The Modulation of Compositional Heterogeneity for Controlling Shear Banding in Co-P Metallic Nanoglasses
Abstract
:1. Introduction
2. Simulation Methodologies
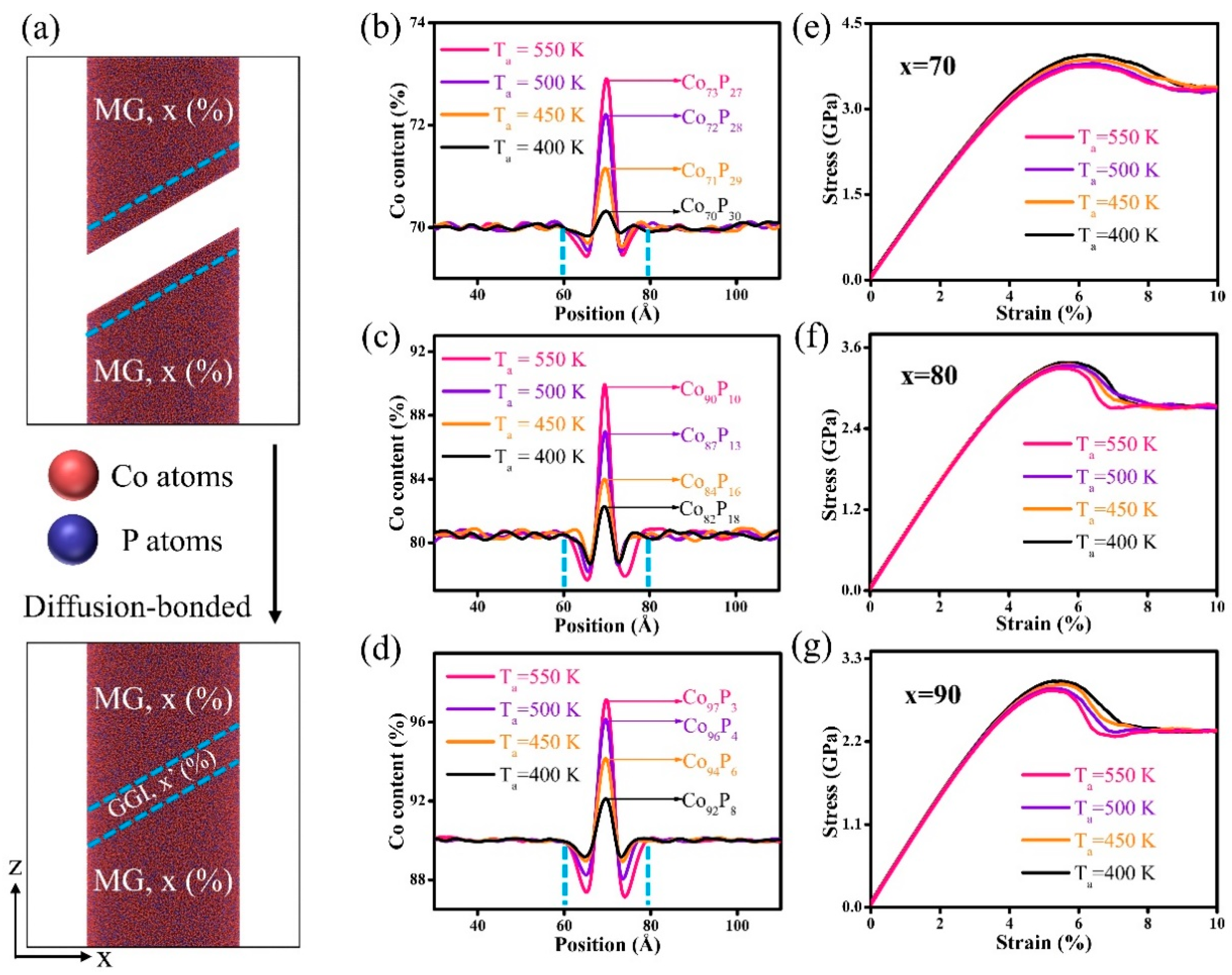
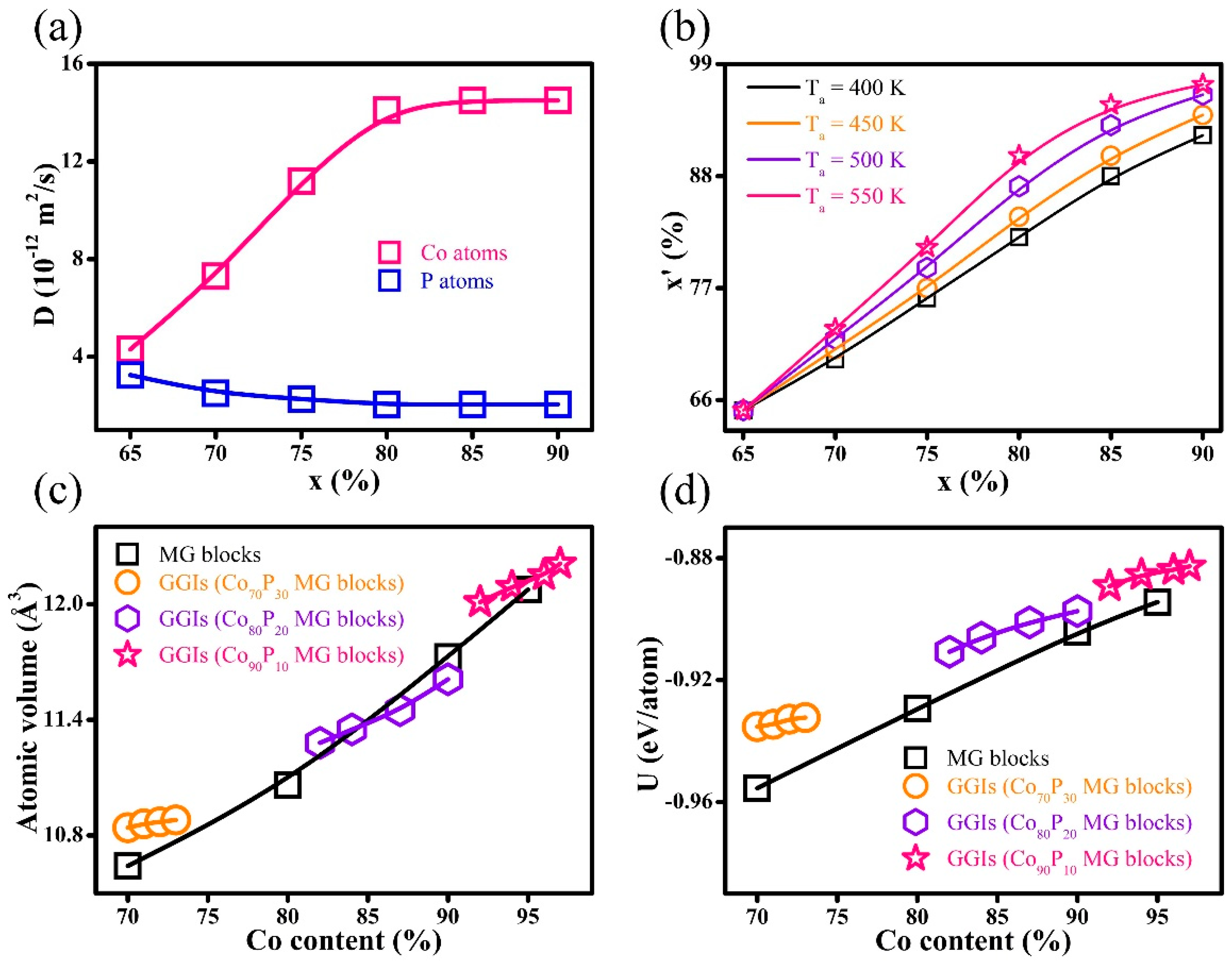
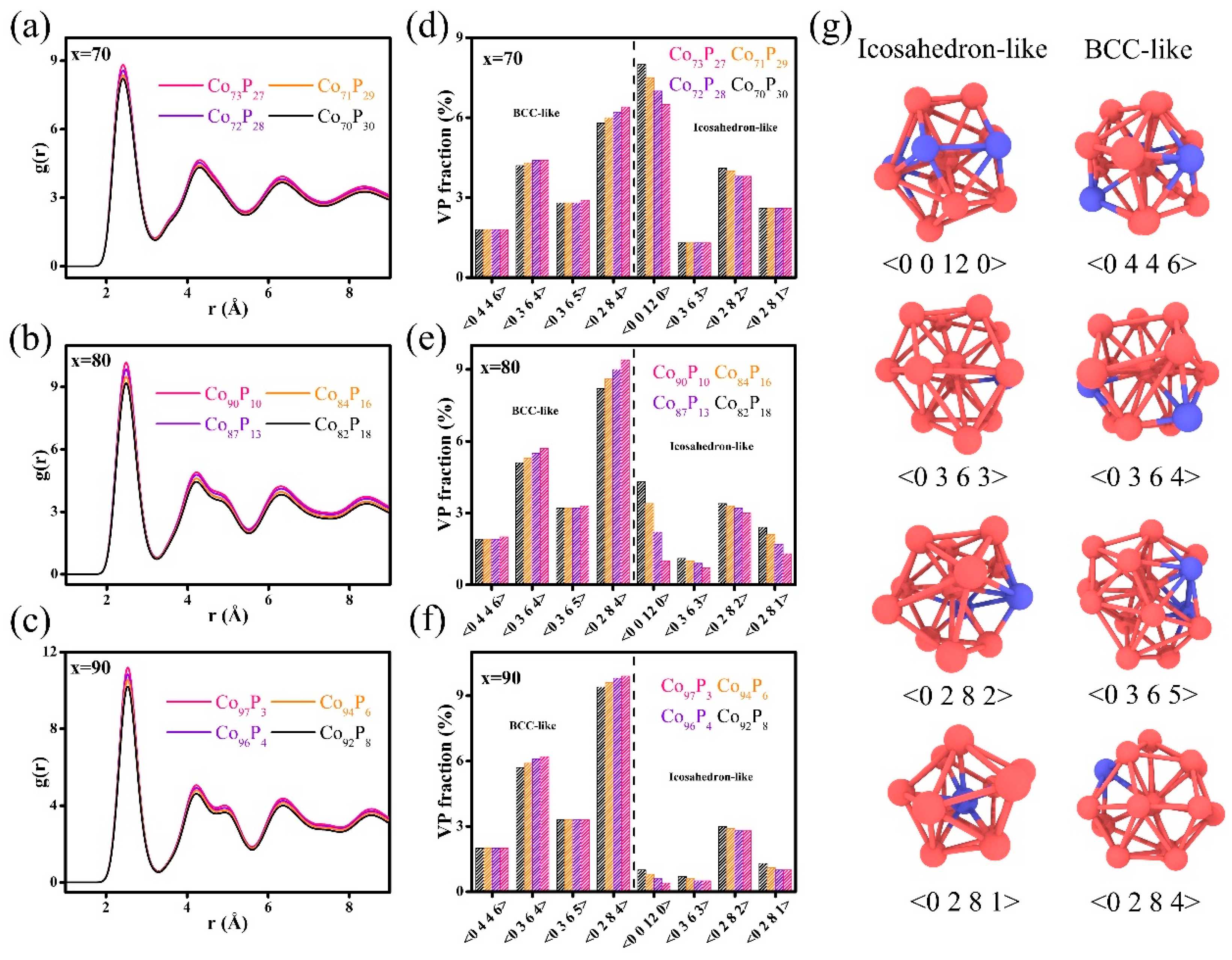
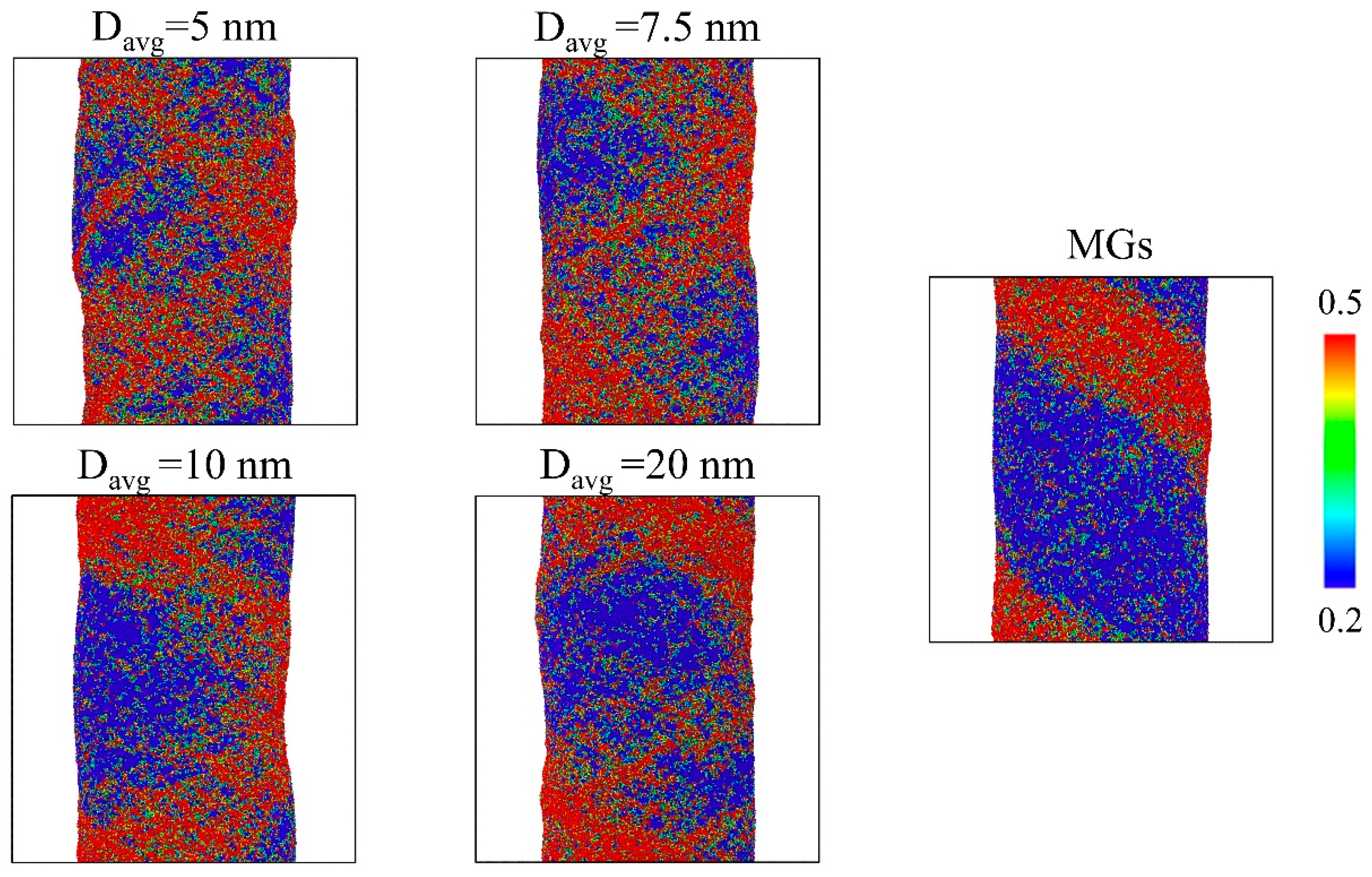
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-Crystalline Structure in Solidified Gold–Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Inoue, A. High Strength Bulk Amorphous Alloys with Low Critical Cooling Rates (Overview). Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.; Koshiba, H.; Kato, H.; Yavari, A.R. Cobalt-Based Bulk Glassy Alloy with Ultrahigh Strength and Soft Magnetic Properties. Nat. Mater. 2003, 2, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.J.; Poon, S.J.; Shiflet, G.J. Mechanical Properties of Iron-Based Bulk Metallic Glasses. J. Mater. Res. 2007, 22, 344–351. [Google Scholar] [CrossRef]
- Peker, A.; Johnson, W.L. A Highly Processable Metallic Glass: Zr41.2Ti13.8Cu12.5Ni10.0Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344. [Google Scholar]
- Ashby, M.F.; Greer, A.L. Metallic Glasses as Structural Materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Lu, J.; Ravichandran, G.; Johnson, W.L. Deformation Behavior of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 Bulk Metallic Glass over a Wide Range of Strain-Rates and Temperatures. Acta Mater. 2003, 51, 3429–3443. [Google Scholar]
- Conner, R.D.; Johnson, W.L.; Paton, N.E.; Nix, W.D. Shear Bands and Cracking of Metallic Glass Plates in Bending. J. Appl. Phys. 2003, 94, 904–911. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic Plasticity or Brittleness of Metallic Glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar] [CrossRef]
