Data-Driven and Machine Learning to Screen Metal–Organic Frameworks for the Efficient Separation of Methane
Abstract
:1. Introduction
2. Model and Methods
2.1. Molecular Model
2.2. Molecular Simulations
2.3. Machine Learning
3. Results and Discussion
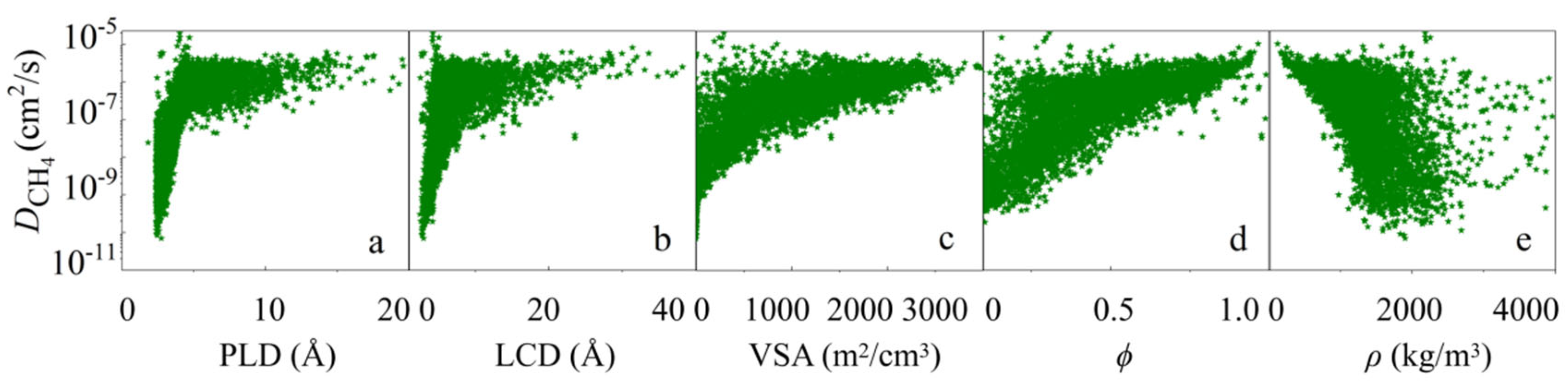
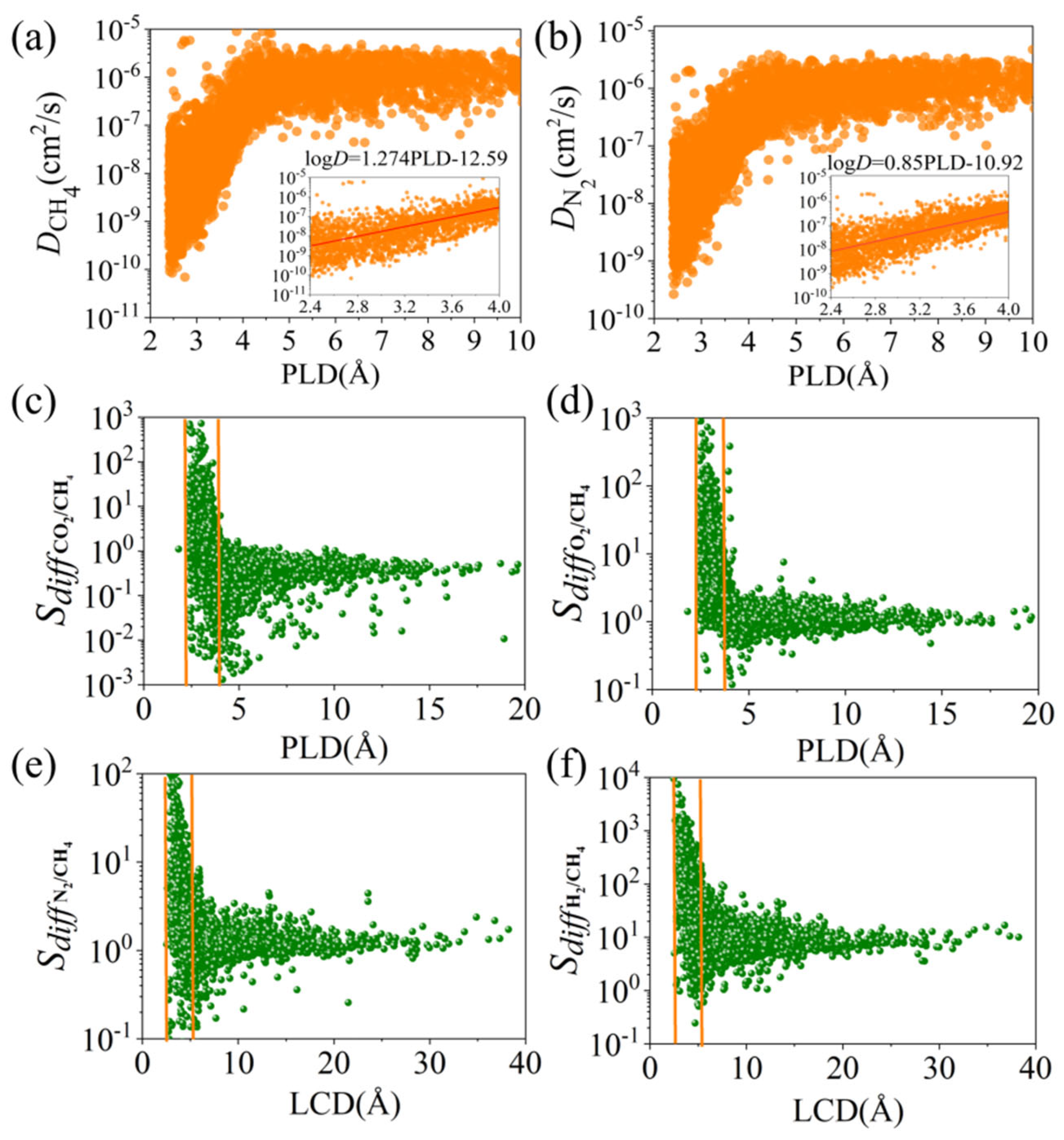
3.1. Statistical Analysis
3.2. Machine Learning
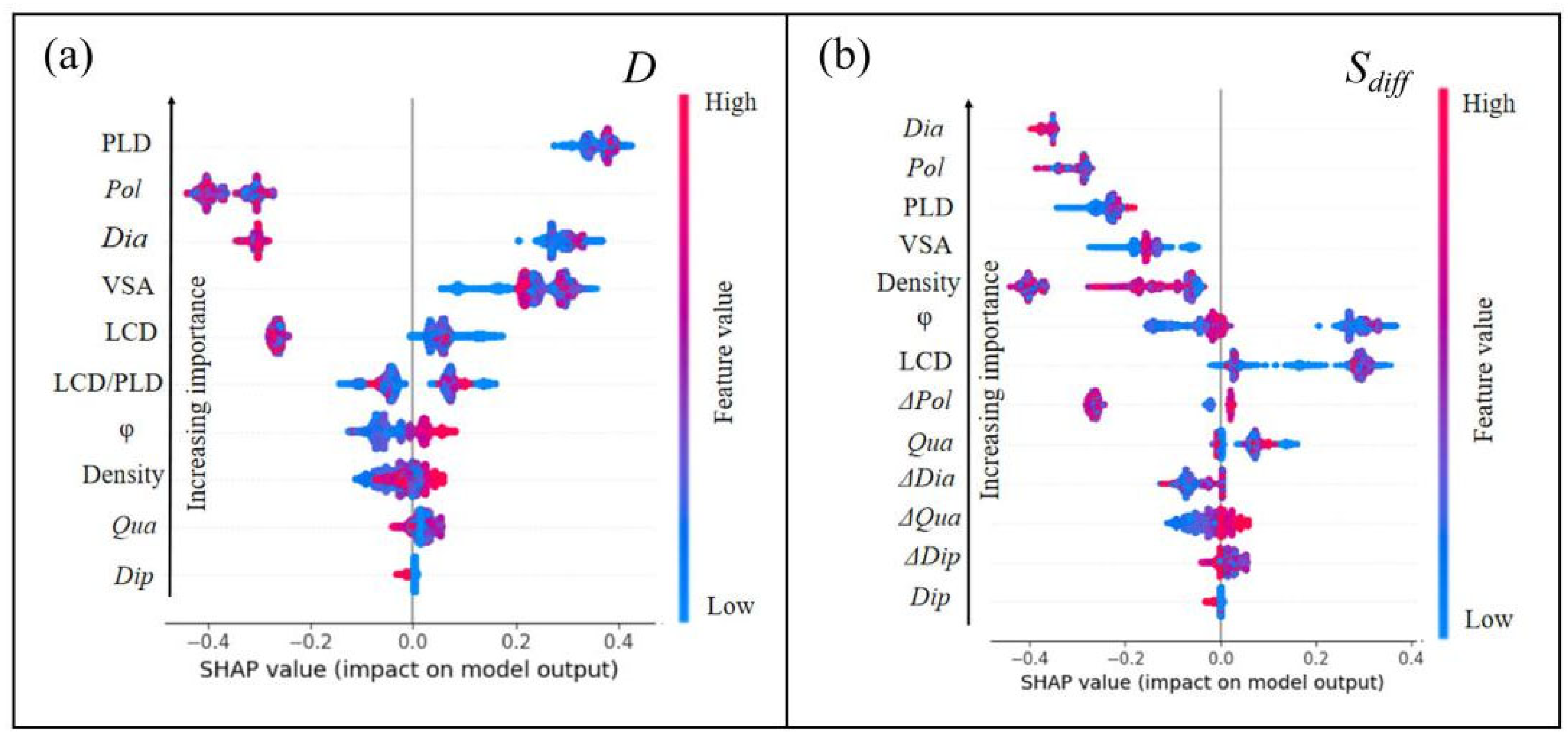
3.3. SHAP Analysis
3.4. Top-Performing Metal–Organic Frameworks (MOFs)
3.5. Design Strategies of MOFs with High Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warmuzinski, K. Harnessing methane emissions from coal mining. Process Saf. Environ. Prot. 2008, 86, 315–320. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Thielemann, T.; Cramer, B.; Schippers, A. Coalbed methane in the Ruhr Basin, Germany: A renewable energy resource. Org. Geochem. 2004, 35, 1537–1549. [Google Scholar] [CrossRef]
- Tutak, M.; Brodny, J. Forecasting methane emissions from hard coal mines including the methane drainage process. Energies 2019, 12, 3840. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, D.; Zeng, S.; Yang, L.; Dong, X.; Zhang, Q.; Xu, Y.; Liu, Z. Significant enhancement in CH4/N2 separation with amine-modified zeolite Y. Fuel 2021, 301, 121077. [Google Scholar] [CrossRef]
