A Tight-Connection g-C3N4/BiOBr (001) S-Scheme Heterojunction Photocatalyst for Boosting Photocatalytic Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.1.1. Preparation of Bulk g-C3N4
2.1.2. Preparation of x-g-C3N4/BiOBr
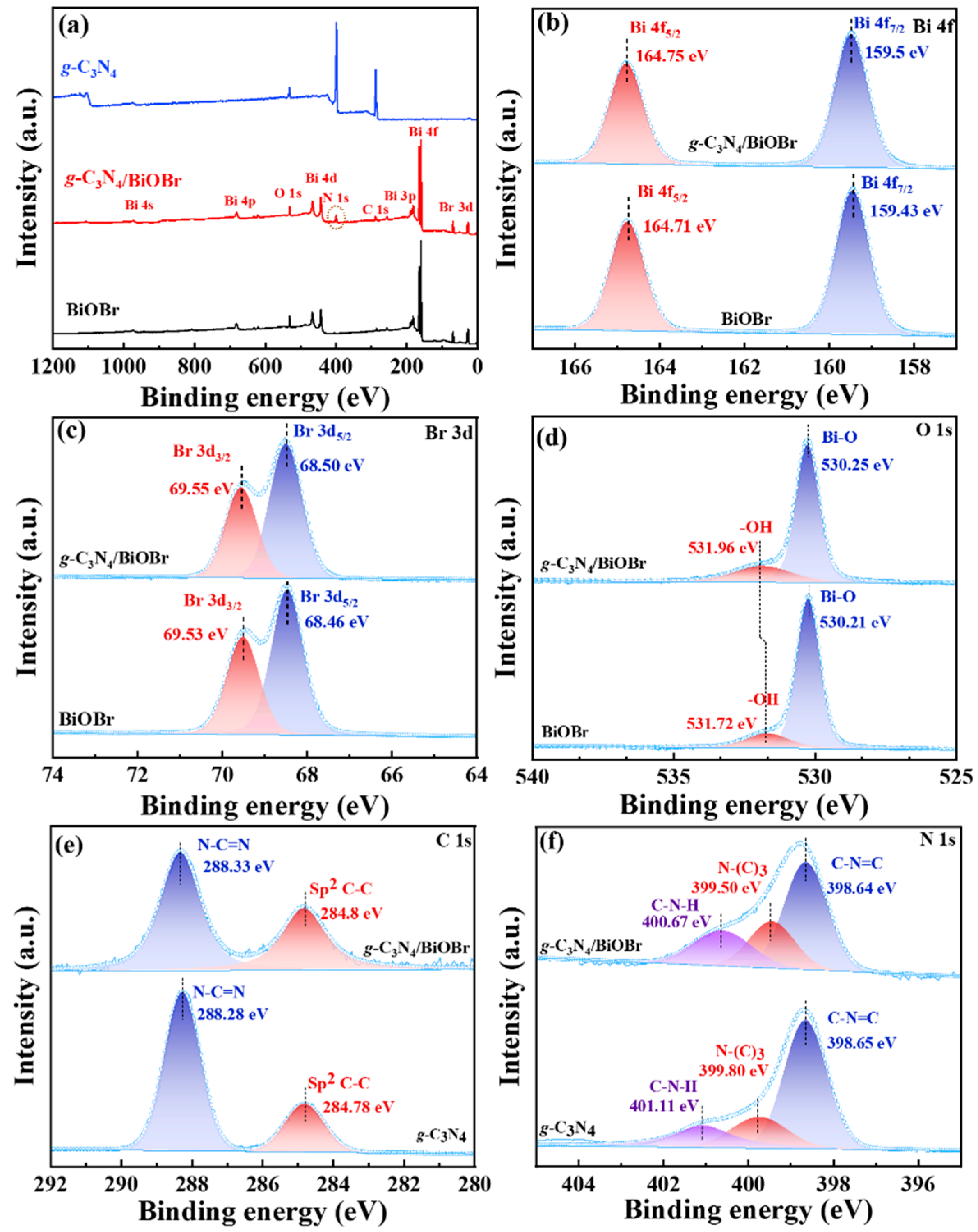
2.2. Characterization
2.3. Photocatalytic Degradation Experiments
2.4. Electrochemical Measurements
3. Results and Discussion
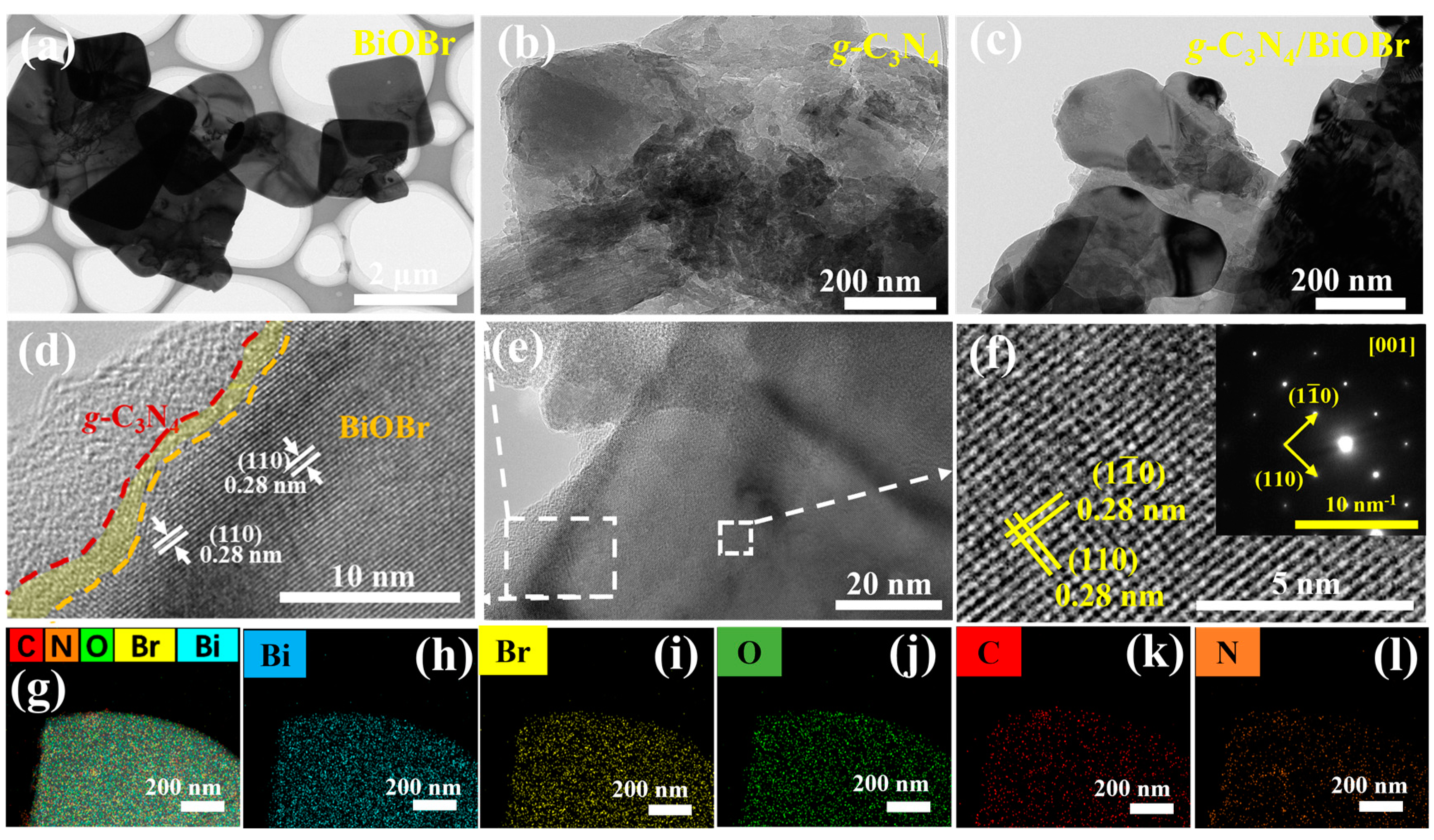
3.1. Structure and Morphology
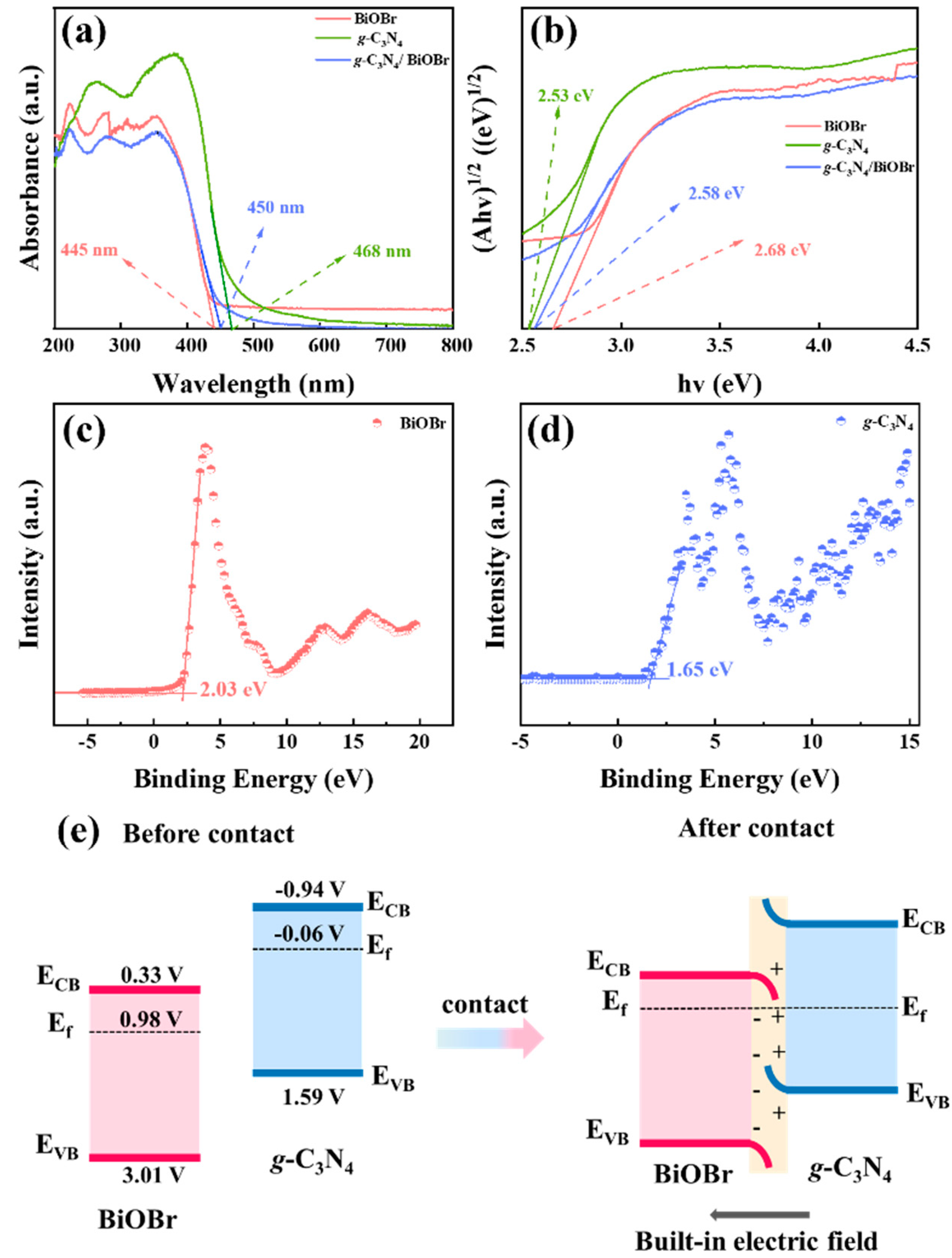
3.2. Band Structure of Catalysts
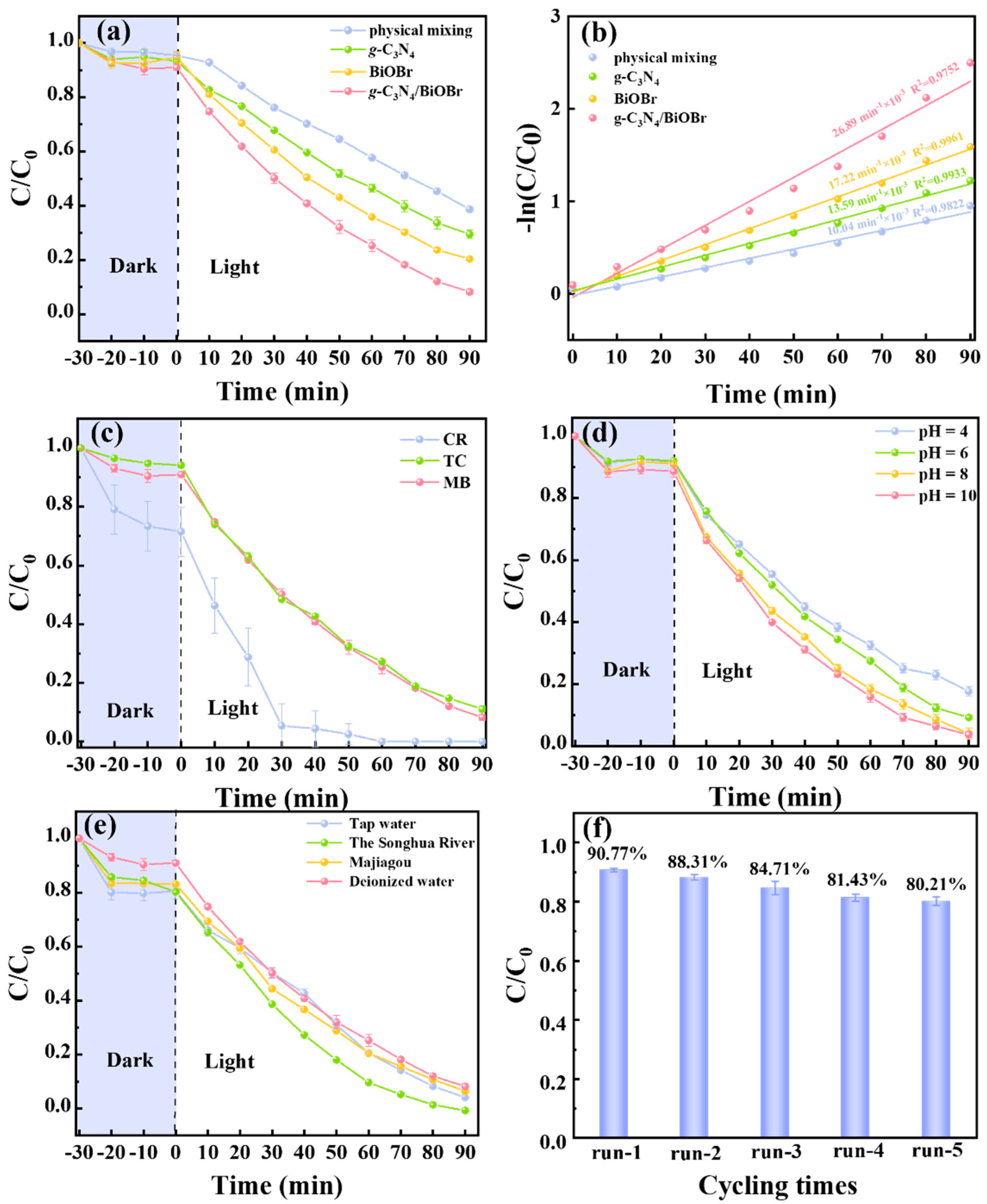
3.3. Photocatalytic Degradation
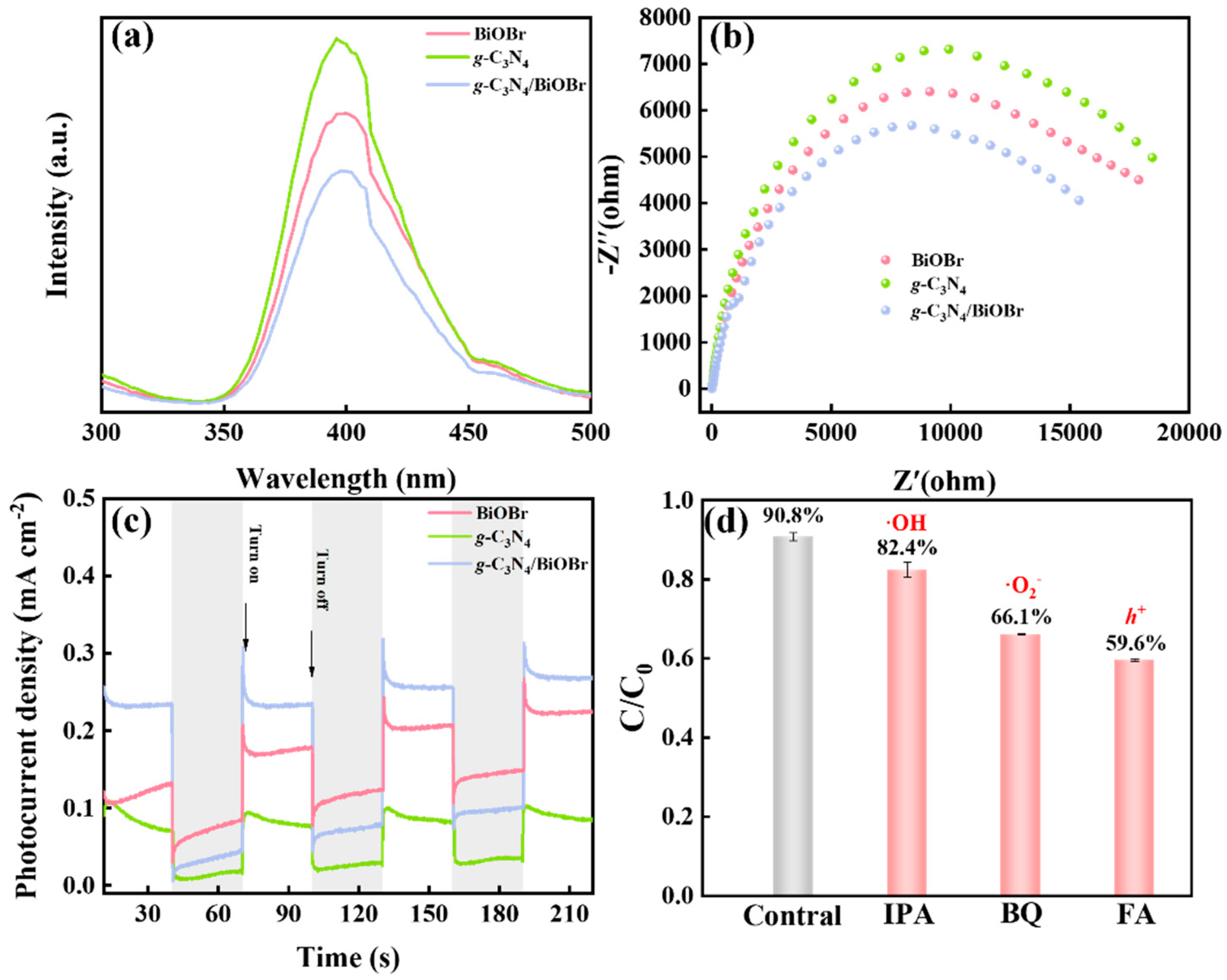
3.4. Photoelectric Properties
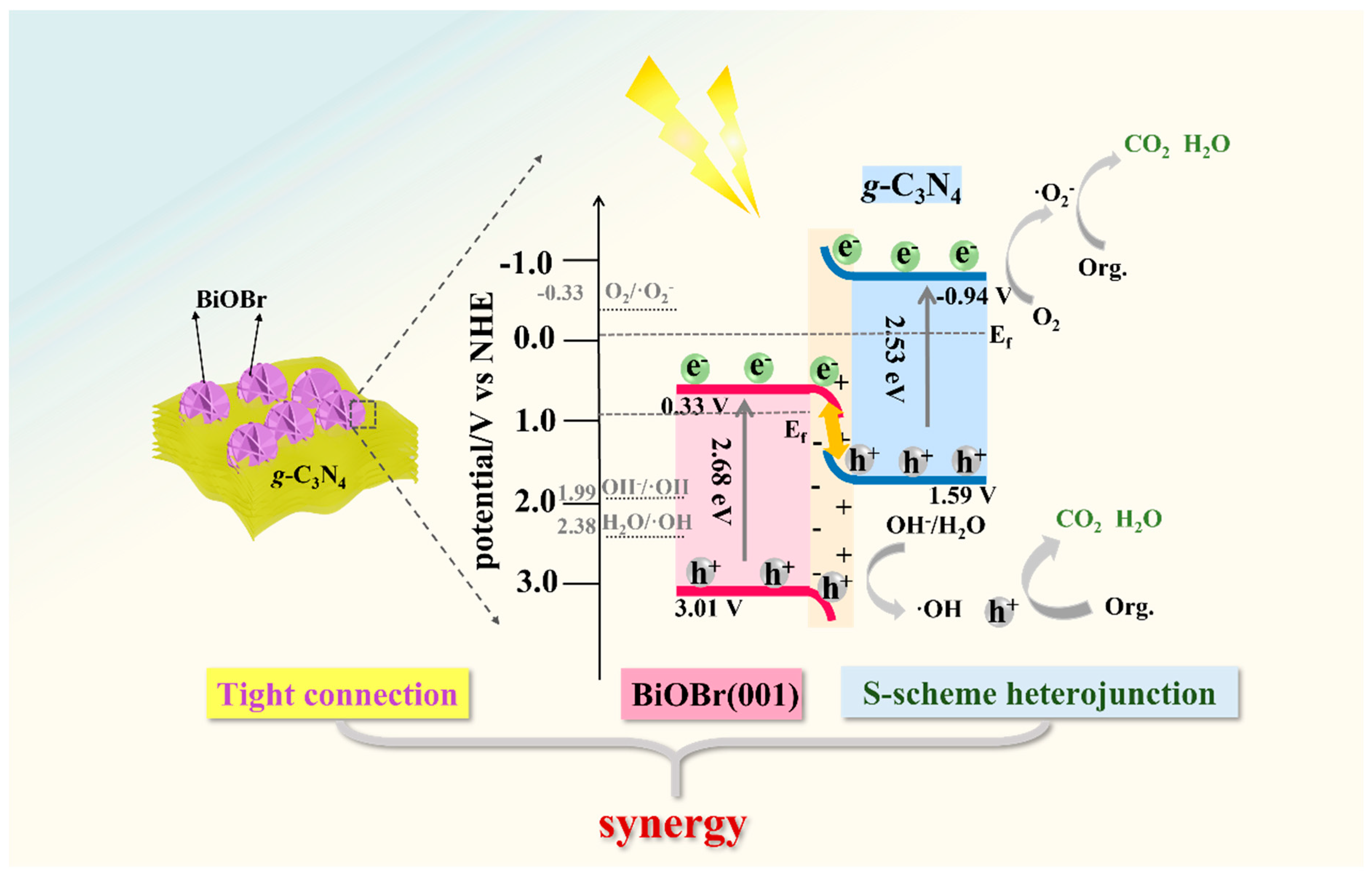
3.5. Photocatalytic Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, N.; Zhao, X.; Gao, M.; Li, Z.; Ding, X.; Li, C.; Liu, K.; Du, X.; Li, W.; Feng, J.; et al. Superior selective adsorption of MgO with abundant oxygen vacancies to removal and recycle reactive dyes. Sep. Purif. Technol. 2021, 275, 119236. [Google Scholar] [CrossRef]
- Gao, M.; Li, W.; Su, X.; Li, Z.; Ding, X.; Du, X.; Ren, Y.; Zhang, H.; Feng, J.; Wei, T. A regenerable Cu2O/BiOBr S-scheme heterojunction photocatalysts for efficient photocatalytic degradation of mixed organic pollutants. Sep. Purif. Technol. 2023, 313, 123447. [Google Scholar] [CrossRef]
- Qing, Y.; Huang, E.; Cao, L.; Gao, H.; Zhao, S.; Li, Y.; Li, W. Facile synthesis of PA photocatalytic membrane based on C-PANI@BiOBr heterostructures with enhanced photocatalytic removal of 17β-estradiol. Chem. Eng. J. 2024, 490, 151617. [Google Scholar] [CrossRef]
- Yi, Q.; Gao, Y.; Zhang, H.; Zhang, H.; Zhang, Y.; Yang, M. Establishment of a pretreatment method for tetracycline production wastewater using enhanced hydrolysis. Chem. Eng. J. 2016, 300, 139–145. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Wu, B.; Xie, H.; Liu, G. Antibiotics and antibiotic resistance genes removal in biological aerated filter. Bioresour. Technol. 2024, 395, 130392. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Yip, A.C.; Lam FL, Y. Bentonite/cerium-modified LDH composite catalyst for catalytic ozonation of high-concentration indigo carmine dye solution. Appl. Catal. A Gen. 2024, 671, 119560. [Google Scholar] [CrossRef]