- Gu, X.J.; McDermott, A.G.; Poon, S.J.; Shiflet, G.J. Critical Poisson’s Ratio for Plasticity in Fe-Mo-C-B-Ln Bulk Amorphous Steel. Appl. Phys. Lett. 2006, 88, 86–89. [Google Scholar] [CrossRef]
- Xie, S.; George, E.P. Size-Dependent Plasticity and Fracture of a Metallic Glass in Compression. Intermetallics 2008, 16, 485–489. [Google Scholar] [CrossRef]
- Gu, X.J.; Poon, S.J.; Shiflet, G.J.; Widom, M. Ductility Improvement of Amorphous Steels: Roles of Shear Modulus and Electronic Structure. Acta Mater. 2008, 56, 88–94. [Google Scholar] [CrossRef]
- Zhao, K.; Xia, X.X.; Bai, H.Y.; Zhao, D.Q.; Wang, W.H. Room Temperature Homogeneous Flow in a Bulk Metallic Glass with Low Glass Transition Temperature. Appl. Phys. Lett. 2011, 98, 141913. [Google Scholar] [CrossRef]
- Raghavan, R.; Murali, P.; Ramamurty, U. On Factors Influencing the Ductile-to-Brittle Transition in a Bulk Metallic Glass. Acta Mater. 2009, 57, 3332–3340. [Google Scholar] [CrossRef]
- Guo, H.; Yan, P.F.; Wang, Y.B.; Tan, J.; Zhang, Z.F.; Sui, M.L.; Ma, E. Tensile Ductility and Necking of Metallic Glass. Nat. Mater. 2007, 6, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Magagnosc, D.J.; Ehrbar, R.; Kumar, G.; He, M.R.; Schroers, J.; Gianola, D.S. Tunable Tensile Ductility in Metallic Glasses. Sci. Rep. 2013, 3, 1096. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Jiang, H.; Liu, C.T.; Ruan, H.H.; Lu, J. Superior Tensile Ductility in Bulk Metallic Glass with Gradient Amorphous Structure. Sci. Rep. 2014, 4, 4757. [Google Scholar] [CrossRef]
