Perspectives on Advanced Lithium–Sulfur Batteries for Electric Vehicles and Grid-Scale Energy Storage
Abstract
:1. Introduction
2. Major Challenges and Solutions
2.1. Shuttle Effect
2.1.1. Electrodes
2.1.2. Electrolytes
2.1.3. Binders
2.1.4. Interlayers
2.2. Reaction Kinetics
2.2.1. Conductivity
2.2.2. Electrocatalysis
2.3. Anodes
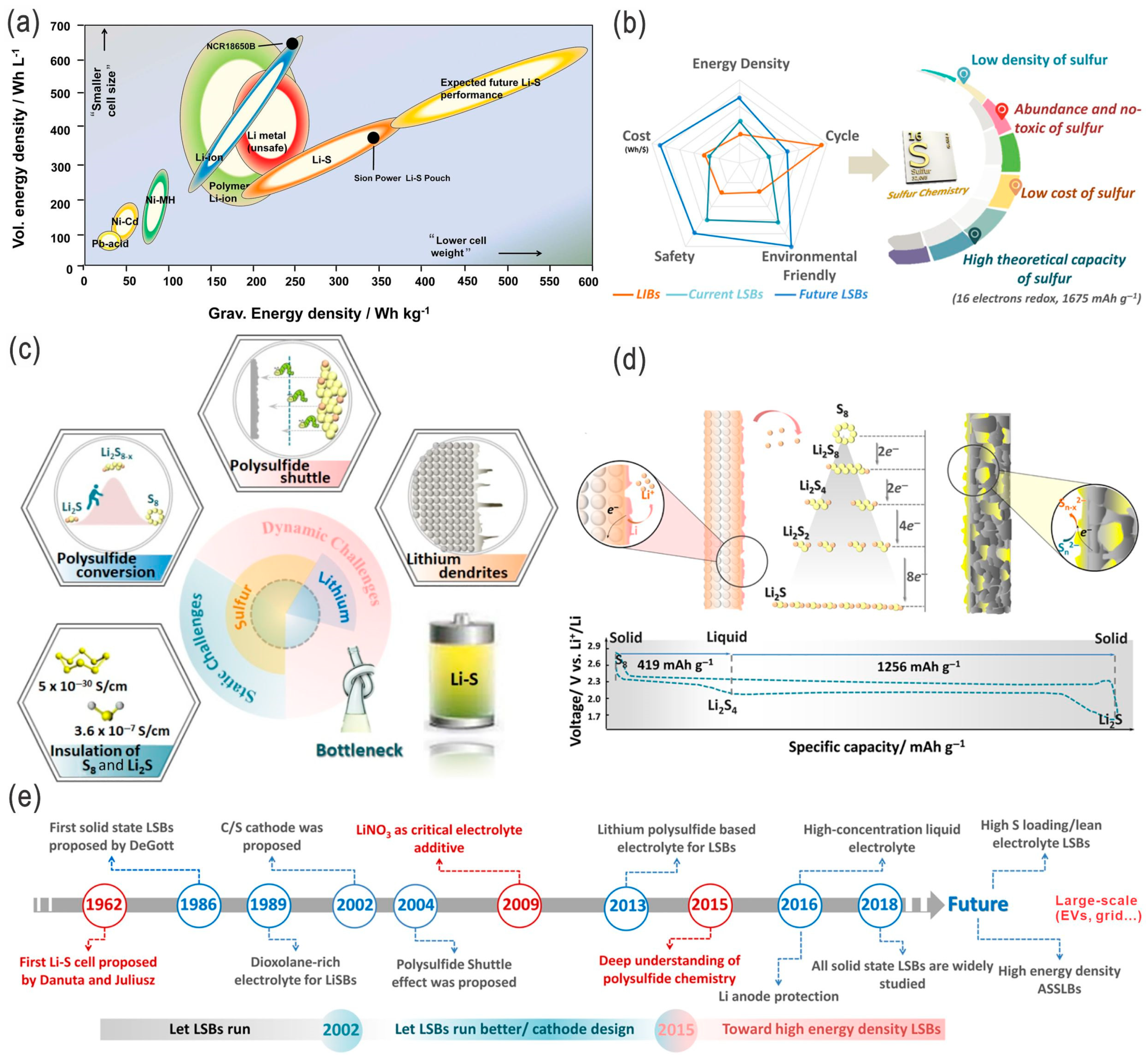
3. Assessments
3.1. Characterizations
3.2. Cost-Effectiveness
3.3. Battery Management and Modeling
3.4. Environmental Adaptability
4. Applications
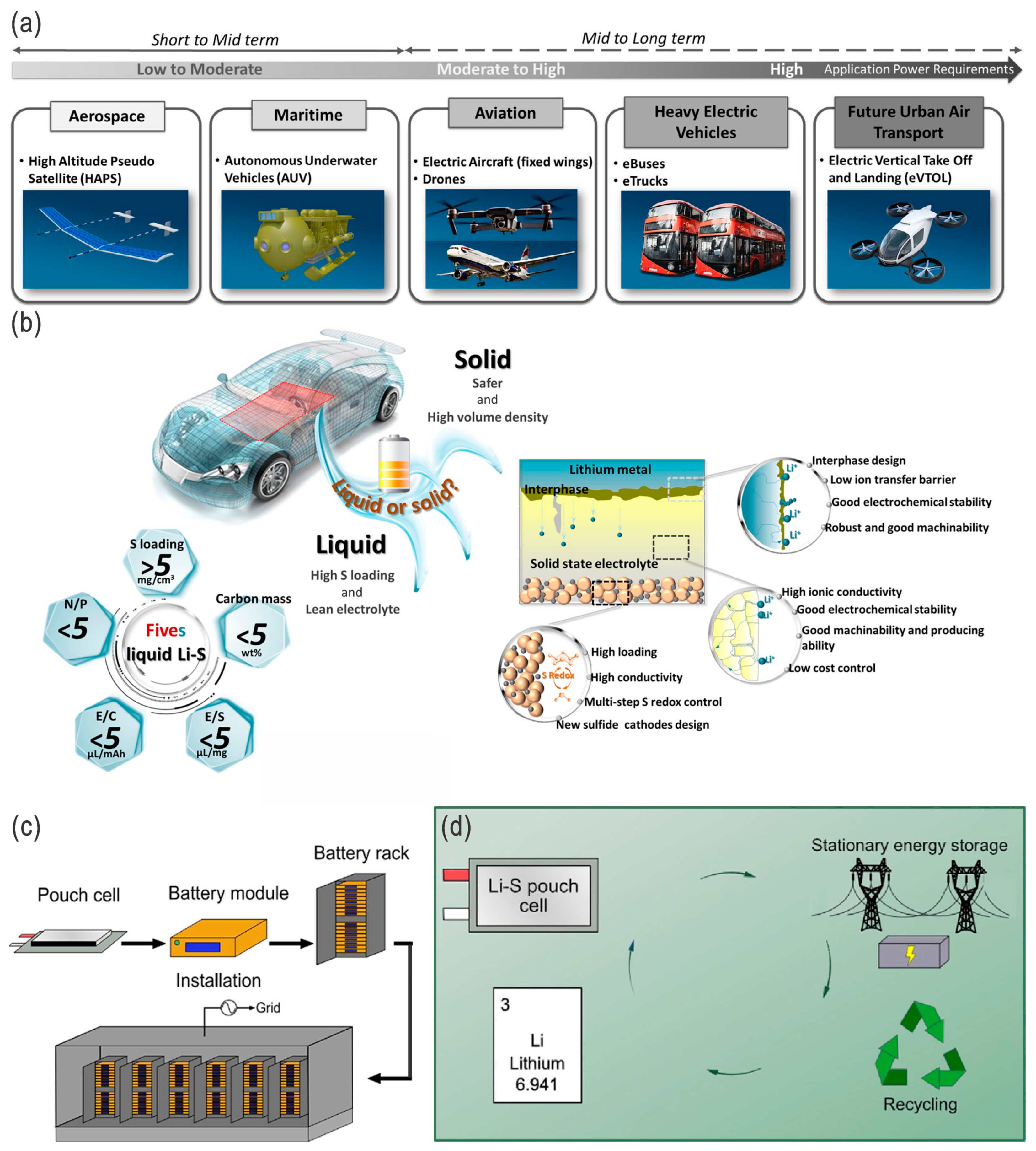
5. Conclusions and Perspectives
- The nanoengineering and nanocompositing of cathodes are of great importance to improve the capacity, energy density, and power density of the full cell. For thick cathodes with desirably high active material loading, 1D carbon nanotubes are superior to carbon black particles due to their enhanced electrical and mechanical properties. Bipolar electrodes with active materials on both sides, rather than unipolar electrodes, directly increase the energy density of the LSBs, although better adhesion properties are required during the cell assembly.
