A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Instruments
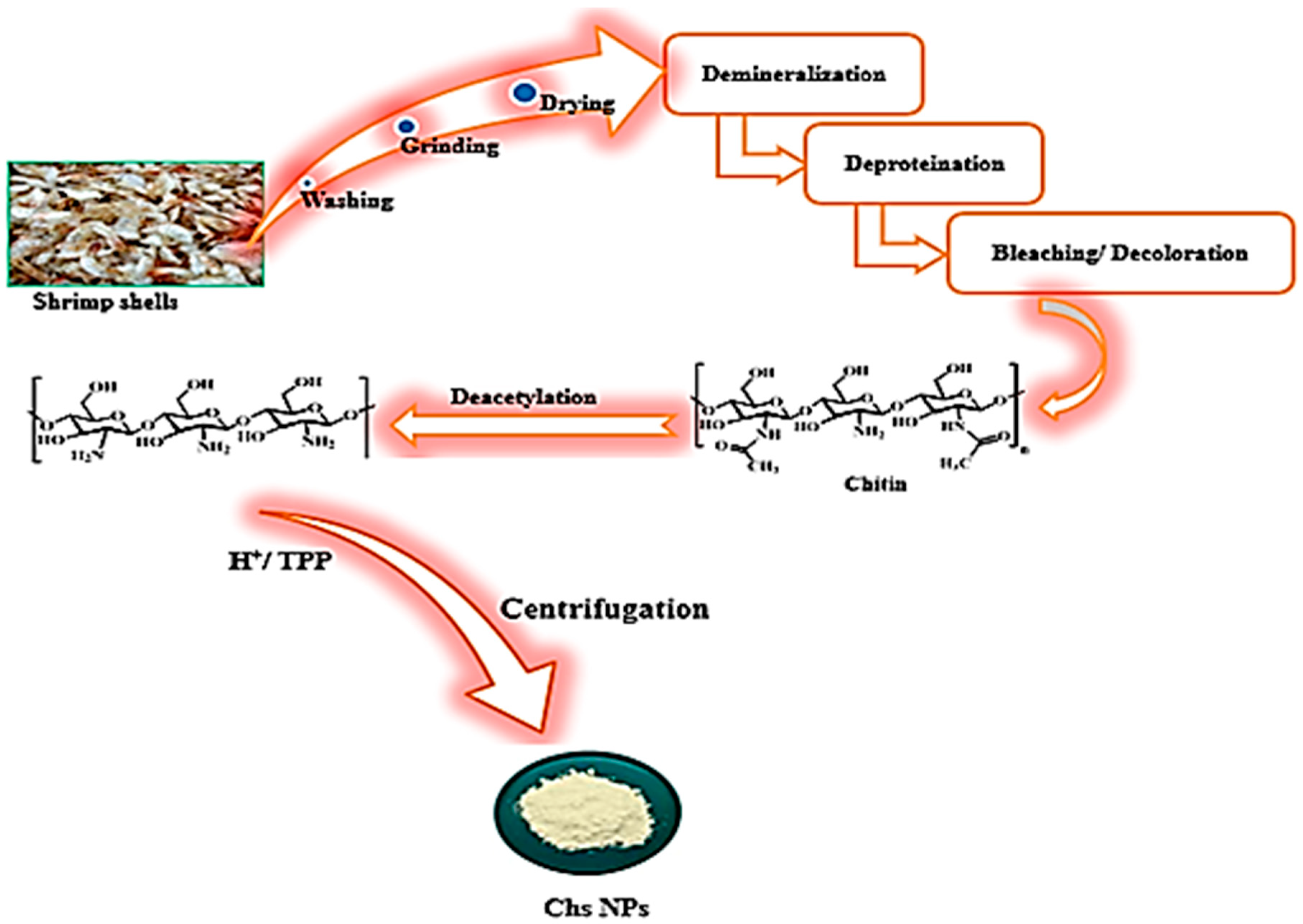
2.3. Preparation of Chs NPs
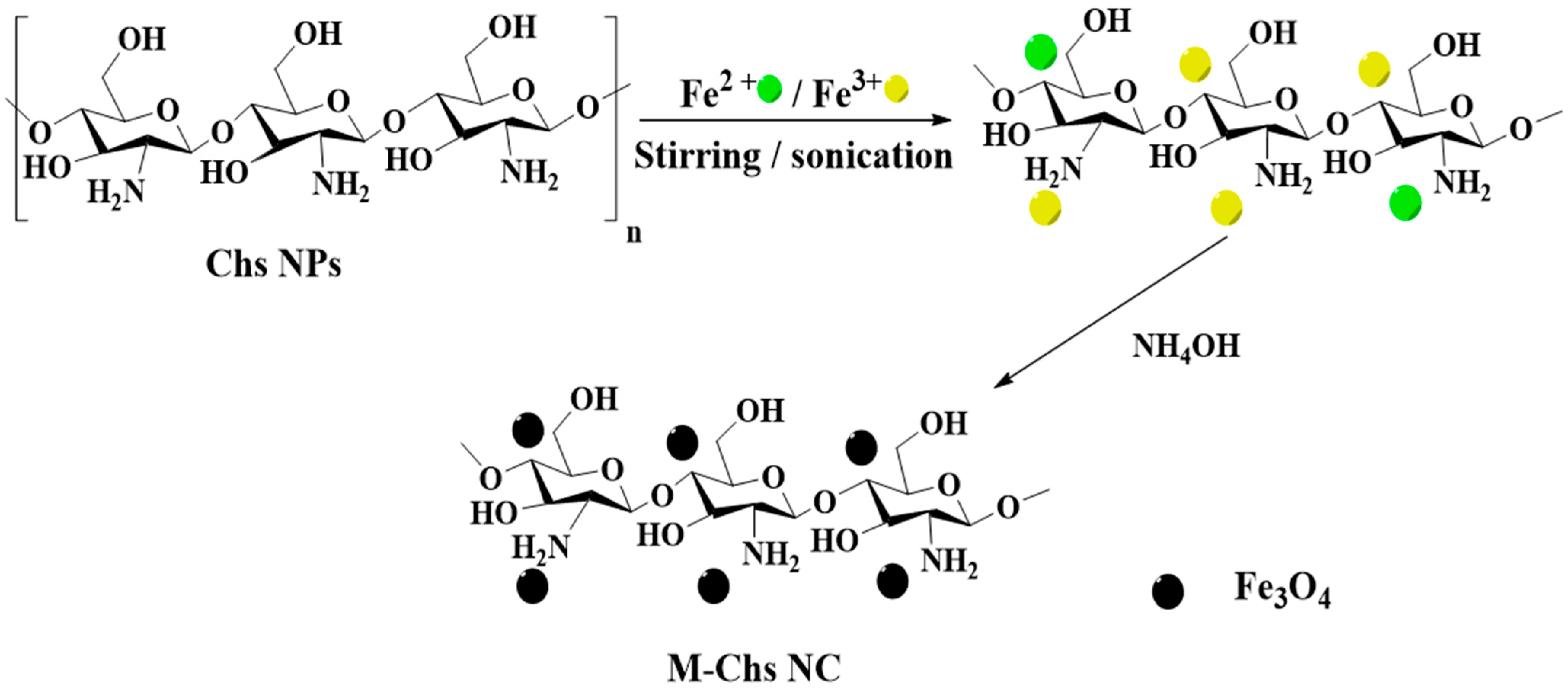
2.4. Synthesis of M-Chs NC
2.5. Preparation of Pharmaceutical Samples
2.6. Preparation of Human Serum Samples
3. Results and Discussion
3.1. Characterization of Extracted Chs NPs and M-Chs NC
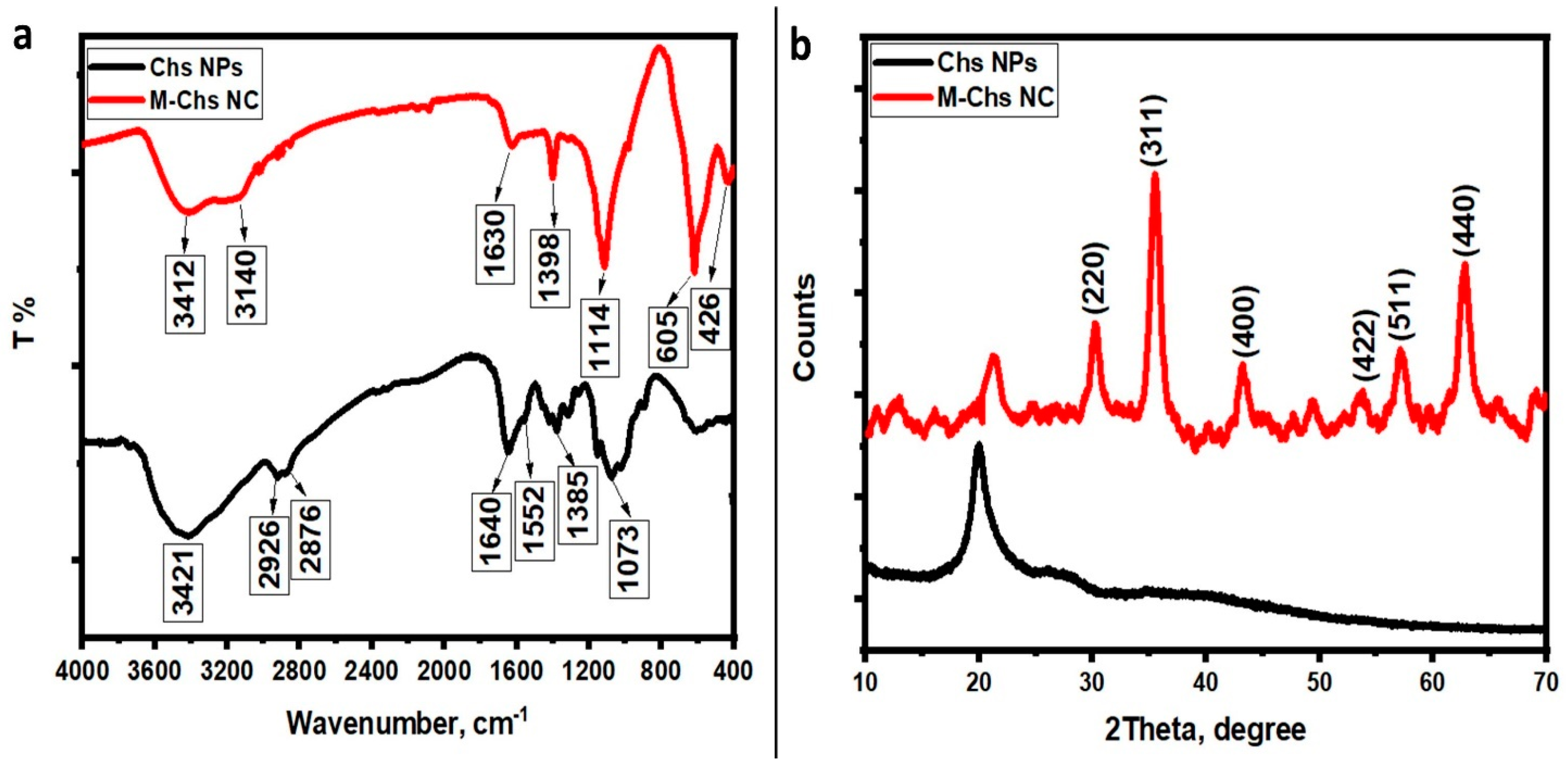
