Antifungal Activity of ZnO Nanoparticles Synthesized from Eichhornia crassipes Extract for Construction Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Eichhornia crassipes Extract
2.2. Synthesis of ZnO Nanoparticles
2.3. Characterization of ZnO Nanomaterials
2.4. Antifungal Activity
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirthika, S.K.; Goel, G.; Matthews, A.; Goel, S. Review of the untapped potentials of antimicrobial materials in the construction sector. Prog. Mater. Sci. 2023, 133, 101065. [Google Scholar] [CrossRef]
- Aburto-Medina, A.; Le, P.H.; MacLaughlin, S.; Ivanova, E. Diversity of experimental designs for the fabrication of antifungal surfaces for the built environment. Appl. Microbiol. Biotechnol. 2021, 105, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Nag, P.K. Sick Building Syndrome and Other Building-Related Illnesses. In Office Buildings; Design Science and Innovation; Springer: Singapore, 2019; pp. 53–103. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Ying, S.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Hublikar, L.V.; Ganachari, S.V.; Raghavendra, N.; Patil, V.B.; Banapurmath, N.R. Green synthesis silver nanoparticles via Eichhornia Crassipes leaves extract and their applications. Curr. Res. Green. Sustain. Chem. 2021, 4, 100212. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Stohlgren, T.J.; Pyšek, P.; Kartesz, J.; Nishino, M.; Pauchard, A.; Winter, M.; Pino, J.; Richardson, D.M.; Wilson, J.; Murray, B.R.; et al. Globalization effects on common plant species. In Encyclopedia of Biodiversity; Academic Press: Waltham, MA, USA, 2013; pp. 700–706. [Google Scholar] [CrossRef]