- Ren, Z.Q.; Churakova, A.A.; Wang, X.; Goel, S.; Liu, S.N.; You, Z.S.; Liu, Y.; Lan, S.; Gunderov, D.V.; Wang, J.T.; et al. Enhanced Tensile Strength and Ductility of Bulk Metallic Glasses Zr52.5Cu17.9Al10Ni14.6Ti5 via High-Pressure Torsion. Mater. Sci. Eng. A 2021, 803, 140485. [Google Scholar] [CrossRef]
- Sarac, B.; Schroers, J. Designing Tensile Ductility in Metallic Glasses. Nat. Commun. 2013, 4, 2158. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Hofmann, D.; Jarvis, D.J.; Fecht, H.J. Low-Density High-Strength Bulk Metallic Glasses and Their Composites: A Review. Adv. Eng. Mater. 2015, 17, 761–780. [Google Scholar] [CrossRef]
- Müller, T.; Bachmaier, A.; Konetschnik, R.; Schöberl, T.; Pippan, R. Mechanical Properties of Electrodeposited Amorphous/Crystalline Multilayer Structures in the Fe-P System. Mater. Sci. Eng. A 2018, 715, 83–91. [Google Scholar] [CrossRef]
- Jing, J.; Krämer, A.; Birringer, R.; Gleiter, H.; Gonser, U. Modified Atomic Structure in a Pd-Fe-Si Nanoglass: A Mössbauer Study. J. Non Cryst. Solids 1989, 113, 167–170. [Google Scholar] [CrossRef]
- Averback, R.S.; Hahn, H.; Höfler, H.J.; Logas, J.C. Processing and Properties of Nanophase Amorphous Metallic Alloys: Ni-Ti. Appl. Phys. Lett. 1990, 57, 1745–1747. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, X.C.; Zheng, G.P. Mechanical Properties and Crystallization Behaviors of Microstructured Co-Fe-P Amorphous Alloys. Metall. Mater. Trans. A 2011, 42, 211–218. [Google Scholar] [CrossRef]
- Gleiter, H. Nanoglasses: A New Kind of Noncrystalline Materials. Beilstein J. Nanotechnol. 2013, 4, 517–533. [Google Scholar] [CrossRef]
- Gleiter, H.; Schimmel, T.; Hahn, H. Nanostructured Solids—From Nano-Glasses to Quantum Transistors. Nano Today 2014, 9, 17–68. [Google Scholar] [CrossRef]
