Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cs-Oleate
2.3. Synthesis of CsPbBr1.5Cl1.5 NCs
2.4. Fabrication of Devices
2.5. Characterization
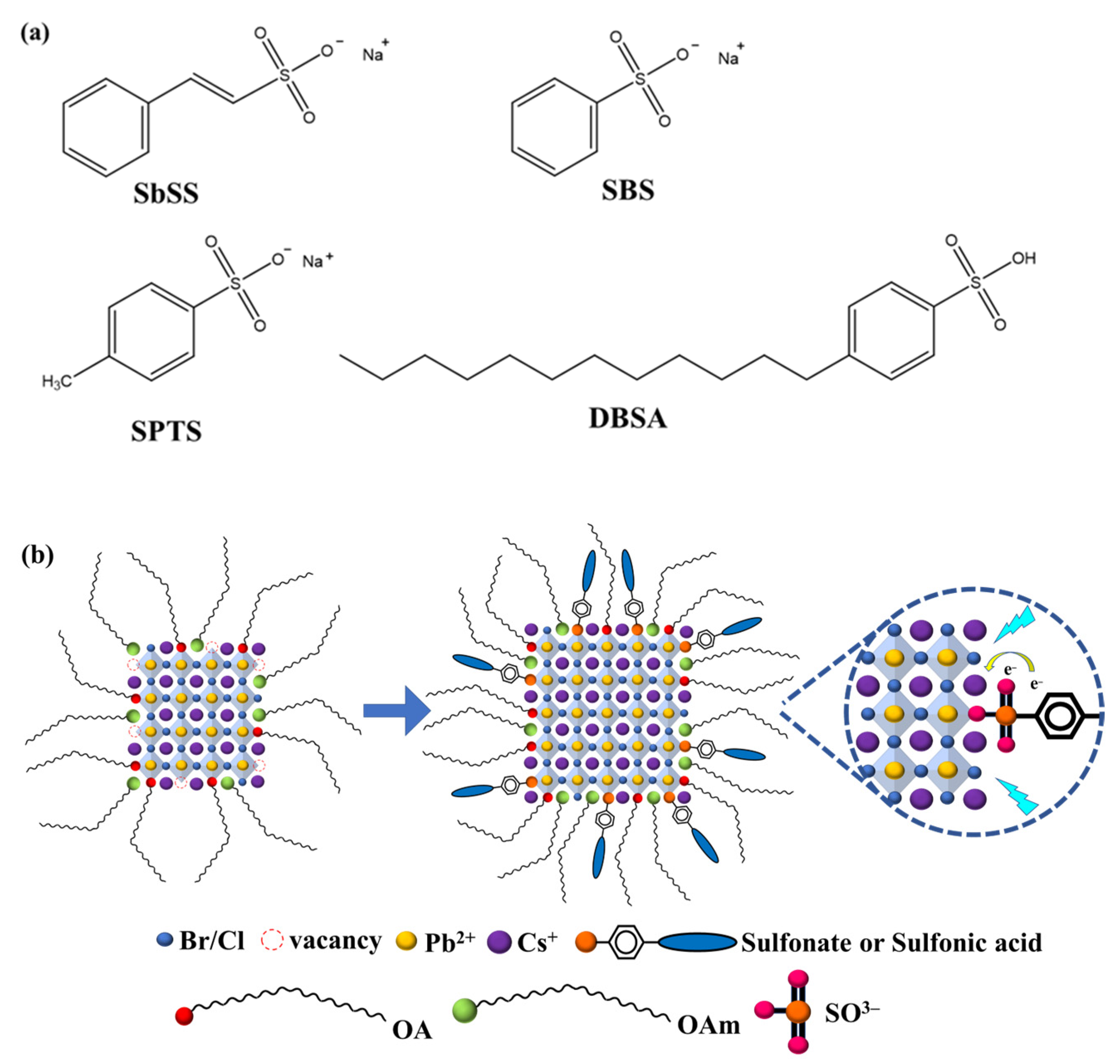
3. Results and Discussion
3.1. TEM and Elemental Distribution Analysis
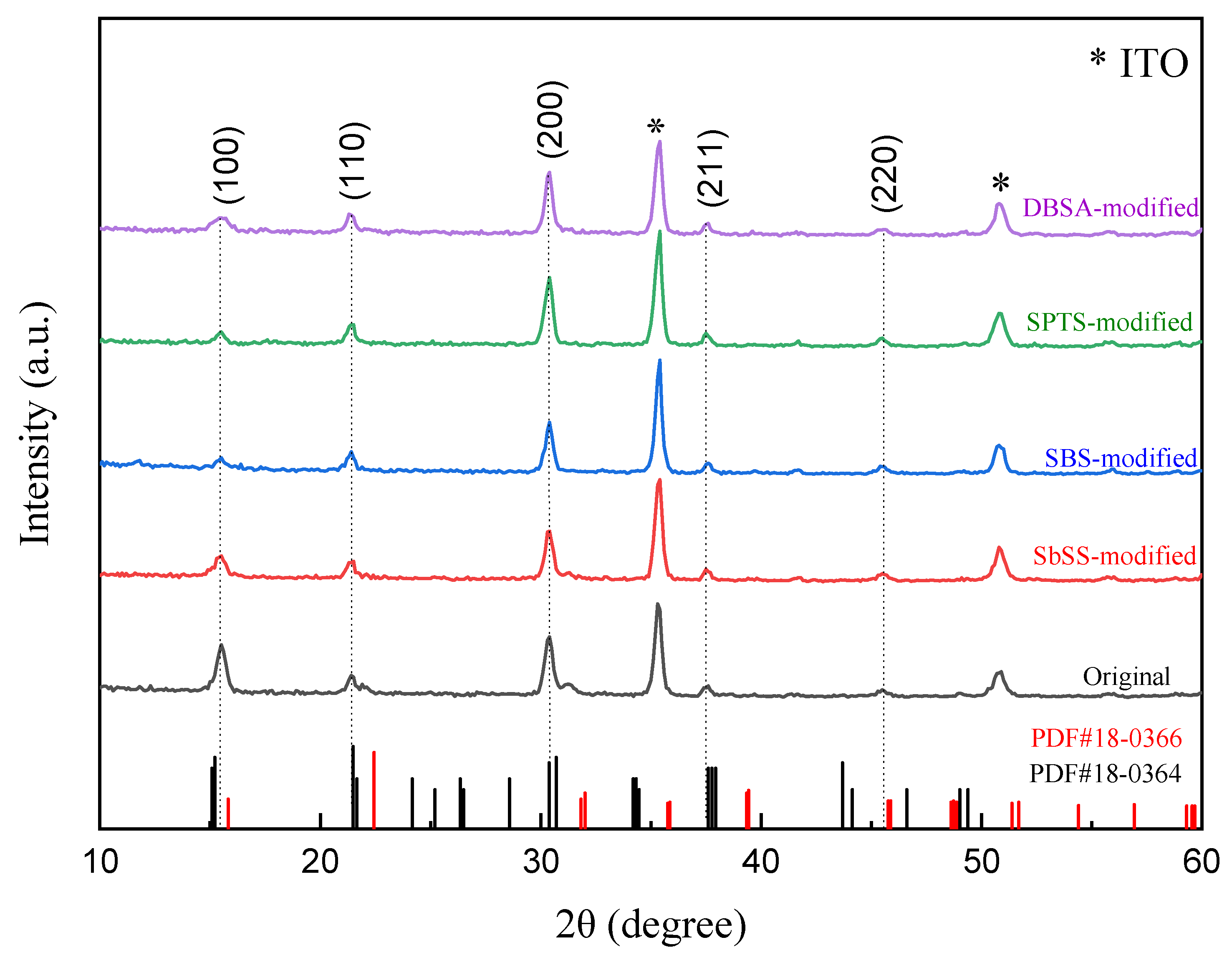
3.2. XRD Analysis
3.3. XPS Analysis
3.4. FT-IR Analysis
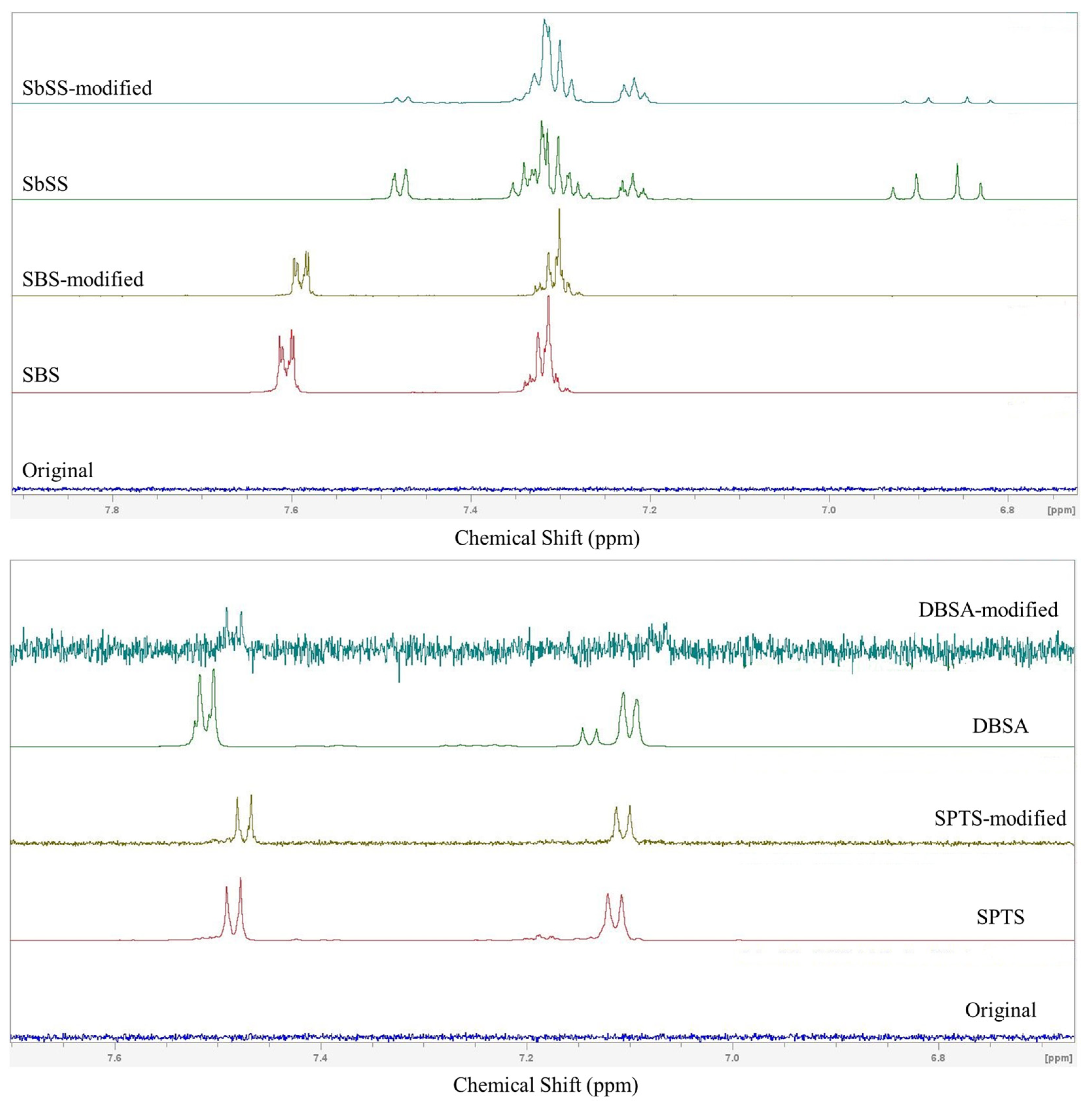
3.5. NMR Analysis
3.6. Optical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yuan, F.; Johnston, A.; Gao, C.; Choubisa, H.; Gao, Y.; Wang, Y.-K.; Sagar, L.K.; Sun, B.; Li, P.; et al. High Color Purity Lead-Free Perovskite Light-Emitting Diodes via Sn Stabilization. Adv. Sci. 2020, 7, 1903213. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-N.; Song, Y.; Yao, J.-S.; Wang, K.-H.; Wang, J.-J.; Zhu, B.-S.; Yao, M.-M.; Rahman, S.U.; Lan, Y.-F.; Fan, F.-J.; et al. Potassium Bromide Surface Passivation on CsPbI3−xBrx Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Su, R.; Du, W.; Liu, X.; Zhao, L.; Ha, S.T.; Xiong, Q. Advances in Small Perovskite-Based Lasers. Small Methods 2017, 1, 1700163. [Google Scholar] [CrossRef]
- Wang, F.; Zou, X.; Xu, M.; Wang, H.; Wang, H.; Guo, H.; Guo, J.; Wang, P.; Peng, M.; Wang, Z.; et al. Recent Progress on Electrical and Optical Manipulations of Perovskite Photodetectors. Adv. Sci. 2021, 8, 2100569. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Vighnesh, K.; Wang, S.; Liu, H.; Rogach, A.L. Hot-Injection Synthesis Protocol for Green-Emitting Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2022, 16, 19618–19625. [Google Scholar] [CrossRef]
