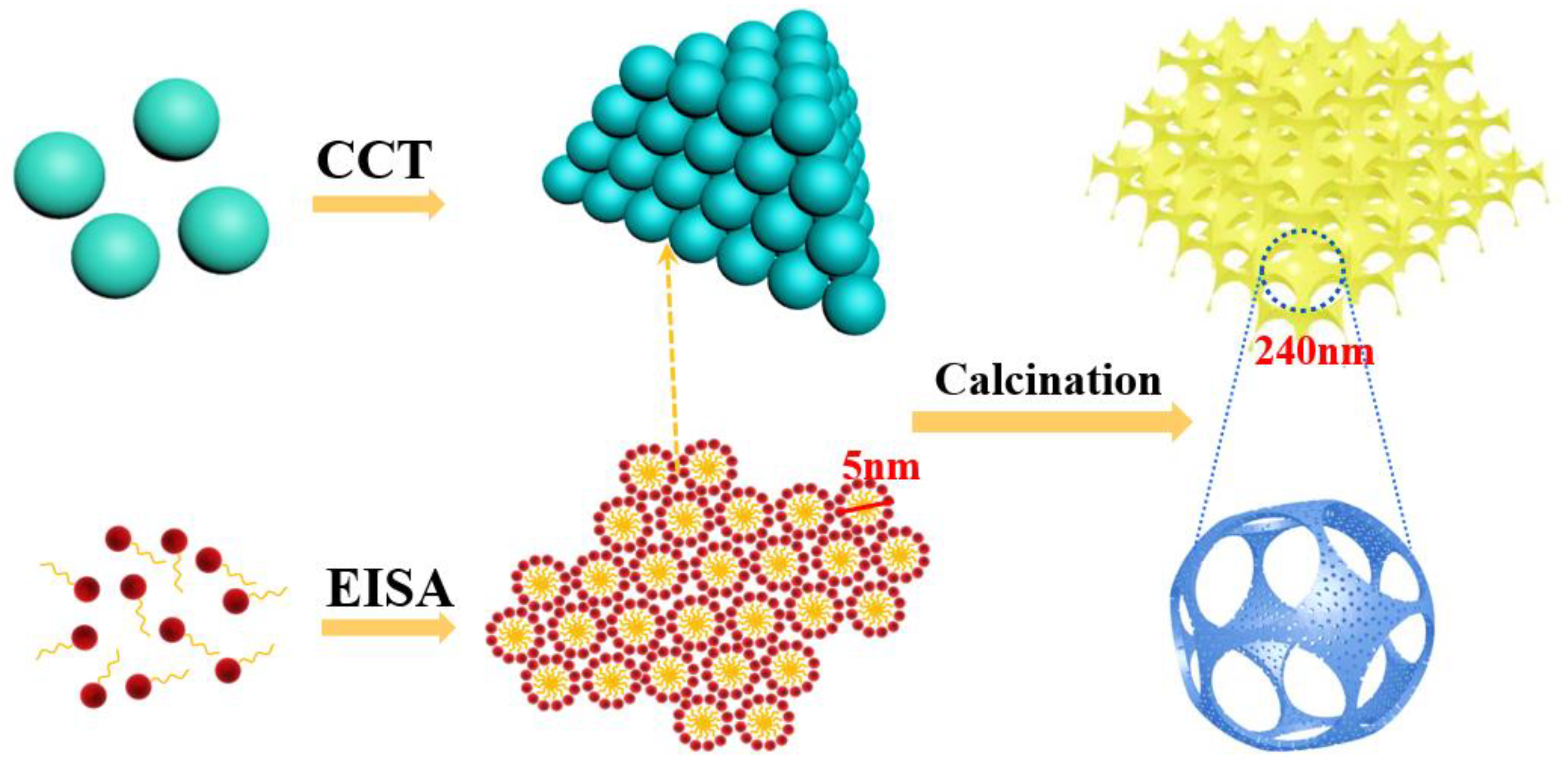
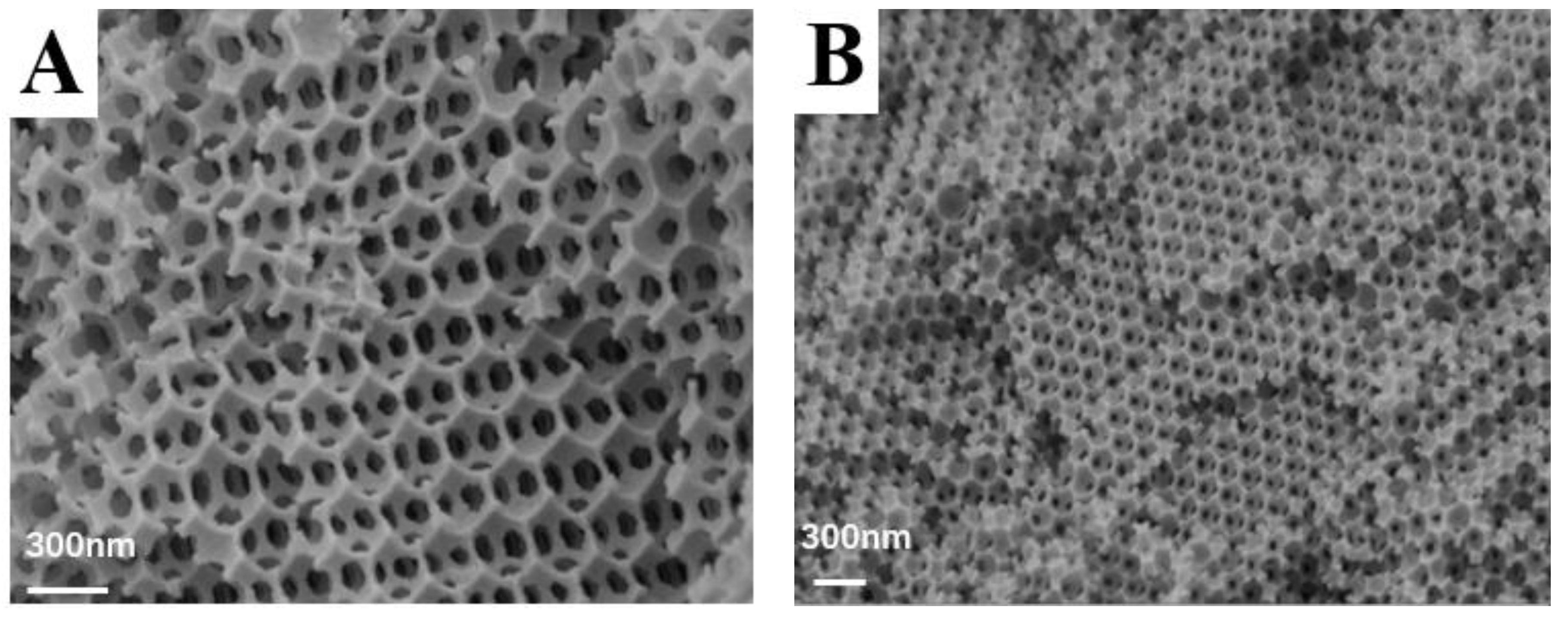
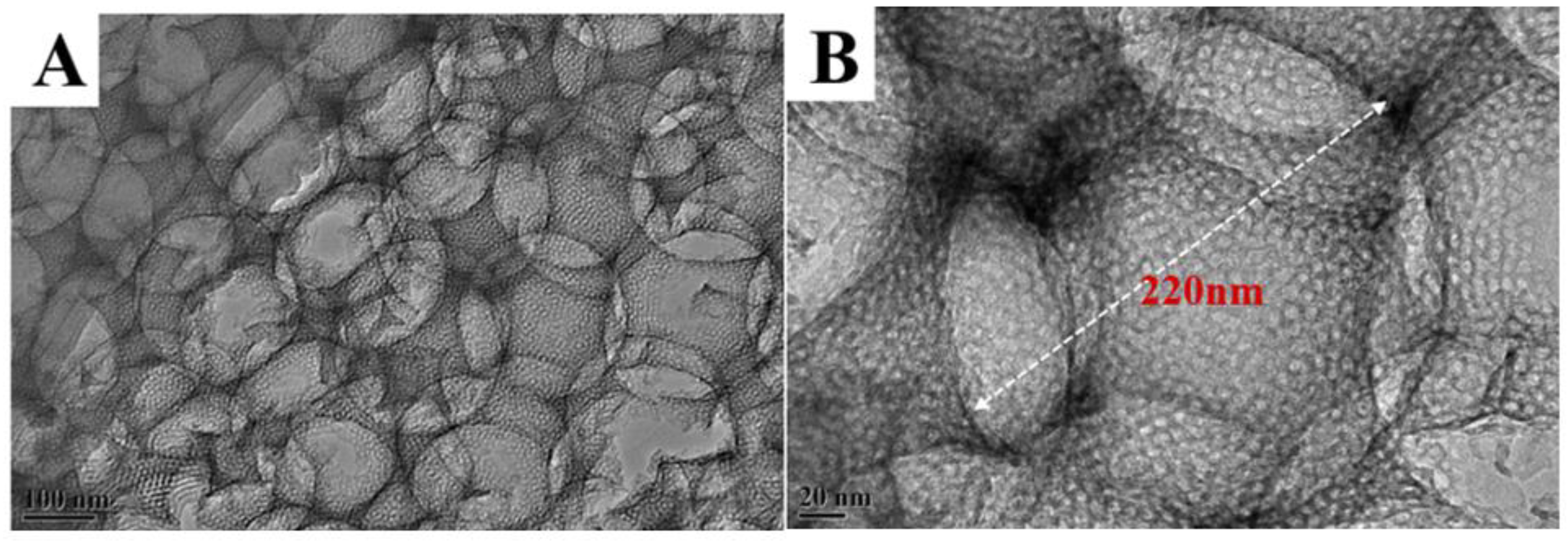
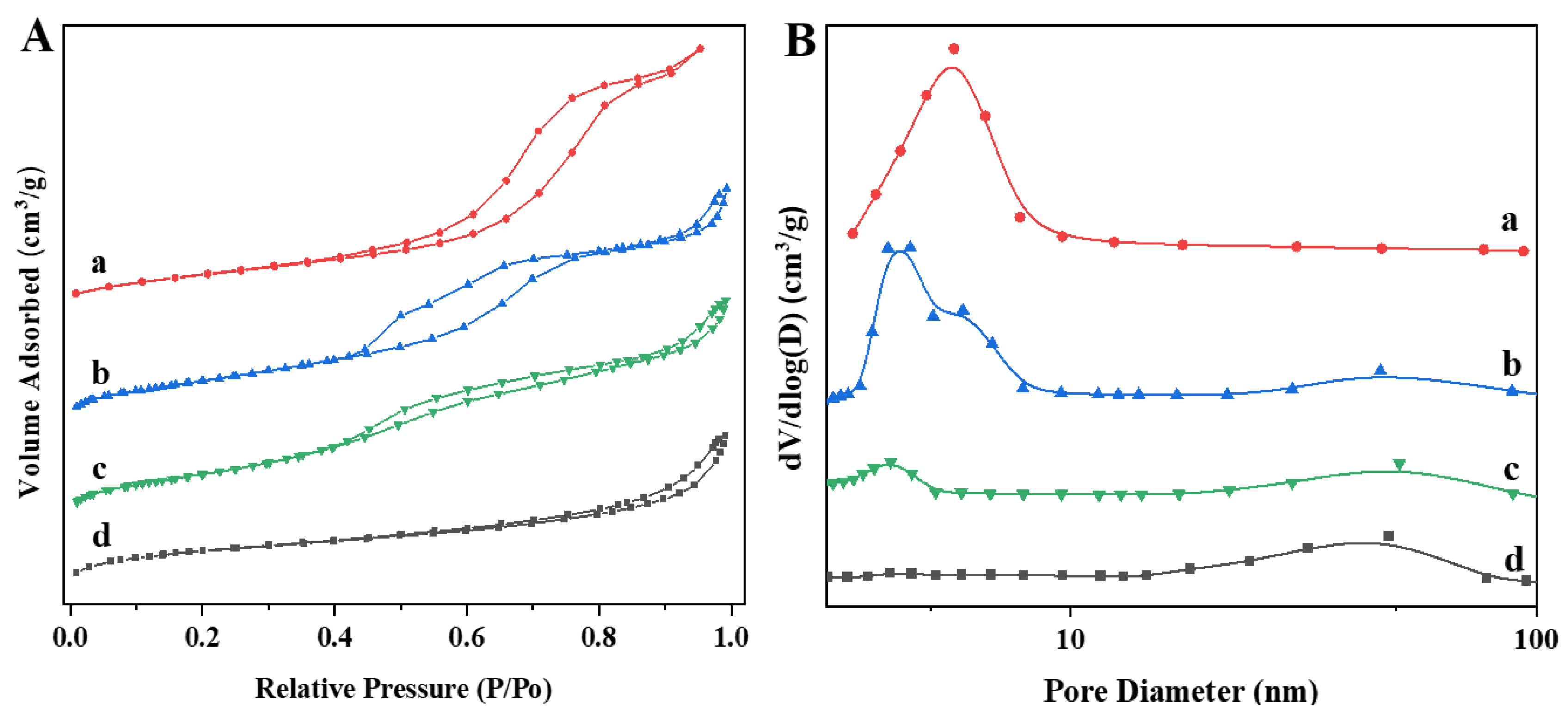
3.1. Physical Properties and Morphology
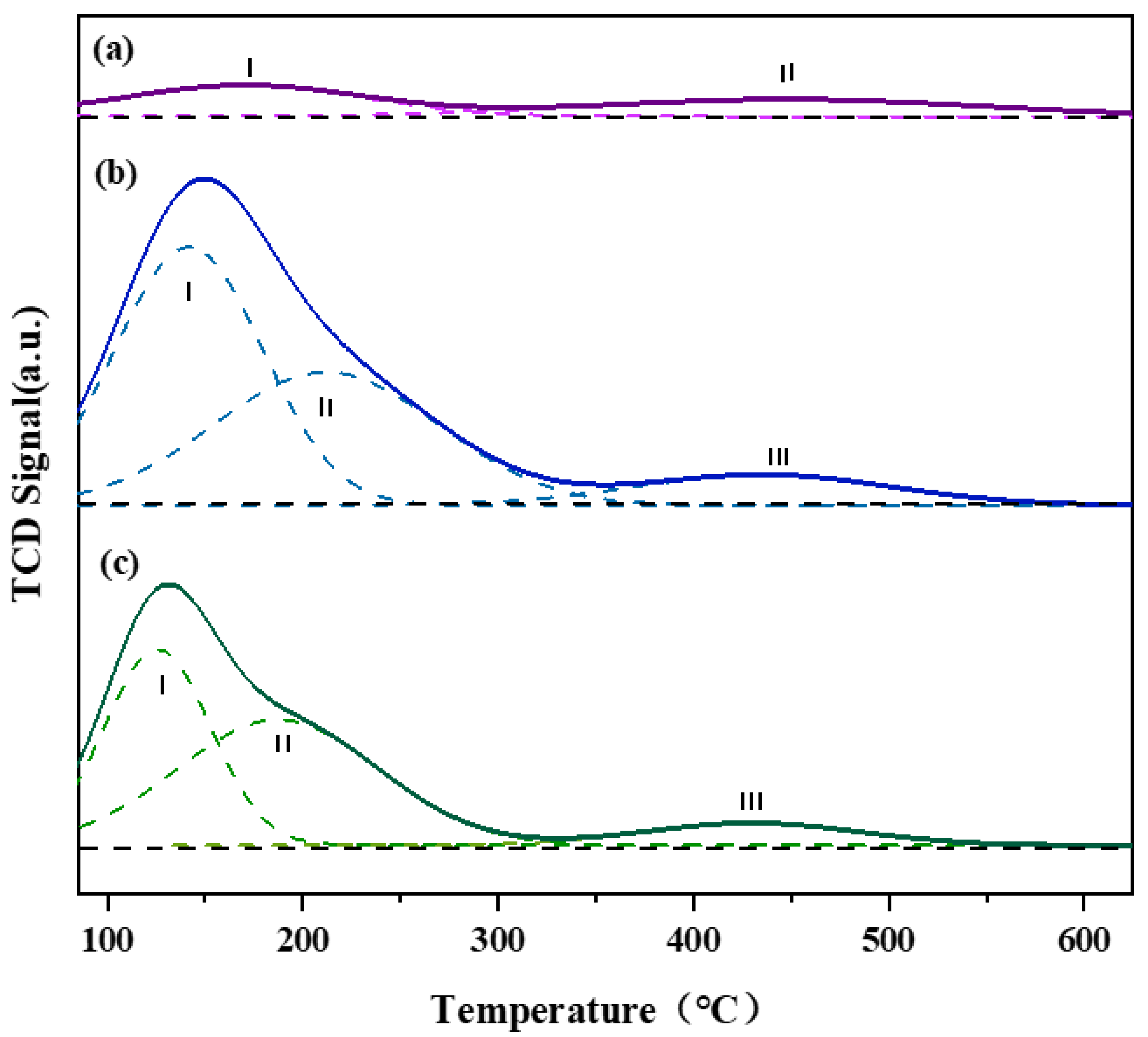
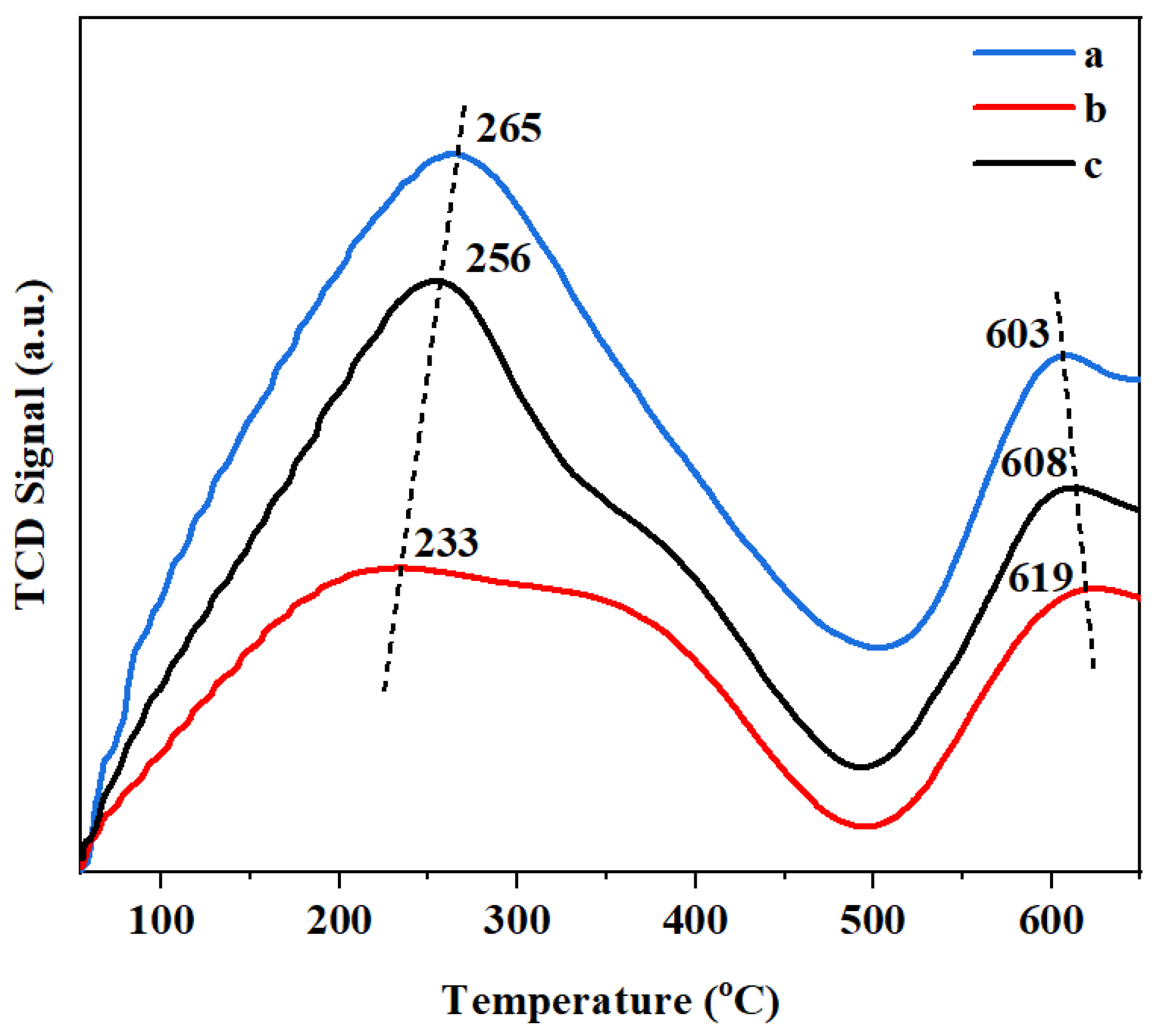
3.2. Surface Property of Support
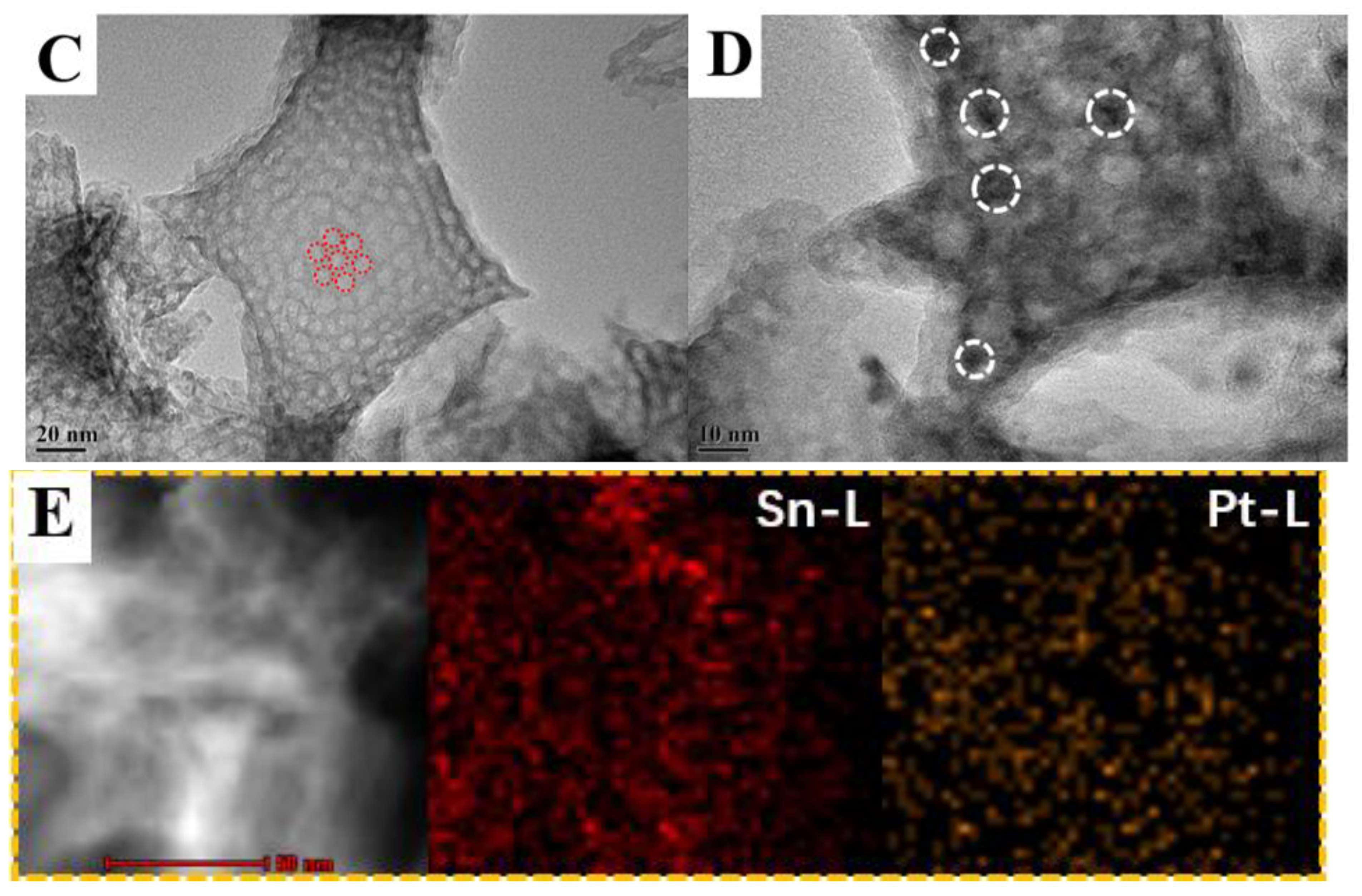
3.3. Structure of Active Sites
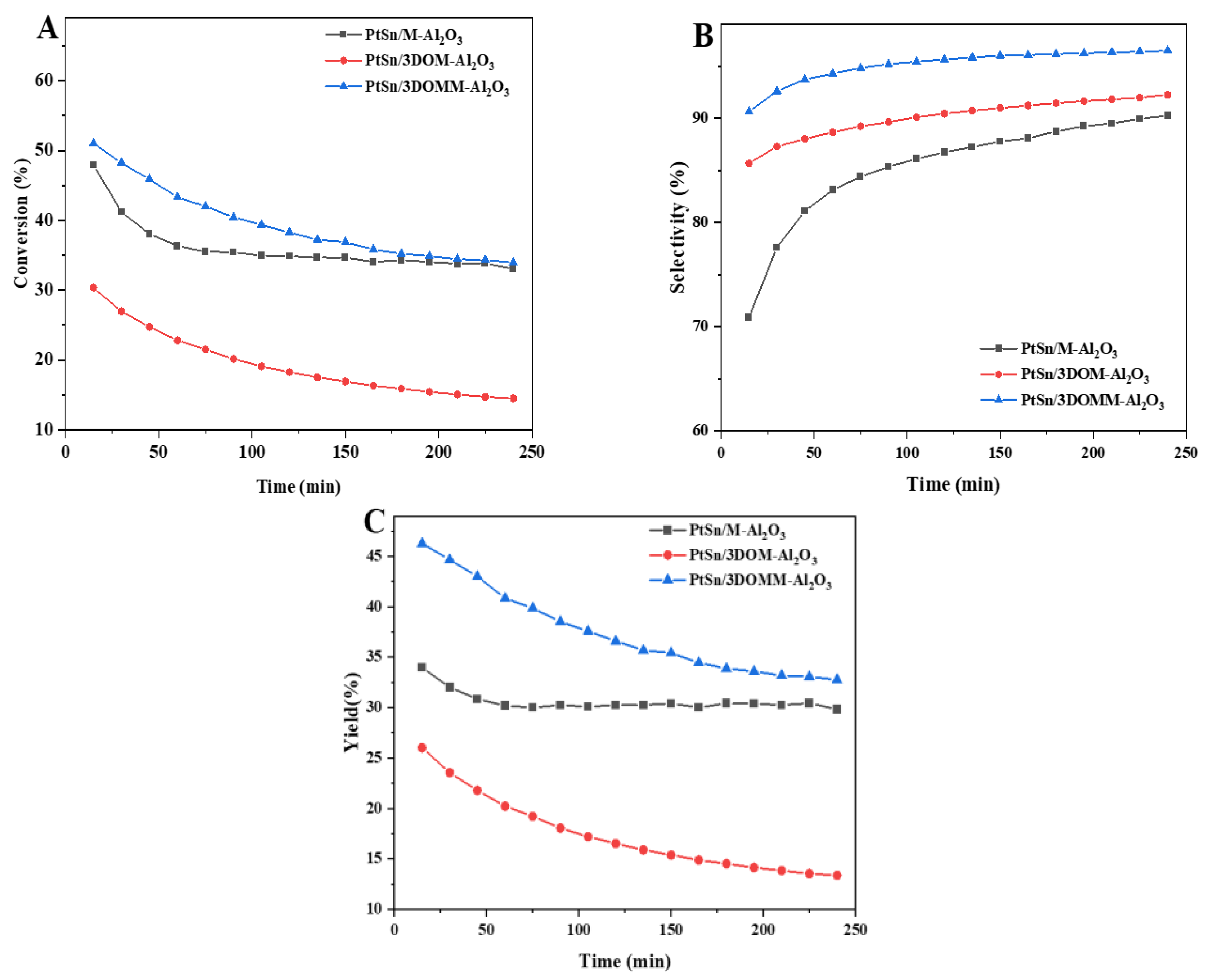
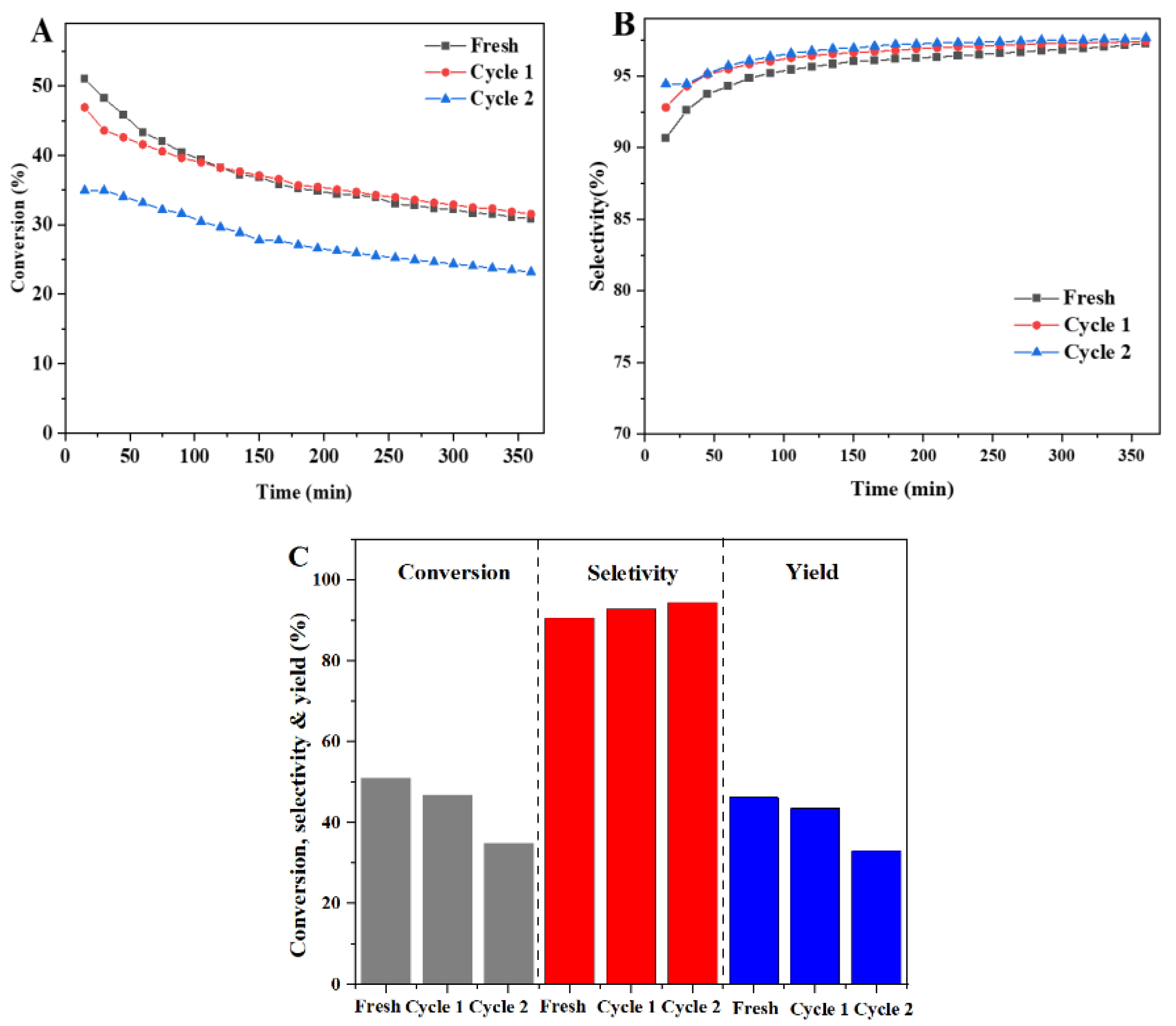
3.4. Catalytic Performance for PDH
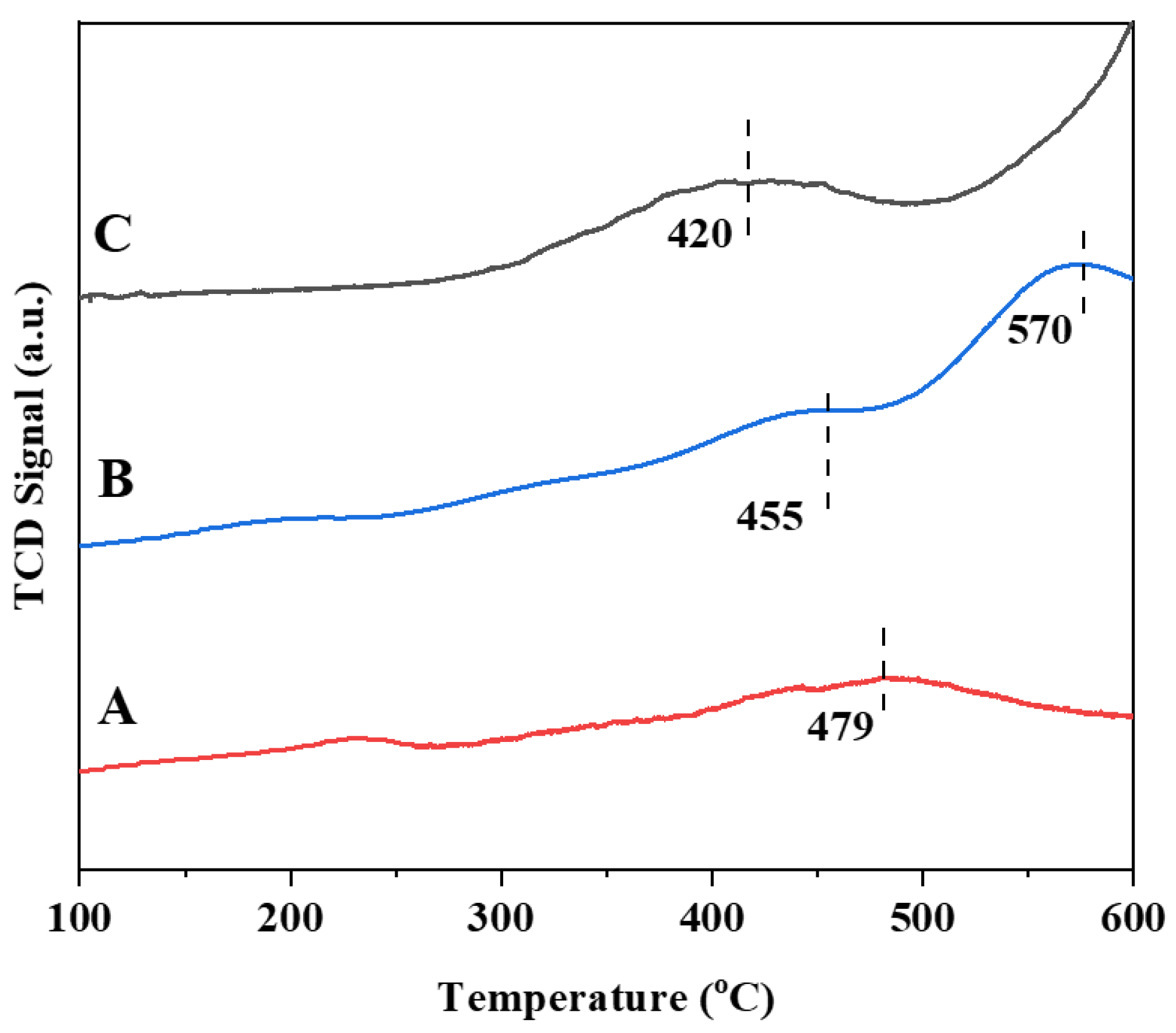
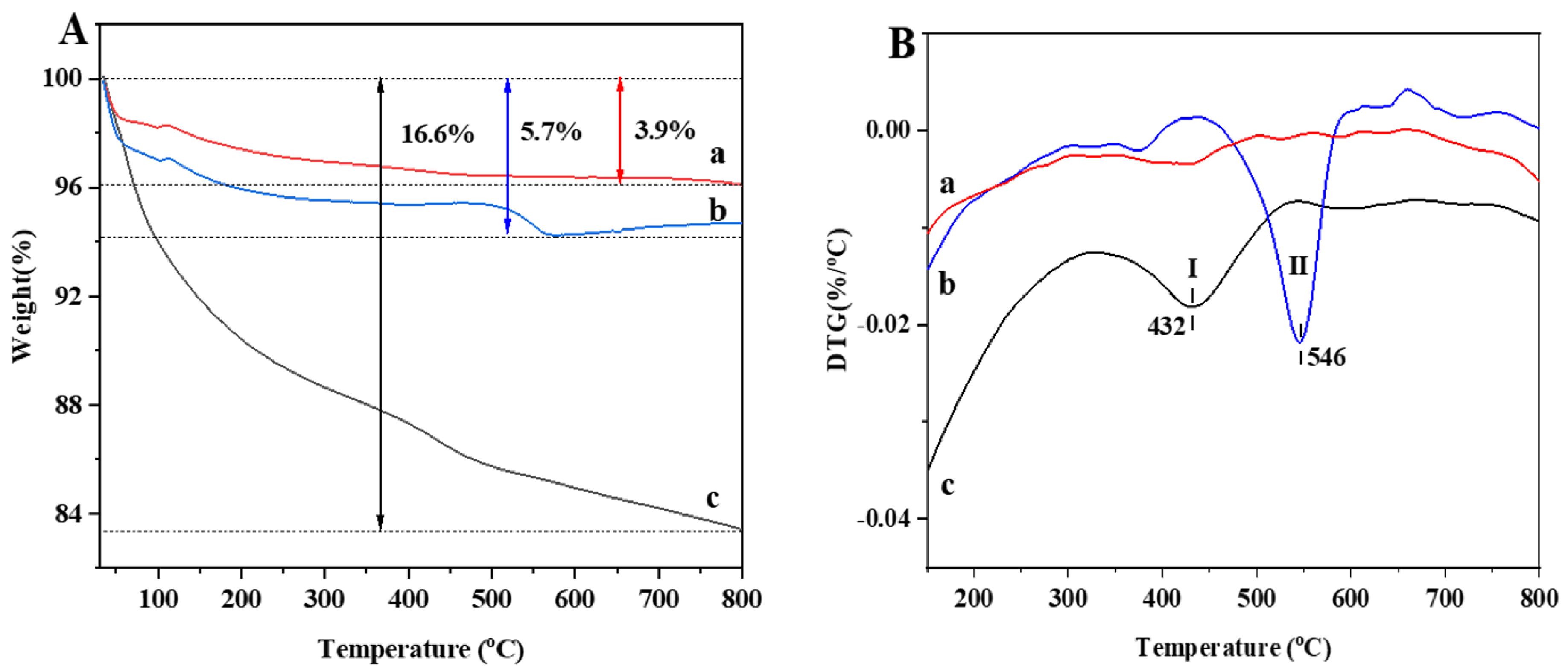
3.5. Coke Analysis of Used Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Chang, X.; Chen, S.; Sun, G.; Zhou, X.; Vovk, E.; Yang, Y.; Deng, W.; Zhao, Z.; Mu, R.; et al. On the Role of Sn Segregation of Pt-Sn Catalysts for Propane Dehydrogenation. ACS Catal. 2021, 11, 4401–4410. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Kaylor, N.; Davis, R.J. Propane dehydrogenation over supported Pt-Sn nanoparticles. J. Catal. 2018, 367, 181–193. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, Y.; Bao, X.; Xie, Z.; Zhu, H. Propane Dehydrogenation over Pt Clusters Localized at the Sn Single-Site in Zeolite Framework. ACS Catal. 2019, 10, 818–828. [Google Scholar] [CrossRef]
- Gómez-Quero, S.; Tsoufis, T.; Rudolf, P.; Makkee, M.; Kapteijn, F.; Rothenberg, G. Kinetics of propane dehydrogenation over Pt–Sn/Al2O3. Catal. Sci. Technol. 2013, 3, 962–971. [Google Scholar] [CrossRef]