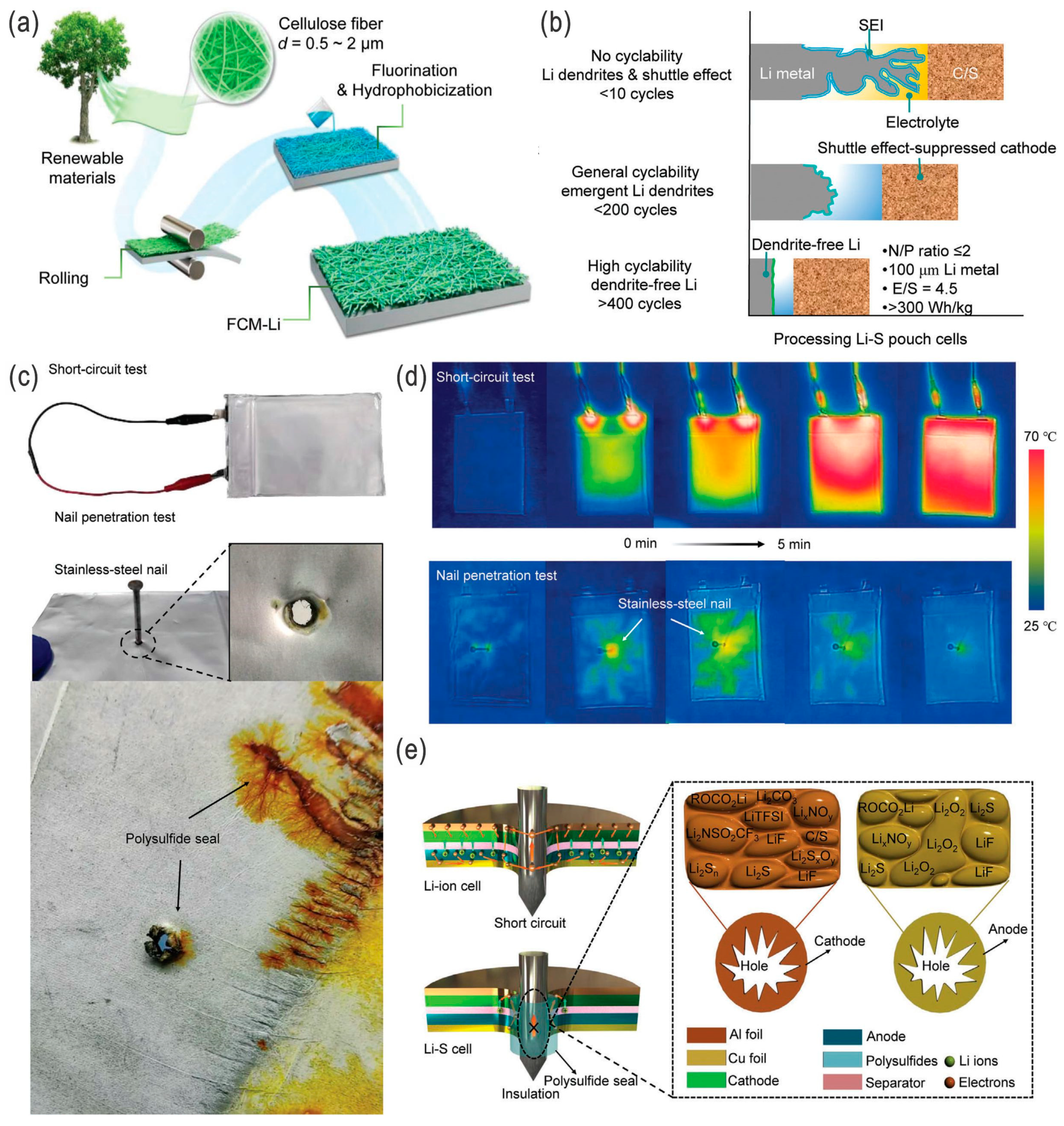
- The introduction of interlayers is a strategy to suppress the polysulfide shuttling effect during cycling; however, these interlayers add weight to the cell and, thus, reduce the practical energy densities. Decreasing the thickness of the interlayers or increasing the functionalities of the interlayers are alternative solutions for a higher practical specific energy, e.g., via coating the composite cathodes or introducing redox-active interlayers, or compositing with separators and/or cathodes for an integrated subsystem. Introduction of the interlayer on the lithium metal anode side is a critical alternative solution to address or suppress the dendrite issue, where the nucleation and growth of lithium are controlled and the soft short-circuit thereof is avoided. However, for large-scale energy storage systems, rigid inorganic interlayers are not versatile, and flexible polymeric or gel-type interlayers, whether all-solid-state or not, are more applicable for these applications, including EVs, stationary energy storage, and smart grids.
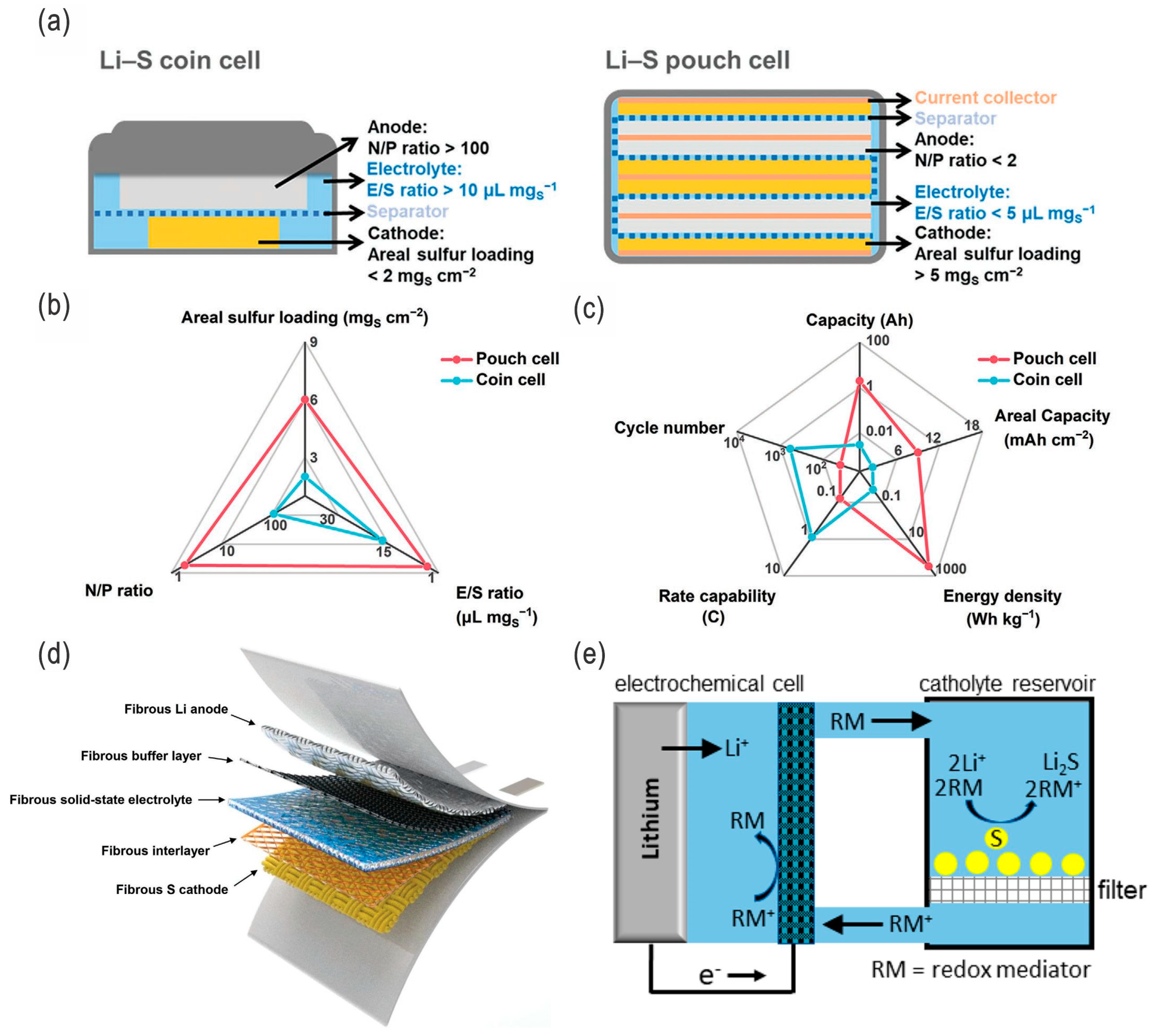
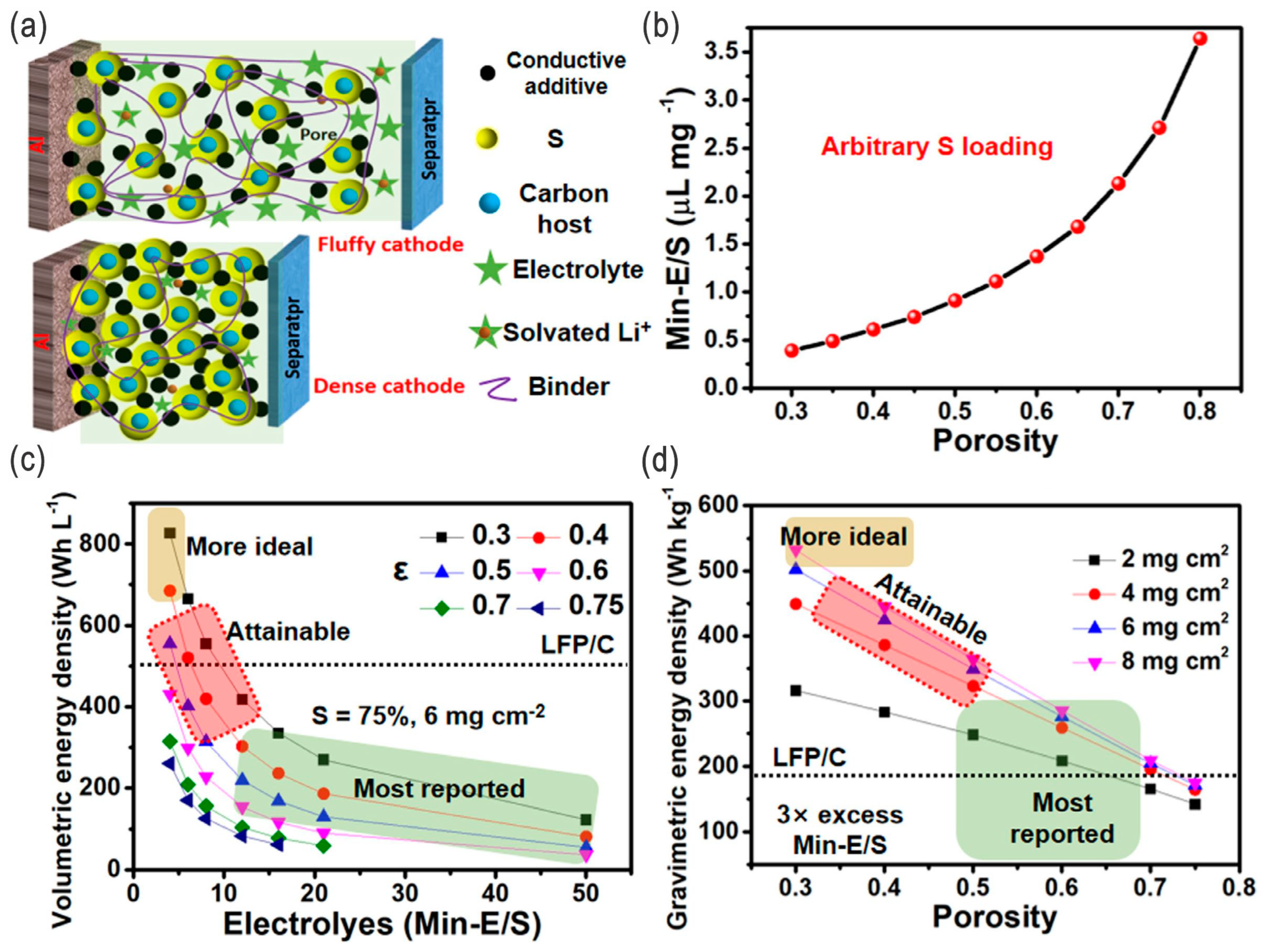
- Lean electrolytes are critical to elevate the energy densities; (extremely) high-concentration electrolytes (e.g., >10 M) and conventional diluted electrolytes (e.g., 1 M) with appropriate fluorides as additives/cosolvents are also alternative strategies. For the high-concentration electrolytes or fluorinated electrolytes, the polysulfides’ dissolution/shuttling and/or the lithium dendrite issue can be effectively alleviated. However, the ultrahigh concentration or high-fluorinated electrolytes/additives will increase the cost of the batteries and, thus, undermine their competiveness. Overall, they should be balanced, and moderate-fluorinated additives may be a more promising candidate.
- LSBs will be safer for EVs than conventional LIBs; owing to the elimination of oxygen from metallic oxide cathodes and the thermal runaway thereof, their cycle lifetime might not be as long as that of conventional LIBs. Although LSBs show higher specific energy than state-of-the-art EV cells, there are many other important factors, such as power (output and charging) and cycle life, which are not sufficiently on par with them.
