Multifunctional Vanadium Nitride-Modified Separator for High-Performance Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of VN@NC
2.3. Preparation of the Modified Separator
2.4. Material Characterization
2.5. Electrochemical Characterization
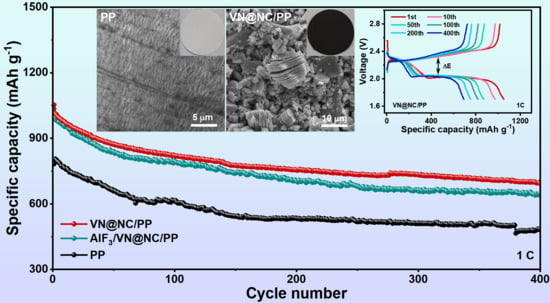
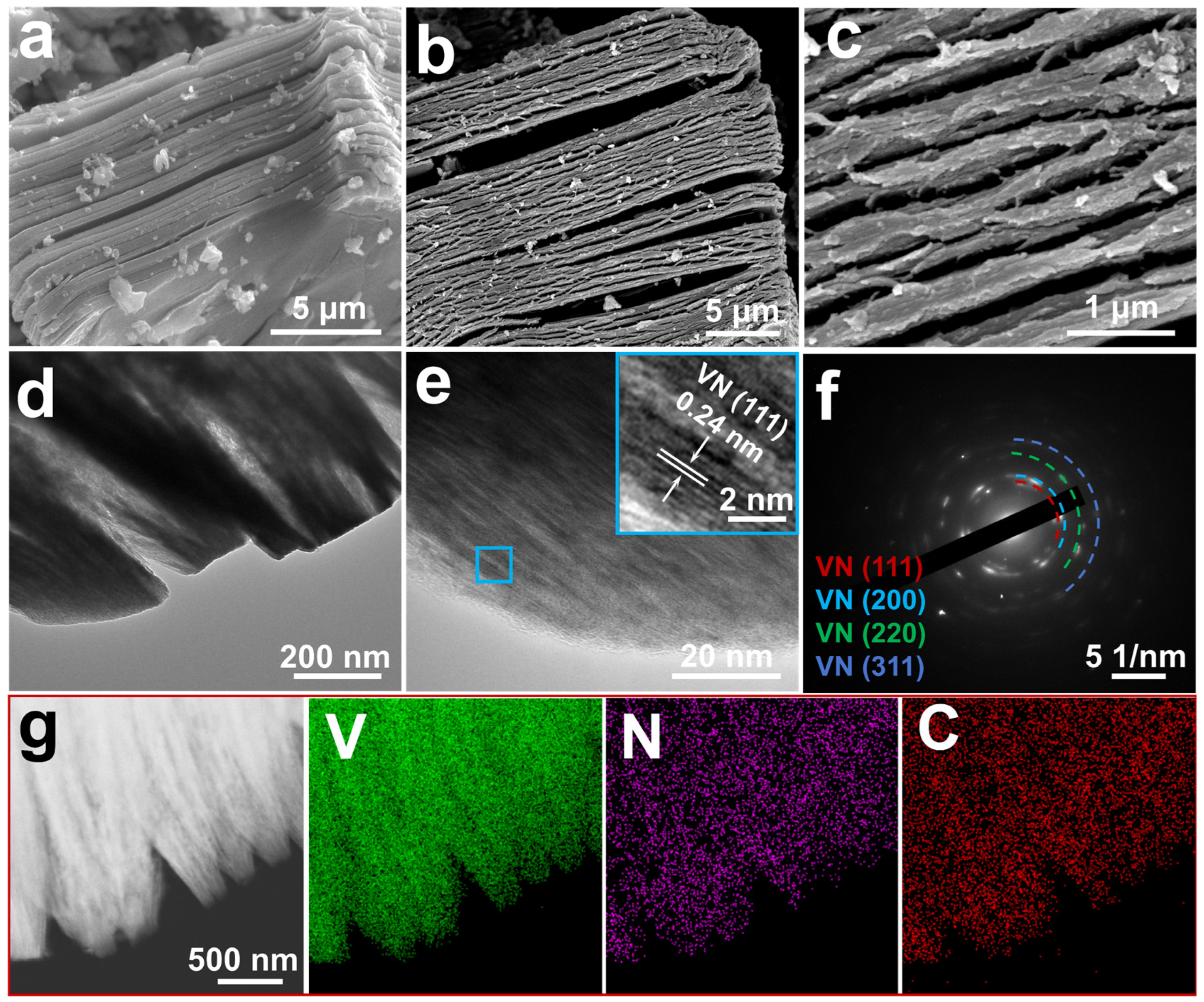
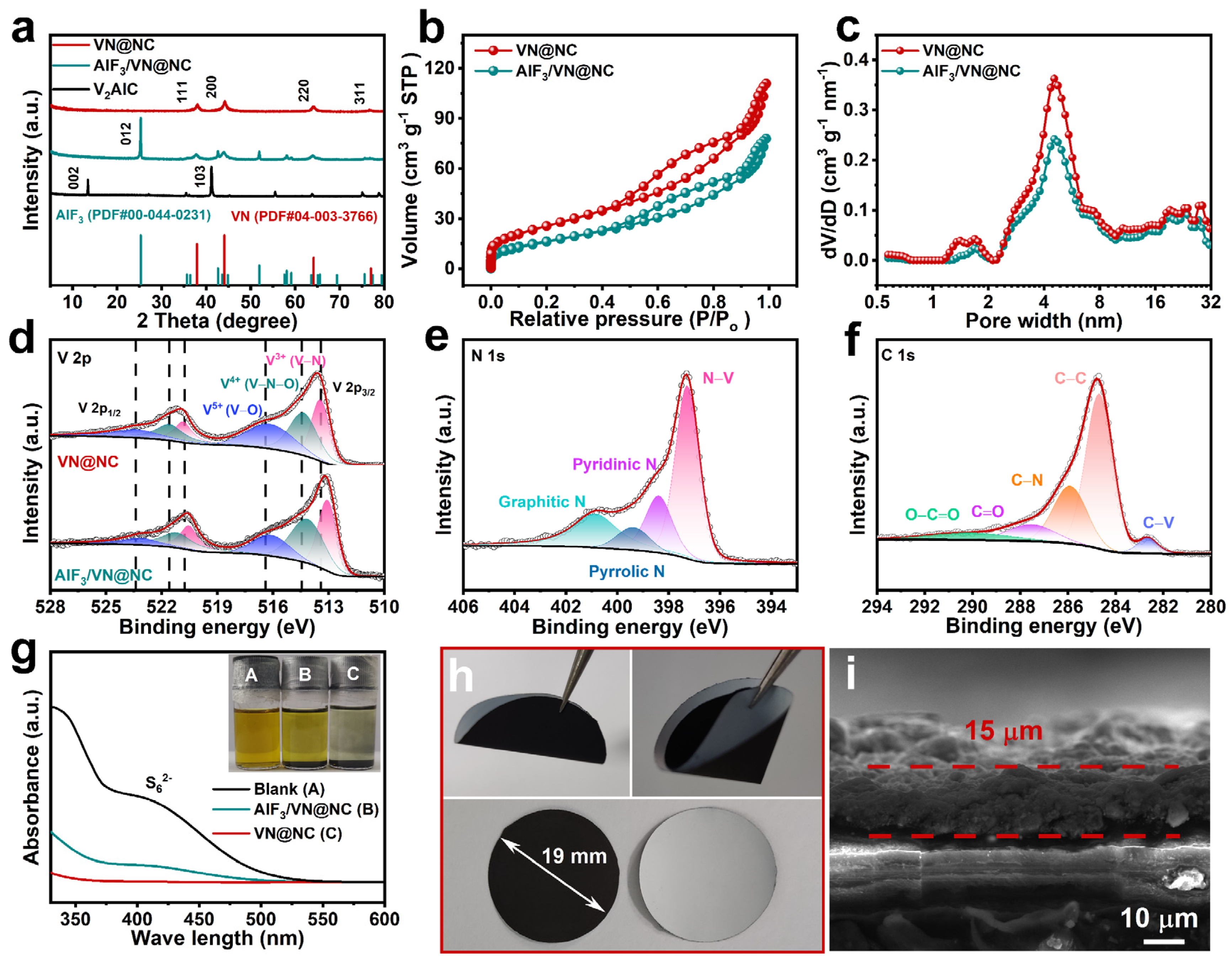
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in lithium-sulfur batteries: From academic research to commercial viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining theory and experiment in lithium-sulfur batteries: Current progress and future perspectives. Mater. Today 2019, 22, 142–158. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, C.; Zhang, L.; Li, Y.; Lu, J. Deciphering the reaction mechanism of lithium-sulfur batteries by in situ/operando synchrotron-based characterization techniques. Adv. Energy Mater. 2019, 9, 1900148. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-sulfur batteries: Attaining the critical metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, Y.; Wu, F.; Li, Y.; Li, L.; Chen, R. Anode interface engineering and architecture design for high-performance lithium-sulfur batteries. Adv. Mater. 2019, 31, 1806532. [Google Scholar] [CrossRef]
- Wang, W.P.; Zhang, J.; Chou, J.; Yin, Y.X.; You, Y.; Xin, S.; Guo, Y.G. Solidifying cathode-electrolyte interface for lithium-sulfur batteries. Adv. Energy Mater. 2020, 11, 2000791. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.; Yan, Y.; Yan, T.; Cheng, C.; Sun, D.; Zhang, L. Utilizing the built-in electric field of p-n junctions to spatially propel the stepwise polysulfide conversion in lithium-sulfur batteries. Adv. Mater. 2021, 33, 2105067. [Google Scholar] [CrossRef]
- Hua, W.; Li, H.; Pei, C.; Xia, J.; Sun, Y.; Zhang, C.; Lv, W.; Tao, Y.; Jiao, Y.; Zhang, B.; et al. Selective catalysis remedies polysulfide shuttling in lithium-sulfur batteries. Adv. Mater. 2021, 33, 2101006. [Google Scholar] [CrossRef]
- Ren, Y.; Zhai, Q.; Wang, B.; Hu, L.; Ma, Y.; Dai, Y.; Tang, S.; Meng, X. Synergistic adsorption-electrocatalysis of 2D/2D heterostructure toward high performance Li-S batteries. Chem. Eng. J. 2022, 439, 135535. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Xiao, Y.; Cao, F.; Zhang, H. Recent progress of separators in lithium-sulfur batteries. Energy Storage Mater. 2021, 40, 439–460. [Google Scholar] [CrossRef]
- Pei, F.; Lin, L.; Fu, A.; Mo, S.; Ou, D.; Fang, X.; Zheng, N. A two-dimensional porous carbon-modified separator for high-energy-density Li-S batteries. Joule 2018, 2, 323–336. [Google Scholar] [CrossRef]