- Ye, S.; Yu, M.; Zhao, M.; Song, J.; Qu, J. Low temperature synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals in open air and their upconversion luminescence. J. Alloys Compd. 2018, 730, 62–70. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals with Tunable Composition and Thickness by Ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Grasmik, V.; Wark, M.; Mudring, A.-V. Microwave-Assisted Synthesis of Perovskite SrSnO3 Nanocrystals in Ionic Liquids for Photocatalytic Applications. Inorg. Chem. 2017, 56, 6920–6932. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, A.; Yu, J.C.; Nam, Y.S.; Choi, Y.; Park, J.; Song, M.H. Surface Ligand Engineering for Efficient Perovskite Nanocrystal-Based Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 8428–8435. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Luan, W.; Liu, M.; Turyanska, L. DDAB-assisted synthesis of iodine-rich CsPbI3 perovskite nanocrystals with improved stability in multiple environments. J. Mater. Chem. C 2020, 8, 2381–2387. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Wu, Y.; Wei, C.; Qin, Z.; Zhang, C.; Sun, Z.; Li, Y.; Wang, Y.; Zeng, H. Surface Halogen Compensation for Robust Performance Enhancements of CsPbX3 Perovskite Quantum Dots. Adv. Opt. Mater. 2019, 7, 1900276. [Google Scholar] [CrossRef]
- Fang, T.; Yuan, S.; Li, X.; Shan, Q.; Zhou, Y.; Xu, B.; Liu, G.; Zhang, S.; Li, X.; Li, W.; et al. Sulfonate Additive Simultaneously Suppresses Interstitials and Vacancies Toward Efficient and Stable Perovskite Quantum Dot LEDs. Adv. Opt. Mater. 2024, 12, 2302253. [Google Scholar] [CrossRef]
- Guo, J.; Lu, M.; Zhang, X.; Sun, S.; Han, C.; Zhang, Y.; Yang, X.; Kershaw, S.V.; Zheng, W.; Rogach, A.L. Highly Stable and Efficient Light-Emitting Diodes Based on Orthorhombic γ-CsPbI3 Nanocrystals. ACS Nano 2023, 17, 9290–9301. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cao, M.; Zhong, Q.; Li, P.; Zhang, X.; Zhang, Q. All-inorganic cesium lead halide perovskite nanocrystals: Synthesis, surface engineering and applications. J. Mater. Chem. C 2019, 7, 757–789. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Akhtarianfar, S.F.; Shojaei, S. Role of Methylammonium Rotation in Hybrid Halide MAPbX3 (X = I, Br, and Cl) Perovskites by a Density Functional Theory Approach: Optical and Electronic Properties. J. Phys. Chem. C 2019, 123, 6725–6734. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, Y.; Zeng, S.; Cui, Y.; Xiao, Y. Surface Regulation of CsPbBr3 Quantum Dots for Standard Blue-Emission with Boosted PLQY. Adv. Opt. Mater. 2020, 8, 2000167. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, S.; Liu, J.; Yin, J.; Yuan, F.; Shen, W.-S.; Yao, K.; Wei, M.; Zhou, C.; Song, K.; et al. Chlorine Vacancy Passivation in Mixed Halide Perovskite Quantum Dots by Organic Pseudohalides Enables Efficient Rec. 2020 Blue Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 793–798. [Google Scholar] [CrossRef]
- Luo, C.; Li, W.; Xiong, D.; Fu, J.; Yang, W. Surface pre-optimization of a mixed halide perovskite toward high photoluminescence quantum yield in the blue spectrum range. Nanoscale 2019, 11, 15206–15215. [Google Scholar] [CrossRef]
- Li, N.; Jia, Y.; Guo, Y.; Zhao, N. Ion Migration in Perovskite Light-Emitting Diodes: Mechanism, Characterizations, and Material and Device Engineering. Adv. Mater. 2022, 34, 2108102. [Google Scholar] [CrossRef]
- Cho, J.; Kamat, P.V. Photoinduced Phase Segregation in Mixed Halide Perovskites: Thermodynamic and Kinetic Aspects of Cl–Br Segregation. Adv. Opt. Mater. 2021, 9, 2001440. [Google Scholar] [CrossRef]
- Park, C.B.; Shin, Y.S.; Yoon, Y.J.; Jang, H.; Son, J.G.; Kim, S.; An, N.G.; Kim, J.W.; Jun, Y.C.; Kim, G.-H.; et al. Suppression of halide migration and immobile ionic surface passivation for blue perovskite light-emitting diodes. J. Mater. Chem. C 2022, 10, 2060–2066. [Google Scholar] [CrossRef]