- For large-scale batteries, the lithium plate or foil may not quite be applicable to achieve controlled lithium nucleation and growth to avoid the dendrite problem as well as to achieve a higher utilization ratio of active materials (e.g., lithium and sulfur) in the topological space, mesh, or (porous) carbon cloth/paper. In ideal conditions, embedding active materials such as sulfur, Li2S, LPSs, and Li in porous carbon cloth/paper sandwiched by polymer-type electrolytes may be the optimal strategy for eventual practical LSBs with high energy/power densities, high cost-effectiveness, scalability, and flexible properties.
- Although LSBs are relatively eco-friendly and safer compared with LIBs, under severe conditions, such as thermal runaway and explosion, LSBs will release more toxic SO2 gas, in addition to the organic pollutants from electrolytes. All-solid-state LSBs that solve both the LPS shuttling and dendritic growth issues would be the ideal choice for EVs and other transport systems as well as grid storage in the future, while in the near future electrodes/electrolytes with high sulfur loading and lean electrolytes in liquid-type LSBs will be more feasible due to their low cost and high energy density.
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D/2D/3D | One/two/three-dimensional |
| CE | Coulombic efficiency |
| CNTs | Carbon nanotubes |
| LIBs | Lithium-ion batteries or Li-ion batteries |
| LPSs | Lithium polysulfides |
| LSBs | Lithium–sulfur batteries or Li–S batteries |
| NC | Nitrogen-doped carbon |
| OCV | Open-circuit voltage |
| PVDF | Polyvinylidene fluoride |
| rGO | Reduced graphene oxide |
| SAC | Single-atom catalyst |
| SEI | Solid–electrolyte interface |
| SSLSBs | Solid-state Li–S batteries |
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; Seh, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Yao, W.; Liao, K.; Lai, T.; Sul, H.; Manthiram, A. Rechargeable metal-sulfur batteries: Key materials to mechanisms. Chem. Rev. 2024, 124, 4935–5118. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, T.; Gao, Y.; Pan, Z.; Hu, R.; Wang, J. Will lithium-sulfur batteries be the next beyond-lithium ion batteries and even much better? InfoMat 2022, 4, e12359. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.Z.; Chung, S.H.; Zu, C.X.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Trento, C. Lithium-Sulfur Batteries vs. Lithium-Ion Batteries: A Comparative Analysis. Available online: https://www.samaterials.com/lithium-sulfur-batteries-lithium-ion-batteries.html (accessed on 3 June 2024).
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Wickerts, S.; Arvidsson, R.; Nordelöf, A.; Svanström, M.; Johansson, P. Prospective life cycle assessment of lithium-sulfur batteries for stationary energy storage. ACS Sustain. Chem. Eng. 2023, 11, 9553–9563. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, X.-Q.; Huang, J.-Q.; Liu, Q.; Zhang, Q. Lithium-anode protection in lithium–sulfur batteries. Trends Chem. 2019, 1, 693–704. [Google Scholar] [CrossRef]
- Hagen, M.; Hanselmann, D.; Ahlbrecht, K.; Maça, R.; Gerber, D.; Tübke, J. Lithium–sulfur cells: The gap between the state-of-the-art and the requirements for high energy battery cells. Adv. Energy Mater. 2015, 5, 1401986. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhao, M.; Hou, L.-P.; Zhang, X.-Q.; Li, B.-Q.; Huang, J.-Q. Toward practical high-energy-density lithium–sulfur pouch cells: A review. Adv. Mater. 2022, 34, 2201555. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, Q.; Zhang, Q.; Cui, Y.; Zheng, Z. Fibrous materials for flexible Li–S battery. Adv. Energy Mater. 2021, 11, 2002580. [Google Scholar] [CrossRef]
- Meyerson, M.L.; Rosenberg, S.G.; Small, L.J. A mediated Li–S flow battery for grid-scale energy storage. ACS Appl. Energy Mater. 2022, 5, 4202–4211. [Google Scholar] [CrossRef]
- Ni, W.; Shi, L.-Y. 14—Graphene–sulfur nanocomposites as cathode materials and separators for lithium–sulfur batteries. In Lithium-Sulfur Batteries; Gupta, R.K., Nguyen, T.A., Song, H., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 289–314. [Google Scholar]
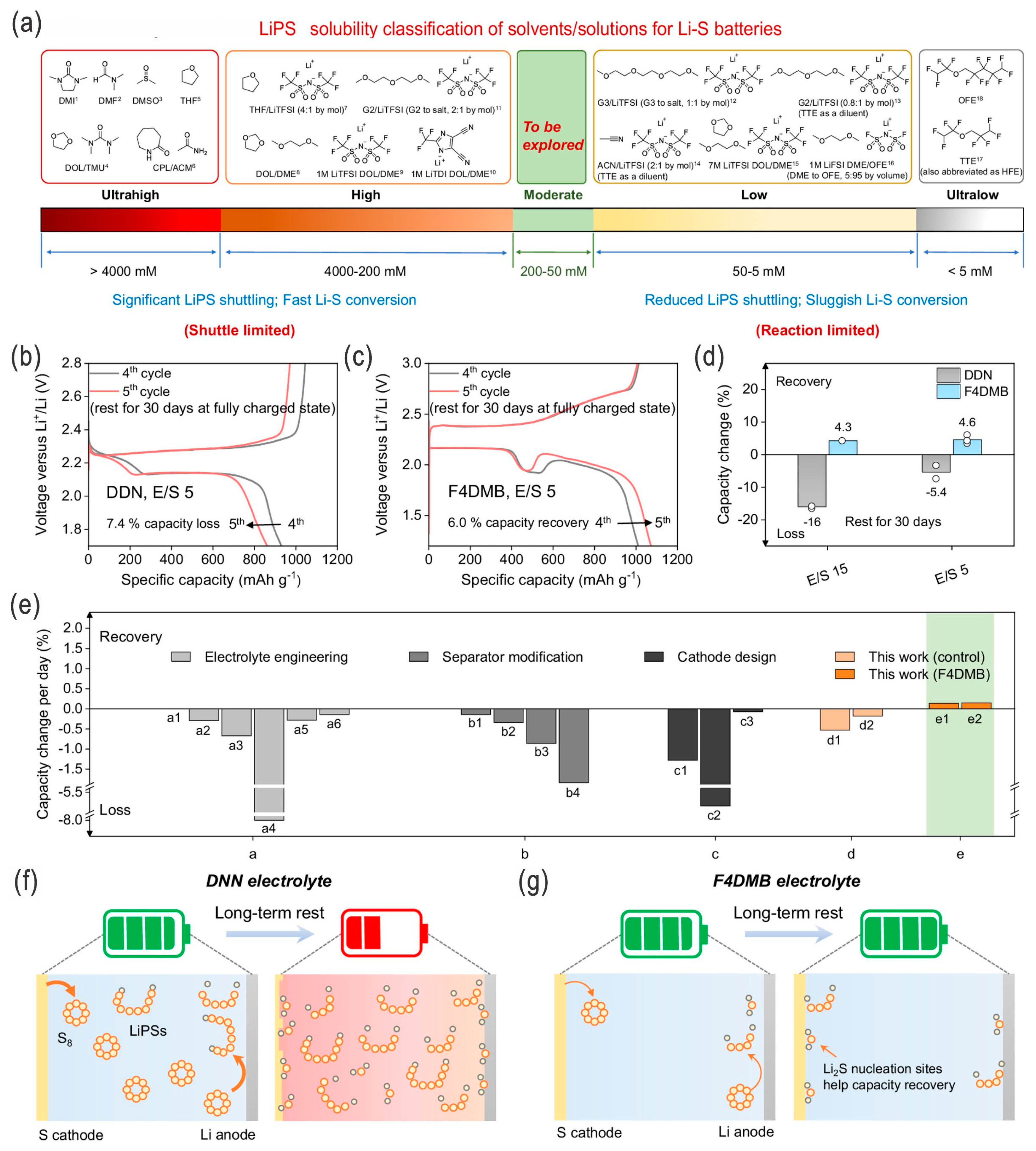
- Wang, X.; Tan, Y.; Shen, G.; Zhang, S. Recent progress in fluorinated electrolytes for improving the performance of Li–S batteries. J. Energy Chem. 2020, 41, 149–170. [Google Scholar] [CrossRef]
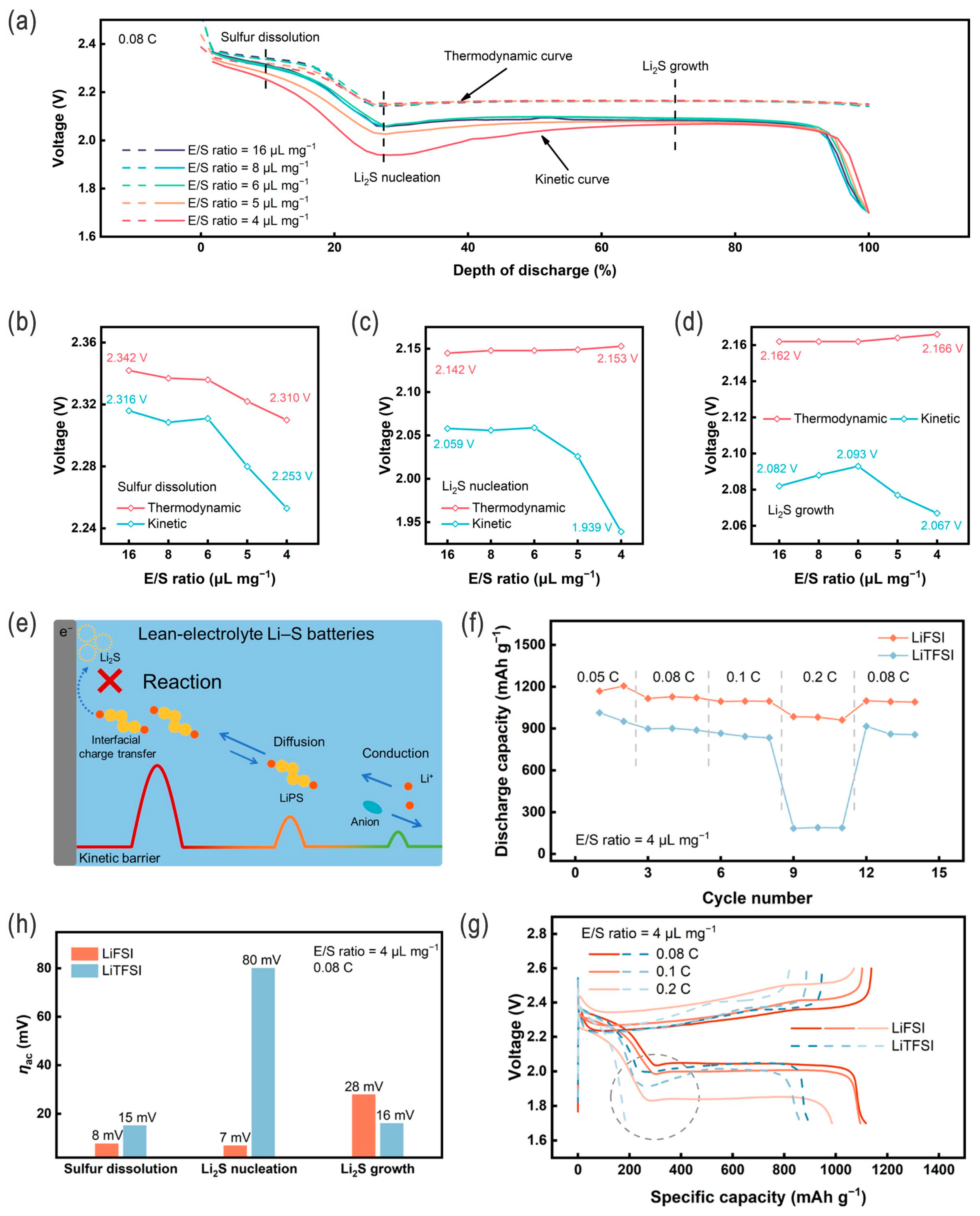
- Zhao, M.; Li, B.-Q.; Peng, H.-J.; Yuan, H.; Wei, J.-Y.; Huang, J.-Q. Lithium–Sulfur batteries under lean electrolyte conditions: Challenges and opportunities. Angew. Chem. Int. Ed. 2020, 59, 12636–12652. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, L.; Pan, F.; Gong, C.; Sun, L.; Gao, H.; Zhang, J.; Zhao, Y.; Wang, G.; Liu, H. Engineering strategies for suppressing the shuttle effect in lithium–sulfur batteries. Nano-Micro Lett. 2023, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Patel, M.; Bhoyate, S.; Prasad, V.; Choi, W. Nanoengineering to achieve high efficiency practical lithium–sulfur batteries. Nanoscale Horiz. 2020, 5, 808–831. [Google Scholar] [CrossRef]
- Ni, W.; Cheng, J.; Li, X.; Guan, Q.; Qu, G.; Wang, Z.; Wang, B. Multiscale sulfur particles confined in honeycomb-like graphene with the assistance of bio-based adhesive for ultrathin and robust free-standing electrode of Li–S batteries with improved performance. RSC Adv. 2016, 6, 9320–9327. [Google Scholar] [CrossRef]