3.1.1. Structural Analysis
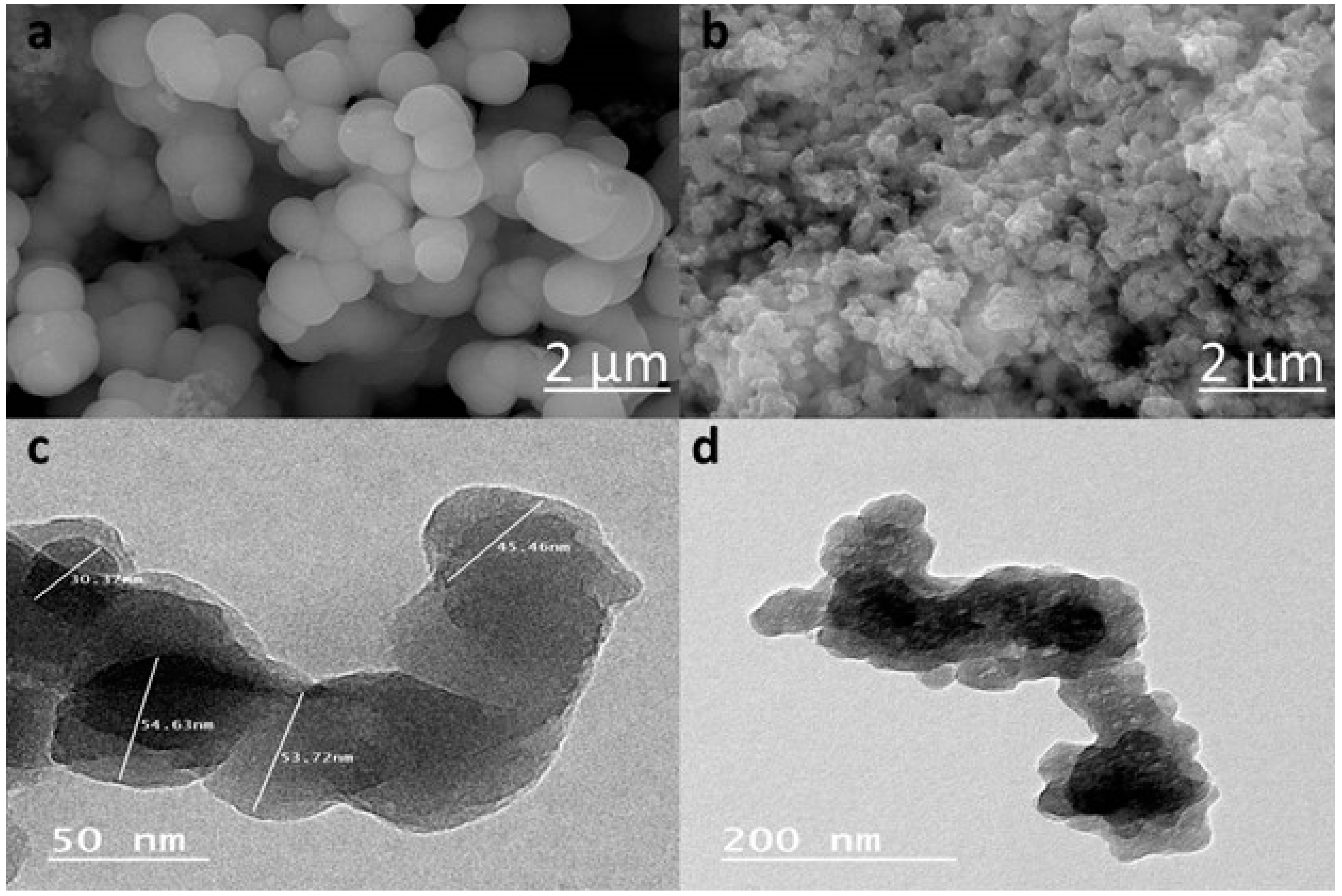
3.1.2. Morphological Analysis
3.2. Electroactive Surface Area Measurements
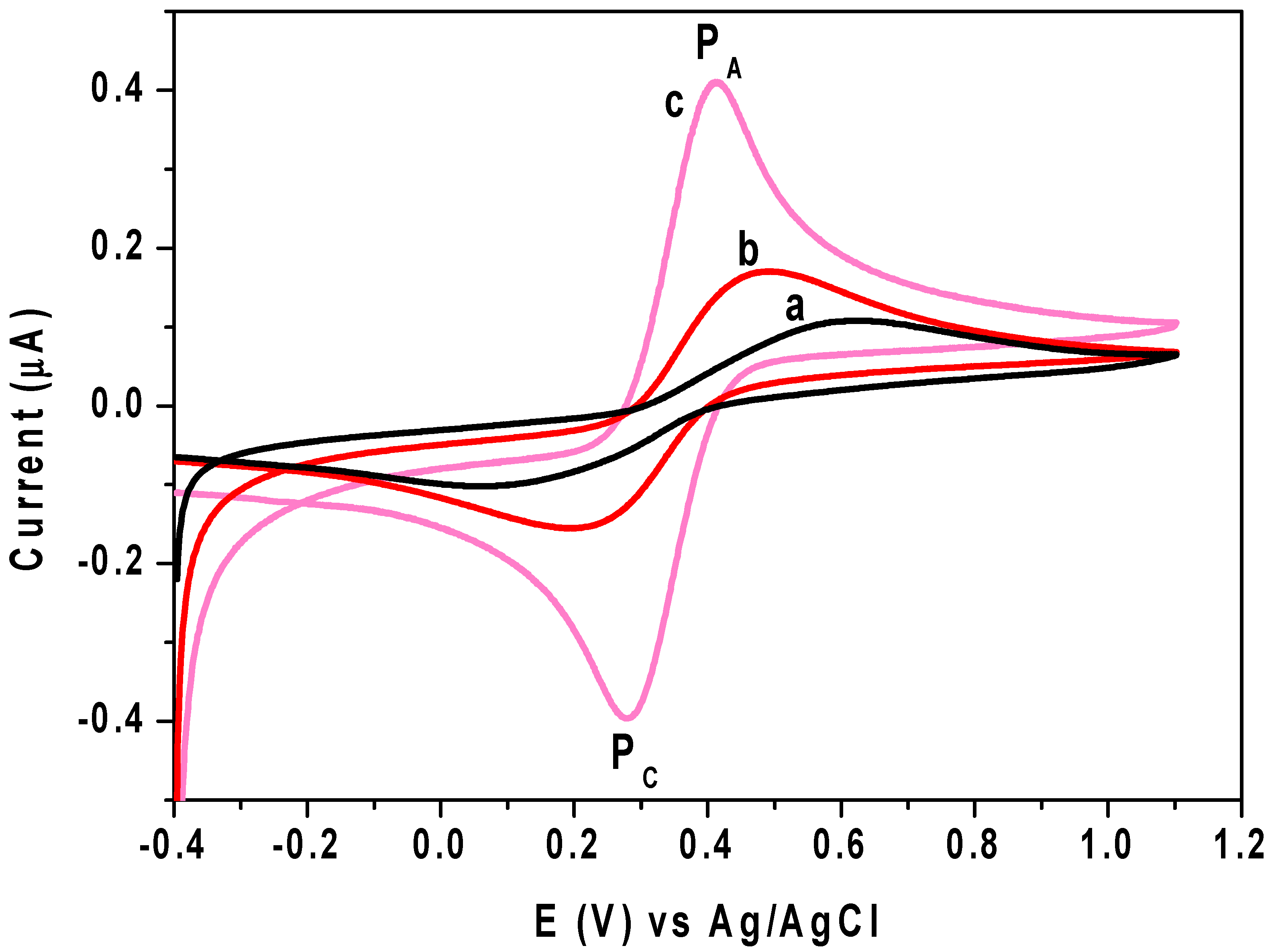
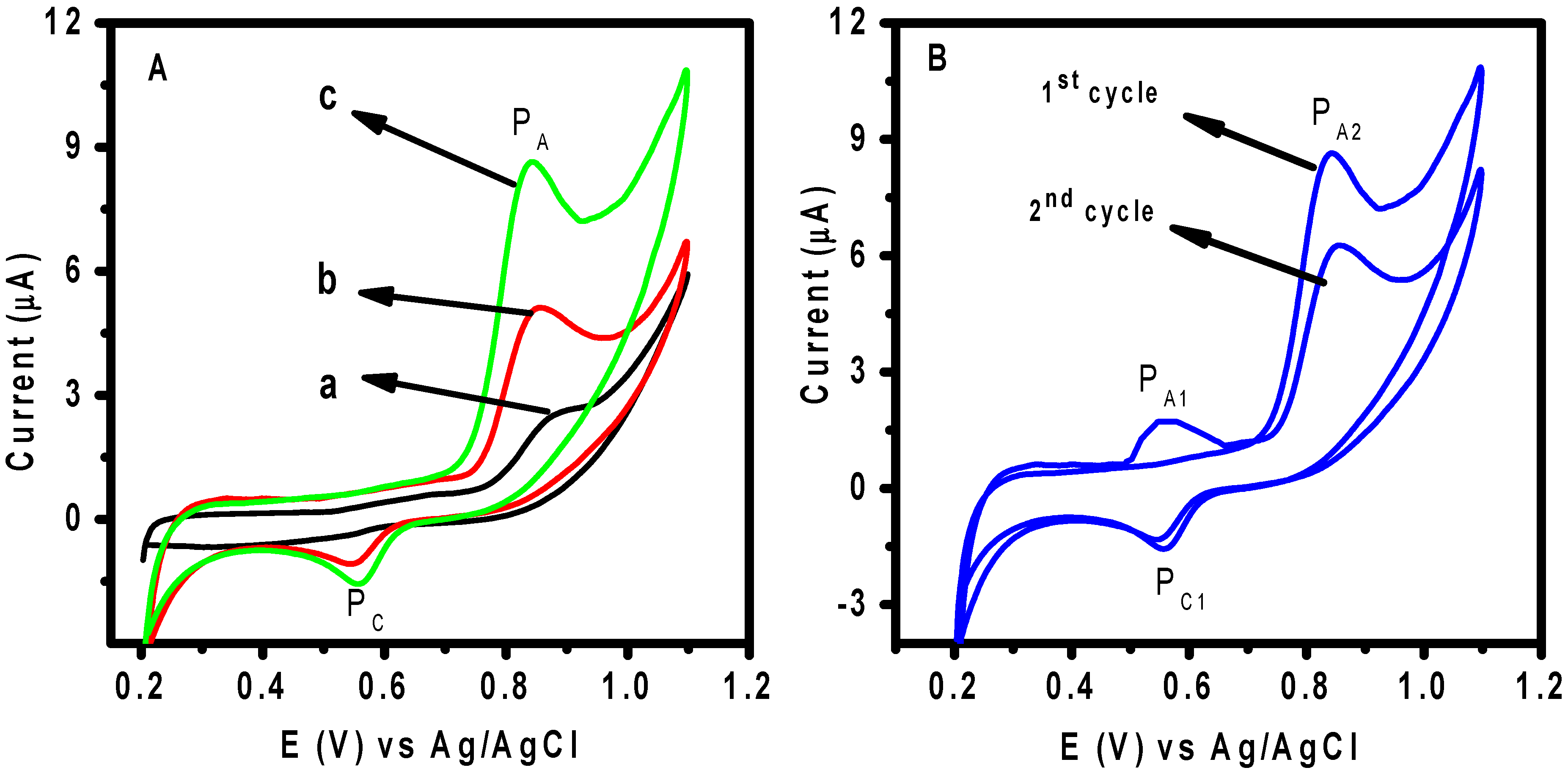
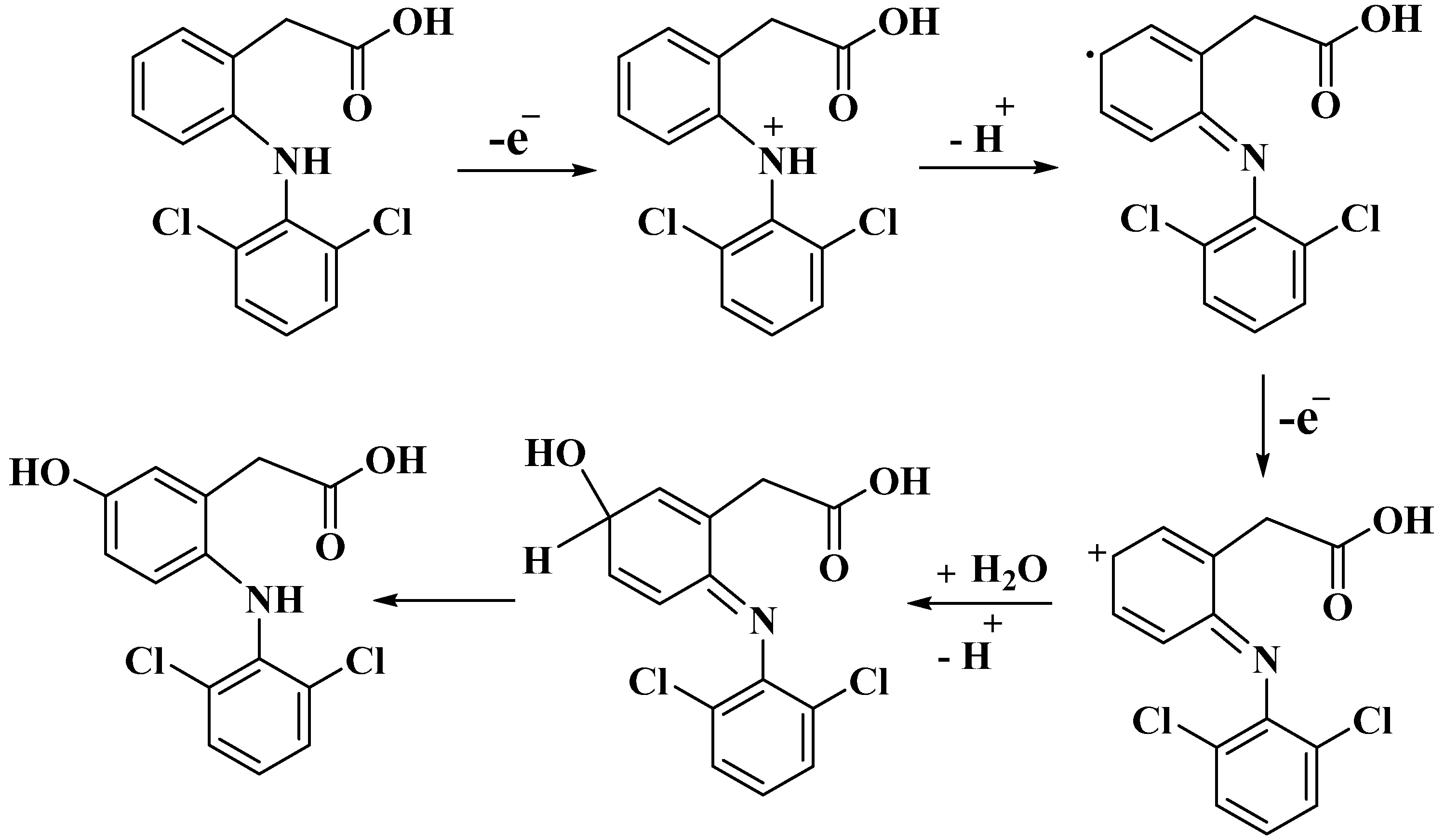
3.3. Electrochemical Behavior of DIC
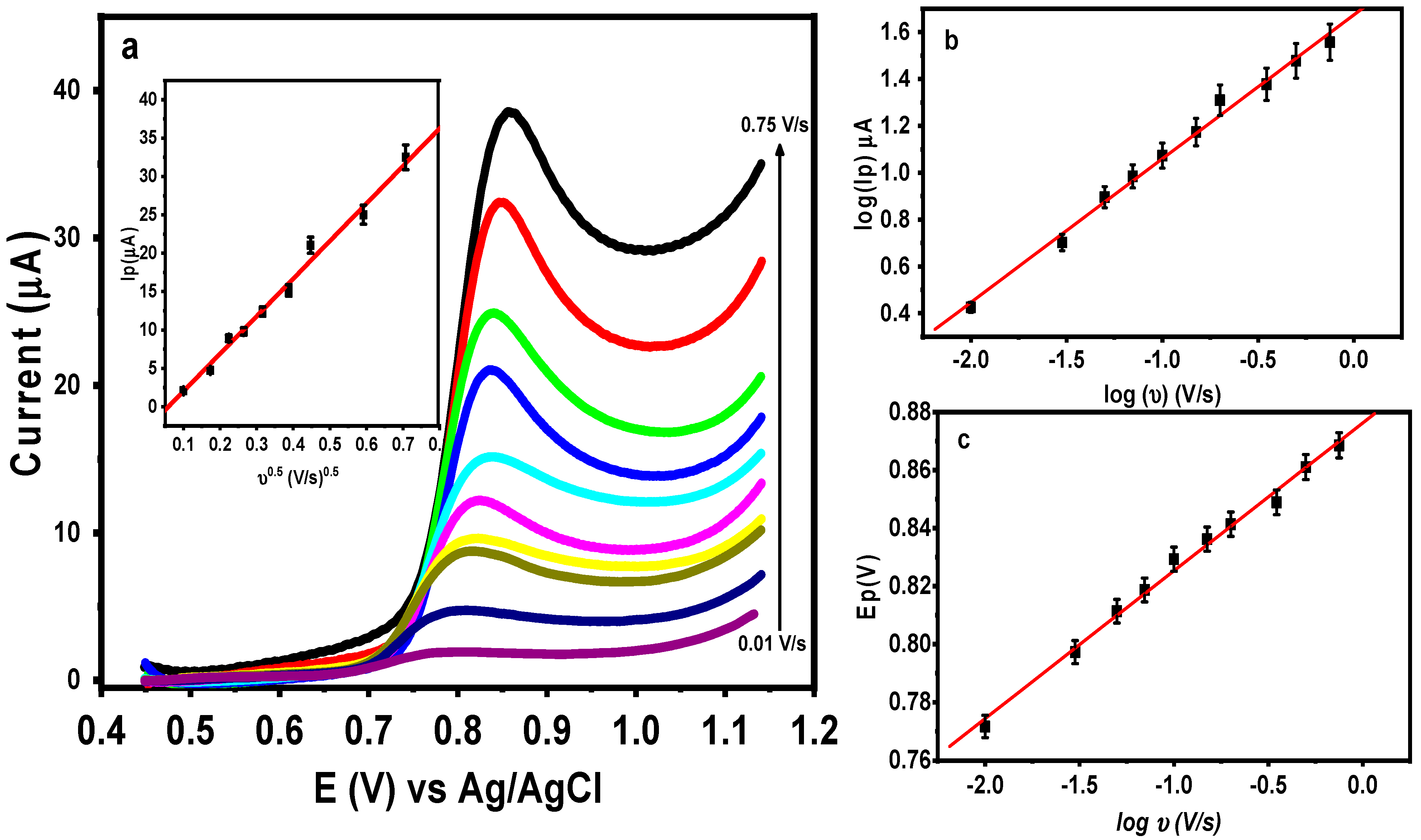
3.4. Effect of Scan Rate
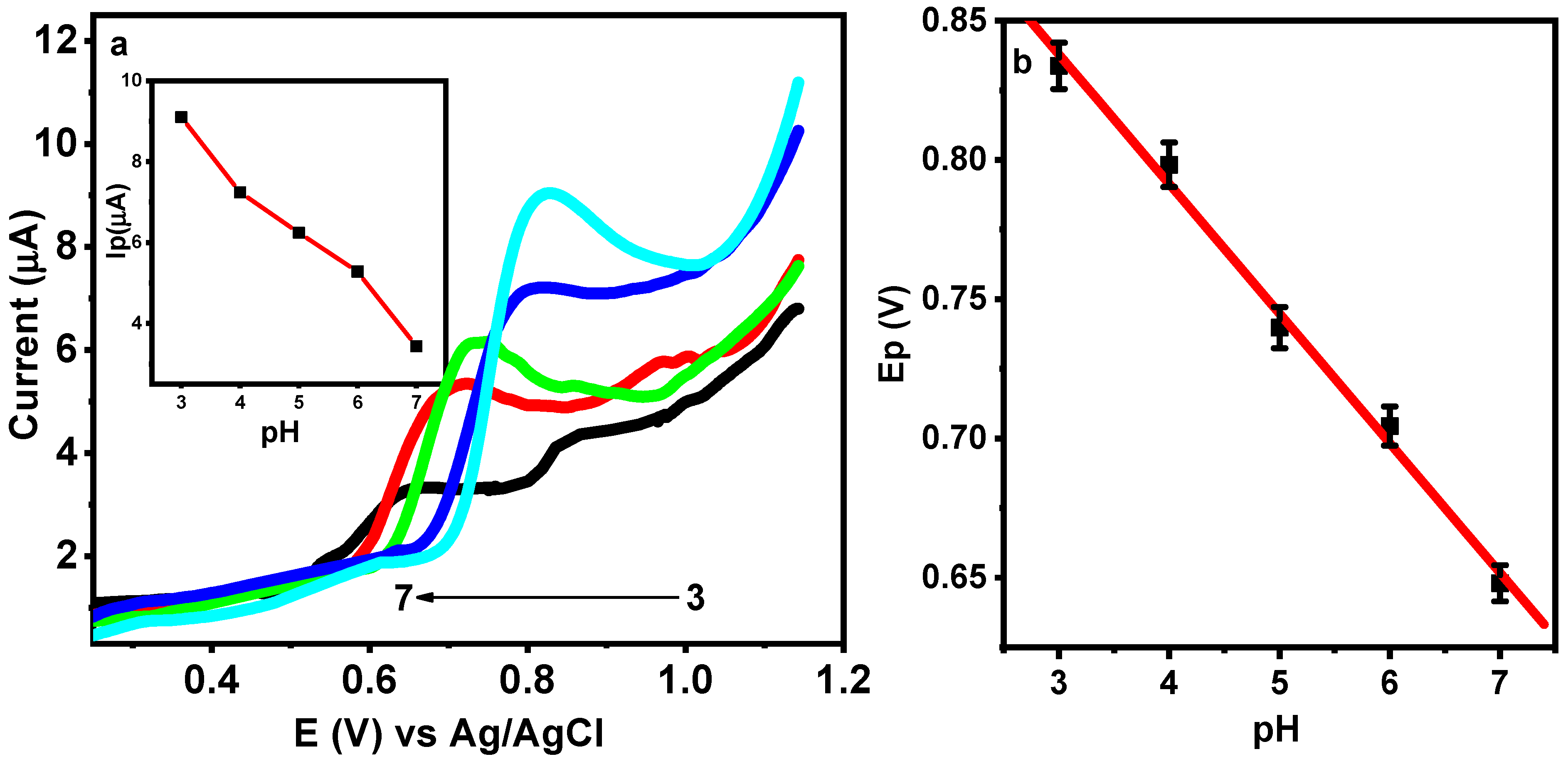