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational design of nanostructured functional interlayer/separator for advanced Li-S batteries. Adv. Funct. Mater. 2018, 28, 1707411. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Zhang, B.; Chen, C.; Lin, Z. Integrating conductivity, immobility, and catalytic ability into high-n carbon/graphene sheets as an effective sulfur host. Adv. Mater. 2019, 32, 1906357. [Google Scholar] [CrossRef]
- Sun, Z.; Vijay, S.; Heenen, H.H.; Eng, A.Y.S.; Tu, W.; Zhao, Y.; Koh, S.W.; Gao, P.; Seh, Z.W.; Chan, K.; et al. Catalytic polysulfide conversion and physiochemical confinement for lithium-sulfur batteries. Adv. Energy Mater. 2020, 10, 1904010. [Google Scholar] [CrossRef]
- Baek, M.; Shin, H.; Char, K.; Choi, J.W. New high donor electrolyte for lithium-sulfur batteries. Adv. Mater. 2020, 32, 2005022. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, Z.; Ai, C.; Liu, S.; Liu, P.; Zhong, Y.; Lin, S.; Deng, S.; Liu, Q.; et al. Coupling a sponge metal fibers skeleton with in situ surface engineering to achieve advanced electrodes for flexible lithium-sulfur batteries. Adv. Mater. 2020, 32, 2003657. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Jin, J.; Li, Y.; Wu, M.; Wen, Z. Constructing dual interfacial modification by synergetic electronic and ionic conductors: Toward high-performance LAGP-based Li-S batteries. Energy Storage Mater. 2019, 23, 299–305. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, Y.; Li, W.; Meng, L.; Bai, Y.; Chen, G. Organic-inorganic multi-scale enhanced interfacial engineering of sulfide solid electrolyte in Li-S battery. Chem. Eng. J. 2020, 396, 125334. [Google Scholar] [CrossRef]
- Hakari, T.; Fujita, Y.; Deguchi, M.; Kawasaki, Y.; Otoyama, M.; Yoneda, Y.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Solid electrolyte with oxidation tolerance provides a high-capacity Li2S-based positive electrode for all-solid-state Li/S batteries. Adv. Funct. Mater. 2021, 32, 2106174. [Google Scholar] [CrossRef]
- Luo, S.; Liu, X.; Zhang, X.; Wang, X.; Wang, Z.; Zhang, Y.; Wang, H.; Ma, W.; Zhu, L.; Zhang, X. Nanostructure of the interphase layer between a single Li dendrite and sulfide electrolyte in all-solid-state Li batteries. ACS Energy Lett. 2022, 7, 3064–3071. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Zheng, P.; Wang, Y.; Ng, S.-W.; Chen, Y.; Deng, Y.; Zheng, Z.; Wang, C. Water-based phytic acid-crosslinked supramolecular binders for lithium-sulfur batteries. Chem. Eng. J. 2020, 395, 124981. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Zhang, Y.; Wang, S.; Xin, F.; Chen, X.; Yu, D. 3D-crosslinked tannic acid/poly(ethylene oxide) complex as a three-in-one multifunctional binder for high-sulfur-loading and high-stability cathodes in lithium-sulfur batteries. Energy Storage Mater. 2019, 17, 293–299. [Google Scholar] [CrossRef]