- Nawaz, Z. Light alkane dehydrogenation to light olefin technologies: A comprehensive review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Nawaz, Z.; Tang, X.P.; Wang, Y.; Wei, F. Parametric Characterization and Influence of Tin on the Performance of Pt-Sn/SAPO-34 Catalyst for Selective Propane Dehydrogenation to Propylene. Ind. Eng. Chem. Res. 2010, 49, 1274–1280. [Google Scholar] [CrossRef]
- Bricker, J.C. Advanced Catalytic Dehydrogenation Technologies for Production of Olefins. Top. Catal. 2012, 55, 1309–1314. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, F.; Bao, X. Interface-Confined Oxide Nanostructures for Catalytic Oxidation Reactions. Acc. Chem. Res. 2013, 46, 1692–1701. [Google Scholar] [CrossRef]
- Zhao, Z.; Dubau, L.; Maillard, F. Evidences of the migration of Pt crystallites on high surface area carbon supports in the presence of reducing molecules. J. Power Sources 2012, 217, 449–458. [Google Scholar] [CrossRef]
- Wang, W.; Yao, S.J.; Deng, S.W.; Wang, Y.B.; Qiu, C.L.; Mao, C.L.; Wang, J.G. Sintering Rate and Mechanism of Supported Pt Nanoparticles by Multiscale Simulation. Langmuir 2021, 37, 12529–12538. [Google Scholar] [CrossRef] [PubMed]
- Nykänen, L.; Honkala, K. Selectivity in Propene Dehydrogenation on Pt and Pt3Sn Surfaces from First Principles. ACS Catal. 2013, 3, 3026–3030. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, M.; Yu, Y.; Zhu, Y.; Sui, Z.; Zhou, X.; Holmen, A.; Chen, D. Size-Dependent Reaction Mechanism and Kinetics for Propane Dehydrogenation over Pt Catalysts. ACS Catal. 2015, 5, 6310–6319. [Google Scholar] [CrossRef]
- Ma, S.; Liu, Z. Zeolite-confined subnanometric PtSn mimicking mortise-and-tenon joinery for catalytic propane dehydrogenation. Nat. Commun. 2022, 13, 2716. [Google Scholar] [CrossRef]