- Luo, C.; Yan, C.; Li, W.; Chun, F.; Xie, M.; Zhu, Z.; Gao, Y.; Guo, B.; Yang, W. Ultrafast Thermodynamic Control for Stable and Efficient Mixed Halide Perovskite Nanocrystals. Adv. Funct. Mater. 2020, 30, 2000026. [Google Scholar] [CrossRef]
- Wang, X.; Bai, T.; Meng, X.; Ji, S.; Zhang, R.; Zheng, D.; Yang, B.; Jiang, J.; Han, K.; Liu, F. Filling Chlorine Vacancy with Bromine: A Two-Step Hot-Injection Approach Achieving Defect-Free Hybrid Halogen Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2022, 14, 46857–46865. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.A.; Huang, Y.; Bricker, N.A.; Juhl, C.S.; Milstein, T.J.; MacKenzie, J.D.; Luscombe, C.K.; Gamelin, D.R. Modular Zwitterion-Functionalized Poly(isopropyl methacrylate) Polymers for Hosting Luminescent Lead Halide Perovskite Nanocrystals. Chem. Mater. 2021, 33, 3779–3790. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.; Chang, Y.; Guo, X.; Zou, D.; Sun, Y.; Zeng, Q.; Liu, X. Ultra-small-size highly efficient and stable CsPbBr3 quantum dots synthesized by using a cesium-dodecyl benzene sulfonic acid solution. Chem. Eng. J. 2023, 473, 145213. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, Y.; Fu, Y.; Zheng, Y.; Shao, Y.; Bai, Y. Understanding and minimizing non-radiative recombination losses in perovskite light-emitting diodes. J. Mater. Chem. C 2022, 10, 13590–13610. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zeng, H. Highly Luminescent and Stable Halide Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 673–681. [Google Scholar] [CrossRef]
- Kuan, C.-H.; Yang, S.-H. Surface ligand engineering of perovskite nanocrystals with a conjugated sulfonate ligand for light-emitting applications. Mater. Adv. 2022, 3, 7824–7832. [Google Scholar] [CrossRef]
