Characterization of Microplastics and Adsorbed/Dissolved Polycyclic Aromatic Hydrocarbons in the Biggest River System in Saitama and Tokyo, Japan
Abstract
:1. Introduction
2. Experimental Method
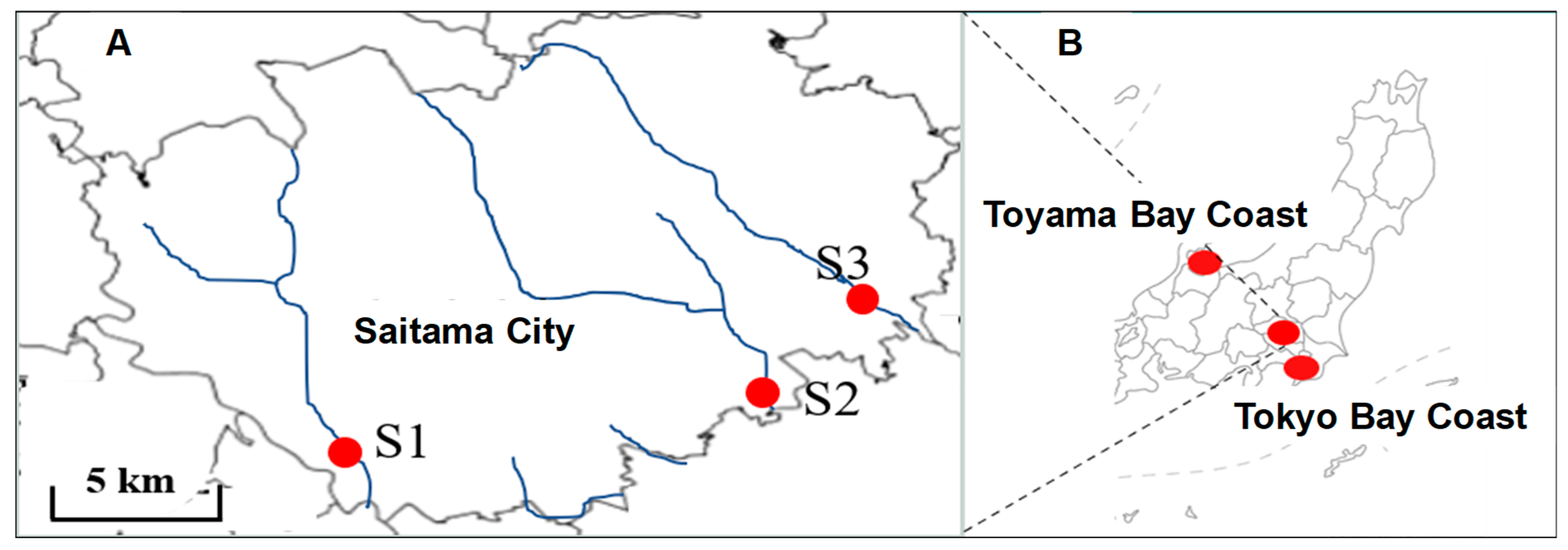
2.1. Study Area
2.2. Sample and Sample Collection
2.3. Sample Pretreatment and Analysis of MPs
2.3.1. Microscopic Analysis
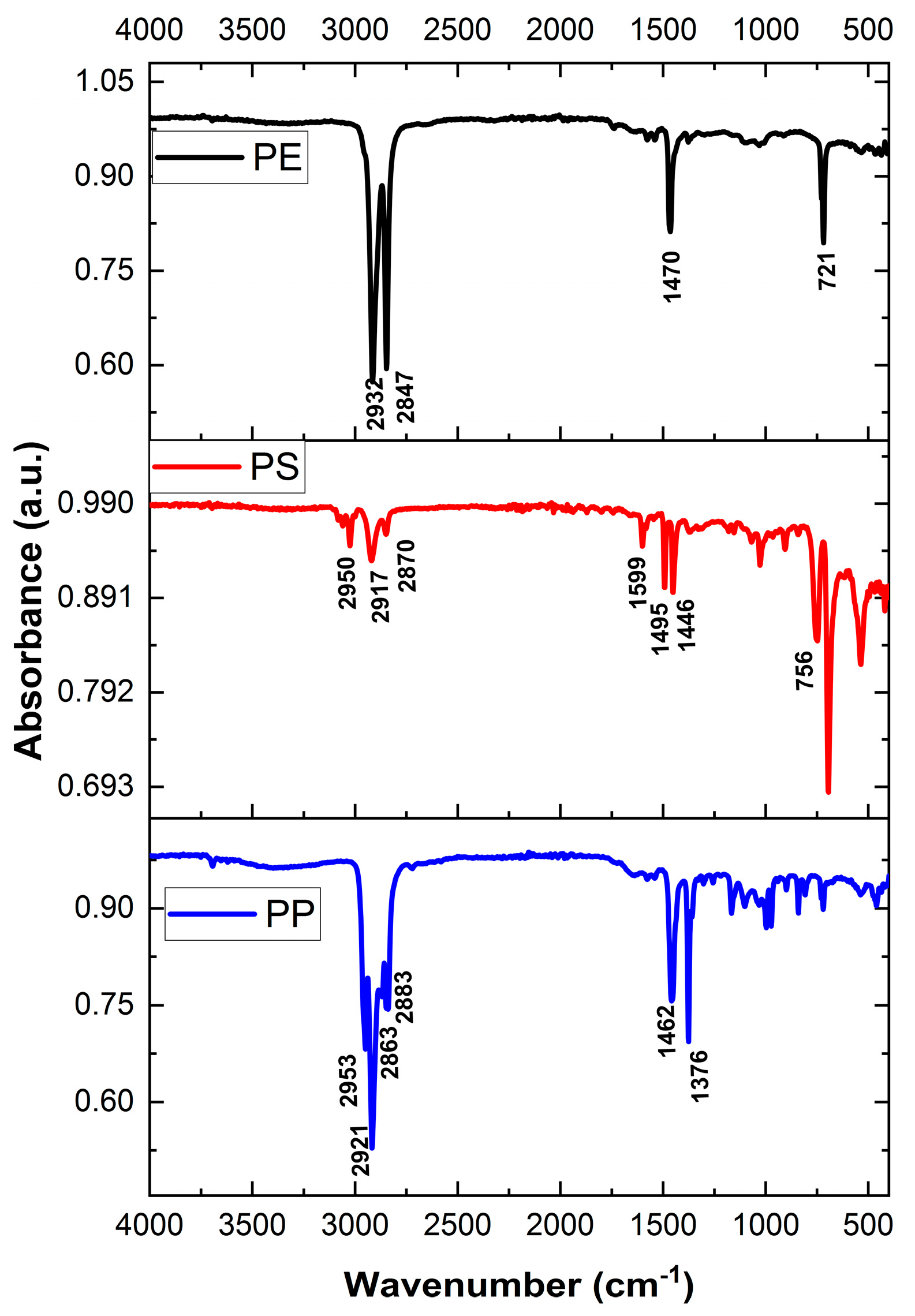
2.3.2. Component Analysis Using FT-IR
2.4. Extraction Method of MPs Adsorped PAHs
2.4.1. Extraction of PAHs in River Water Suspension Method
2.4.2. Liquid–Liquid Extraction Method
2.5. Analytical Quality Control
2.5.1. Cleaning of Appliances
2.5.2. Calibration Curve, Detection Limit, Quantification Limit
2.5.3. Addition Recovery Test
Solvent Extraction Method
Liquid–Liquid Extraction Method
2.5.4. Operation Blank Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. River MPs
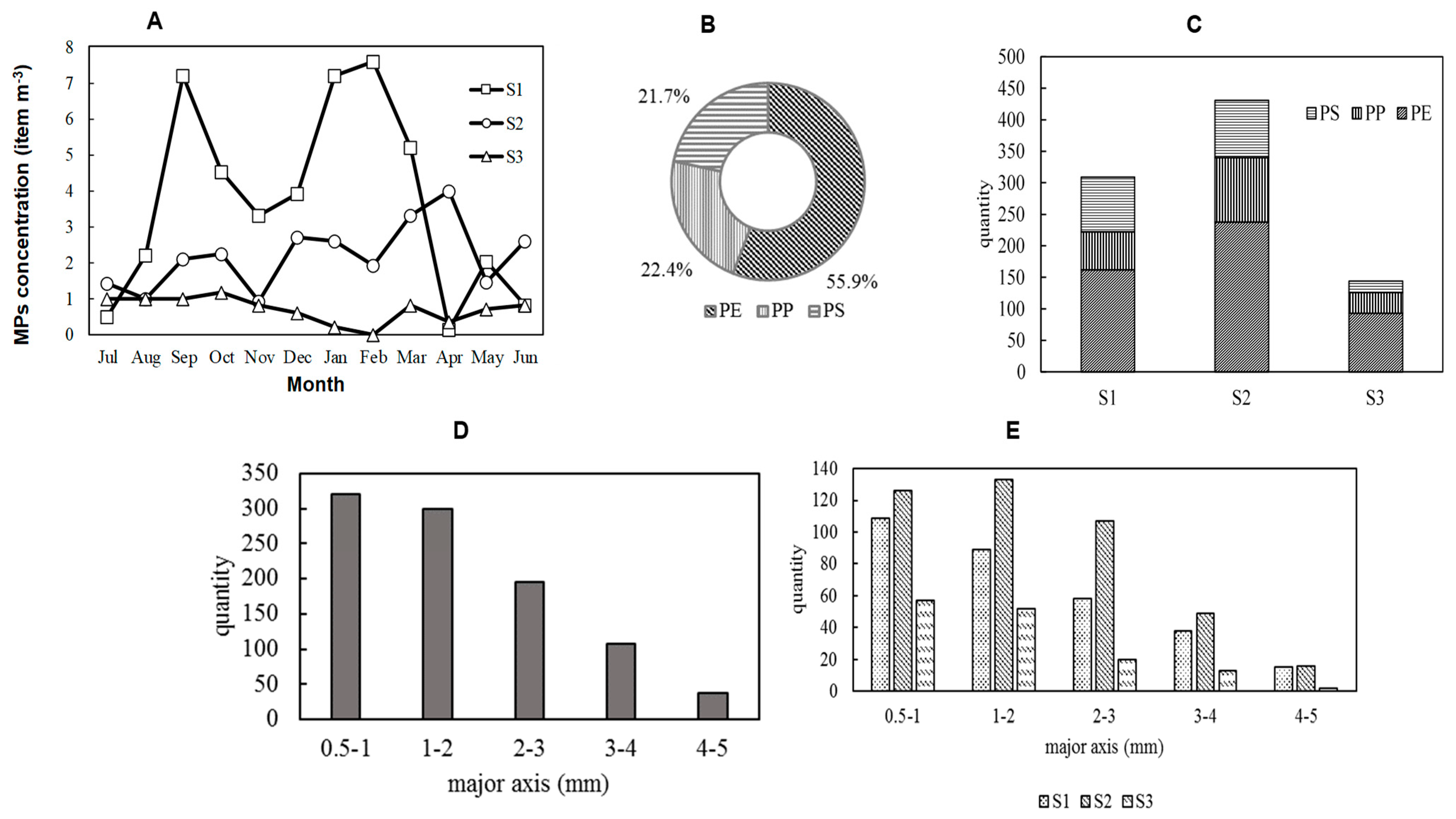
3.1.1. River MPs Concentration
3.1.2. Seasonal Fluctuations in River Microplastics
3.1.3. Polymer Properties of River Microplastics
3.1.4. Particle Size Characteristics of River Microplastics
3.2. River MPs Adsorbed PAHs
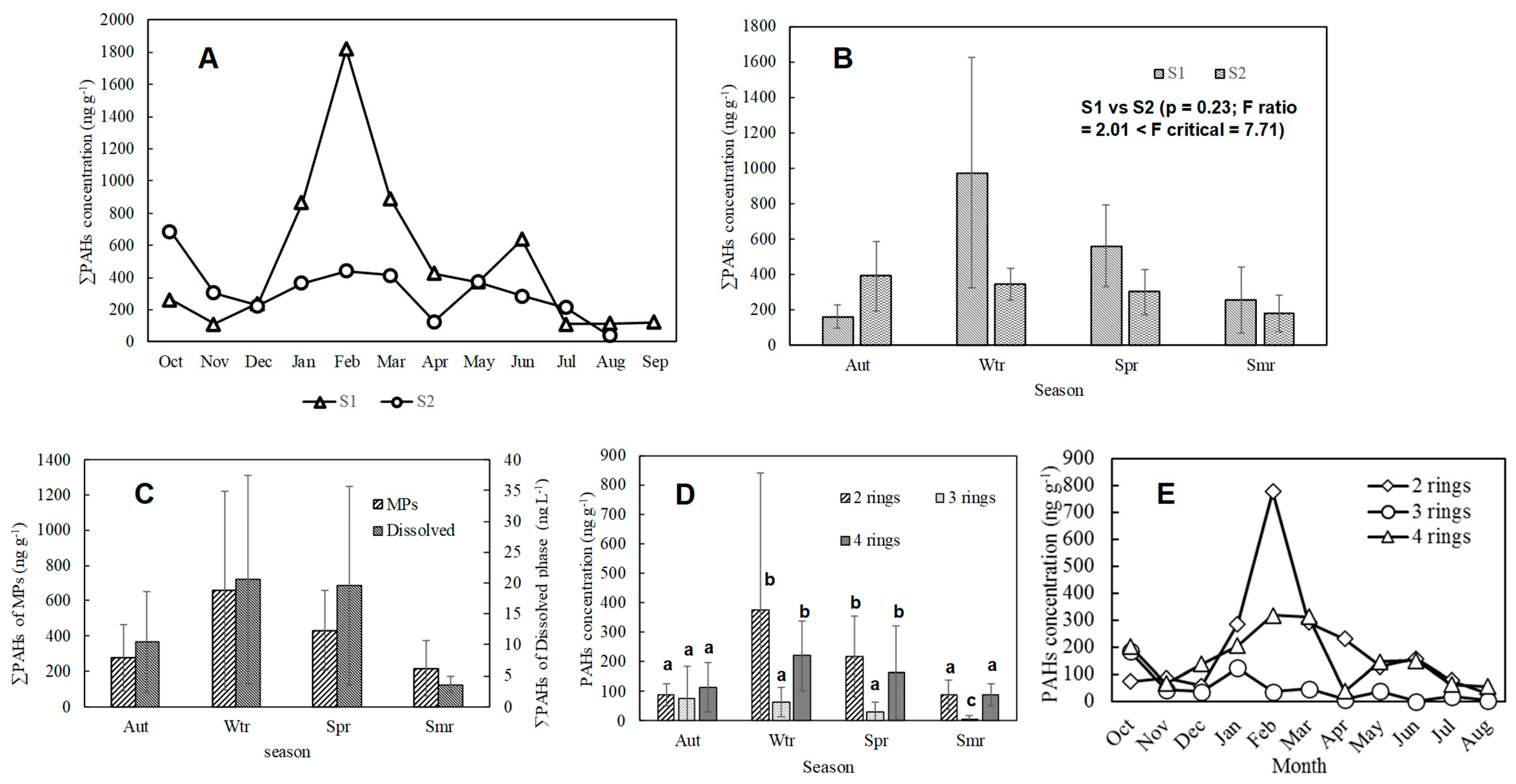
3.2.1. Quantitative Results and Component Characteristics of River MPs’ Adsorption PAHs
Isomeric Ratios
3.2.2. Seasonal Variation of River MPs’ Adsorbed PAHs
