Advancing Silver Bismuth Sulfide Quantum Dots for Practical Solar Cell Applications
Abstract
:1. Introduction
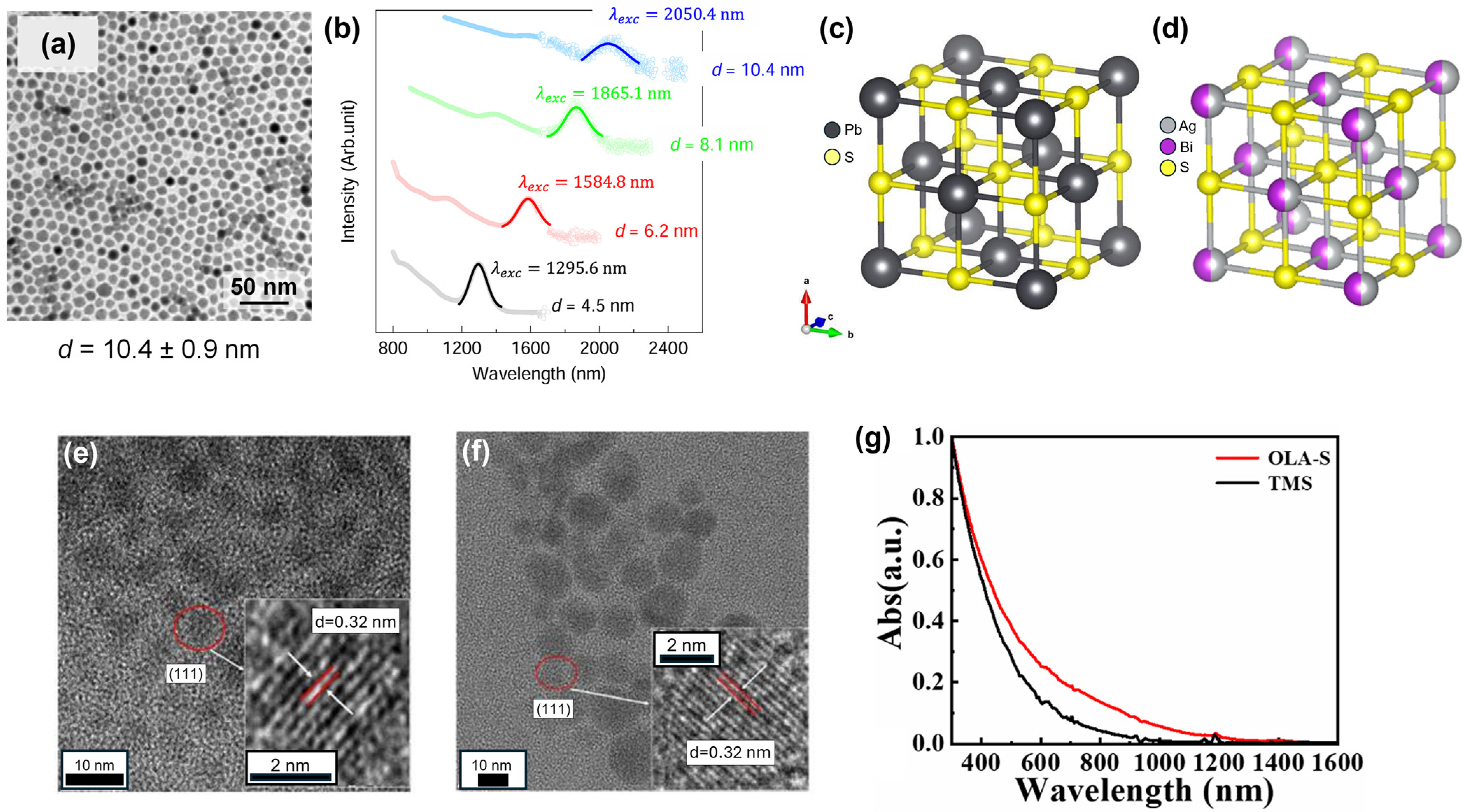
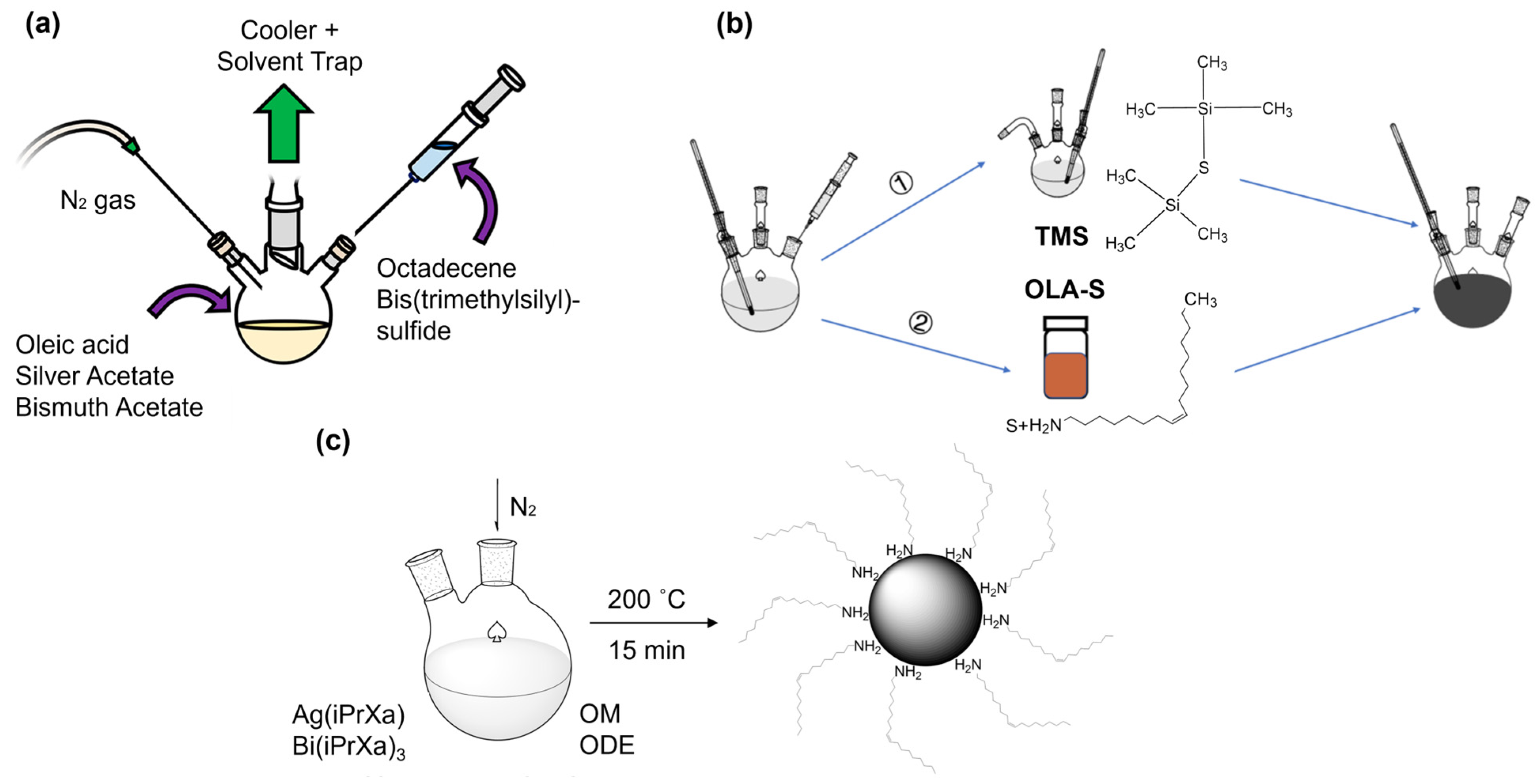
2. Synthesis Method
| Material | Method | Precursor | Size (nm) | Application | Ref |
|---|---|---|---|---|---|
| AgBiS2 QDs | Chemical and sonochemical deposition | AgNO3, Bi(NO3)3, and Na2S2O3 in HNO3 | 4.2 | Photoconductor | [27] |
| AgBiS2 NCs | Chemical synthesis and solvothermal | Ag(OAc), Bi(OAc), and sulfur in OA | ~11 | Thermoelectric | [24] |
| AgBiS2 QDs | Hot injection | AgNO3, Bi(NO3)3, and sulfur in OLA | 8.5 ± 1.2 | - | [28] |
| AgBiS2 NCs | Solvothermal | Ag(OAc), BiCl3, and CS2 in OA, CH, and 1-decanol | 7.6 | Quantum dot-sensitized solar cells | [29] |
| AgBiS2 NCs | Hot injection | Ag(OAc), Bi(OAc) in OA, and (TMS)2S in ODE | 4.62 | Solar cells | [15] |
| AgBiS2 thin film | Molecular precursor | AgNO3, Bi(NO3)3·5(H2O), and SC(NH2)2 in DMSO | 11–67 | Photoconductor | [30] |
| AgBiS2 NCs | Chemical synthesis (room temperature) | AgNO3 and Bi(NO3)3·5(H2O) in OA; sulfur powder in OCT/DDA | 7–15 | Photodetector | [31] |
| AgBiS2 thin films | Molecular precursor | Ag(OAc) and Bi2O3 in DTCA | ~20–40 | Light absorbers | [32] |
| AgBiS2 nano ink | Hot injection | Ag(OAc) and Bi(OAc) in OA; sulfur in OLA. | 8.75 | Photodetector | [33] |
| AgBiS2 NCs | Hot injection | Ag(iPrXa), Bi(iPrXa)3 in OLA and ODE | 4.1–7.6 | - | [34] |
| AgBiS2 QDs | Cation exchange | Ag2S synthesis: AgNO3 in toluene and OLA; St2S in toluene. Cation exchange: Ag2S QDs in toluene added by Bi(neo)3 in TOP | 3 | Solar cell | [35] |
2.1. Sonochemical Synthesis
2.2. Solvothermal
2.3. Hot Injection
2.4. Room Temperature Chemical Synthesis in Ambient Atmosphere
2.5. Successive Ionic Layer Adsorption and Reaction (SILAR)
2.6. Chemical Bath Deposition
2.7. Synthesis by Reacting Molecular Precursor in Organic Solvent
3. Ligand Exchange
4. Device Fabrication
| Device Structure | Ligand | PCE (%) | VOC (V) | JSC (mA/cm2) | FF | Ref |
|---|---|---|---|---|---|---|
| ITO/ZnO/AgBiS2/PTB7/MoOx/Ag | TMAI | 6.31 | 0.45 | 22.1 | 0.63 | [15] |
| ITO/ZnO/AgBiS2/P3HT/Au | TMAI | 4.3 | 0.46 | 16.7 | 0.56 | [45] |
| ITO/ZnO/AgBiS2/PTB7/MoOx/Ag | TMAI | 5.75 | 0.51 | 17.63 | 0.64 | [56] |
| FTO/ZnO/AgBiS2/P3HT/MoO3/Al | No ligand exchange | 1.24 | 0.21 | 15.68 | 0.37 | [54] |
| ITO/ZnO/AgBiS2/PCE-10/MoO3/Ag | TMAI | 4.57 | 0.44 | 18.87 | 0.54 | [58] |
| ITO/PEDOT:PSS/AgBiS2/C60/BCP/Cu | No ligand exchange | 2.04 | 0.28 | 16.85 | 0.2 | [52] |
| ITO/NiO/AgBiS2/ZnO/Al | TMAI-MPA | 4.74 | 0.46 | 16.46 | 0.58 | [66] |
| ITO/ZnO NP/AgBiS2-TMAI/PTAA:C60F48/Ag | TMAI | 4.4 | 0.44 | 18.5 | 0.56 | [68] |
| ITO/SnO2/AgBiS2/PTAA/MoO3/Ag | MPA | 7.3 | 0.45 | 24.9 | 0.61 | [60] |
| ITO/ZnO/AgBiS2/PBDB-T-2F:BTP-4Cl/MoO3/Ag | ME | 9.1 | 0.49 | 27.07 | 0.68 | [16] |
| ITO/SnO2/AgBiS2/PTAA/MoO3/Ag | MPA | 9.17 | 0.495 | 27.11 | 0.68 | [69] |
| ITO/ZnO/AgBiS2/QPB/PTB7/MoO3 | TMAI | 6.78 | 0.47 | 21.5 | 0.67 | [67] |
| ITO/ZnO/AgBiS2/PTB7/MoOx/Ag | AgX and NaX (X = Br, I) | 8.11 | 0.54 | 22.33 | 0.67 | [62] |
| ITO/SnO2/AgBiS2/PTAA/MoO3/Ag | MPA | 7.35 | 0.486 | 23.81 | 0.64 | [35] |
5. Comparison to PbS QD Development
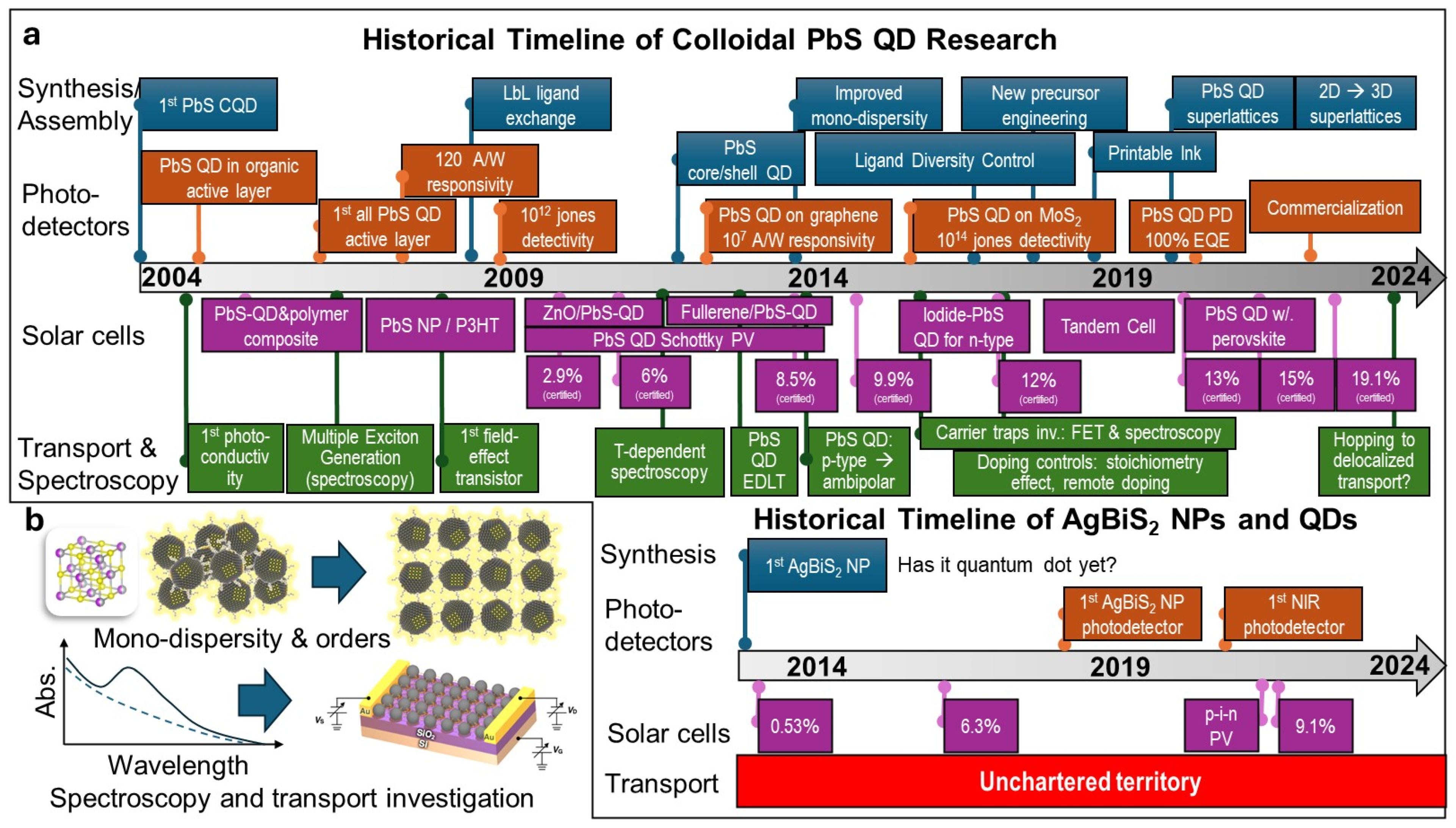
6. Conclusions and Future Outlook
- Improving the orders of the nanocrystal assemblies
- Improving Electronic Properties
- Enhancing Light Absorption and Photocurrent
- Device Architecture Sophistication, Stability, and Environmental Compatibility
Author Contributions
Funding
Conflicts of Interest
References
- Jean, J.; Brown, P.R.; Jaffe, R.L.; Buonassisi, T.; Bulović, V. Pathways for Solar Photovoltaics. Energy Environ. Sci. 2015, 8, 1200–1219. [Google Scholar] [CrossRef]
- Yu, K.; Schanze, K.S. Commemorating The Nobel Prize in Chemistry 2023 for the Discovery and Synthesis of Quantum Dots. ACS Cent. Sci. 2023, 9, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar Light Harvesting with Multinary Metal Chalcogenide Nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- NREL Best Research Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 17 June 2024).
- Klimov, V. (Ed.) Nanocrystal Quantum Dots, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315218632. [Google Scholar]