- Vanathi, P.; Periakaruppan, R.; Narendhran, S.; Sivaraj, R.; Rahman, P.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Jaithon, T.; Ruangtong, J.; T-Thienprasert, J.; T-Thienprasert, N.P. Effects of Waste-Derived ZnO Nanoparticles against Growth of Plant Pathogenic Bacteria and Epidermoid Carcinoma Cells. Crystals 2022, 12, 779. [Google Scholar] [CrossRef]
- López-González, R.L.; Gutiérrez, M.; Lezama, R.; Gómez-Cornelio, S.; Lobato, C.; Gómez-Rivera, A.; Quintana-Owen, P.; Alvarez-Lemus, M. ZnO nanomaterials with enhanced antimicrobial activity obtained by Eichhornia crassipes aqueous extract-mediated synthesis. J. Chem. Technol. Biotechnol. 2024. [Google Scholar] [CrossRef]
- López-González, R.L.; De La Fuente, O.; Lezama, R.; Del Carmen Uribe López, M.; Quintana Owen, P.; Hidalgo, M.; Alvarez-Lemus, M. Effect of phenol concentration on the photocatalytic performance of ZnO nanoparticles. J. Chem. Technol. Biotechnol. 2023, 98, 1826–1836. [Google Scholar] [CrossRef]
- Gómez-Cornelio, S.; Ortega-Morales, O.; Morón-Ríos, A.; Reyes-Estebanez, M.; Rosa-García, S. Changes in fungal community composition of biofilms on limestone across a chronosequence in Campeche, Mexico. Act. Bot. Mex. 2016, 117, 59–77. [Google Scholar] [CrossRef]
- Sutton, B.C. The Coelomycetes; CABI Published: London, UK, 1980; 696p. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS-KNAW Fungal Biodiversity Center: London, UK, 2011; 997p. [Google Scholar]
- Soria-Castro, M.; De La Rosa-García, S.; Quintana, P.; Gómez-Cornelio, S.; Sierra-Fernández, A.; Gómez-Ortíz, N. Broad spectrum antimicrobial activity of Ca(Zn(OH)3)2·2H2O and ZnO nanoparticles synthesized by the sol–gel method. J. Sol-Gel. Sci. Technol. 2018, 89, 284–294. [Google Scholar] [CrossRef]