- Yoo, G.; Kim, S.; Chanthad, C.; Cho, M.; Lee, Y. Elastic rubber-containing multifunctional binder for advanced Li-S batteries. Chem. Eng. J. 2021, 405, 126628. [Google Scholar] [CrossRef]
- Lingappan, N.; Lee, W.; Passerini, S.; Pecht, M. A comprehensive review of separator membranes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113726. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Iqbal, N.; Ghani, U.; Liao, W.; He, X.; Lu, Y.; Wang, Z.; Li, T. Synergistically engineered 2D MXenes for metal-ion/Li–S batteries: Progress and outlook. Mater. Today Adv. 2022, 16, 100303. [Google Scholar] [CrossRef]
- Xia, S.; Song, J.; Zhou, Q.; Liu, L.; Ye, J.; Wang, T.; Chen, Y.; Liu, Y.; Wu, Y.; van Ree, T. A separator with double coatings of Li4Ti5O12 and conductive carbon for Li-S battery of good electrochemical performance. Adv. Sci. 2023, 10, 2301386. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, Z.W.; Huang, J.Q.; Zhang, G.; Xie, J.; Xu, W.T.; Shi, J.L.; Chen, X.; Cheng, X.B.; Zhang, Q. A cooperative interface for highly efficient lithium-sulfur batteries. Adv. Mater. 2016, 28, 9551–9558. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, J.; Lu, X.; Kang, L.; Tan, C.; Xu, R.; Yuan, L.; Brett, D.J.L.; Shearing, P.R.; Wang, F.R.; et al. Precisely visit the performance modulation of functionalized separator in Li-S batteries via consecutive multiscale analysis. Energy Storage Mater. 2022, 49, 85–92. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.J.; Li, B.Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.Q.; Zhang, Q. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium-sulfur batteries. Adv. Energy Mater. 2018, 9, 1802768. [Google Scholar] [CrossRef]
- Hu, X.; Zhong, L.; Shu, C.; Fang, Z.; Yang, M.; Li, J.; Yu, D. Versatile, aqueous soluble C2N quantum dots with enriched active edges and oxygenated groups. J. Am. Chem. Soc. 2020, 142, 4621–4630. [Google Scholar] [CrossRef]
- Liu, B.; Torres, J.F.; Taheri, M.; Xiong, P.; Lu, T.; Zhu, J.; Liu, Y.; Yu, G.; Tricoli, A. Dualion flux management for stable high areal capacity lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2103444. [Google Scholar] [CrossRef]
- Fang, D.; Wang, Y.; Liu, X.; Yu, J.; Qian, C.; Chen, S.; Wang, X.; Zhang, S. Spider-web-inspired nanocomposite-modified separator: Structural and chemical cooperativity inhibiting the shuttle effect in Li-S batteries. ACS Nano 2019, 13, 1563–1573. [Google Scholar] [CrossRef]
- Fu, S.; Wang, H.; Zhong, Y.; Schaefer, S.; Li, M.; Wu, M.; Wang, H. High-mass-loading Li-S batteries catalytically activated by cerium oxide: Performance and failure analysis under lean electrolyte conditions. Adv. Mater. 2023, 35, 2302771. [Google Scholar] [CrossRef]