- Yang, D.; Ni, W.; Cheng, J.; Wang, Z.; Wang, T.; Guan, Q.; Zhang, Y.; Wu, H.; Li, X.; Wang, B. Flexible three-dimensional electrodes of hollow carbon bead strings as graded sulfur reservoirs and the synergistic mechanism for lithium–sulfur batteries. Appl. Surf. Sci. 2017, 413, 209–218. [Google Scholar] [CrossRef]
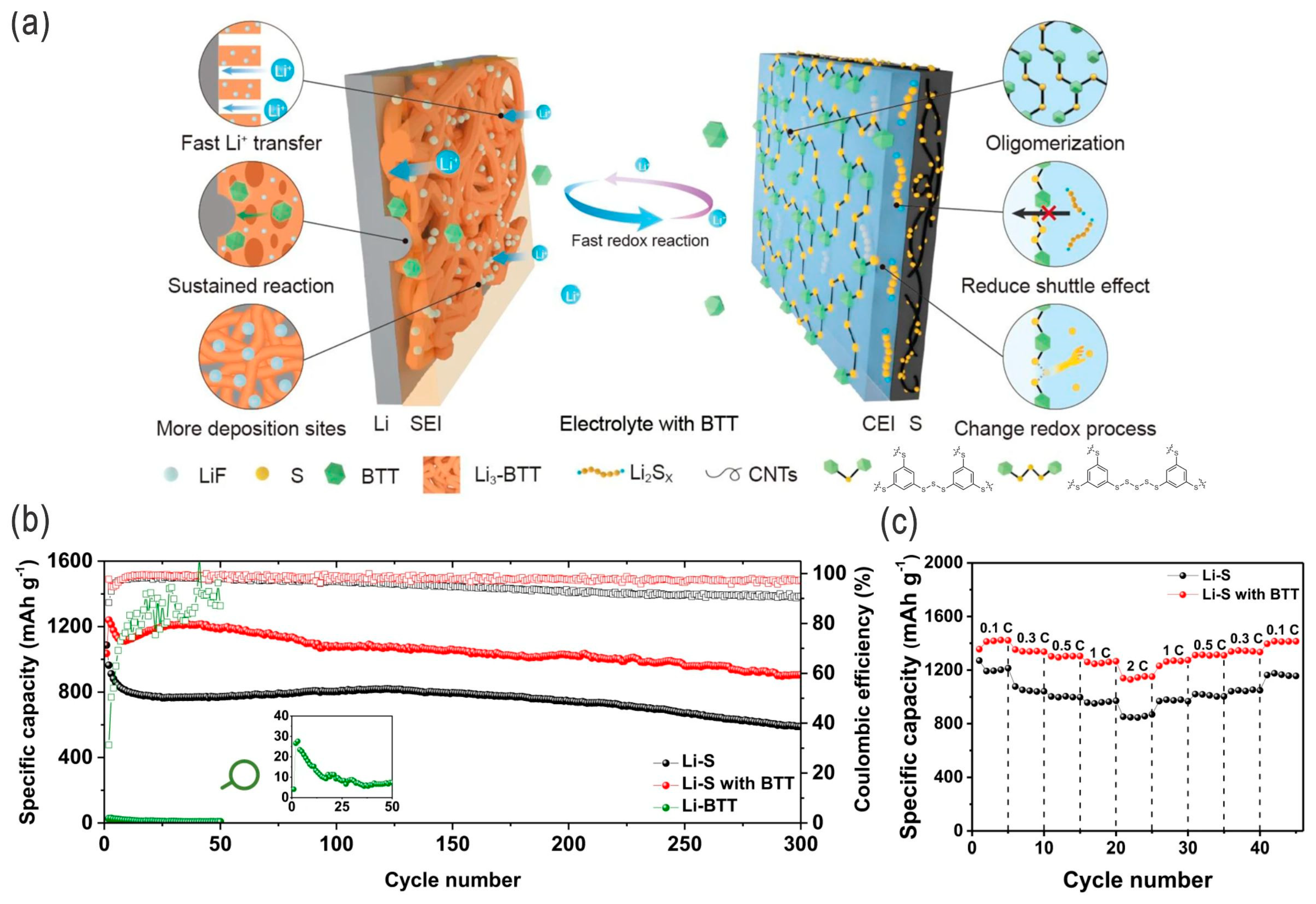
- Ji, Z.; Feng, L.; Zhu, Z.; Fu, X.; Yang, W.; Wang, Y. Polymeric interface engineering in lithium-sulfur batteries. Chem. Eng. J. 2023, 455, 140462. [Google Scholar] [CrossRef]
- Zhong, M.E.; Guan, J.; Sun, J.; Shu, X.; Ding, H.; Chen, L.; Zhou, N.; Xiao, Z. A cost- and energy density-competitive lithium-sulfur battery. Energy Storage Mater. 2021, 41, 588–598. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.-H.; Zhang, W.; Manthiram, A.; Yu, G. Nanostructured host materials for trapping sulfur in rechargeable Li–S batteries: Structure design and interfacial chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, S.; Hou, Y. Sulfur hosts against the shuttle effect. Small Methods 2018, 2, 1700345. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.-M. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium–sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Ding, N.; Yang, J.; Li, X.; Liu, Z.; Zong, Y. Engineering high-performance sulfur electrode from industrial conductive carbons. ACS Sustain. Chem. Eng. 2019, 7, 5515–5523. [Google Scholar] [CrossRef]
- Song, M.-K.; Zhang, Y.; Cairns, E.J. A Long-Life, High-rate lithium/sulfur cell: A multifaceted approach to enhancing cell performance. Nano Lett. 2013, 13, 5891–5899. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Dong, Y.F.; Li, H.J.; Zhao, Z.B.; Wu, H.B.; Hao, C.; Liu, S.H.; Qiu, J.S.; Lou, X.W. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002. [Google Scholar] [CrossRef]
- Luo, C.; Hu, E.; Gaskell, K.J.; Fan, X.; Gao, T.; Cui, C.; Ghose, S.; Yang, X.-Q.; Wang, C. A chemically stabilized sulfur cathode for lean electrolyte lithium sulfur batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 14712–14720. [Google Scholar] [CrossRef]
- Li, Z.; Sami, I.; Yang, J.; Li, J.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Jo, S.-C.; Hong, J.-W.; Choi, I.-H.; Kim, M.-J.; Kim, B.G.; Lee, Y.-J.; Choi, H.Y.; Kim, D.; Kim, T.; Baeg, K.-J.; et al. Multimodal capturing of polysulfides by phosphorus-doped carbon composites for flexible high-energy-density lithium–sulfur batteries. Small 2022, 18, 2200326. [Google Scholar] [CrossRef]
- Monisha, M.; Permude, P.; Ghosh, A.; Kumar, A.; Zafar, S.; Mitra, S.; Lochab, B. Halogen-free flame-retardant sulfur copolymers with stable Li–S battery performance. Energy Storage Mater. 2020, 29, 350–360. [Google Scholar] [CrossRef]
- Boenke, T.; Kirchhoff, S.; Reuter, F.S.; Schmidt, F.; Weller, C.; Dörfler, S.; Schwedtmann, K.; Härtel, P.; Abendroth, T.; Althues, H.; et al. The role of polysulfide-saturation in electrolytes for high power applications of real world Li-S pouch cells. Nano Res. 2023, 16, 8313–8320. [Google Scholar] [CrossRef]
- Qie, L.; Zu, C.; Manthiram, A. A high energy lithium-sulfur battery with ultrahigh-loading lithium polysulfide cathode and its failure mechanism. Adv. Energy Mater. 2016, 6, 1502459. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.; Kim, H.; Park, H.; Kim, H.; Kim, J.; Agostini, M.; Sun, Y.-K.; Hwang, J.-Y. Heterostructured nickel–cobalt metal alloy and metal oxide nanoparticles as a polysulfide mediator for stable lithium–sulfur full batteries with lean electrolyte. Carbon Energy 2024, e472. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller sulfur molecules promise better lithium-sulfur batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Q.; An, Y.; Anasori, B.; Wang, H.; Xu, B. Ultra-microporous carbons encapsulate small sulfur molecules for high performance lithium-sulfur battery. Nano Energy 2017, 33, 402–409. [Google Scholar] [CrossRef]
- Pai, R.; Singh, A.; Tang, M.H.; Kalra, V. Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li-S batteries. Commun. Chem. 2022, 5, 17. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
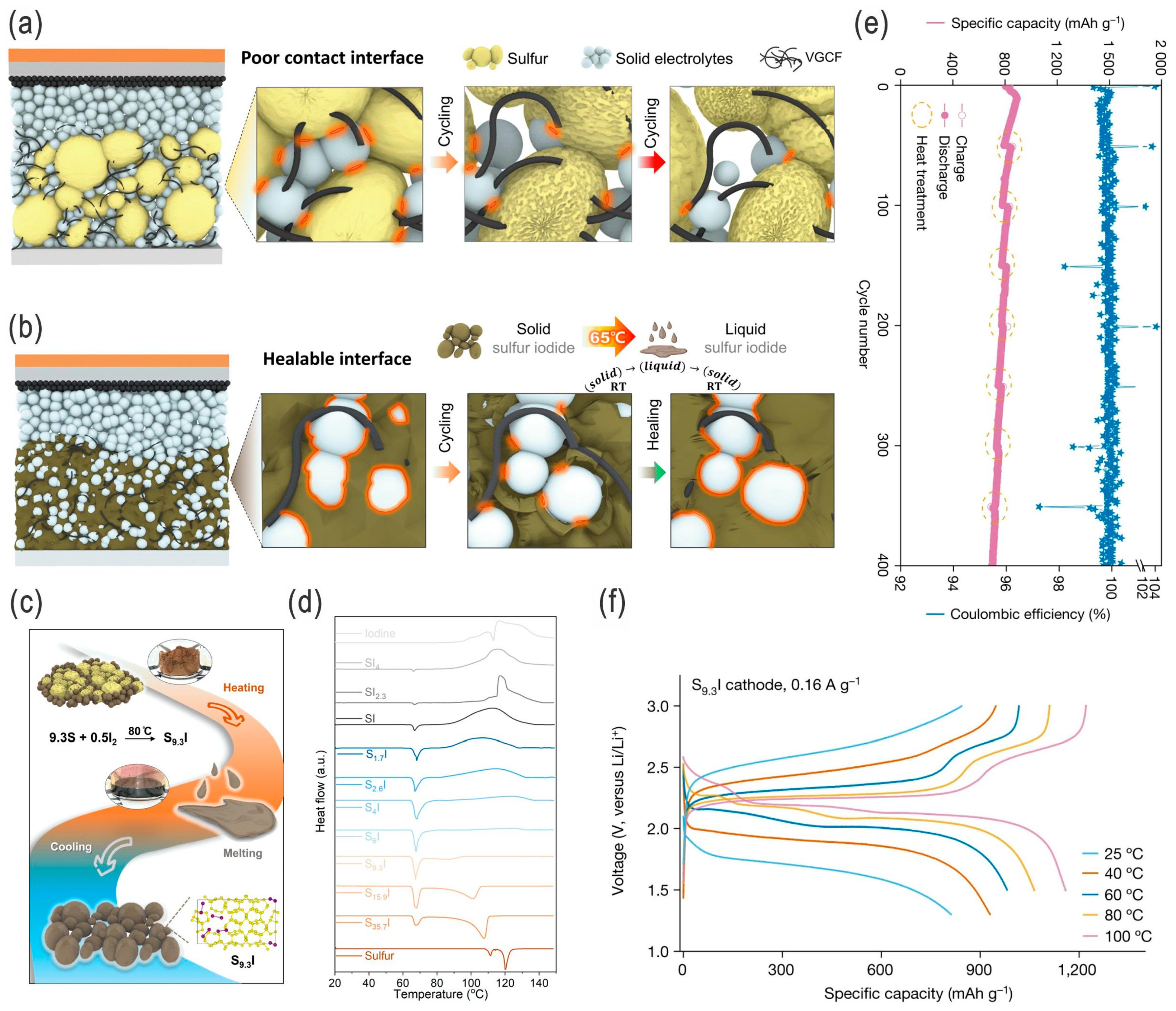
- Zhou, J.; Holekevi Chandrappa, M.L.; Tan, S.; Wang, S.; Wu, C.; Nguyen, H.; Wang, C.; Liu, H.; Yu, S.; Miller, Q.R.S.; et al. Healable and conductive sulfur iodide for solid-state Li–S batteries. Nature 2024, 627, 301–305. [Google Scholar] [CrossRef]
- Judez, X.; Martinez-Ibañez, M.; Santiago, A.; Armand, M.; Zhang, H.; Li, C. Quasi-solid-state electrolytes for lithium sulfur batteries: Advances and perspectives. J. Power Sources 2019, 438, 226985. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Wang, Z.; Qiu, J. Development of quasi-solid-state anode-free high-energy lithium sulfide-based batteries. Nat. Commun. 2022, 13, 4415. [Google Scholar] [CrossRef]
- Ding, B.; Wang, J.; Fan, Z.; Chen, S.; Lin, Q.; Lu, X.; Dou, H.; Kumar Nanjundan, A.; Yushin, G.; Zhang, X.; et al. Solid-state lithium–sulfur batteries: Advances, challenges and perspectives. Mater. Today 2020, 40, 114–131. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Adams, B.D.; Carino, E.V.; Connell, J.G.; Han, K.S.; Cao, R.; Chen, J.; Zheng, J.; Li, Q.; Mueller, K.T.; Henderson, W.A.; et al. Long term stability of Li-S batteries using high concentration lithium nitrate electrolytes. Nano Energy 2017, 40, 607–617. [Google Scholar] [CrossRef]