- Philippe, S.; Souaïbou, F.; Jean-Pierre, N.; Boniface, Y.; Paulin, A.; Issaka, Y.; Chantal, M.; Dominique, S.K. Chemical composition and antifungal activity of essential oil of fresh leaves of Cinnamomum zeylanicum from Benin against six mycotoxigenic fungi isolated from a traditional cheese wagashi. Res. J. Biol. Sci. 2012, 1, 22–27. [Google Scholar]
- Hammer, O.; Harper, A.T.; Ryan, D.P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electronica. 2001, 4, 1–9. [Google Scholar]
- Basnet, P.; Inakhunbi-Chanu, T.; Samanta, D.; Chatterjee, S. A review on bio-synthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J. Photochem. Photobiol. B 2018, 183, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Bharath, L. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Matinise, N.; Fuku, X.; Kaviyarasu, K.; Mayedwa, N.; Mâaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Fakhar-e-Alam, M.; Amjad, I.; Saadullah, M.; Tahir, M.; Jawad, M.; Asif, M.; Atif, M.; Zara, S.; Rashad, M. Antitumor activity of zinc oxide nanoparticles fused with green extract of Nigella sativa. J. Saudi Chem. Soc. 2024, 28, 101814. [Google Scholar] [CrossRef]
- Mankomal, N.; Harpreet, K. Synergistic Effect of biochar impregnated with ZnO nano-flowers for effective removal of organic pollutants from wastewater. Appl. Surf. Sci. 2022, 12, 100339. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Gomez-Zavaglia, A.; Guerrero, A.; Romero, A. Green synthesis of ZnO nanoparticles using polyphenol extracts from pepper waste (Capsicum annuum). J. Clean. Prod. 2022, 350, 131541. [Google Scholar] [CrossRef]
- Nasiri, S.; Rabiei, M.; Palevičius, A.; Janušas, G.; Vilkauskas, A.; Venkatramaiah, N.; Monshi, A. Modified Scherrer equation to calculate crystal size by XRD with high accuracy, examples Fe2O3, TiO2 and V2O5. Nano-Trends. 2023, 3, 100015. [Google Scholar] [CrossRef]