- Aslam, M.K.; Jamil, S.; Hussain, S.; Xu, M. Effects of catalysis and separator functionalization on high-energy lithium-sulfur batteries: A complete review. Energy Environ. Mater. 2023, 6, e12420. [Google Scholar] [CrossRef]
- Gai, L.; Zhao, C.; Zhang, Y.; Hu, Z.; Shen, Q. Constructing a multifunctional mesoporous composite of metallic cobalt nanoparticles and nitrogen-doped reduced graphene oxides for high-performance lithium-sulfur batteries. Carbon Energy 2022, 4, 142–154. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Q.; Xu, H.; Zhang, Y.; Zhang, B.; Zhang, Z.; Lin, Z.; Zhang, S. Ni/SiO2/graphene-modified separator as a multifunctional polysulfide barrier for advanced lithium-sulfur batteries. Nano Energy 2020, 76, 105033. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Z.; Guo, T.; Zhou, Y.; Liu, M.; Xia, X.; Sun, J.; Lu, L.; Ouyang, X.; Wang, X.; et al. Strong chemical interaction between lithium polysulfides and flame-retardant polyphosphazene forLSBswith enhanced safety and electrochemical performance. Adv. Mater. 2021, 33, 2007549. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Fang, Z.; Zhao, S.; He, Y.-s.; Zhang, W.; Mu, J.; Zhang, L.; Ma, Z.-F. Constructing a catalytic reservoir using cobalt nanoparticles-MoS2@nitrogen doped carbon nanotubes on the separator to immobilize polysulfides and accelerate their conversion for lithium-sulfur batteries. Chem. Eng. J. 2022, 446, 136943. [Google Scholar] [CrossRef]
- Qian, J.; Xing, Y.; Yang, Y.; Li, Y.; Yu, K.; Li, W.; Zhao, T.; Ye, Y.; Li, L.; Wu, F.; et al. Enhanced electrochemical kinetics with highly dispersed conductive and electrocatalytic mediators for lithium-sulfur batteries. Adv. Mater. 2021, 33, 2100810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Ye, J.; Xia, G.; Fu, Z.; Hu, C. A trifunctional modified separator based on Fe tetraaminophthalocyanine@rGO for lithium-sulfur batteries. Chem. Eng. J. 2021, 405, 126947. [Google Scholar] [CrossRef]
- Zeng, P.; Huang, L.; Zhang, X.; Zhang, R.; Wu, L.; Chen, Y. Long-life and high-areal-capacityLSBsrealized by a honeycomb-like N, P dual-doped carbon modified separator. Chem. Eng. J. 2018, 349, 327–337. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, S.; Guo, J.; Dai, Y.; Zheng, W.; Jiang, X.; Wu, X.; Xiao, W.; He, G.; Li, X. Position difference between Mo clusters and N sites induced highly synergistic electrocatalysis in integrated electrode-separator membranes with crosslinked hierarchically porous interface. Energy Storage Mater. 2022, 45, 370–379. [Google Scholar] [CrossRef]
- Hu, S.; Yi, M.; Wu, H.; Wang, T.; Ma, X.; Liu, X.; Zhang, J. Ionic-liquid-assisted synthesis of N, F, and B Co-doped CoFe2O4−x on multiwalled carbon nanotubes with enriched oxygen vacancies for Li-S batteries. Adv. Funct. Mater. 2021, 32, 2111084. [Google Scholar] [CrossRef]