3.5. Effect of pH
3.6. Chronoamperometric Study
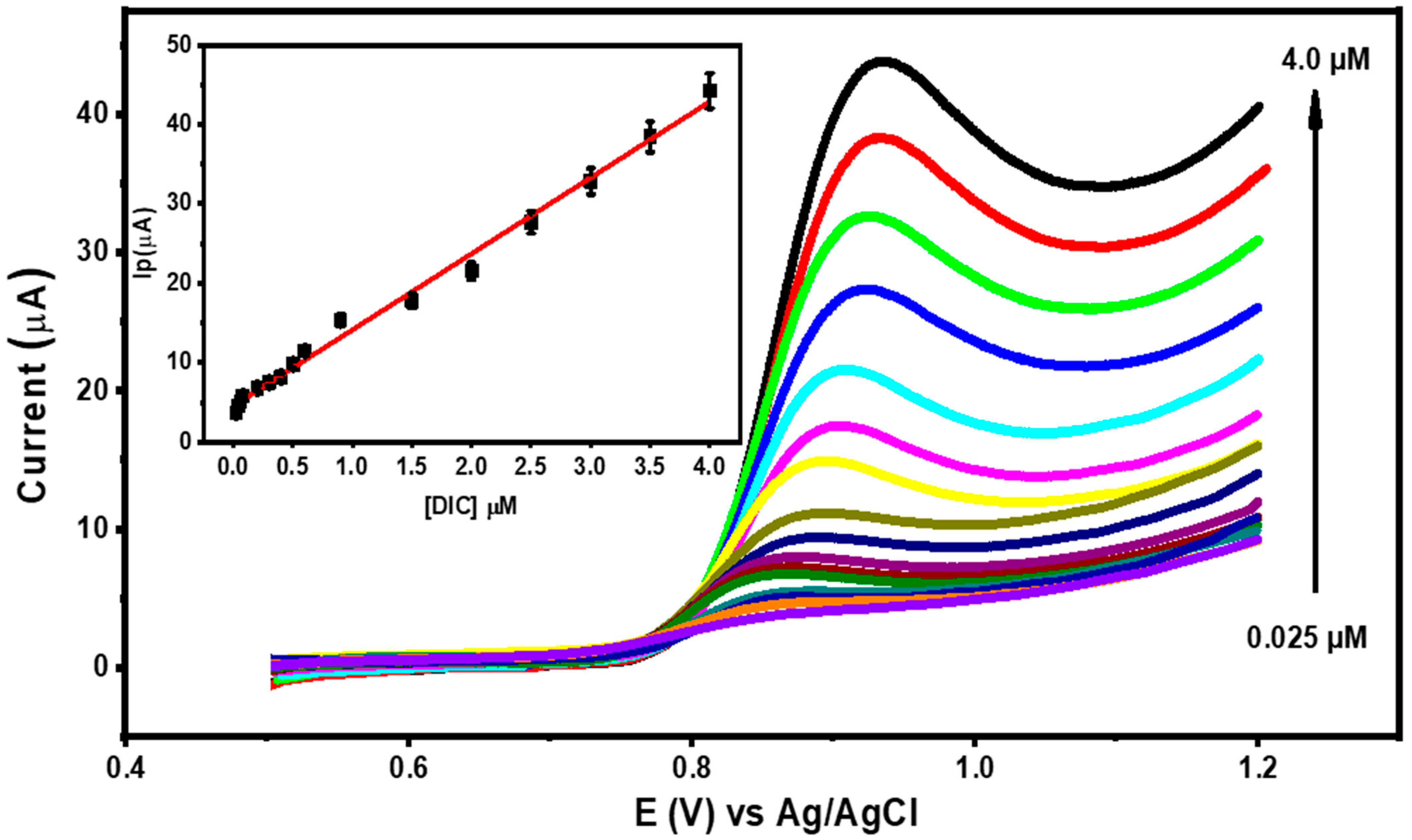
3.7. Calibration Curve and Detection Limit
3.8. Effect of Interferences
3.9. Repeatability and Stability
3.10. Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berto, S.; Cagno, E.; Prenesti, E.; Aragona, G.; Bertinetti, S.; Giacomino, A.; Inaudi, P.; Malandrino, M.; Terranova, E.; Abollino, O. Voltammetric