- Gleiter, H. Nanoglasses: A New Kind of Noncrystalline Material and the Way to an Age of New Technologies? Small 2016, 12, 2225–2233. [Google Scholar] [CrossRef]
- Chen, N.; Louzguine-Luzgin, D.V.; Yao, K. A New Class of Non-Crystalline Materials: Nanogranular Metallic Glasses. J. Alloys Compd. 2017, 707, 371–378. [Google Scholar] [CrossRef]
- Li, T.; Li, N.; Kuang, B.; Zheng, G. Molecular Dynamics Simulation on the Mechanical Properties of Zr-Cu Metallic Nanoglasses with Heterogeneous Chemical Compositions. Front. Mater. 2024, 11, 1355522. [Google Scholar] [CrossRef]
- Wang, X.D.; Cao, Q.P.; Jiang, J.Z.; Franz, H.; Schroers, J.; Valiev, R.Z.; Ivanisenko, Y.; Gleiter, H.; Fecht, H.J. Atomic-Level Structural Modifications Induced by Severe Plastic Shear Deformation in Bulk Metallic Glasses. Scr. Mater. 2011, 64, 81–84. [Google Scholar] [CrossRef]
- Shao, H.; Xu, Y.; Shi, B.; Yu, C.; Hahn, H.; Gleiter, H.; Li, J. High Density of Shear Bands and Enhanced Free Volume Induced in Zr70Cu20Ni10 Metallic Glass by High-Energy Ball Milling. J. Alloys Compd. 2013, 548, 77–81. [Google Scholar] [CrossRef]
- Fang, J.X.; Vainio, U.; Puff, W.; Würschum, R.; Wang, X.L.; Wang, D.; Ghafari, M.; Jiang, F.; Sun, J.; Hahn, H.; et al. Atomic Structure and Structural Stability of Sc75Fe25 Nanoglasses. Nano Lett. 2012, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Chen, N.; Liu, P.; Wang, Z.; Louzguine-Luzgin, D.V.; Chen, M.W.; Perepezko, J.H. The Ultrastable Kinetic Behavior of an Au-Based Nanoglass. Acta Mater. 2014, 79, 30–36. [Google Scholar] [CrossRef]
- Ghafari, M.; Mu, X.; Bednarcik, J.; Hutchison, W.D.; Gleiter, H.; Campbell, S.J. Magnetic Properties of Iron Clusters in Sc75Fe25 Nanoglass. J. Magn. Magn. Mater. 2020, 494, 165819. [Google Scholar] [CrossRef]