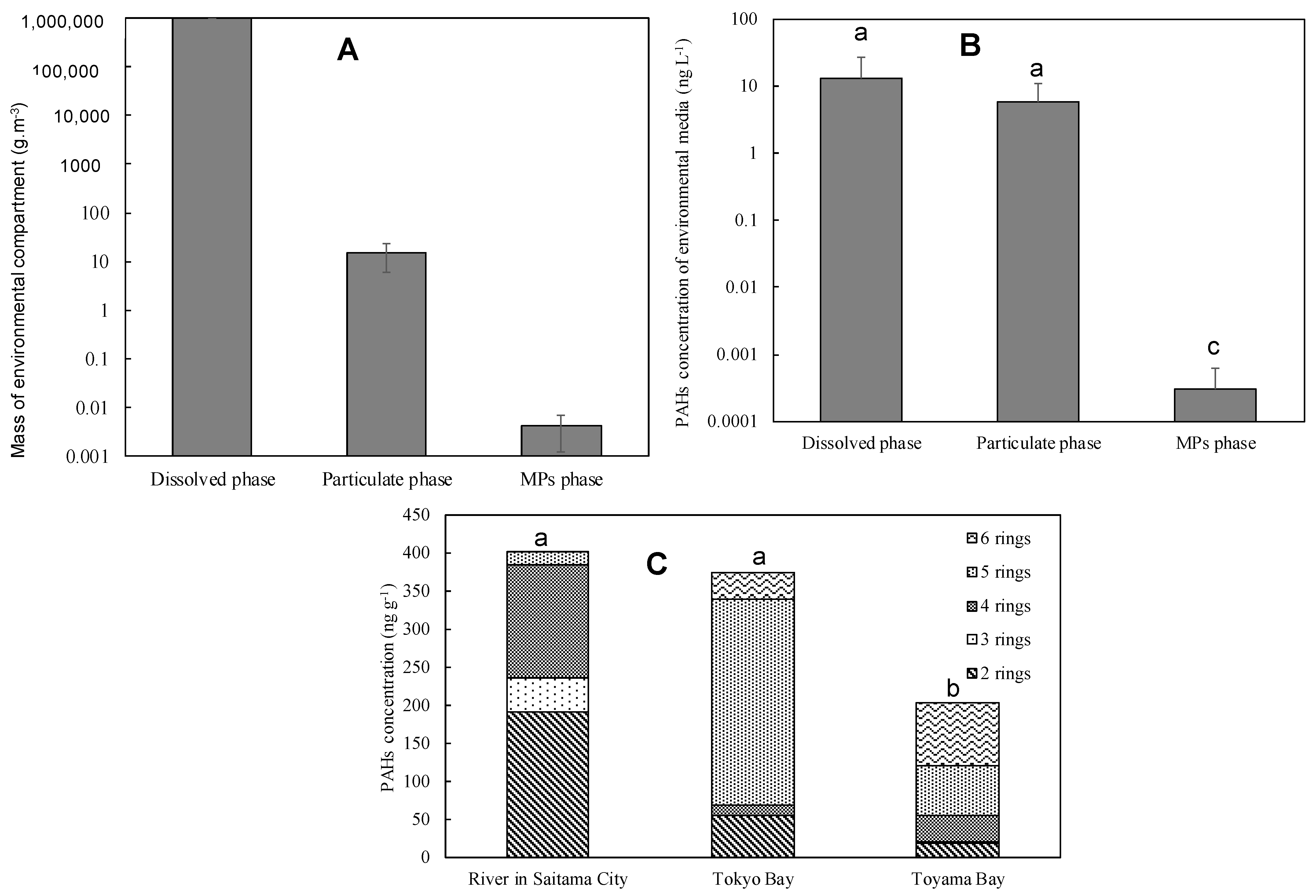
3.2.3. Underwater PAHs Distribution
Comparing the Masses of PAHs Dissolved, Particulate, and Adsorbed MPs in River Water
3.2.4. Comparison of River and Coastal Microplastic Adsorption PAHs
4. Conclusions and Recommendation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Enyoh, C.E.; Wirnkor, V.A.; Ngozi, V.E.; Chukwuemeka, I.S. Macrodebris and microplastics pollution in Nigeria: First report on abundance, distribution and composition. Environ. Anal. Health Toxicol. 2019, 34, e2019012. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2018, 244, 958–965. [Google Scholar] [CrossRef]
- Enamul Kabir, A.H.M.; Sekine, M.; Imai, T.; Yamamoto, K. Microplastics Pollution in the Seto Inland Sea and Sea of Japan Surrounded Yamaguchi Prefecture Areas, Japan: Abundance, Characterization and Distribution, and Potential Occurrences. J. Water Environ. Technol. 2020, 18, 175–194. [Google Scholar] [CrossRef]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, S.J. Accumulation of polystyrene microplastics in the gastrointestinal tracts of juvenile fish from the English Channel. Environ. Sci. Technol. 2013, 47, 12954–12961. [Google Scholar]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Ohiagu, F.O.; Verla, A.W.; Wang, Q.; Shafea, L.; Verla, E.N.; Isiuku, B.O.; Chowdhury, T.; Ibe, F.C.; Chowdhury, M.A.H. “Plasti-remediation”: Advances in the potential use of environmental plastics for pollutant removal. Environ. Technol. Innov. 2021, 23, 101791. [Google Scholar] [CrossRef]