- Parida, V.K.; Srivastava, S.K.; Chowdhury, S.; Gupta, A.K. Facile synthesis of 2D/0D Bi2O3/MnO2 Z-scheme heterojunction for enhanced visible light-assisted photocatalytic degradation of acetaminophen. Chem. Eng. J. 2023, 472, 144969. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Zhang, R.; Li, Z.; Tian, B.; Ma, H.; Zheng, Z.; Zhou, B.; Ji, M.; Shi, C.; et al. Supramolecularly engineered S-scheme SubPc-Br/MoS2 photocatalyst nanosheets for enhanced photocatalytic degradation of antibiotics. Chem. Eng. J. 2023, 477, 147193. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Fang, M.-J.; Hsu, Y.-J. Modulation of interfacial charge dynamics of semiconductor heterostructures for advanced photocatalytic applications. Coord. Chem. Rev. 2021, 438, 213876. [Google Scholar] [CrossRef]
- Gao, M.; Li, Z.; Su, X.; Zhang, X.; Chang, J.; Geng, D.; Lu, Y.; Zhang, H.; Wei, T.; Feng, J. 2D/2D MgO/g-C3N4 S-scheme heterogeneous tight with Mg–N bonds for efficient photo-Fenton degradation: Enhancing both oxygen vacancy and charge migration. Chemosphere 2023, 343, 140285. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, J.; Wang, S.; Wang, X.; Fang, M.; Zuo, Q.; Kong, M.; Liu, Z.; Tan, X. Stress modulation on photodegradation of tetracycline by Sn-doped BiOBr. J. Environ. Chem. Eng. 2022, 10, 107675. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, D.; Liu, C.; Zhou, X.; Yang, H.; Qu, J.; He, D. Efficient photocatalytic water disinfection by a novel BP/BiOBr S-scheme heterojunction photocatalyst. Chem. Eng. J. 2023, 468, 143581. [Google Scholar] [CrossRef]