- Lei, D.; Shi, K.; Ye, H.; Wan, Z.; Wang, Y.; Shen, L.; Li, B.; Yang, Q.-H.; Kang, F.; He, Y.-B. Progress and perspective of solid-state lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707570. [Google Scholar] [CrossRef]
- Pang, Q.; Shyamsunder, A.; Narayanan, B.; Kwok, C.Y.; Curtiss, L.A.; Nazar, L.F. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat. Energy 2018, 3, 783–791. [Google Scholar] [CrossRef]
- Cheng, L.; Curtiss, L.A.; Zavadil, K.R.; Gewirth, A.A.; Shao, Y.; Gallagher, K.G. Sparingly solvating electrolytes for high energy density lithium–sulfur batteries. ACS Energy Lett. 2016, 1, 503–509. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Z.; Wang, J.; Zheng, X.; Ye, Y.; Gong, H.; Xiao, X.; Yang, Y.; Chen, Y.; Bone, S.E.; et al. Electrolytes with moderate lithium polysulfide solubility for high-performance long-calendar-life lithium–sulfur batteries. Proc. Natl. Acad. Sci. USA 2023, 120, e2301260120. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Peng, H.-J.; Nie, J.-Q.; Liu, X.-Y.; Zhang, Q.; Wei, F. Polysulfide shuttle control: Towards a lithium-sulfur battery with superior capacity performance up to 1000 cycles by matching the sulfur/electrolyte loading. J. Power Sources 2014, 253, 263–268. [Google Scholar] [CrossRef]
- Lee, C.-W.; Pang, Q.; Ha, S.; Cheng, L.; Han, S.-D.; Zavadil, K.R.; Gallagher, K.G.; Nazar, L.F.; Balasubramanian, M. Directing the lithium–sulfur reaction pathway via sparingly solvating electrolytes for high energy density batteries. ACS Cent. Sci. 2017, 3, 605–613. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Cheng, Q.; Li, X.-Y.; Li, Z.; Song, Y.-W.; Sun, F.; Zhao, M.; Zhang, X.-Q.; Li, B.-Q.; Huang, J.-Q. Cathode kinetics evaluation in lean-electrolyte lithium–sulfur batteries. J. Am. Chem. Soc. 2023, 145, 16449–16457. [Google Scholar] [CrossRef]
- Baek, M.; Shin, H.; Char, K.; Choi, J.W. New high donor electrolyte for lithium–sulfur batteries. Adv. Mater. 2020, 32, 2005022. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Liu, X.; Luo, J. Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 2019, 52, 3223–3232. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, S.Y.; Lee, J.S. Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles. npj Comput. Mater. 2022, 8, 103. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, G.; Fan, X.; Chen, J.; Li, Q.; Wang, H.; Yang, Y.; DeMella, K.C.; Raghavan, S.R.; Wang, C. High-fluorinated electrolytes for Li–S batteries. Adv. Energy Mater. 2019, 9, 1803774. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, X.; Ji, G.; Wang, H.; Hou, S.; DeMella, K.C.; Raghavan, S.R.; Wang, J.; Xu, K.; Wang, C. Manipulating electrolyte and solid electrolyte interphase to enable safe and efficient Li-S batteries. Nano Energy 2018, 50, 431–440. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Zhu, C.; Ma, J. Armor-like inorganic-rich cathode electrolyte interphase enabled by the pentafluorophenylboronic acid additive for high-voltage Li||NCM622 batteries. Angew. Chem. Int. Ed. 2023, 62, e202300057. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, W.; Si, Y.; Wang, D.; Fu, Y.; Manthiram, A. Artificial dual solid-electrolyte interfaces based on in situ organothiol transformation in lithium sulfur battery. Nat. Commun. 2021, 12, 3031. [Google Scholar] [CrossRef]
- Jin, C.; Huang, Y.; Li, L.; Wei, G.; Li, H.; Shang, Q.; Ju, Z.; Lu, G.; Zheng, J.; Sheng, O.; et al. A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries. Nat. Commun. 2023, 14, 8269. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Hwa, Y.; Cairns, E.J.; Helms, B.A. Redox-active supramolecular polymer binders for lithium–sulfur batteries that adapt their transport properties in operando. Chem. Mater. 2016, 28, 7414–7421. [Google Scholar] [CrossRef]
- Huang, Y.; Shaibani, M.; Gamot, T.D.; Wang, M.; Jovanović, P.; Dilusha Cooray, M.C.; Mirshekarloo, M.S.; Mulder, R.J.; Medhekar, N.V.; Hill, M.R.; et al. A saccharide-based binder for efficient polysulfide regulations in Li-S batteries. Nat. Commun. 2021, 12, 5375. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, K.; Fan, Y.; Yuan, M.; Liu, B.; Liu, W.; Shi, F.; Liu, Y.; Chen, W.; Lopez, J.; et al. An aqueous inorganic polymer binder for high performance lithium–sulfur batteries with flame-retardant properties. ACS Cent. Sci. 2018, 4, 260–267. [Google Scholar] [CrossRef]
- Senthil, C.; Kim, S.-S.; Jung, H.Y. Flame retardant high-power Li-S flexible batteries enabled by bio-macromolecular binder integrating conformal fractions. Nat. Commun. 2022, 13, 145. [Google Scholar] [CrossRef]
- Shaibani, M.; Mirshekarloo, M.S.; Singh, R.; Easton, C.D.; Cooray, M.C.D.; Eshraghi, N.; Abendroth, T.; Dörfler, S.; Althues, H.; Kaskel, S.; et al. Expansion-tolerant architectures for stable cycling of ultrahigh-loading sulfur cathodes in lithium-sulfur batteries. Sci. Adv. 2020, 6, eaay2757. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, S.; Yu, D.; Zhang, Q.; Liu, K.; Han, J.; Guo, Z.; Liu, J.; Wang, L. Cationic-polymer-functionalized separator as a high-efficiency polysulfide shuttle barrier for long-life Li–S battery. ACS Appl. Energy Mater. 2021, 4, 2914–2921. [Google Scholar] [CrossRef]
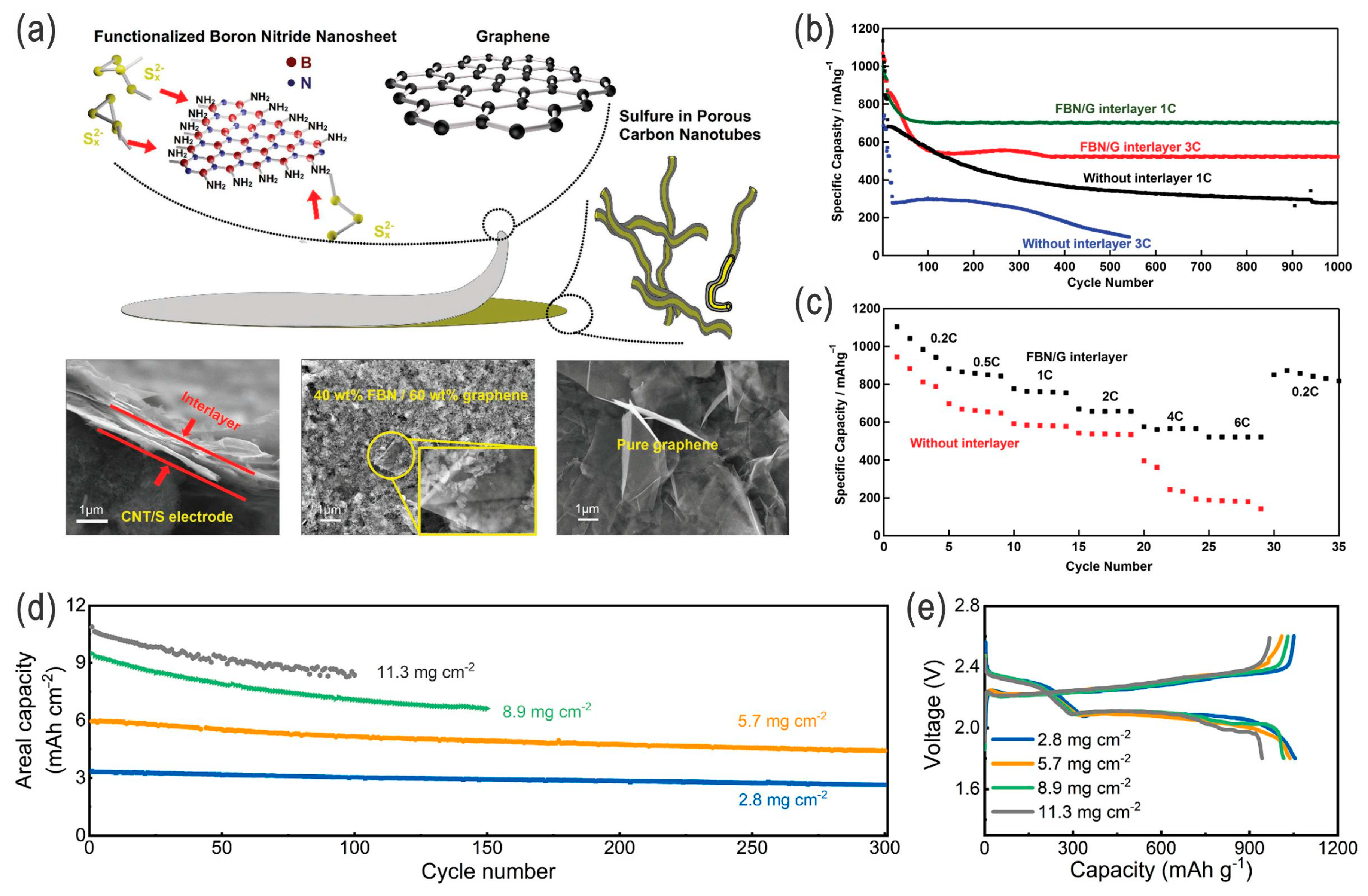
- Fan, Y.; Yang, Z.; Hua, W.; Liu, D.; Tao, T.; Rahman, M.M.; Lei, W.; Huang, S.; Chen, Y. Functionalized boron nitride nanosheets/graphene interlayer for fast and long-life lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1602380. [Google Scholar] [CrossRef]