Study for the Determination of Diclofenac in Aqueous Solutions Using Electro-Activated Carbon Electrodes. Appl. Sci. 2022, 12, 7983. [Google Scholar] [CrossRef]
- Farzaneh Nasiri, G.H.R. Deiminiat, A new electrochemical sensing platform for quantitative determination of diclofenac based on gold nanoparticles decorated multiwalled carbon nanotubes/graphene oxide nanocomposite film. Int. J. Environ. Anal. Chem. 2019, 101, 153–166. [Google Scholar] [CrossRef]
- Eteya, M.M.; Rounaghi, G.H.; Deiminiat, B. Fabrication of a new electrochemical sensor based on AuPt bimetallic nanoparticles decorated multi-walled carbon nanotubes for determination of diclofenac. Microchem. J. 2019, 144, 254–260. [Google Scholar] [CrossRef]
- Tubino, M.; de Souza, R.L. Determination of diclofenac in pharmaceutical preparations by diffuse reflectance photometry. Talanta 2006, 68, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Barde, L.N. Simultaneous Spectrophotometric Determination of Metaxalone and Diclofenac Potassium in Combined Tablet Dosage form. Int. J. Chem. Sci 2009, 7, 539–545. Available online: https://www.researchgate.net/publication/267246092 (accessed on 25 January 2023).