- Cheng, J.; Li, T.; Ullah, S.; Luo, F.; Wang, H.; Yan, M.; Zheng, G. Giant Magnetocaloric Effect in Nanostructured Fe-Co-P Amorphous Alloys Enabled through Pulse Electrodeposition. Nanotechnology 2020, 31, 385704. [Google Scholar] [CrossRef]
- Li, T.; Shen, Y.; Zheng, G. Characterization on the Glass Forming Ability of Metallic Nano-Glasses by the Dynamic Scaling for Mechanical Loss in Supercooled Liquid State. Scr. Mater. 2021, 203, 114109. [Google Scholar] [CrossRef]
- Li, T.; Zheng, G. The Anelastic Behaviors of Co–Fe–Ni–P Metallic Nano-Glasses: Studies on the Viscous Glass–Glass Interfaces. Metall. Mater. Trans. A 2022, 53, 3736–3748. [Google Scholar] [CrossRef]
- Li, T.; Zheng, G. The Influences of Glass–Glass Interfaces and Ni Additions on Magnetic Properties of Transition-Metal Phosphide Nano-Glasses. AIP Adv. 2022, 12, 085229. [Google Scholar] [CrossRef]
- Yang, Q.; Pei, C.-Q.; Yu, H.-B.; Feng, T. Metallic Nanoglasses with Promoted β-Relaxation and Tensile Plasticity. Nano Lett. 2021, 21, 6051–6056. [Google Scholar] [CrossRef]
- Adibi, S.; Branicio, P.S.; Zhang, Y.W.; Joshi, S.P. Composition and Grain Size Effects on the Structural and Mechanical Properties of CuZr Nanoglasses. J. Appl. Phys. 2014, 116, 043522. [Google Scholar] [CrossRef]
- Adibi, S.; Branicio, P.S.; Joshi, S.P. Suppression of Shear Banding and Transition to Necking and Homogeneous Flow in Nanoglass Nanopillars. Sci. Rep. 2015, 5, 15611. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Jiang, F.; Hahn, H.; Li, J.; Gleiter, H.; Sun, J.; Fang, J.X. Plasticity of a Scandium-Based Nanoglass. Scr. Mater. 2015, 98, 40–43. [Google Scholar] [CrossRef]