- Lashof, D.A.; Ahuja, D.R. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- Yin, Z.; Linga, P. Methane hydrates: A future clean energy resource. Chin. J. Chem. Eng. 2019, 27, 2026–2036. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Tyagi, B.; Vijay, V.K.; Vijay, V.; Thakur, I.S.; Kamyab, H.; Nguyen, D.D.; Kumar, A. Advances in biogas valorization and utilization systems: A comprehensive review. J. Clean. Prod. 2020, 273, 123052. [Google Scholar] [CrossRef]
- Gomes, V.G.; Yee, K.W.K. Pressure swing adsorption for carbon dioxide sequestration from exhaust gases. Sep. Purif. Technol. 2002, 28, 161–171. [Google Scholar] [CrossRef]
- Wiheeb, A.D.; Helwani, Z.; Kim, J.; Othman, M.R. Pressure swing adsorption technologies for carbon dioxide capture. Sep. Purif. Rev. 2016, 45, 108–121. [Google Scholar] [CrossRef]
- Mel, M.; Ibrahim MM, A.; Setyobudi, R.H. Preliminary study of biogas upgrading and purification by pressure swing adsorption. Proc. AIP Conf. 2016, 1755, 130010. [Google Scholar]
- Lin, R.B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; An, B.; Lin, W. Metal–organic frameworks in solid–gas phase catalysis. ACS Catal. 2018, 9, 130–146. [Google Scholar] [CrossRef]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal organic frameworks as drug targeting delivery vehicles in the treatment of cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Jiang, X.L.; Xu, H.; Zhao, B. Applications of MOF as luminescent sensors for environmental pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Q.; Yin, X.B. Metal–organic frameworks with multiple luminescence emissions: Designs and applications. Acc. Chem. Res. 2020, 53, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, H.; Zhang, X.; Zhao, H.; Li, F.; Gu, Y. Designed metal-organic frameworks with potential for multi-component hydrocarbon separation. Coord. Chem. Rev. 2023, 484, 215111. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, W.; Deng, X.; Cai, C.; Yan, Y.; Liang, H.; Liu, Z.; Qiao, Z. Machine-learning-assisted high-throughput computational screening of high performance metal–organic frameworks. Mol. Syst. Des. Eng. 2020, 5, 725–742. [Google Scholar] [CrossRef]
- Anbia, M.; Hoseini, V.; Sheykhi, S. Sorption of methane, hydrogen and carbon dioxide on metal-organic framework, iron terephthalate (MOF-235). J. Ind. Eng. Chem. 2012, 18, 1149–1152. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.; Pham, T.; Lan, P.C.; Xing, H.; Forrest, K.A.; Wojtas, L.; Space, B.; Ma, S. A metal–organic framework based methane Nano-trap for the capture of coal-mine methane. Angew. Chem. Int. Ed. 2019, 58, 10138–10141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, Q.; Li, Y.N.; Yang, L.; Gao, Q.; Li, Y.N.; Liu, Q.N.; Liu, X.T.; Wang, S.; Chen, L.Z. Efficient purification of CH4 from ternary mixtures by a microporous heterometal-organic framework. Sep. Purif. Technol. 2024, 335, 126235. [Google Scholar] [CrossRef]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C.; Mecha, A.C. Potential of wood ash in purification of biogas. Energy Sustain. Dev. 2021, 65, 45–52. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.; Cao, H.; Ji, W.; Duan, J.; Jing, S.; Jin, W. Finely Tuned Porous Coordination Polymers To Boost Methane Separation Efficiency. Chem. Eur. J. 2019, 25, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, E.L.; Li, M. Purification of coal mine methane on carbon molecular sieve by vacuum pressure swing adsorption. Sep. Sci. Technol. 2016, 51, 909–916. [Google Scholar] [CrossRef]
- Tu, S.; Yu, L.; Lin, D.; Chen, Y.; Wu, Y.; Zhou, X.; Li, Z.; Xia, Q. Robust nickel-based metal–organic framework for highly efficient methane purification and capture. ACS Appl. Mater. Interfaces 2022, 14, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Xue, M.; Fan, L.; Huang, L.; Guo, L.; Wei, G.; Chen, B.; Qiu, S. Highly selective sieving of small gas molecules by using an ultra-microporous metal—Organic framework membrane. Energy Environ. Sci. 2014, 7, 4053–4060. [Google Scholar] [CrossRef]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Yildirim, T.; Sholl, D.S.; Snurr, R.Q. Computation-ready, experimental metal—Organic frameworks: A tool to enable high-throughput screening of nanoporous crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Willems, T.F.; Rycroft, C.H.; Kazi, M.; Meza, J.C.; Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 2012, 149, 134–141. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Chokbunpiam, T.; Chanajaree, R.; Caro, J.; Janke, W.; Remsungnen, T.; Hannongbua, S.; Fritzsche, S. Separation of nitrogen dioxide from the gas mixture with nitrogen by use of ZIF materials; computer simulation studies. Comput. Mater. Sci. 2019, 168, 246–252. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 2013, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Luo, S.; Zhao, G.; Wu, H. Predicting hard rock pillar stability using GBDT, XGBoost, and LightGBM algorithms. Mathematics 2020, 8, 765. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. Lightgbm: A highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 2017, 3149–3157, 3149–3157. [Google Scholar]
- Bergstra, J.; Komer, B.; Eliasmith, C.; Yamins, D.; Cox, D.D. Hyperopt: A python library for model selection and hyperparameter optimization. Comput. Sci. Discov. 2015, 8, 014008. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 4768–4777, 4768–4777. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Vassallo, A.; Wu, J. Toward the inverse design of MOF membranes for efficient D2/H2 separation by combination of physics-based and data-driven modeling. J. Membr. Sci. 2020, 598, 117675. [Google Scholar] [CrossRef]
- Qiao, Z.; Peng, C.; Zhou, J.; Jiang, J. High-throughput computational screening of 137953 metal–organic frameworks for membrane separation of a CO2/N2/CH4 mixture. J. Mater. Chem. A 2016, 4, 15904–15912. [Google Scholar] [CrossRef]
- Yu, S.; Bo, J.; Meijun, Q. Molecular dynamic simulation of self-and transport diffusion for CO2/CH4/N2 in low-rank coal vitrinite. Energy Fuels 2018, 32, 3085–3096. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Duan, Y.; Wei, Y.; Dong, C.; Ding, L.; Qiao, Z.; Wang, H. Selective gas diffusion in two-dimensional MXene lamellar membranes: Insights from molecular dynamics simulations. J. Mater. Chem. A 2018, 6, 11734–11742. [Google Scholar] [CrossRef]
- Tang, H.; Jiang, J. In silico screening and design strategies of ethane-selective metal–organic frameworks for ethane/ethylene separation. AIChE J. 2021, 67, e17025. [Google Scholar] [CrossRef]

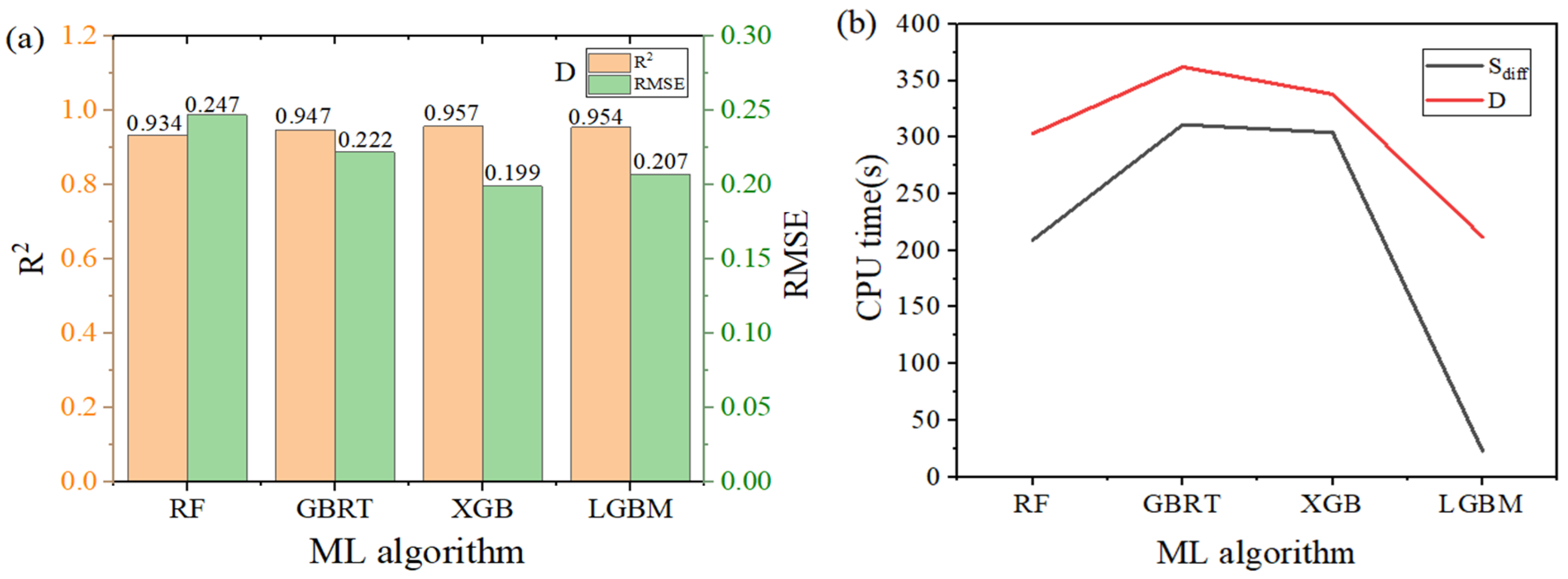

| Indicators | Algorithm | R2 | MAE | RMSE |
|---|---|---|---|---|
| D | RF | 0.934 | 0.151 | 0.247 |
| LGBM | 0.954 | 0.127 | 0.207 | |
| XGB | 0.957 | 0.119 | 0.199 | |
| GBRT | 0.947 | 0.129 | 0.222 | |
| S | RF | 0.887 | 0.155 | 0.264 |
| LGBM | 0.931 | 0.127 | 0.207 | |
| XGB | 0.928 | 0.123 | 0.210 | |
| GBRT | 0.907 | 0.135 | 0.240 |
| Gas Mixture i/j | CSD Cord | LCD [Å] | ϕ | PLD [Å] | ρ [kg/m3] | Di [cm2/s] | Dj [cm2/s] | Sdiff(i/j) |
|---|---|---|---|---|---|---|---|---|
| He/CH4 | ELUQIM04 | 2.92 | 0.04 | 2.44 | 1764.65 | 9.03 × 10−6 | 1.47 × 10−10 | 61,406.36 |
| ELUQIM05 | 2.90 | 0.04 | 2.43 | 1773.83 | 8.42 × 10−6 | 1.76 × 10−10 | 47,815.36 | |
| ELUQIM06 | 2.89 | 0.04 | 2.41 | 1779.43 | 9.74 × 10−6 | 8.11 × 10−10 | 12,015.02 | |
| H2/CH4 | ELUQIM05 | 2.90 | 0.04 | 2.43 | 1773.83 | 7.08 × 10−6 | 1.76 × 10−10 | 40,173.07 |
| ELUQIM04 | 2.92 | 0.04 | 2.44 | 1764.65 | 4.14 × 10−6 | 1.47 × 10−10 | 28,148.27 | |
| FAPYEA04 | 2.47 | 0.00 | 2.40 | 1583.54 | 2.83 × 10−6 | 3.01 × 10−10 | 9397.11 | |
| CO2/CH4 | XEKDUO | 2.98 | 0.02 | 2.75 | 1903.55 | 1.70 × 10−7 | 6.92 × 10−11 | 2463.01 |
| ELUQIM05 | 2.90 | 0.04 | 2.43 | 1773.83 | 3.91 × 10−7 | 1.76 × 10−10 | 2220.35 | |
| HIQPEE | 3.84 | 0.15 | 3.12 | 1440.14 | 1.24 × 10−6 | 5.69 × 10−10 | 2185.18 | |
| O2/CH4 | FAPYEA04 | 2.47 | 0.00 | 2.40 | 1583.54 | 2.92 × 10−7 | 3.01 × 10−10 | 968.16 |
| ELUQIM05 | 2.90 | 0.04 | 2.43 | 1773.83 | 1.58 × 10−7 | 1.76 × 10−10 | 898.78 | |
| GUXQAS | 2.79 | 0.02 | 2.52 | 1598.28 | 1.16 × 10−7 | 1.30 × 10−10 | 893.53 | |
| H2S/CH4 | GUXQAS | 2.79 | 0.02 | 2.52 | 1598.28 | 1.70 × 10−9 | 1.30 × 10−10 | 13.04 |
| RUPZIM | 3.48 | 0.11 | 3.25 | 1549.49 | 1.51 × 10−7 | 1.39 × 10−8 | 10.87 | |
| GUXPUL | 2.79 | 0.02 | 2.58 | 1595.02 | 1.18 × 10−9 | 1.10 × 10−10 | 10.70 | |
| N2/CH4 | FAPYEA04 | 2.47 | 0.00 | 2.40 | 1583.54 | 1.11 × 10−7 | 3.01 × 10−10 | 369.05 |
| PARFOF | 2.77 | 0.05 | 2.46 | 1541.02 | 4.78 × 10−7 | 2.16 × 10−9 | 221.18 | |
| HIWXER01 | 3.29 | 0.13 | 2.76 | 2533.00 | 1.80 × 10−7 | 1.27 × 10−9 | 141.34 |
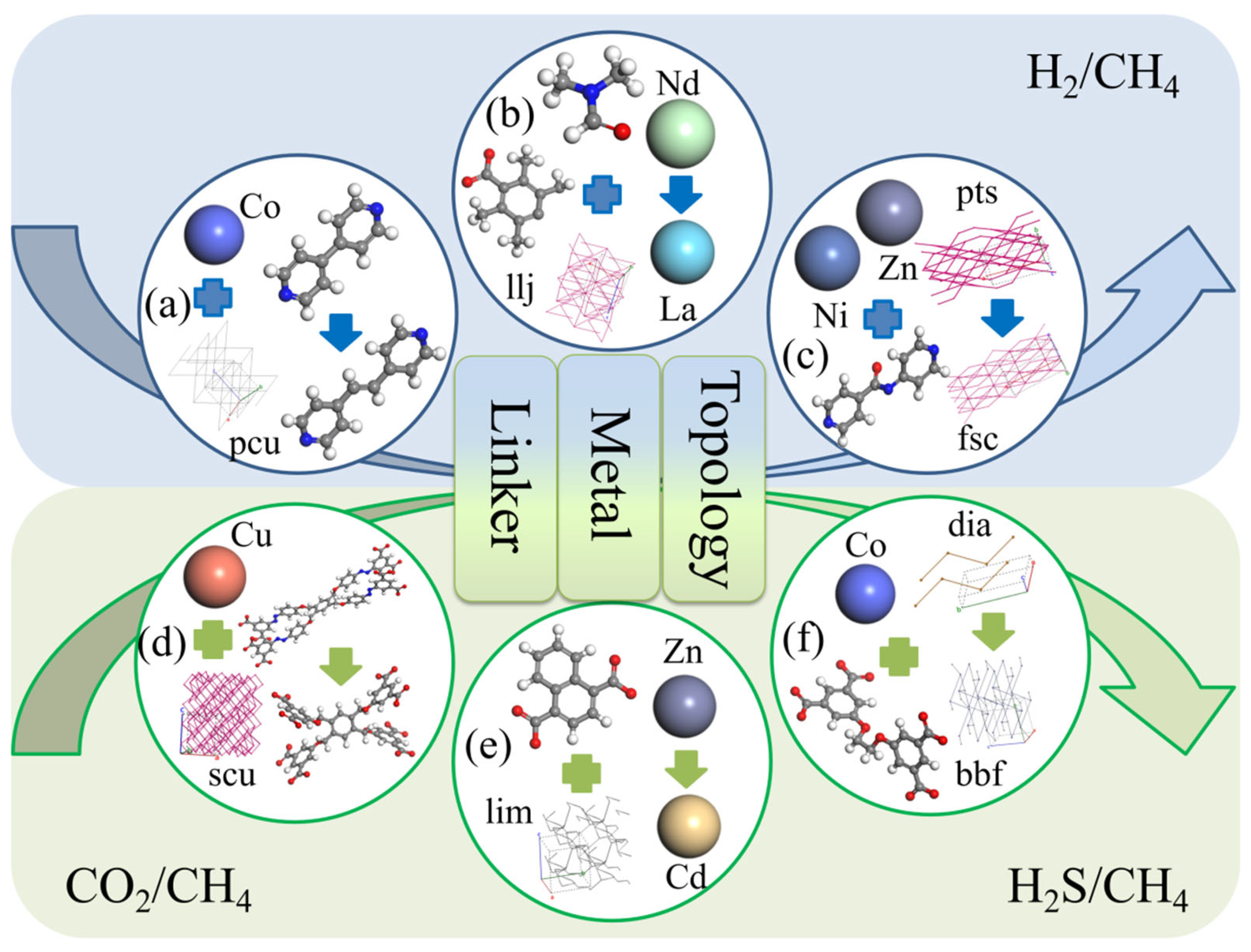
| Gas Mixture (i/j) | NO. | CSD Cord | Metal Center | Organic Links | Top Structure | Sdiff(i/j) |
|---|---|---|---|---|---|---|
| H2/CH4 | a | GIRDUI | Co | MGFJDEHFNMWYBD | pcu | 7.93 |
| GIRGUL | Co | MTAVBTGOXNGCJR | pcu | 643.48 | ||
| b | CIMTAV | La | BVKZGUZCCUSVTD | llj | 4.58 | |
| CIMTEZ | Nd | BVKZGUZCCUSVTD | llj | 12.97 | ||
| c | EBELUU | NiZn | JEVCWSUVFOYBFI | fsc | 5.36 | |
| EBEMEF | NiZn | JEVCWSUVFOYBFI | pts | 147.93 | ||
| CO2/CH4 | d | CAJQEL | Cu | GRYHAGOZZMMYAO | scu | 0.31 |
| CAJQIP | Cu | DUKMDOUQAIDJRW | scu | 2.24 | ||
| e | HEBTEP | Zn | ABMFBCRYHDZLRD | lim | 21.93 | |
| SETFUT | Cd | ABMFBCRYHDZLRD | lim | 1.22 | ||
| H2S/CH4 | f | ISIKIF | Co | GEBVRXNOWAYDCP | dia | 16.07 |
| ISIKOL | Co | GEBVRXNOWAYDCP | bbf | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Huang, X.; Xu, F.; Wang, W.; Li, H.; Gong, L.; Zhao, Y.; Guo, S.; Liang, H.; Qiao, Z. Data-Driven and Machine Learning to Screen Metal–Organic Frameworks for the Efficient Separation of Methane. Nanomaterials 2024, 14, 1074. https://doi.org/10.3390/nano14131074
Guan Y, Huang X, Xu F, Wang W, Li H, Gong L, Zhao Y, Guo S, Liang H, Qiao Z. Data-Driven and Machine Learning to Screen Metal–Organic Frameworks for the Efficient Separation of Methane. Nanomaterials. 2024; 14(13):1074. https://doi.org/10.3390/nano14131074
Chicago/Turabian StyleGuan, Yafang, Xiaoshan Huang, Fangyi Xu, Wenfei Wang, Huilin Li, Lingtao Gong, Yue Zhao, Shuya Guo, Hong Liang, and Zhiwei Qiao. 2024. "Data-Driven and Machine Learning to Screen Metal–Organic Frameworks for the Efficient Separation of Methane" Nanomaterials 14, no. 13: 1074. https://doi.org/10.3390/nano14131074






