Interplay of Kinetic and Thermodynamic Factors in the Stationary Composition of Vapor–Liquid–Solid IIIVxV1−x Nanowires
Abstract
:1. Introduction
2. Model
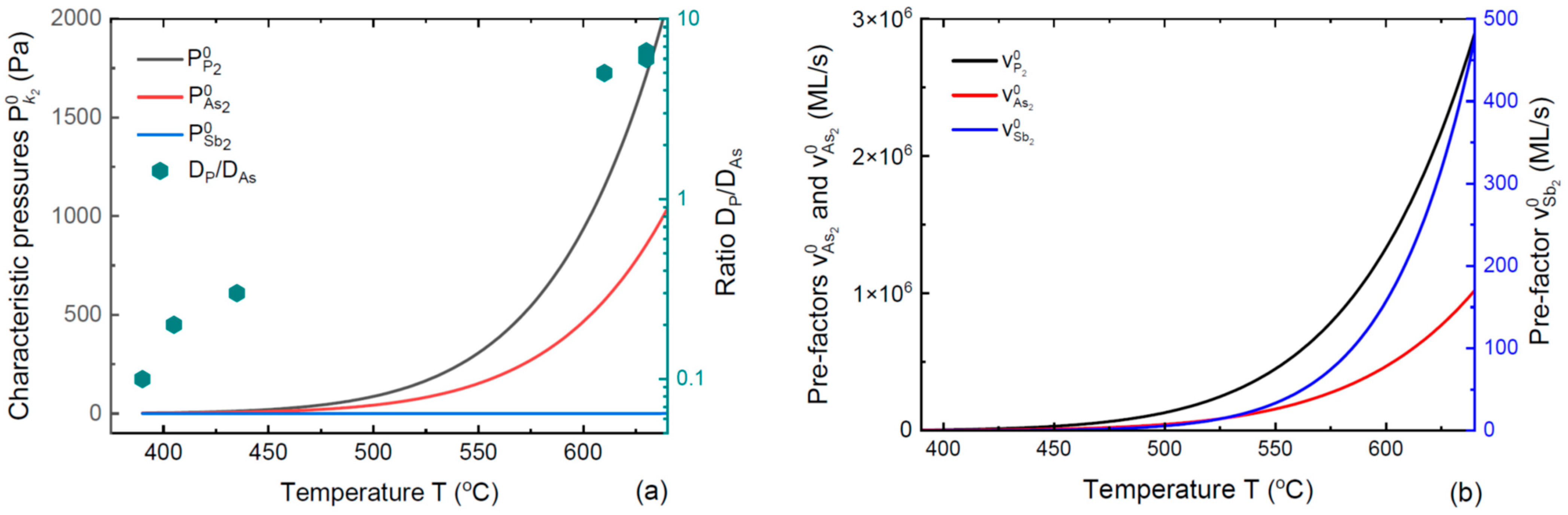
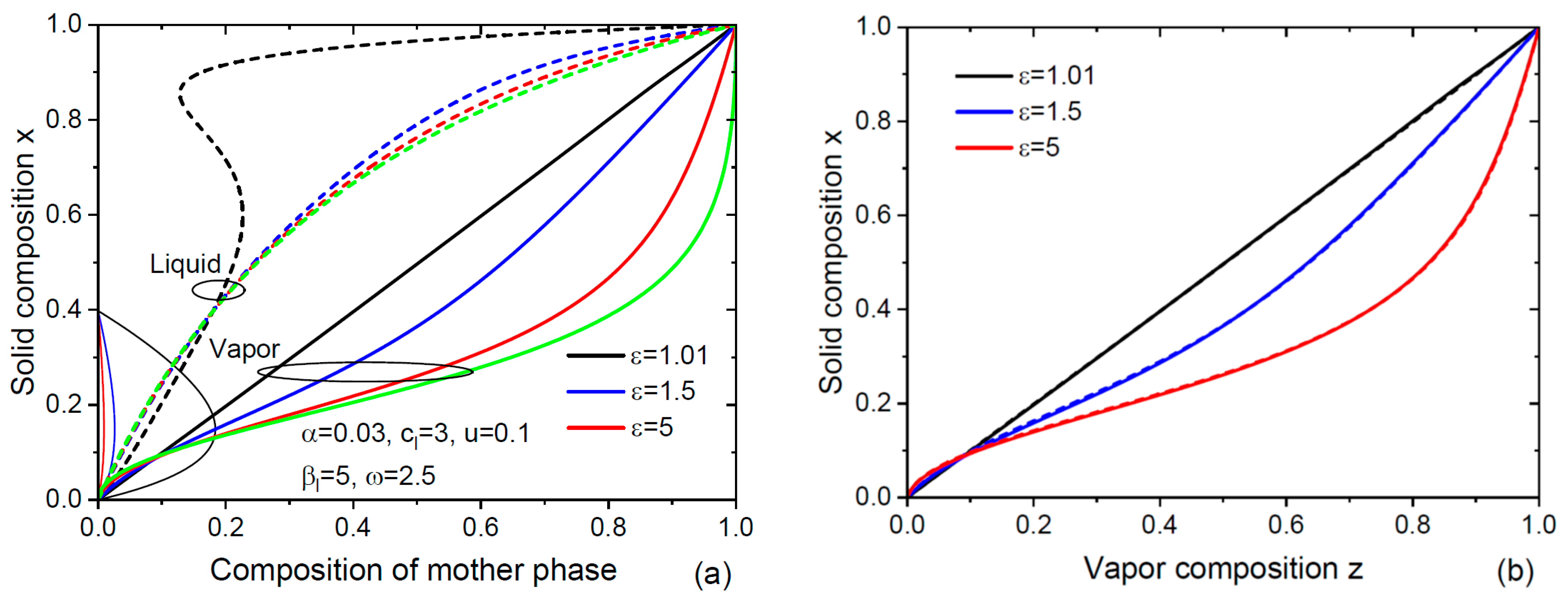
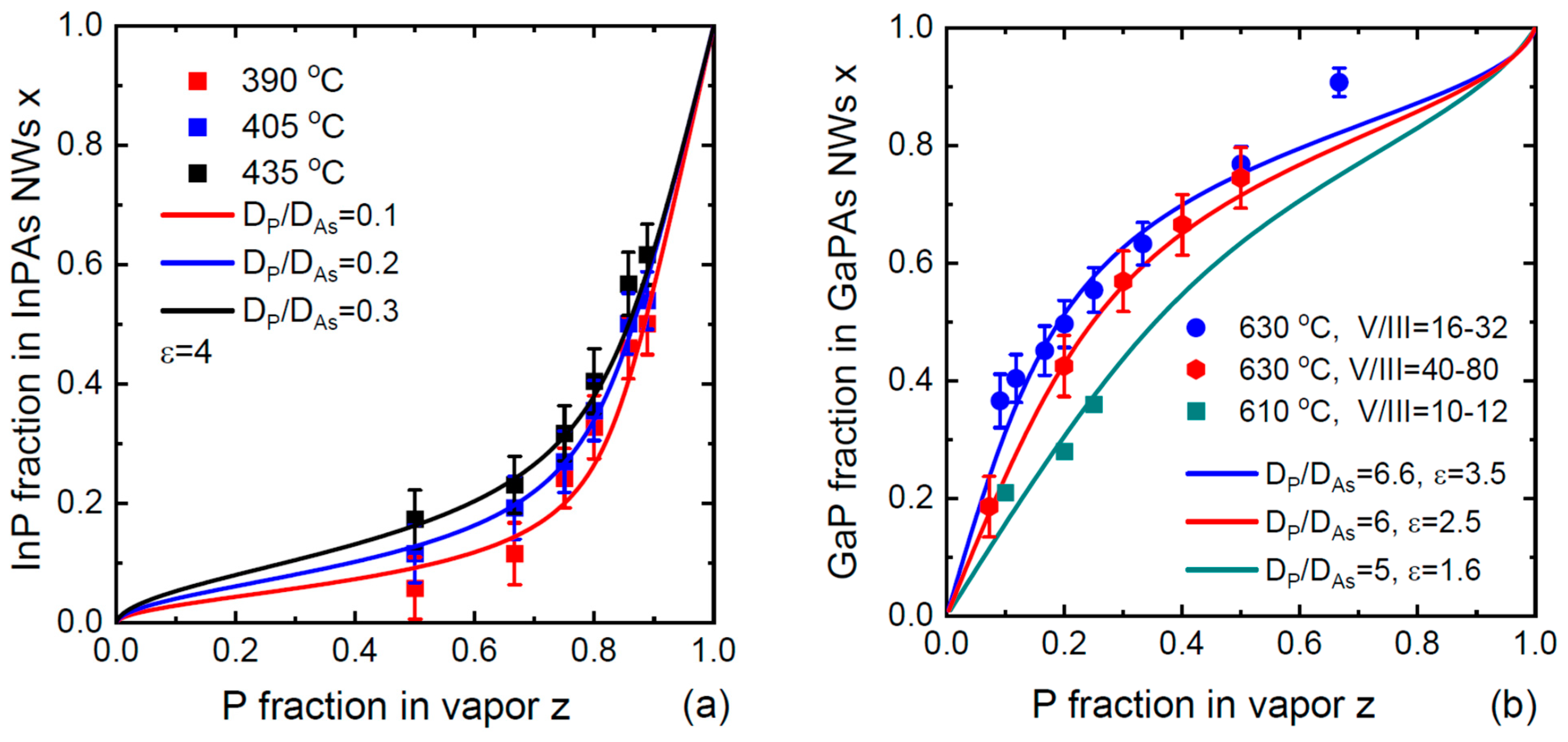
3. Results and Discussion
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McIntyre, P.C.; Fontcuberta i Morral, A. Semiconductor nanowires: To grow or not to grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Boras, G.; Yu, X.; Liu, H. III–V ternary nanowires on Si substrates: Growth, characterization and device applications. J. Semicond. 2019, 40, 101301. [Google Scholar] [CrossRef]
- Martelli, F. III–V ternary nanowires. In Advances in III–V Semiconductor Nanowires and Nanodevices; Li, J., Wang, D., LaPierre, R.R., Eds.; Bentham Science: Sharjah, United Arab Emirates, 2011; pp. 105–128. [Google Scholar]
- Ren, D.; Ahtapodov, L.; Nilsen, J.S.; Yang, J.; Gustafsson, A.; Huh, J.; Conibeer, G.J.; Van Helvoort, A.T.; Fimland, B.O.; Weman, H. Single-mode near-infrared lasing in a GaAsSb-based nanowire superlattice at room temperature. Nano Lett. 2018, 18, 2304. [Google Scholar] [CrossRef] [PubMed]