- Ren, L.; Qiao, L.; Pato, A.H.; Liu, J.; Wang, Y.; Lu, X.; Zhao, Y.; Wang, Q.; Liu, W.; Xu, H.; et al. Suppression of long-chain lithium polysulfide formation through a selenium-doped linear sulfur copolymer cathode for high-performance lithium–organosulfur batteries. J. Mater. Chem. A 2024, 12, 4249–4257. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Zhang, T.; Ye, H.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Simultaneous cobalt and phosphorous doping of MoS2 for improved catalytic performance on polysulfide conversion in lithium–sulfur batteries. Adv. Energy Mater. 2019, 9, 1902096. [Google Scholar] [CrossRef]
- Ren, L.; Liu, J.; Zhao, Y.; Wang, Y.; Lu, X.; Zhou, M.; Zhang, G.; Liu, W.; Xu, H.; Sun, X. Regulating electronic structure of Fe-N4 single atomic catalyst via neighboring sulfur doping for high performance lithium-sulfur batteries. Adv. Funct. Mater. 2023, 33, 2210509. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, J.; Yang, C.; Zhang, C.; Hu, J.; Chang, S.; Dong, H.; Wu, C.; Hong, Y.; Zhang, L. Promoting reversible redox kinetics by separator architectures based on CoS2/HPGC interlayer as efficient polysulfide-trapping shield for Li-S batteries. Small 2020, 16, 2002046. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, J.; Cao, Y.; Meng, Y.; Wu, Z.; Zhan, L.; Wang, Y.; Zhang, Y.; Manke, I.; Chen, N.; et al. Dynamic intercalation–conversion site supported ultrathin 2D mesoporous SnO2/SnSe2 hybrid as bifunctional polysulfide immobilizer and lithium regulator for lithium-sulfur chemistry. ACS Nano 2022, 16, 10783–10797. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, C.; Hua, J.; Hong, X.; Sun, C.; Li, H.W.; Xu, X.; Mai, L. Engineering oxygen vacancies in a polysulfide-blocking layer with enhanced catalytic ability. Adv. Mater. 2020, 32, 1907444. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and catalytic conversion of polysulfides by in situ built TiO2-MXene heterostructures for lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Xi, K.; He, D.; Harris, C.; Wang, Y.; Lai, C.; Li, H.; Coxon, P.R.; Ding, S.; Wang, C.; Kumar, R.V. Enhanced sulfur transformation by multifunctional FeS2/FeS/S composites for high-volumetric capacity cathodes in lithium-sulfur batteries. Adv. Sci. 2019, 6, 1800815. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yu, D.; Suo, L.; Wang, C.; Wang, Z.; Xu, G.; Xiao, X.; Ge, M.; Ko, M.; Chen, Y.; et al. Manipulating sulfur mobility enables advanced Li-S batteries. Matter 2019, 1, 1047–1060. [Google Scholar] [CrossRef]
- Huang, L.; Shen, S.; Zhong, Y.; Zhang, Y.; Zhang, L.; Wang, X.; Xia, X.; Tong, X.; Zhou, J.; Tu, J. Multifunctional hyphae carbon powering lithium-sulfur batteries. Adv. Mater. 2021, 34, 2107415. [Google Scholar] [CrossRef] [PubMed]
- Razaq, R.; Din, M.M.U.; Småbråten, D.R.; Eyupoglu, V.; Janakiram, S.; Sunde, T.O.; Allahgoli, N.; Rettenwander, D.; Deng, L. Synergistic effect of bimetallic mof modified separator for long cycle life lithium-sulfur batteries. Adv. Energy Mater. 2023, 14, 2302897. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. Long-life, high-rate lithium-sulfur cells with a carbon-free VN host as an efficient polysulfide adsorbent and lithium dendrite inhibitor. Adv. Energy Mater. 2019, 10, 1903241. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Gong, W.; Meng, X.; Ma, J.; Zhang, J.; Zheng, L.; Abruña, H.D.; Geng, J. Kinetic enhancement of sulfur cathodes by N-doped porous graphitic carbon with bound VN nanocrystals. Small 2020, 16, 2004950. [Google Scholar] [CrossRef]
- Zhang, J.; Xi, W.; Yu, F.; Zhang, Y.; Wang, R.; Gong, Y.; He, B.; Wang, H.; Jin, J. Constructing MoS2-SnS heterostructures on N-doped carbon nanosheets for enhanced catalytic conversion of polysulfides in lithium-sulfur batteries. Chem. Eng. J. 2023, 475, 146009. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Z.; Sun, Y.; Wu, J.; Pan, X.; Sun, J. Steering bidirectional sulfur redox via geometric/electronic mediator comodulation for Li-S batteries. ACS Nano 2023, 17, 6002–6010. [Google Scholar] [CrossRef]
- Jing, E.; Chen, L.; Xu, S.; Tian, W.; Zhang, D.; Wang, N.; Bai, Z.; Zhou, X.; Liu, S.; Duan, D.; et al. Dual redox catalysis of VN/nitrogen-doped graphene nanocomposites for high-performance lithium-sulfur batteries. J. Energy Chem. 2022, 64, 574–582. [Google Scholar] [CrossRef]
- Ye, C.; Jiao, Y.; Jin, H.; Slattery, A.D.; Davey, K.; Wang, H.; Qiao, S.Z. 2D MoN-VN heterostructure to regulate polysulfides for highly efficient lithium-sulfur batteries. Angew. Chem. Int. Ed. 2018, 130, 16945–16949. [Google Scholar] [CrossRef]
- Wang, S.; Feng, S.; Liang, J.; Su, Q.; Zhao, F.; Song, H.; Zheng, M.; Sun, Q.; Song, Z.; Jia, X.; et al. Insight into MoS2-MoN heterostructure to accelerate polysulfide conversion toward high-energy-density lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003314. [Google Scholar] [CrossRef]
- Chu, R.; Nguyen, T.T.; Bai, Y.; Kim, N.H.; Lee, J.H. Uniformly controlled treble boundary using enriched adsorption sites and accelerated catalyst cathode for robust lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2102805. [Google Scholar] [CrossRef]