- Guo, J.; Liao, X.; Lee, M.-H.; Hyett, G.; Huang, C.-C.; Hewak, D.W.; Mailis, S.; Zhou, W.; Jiang, Z. Experimental and DFT insights of the Zn-doping effects on the visible-light photocatalytic water splitting and dye decomposition over Zn-doped BiOBr photocatalysts. Appl. Catal. B Environ. 2019, 243, 502–512. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Zhang, L.; Shen, J.; Meng, L.; Wang, X. Restructuring surface frustrated Lewis acid-base pairs of BiOBr through isomorphous Sn doping for boosting photocatalytic CO2 reduction. Chem. Eng. J. 2023, 464, 142536. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Li, T.; Xing, X.; Fan, W.; Zhang, L.; Hu, C. Enhanced internal electric field in S-doped BiOBr for intercalation, adsorption and degradation of ciprofloxacin by photoinitiation. Appl. Catal. B Environ. 2022, 302, 120824. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Yu, J.C. Photocatalytic degradation of ibuprofen on S-doped BiOBr. Chemosphere 2021, 278, 130376. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Y.; Qu, Y.; Jing, L.Q. Recent advances in BiOBr-based photocatalysts for environmental remediation. Chin. Chem. Lett. 2021, 32, 3265–3276. [Google Scholar] [CrossRef]
- Wei, X.-X.; Cui, H.; Guo, S.; Zhao, L.; Li, W. Hybrid BiOBr–TiO2 nanocomposites with high visible light photocatalytic activity for water treatment. J. Hazard. Mater. 2013, 263, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Wang, Y.; Chen, Y.; Jiang, X.; Lin, M.; Lin, C.; Lin, T.; Gao, M.; Zhao, C.; Wu, X. Improved piezo-photocatalysis for aquatic multi-pollutant removal via BiOBr/BaTiO3 heterojunction construction. Chem. Eng. J. 2024, 488, 151002. [Google Scholar] [CrossRef]
- Zuo, S.; Huang, S.; Wang, Y.; Wan, J.; Yan, Z.; Ma, Y.; Wang, S. Surface modify BiOBr/Mn-Ti3C2Tx to enhance the carrier separation efficiency and weak electron utilization for photo-assisted peroxymonosulfate activation to degrade emerging contaminants. Chem. Eng. J. 2023, 475, 146230. [Google Scholar] [CrossRef]
- Li, Z.; Wen, C.; Li, D.; Fang, Z.; Lin, Z.; Liu, D.; Wang, Y.; Zhang, X.; Chen, P.; Lv, W.; et al. Insights into nitrogen-doped BiOBr with oxygen vacancy and carbon quantum dots photocatalysts for the degradation of sulfonamide antibiotics: Actions to promote exciton dissociation and carrier migration. Chem. Eng. J. 2024, 492, 152449. [Google Scholar] [CrossRef]