- Haffouz, S.; Zeuner, K.D.; Dalacu, D.; Poole, P.J.; Lapointe, J.; Poitras, D.; Mnaymneh, K.; Wu, X.; Couillard, M.; Korkusinski, M.; et al. Bright single InAsP quantum dots at telecom wavelengths in position-controlled InP nanowires: The role of the photonic waveguide. Nano Lett. 2018, 18, 3047. [Google Scholar] [CrossRef]
- Singh, R.; Bester, G. Nanowire quantum dots as an ideal source of entangled photon pairs. Phys. Rev. Lett. 2009, 103, 063601. [Google Scholar]
- Leandro, L.; Gunnarsson, C.P.; Reznik, R.; Jöns, K.D.; Shtrom, I.; Khrebtov, A.; Kasama, T.; Zwiller, V.; Cirlin, G.; Akopian, N. Nanowire quantum dots tuned to atomic resonances. Nano Lett. 2018, 18, 7217. [Google Scholar] [CrossRef]
- Dalacu, D.; Poole, P.J.; Williams, R.L. Nanowire-based sources of non-classical light. Nanotechnology 2019, 30, 232001. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89. [Google Scholar] [CrossRef]
- Colombo, C.; Spirkoska, D.; Frimmer, M.; Abstreiter, G.; Fontcuberta i Morral, A. Ga-assisted catalyst-free growth mechanism of GaAs nanowires by molecular beam epitaxy. Phys. Rev. B 2008, 77, 155326. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Glas, F. Vapor–liquid–solid growth of semiconductor nanowires. In Fundamental Properties of Semiconductor Nanowires; Fukata, N., Rurali, R., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Borg, B.M.; Dick, K.A.; Eymery, J.; Wernersson, L.