- Soto-Robles, C.; Luque, P.; Gómez-Gutiérrez, C.; Nava, O.; Vilchis-Néstor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results. Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green Synthesis of ZnO Nanoparticles Using Solanum Nigrum Leaf Extract and Their Antibacterial Activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green Synthesis of Multifunctional Zinc Oxide (ZnO) Nanoparticles Using Cassia Fistula Plant Extract and Their Photodegradative, Antioxidant and Antibacterial Activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cayratia Pedata Leaf Extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah-Akhtar, N.; Khattak, A.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Sedefoglu, N. Green synthesis of ZnO nanoparticles by Myrtus communis plant extract with investigation of effect of precursor, calcination temperature and study of photocatalytic performance. Ceram. Int. 2024, 50, 9884–9895. [Google Scholar] [CrossRef]
- Padmasree, G.; Anuradha, K.; Kumar, A.A.; Pragadeeswari, G. Studies on antibacterial efficacy of ZnO nanoparticles using Tanner’s Cassia flower extract from green synthesis. Mater. Today Proc. 2023, 92, 658–661. [Google Scholar] [CrossRef]
- Kumar, S.; Rang Rao, V.; Nageswara Rao, G. Effect of morphology, crystallite size and optical band gap on photocatalytic activity of ZnO nanostructures for decolorization of R6G. Mater. Today Proc. 2022, 62, 5494–5502. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.; Hidalgo, M.; Mací-As, M. High UV-photocatalytic activity of ZnO and Ag/ZnO synthesized by a facile method. Catal. Today. 2017, 284, 121–128. [Google Scholar] [CrossRef]