- Li, B.-Q.; Kong, L.; Zhao, C.-X.; Jin, Q.; Chen, X.; Peng, H.-J.; Qin, J.-L.; Chen, J.-X.; Yuan, H.; Zhang, Q.; et al. Expediting redox kinetics of sulfur species by atomic-scale electrocatalysts in lithium–sulfur batteries. InfoMat 2019, 1, 533–541. [Google Scholar] [CrossRef]
- Pei, H.; Yang, C.; Wang, P.; Lin, J.; Yin, L.; Zhou, X.; Xie, X.; Ye, Y. Efficient thermal management of lithium-sulfur batteries by highly thermally conductive LBL-assembled composite separators. Electrochim. Acta 2022, 407, 139807. [Google Scholar] [CrossRef]
- Wang, M.; Emre, A.E.; Kim, J.-Y.; Huang, Y.; Liu, L.; Cecen, V.; Huang, Y.; Kotov, N.A. Multifactorial engineering of biomimetic membranes for batteries with multiple high-performance parameters. Nat. Commun. 2022, 13, 278. [Google Scholar] [CrossRef]
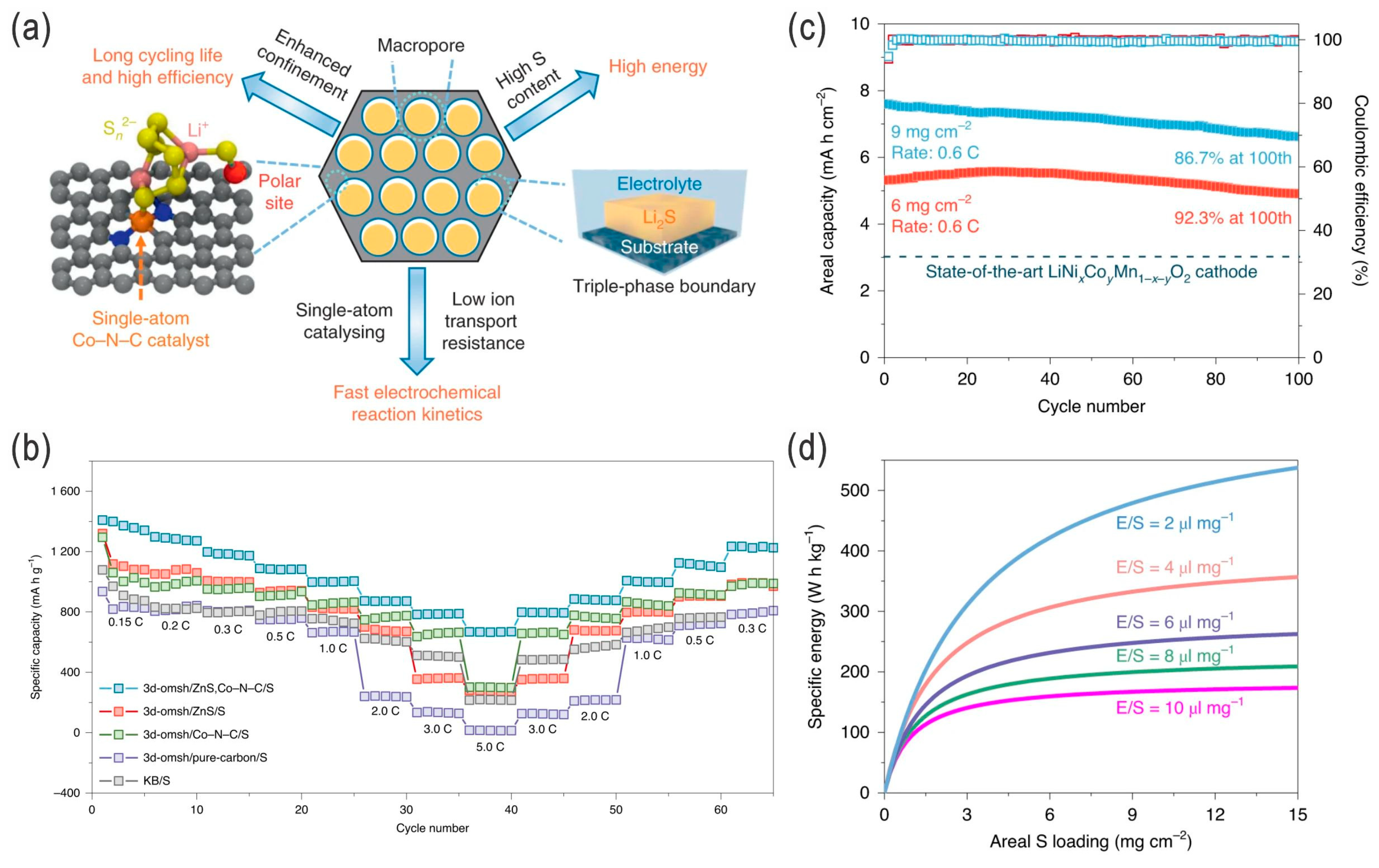
- Zhao, C.; Xu, G.-L.; Yu, Z.; Zhang, L.; Hwang, I.; Mo, Y.-X.; Ren, Y.; Cheng, L.; Sun, C.-J.; Ren, Y.; et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 2021, 16, 166–173. [Google Scholar] [CrossRef]
- Li, G.; Lei, W.; Luo, D.; Deng, Y.P.; Wang, D.; Chen, Z. 3D porous carbon sheets with multidirectional ion pathways for fast and durable lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702381. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C. One dimensional nanostructures contribute better Li–S and Li–Se batteries: Progress, challenges and perspectives. Energy Storage Mater. 2019, 23, 190–224. [Google Scholar] [CrossRef]
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 17204–17241. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, Z.-S.; Chen, J. Two-dimensional materials for advanced Li-S batteries. Energy Storage Mater. 2019, 22, 284–310. [Google Scholar] [CrossRef]
- Liu, Y.; Barnscheidt, Y.; Peng, M.; Bettels, F.; Li, T.; He, T.; Ding, F.; Zhang, L. A biomass-based integral approach enables Li-S full pouch cells with exceptional power density and energy density. Adv. Sci. 2021, 8, 2101182. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Zhang, B.; Fu, L.; Wan, M.; Li, G.; Cai, Z.; Tu, S.; Duan, X.; Seh, Z.W.; et al. Manipulating redox kinetics of sulfur species using Mott–Schottky electrocatalysts for advanced lithium–sulfur batteries. Nano Lett. 2021, 21, 6656–6663. [Google Scholar] [CrossRef]
- Li, H.; Meng, R.; Ye, C.; Tadich, A.; Hua, W.; Gu, Q.; Johannessen, B.; Chen, X.; Davey, K.; Qiao, S.-Z. Developing high-power Li||S batteries via transition metal/carbon nanocomposite electrocatalyst engineering. Nat. Nanotechnol. 2024. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Liu, L.; Koch, J.; Rosebrock, M.; Li, T.; Bettels, F.; He, T.; Pfnür, H.; Bigall, N.C.; et al. Nitrogen doping improves the immobilization and catalytic effects of Co9S8 in Li-S batteries. Adv. Funct. Mater. 2020, 30, 2002462. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, M.; Wang, C. Highly efficient flexible Li–S full batteries with hollow Ru–RuO2−x nanofibers as robust polysulfide anchoring-catalysts and lithium dendrite inhibitors. J. Mater. Chem. A 2022, 10, 8826–8836. [Google Scholar] [CrossRef]
- Deng, S.; Guo, T.; Heier, J.; Zhang, C. Unraveling polysulfide’s adsorption and electrocatalytic conversion on metal oxides for li-s batteries. Adv. Sci. 2023, 10, 2204930. [Google Scholar] [CrossRef]
- Zhou, T.; Liang, J.; Ye, S.; Zhang, Q.; Liu, J. Fundamental, application and opportunities of single atom catalysts for Li-S batteries. Energy Storage Mater. 2023, 55, 322–355. [Google Scholar] [CrossRef]
- Kwok, C.Y.; Xu, S.; Kochetkov, I.; Zhou, L.; Nazar, L.F. High-performance all-solid-state Li2S batteries using an interfacial redox mediator. Energy Environ. Sci. 2023, 16, 610–618. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. A Li2S-TiS2-electrolyte composite for stable Li2S-based lithium–sulfur batteries. Adv. Energy Mater. 2019, 9, 1901397. [Google Scholar] [CrossRef]
- Liu, D.D.; Chen, C.; Xiong, X.H. Research progress on artificial protective films for lithium metal anodes. Acta Phys.-Chim. Sin. 2021, 37, 2008078. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards practical lithium-metal anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef]
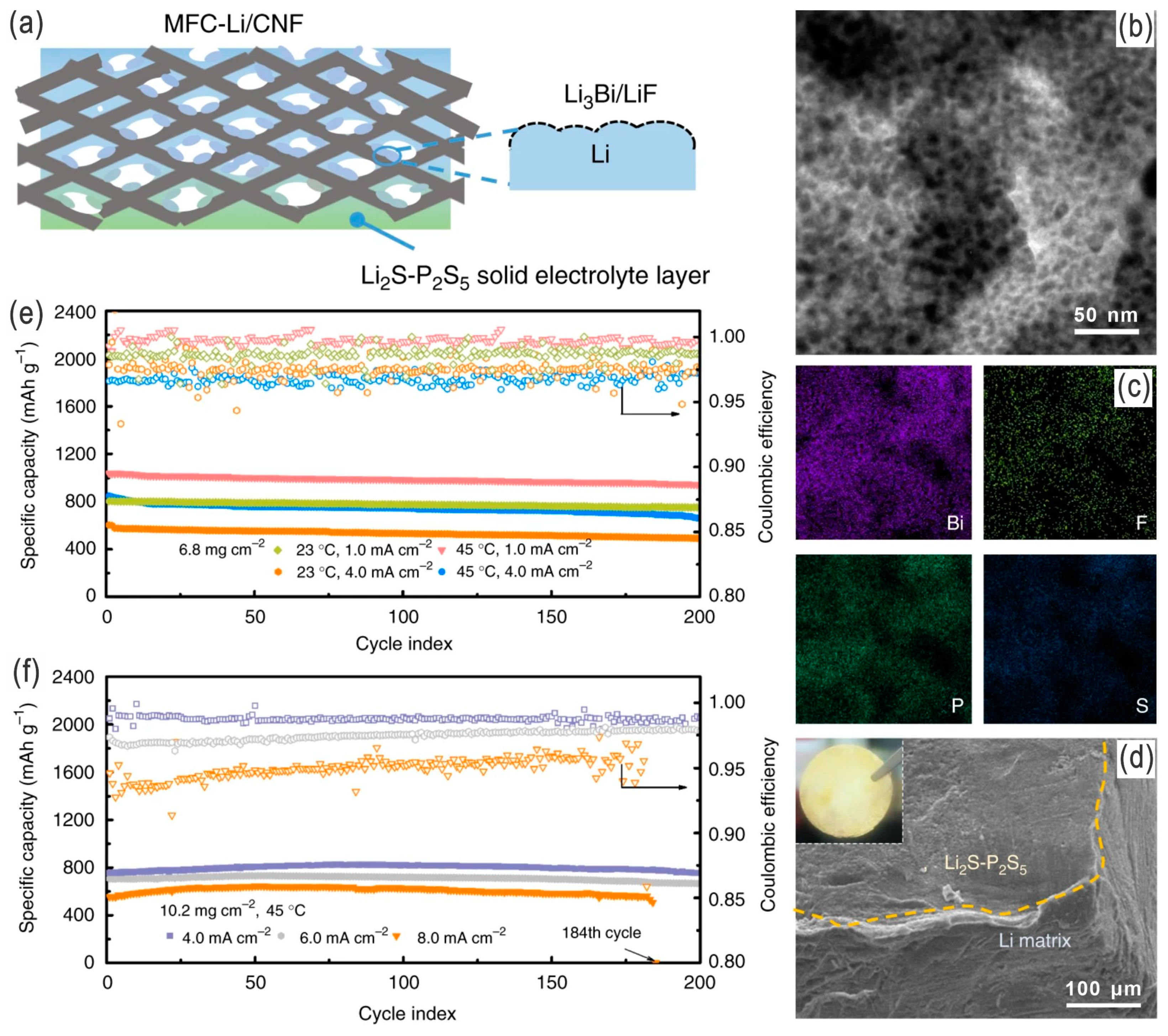
- Ren, Y.X.; Zeng, L.; Jiang, H.R.; Ruan, W.Q.; Chen, Q.; Zhao, T.S. Rational design of spontaneous reactions for protecting porous lithium electrodes in lithium–sulfur batteries. Nat. Commun. 2019, 10, 3249. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Y.; Liu, Y.; Wang, S.; Hu, A.; Lei, T.; Li, Y.; Li, P.; Chen, D.; Xia, L.; et al. Ultralong cycling and safe lithium–sulfur pouch cells for sustainable energy storage. Adv. Mater. 2024, 36, 2312880. [Google Scholar] [CrossRef]
- Eroglu, D.; Zavadil, K.R.; Gallagher, K.G. Critical link between materials chemistry and cell-level design for high energy density and low cost lithium-sulfur transportation battery. J. Electrochem. Soc. 2015, 162, A982. [Google Scholar] [CrossRef]
- Wolff, D.; Canals Casals, L.; Benveniste, G.; Corchero, C.; Trilla, L. The effects of lithium sulfur battery ageing on second-life possibilities and environmental life cycle assessment studies. Energies 2019, 12, 2440. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Chung, S.-H.; Chang, C.-H.; Manthiram, A. Progress on the critical parameters for lithium–sulfur batteries to be practically viable. Adv. Funct. Mater. 2018, 28, 1801188. [Google Scholar] [CrossRef]