- Carey, G.H.; Levina, L.; Comin, R.; Voznyy, O.; Sargent, E.H. Record Charge Carrier Diffusion Length in Colloidal Quantum Dot Solids via Mutual Dot-to-Dot Surface Passivation. Adv. Mater. 2015, 27, 3325–3330. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of Monodisperse Iron Oxide Nanocrystal Formation by “Heating-Up” Process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of Semiconductor Nanocrystals, Focusing on Nontoxic and Earth-Abundant Materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef] [PubMed]
- Aresti, M.; Saba, M.; Piras, R.; Marongiu, D.; Mula, G.; Quochi, F.; Mura, A.; Cannas, C.; Mureddu, M.; Ardu, A.; et al. Colloidal Bi2S3 Nanocrystals: Quantum Size Effects and Midgap States. Adv. Funct. Mater. 2014, 24, 3341–3350. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Chen, J.; Cai, S.; Long, Z.; Fang, X. Design Principles and Material Engineering of ZnS for Optoelectronic Devices and Catalysis. Adv. Funct. Mater. 2018, 28, 1802029. [Google Scholar] [CrossRef]
- Miranti, R.; Septianto, R.D.; Kikitsu, T.; Hashizume, D.; Matsushita, N.; Iwasa, Y.; Bisri, S.Z. π-SnS Colloidal Nanocrystals with Size-Dependent Band Gaps. J. Phys. Chem. C 2022, 126, 5323–5332. [Google Scholar] [CrossRef]
- Abuelwafa, A.A.; Matiur, R.M.; Putri, A.A.; Soga, T. Synthesis, Structure, and Optical Properties of the Nanocrystalline Bismuth Oxyiodide (BiOI) for Optoelectronic Application. Opt. Mater. 2020, 109, 110413. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, K.; Jiang, X.; Wang, Y.; Ge, Y.; Wu, L.; Xu, S.; Bao, Q.; Zhang, H. Nonlinear Optical Absorption and Ultrafast Carrier Dynamics of Copper Antimony Sulfide Semiconductor Nanocrystals. J. Mater. Chem. C 2018, 6, 8977–8983. [Google Scholar] [CrossRef]
- Bernechea, M.; Miller, N.C.; Xercavins, G.; So, D.; Stavrinadis, A.; Konstantatos, G. Solution-Processed Solar Cells Based on Environmentally Friendly AgBiS2 Nanocrystals. Nat. Photonics 2016, 10, 521–525. [Google Scholar] [CrossRef]
- Kim, C.; Kozakci, I.; Kim, J.; Lee, S.Y.; Lee, J. Highly Efficient (>9%) Lead-Free AgBiS2 Colloidal Nanocrystal/Organic Hybrid Solar Cells. Adv. Energy Mater. 2022, 12, 2200262. [Google Scholar] [CrossRef]
- Öberg, V.A.; Johansson, M.B.; Zhang, X.; Johansson, E.M.J. Cubic AgBiS2Colloidal Nanocrystals for Solar Cells. ACS Appl. Nano Mater. 2020, 3, 4014–4024. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, H.; Kim, G.; Kang, J.; Lee, C.B.; Bae, S.Y.; Song, H.; Kim, K.; Kim, J.H.; Jung, I.H.; et al. Dual Facet Passivation of Silver Halometallate for Eco-Friendly Silver Bismuth Sulfide near IR Photodetector. Chem. Eng. J. 2023, 473, 145246. [Google Scholar] [CrossRef]
- Huang, Y.T.; Nodari, D.; Furlan, F.; Zhang, Y.; Rusu, M.; Dai, L.; Andaji-Garmaroudi, Z.; Darvill, D.; Guo, X.; Rimmele, M.; et al. Fast Near-Infrared Photodetectors Based on Nontoxic and Solution-Processable AgBiS2. Small 2023, 20, e2310199. [Google Scholar] [CrossRef]
- Huang, P.C.; Yang, W.C.; Lee, M.W. AgBiS2 Semiconductor-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 18308–18314. [Google Scholar] [CrossRef]
- Kang, J.; Oh, J.T.; Kim, H.; Kim, H.; Choi, H.; Jung, I.H. Environmentally Friendly AgBiS2 Nanocrystal-Based High-Performance Quantum-Dot Photodetectors. Appl. Surf. Sci. 2022, 597, 153661. [Google Scholar] [CrossRef]
- Chen, D.; Shivarudraiah, S.B.; Geng, P.; Ng, M.; Li, C.-H.A.; Tewari, N.; Zou, X.; Wong, K.S.; Guo, L.; Halpert, J.E. Solution-Processed, Inverted AgBiS2 Nanocrystal Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 1634–1642. [Google Scholar] [CrossRef]
- Manimozhi, T.; Archana, J.; Navaneethan, M.; Ramamurthi, K. Shape-Controlled Synthesis of AgBiS2 Nano-/Microstructures Using PEG-Assisted Facile Solvothermal Method and Their Functional Properties. Appl. Surf. Sci. 2019, 487, 664–673. [Google Scholar] [CrossRef]
- Guin, S.; Biswas, K. Cation Disorder and Bond Anharmonicity Optimize the Thermoelectric Properties in Kinetically Stabilized Rocksalt AgBiS2 Nanocrystals. Chem. Mater. 2013, 25, 3225–3231. [Google Scholar] [CrossRef]
- Septianto, R.D.; Miranti, R.; Kikitsu, T.; Hikima, T.; Hashizume, D.; Matsushita, N.; Iwasa, Y.; Bisri, S.Z. Enabling Metallic Behaviour in Two-Dimensional Superlattice of Semiconductor Colloidal Quantum Dots. Nat. Commun. 2023, 14, 2670. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, X.; Shen, X.; Ding, S.; Feng, H.; Wu, G.; Zhang, Y. Optimizing the Synthetic Conditions of “Green” Colloidal AgBiS2 Nanocrystals Using a Low-Cost Sulfur Source. Nanomaterials 2022, 12, 3742. [Google Scholar] [CrossRef]