- Franke, O.; Leisen, D.; Gleiter, H.; Hahn, H. Thermal and Plastic Behavior of Nanoglasses. J. Mater. Res. 2014, 29, 1210–1216. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, F.; Hahn, H.; Li, J.; Gleiter, H.; Sun, J.; Fang, J. Sample Size Effects on Strength and Deformation Mechanism of Sc75Fe25 Nanoglass and Metallic Glass. Scr. Mater. 2016, 116, 95–99. [Google Scholar] [CrossRef]
- Nandam, S.H.; Ivanisenko, Y.; Schwaiger, R.; Śniadecki, Z.; Mu, X.; Wang, D.; Chellali, R.; Boll, T.; Kilmametov, A.; Bergfeldt, T.; et al. Cu-Zr Nanoglasses: Atomic Structure, Thermal Stability and Indentation Properties. Acta Mater. 2017, 136, 181–189. [Google Scholar] [CrossRef]
- Nandam, S.H.; Adjaoud, O.; Schwaiger, R.; Ivanisenko, Y.; Chellali, M.R.; Wang, D.; Albe, K.; Hahn, H. Influence of Topological Structure and Chemical Segregation on the Thermal and Mechanical Properties of Pd-Si Nanoglasses. Acta Mater. 2020, 193, 252–260. [Google Scholar] [CrossRef]
- Li, T.; Ma, K.; Zheng, G. The Effects of Glass-Glass Interfaces on Thermodynamic and Mechanical Properties of Co-Fe-P Metallic Nano-Glasses. J. Mater. Res. 2021, 36, 4951–4962. [Google Scholar] [CrossRef]
- Li, T.; Zheng, G. Atomistic Simulation on the Mechanical Properties of Diffusion Bonded Zr-Cu Metallic Glasses with Oxidized Interfaces. Metall. Mater. Trans. A 2021, 52, 1939–1946. [Google Scholar] [CrossRef]
- Adjaoud, O.; Albe, K. Interfaces and Interphases in Nanoglasses: Surface Segregation Effects and Their Implications on Structural Properties. Acta Mater. 2016, 113, 284–292. [Google Scholar] [CrossRef]
- Zheng, K.; Branicio, P. Synthesis of Metallic Glass Nanoparticles by Inert Gas Condensation. Phys. Rev. Mater. 2020, 4, 076001. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Mu, X.; Goel, S.; Feng, T.; Ivanisenko, Y.; Hahn, H.; Gleiter, H. Surface Segregation of Primary Glassy Nanoparticles of Fe90Sc10 Nanoglass. Mater. Lett. 2016, 181, 248–252. [Google Scholar] [CrossRef]
- Li, T.; Li, N.; Zhang, S.; Zheng, G. Mechanical Property Dependence on Compositional Heterogeneity in Co-P Metallic Nanoglasses. Sci. Rep. 2024, 14, 7458. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1997, 117, 1–19. [Google Scholar] [CrossRef]
- Pak, H.; Doyama, M. The Calculation of a Vacancy and Divacancies in α-Iron. J. Fac. Eng. Univ. Tokyo B 1969, 30, 111–115. [Google Scholar]
- Van Hoang, V.; Hung, N.H.; Anh, N.H.T. Computer Simulation of the Effects of B, P Concentration on the Pore Distribution in the Amorphous Co-B, Co-P Alloys. J. Metastable Nanocryst. Mater. 2003, 18, 43–48. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A Tool for Manipulating and Converting Atomic Data Files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Zheng, G.P.; Gross, D.; Li, M. The Effect of Microstructure on Magnetic Phase Transitions in an Ising Model. Phys. A 2005, 355, 355–373. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and Analysis of Atomistic Simulation Data with OVITO-the Open Visualization Tool. Model. Simul. Mat. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Cao, A.J.; Ma, E. Correlation between the Elastic Modulus and the Intrinsic Plastic Behavior of Metallic Glasses: The Roles of Atomic Configuration and Alloy Composition. Acta Mater. 2009, 57, 3253–3267. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.M.; Zhang, J.C.; Zheng, G.P.; Wang, X.Y. The Prominent Combination of Ultrahigh Strength and Superior Tensile Plasticity in Cu–Zr Nanoglass Connected by Oxide Interfaces: A Molecular Dynamics Study. J. Alloys Compd. 2019, 801, 318–326. [Google Scholar] [CrossRef]