- Ren, G.; Shi, M.; Li, Z.; Zhang, Z.; Meng, X. Electronic metal-support interaction via defective-induced platinum modified BiOBr for photocatalytic N2 fixation. Appl. Catal. B Environ. 2023, 327, 122462. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Zhou, Y.; Zeng, L.; Huang, Y.; Lan, S.; Zhu, M. Piezo-enhanced charge carrier separation over plasmonic Au-BiOBr for piezo-photocatalytic carbamazepine removal. Appl. Catal. B Environ. 2022, 311, 121369. [Google Scholar] [CrossRef]
- Tian, J.; Zhong, K.; Zhu, X.; Yang, J.; Mo, Z.; Liu, J.; Dai, J.; She, Y.; Song, Y.; Li, H.; et al. Highly exposed active sites of Au nanoclusters for photocatalytic CO2 reduction. Chem. Eng. J. 2023, 451, 138392. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Wang, B.; Zhu, X.; Yang, J.; Liu, P.; Zhu, W.; Chen, Z.; Xia, J. Synchronous activation of Ag nanoparticles and BiOBr for boosting solar-driven CO2 reduction. Chin. Chem. Lett. 2023, 34, 107962. [Google Scholar] [CrossRef]
- Allagui, L.; Chouchene, B.; Gries, T.; Medjahdi, G.; Girot, E.; Framboisier, X.; Amara, A.B.H.; Balan, L.; Schneider, R. Core/shell rGO/BiOBr particles with visible photocatalytic activity towards water pollutants. Appl. Surf. Sci. 2019, 490, 580–591. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Chen, P.; Zheng, H.; Shi, J.; Shu, H.; Liu, Y. Photocatalytic degradation of tetracycline by using a regenerable (Bi)BiOBr/rGO composite. J. Clean. Prod. 2022, 339, 130771. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Z.; Xia, J.; Di, J.; Liu, Y.; Chen, R.; Yin, S.; Zhang, S.; Li, H. Enhanced photocatalytic performance of carbon quantum dots/BiOBr composite and mechanism investigation. Chin. Chem. Lett. 2018, 29, 805–810. [Google Scholar] [CrossRef]
- Wu, M.; Wang, H.; Zhang, B.; Wang, J.; Fan, M.; Dong, L.; Li, B.; Chen, Z.; Chen, G. Dual electron reservoirs: Synergistic photocatalytic degradation of antibiotics by oxygen vacancy-rich BiOBr nanoflowers and magnetic carbon nanotubes. Surf. Interfaces 2024, 45, 103947. [Google Scholar] [CrossRef]