- Lee, B.-J.; Zhao, C.; Yu, J.-H.; Kang, T.-H.; Park, H.-Y.; Kang, J.; Jung, Y.; Liu, X.; Li, T.; Xu, W.; et al. Development of high-energy non-aqueous lithium-sulfur batteries via redox-active interlayer strategy. Nat. Commun. 2022, 13, 4629. [Google Scholar] [CrossRef]
- Xu, J.; Liu, K.; Khan, M.A.; Wang, H.; He, T.; Zhao, H.; Ye, D.; Tang, Y.; Zhang, J. Sub-zero temperature electrolytes for lithium-sulfur batteries: Functional mechanisms, challenges and perspectives. Chem. Eng. J. 2022, 443, 136637. [Google Scholar] [CrossRef]
- Kulisch, J.; Sommer, H.; Brezesinski, T.; Janek, J. Simple cathode design for Li–S batteries: Cell performance and mechanistic insights by in operando X-ray diffraction. Phys. Chem. Chem. Phys. 2014, 16, 18765–18771. [Google Scholar] [CrossRef]
- Fu, Y.; Singh, R.K.; Feng, S.; Liu, J.; Xiao, J.; Bao, J.; Xu, Z.; Lu, D. Understanding of low-porosity sulfur electrode for high-energy lithium–sulfur batteries. Adv. Energy Mater. 2023, 13, 2203386. [Google Scholar] [CrossRef]
- Enabling Fast Charging Batteries with 3D Lithium Metal Architectures and Sulfurized Carbon Cathodes. Available online: https://arpa-e.energy.gov/technologies/projects/enabling-fast-charging-batteries-3d-lithium-metal-architectures-and (accessed on 3 June 2024).
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A review on electric vehicle battery modelling: From lithium-ion toward lithium–sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef]
- Propp, K.; Marinescu, M.; Auger, D.J.; O’Neill, L.; Fotouhi, A.; Somasundaram, K.; Offer, G.J.; Minton, G.; Longo, S.; Wild, M.; et al. Multi-temperature state-dependent equivalent circuit discharge model for lithium-sulfur batteries. J. Power Sources 2016, 328, 289–299. [Google Scholar] [CrossRef]
- Zeng, Q.; Zou, Z.; Chen, J.; Jiang, Y.; Zeng, L.; Li, C. Closed-loop modeling to evaluate the performance of a scaled-up lithium–sulfur battery in electric vehicle applications. Appl. Sci. 2021, 11, 9593. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Electric vehicle battery parameter identification and SOC observability analysis: NiMH and Li-S case studies. IET Power Electron. 2017, 10, 1289–1297. [Google Scholar] [CrossRef]
- Stroe, D.I.; Knap, V.; Swierczynski, M.; Schaltz, E. Electrochemical impedance spectroscopy-based electric circuit modeling of lithium–sulfur batteries during a discharging state. IEEE Trans. Ind. Appl. 2019, 55, 631–637. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Lithium–sulfur battery state-of-charge observability analysis and estimation. IEEE Trans. Power Electron. 2018, 33, 5847–5859. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, J.; Pilla, S.; Rao, A.M.; Xu, B. Application of a new type of lithium-sulfur battery and reinforcement learning in plug-in hybrid electric vehicle energy management. J. Energy Storage 2023, 59, 106546. [Google Scholar] [CrossRef]
- Li, X.; Lushington, A.; Sun, Q.; Xiao, W.; Liu, J.; Wang, B.; Ye, Y.; Nie, K.; Hu, Y.; Xiao, Q.; et al. Safe and durable high-temperature lithium–sulfur batteries via molecular layer deposited coating. Nano Lett. 2016, 16, 3545–3549. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Nie, T.; Zhu, A.; Xu, J.; Cao, Y.; Hu, S.; Zhang, X.; Niu, D. Interwoven N-doped bimetallic/carbon nanosheets as an efficient electrocatalyst for wide-temperature lithium-sulfur batteries. Chem. Eng. J. 2024, 484, 149171. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, T.; Gao, X.; Yuan, C. Life cycle assessment of lithium sulfur battery for electric vehicles. J. Power Sources 2017, 343, 284–295. [Google Scholar] [CrossRef]
- Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative life cycle assessment of Li-Sulphur and Li-ion batteries for electric vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086. [Google Scholar] [CrossRef]
- Accardo, A.; Garofalo, A.; Dotelli, G.; Spessa, E. Prospective LCA of Next-Generation Cells for Electric Vehicle Applications. IEEE Access 2024, 12, 19584–19597. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, B.; Li, S.; Yu, Y.; Li, X.; Chang, Z.; Wu, H.; Hu, Y.; Huang, K.; Liu, L.; et al. Life cycle environmental impact assessment for battery-powered electric vehicles at the global and regional levels. Sci. Rep. 2023, 13, 7952. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Lu, J. Lithium-Sulfur Batteries for Commercial Applications. Chem 2018, 4, 3–7. [Google Scholar] [CrossRef]
- Benveniste, G.; Rallo, H.; Canals Casals, L.; Merino, A.; Amante, B. Comparison of the state of Lithium-Sulphur and lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef]
- Iclodean, C.; Varga, B.; Burnete, N.; Cimerdean, D.; Jurchiş, B. Comparison of different battery types for electric vehicles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 252, 012058. [Google Scholar] [CrossRef]
- Siczek, K.; Siczek, K.; Piersa, P.; Adrian, Ł.; Szufa, S.; Obraniak, A.; Kubiak, P.; Zakrzewicz, W.; Bogusławski, G. The comparative study on the Li-S and Li-ion batteries cooperating with the photovoltaic array. Energies 2020, 13, 5109. [Google Scholar] [CrossRef]
- Amos, J. Solar Plane Makes Record Flight. Available online: http://news.bbc.co.uk/1/hi/sci/tech/7577493.stm (accessed on 3 June 2024).