- Ritter, Y.; Sopu, D.; Gleiter, H.; Albe, K. Structure, Stability and Mechanical Properties of Internal Interfaces in Cu64Zr36 Nanoglasses Studied by MD Simulations. Acta Mater. 2011, 59, 6588–6593. [Google Scholar] [CrossRef]
- Şopu, D.; Albe, K. Influence of Grain Size and Composition, Topology and Excess Free Volume on the Deformation Behavior of Cu-Zr Nanoglasses. Beilstein J. Nanotechnol. 2015, 6, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.L.; Langer, J.S. Dynamics of Viscoplastic Deformation in Amorphous Solids. Phys. Rev. 1998, 57, 7192. [Google Scholar] [CrossRef]
- Shimizu, F.; Ogata, S.; Li, J. Theory of Shear Banding in Metallic Glasses and Molecular Dynamics Calculations. Mater. Trans. 2007, 48, 2923–2927. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, N.; Yu, T.; Zheng, G. The Modulation of Compositional Heterogeneity for Controlling Shear Banding in Co-P Metallic Nanoglasses. Nanomaterials 2024, 14, 993. https://doi.org/10.3390/nano14120993
Li T, Li N, Yu T, Zheng G. The Modulation of Compositional Heterogeneity for Controlling Shear Banding in Co-P Metallic Nanoglasses. Nanomaterials. 2024; 14(12):993. https://doi.org/10.3390/nano14120993
Chicago/Turabian StyleLi, Tian, Nana Li, Tianlai Yu, and Guangping Zheng. 2024. "The Modulation of Compositional Heterogeneity for Controlling Shear Banding in Co-P Metallic Nanoglasses" Nanomaterials 14, no. 12: 993. https://doi.org/10.3390/nano14120993