- Zak, A.K.; Abrishami, M.E.; Majid, W.A.; Yousefi, R.; Hosseini, S.M. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, J.; Yang, Y.; Wei, T.; Tan, R.; Song, W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Li, D.W.; Yang, C.S. Fungal contamination as a major contributor to sick building syndrome. Adv. Appl. Microbiol. 2004, 55, 31–112. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L. Associations between Fungal Species and Water-Damaged Building Materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Ponder, M.M.; Garcia-Diaz, J. Phoma Infections: Classification, Potential Food Sources, and Their Clinical Impact. Microorganisms 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids–A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Trzcińska-Wencel, J.; Wypij, M.; Terzyk, A.P.; Rai, M.; Golińska, P. Biofabrication of novel silver and zinc oxide nanoparticles from Fusarium solani IOR 825 and their potential application in agriculture as biocontrol agents of phytopathogens, and seed germination and seedling growth promoters. Front. Chem. 2023, 11, 1235437. [Google Scholar] [CrossRef]
- De la Rosa-García, S.C.; Fuentes, A.F.; Gómez-Cornelio, S.; Zagada-Domínguez, U.; Quintana, P. Structural characterization of antifungal CaZn2(OH)6·2H2O nanoparticles obtained via mechanochemical processing. J. Mater. Sci. 2018, 53, 13758–13768. [Google Scholar] [CrossRef]
- De Maria, V.P.K.; De Paiva, F.F.G.; Tamashiro, J.R.; Silva, L.H.P.; Da Silva Pinho, G.; Rubio-Marcos, F.; Kinoshita, Â. Advances in ZnO nanoparticles in building material: Antimicrobial and photocatalytic applications—Systematic literature review. Constr. Build. Mater. 2024, 417, 135337. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.; Felder, E.; Tahir, M.N.; Kaltbeitzel, A.; Heinrich, U.R.; Brochhausen, C.; Mailänder, V.; Tremel, W.; Brieger, J. Genotoxic effects of zinc oxide nanoparticles. Nanoscale 2015, 7, 8931–8938. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Pang, Y.L.; Lim, S.; Chong, W.C. Facile green synthesis of ZnO nanoparticles using natural-based materials: Properties, mechanism, surface modification and application. J. Environ. Chem. Eng. 2021, 9, 105417. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Hanagata, N.; Su, H.; Chen, H.; Fujita, D. Challenge to assess the toxic contribution of metal cation released from nanomaterials for nanotoxicology—The case of ZnO nanoparticles. Nanoscale 2013, 5, 4763. [Google Scholar] [CrossRef]
- De Lucas-Gil, E.; Del Campo, A.; Pascual, L.; Monte-Serrano, M.; Menéndez, J.; Fernández, J.; Rubio-Marcos, F. The fight against multidrug-resistant organisms: The role of ZnO crystalline defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 575–581. [Google Scholar] [CrossRef]
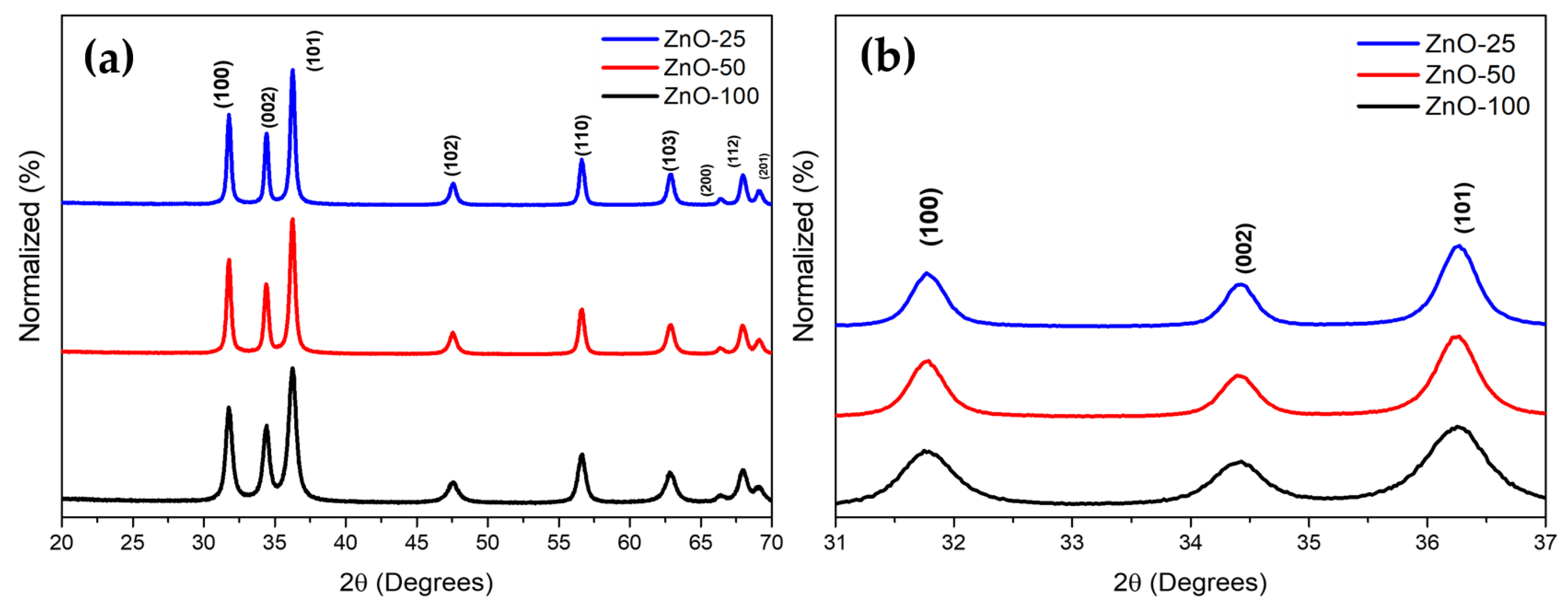
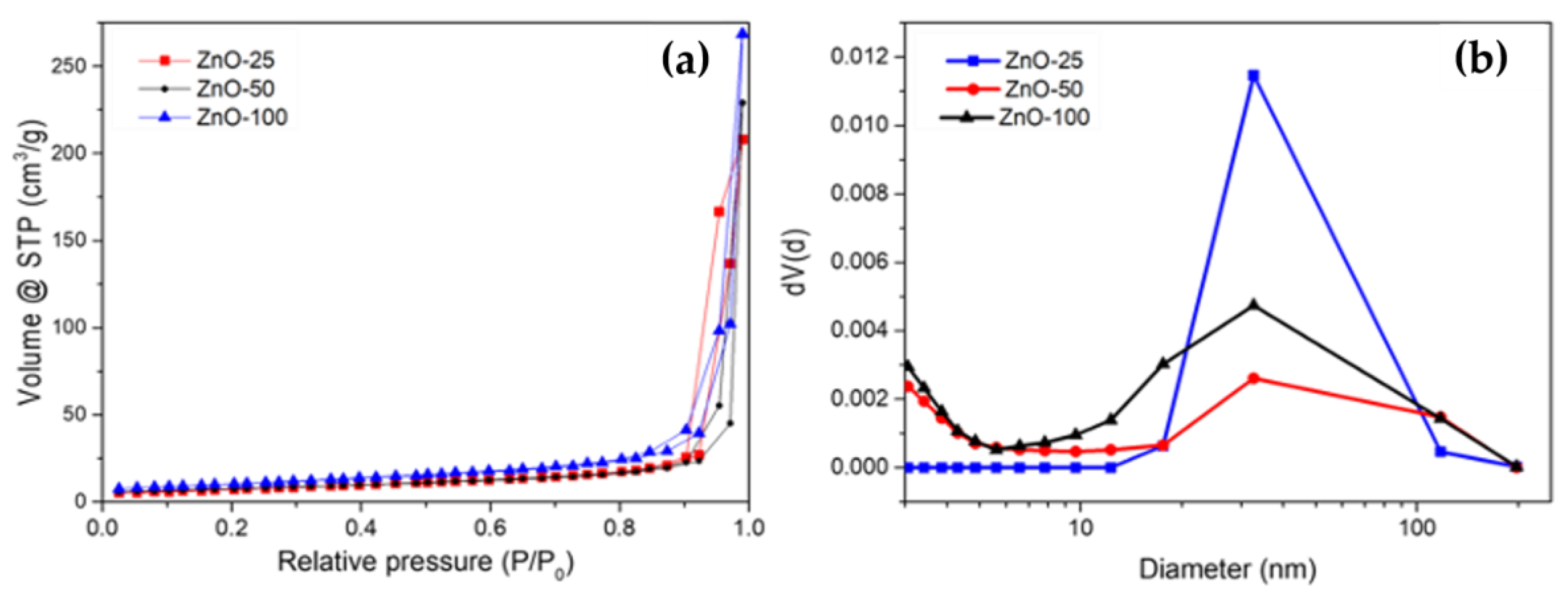
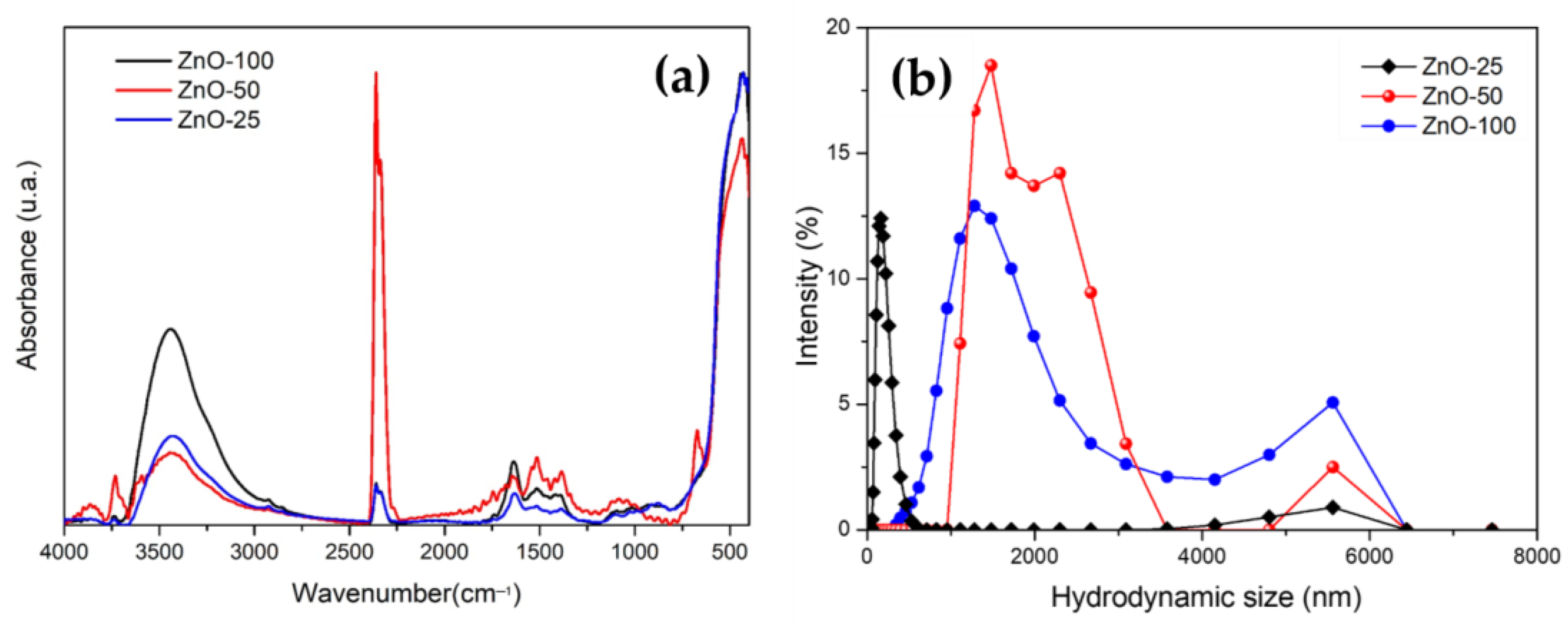
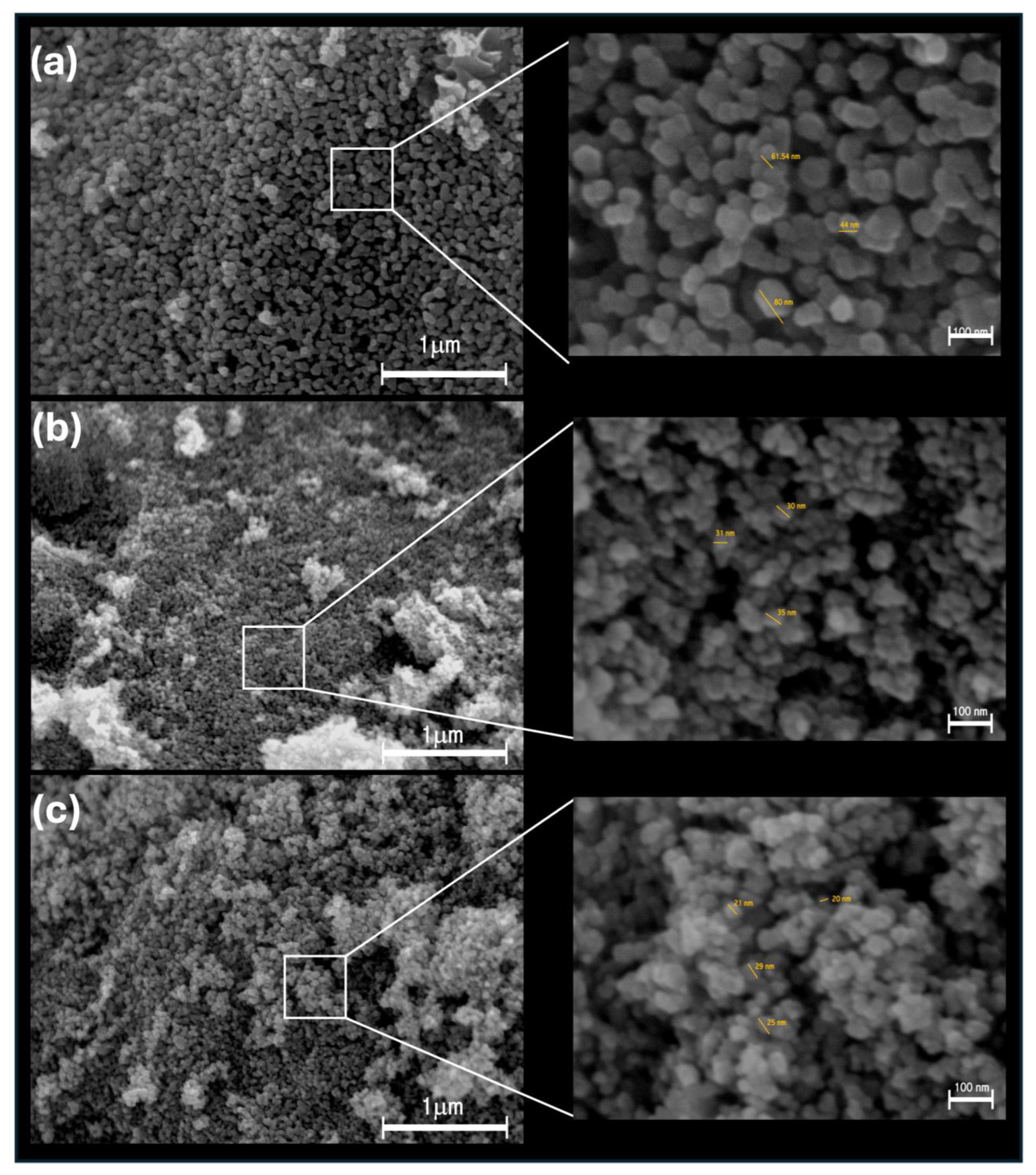

| Sample | Crystallite Size (nm) | Eg (eV) | Zavg (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| ZnO-25 | 20.03 | 3.26 | 163 | 0.23 | 17.40 |
| ZnO-50 | 17.79 | 3.22 | 3406 | 0.51 | 3.52 |
| ZnO-100 | 11.80 | 3.27 | 1542 | 0.38 | 1.40 |
| Sample | Surface Area m2/g | C | R | DFHH (des) | Pore Width (nm) |
|---|---|---|---|---|---|
| ZnO-25 | 26 | 70.71 | 0.99 | 2.27 | 33 |
| ZnO-50 | 26 | 264.13 | 0.99 | 2.34 | 32 |
| ZnO-100 | 36 | 172.48 | 1.00 | 2.35 | 32 |
| Strain | ZnO-100 | ZnO-50 | ZnO-25 | |||
|---|---|---|---|---|---|---|
| MFC/MIC (μg/mL) | MA | MFC/MIC (μg/mL) | MA | MFC/MIC (μg/mL) | MA | |
| Phoma sp. (H4) | 312/156 | FC | 312/156 | FC | 78/78 | FC |