- Gould, T.D.; Lubers, A.M.; Corpuz, A.R.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Controlling Nanoscale Properties of Supported Platinum Catalysts through Atomic Layer Deposition. ACS Catal. 2015, 5, 1344–1352. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, Y.; Zhou, X.; Sui, Z.; Chen, D. First-Principles Calculations of Propane Dehydrogenation over PtSn Catalysts. ACS Catal. 2012, 2, 1247–1258. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.H.B.; Weckhuysen, B.M.; Datye, A.K. Role of Sn in the Regeneration of Pt/γ-Al2O3 Light Alkane Dehydrogenation Catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef]
- Sun, P.; Siddiqi, G.; Vining, W.C.; Chi, M.; Bell, A.T. Novel Pt/Mg(In)(Al)O Catalysts for Ethane and Propane Dehydrogenation. J. Catal. 2011, 282, 165–174. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Beale, A.M.; Maaijen, K.; Weng, T.C.; Glatzel, P.; Weckhuysen, B.M. A combined in situ time-resolved UV–Vis, Raman and high-energy resolution X-ray absorption spectroscopy study on the deactivation behavior of Pt and PtSn propane dehydrogenation catalysts under industrial reaction conditions. J. Catal. 2010, 276, 268–279. [Google Scholar] [CrossRef]
- Prakash, N.; Lee, M.; Yoon, S.; Jung, K. Role of acid solvent to prepare highly active PtSn/θ-Al2O3 catalysts in dehydrogenation of propane to propylene. Catal. Today 2017, 293–294, 33–41. [Google Scholar] [CrossRef]
- Barias, O.A.; Holmen, A.; Blekkan, E.A. Propane dehydrogenation over supported platinum catalysts: Effect of tin as a promoter. Catal. Today. 1995, 24, 361–364. [Google Scholar] [CrossRef]
- Lee, J.; Jang, E.J.; Kwak, J.H. Acid-base properties of Al2O3: Effects of morphology, crystalline phase, and additives. J. Catal. 2017, 345, 135–148. [Google Scholar] [CrossRef]