- Arancibia, J.A.; Boldrini, M.A.; Escandar, G.M. Spectrofluorimetric determination of diclofenac in the presence of α-cyclodextrin. Talanta 2000, 52, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Davarani, S.S.H.; Pourahadi, A.; Nojavan, S.; Banitaba, M.H.; Nasiri-Aghdam, M. Electro membrane extraction of sodium diclofenac as an acidic compound from wastewater, urine, bovine milk, and plasma samples and quantification by high-performance liquid chromatography. Anal. Chim. Acta 2012, 722, 55–62. [Google Scholar] [CrossRef]
- Song, X.Y.; Shi, Y.P.; Chen, J. A novel extraction technique based on carbon nanotubes reinforced hollow fiber solid/liquid microextraction for the measurement of piroxicam and diclofenac combined with high performance liquid chromatography. Talanta 2012, 100, 153–161. [Google Scholar] [CrossRef]
- Koutsouba, V.; Heberer, T.; Fuhrmann, B.; Schmidt-Baumler, K.; Tsipi, D.; Hiskia, A. Determination of polar pharmaceuticals in sewage water of Greece by gas chromatography–mass spectrometry. Chemosphere 2003, 51, 69–75. [Google Scholar] [CrossRef]
- Česen, M.; Heath, E. Disk-based solid phase extraction for the determination of diclofenac and steroidal estrogens E1, E2 and EE2 listed in the WFD watch list by GC–MS. Sci. Total Environ. 2017, 590–591, 832–837. [Google Scholar] [CrossRef]
- Roscher, J.; Vogel, M.; Karst, U. Identification of ultraviolet transformation products of diclofenac by means of liquid chromatography and mass spectrometry. J. Chromatogr. A 2016, 1457, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, S.; Pasquini, B.; Caprini, C.; del Bubba, M.; Squarcialupi, L.; Colotta, V.; Furlanetto, S. A comprehensive strategy in the development of a cyclodextrin-modified microemulsion electrokinetic chromatographic method for the assay of diclofenac and its impurities: Mixture-process variable experiments and quality by design. J. Chromatogr. A 2016, 1466, 189–198. [Google Scholar] [CrossRef]
- Kaale, E.; Nyamweru, B.C.; Manyanga, V.; Chambuso, M.; Layloff, T. The development and validation of a Thin Layer Chromatography densitometry method for the analysis of diclofenac sodium tablets. Int. J. Chem. Anal. Sci. 2013, 4, 73–79. [Google Scholar] [CrossRef]
- de Carvalho, R.C.; Betts, A.J.; Cassidy, J.F. Diclofenac determination using CeO2 nanoparticle modified screen-printed electrodes—A study of background correction. Microchem. J. 2020, 158, 105258. [Google Scholar] [CrossRef]
- Pourghobadi, R.; Baezzat, M.R. Silica Nanoparticles Modified Carbon Paste Electrode as a Voltammetric Sensor for Determination of Diclofenac. Anal. Bioanal. Chem. Res. 2017, 4, 261–268. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Bagheri, S.; Karim, M.A.; Kadri, N.A.; Basirun, W.J. Immobilized copper ions on MWCNTS-Chitosan thin film: Enhanced amperometric sensor for electrochemical determination of diclofenac sodium in aqueous solution. Int. J. Hydrogen Energy 2017, 42, 19951–19960. [Google Scholar] [CrossRef]
- Fard, G.P.; Alipour, E.; Sabzi, R.E.A. Modification of a disposable pencil graphite electrode with multiwalled carbon nanotubes: Application to electrochemical determination of diclofenac sodium in some pharmaceutical and biological samples. Anal. Methods 2016, 8, 3966–3974. [Google Scholar] [CrossRef]
- MGoodarzian, M.; Khalilzade, M.A.; Karimi, F.; Gupta, V.K.; Keyvanfard, M.; Bagheri, H.; Fouladgar, M. Square wave voltammetric determination of diclofenac in liquid phase using a novel ionic liquid multiwall carbon nanotubes paste electrode. J. Mol. Liq. 2014, 197, 114–119. [Google Scholar] [CrossRef]
- Mokhtari, A.; Karimi-Maleh, H.; Ensafi, A.A.; Beitollahi, H. Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sens. Actuators B Chem. 2012, 169, 96–105. [Google Scholar] [CrossRef]
- Lisboa, T.P.; de Oliveira, W.B.V.; de Souza, C.C.; Oliveira, R.S.; Matos, M.A.C.; Matos, R.C. Development of a 3D disposable device for the electrochemical determination of diclofenac in different matrices. Anal. Bioanal. Chem. 2023, 415, 357–366. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non-steroidal anti-inflammatory drug diclofenac. Mater. Sci. Eng.: C 2012, 32, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Killedar, L.; Ilager, D.; Shetti, N.P.; Aminabhavi, T.M.; Raghava Reddy, K. Synthesis of ruthenium doped titanium dioxide nanoparticles for the electrochemical detection of diclofenac sodium. J. Mol. Liq. 2021, 340, 116891. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sens. Actuators B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Abou-Krisha, M.M.; Alhamzani, A.G.; Yousef, T.A. An effective, novel, and cheap carbon paste electrode for naproxen estimation. Rev. Anal. Chem. 2022, 41, 168–179. [Google Scholar] [CrossRef]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J.S. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzym. Microb. Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; Franco, L.O.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Abdelkrim, E.K. Chitosan as a sustainable organocatalyst: A concise overview. ChemSusChem 2015, 8, 217–244. [Google Scholar] [CrossRef]