- Sun, R.; Bai, Y.; Bai, Z.; Peng, L.; Luo, M.; Qu, M.; Gao, Y.; Wang, Z.; Sun, W.; Sun, K. Phosphorus vacancies as effective polysulfide promoter for high-energy-density lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2102739. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Zhou, B.; Jiao, Y.; Yan, Y.; Du, X.; Huang, J.; Wu, C.; Wang, X.; et al. Carbon quantum dots-modified interfacial interactions and ion conductivity for enhanced high current density performance in lithium-sulfur batteries. Adv. Energy Mater. 2018, 9, 1802955. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium-sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Deng, Y.; Huang, Z.; Zhou, G.; Lv, W.; He, Y.B.; Wan, Y.; Kang, F.; Yang, Q.H. Optimized catalytic WS2-WO3 heterostructure design for accelerated polysulfide conversion in lithium–sulfur batteries. Adv. Energy Mater. 2020, 10, 2000091. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Wang, X.; Chen, Z. Engineering the conductive network of metal oxide-based sulfur cathode toward efficient and longevous lithium–sulfur batteries. Adv. Energy Mater. 2020, 10, 2002076. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Shi, H.; Olsson, E.; Cai, Q.; Wu, Z.S.; Liu, J.; Lu, G.Q. Molecular-level design of pyrrhotite electrocatalyst decorated hierarchical porous carbon spheres as nanoreactors for lithium-sulfur batteries. Adv. Energy Mater. 2020, 10, 2000651. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, D.; Tang, P.; Zhang, C.; Jacas Biendicho, J.; Zhang, Y.; Llorca, J.; Wang, X.; Li, J.; Heggen, M.; et al. Atomically dispersed Fe in a C2N based catalyst as a sulfur host for efficient lithium-sulfur batteries. Adv. Energy Mater. 2020, 11, 2003507. [Google Scholar] [CrossRef]
- Mo, Y.; Lin, J.; Li, S.; Yu, J. Anchoring Mo2C nanoparticles on vertical graphene nanosheets as a highly efficient catalytic interlayer for Li-S batteries. Chem. Eng. J. 2022, 433, 134306. [Google Scholar] [CrossRef]
- Shen, C.; Xie, J.; Zhang, M.; Andrei, P.; Hendrickson, M.; Plichta, E.J.; Zheng, J.P. Self-discharge behavior of lithium-sulfur batteries at different electrolyte/sulfur ratios. J. Electrochem. Soc. 2019, 166, A5287–A5294. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Liu, L.; Zhu, X.; Guo, Z.; Guo, W.; Han, Q.; He, J.; Zhao, Y. Uniform coverage of high-loading sulfur on cross-linked carbon nanofibers for high reaction kinetics in Li–S batteries with low electrolyte/sulfur ratio. J. Mater. Chem. A 2022, 10, 1433–1441. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Liao, C.; Han, L.; Song, L.; Hu, Y.; Mu, X.; Kan, Y. A flame retardant separator modified by MOFs-derived hybrid for safe and efficient Li-S batteries. J. Energy Chem. 2022, 64, 372–384. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, S.; Jia, X.; Yang, J.; Li, Y.; Shao, D.; Feng, L.; Liao, J.; Song, H. MXene derivative Ta4C3-Ta2O5 heterostructure as bi-functional barrier for Li-S batteries. J. Mater. Sci. Technol. 2023, 151, 89–98. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Wu, X.; Yuan, H.; Yu, Y.; Tan, Y. Boosting adsorption and catalysis of polysulfides by multifunctional separator for lithium–sulfur batteries. ACS Energy Lett. 2022, 7, 4190–4197. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Li, G.; Liu, C.; Cao, L.; Zhang, Y.; Bao, W.; Wang, H.; Yao, Y.; Liu, S.; et al. Ion selective covalent organic framework enabling enhanced electrochemical performance of lithium–sulfur batteries. Nano Lett. 2021, 21, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Saroha, R.; Oh, J.H.; Lee, J.S.; Kang, Y.C.; Jeong, S.M.; Kang, D.-W.; Cho, C.; Cho, J.S. Hierarchically porous nanofibers comprising multiple core–shell Co3O4@graphitic carbon nanoparticles grafted within N-doped CNTs as functional interlayers for excellent Li–S batteries. Chem. Eng. J. 2021, 426, 130805. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, Y.; Pei, H.; Zhao, M.; Wang, Y.; Zhou, X.; Gamal Mohamed, M.; Kuo, S.-W.; Ye, Y. Metalloporphyrin conjugated porous polymer in-situ grown on a Celgard separator as multifunctional polysulfide barrier and catalyst for high-performance Li-S batteries. Chem. Eng. J. 2023, 473, 144733. [Google Scholar] [CrossRef]
- Seon, Y.H.; Saroha, R.; Cho, J.S. Hierarchically porous N-doped C nanofibers comprising TiO2 quantum dots and ZIF-8-derived hollow C nanocages as ultralight interlayer for stable Li–S batteries. Compos. Part B 2022, 237, 109856. [Google Scholar] [CrossRef]
- Luo, M.; Bai, Y.; Sun, R.; Qu, M.; Wang, M.; Yang, Z.; Wang, Z.; Sun, W.; Sun, K. Enhancing anchoring and catalytic conversion of polysulfides by nitrogen deficient cobalt nitride for advanced lithium-sulfur batteries. J. Energy Chem. 2022, 73, 407–415. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, Y.; Zhang, X.; Shen, M.; Liu, X.; Gao, X.; Hou, L.; Yuan, C. Multifunctional Vanadium Nitride-Modified Separator for High-Performance Lithium–Sulfur Batteries. Nanomaterials 2024, 14, 656. https://doi.org/10.3390/nano14080656
Liu S, Liu Y, Zhang X, Shen M, Liu X, Gao X, Hou L, Yuan C. Multifunctional Vanadium Nitride-Modified Separator for High-Performance Lithium–Sulfur Batteries. Nanomaterials. 2024; 14(8):656. https://doi.org/10.3390/nano14080656
Chicago/Turabian StyleLiu, Sen, Yang Liu, Xu Zhang, Maoqiang Shen, Xuesen Liu, Xinyue Gao, Linrui Hou, and Changzhou Yuan. 2024. "Multifunctional Vanadium Nitride-Modified Separator for High-Performance Lithium–Sulfur Batteries" Nanomaterials 14, no. 8: 656. https://doi.org/10.3390/nano14080656
APA StyleLiu, S., Liu, Y., Zhang, X., Shen, M., Liu, X., Gao, X., Hou, L., & Yuan, C. (2024). Multifunctional Vanadium Nitride-Modified Separator for High-Performance Lithium–Sulfur Batteries. Nanomaterials, 14(8), 656. https://doi.org/10.3390/nano14080656