- Pejova, B.; Grozdanov, I.; Nesheva, D.; Petrova, A. Size-Dependent Properties of Sonochemically Synthesized Three-Dimensional Arrays of Close-Packed Semiconducting AgBiS2 Quantum Dots. Chem. Mater. 2008, 20, 2551–2565. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, X.; Ji, S.; Jia, C.; Ye, C. The Synthesis of Monodispersed AgBiS2 Quantum Dots with a Giant Dielectric Constant. CrystEngComm 2013, 15, 7644–7648. [Google Scholar] [CrossRef]
- Liang, N.; Chen, W.; Dai, F.; Wu, X.; Zhang, W.; Li, Z.; Shen, J.; Huang, S.; He, Q.; Zai, J.; et al. Homogenously Hexagonal Prismatic AgBiS2 Nanocrystals: Controlled Synthesis and Application in Quantum Dot-Sensitized Solar Cells. CrystEngComm 2015, 17, 1902–1905. [Google Scholar] [CrossRef]
- Gu, E.; Lin, X.; Tang, X.; Matt, G.J.; Osvet, A.; Hou, Y.; Jäger, S.; Xie, C.; Karl, A.; Hock, R.; et al. Single Molecular Precursor Ink for AgBiS2 Thin Films: Synthesis and Characterization. J. Mater. Chem. C 2018, 6, 7642–7651. [Google Scholar] [CrossRef]
- Mak, C.H.; Qian, J.; Rogée, L.; Lai, W.K.; Lau, S.P. Facile Synthesis of AgBiS2 Nanocrystals for High Responsivity Infrared Detectors. RSC Adv. 2018, 8, 39203–39207. [Google Scholar] [CrossRef]
- Van Embden, J.; Della Gaspera, E. Ultrathin Solar Absorber Layers of Silver Bismuth Sulfide from Molecular Precursors. ACS Appl. Mater. Interfaces 2019, 11, 16674–16682. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kim, D.; Oshima, Y.; Sato, H.; Park, J.; Kim, H. Synthesis and Application of AgBiS2 and Ag2S Nanoinks for the Production of IR Photodetectors. ACS Omega 2021, 6, 20710–20718. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulou, E.; Knez, D.; Sigl, M.; Kothleitner, G.; Trimmel, G.; Rath, T. A Colloidal Synthesis Route Towards AgBiS2 Nanocrystals Based on Metal Xanthate Precursors. ChemNanoMat 2023, 9, e202200414. [Google Scholar] [CrossRef]
- Senina, A.; Prudnikau, A.; Wrzesińska-Lashkova, A.; Vaynzof, Y.; Paulus, F. Cation Exchange Synthesis of AgBiS2 Quantum Dots for Highly Efficient Solar Cells. Nanoscale 2024, 16, 9325–9334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Mei, X.; Chen, J.; Johansson, E.M.J.; Zhang, X. Colloidal Quantum Dot for Infrared-Absorbing Solar Cells: State-Ofthe-Art and Prospects. Nano Res. Energy 2024, 3, e9120095. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Z.; Wang, Y.; Lu, K.; Yang, F.; Gu, M.; Xu, Y.; Chen, S.; Ling, X.; Zhang, Y.; et al. Towards Scalable Synthesis of High-Quality PbS Colloidal Quantum Dots for Photovoltaic Applications. J. Mater. Chem. C 2019, 7, 1575–1583. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Cademartiri, L.; Montanari, E.; Calestani, G.; Migliori, A.; Guagliardi, A.; Ozin, G.A. Size-Dependent Extinction Coefficients of PbS Quantum Dots. J. Am. Chem. Soc. 2006, 128, 10337–10346. [Google Scholar] [CrossRef]
- Murray, C.; Norris, D.; Bawendi, M.; Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Peterson, J.J.; Krauss, T.D. Photobrightening and Photodarkening in PbS Quantum Dots. Phys. Chem. Chem. Phys. 2006, 8, 3851–3856. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Yu, Y.; Zhang, L.; Zhang, G.; Jiang, S.; Liu, W.; Song, H.; Tang, J. Effect of Ligand Passivation on Morphology, Optical and Photoresponse Properties of CdS Colloidal Quantum Dots Thin Film. J. Mater. Sci. Mater. Electron. 2014, 25, 1499–1504. [Google Scholar] [CrossRef]
- Liu, J.; Yu, H.; Wu, Z.; Wang, W.; Peng, J.; Cao, Y. Size-Tunable near-Infrared PbS Nanoparticles Synthesized from Lead Carboxylate and sulfurwith Oleylamine as Stabilizer. Nanotechnology 2008, 19, 345602. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Tian, X.; Shaw, S.; Petersen, R.E.; Cademartiri, L. Sulfur in Oleylamine as a Powerful and Versatile Etchant for Oxide, Sulfide, and Metal Colloidal Nanoparticles. Phys. Status Solidi Appl. Mater. Sci. 2017, 214, 1600543. [Google Scholar] [CrossRef]
- Hu, L.; Patterson, R.J.; Zhang, Z.; Hu, Y.; Li, D.; Chen, Z.; Yuan, L.; Teh, Z.L.; Gao, Y.; Conibeer, G.J.; et al. Enhanced Optoelectronic Performance in AgBiS2 Nanocrystals Obtained via an Improved Amine-Based Synthesis Route. J. Mater. Chem. C 2018, 6, 731–737. [Google Scholar] [CrossRef]