-E. Enhanced Sb incorporation in InAsSb nanowires grown by metalorganic vapor phase epitaxy. Appl. Phys. Lett. 2011, 98, 113104. [Google Scholar] [CrossRef]
- Namazi, L.; Ghalamestani, S.G.; Lehmann, S.; Zamani, R.R.; Dick, K.A. Direct nucleation, morphology and compositional tuning of InAs1−xSbx nanowires on InAs (111)B substrates. Nanotechnology 2017, 28, 165601. [Google Scholar] [CrossRef]
- Zhuang, Q.D.; Alradhi, H.; Jin, Z.M.; Chen, X.R.; Shao, J.; Chen, X.; Sanchez, A.M.; Cao, Y.C.; Liu, J.Y.; Yates, P.; et al. Optically-efficient InAsSb nanowires for silicon-based mid-wavelength infrared optoelectronics. Nanotechnology 2017, 28, 105710. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Pan, D.; Liu, L.; Tong, S.; Zhuo, R.; Zhao, J. Large-composition-range pure-phase homogeneous InAs1–xSbx nanowires. J. Phys. Chem. Lett. 2022, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Caroff, P.; Wong-Leung, J.; Tan, H.H.; Jagadish, C. Controlling the morphology, composition and crystal structure in gold-seeded GaAs1−xSbx nanowires. Nanoscale 2015, 7, 4995. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.I.; Bjork, M.T.; Jeppesen, S.; Wagner, J.B.; