Aluminum-Crosslinked Nanocellulose Scaffolds for Fluoride Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nitro-Oxidized Cellulose Nanofibers (NOCNF)
2.3. Preparation of Al-CNF Composite Suspension
2.4. Characterization of NOCNF and Al-CNF
2.4.1. Conductometric Titration
2.4.2. Fourier-Transform Infrared Spectrometry
2.4.3. X-ray Photoelectron Spectroscopy
2.4.4. Zeta Potential
2.4.5. UV–VIS Analysis
2.4.6. Scanning Electron Microscopy
2.4.7. Transmission Electron Microscopy
2.4.8. Atomic Force Microscopy
2.4.9. Wide-Angle X-ray Diffraction
2.4.10. 13C and 27Al Cross-Polarization Magic-Angle Spinning Nuclear Magnetic Resonance
2.4.11. Thermogravimetric Analysis
2.4.12. The Fluoride Adsorption Study
3. Results and Discussion
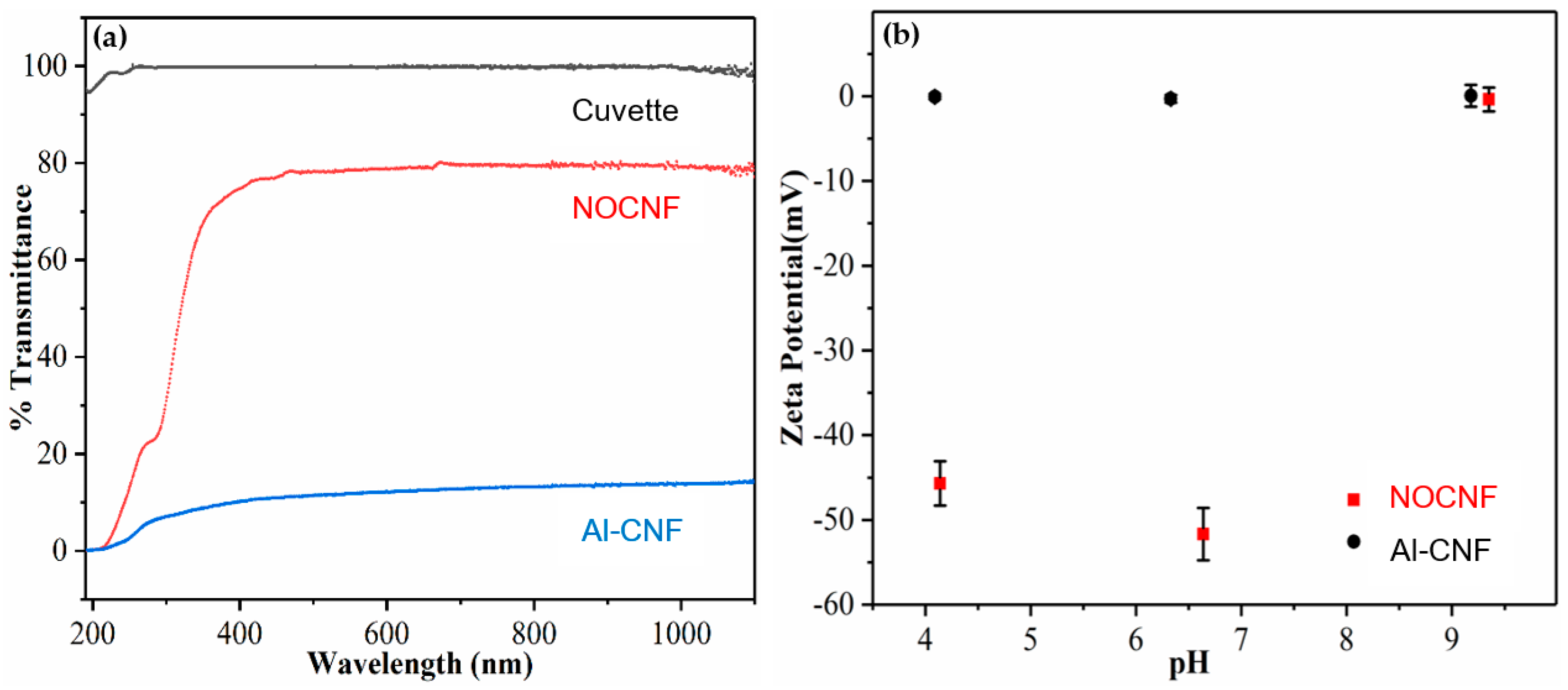
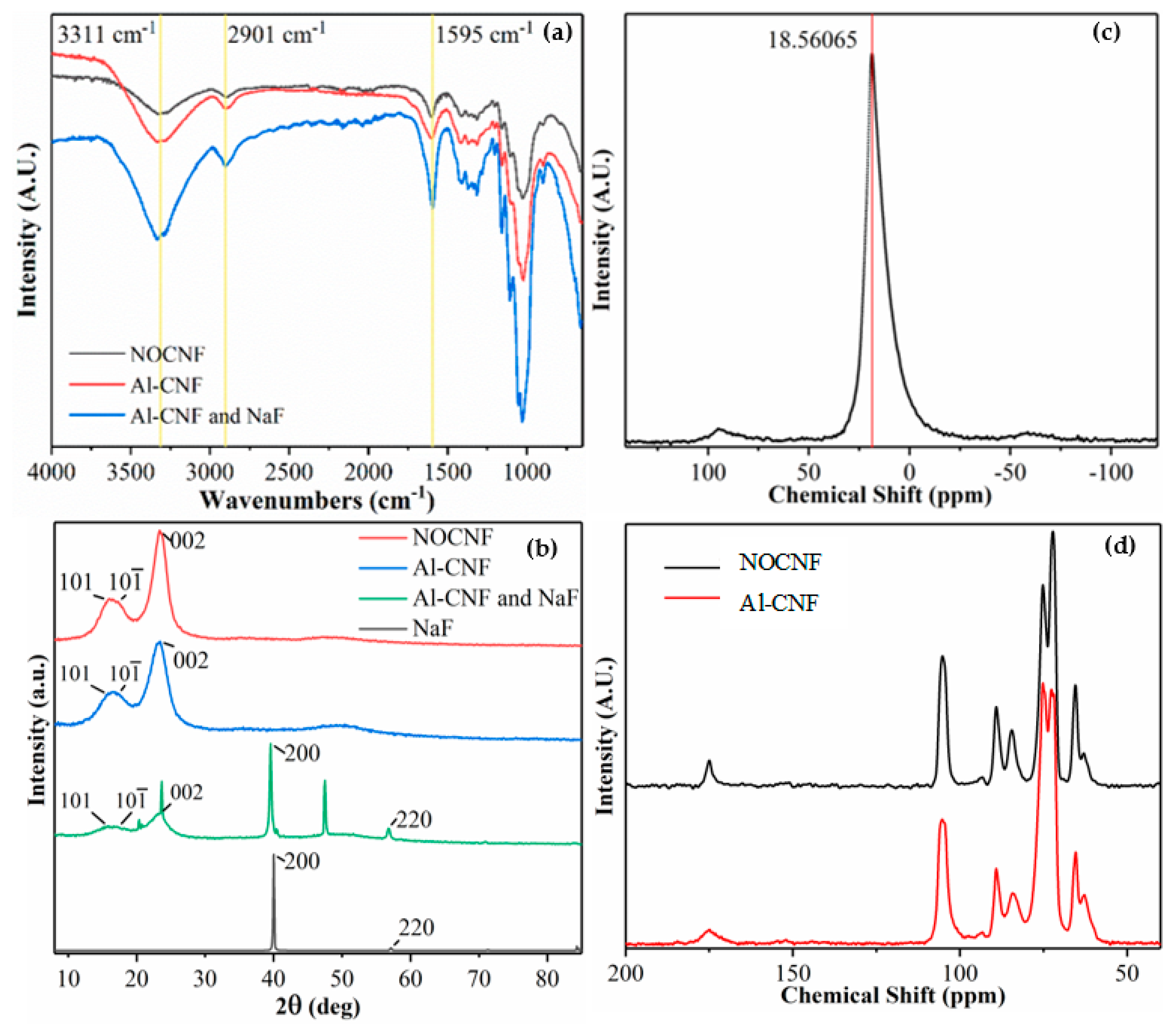
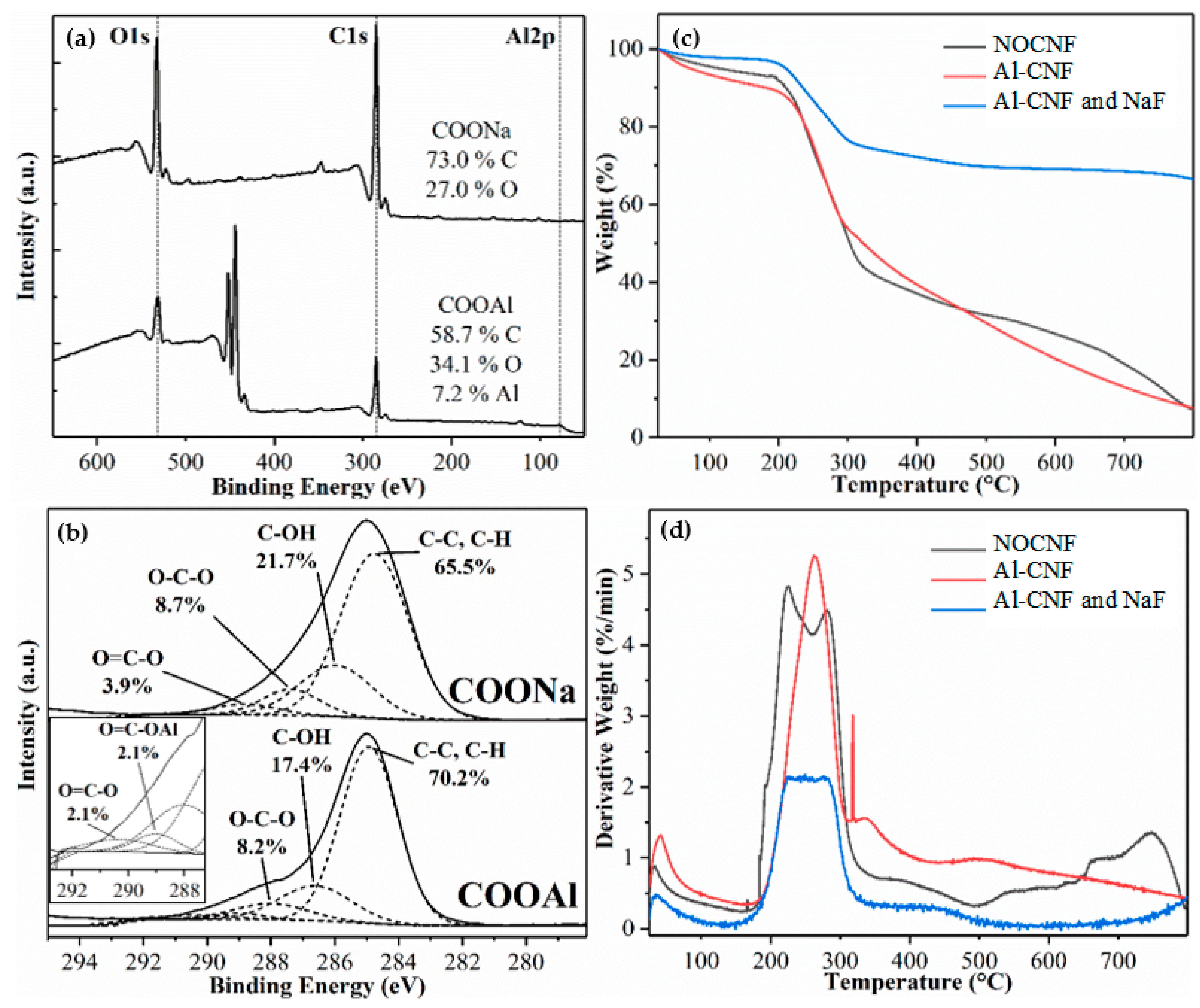
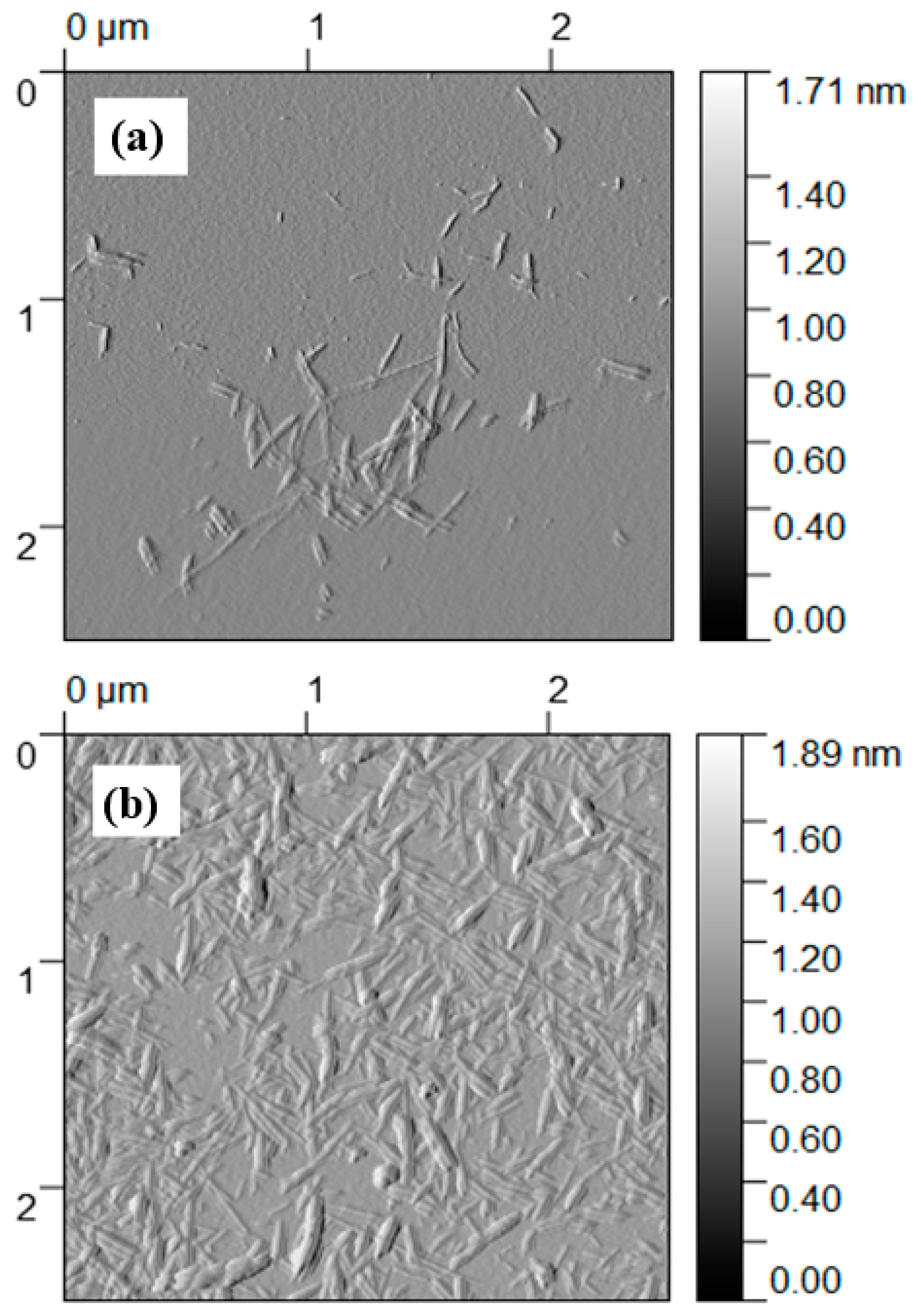
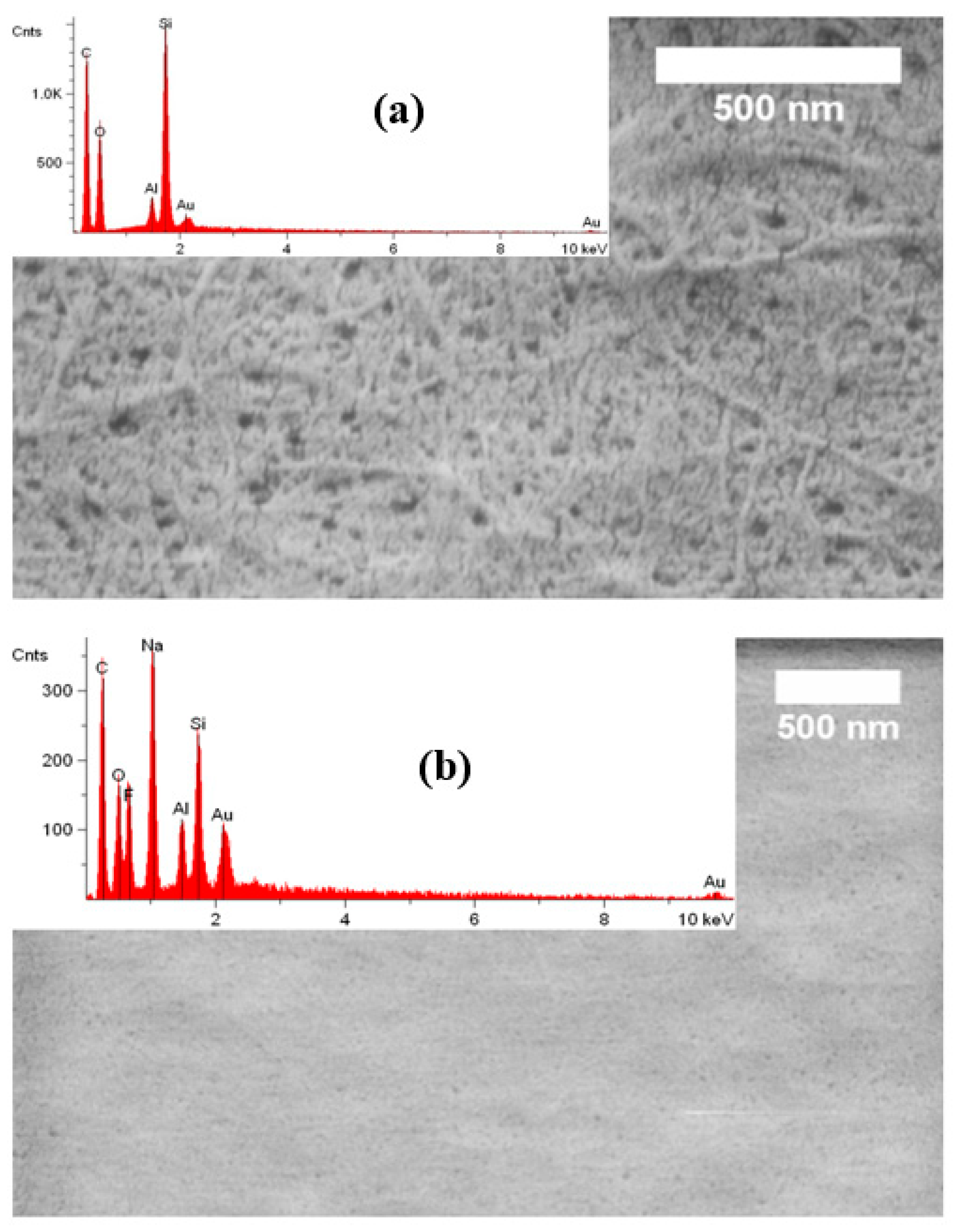
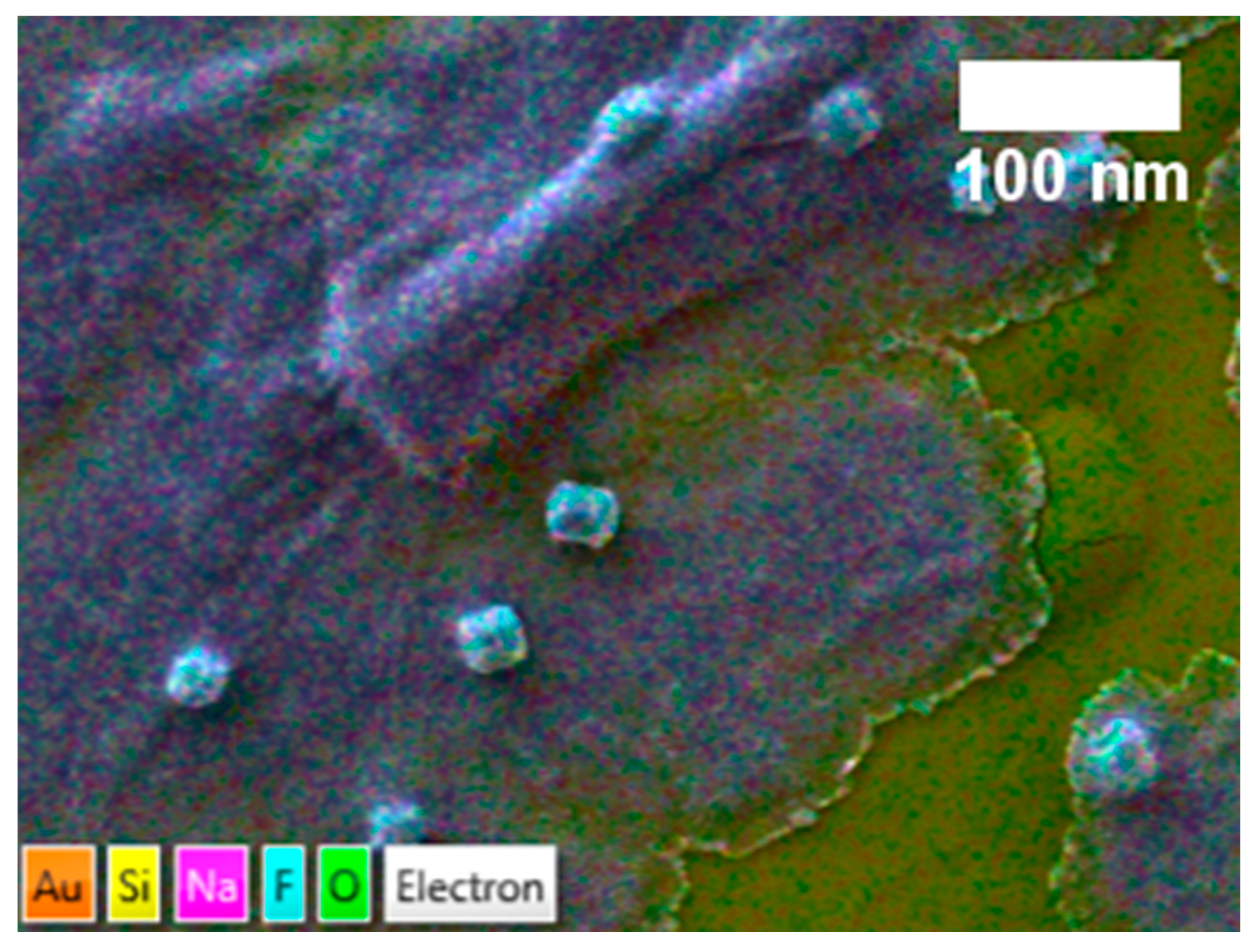
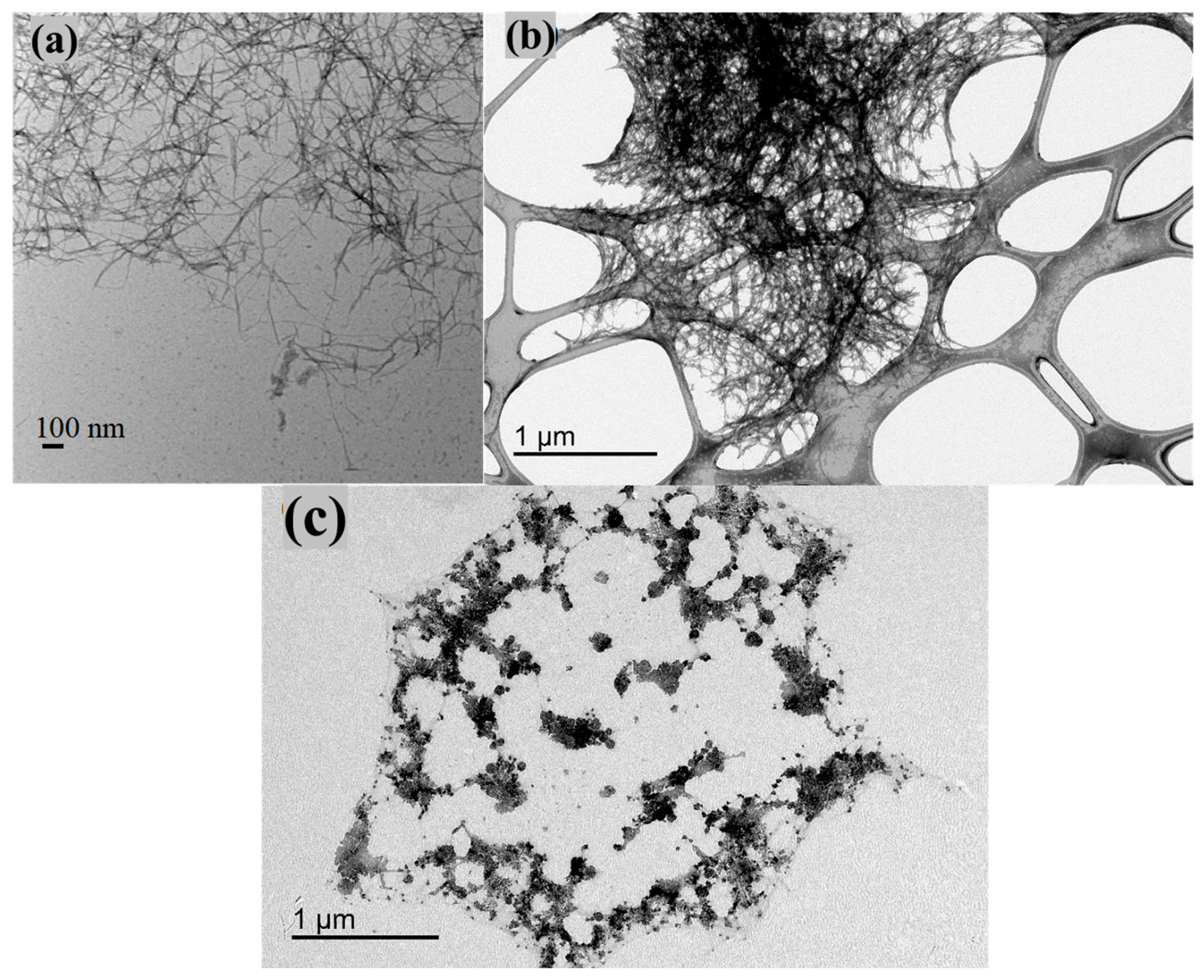
3.1. Structure and Functionality of NOCNF and Al-CNF
3.2. Fluoride Adsorption Study Using Al-CNF
3.3. Sequential Applicability and Comparative Studies of Al-CNF
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Drinking Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 20 May 2024).
- NIH. Fluoridated Water. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/myths/fluoridated-water-fact-sheet (accessed on 20 May 2024).
- Guissouma, W.; Hakami, O.; Al-Rajab, A.J.; Tarhouni, J. Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere 2017, 177, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315. [Google Scholar] [CrossRef]
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for sustainable water purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Lindström, T.; Khan, M.; Rezaei, M.; Hsiao, B.S. Nanocellulose preparation from diverse plant feedstocks, processes, and chemical treatments: A review emphasizing non-woods. BioResources 2024, 19, 1865–1924. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Onyango, M.S.; Kojima, Y.; Aoyi, O.; Bernardo, E.C.; Matsuda, H. Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci. 2004, 279, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Reinwald, M.; Jeihanipour, A.; Schäfer, A.I. Removal of fluoride and natural organic matter from natural tropical brackish waters by nanofiltration/reverse osmosis with varying water chemistry. Chemosphere 2019, 217, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Ku, Y.; Chiou, H.-M. The Adsorption of Fluoride Ion from Aqueous Solution by Activated Alumina. Water Air Soil Pollut. 2002, 133, 349–361. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- López Valdivieso, A.; Reyes Bahena, J.L.; Song, S.; Herrera Urbina, R. Temperature effect on the zeta potential and fluoride adsorption at the α-Al2O3/aqueous solution interface. J. Colloid Interface Sci. 2006, 298, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the Interpretation of X-ray diffraction powder patterns in terms of the nanostructure of Cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Haouas, M.; Taulelle, F.; Martineau, C. Recent advances in application of 27Al NMR spectroscopy to materials science. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 94, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
- Van Der Kamp, K.A.; Qiang, D.M.; Aburub, A.; Wurster, D.E. Modified Langmuir-like Model for Modeling the Adsorption from Aqueous Solutions by Activated Carbons. Langmuir 2005, 21, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ayoob, S.; Gupta, A.K.; Bhakat, P.B.; Bhat, V.T. Investigations on the kinetics and mechanisms of sorptive removal of fluoride from water using alumina cement granules. Chem. Eng. J. 2008, 140, 6–14. [Google Scholar] [CrossRef]
- Sujana, M.G.; Thakur, R.S.; Rao, S.B. Removal of Fluoride from Aqueous Solution by Using Alum Sludge. J. Colloid Interface Sci. 1998, 206, 94–101. [Google Scholar] [CrossRef]
- Tripathy, S.S.; Bersillon, J.-L.; Gopal, K. Removal of fluoride from drinking water by adsorption onto alum-impregnated activated alumina. Sep. Purif. Technol. 2006, 50, 310–317. [Google Scholar] [CrossRef]
- Islam, M.; Patel, R.K. Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J. Hazard. Mater. 2007, 143, 303–310. [Google Scholar] [CrossRef]
- Eskandarpour, A.; Onyango, M.S.; Ochieng, A.; Asai, S. Removal of fluoride ions from aqueous solution at low pH using schwertmannite. J. Hazard. Mater. 2008, 152, 571–579. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.K.N.; Labhsetwar, N.; Rayalu, S. Defluoridation of drinking water using chitosan based mesoporous alumina. Microporous Mesoporous Mater. 2011, 142, 454–463. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Reddy, A.S.; Krishnaiah, A. Synthesis and characterization of glutaraldehyde-crosslinked calcium alginate for fluoride removal from aqueous solutions. J. Appl. Polym. Sci. 2011, 120, 3443–3452. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, S.; Cao, A.; Zhao, D.; Zhang, X.; Xu, C.; Luan, Z.; Ruan, D.; Liang, J.; Wu, D.; et al. Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem. Phys. Lett. 2001, 350, 412–416. [Google Scholar] [CrossRef]
- Gai, W.Z.; Deng, Z.Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Jaroniec, M. Adsorption on heterogeneous surfaces: The exponential equation for the overall adsorption isotherm. Surf. Sci. 1975, 50, 553–564. [Google Scholar] [CrossRef]
- Douglas, L.M.; Vermeulen, T. Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 1981, 85, 3247–3250. [Google Scholar]
- Meenakshi, S.; Sundaram, C.S.; Sukumar, R. Enhanced fluoride sorption by mechanochemically activated kaolinites. J. Hazard. Mater. 2008, 153, 164–172. [Google Scholar] [CrossRef]
- Aoudj, S.; Drouiche, N.; Hecini, M.; Ouslimane, T.; Palaouane, B. Coagulation as a post-treatment method for the defluoridation of photovoltaic cell manufacturing wastewater. Procedia Eng. 2012, 33, 111–120. [Google Scholar] [CrossRef]
- Sivasankar, V.; Rajkumar, S.; Murugesh, S.; Darchen, A. Influence of shaking or stirring dynamic methods in the defluoridation behavior of activated tamarind fruit shell carbon. Chem. Eng. J. 2012, 197, 162–172. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Ghareeb, H.O.; Dannoun, E.M.A.; Hussen, S.A.; Hadi, J.M.; Ahmed, K.K.; Hussein, A.M. Characteristics of Poly(vinyl alcohol) (PVA) based composites integrated with green synthesized Al3+-metal complex: Structural, optical, and localized density of state analysis. Polymers 2021, 13, 1316. [Google Scholar] [CrossRef]
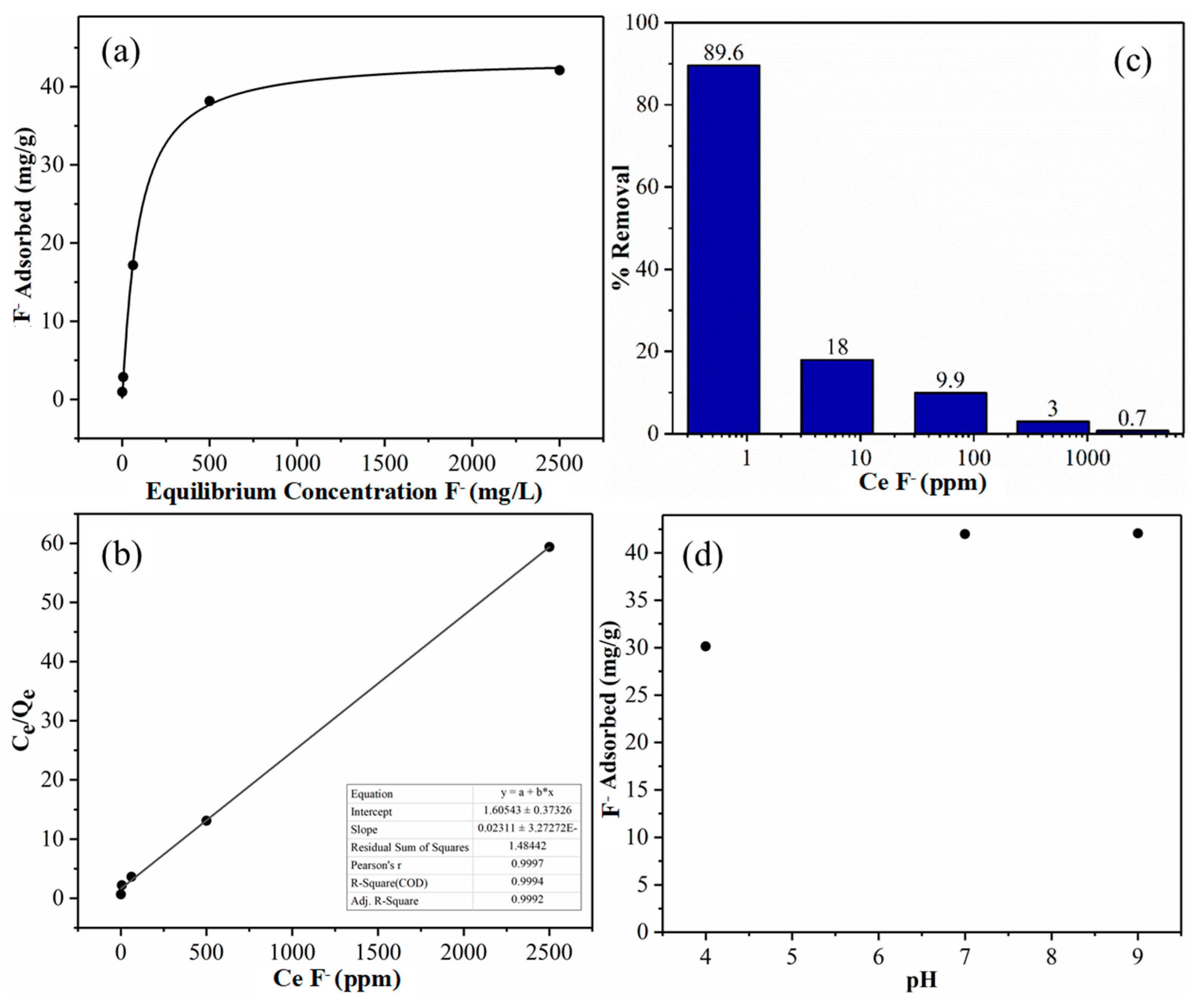
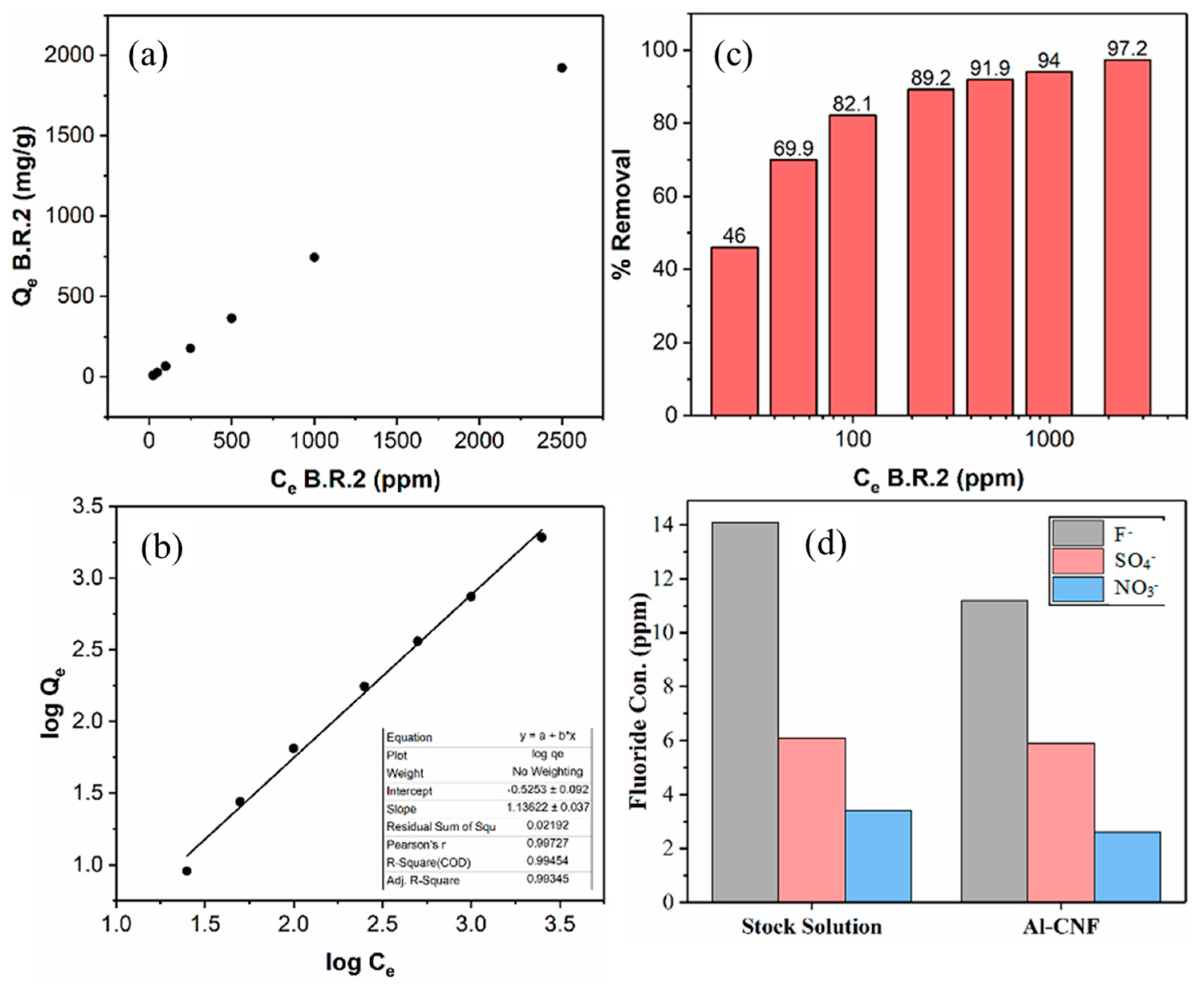
| Calc. Eqlbrm. Con. of Fluoride, Ce (ppm F−) | Con. of Water + Fluoride Sol. 1:1 (ppm F−) | Con. of Al-CNF (0.0760 wt%) + Fluoride Solution 1:1 (3 mL each) (ppm F−) | Diff. in Con. (ppm) | Percent Removal (%) | Fluoride Removed (mg) | Quantity of Al-CNF (g) | Qe (mg/g) | Ce/Qe |