- Oh, J.T.; Cho, H.; Bae, S.Y.; Lim, S.J.; Kang, J.; Jung, I.H.; Choi, H.; Kim, Y. Improved Size Distribution of AgBiS2 Colloidal Nanocrystals by Optimized Synthetic Route Enhances Photovoltaic Performance. Int. J. Energy Res. 2020, 44, 11006–11014. [Google Scholar] [CrossRef]
- Akgul, M.Z.; Figueroba, A.; Pradhan, S.; Bi, Y.; Konstantatos, G. Low-Cost RoHS Compliant Solution Processed Photovoltaics Enabled by Ambient Condition Synthesis of AgBiS2 Nanocrystals. ACS Photonics 2020, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Si, H.-Y.; Yuan, D.; Chen, J.-S.; Chow, G.-M. Synthesis of PbS Nanocrystals from Sulfur–Amine Solutions at Room Temperature. RSC Adv. 2011, 1, 817–822. [Google Scholar] [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; Revaprasadu, N.; O’Brien, P. Synthesis of CdS and PbS Nanoparticles by the Thermal Decomposition of Ethyl Xanthate Complexes in Castor Oil Using the Heat-up Technique. Mater. Sci. Semicond. Process. 2021, 122, 105493. [Google Scholar] [CrossRef]
- De Trizio, L.; Manna, L. Forging Colloidal Nanostructures via Cation Exchange Reactions. Chem. Rev. 2016, 116, 10852–10887. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Jiang, L.; Qi, Y.; Li, R.; Li, Y.; Xu, Y.; Liu, H.; Lin, Q. Chemical Bath Deposition of AgBiS2 Films for Visible and X-Ray Detection. Appl. Mater. Today 2022, 26, 101262. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Peng, J.; Cui, L.; Li, R.; Xu, Y.; Li, W.; Li, Y.; Tian, X.; Lin, Q. Solution-Processed AgBiS2 Photodetectors from Molecular Precursors. J. Mater. Chem. C 2020, 8, 2436–2441. [Google Scholar] [CrossRef]
- Uhl, A.R.; Katahara, J.K.; Hillhouse, H.W. Molecular-Ink Route to 13.0% Efficient Low-Bandgap CuIn(S,Se)2 and 14.7% Efficient Cu(In,Ga)(S,Se)2 Solar Cells. Energy Environ. Sci. 2016, 9, 130–134. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, L.; Zhang, W.; Li, X.; Fang, J. In Situ Grown Silver Bismuth Sulfide Nanorod Arrays and Their Application to Solar Cells. CrystEngComm 2019, 21, 3137–3141. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. PbS Nanostructures: A Review of Recent Advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Oh, J.T.; Bae, S.Y.; Ha, S.R.; Cho, H.; Lim, S.J.; Boukhvalov, D.W.; Kim, Y.; Choi, H. Water-Resistant AgBiS2 Colloidal Nanocrystal Solids for Eco-Friendly Thin Film Photovoltaics. Nanoscale 2019, 11, 9633–9640. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Oh, J.T.; Park, J.Y.; Ha, S.R.; Choi, J.; Choi, H.; Kim, Y. Improved Eco-Friendly Photovoltaics Based on Stabilized AgBiS2Nanocrystal Inks. Chem. Mater. 2020, 32, 10007–10014. [Google Scholar] [CrossRef]
- Ming, S.; Liu, X.; Zhang, W.; Xie, Q.; Wu, Y.; Chen, L.; Wang, H.Q. Eco-Friendly and Stable Silver Bismuth Disulphide Quantum Dot Solar Cells via Methyl Acetate Purification and Modified Ligand Exchange. J. Clean. Prod. 2020, 246, 118966. [Google Scholar] [CrossRef]
- Bae, S.Y.; Yang, J.; Oh, J.T.; Lee, C.B.; Song, H.; Lee, B.R.; Jin, H.M.; Kim, K.; Hong, J.P.; Kim, Y.; et al. Understanding the Cation-Selective Ligand Passivation for AgBiS2 Nanocrystal Photovoltaics. Chem. Eng. J. 2023, 474, 145674. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, L.; Wang, Z.; Konstantatos, G. Environmentally Friendly AgBiS2 Nanocrystal Inks for Efficient Solar Cells Employing Green Solvent Processing. Adv. Energy Mater. 2022, 12, 2200700. [Google Scholar] [CrossRef]
- Kelley, M.L.; Ahmed, F.; Abiodun, S.L.; Usman, M.; Jewel, M.U.; Hussain, K.; Zur Loye, H.C.; Chandrashekhar, M.V.S.; Greytak, A.B. Photoconductive Thin Films Composed of Environmentally Benign AgBiS2 Nanocrystal Inks Obtained through a Rapid Phase Transfer Process. ACS Appl. Electron. Mater. 2021, 3, 1550–1555. [Google Scholar] [CrossRef]
- Kim, D.; Cho, G.; Kim, Y.H.; Kwon, J.H.; Oh, Y.; Yang, M.; Jee, S.; Lee, I.S.; Si, M.J.; Jung, Y.; et al. Multi-Facet Passivation of Ternary Colloidal Quantum Dot Enabled by Quadruple-Ligand Ensemble toward Efficient Lead-Free Optoelectronics. Adv. Energy Mater. 2024, 14, 2302579. [Google Scholar] [CrossRef]
- Pejova, B.; Nesheva, D.; Aneva, Z.; Petrova, A. Photoconductivity and Relaxation Dynamics in Sonochemically Synthesized Assemblies of AgBiS2 Quantum Dots. J. Phys. Chem. C 2011, 115, 37–46. [Google Scholar] [CrossRef]