- Sun, S.; Jia, P.; Huang, Q.; Lu, M.; Lu, P.; Qin, F.; Sun, G.; Gao, Y.; Bai, X.; Wu, Z.; et al. Spectrally stable and efficient pure-blue light-emitting diodes based on CsPb(Br/Cl)3 nanocrystals with surface passivation by organic sulfonate. Mater. Today Phys. 2022, 28, 100899. [Google Scholar] [CrossRef]
- Davis, A.H.; Li, S.; Lin, H.; Chu, C.; Franck, J.M.; Leem, G.; Maye, M.M.; Zheng, W. Ligand-mediated synthesis of chemically tailored two-dimensional all-inorganic perovskite nanoplatelets under ambient conditions. J. Mater. Chem. C 2021, 9, 14226–14235. [Google Scholar] [CrossRef]
- Feng, X.; Ju, H.; Song, T.; Fang, T.; Liu, W.; Huang, W. Highly Efficient Photocatalytic Degradation Performance of CsPb(Br1−xClx)3-Au Nanoheterostructures. ACS Sustain. Chem. Eng. 2019, 7, 5152–5156. [Google Scholar] [CrossRef]
- Long, Z.; Yang, S.; Pi, J.; Zhou, D.; Wang, Q.; Yang, Y.; Wu, H.; Qiu, J. All-inorganic halide perovskite (CsPbX3, X = Cl, Br, I) quantum dots synthesized via fast anion hot injection by using trimethylhalosilanes. Ceram. Int. 2022, 48, 35474–35479. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Shang, Y.; Zhou, W.; Zhang, W.; Yang, S. Surface Passivation of Perovskite Film by Sodium Toluenesulfonate for Highly Efficient Solar Cells. Sol. RRL 2020, 4, 2000113. [Google Scholar] [CrossRef]
- Cao, J.; Yin, Z.; Pang, Q.; Lu, Y.; Nong, X.; Zhang, J.Z. Modulating optical properties and interfacial electron transfer of CsPbBr3 perovskite nanocrystals via indium ion and chlorine ion co-doping. J. Chem. Phys. 2021, 155, 234701. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Braterman, P.S. High affinity of dodecylbenzene sulfonate for layered double hydroxide and resulting morphological changes. J. Mater. Chem. 2003, 13, 268–273. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Chemical changes of heat treated pine and eucalypt wood monitored by FTIR. Maderas-Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, L.; Chen, Y.; Geng, C.; Shi, S.; Zhang, Z.; Bi, W.; Xu, S. Amine- and Acid-Free Synthesis of Stable CsPbBr3 Perovskite Nanocrystals. Chem. Mater. 2020, 32, 1904–1913. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y.-H.; Xu, H.; Nagane, S.; Wexler, R.B.; Kim, D.-H.; et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Niu, J.; Zhang, H.; Lou, S.; Gao, C.; Zhan, Y.; Zhang, X.; Jin, Q.; Zheng, L. Temperature-dependent photoluminescence spectra and decay dynamics of MAPbBr3 and MAPbI3 thin films. AIP Adv. 2018, 8, 095108. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Wang, H.-Y.; Zhang, Z.-Y.; Zhang, Y.; Sun, C.; Yue, Y.-Y.; Wang, L.; Chen, Q.-D.; Sun, H.-B. Photoluminescence quenching of inorganic cesium lead halides perovskite quantum dots (CsPbX3) by electron/hole acceptor. Phys. Chem. Chem. Phys. 2017, 19, 1920–1926. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-H.; Yang, S.-H.; Tsai, W.-C.; Hsu, H.-C. Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands. Nanomaterials 2024, 14, 1049. https://doi.org/10.3390/nano14121049
Huang S-H, Yang S-H, Tsai W-C, Hsu H-C. Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands. Nanomaterials. 2024; 14(12):1049. https://doi.org/10.3390/nano14121049
Chicago/Turabian StyleHuang, Shu-Han, Sheng-Hsiung Yang, Wen-Cheng Tsai, and Hsu-Cheng Hsu. 2024. "Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands" Nanomaterials 14, no. 12: 1049. https://doi.org/10.3390/nano14121049
APA StyleHuang, S.-H., Yang, S.-H., Tsai, W.-C., & Hsu, H.-C. (2024). Enhancing Optical and Thermal Stability of Blue-Emitting Perovskite Nanocrystals through Surface Passivation with Sulfonate or Sulfonic Acid Ligands. Nanomaterials, 14(12), 1049. https://doi.org/10.3390/nano14121049