- Liu, T.; Hu, K.; Li, Y.; Wang, Y.; Han, D.; Wang, Z.; Gu, F. The Z-Scheme MIL-88B(Fe)/BiOBr Heterojunction Promotes Fe(III)/Fe(II) Cycling and Photocatalytic-Fenton-Like Synergistically Enhances the Degradation of Ciprofloxacin. Small 2024, 2309541. [Google Scholar] [CrossRef]
- Low, B.Q.L.; Jiang, W.; Yang, J.; Zhang, M.; Wu, X.; Zhu, H.; Zhu, H.; Heng, J.Z.X.; Tang, K.Y.; Wu, W.-Y.; et al. 2D/2D Heterojunction of BiOBr/BiOI Nanosheets for In Situ H2O2 Production and Activation toward Efficient Photocatalytic Wastewater Treatment. Small Methods 2023, 8, 2301368. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Yan, J.; Xiong, Z.; Lou, X.; Ye, C.; Chen, J.; Qiu, T. Facile one-pot synthesis of a BiOBr/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity for tetracycline degradation. Chin. J. Chem. Eng. 2023, 53, 222–231. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Yin, Z.; Li, Z.; Dong, X.; Song, Z.; Chen, D.; Hu, R.; Wang, Q.; Han, J.; et al. “One stone four birds” design atom co-sharing BiOBr/Bi2S3 S-scheme heterojunction photothermal synergistic enhanced full-spectrum photocatalytic activity. Appl. Catal. B Environ. 2024, 344, 123601. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, X.; Zhang, K.; Ma, Y.; Wang, H.; Li, H.; Huang, H.; Sun, X.; Ma, T. Construction of a 2D/2D Crystalline Porous Materials Based S-Scheme Heterojunction for Efficient Photocatalytic H2 Production. Adv. Energy Mater. 2024, 14, 2303638. [Google Scholar] [CrossRef]
- Zu, D.; Ying, Y.; Wei, Q.; Xiong, P.; Ahmed, M.S.; Lin, Z.; Li, M.M.-J.; Li, M.; Xu, Z.; Chen, G.; et al. Oxygen Vacancies Trigger Rapid Charge Transport Channels at the Engineered Interface of S-scheme Heterojunction for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. 2024, e202405756. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Xu, Q.; Wageh, S.; Al-Ghamdi, A.A.; Li, X. Design principle of S-scheme heterojunction photocatalyst. J. Mater. Sci. Technol. 2022, 124, 171–173. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Gu, X.; Lin, S.; Qi, K.; Yan, Y.; Li, R.; Popkov, V.; Almjasheva, O. Application of tungsten oxide and its composites in photocatalysis. Sep. Purif. Technol. 2024, 345, 127299. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Jang, H.W. Recent Advances in Bismuth-Based Nanomaterials for Photoelectrochemical Water Splitting. ChemSusChem 2017, 10, 3001–3018. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Liu, B. Ultrathin nanoflake-assembled hierarchical BiOBr microflower with highly exposed {001} facets for efficient photocatalytic degradation of gaseous ortho-dichlorobenzene. Appl. Catal. B Environ. 2021, 281, 119478. [Google Scholar] [CrossRef]
- Dou, X.C.; Zhang, C.L.; Shi, H.F. The simultaneous promotion of Cr (VI) photoreduction and tetracycline removal over 3D/2D Cu2O/BiOBr S-scheme nanostructures. Sep. Purif. Technol. 2022, 282, 120023. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Zhang, J.; Liu, Q.; Liang, C.; Zhu, G. Facile constructing novel 2D porous g-C3N4/BiOBr hybrid with enhanced visible-light-driven photocatalytic activity. Sep. Purif. Technol. 2017, 178, 6–17. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Jia, J.; Tian, J.; Li, Z.; Wang, X.; Xing, Z. Oxygen vacancy-mediated sandwich-structural TiO2−x/ultrathin g-C3N4/TiO2−x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Hazard. Mater. 2021, 408, 124432. [Google Scholar] [CrossRef]
- Luo, Z.; Ye, X.; Zhang, S.; Xue, S.; Yang, C.; Hou, Y.; Xing, W.; Yu, R.; Sun, J.; Yu, Z.; et al. Unveiling the charge transfer dynamics steered by built-in electric fields in BiOBr photocatalysts. Nat. Commun. 2022, 13, 2230. [Google Scholar] [CrossRef]
- Gao, M.; Feng, J.; He, F.; Zeng, W.; Wang, X.; Ren, Y.; Wei, T. Carbon microspheres work as an electron bridge for degrading high concentration MB in CoFe2O4@carbon microsphere/g-C3N4 with a hierarchical sandwich-structure. Appl. Surf. Sci. 2020, 507, 145167. [Google Scholar] [CrossRef]