- Sun, B.; Ouellette, O.; García de Arquer, F.P.; Voznyy, O.; Kim, Y.; Wei, M.; Proppe, A.H.; Saidaminov, M.I.; Xu, J.; Liu, M.; et al. Multibandgap Quantum Dot Ensembles for Solar-Matched Infrared Energy Harvesting. Nat. Commun. 2018, 9, 4003. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, D.; Voznyy, O.; Levina, L.; Hoogland, S.; Kemp, K.W.; Ip, A.H.; Thon, S.M.; Sargent, E.H. Engineering Colloidal Quantum Dot Solids within and beyond the Mobility-Invariant Regime. Nat. Commun. 2014, 5, 3803. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.T.; Bae, S.Y.; Yang, J.; Ha, S.R.; Song, H.; Lee, C.B.; Shome, S.; Biswas, S.; Lee, H.M.; Seo, Y.H.; et al. Ultra-Stable All-Inorganic Silver Bismuth Sulfide Colloidal Nanocrystal Photovoltaics Using Pin Type Architecture. J. Power Sources 2021, 514, 230585. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.; Shin, D.; Kim, T.; Choi, M.; Kim, J.; Lee, D.C.; Jeong, S. Quantum Dot/Polymer Bulk Heterostructure Interlayer for Enhanced Charge Collection in AgBiS2 Quantum Dot Photovoltaics. Adv. Opt. Mater. 2022, 10, 2201086. [Google Scholar] [CrossRef]
- Becker-Koch, D.; Albaladejo-Siguan, M.; Kress, J.; Kumar, R.; Hofstetter, Y.J.; An, Q.; Bakulin, A.A.; Paulus, F.; Vaynzof, Y. Oxygen-Induced Degradation in AgBiS2 Nanocrystal Solar Cells. Nanoscale 2022, 14, 3020–3030. [Google Scholar] [CrossRef]
- Wang, Y.; Kavanagh, S.R.; Burgués-Ceballos, I.; Walsh, A.; Scanlon, D.; Konstantatos, G. Cation Disorder Engineering Yields AgBiS2 Nanocrystals with Enhanced Optical Absorption for Efficient Ultrathin Solar Cells. Nat. Photonics 2022, 16, 235–241. [Google Scholar] [CrossRef]
- Balazs, D.M.; Bijlsma, K.I.; Fang, H.-H.; Dirin, D.N.; Döbeli, M.; Kovalenko, M.V.; Loi, M.A. Stoichiometric Control of the Density of States in PbS Colloidal Quantum Dot Solids. Sci. Adv. 2017, 3, eaao1558. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, K.; Han, L.; Liu, Z.; Shi, G.; Fang, H.; Chen, S.; Wu, T.; Yang, F.; Gu, M.; et al. In Situ Passivation for Efficient PbS Quantum Dot Solar Cells by Precursor Engineering. Adv. Mater. 2018, 30, 1704871. [Google Scholar] [CrossRef]
- Klem, E.J.D.; MacNeil, D.D.; Cyr, P.W.; Levina, L.; Sargent, E.H. Efficient Solution-Processed Infrared Photovoltaic Cells: Planarized All-Inorganic Bulk Heterojunction Devices via Inter-Quantum-Dot Bridging during Growth from Solution. Appl. Phys. Lett. 2007, 90, 183113. [Google Scholar] [CrossRef]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Cha, D.; et al. Colloidal-Quantum-Dot Photovoltaics Using Atomic-Ligand Passivation. Nat. Mater. 2011, 10, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, R.J.; Beard, M.C.; Johnson, J.C.; Yu, P.; Micic, O.I.; Nozik, A.J.; Shabaev, A.; Efros, A.L. Highly Efficient Multiple Exciton Generation in Colloidal PbSe and PbS Quantum Dots. Nano Lett. 2005, 5, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Klem, E.; Shukla, H.; Hinds, S.; MacNeil, D.; Levina, L.; Sargent, E. Impact of Dithiol Treatment and Air Annealing on the Conductivity, Mobility, and Hole Density in PbS Colloidal Quantum Dot Solids. Appl. Phys. Lett. 2008, 92, 212105. [Google Scholar] [CrossRef]
- Bisri, S.Z.; Piliego, C.; Yarema, M.; Heiss, W.; Loi, M.A. Low Driving Voltage and High Mobility Ambipolar Field-Effect Transistors with PbS Colloidal Nanocrystals. Adv. Mater. 2013, 25, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.M.; Nugraha, M.I.; Bisri, S.Z.; Sytnyk, M.; Heiss, W.; Loi, M.A. Reducing Charge Trapping in PbS Colloidal Quantum Dot Solids. Appl. Phys. Lett. 2014, 104, 112104. [Google Scholar] [CrossRef]
- Wang, X.; Koleilat, G.I.; Tang, J.; Liu, H.; Kramer, I.J.; Debnath, R.; Brzozowski, L.; Barkhouse, D.A.R.; Levina, L.; Hoogland, S.; et al. Tandem Colloidal Quantum Dot Solar Cells Employing a Graded Recombination Layer. Nat. Photonics 2011, 5, 480–484. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; García de Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% Certified Colloidal Quantum Dot Solar Cells via Solvent-Polarity-Engineered Halide Passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Johnston, K.W.; Pattantyus-Abraham, A.G.; Clifford, J.P.; Myrskog, S.H.; Hoogland, S.; Shukla, H.; Klem, E.J.D.; Levina, L.; Sargent, E.H. Efficient Schottky-Quantum-Dot Photovoltaics: The Roles of Depletion, Drift, and Diffusion. Appl. Phys. Lett. 2008, 92, 122111. [Google Scholar] [CrossRef]