- Shi, L.; Deng, G.; Li, W.; Miao, S.; Wang, Q.; Zhang, W.; Lu, A. Al2O3 Nanosheets Rich in Pentacoordinate Al3+ Ions Stabilize Pt-Sn Clusters for Propane Dehydrogenation. Angew. Chem. Inter. Ed. 2015, 54, 13994–13998. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wang, H.; Duan, X.; Sui, Z.; Zhou, X.; Coppens, M.; Yuan, W. Pore network modeling of catalyst deactivation by coking, from single site to particle, during propane dehydrogenation. AIChE J. 2019, 65, 140–150. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, Y.; Zhang, Y.; Zhang, P.; Yu, Q.; Mei, X.; Liu, X.; Zhao, Z.; Liu, J. Synergetic Effect of K Sites and Pt Nanoclusters in an Ordered Hierarchical Porous Pt-KMnOx/Ce0.25Zr0.75O2 Catalyst for Boosting Soot Oxidation. ACS Catal. 2020, 10, 7123–7135. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, Q.; Mei, X.; Liu, J.; Wei, Y.; Zhao, Z.; Wu, D.; Li, J. Fabrication of Spinel-Type PdxCo3–xO4 Binary Active Sites on 3D Ordered Meso-macroporous Ce-Zr-O2 with Enhanced Activity for Catalytic Soot Oxidation. ACS Catal. 2018, 8, 7915–7930. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.; Jing, F.; Luo, S.; Chu, W. Facile one-pot synthesized ordered mesoporous Mg-SBA-15 supported PtSn catalysts for propane dehydrogenation. Appl. Catal. A 2017, 533, 17–27. [Google Scholar] [CrossRef]
- McManus, J.; Ashbrook, S.E.; MacKenzie, K.J.D.; Wimperis, S. 27Al multiple-quantum MAS and 27Al{1H} CPMAS NMR study of amorphous aluminosilicates. J. Non-Cryst. Solids 2001, 282, 278–290. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.W.; Kim, D.H.; Peden, C.H.; Allard, L.F.; Szanyi, J. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on gamma-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef]