- Gioia, R.; Dachs, J.; Nizzetto, L.; Berrojalbiz, N.; Galbán, C.; Del Vento, S.; Méjanelle, L.; Jones, K.C. Sources, Transport and Fate of Organic Pollutants in the Oceanic Environment. In Persistent Pollution—Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2011; pp. 111–139. [Google Scholar]
- Bouhroum, R.; Boulkamh, A.; Asia, L.; Lebarillier, S.; Ter Halle, A.; Syakti, A.D.; Doumenq, P.; Malleret, L.; Wong-Wah-Chung, P. Concentrations and fingerprints of PAHs and PCBs adsorbed onto marine plastic debris from the Indonesian Cilacap coast and the North Atlantic gyre. Reg. Stud. Mar. Sci. 2019, 29, 100611. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Koudryashova, Y.; Chizhova, T.; Tishchenko, P.; Hayakawa, K. Seasonal Variability of Polycyclic Aromatic Hydrocarbons (PAHs) in a Coastal Marine Area in the Northwestern Region of the Sea of Japan/East Sea (Possiet Bay). Ocean Sci. J. 2019, 54, 635–655. [Google Scholar] [CrossRef]
- Singh, V.; Negi, R.; Jacob, M.; Gayathri, A.; Rokade, A.; Sarma, H.; Kalita, J.; Tasfia, S.T.; Bharti, R.; Wakid, A.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in aquatic ecosystem exposed to the 2020 Baghjan oil spill in upper Assam, India: Short-term toxicity and ecological risk assessment. PLoS ONE 2023, 18, e0293601. [Google Scholar] [CrossRef]
- Rabin, M.H.; Wang, Q.; Wang, W.; Enyoh, C.E. Pollution Characteristics, Source Apportionment, and Health Risk of Polycyclic Aromatic Hydrocarbons (PAHs) of Fine Street Dust during and after COVID-19 Lockdown in Bangladesh. Processes 2022, 10, 2575. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef]
- Xia, F.; Yao, Q.; Zhang, J.; Wang, D. Effects of seasonal variation and resuspension on microplastics in river sediments. Environ. Pollut. 2021, 286, 117403. [Google Scholar] [CrossRef]
- Qian, Y.; Shang, Y.; Zheng, Y.; Jia, Y.; Wang, F. Temporal and spatial variation of microplastics in Baotou section of Yellow River, China. J. Environ. Manag. 2023, 338, 117803. [Google Scholar] [CrossRef]
- Sankoda, K.; Yamada, Y. Occurrence, distribution, and possible sources of microplastics in the surface river water in the Arakawa River watershed. Environ. Sci. Pollut. Res. 2021, 28, 27474–27480. [Google Scholar] [CrossRef]
- Alex, K.T.M. Raging River: Tracing the Arakawa, Japan’s Most Dangerous Water Source. 2022. Available online: https://www.japantimes.co.jp/life/2022/02/09/environment/arakawa-river-flooding/ (accessed on 22 September 2021).
- Casila, J.C. Effects of Tidal Patterns and Physico-Chemical Water Quality Variation on Scum Generation and Odor in Multi-branched Urban Estuaries. 2019. Available online: https://tokyo-metro-u.repo.nii.ac.jp/?action=repository_action_common_download&item_id=8188&item_no=1&attribute_id=18&file_no=1 (accessed on 22 September 2021).
- Enyoh, C.E.; Wang, Q.; Momimul, R.H.; Senlin, L. Preliminary characterization and probabilistic risk assessment of microplastics and potentially toxic elements (PTEs) in garri (cassava flake), a common staple food consumed in West Africa. Environ. Anal. Health Toxicol. 2023, 38, e2023005. [Google Scholar] [CrossRef]
- Chae, D.H.; Kim, I.S.; Kim, S.K.; Song, Y.K.; Shim, W.J. Abundance and Distribution Characteristics of Microplastics in Surface Seawaters of the Incheon/Kyeonggi Coastal Region. Arch. Environ. Contam. Toxicol. 2015, 69, 269–278. [Google Scholar] [CrossRef]
- Kwan, C.P.; Hung, P.L.; Fok, L. River Microplastic Contamination and Dynamics upon a Rainfall Event in Hong Kong, China. Environ. Process. 2019, 6, 253–264. [Google Scholar]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic Abundance and Distribution in the Open Water and Sediment of the Ottawa River, Canada, and Its Tributaries. 2017. Available online: https://www.facetsjournal.com/doi/10.1139/facets-2016-0070 (accessed on 22 January 2020).