- Li, H.; Deng, F.; Zheng, Y.; Hua, L.; Qu, C.; Luo, X. Visible-light-driven Z-scheme rGO/Bi2S3–BiOBr heterojunctions with tunable exposed BiOBr (102) facets for efficient synchronous photocatalytic degradation of 2-nitrophenol and Cr(vi) reduction. Environ. Sci. Nano 2019, 6, 3670–3683. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Shen, Y.; Liu, S.; Ma, H.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Feng, J.; Ren, Y.; Ma, J. Impact of Crystal Types of AgFeO2 Nanoparticles on the Peroxymonosulfate Activation in the Water. Environ. Sci. Technol. 2019, 53, 4500–4510. [Google Scholar] [CrossRef]
- Sá, J.; Agüera, C.A.; Gross, S.; Anderson, J.A. Photocatalytic nitrate reduction over metal modified TiO2. Appl. Catal. B Environ. 2009, 85, 192–200. [Google Scholar] [CrossRef]
- Su, X.; Cao, N.; Feng, J.; Li, W.; Ding, X.; Li, Z.; Gao, M.; Hu, L.; Zhang, H.; Ren, Y. The enhanced photocatalytic performance of the amorphous carbon/MgO nanofibers: Insight into the role of the oxygen vacancies and 1D morphology. Appl. Surf. Sci. 2023, 616, 156470. [Google Scholar] [CrossRef]
- Yang, W.; Sun, K.; Wan, J.; Ma, Y.-A.; Liu, J.; Zhu, B.; Liu, L.; Fu, F. Boosting holes generation and O2 activation by bifunctional NiCoP modified Bi4O5Br2 for efficient photocatalytic aerobic oxidation. Appl. Catal. B Environ. 2023, 320, 121978. [Google Scholar] [CrossRef]
- Lu, W.; Xu, L.; Shen, X.; Meng, L.; Pan, Y.; Zhang, Y.; Han, J.; Mei, X.; Qiao, W.; Gan, L. Highly efficient activation of sulfite by p-type S-doped g-C3N4 under visible light for emerging contaminants degradation. Chem. Eng. J. 2023, 472, 144708. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Lu, X.; Xu, W.; Gong, Q.; Ding, J.; Dan, B.; Xie, P. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2019, 358, 1091–1100. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Wu, D.; Cheng, X.; Ren, H. UV/Nitrate photocatalysis for degradation of Methylene blue in wastewater: Kinetics, transformation products, and toxicity assessment. Environ. Technol. Innov. 