|---|---|---|---|---|---|---|---|---|
| 0.625 | 0.4072 | 0.04239 | 0.3648 | 89.6 | 0.002189 | 0.00228 | 0.960 | 0.651 |
| 6.25 | 6.040 | 4.954 | 1.086 | 18.0 | 0.006516 | 0.00228 | 2.86 | 2.19 |
| 62.5 | 65.61 | 59.09 | 6.52 | 9.9 | 0.0391 | 0.00228 | 17.2 | 3.64 |
| 500 | 481.6 | 467.1 | 14.5 | 3.0 | 0.0870 | 0.00228 | 38.2 | 13.1 |
| 2500 | 2243 | 2227 | 16 | 0.7 | 0.096 | 0.00228 | 42.1 | 59.3 |
| Adsorbent | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Alum sludge | 5.4 | [21] |
| Activated alumina (γ-Al2O3) | 16.3 | [12] |
| Alum-impregnated activated alumina | 40.7 | [22] |
| Quick lime | 16.7 | [23] |
| Schwertmannite | 55.3 | [24] |
| Chitosan-based mesoporous alumina | 8.3 | [25] |
| Glutaraldehyde-crosslinked calcium alginate | 73.6 | [26] |
| Amorphous alumina supported on carbon nanotubes | 28.7 | [27] |
| Al-CNF | 43.6 | This study |
| pH | Dissolved Al3+ (ppb) |
|---|---|
| Acidic | 560 |
| Basic | 11.8 |
| Neutral | 2.7 |
| Calc. Equilibrium Con. of B.R.2, Ce (ppm) | Absorbance Measured at 518 nm of Water + B.R.2 Solution 1:1 (3 mL Each) | Absorbance Measured at 518 nm of Flocculated Al-CNF (0.1264 wt%) + B.R.2 Sol. 1:1 (3 mL Each) | Con. of Flocculated Al-CNF (0.126 wt%) + B.R.2 Sol. 1:1 (3 mL each) | Conc. Diff. (ppm) | Eff. (%) | B.R.2 Removed (mg) | Quantity of Flocculated Al-CNF (g) | Qe (mg/g) | Log Ce | Log Qe |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 0.1220 | 0.0147 | 13.51 | 11.49 | 46.0 | 0.035 | 0.00379 | 9.090 | 1.398 | 0.959 |
| 50 | 0.2447 | 0.0220 | 15.06 | 34.94 | 69.9 | 0.105 | 0.00379 | 27.64 | 1.699 | 1.442 |
| 100 | 0.5039 | 0.0354 | 17.91 | 82.09 | 82.1 | 0.246 | 0.00379 | 64.94 | 2.000 | 1.813 |
| 250 | 1.1850 | 0.0786 | 27.11 | 222.89 | 89.2 | 0.669 | 0.00379 | 176.3 | 2.398 | 2.246 |
| 500 | 2.2398 * | 0.1411 | 40.40 | 459.60 | 91.9 | 1.379 | 0.00379 | 363.6 | 2.699 | 2.561 |
| 1000 | 4.6762 * | 0.2340 | 60.17 | 939.83 | 94.0 | 2.819 | 0.00379 | 743.5 | 3.000 | 2.871 |
| 2500 | 10.9905 * | 0.2787 | 69.68 | 2430.32 | 97.2 | 7.291 | 0.00379 | 1923 | 3.398 | 3.284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.I.; Sharma, S.K.; Sharma, P.R.; Alhamzani, A.G.; Hsiao, B.S. Aluminum-Crosslinked Nanocellulose Scaffolds for Fluoride Removal. Nanomaterials 2024, 14, 1032. https://doi.org/10.3390/nano14121032
Johnson KI, Sharma SK, Sharma PR, Alhamzani AG, Hsiao BS. Aluminum-Crosslinked Nanocellulose Scaffolds for Fluoride Removal. Nanomaterials. 2024; 14(12):1032. https://doi.org/10.3390/nano14121032
Chicago/Turabian StyleJohnson, Ken I., Sunil K. Sharma, Priyanka R. Sharma, Abdulrahman G. Alhamzani, and Benjamin S. Hsiao. 2024. "Aluminum-Crosslinked Nanocellulose Scaffolds for Fluoride Removal" Nanomaterials 14, no. 12: 1032. https://doi.org/10.3390/nano14121032