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L.; et al. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar] [CrossRef]
- Alam, F.C.; Sembiring, E.; Muntalif, B.S.; Suendo, V. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G. Sorption behaviors of phenanthrene, nitrobenzene, and naphthalene on mesoplastics and microplastics. Environ. Sci. Pollut. Res. 2019, 26, 12563–12573. [Google Scholar] [CrossRef]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef]
- Jeong, H.; Kusano, T.; Addai-Arhin, S.; Nugraha, W.C.; Novirsa, R.; Dinh, Q.P.; Shirosaki, T.; Fujita, E.; Kameda, Y.; Cho, H.S.; et al. Differences in microplastic distributions on the surface freshwater collected using 100- and 355-μm meshes. Environ. Monit. Contam. Res. 2022, 2, 22–34. [Google Scholar] [CrossRef]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef]
- Shi, J.; Sanganyado, E.; Wang, L.; Li, P.; Li, X.; Liu, W. Organic pollutants in sedimentary microplastics from eastern Guangdong: Spatial distribution and source identification. Ecotoxicol. Environ. Saf. 2020, 193, 110356. [Google Scholar] [CrossRef]
- Capriotti, M.; Cocci, P.; Bracchetti, L.; Cottone, E.; Scandiffio, R.; Caprioli, G.; Sagratini, G.; Mosconi, G.; Bovolin, P.; Palermo, F.A. Microplastics and their associated organic pollutants from the coastal waters of the central Adriatic Sea (Italy): Investigation of adipogenic effects in vitro. Chemosphere 2021, 263, 128090. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.J.; Shi, L.; Liu, L.Y.; Zeng, E.Y. Polycyclic aromatic hydrocarbons affiliated with microplastics in surface waters of Bohai and Huanghai Seas, China. Environ. Pollut. 2018, 241, 834–840. [Google Scholar] [CrossRef]
- Jameson, C.W. Chapter 7—Polycyclic Aromatic Hydrocarbons and Associated Occupational Exposures. Part 1. Concordance between Cancer in Humans and in Experimental Animals; International Agency for Research on Cancer: Lyon, France, 2022; pp. 59–63. [Google Scholar]
- Martínez-Álvarez, I.; Le Menach, K.; Devier, M.H.; Cajaraville, M.P.; Orbea, A.; Budzinski, H. Sorption of benzo(a)pyrene and of a complex mixture of petrogenic polycyclic aromatic hydrocarbons onto polystyrene microplastics. Front. Environ. Chem. 2022, 3, 958607. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhan, C.; Liu, H.; Zhang, L.; Hu, T.; Xing, X.; Qu, C. Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Street Dust of Huanggang, Central China: Status, Sources and Human Health Risk Assessment. Aerosol Air Qual. Res. 2019, 19, 221–233. [Google Scholar] [CrossRef]
- Lawal, A.T.; Fantke, P. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Wang, Q. Adsorption and toxicity characteristics of ciprofloxacin on differently prepared polyethylene terephthalate microplastics from both experimental and theoretical perspectives. J. Water Process Eng. 2023, 53, 103909. [Google Scholar] [CrossRef]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef]

| PAHs | IDL (ng/mL) | IQL (ng/mL) | Solvent Extraction Method | Liquid–Liquid Extraction Method | ||
|---|---|---|---|---|---|---|
| LOD (ng/g) | LOQ (ng/g) | LOD (ng/L) | LOQ (ng/L) | |||
| Nap | 0.64 | 2.12 | 2.55 | 8.49 | 0.13 | 0.42 |
| Ac | 0.45 | 1.5 | 1.8 | 6 | 0.09 | 0.3 |
| Ace | 0.28 | 0.95 | 1.14 | 3.79 | 0.06 | 0.19 |
| Flu | 0.31 | 1.02 | 1.23 | 4.08 | 0.06 | 0.2 |
| Phe | 0.37 | 1.23 | 1.48 | 4.93 | 0.07 | 0.25 |
| Ant | 1.09 | 3.63 | 4.36 | 14.53 | 0.22 | 0.73 |
| FA | 0.67 | 2.23 | 2.68 | 8.94 | 0.13 | 0.45 |
| Pyr | 0.57 | 1.92 | 2.3 | 7.66 | 0.11 | 0.38 |
| BaA | 1.53 | 5.11 | 6.13 | 20.45 | 0.31 | 1.02 |
| Chry | 1.23 | 4.09 | 4.91 | 16.36 | 0.25 | 0.82 |
| BkF | 1.04 | 3.48 | 4.17 | 13.91 | 0.21 | 0.7 |
| BbF | 2.32 | 7.72 | 9.26 | 30.88 | 0.46 | 1.54 |