- Gao, X.; Lu, W.; Hu, S.; Li, W.; Lu, A. Rod-shaped porous alumina-supported Cr2O3 catalyst with low acidity for propane dehydrogenation. Chin. J. Catal. 2019, 40, 184–191. [Google Scholar] [CrossRef]
- Yu, H.L.; Xu, H.Y.; Ge, Q.J.; Li, W.Z. Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation. J. Mol. Catal. A Chem. 2007, 266, 80–87. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Jia, M.; Gao, X.; Yu, J. ZSM-5 zeolites with different SiO2/Al2O3 ratios as fluid catalytic cracking catalyst additives for residue cracking. Chin. J. Catal. 2015, 36, 806–812. [Google Scholar] [CrossRef]
- Jin, Q.; Shen, Y.; Zhu, S. Praseodymium Oxide Modified CeO2/Al2O3 Catalyst for Selective Catalytic Reduction of NO by NH3. Chin. J. Chem. 2016, 34, 1283–1290. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.M.; Zhang, Y.W.; Bai, L.Y.; Tang, M.H. Effect of Preparation Processes on Catalytic Performance of PtSnNa/ZSM-5 for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2009, 48, 5598–5603. [Google Scholar] [CrossRef]
- Tasbihi, M.; Feyzi, F.; Amlashi, M.A.; Abdullah, A.Z.; Mohamed, A.R. Effect of the addition of potassium and lithium in Pt-Sn/Al2O3 catalysts for the dehydrogenation of isobutane. Fuel Process. Technol. 2007, 88, 883–889. [Google Scholar] [CrossRef]
- He, S.; Sun, C.; Bai, Z.; Dai, X.; Wang, B. Dehydrogenation of long chain paraffins over supported Pt-Sn-K/Al2O3 catalysts: A study of the alumina support effect. Appl. Catal. A 2009, 356, 88–98. [Google Scholar] [CrossRef]