- Hou, Y.-X.; Li, Y.; Zhang, Z.-C.; Li, J.-Q.; Qi, D.-H.; Chen, X.-D.; Wang, J.-J.; Yao, B.-W.; Yu, M.-X.; Lu, T.-B.; et al. Large-Scale and Flexible Optical Synapses for Neuromorphic Computing and Integrated Visible Information Sensing Memory Processing. ACS Nano 2021, 15, 1497–1508. [Google Scholar] [CrossRef]
- Russ, B.; Eisler, C.N. The Future of Quantum Technologies: Superfluorescence from Solution-Processed, Tunable Materials. Nanophotonics 2024, 13, 1943–1951. [Google Scholar] [CrossRef]
- Meng, L.; Zeng, T.; Jin, Y.; Xu, Q.; Wang, X. Surface-Modified Substrates for Quantum Dot Inks in Printed Electronics. ACS Omega 2019, 4, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Gao, J.; Lloyd, M.T.; Semonin, O.E.; Beard, M.C.; Nozik, A.J. Stability Assessment on a 3% Bilayer PbS/ZnO Quantum Dot Heterojunction Solar Cell. Adv. Mater. 2010, 22, 3704–3707. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bharti, P.; Pradhan, B. Performance Optimization of Efficient PbS Quantum Dots Solar Cells through Numerical Simulation. Sci. Rep. 2023, 13, 10511. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Bin Na, H.; Yu, T.; Yu, J.H.; Kim, Y.W.; Wu, F.; Zhang, J.Z.; Hyeon, T. Generalized and Facile Synthesis of Semiconducting Metal Sulfide Nanocrystals. J. Am. Chem. Soc. 2003, 125, 11100–11105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Huo, N.; Li, F.; Gu, M.; Ling, X.; Zhang, Y.; Lu, K.; Han, L.; Fang, H.; et al. Room-Temperature Direct Synthesis of Semi-Conductive PbS Nanocrystal Inks for Optoelectronic Applications. Nat. Commun. 2019, 10, 5136. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Howard, I.; Fischer, A.; Hoogland, S.; Clifford, J.; Klem, E.; Levina, L.; Sargent, E.H. Ultrasensitive Solution-Cast Quantum Dot Photodetectors. Nature 2006, 442, 180–183. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.A.; Konstantatos, G.; Zhang, S.; Cyr, P.W.; Klem, E.J.D.; Levina, L.; Sargent, E.H. Solution-Processed PbS Quantum Dot Infrared Photodetectors and Photovoltaics. Nat. Mater. 2005, 4, 138–142. [Google Scholar] [CrossRef]
- Clifford, J.P.; Konstantatos, G.; Johnston, K.W.; Hoogland, S.; Levina, L.; Sargent, E.H. Fast, Sensitive and Spectrally Tuneable Colloidal-Quantum-Dot Photodetectors. Nat. Nanotechnol. 2009, 4, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Law, M.; Song, Q.; Perkins, C.L.; Beard, M.C.; Nozik, A.J. Structural, Optical, and Electrical Properties of Self-Assembled Films of PbSe Nanocrystals Treated with 1,2-Ethanedithiol. ACS Nano 2008, 2, 271–280. [Google Scholar] [CrossRef]
- Konstantatos, G.; Badioli, M.; Gaudreau, L.; Osmond, J.; Bernechea, M.; de Arquer, F.P.G.; Gatti, F.; Koppens, F.H.L. Hybrid Graphene–Quantum Dot Phototransistors with Ultrahigh Gain. Nat. Nanotechnol. 2012, 7, 363–368. [Google Scholar] [CrossRef]
- Zarghami, M.H.; Liu, Y.; Gibbs, M.; Gebremichael, E.; Webster, C.; Law, M. P-Type PbSe and PbS Quantum Dot Solids Prepared with Short-Chain Acids and Diacids. ACS Nano 2010, 4, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Sahu, A.; Norris, D.J.; Frisbie, C.D. Size- and Temperature-Dependent Charge Transport in PbSe Nanocrystal Thin Films. Nano Lett. 2011, 11, 3887–3892. [Google Scholar] [CrossRef]
- Nagpal, P.; Klimov, V.I. Role of Mid-Gap States in Charge Transport and Photoconductivity in Semiconductor Nanocrystal Films. Nat. Commun. 2011, 2, 486. [Google Scholar] [CrossRef] [PubMed]
- Piliego, C.; Protesescu, L.; Bisri, S.Z.; Kovalenko, M.V.; Loi, M.A. 5.2% Efficient PbS Nanocrystal Schottky Solar Cells. Energy Environ. Sci. 2013, 6, 3054. [Google Scholar] [CrossRef]
- Balazs, D.M.; Loi, M.A. Lead-Chalcogenide Colloidal-Quantum-Dot Solids: Novel Assembly Methods, Electronic Structure Control, and Application Prospects. Adv. Mater. 2018, 30, 1800082. [Google Scholar] [CrossRef]
- Brown, P.R.; Kim, D.; Lunt, R.R.; Zhao, N.; Bawendi, M.G.; Grossman, J.C.; Bulović, V. Energy Level Modification in Lead Sulfide Quantum Dot Thin Films through Ligand Exchange. ACS Nano 2014, 8, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, M.I.; Häusermann, R.; Bisri, S.Z.; Matsui, H.; Sytnyk, M.; Heiss, W.; Takeya, J.; Loi, M.A. High Mobility and Low Density of Trap States in Dual-Solid-Gated PbS Nanocrystal Field-Effect Transistors. Adv. Mater. 2015, 27, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.G.; Piveteau, L.; Bisri, S.Z.; Kovalenko, M.V.; Loi, M.A. Double Gate PbS Quantum Dot Field-Effect Transistors for Tuneable Electrical Characteristics. Adv. Electron. Mater. 2016, 2, 1500467. [Google Scholar] [CrossRef]