| BaP | 4.17 | 13.9 | 16.69 | 55.62 | 0.83 | 2.78 |
| IP | 2.27 | 7.56 | 9.07 | 30.25 | 0.45 | 1.51 |
| DahA | 7.14 | 23.79 | 28.55 | 95.17 | 1.43 | 4.76 |
| BP | 3.72 | 12.42 | 14.9 | 49.66 | 0.74 | 2.48 |
| PAHs | Solvent Extraction Method: MPs (n = 2) | Solvent Extraction Method: Suspension (n = 2) | Liquid–Liquid Extraction Method (n = 2) | |||
|---|---|---|---|---|---|---|
| Recovery Rate (%) | % RSD | Recovery Rate (%) | % RSD | Recovery Rate (%) | % RSD | |
| Nap | 73.0 | 18.1 | 60.0 | 10.2 | 77.2 | 0.6 |
| Ac | 59.4 | 15.2 | 51.8 | 9.6 | 69.0 | 1.2 |
| Ace | 66.6 | 14.9 | 30.2 | 3.2 | 35.5 | 0.9. |
| Flu | 75.3. | 10.4 | 45.0 | 9.7 | 57.2 | 1.3 |
| Phe | 62.3 | 12.9 | 71.1 | 12.2 | 118.9 | 10.4 |
| Ant | 76.9 | 13.4 | 77.1 | 10.1. | 112.1 | 0.5 |
| FA | 72.3 | 11.1 | 81.5 | 6.7 | 125.1 | 6.2 |
| Py | 86.9 | 14.6 | 86.0 | 7.3 | 144.5 | 5.5 |
| BaA | 54.4 | 4.9 | 128.5 | 0.2 | 110.7 | 5.5 |
| Chry | 61.0 | 9.7 | 149.0 | 1.2 | 132.6 | 2.2 |
| BbF | 30.1 | 6.0 | 102.2 | 2.0 | 98.5 | 1.7 |
| BkF | 47.3 | 8.2 | 116.0 | 9.8 | 126.0 | 5.4 |
| BaP | 38.1 | 14.5 | 117.6 | 9.0 | 105.3 | 5.6 |
| IP | 32.3 | 2.7 | 121.0 | 2.2 | 107.0 | 1.6 |
| DahA | 31.1 | 32.2 | 120.2 | 3.2 | 106.8 | 0.2 |
| BP | 36.2 | 5.3 | 86.8 | 4.2 | 104.4 | 2.8 |
| d-Chry | 51.1 | 1.6 | 89.6 | 29.3 | 72.3 | 0.1 |
| d-Ace | 59.9 | 18.6 | 45.5 | 29.2 | 37.6 | 5.0 |
| d-Nap | 54.0 | 14.4 | 42.4 | 29.9 | 29.9 | 5.2. |
| d-peryrene | 39.5 | 1.6 | 72.5 | 33.5 | 64.4 | 2.3 |
| d-Phe | 82.3 | 18.7 | 58.7 | 25.6 | 63.3 | 10.2 |
| PAHs | Solvent Extraction Method: MPs (n = 3) | Solvent Extraction Method: Suspension (n = 3) | Liquid–Liquid Extraction Method (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | % RSD | Average | Standard Deviation | % RSD | Average | Standard Deviation | % RSD | |
| Nap | 2.24 | 0.17 | 7.64 | 7.33 | 6.50 | 88.57 | 0.66 | 0.51 | 78.05 |
| Ac | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| Ace | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| Flu | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| Phe | 6.55 | 1.03 | 15.65 | 5.77 | 1.93 | 33.47 | 6.09 | 1.41 | 23.14 |
| Ant | 2.05 | 2.05 | 100.0 | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| FA | 3.33 | 0.27 | 8.04 | 1.48 | 1.48 | 100.0 | ―――― | ―――― | ―――― |
| Py | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| BaA | 3.76 | 0.68 | 17.96 | 7.23 | 0.94 | 12.96 | ―――― | ―――― | ―――― |
| Chry | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| BbF | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| BkF | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| BaP | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| IP | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| DahA | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| BP | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― | ―――― |
| S1 | S2 | S3 | Mean ± SDV | CV (%) | |
|---|---|---|---|---|---|
| Number density (Pieces/m3) | 3.7 | 2.2 | 0.74 | 2.21 ± 1.48 | 66.87 |
| Flow rate (m3/s) | 3.1 | 4.7 | 3.4 | 3.73 ± 0.85 | 22.78 |
| Flow velocity (m/s) | 0.18 | 0.35 | 0.32 | 0.28 ± 0.09 | 32.03 |
| MPs flow rate (Pieces/s) | 10.8 | 9.21 | 2.87 | 7.63 ± 4.20 | 55.01 |
| S1 | S2 | S3 | |||||
|---|---|---|---|---|---|---|---|
| PAHs | Average | Range | Number of Detections | Average | Range | Number of Detections | |
| Nap | 28.282 | 45.3–1387 | 12/12 | 101 | 1.47–264 | 12/12 | ND |
| Ac | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| Ace | 14.5 | ND–92.3 | 3/12 | 12.4 | ND-51.0 | 6/12 | ND |
| Flu | 2.89 | ND–18.8 | 2/12 | 14.6 | ND-48.5 | 6/12 | ND |
| Phe | 11.0 | ND–75.5 | 5/12 | 21.7 | ND–166 | 7/12 | ND |
| Ant | 4.95 | ND–36.4 | 3/12 | 6.72 | ND-46.9 | 6/12 | ND |
| FA | 56.8 | ND–240 | 10/12 | 37.5 | 12.7–108 | 12/12 | ND |