- Xu, C.; Nie, J.; Wu, W.; Fu, L.; Lin, B. Design of self-healable supramolecular hybrid network based on carboxylated styrene butadiene rubber and nano-chitosan. Carbohydr. Polym. 2019, 205, 410–419. [Google Scholar] [CrossRef]
- Tao, F.; Ma, S.; Tao, H.; Jin, L.; Luo, Y.; Zheng, J.; Xiang, W.; Deng, H. Chitosan-based drug delivery systems: From synthesis strategy to osteomyelitis treatment—A review. Carbohydr. Polym. 2021, 251, 117063. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Juska, V.B.; Pemble, M.E. A dual-enzyme, micro-band array biosensor based on the electrodeposition of carbon nanotubes embedded in chitosan and nanostructured Au-foams on microfabricated gold band electrodes. Analyst 2020, 145, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; Moreira, K.d.S.; de Oliveira, A.L.B.; Mota, G.F.; Souza, J.E.D.S.; Falcão, I.R.D.A.; et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: How to choose the best strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. Biotech 2013, 4, 167–175. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Kahdestani, S.A.; Shahriari, M.H.; Abdouss, M. Synthesis and characterization of chitosan nanoparticles containing teicoplanin using sol–gel. Polymer. Bulletin. 2021, 78, 1133–1148. [Google Scholar] [CrossRef]
- Atangana, E.; Chiweshe, T.T.; Roberts, H. Modification of Novel Chitosan-Starch Cross-Linked Derivatives Polymers: Synthesis and Characterization. J. Polym. Environ. 2019, 27, 979–995. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.-L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2019, 45, 51–63. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Alsoghier, H.M.; Alhamzani, A.G.; Abou-Krisha, M.M.; Yousef, T.A.; Assaf, H.F. A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode. Nanomaterials 2022, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Abo-Bakr, A.M.; Abd-Elsabour, M.; Abou-Krisha, M.M. An Efficient Novel Electrochemical Sensor for Simultaneous Determination of Vitamin C and Aspirin Based on a PMR/Zn-Al LDH/GCE. Electroanalysis 2021, 33, 2476–2489. [Google Scholar] [CrossRef]
- Da Cunha, C.E.P.; Rodrigues, E.S.B.; Fernandes Alecrim, M.; Thomaz, D.V.; Macêdo, I.Y.L.; Garcia, L.F.; de Oliveira Neto, J.R.; Moreno, E.K.G.; Ballaminut, N.; de Souza Gil, E. Voltammetric Evaluation of Diclofenac Tablets Samples through Carbon Black-Based Electrodes. Pharmaceuticals 2019, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Asadollahzadeh, H.; Shahidi, M.; Rastakhiz, N.; Mohammadi, S.Z. Sensitive determination of hydroxylamine by using modified electrode by La2O3–Co3O4 nanocomposite and ionic liquid. Mater. Chem. Phys. 2022, 286, 126209. [Google Scholar] [CrossRef]

| Method | Working Electrode | Linear Range [µM] | LOD [µM] | Reference |
|---|---|---|---|---|
| DPV | GCE-anodic act SPCE-anodic act | 0.01–0.05 0.067–0.49 | 0.0053 0.024 | [1] |
| DPV | Silica NPs-CPE | 0.1–500 | 0.046 | [15] |
| DPV | Cu/CTS/MWCNTs/GCE | 0.3–200 | 0.021 | [16] |
| DPV | MWCNTs/PGE | 0.047–12.95 | 0.017 | [17] |
| SWV | IL/CNTPE | 0.3–750 | 0.09 | [18] |
| SWV | MWCNT–IL/CCE | 0.05–50 | 0.018 | [19] |
| SWV | IL/CNTPE | 0.5–300 | 0.20 | [20] |
| DPV | MWCNTs/Cu(OH)2NP/ILNC/GCE | 0.18–119 | 0.04 | [21] |
| DPV | VFMCNTPE | 5–600 | 2.0 | [22] |
| DPV | EPP graphite electrode | 0.01–1 | 0.0062 | [23] |
| DPV | M-Chs NC/CPE | 0.025 | 0.007 | This work |
| Sample | DIC Added (μM) | DIC Found (μM) | Recovery% |
|---|---|---|---|
| VoltarenTM (100 mg) | 5 | 4.96 | 99.20 |
| 10 | 9.82 | 98.20 | |
| 15 | 15.20 | 101.3 | |
| Human serum | 5 | 4.85 | 97.0 |
| 10 | 10.27 | 102.7 | |
| 15 | 14.83 | 98.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elsabour, M.; Abou-Krisha, M.M.; Kenawy, S.H.; Yousef, T.A. A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation. Nanomaterials 2023, 13, 1079. https://doi.org/10.3390/nano13061079
Abd-Elsabour M, Abou-Krisha MM, Kenawy SH, Yousef TA. A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation. Nanomaterials. 2023; 13(6):1079. https://doi.org/10.3390/nano13061079
Chicago/Turabian StyleAbd-Elsabour, Mohamed, Mortaga M. Abou-Krisha, Sayed H. Kenawy, and Tarek A. Yousef. 2023. "A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation" Nanomaterials 13, no. 6: 1079. https://doi.org/10.3390/nano13061079
APA StyleAbd-Elsabour, M., Abou-Krisha, M. M., Kenawy, S. H., & Yousef, T. A. (2023). A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation. Nanomaterials, 13(6), 1079. https://doi.org/10.3390/nano13061079