- Bisri, S.Z.; Degoli, E.; Spallanzani, N.; Krishnan, G.; Kooi, B.J.; Ghica, C.; Yarema, M.; Heiss, W.; Pulci, O.; Ossicini, S.; et al. Determination of the Electronic Energy Levels of Colloidal Nanocrystals Using Field-Effect Transistors and Ab-Initio Calculations. Adv. Mater. 2014, 26, 5639–5645. [Google Scholar] [CrossRef]
- Liu, L.; Bisri, S.Z.; Ishida, Y.; Hashizume, D.; Aida, T.; Iwasa, Y. Ligand and Solvent Effects on Hole Transport in Colloidal Quantum Dot Assemblies for Electronic Devices. ACS Appl. Nano Mater. 2018, 1, 5217–5225. [Google Scholar] [CrossRef]
- Maiti, S.; André, A.; Banerjee, R.; Hagenlocher, J.; Konovalov, O.; Schreiber, F.; Scheele, M. Monitoring Self-Assembly and Ligand Exchange of PbS Nanocrystal Superlattices at the Liquid/Air Interface in Real Time. J. Phys. Chem. Lett. 2018, 9, 739–744. [Google Scholar] [CrossRef]
- Scheele, M.; Brütting, W.; Schreiber, F. Coupled Organic-Inorganic Nanostructures (COIN). Phys. Chem. Chem. Phys. 2015, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.M.; Dirin, D.N.; Fang, H.-H.; Protesescu, L.; ten Brink, G.H.; Kooi, B.J.; Kovalenko, M.V.; Loi, M.A. Counterion-Mediated Ligand Exchange for PbS Colloidal Quantum Dot Superlattices. ACS Nano 2015, 9, 11951–11959. [Google Scholar] [CrossRef] [PubMed]
- Bederak, D.; Balazs, D.M.; Sukharevska, N.V.; Shulga, A.G.; Abdu-Aguye, M.; Dirin, D.N.; Kovalenko, M.V.; Loi, M.A. Comparing Halide Ligands in PbS Colloidal Quantum Dots for Field-Effect Transistors and Solar Cells. ACS Appl. Nano Mater. 2018, 1, 6882–6889. [Google Scholar] [CrossRef]
- Septianto, R.D.; Liu, L.; Iskandar, F.; Matsushita, N.; Iwasa, Y.; Bisri, S.Z. On-Demand Tuning of Charge Accumulation and Carrier Mobility in Quantum Dot Solids for Electron Transport and Energy Storage Devices. NPG Asia Mater. 2020, 12, 33. [Google Scholar] [CrossRef]
- Liu, L.; Bisri, S.Z.; Ishida, Y.; Aida, T.; Iwasa, Y. Tunable Electronic Properties by Ligand Coverage Control in PbS Nanocrystal Assemblies. Nanoscale 2019, 11, 20467–20474. [Google Scholar] [CrossRef] [PubMed]
- Whitham, K.; Yang, J.; Savitzky, B.H.; Kourkoutis, L.F.; Wise, F.; Hanrath, T. Charge Transport and Localization in Atomically Coherent Quantum Dot Solids. Nat. Mater. 2016, 15, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Abelson, A.; Qian, C.; Crawford, Z.; Zimanyi, G.T.; Law, M. High-Mobility Hole Transport in Single-Grain PbSe Quantum Dot Superlattice Transistors. Nano Lett. 2022, 22, 9578–9585. [Google Scholar] [CrossRef]
- Abelson, A.; Qian, C.; Salk, T.; Luan, Z.; Fu, K.; Zheng, J.G.; Wardini, J.L.; Law, M. Collective Topo-Epitaxy in the Self-Assembly of a 3D Quantum Dot Superlattice. Nat. Mater. 2020, 19, 49–55. [Google Scholar] [CrossRef]
- Balazs, D.M.; Matysiak, B.M.; Momand, J.; Shulga, A.G.; Ibáñez, M.; Kovalenko, M.V.; Kooi, B.J.; Loi, M.A. Electron Mobility of 24 Cm2 V−1 S−1 in PbSe Colloidal-Quantum-Dot Superlattices. Adv. Mater. 2018, 30, 1802265. [Google Scholar] [CrossRef]
- Lan, X.; Chen, M.; Hudson, M.H.; Kamysbayev, V.; Wang, Y.; Guyot-Sionnest, P.; Talapin, D.V. Quantum Dot Solids Showing State-Resolved Band-like Transport. Nat. Mater. 2020, 19, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, K.; Speirs, M.; Gomulya, W.; Jarzab, D.; Manca, M.; Mikhnenko, O.V.; Yarema, M.; Kooi, B.J.; Heiss, W.; Loi, M.A. Exploring the Origin of the Temperature-Dependent Behavior of PbS Nanocrystal Thin Films and Solar Cells. Adv. Funct. Mater. 2012, 22, 1598–1605. [Google Scholar] [CrossRef]
- Speirs, M.J.; Balazs, D.M.; Fang, H.-H.; Lai, L.-H.; Protesescu, L.; Kovalenko, M.V.; Loi, M.A. Origin of the Increased Open Circuit Voltage in PbS–CdS Core–Shell Quantum Dot Solar Cells. J. Mater. Chem. A 2015, 3, 1450–1457. [Google Scholar] [CrossRef]
- Gilmore, R.H.; Winslow, S.W.; Lee, E.M.Y.; Ashner, M.N.; Yager, K.G.; Willard, A.P.; Tisdale, W.A. Inverse Temperature Dependence of Charge Carrier Hopping in Quantum Dot Solids. ACS Nano 2018, 12, 7741–7749. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.C.; Nguyen, Q.; Smilgies, D.-M.; Tisdale, W.A. Impact of Size Dispersity, Ligand Coverage, and Ligand Length on the Structure of PbS Nanocrystal Superlattices. Chem. Mater. 2018, 30, 807–816. [Google Scholar] [CrossRef]