| Pyr | ND | ND–432 | 9/12 | 53.3 | 4.60–105 | 12/12 | ND |
| BaA | ND | ND–ND | 0/12 | 7.64 | ND–33.3 | 4/12 | ND |
| Chry | ND | ND–93.5 | 4/12 | 36.0 | ND–142 | 7/12 | ND |
| BkF | 22.6 | ND–17.4 | 1/12 | 3.33 | ND–40.0 | 1/12 | ND |
| BbF | 1.45 | ND–146 | 1/12 | ND | ND–ND | 0/12 | ND |
| BaP | 12.2 | ND–ND | 0/12 | 9.86 | ND–118 | 1/12 | ND |
| IP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| DahA | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| BP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| ∑16PAHs | 488 | 113–1820 | 12/12 | 304 | 40.0–670 | 12/12 | ND |
| S1 | S2 | S3 | |||||
|---|---|---|---|---|---|---|---|
| PAHs | Average | Range | Number of Detections | Average | Range | Number of Detections | |
| Nap | 4.45 | ND–20.3 | 11/12 | 4.07 | ND–18.2 | 11/12 | ND |
| Ac | 0.285 | ND–1.28 | 3/12 | 0.468 | ND–3.09 | 3/12 | ND |
| Ace | ND | ND–ND | 0/12 | 0.0803 | ND–0.59 | 2/12 | ND |
| Flu | 0.0361 | ND–0.433 | 1/12 | ND | ND–ND | 0/12 | ND |
| Phe | 4.34 | ND–18.0 | 11/12 | 1.76 | ND-9.18 | 9/12 | ND |
| Ant | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| FA | 1.55 | ND–11.3 | 6/12 | 0.738 | ND–3.25 | 6/12 | ND |
| Pyr | 5.60 | ND–27.0 | 11/12 | 2.61 | ND-10.5 | 11/12 | ND |
| BaA | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| Chry | 0.183 | ND–1.32 | 2/12 | 0.0653 | ND–0.794 | 1/12 | ND |
| BkF | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| BbF | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| BaP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| IP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| DahA | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| BP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 | ND |
| ∑16PAHs | 16.5 | 2.09–53.7 | 12/12 | 9.79 | 2.25–21.8 | 12/12 | ND |
| S1 | S2 | |||||
|---|---|---|---|---|---|---|
| PAHs | Average | Range | Number of Detections | Average | Range | Number of Detections |
| Nap | 217 | ND–956 | 7/12 | 280 | ND–1251 | 10/12 |
| Ac | 0.89 | ND–10.7 | 1/12 | 15.0 | ND–180 | 1/12 |
| Ace | 2.38 | ND–28.6 | 1/12 | 30.8 | ND–233 | 3/12 |
| Flu | ND | ND–ND | 0/12 | 24.6 | ND–268 | 2/12 |
| Phe | 43.2 | ND–141 | 4/12 | 54.3 | ND–332 | 4/12 |
| Ant | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 |
| FA | 8.07 | ND–46.9 | 4/12 | 21.7 | ND–112 | 6/12 |
| Pyr | ND | ND–ND | 0/12 | 11.6 | ND–ND | 2/12 |
| BaA | 8.51 | ND–ND | 1/12 | 2.74 | ND–127 | 1/12 |
| Chry | 12.4 | ND–102 | 3/12 | 29.4 | ND–32.9 | 3/12 |
| BkF | 9.10 | ND–101 | 2/12 | 5.53 | ND–163 | 1/12 |
| BbF | 23.8 | ND–85.1 | 2/12 | 13.2 | ND–66.4 | 1/12 |
| BaP | 58.8 | ND–146 | 2/12 | 13.9 | ND–158 | 1/12 |
| IP | ND | ND–502 | 0/12 | ND | ND–167 | 0/12 |
| DahA | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 |
| BP | ND | ND–ND | 0/12 | ND | ND–ND | 0/12 |
| ∑16PAHs | 377 | 16.4–956 | 12/12 | 502 | 18.3–1464 | 12/12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yamada, Y.; Enyoh, C.E.; Wang, W.; Sankoda, K. Characterization of Microplastics and Adsorbed/Dissolved Polycyclic Aromatic Hydrocarbons in the Biggest River System in Saitama and Tokyo, Japan. Nanomaterials 2024, 14, 1030. https://doi.org/10.3390/nano14121030
Wang Q, Yamada Y, Enyoh CE, Wang W, Sankoda K. Characterization of Microplastics and Adsorbed/Dissolved Polycyclic Aromatic Hydrocarbons in the Biggest River System in Saitama and Tokyo, Japan. Nanomaterials. 2024; 14(12):1030. https://doi.org/10.3390/nano14121030
Chicago/Turabian StyleWang, Qingyue, Yojiro Yamada, Christian Ebere Enyoh, Weiqian Wang, and Kenshi Sankoda. 2024. "Characterization of Microplastics and Adsorbed/Dissolved Polycyclic Aromatic Hydrocarbons in the Biggest River System in Saitama and Tokyo, Japan" Nanomaterials 14, no. 12: 1030. https://doi.org/10.3390/nano14121030