| Stachybotrys sp. (H7) | 2500/1250 | FC | 5000/2500 | FC | 1250/1250 | FC |
| Fusarium sp. (H8) | 625/625 | FC | 1250/625 | FC | 625/312 | FC |
| Aspergillus sp. (H10) | 625/312 | FC | 625/312 | FC | 625/625 | FC |
| Strain | Concentration of ZnO-25 (μg/mL) | Itraconazole (mg/mL) | ||
|---|---|---|---|---|
| 1250 | 625 | 312 | 6.6 | |
| Phoma sp. (H4) | 84.1 ± 1.32 | 80.8 ± 0.57 | 72.9 ± 5.19 | 12 ± 0 |
| Stachybotrys sp. (H7) | 77.1 ± 0.57 | 70.0 ± 1.73 | 65.6 ± 3.12 | 47 ± 4.04 |
| Fusarium sp. (H8) | 64.5 ± 4.75 | 53.9 ± 1.89 | 54.6 ± 3.18 | 14 ± 0 |
| Aspergillus sp. (H10) | 74.5 ± 0.76 | 68.7 ± 4.59 | 64.5 ± 0.28 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas Hernández, R.; Alvarez Lemus, M.A.; De la Rosa García, S.; López González, R.; Quintana, P.; García Zaleta, D.; Velázquez Vázquez, V.; Gómez Cornelio, S. Antifungal Activity of ZnO Nanoparticles Synthesized from Eichhornia crassipes Extract for Construction Applications. Nanomaterials 2024, 14, 1007. https://doi.org/10.3390/nano14121007
Vargas Hernández R, Alvarez Lemus MA, De la Rosa García S, López González R, Quintana P, García Zaleta D, Velázquez Vázquez V, Gómez Cornelio S. Antifungal Activity of ZnO Nanoparticles Synthesized from Eichhornia crassipes Extract for Construction Applications. Nanomaterials. 2024; 14(12):1007. https://doi.org/10.3390/nano14121007
Chicago/Turabian StyleVargas Hernández, Rocío, Mayra A. Alvarez Lemus, Susana De la Rosa García, Rosendo López González, Patricia Quintana, David García Zaleta, Viridiana Velázquez Vázquez, and Sergio Gómez Cornelio. 2024. "Antifungal Activity of ZnO Nanoparticles Synthesized from Eichhornia crassipes Extract for Construction Applications" Nanomaterials 14, no. 12: 1007. https://doi.org/10.3390/nano14121007