- Im, J.; Shin, H.; Jang, H.; Kim, H.; Choi, M. Maximizing the catalytic function of hydrogen spillover in platinum-encapsulated aluminosilicates with controlled nanostructures. Nat. Commun. 2014, 5, 3370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Liu, H.; Wang, Y.; Xu, Y.; Wu, P. Effect of La addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. Appl. Catal. A Gen. 2007, 333, 202–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J.; Zhou, S.; Sheng, X.; Zhang, Z.; Xiang, S. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation. J. Mol. Catal. A Chem. 2014, 381, 138–144. [Google Scholar] [CrossRef]
- Cesar, L.G.; Yang, C.; Lu, Z.; Ren, Y.; Zhang, G.; Miller, J.T. Identification of a Pt3Co Surface Intermetallic Alloy in Pt–Co Propane Dehydrogenation Catalysts. ACS Catal. 2019, 9, 5231–5244. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, M.; Zhao, Z.; Zhao, Q. K-modified Sn-containing dendritic mesoporous silica nanoparticles with tunable size and SnOx-silica interaction for the dehydrogenation of propane to propylene. Chem. Eng. J. 2020, 380, 122423. [Google Scholar] [CrossRef]
- Long, L.; Xia, K.; Lang, W.; Shen, L.; Yang, Q.; Yan, X.; Guo, Y. The comparison and optimization of zirconia, alumina, and zirconia-alumina supported PtSnIn trimetallic catalysts for propane dehydrogenation reaction. J. Ind. Eng. Chem. 2017, 51, 271–280. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, R.; Zha, S.; Li, L.; Chen, S.; Zang, K.; Luo, J.; Li, Z.; Purdy, S.C.; Kropf, A.J.; et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 2018, 9, 4454. [Google Scholar] [CrossRef]
- Ma, R.; Yang, T.; Gao, J.; Kou, J.; Zhu Chen, J.; He, Y.; Miller, J.T.; Li, D. Composition tuning of Ru-based phosphide for enhanced propane selective dehydrogenation. ACS Catal. 2020, 10, 10243–10252. [Google Scholar] [CrossRef]