2022, 25, 102198. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, J.; Yao, Y.; Shen, H.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Preparation, characterization and photodegradation mechanism of 0D/2D Cu2O/BiOCl S-scheme heterojunction for efficient photodegradation of tetracycline. Sep. Purif. Technol. 2022, 291, 120965. [Google Scholar]
- Yang, Q.; Tan, G.; Yin, L.; Liu, W.; Zhang, B.; Feng, S.; Bi, Y.; Liu, T.; Wang, Z.; Ren, H.; et al. Full-spectrum broad-spectrum degradation of antibiotics by BiVO4@BiOCl crystal plane S-type and Z-type heterojunctions. Chem. Eng. J. 2023, 467, 143405. [Google Scholar] [CrossRef]
- Shi, W.; Hao, C.; Fu, Y.; Guo, F.; Tang, Y.; Yan, X. Enhancement of synergistic effect photocatalytic/persulfate activation for degradation of antibiotics by the combination of photo-induced electrons and carbon dots. Chem. Eng. J. 2022, 433, 133741. [Google Scholar] [CrossRef]
- Lian, Z.; Wu, T.; Zhang, X.; Cai, S.; Xiong, Y.; Yang, R. Synergistic degradation of tetracycline from Mo2C/MoOx films mediated peroxymonosulfate activation and visible-light triggered photocatalysis. Chem. Eng. J. 2023, 469, 143774. [Google Scholar] [CrossRef]
- Hang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, W.; Hu, L.; Gao, M.; Feng, J. A Tight-Connection g-C3N4/BiOBr (001) S-Scheme Heterojunction Photocatalyst for Boosting Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2024, 14, 1071. https://doi.org/10.3390/nano14131071
Zhang X, Li W, Hu L, Gao M, Feng J. A Tight-Connection g-C3N4/BiOBr (001) S-Scheme Heterojunction Photocatalyst for Boosting Photocatalytic Degradation of Organic Pollutants. Nanomaterials. 2024; 14(13):1071. https://doi.org/10.3390/nano14131071
Chicago/Turabian StyleZhang, Xinyi, Weixia Li, Liangqing Hu, Mingming Gao, and Jing Feng. 2024. "A Tight-Connection g-C3N4/BiOBr (001) S-Scheme Heterojunction Photocatalyst for Boosting Photocatalytic Degradation of Organic Pollutants" Nanomaterials 14, no. 13: 1071. https://doi.org/10.3390/nano14131071