- Boehme, S.C.; Vanmaekelbergh, D.; Evers, W.H.; Siebbeles, L.D.A.; Houtepen, A.J. In Situ Spectroelectrochemical Determination of Energy Levels and Energy Level Offsets in Quantum-Dot Heterojunctions. J. Phys. Chem. C 2016, 120, 5164–5173. [Google Scholar] [CrossRef]
- Gudjonsdottir, S.; van der Stam, W.; Kirkwood, N.; Evers, W.H.; Houtepen, A.J. The Role of Dopant Ions on Charge Injection and Transport in Electrochemically Doped Quantum Dot Films. J. Am. Chem. Soc. 2018, 140, 6582–6590. [Google Scholar] [CrossRef]
- Liu, L.; Septianto, R.D.; Bisri, S.Z.; Ishida, Y.; Aida, T.; Iwasa, Y. Evidence of Band Filling in PbS Colloidal Quantum Dot Square Superstructures. Nanoscale 2021, 13, 14001–14007. [Google Scholar] [CrossRef]
- Talgorn, E.; Gao, Y.; Aerts, M.; Kunneman, L.T.; Schins, J.M.; Savenije, T.J.; van Huis, M.A.; van der Zant, H.S.J.; Houtepen, A.J.; Siebbeles, L.D.a. Unity Quantum Yield of Photogenerated Charges and Band-like Transport in Quantum-Dot Solids. Nat. Nanotechnol. 2011, 6, 733–739. [Google Scholar] [CrossRef]
- Gao, Y.; Aerts, M.; Sandeep, C.S.S.; Talgorn, E.; Savenije, T.J.; Kinge, S.; Siebbeles, L.D.A.; Houtepen, A.J. Photoconductivity of PbSe Quantum-Dot Solids: Dependence on Ligand Anchor Group and Length. ACS Nano 2012, 6, 9606–9614. [Google Scholar] [CrossRef]
- Jo, J.W.; Kim, Y.; Choi, J.; de Arquer, F.P.G.; Walters, G.; Sun, B.; Ouellette, O.; Kim, J.; Proppe, A.H.; Quintero-Bermudez, R.; et al. Enhanced Open-Circuit Voltage in Colloidal Quantum Dot Photovoltaics via Reactivity-Controlled Solution-Phase Ligand Exchange. Adv. Mater. 2017, 29, 1703627. [Google Scholar] [CrossRef] [PubMed]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2024, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, N.F.; Smith, D.W. Quantum Confinement of PbS Microcrystals in Glass. J. Non. Cryst. Solids 1994, 180, 25–31. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Lian, L.; Zhang, Z.; Liu, Y.; Song, L.; Geng, C.; Zhang, J.; Xu, S. Stability Enhancement of PbS Quantum Dots by Site-Selective Surface Passivation for near-Infrared LED Application. Nano Res. 2021, 14, 628–634. [Google Scholar] [CrossRef]
- Abel, K.A.; Shan, J.; Boyer, J.-C.; Harris, F.; van Veggel, F.C.J.M. Highly Photoluminescent PbS Nanocrystals: The Beneficial Effect of Trioctylphosphine. Chem. Mater. 2008, 20, 3794–3796. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D. V Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Akhil, S.; Kusuma, J.; Balakrishna, R.G. Green AgBiSe2/AgBiS2 Core Shell Quantum Dots for Stable Solar Cells by Robust SILAR Method. J. Clean. Prod. 2022, 366, 132760. [Google Scholar] [CrossRef]
- Stavrinadis, A.; Rath, A.K.; de Arquer, F.P.G.; Diedenhofen, S.L.; Magén, C.; Martinez, L.; So, D.; Konstantatos, G. Heterovalent Cation Substitutional Doping for Quantum Dot Homojunction Solar Cells. Nat. Commun. 2013, 4, 2981. [Google Scholar] [CrossRef]
- Crisp, R.W.; Kirkwood, N.; Grimaldi, G.; Kinge, S.; Siebbeles, L.D.A.; Houtepen, A.J. Highly Photoconductive InP Quantum Dots Films and Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6569–6576. [Google Scholar] [CrossRef]
- Albe, V.; Jouanin, C.; Bertho, D. Confinement and Shape Effects on the Optical Spectra of Small CdSe Nanocrystals. Phys. Rev. B 1998, 58, 4713–4720. [Google Scholar] [CrossRef]
- Beard, M.C.; Luther, J.M.; Semonin, O.E.; Nozik, A.J. Third Generation Photovoltaics Based on Multiple Exciton Generation in Quantum Confined Semiconductors. Acc. Chem. Res. 2013, 46, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal Quantum Dot Solar Cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawaddah, F.A.N.; Bisri, S.Z. Advancing Silver Bismuth Sulfide Quantum Dots for Practical Solar Cell Applications. Nanomaterials 2024, 14, 1328. https://doi.org/10.3390/nano14161328
Mawaddah FAN, Bisri SZ. Advancing Silver Bismuth Sulfide Quantum Dots for Practical Solar Cell Applications. Nanomaterials. 2024; 14(16):1328. https://doi.org/10.3390/nano14161328
Chicago/Turabian StyleMawaddah, Fidya Azahro Nur, and Satria Zulkarnaen Bisri. 2024. "Advancing Silver Bismuth Sulfide Quantum Dots for Practical Solar Cell Applications" Nanomaterials 14, no. 16: 1328. https://doi.org/10.3390/nano14161328
APA StyleMawaddah, F. A. N., & Bisri, S. Z. (2024). Advancing Silver Bismuth Sulfide Quantum Dots for Practical Solar Cell Applications. Nanomaterials, 14(16), 1328. https://doi.org/10.3390/nano14161328