- Vázquez-Zavala, A.; Ostoa-Montes, A.; Acosta, D.; Gómez-Cortés, A. Characterization of structure and catalytic activity of Pt-Sn catalysts supported in Al2O3, SiO2 and TiO2. Appl. Surf. Sci. 1998, 136, 62–72. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Sui, Z.; Zhu, Y.; Ye, G.; Chen, D.; Zhou, X.; Yuan, W. Coke Formation on Pt–Sn/Al2O3 Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2018, 57, 8647–8654. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Jiang, F.; Wang, T.; Ma, X.; Gong, J. Propane dehydrogenation over Pt–Cu bimetallic catalysts: The nature of coke deposition and the role of copper. Nanoscale 2014, 6, 10000. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Aly, M.; Fornero, E.L.; Leon-Garzon, A.R.; Galvita, V.V.; Saeys, M. Effect of Boron Promotion on Coke Formation during Propane Dehydrogenation over Pt/γ-Al2O3 Catalysts. ACS Catal. 2020, 10, 5208–5216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Huang, L.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352–361. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Talsi, V.P.; Gulyaeva, T.I.; Kazakov, M.O.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of γ-Al2O3 hydrothermal treatment on the formation and properties of platinum sites in Pt/γ-Al2O3 catalysts. Appl. Catal. A General 2014, 469, 472–482. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Lv, X.; Chen, L.; Yuan, Z. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. J. Catal. 2020, 385, 61–69. [Google Scholar] [CrossRef]

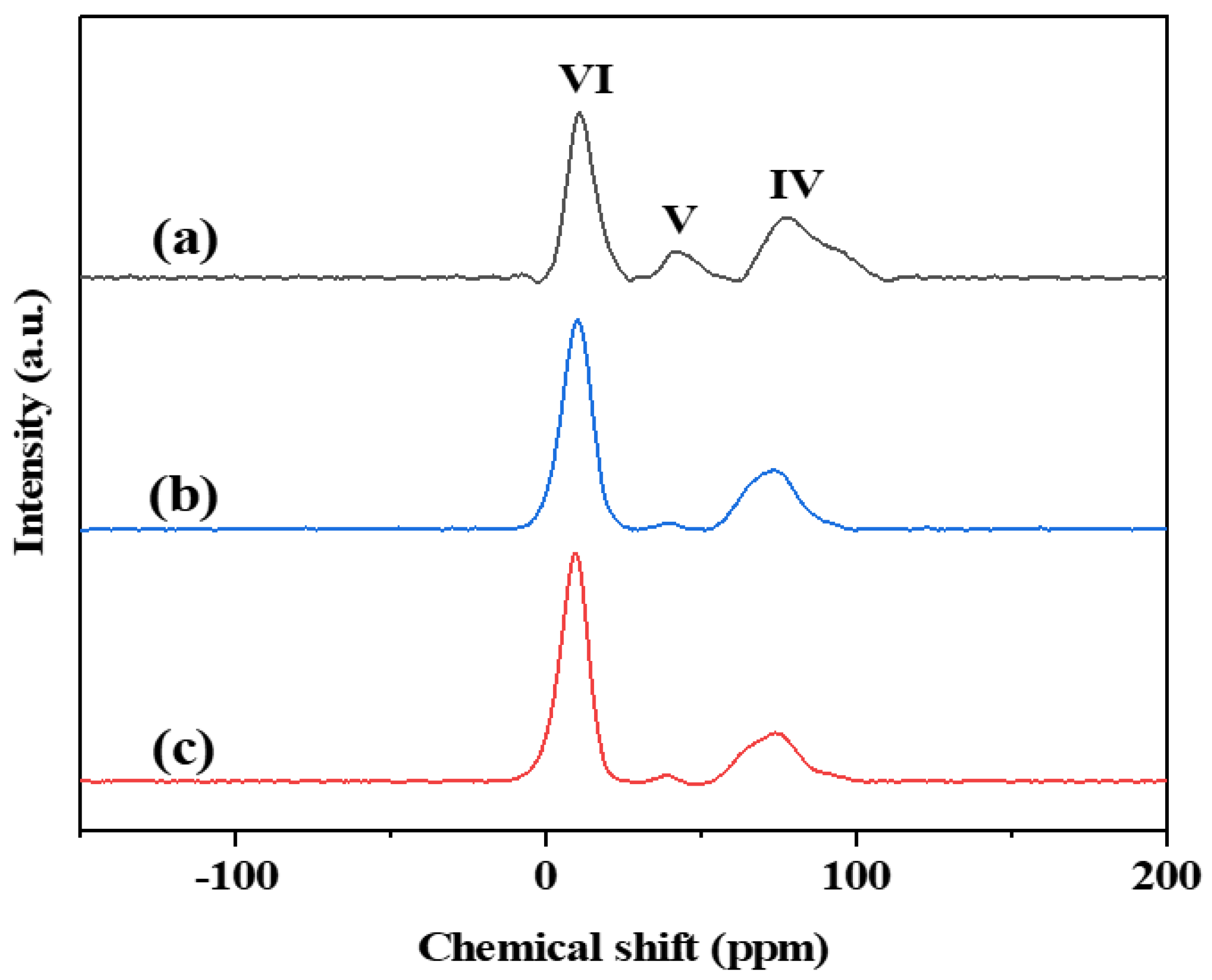

| Catalyst | Peak Area (%) | ||
|---|---|---|---|