- Sino Power INFORMATION BRIEF: Transitioning from Lithium-Sulfur to Licerion Technology. Available online: https://sionpower.com/files/IB_Li–S2Licerion.pdf (accessed on 3 June 2024).
- Kane, M. OXIS to Offer 450 Wh/kg Quasi Solid-State Li-S Cells in Fall 2021. Available online: https://insideevs.com/news/502505/oxis-450-whkg-solidstate-cells/ (accessed on 3 June 2024).
- Lithium-Sulfur Batteries One Step Closer to Commercialisation in the UK: A Faraday Success Story. Available online: https://www.innovationnewsnetwork.com/lithium-sulfur-batteries-one-step-closer-to-commercialisation-in-the-uk/41609/ (accessed on 3 June 2024).
- OXIS Energy to Supply Solid-State Li-S Batteries for Trials and Testing by This Fall; 600 Wh/kg and 900 Wh/L by 2026. Available online: https://www.greencarcongress.com/2021/04/20210421-oxis.html (accessed on 3 June 2024).
- Alcock, C. Patents for Sale as Battery Specialist Oxis Collapses. Available online: https://www.futureflight.aero/news-article/2021-05-27/patents-sale-battery-specialist-oxis-collapses (accessed on 3 June 2024).
- Avalos, G. Lyten Launches San Jose Pilot Production for Cutting-Edge Batteries. Available online: https://www.mercurynews.com/2023/06/14/lyten-san-jose-battery-economy-tech-jobs-real-estate-green-car-energy/ (accessed on 3 June 2024).
- Li-S Energy Achieves 45% Increase in Volumetric Energy Density with New Semi-Solid State Lithium Sulfur Battery. Available online: https://www.businesswire.com/news/home/20230529005039/en/ (accessed on 3 June 2024).
- Li-S Energy Achieves 45% Increase in Volumetric Energy Density with New 20-Layer Semi-Solid State Lithium Sulfur Battery. Available online: https://chargedevs.com/newswire/li-s-energy-claims-to-boost-energy-density-by-45-with-new-20-layer-lithium-sulfur-battery/ (accessed on 3 June 2024).
- Zeta Energy Corporation. Available online: https://zetaenergy.com/ (accessed on 3 June 2024).
- Davies, D.M.; Verde, M.G.; Mnyshenko, O.; Chen, Y.R.; Rajeev, R.; Meng, Y.S.; Elliott, G. Combined economic and technological evaluation of battery energy storage for grid applications. Nat. Energy 2019, 4, 42–50. [Google Scholar] [CrossRef]
- Comello, S.; Reichelstein, S. The emergence of cost effective battery storage. Nat. Commun. 2019, 10, 2038. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, W.; Yang, F.; Zheng, P.; Qiao, R.; Li, Z. Recent progress in polysulfide redox-flow batteries. Batter. Supercaps 2019, 2, 627–637. [Google Scholar] [CrossRef]
- Pan, H.; Wei, X.; Henderson, W.A.; Shao, Y.; Chen, J.; Bhattacharya, P.; Xiao, J.; Liu, J. On the way toward understanding solution chemistry of lithium polysulfides for high energy Li–S redox flow batteries. Adv. Energy Mater. 2015, 5, 1500113. [Google Scholar] [CrossRef]
- Khan, I.A.; Alzahrani, A.S.; Ali, S.; Mansha, M.; Tahir, M.N.; Khan, M.; Qayyum, H.A.; Khan, S.A. Development of membranes and separators to inhibit cross-shuttling of sulfur in polysulfide-based redox flow batteries: A review. Chem. Rec. 2024, 24, e202300171. [Google Scholar] [CrossRef] [PubMed]
- Dörfler, S.; Walus, S.; Locke, J.; Fotouhi, A.; Auger, D.J.; Shateri, N.; Abendroth, T.; Härtel, P.; Althues, H.; Kaskel, S. Recent progress and emerging application areas for lithium–sulfur battery technology. Energy Technol. 2021, 9, 2000694. [Google Scholar] [CrossRef] [PubMed]
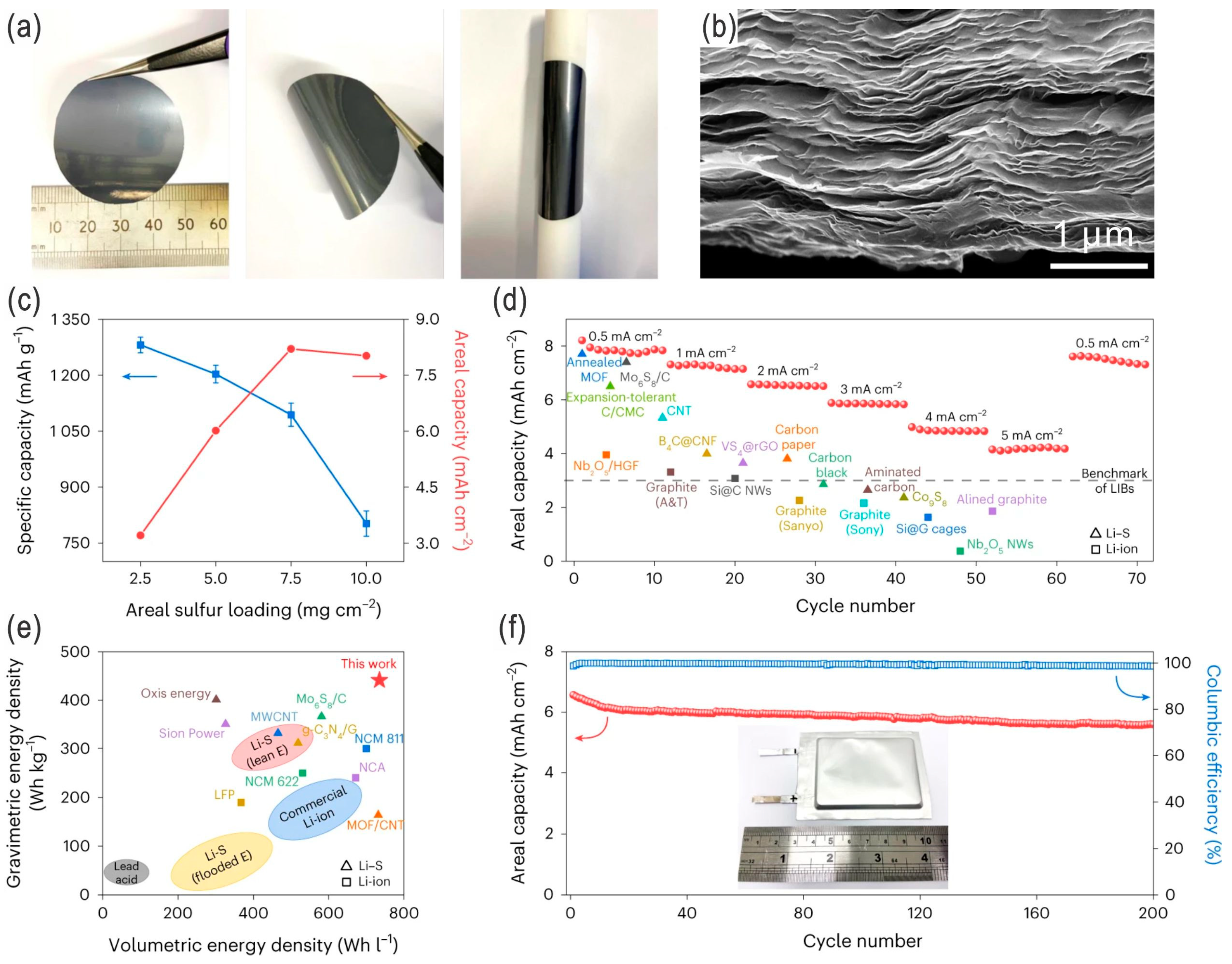



| Sulfur Content (wt.%) | Areal Sulfur Loading (mg cm−2) | Electrode Size (cm2) | E/S Ratio | ||
|---|---|---|---|---|---|
| ≥70 | ≥6.5 | ≥38.9 | ≤5 | ||
| Lithium thickness (μm) | Areal lithium loading (mg cm−2) | Electrode size (cm2) | N/P ratio | ||
| ≤50 | ≤2.67 | ≥38.9 | ≤1.59 | ||
| C-rate | Reversible capacity (mAh g−1) | Retained capacity (mAh g−1) | Cycle No. | Capacity retention (%) | CE (%) |
| 0.1 C | ≥1000 | – | – | – | – |
| 0.2 C | ≥800 | ≥650 | ≥200 | ≥81.25 | ≥98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W. Perspectives on Advanced Lithium–Sulfur Batteries for Electric Vehicles and Grid-Scale Energy Storage. Nanomaterials 2024, 14, 990. https://doi.org/10.3390/nano14120990
Ni W. Perspectives on Advanced Lithium–Sulfur Batteries for Electric Vehicles and Grid-Scale Energy Storage. Nanomaterials. 2024; 14(12):990. https://doi.org/10.3390/nano14120990
Chicago/Turabian StyleNi, Wei. 2024. "Perspectives on Advanced Lithium–Sulfur Batteries for Electric Vehicles and Grid-Scale Energy Storage" Nanomaterials 14, no. 12: 990. https://doi.org/10.3390/nano14120990