| VI | V | IV | |
| PtSn/3DOMM-Al2O3 | 52 | 9 | 39 |
| PtSn/3DOM-Al2O3 | 67 | 1 | 32 |
| PtSn/M-Al2O3 | 71 | 1 | 28 |
| Catalyst | TM (°C) | Peak Area (%) | ||||
|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |
| PtSn/3DOM-Al2O3 | 169 | 447 | / | 53 | 47 | / |
| PtSn/3DOMM-Al2O3 | 125 | 192 | 430 | 41 | 50 | 9 |
| PtSn/M-Al2O3 | 141 | 211 | 435 | 51 | 39 | 10 |
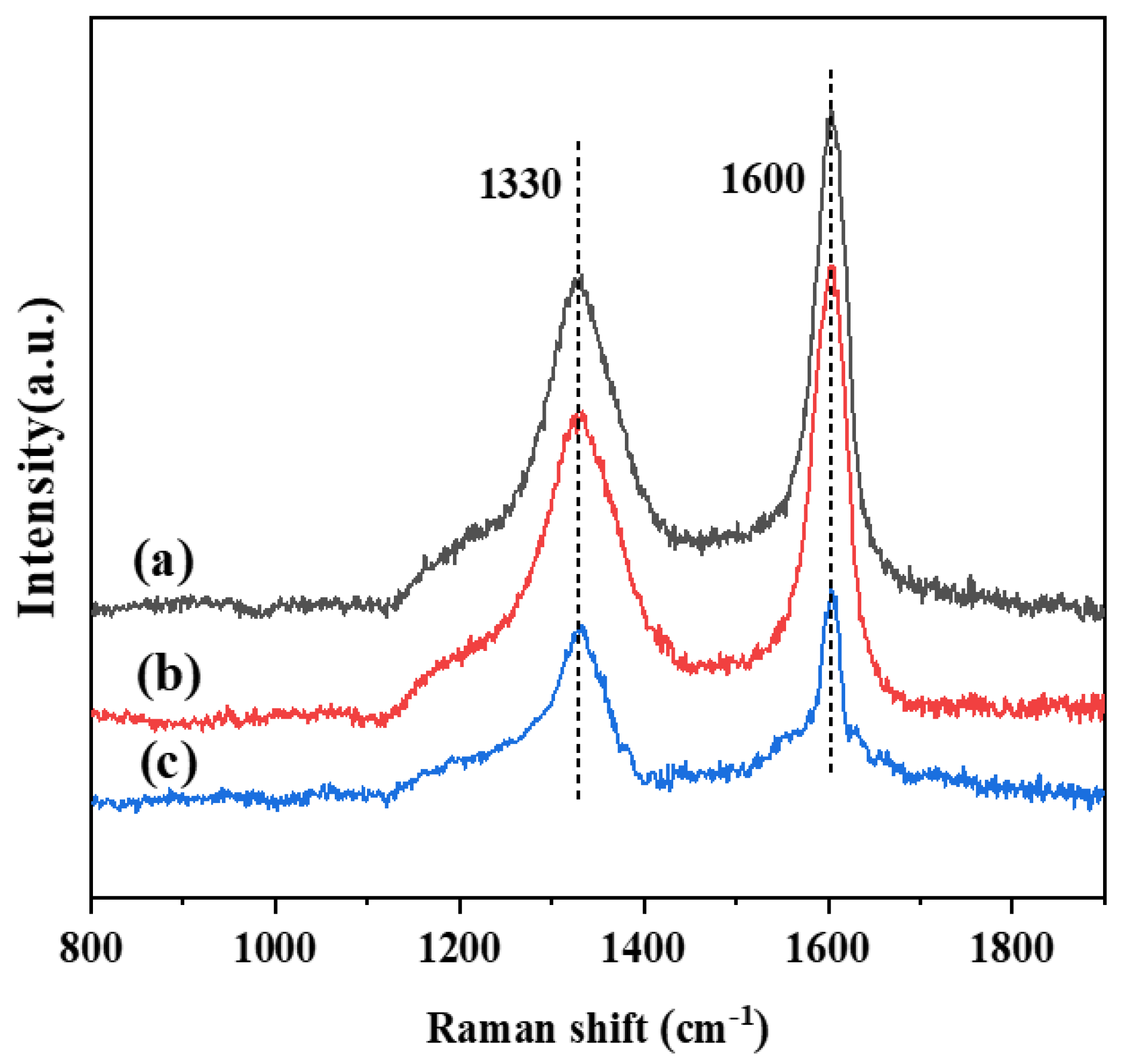
| Catalyst | ID/IG |
|---|---|
| PtSn/3DOM-Al2O3 | 0.84 |
| PtSn/3DOMM-Al2O3 | 0.73 |
| PtSn/M-Al2O3 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Feng, B.; Lian, Q.; Xie, C.; Xiong, J.; Song, W.; Liu, J.; Wei, Y. Ordered Hierarchical Porous Structure of PtSn/3DOMM-Al2O3 Catalyst for Promoting Propane Non-Oxidative Dehydrogenation. Nanomaterials 2023, 13, 728. https://doi.org/10.3390/nano13040728
Sun Y, Feng B, Lian Q, Xie C, Xiong J, Song W, Liu J, Wei Y. Ordered Hierarchical Porous Structure of PtSn/3DOMM-Al2O3 Catalyst for Promoting Propane Non-Oxidative Dehydrogenation. Nanomaterials. 2023; 13(4):728. https://doi.org/10.3390/nano13040728
Chicago/Turabian StyleSun, Yuanqing, Bohan Feng, Qian Lian, Chengshu Xie, Jing Xiong, Weiyu Song, Jian Liu, and Yuechang Wei. 2023. "Ordered Hierarchical Porous Structure of PtSn/3DOMM-Al2O3 Catalyst for Promoting Propane Non-Oxidative Dehydrogenation" Nanomaterials 13, no. 4: 728. https://doi.org/10.3390/nano13040728
APA StyleSun, Y., Feng, B., Lian, Q., Xie, C., Xiong, J., Song, W., Liu, J., & Wei, Y. (2023). Ordered Hierarchical Porous Structure of PtSn/3DOMM-Al2O3 Catalyst for Promoting Propane Non-Oxidative Dehydrogenation. Nanomaterials, 13(4), 728. https://doi.org/10.3390/nano13040728








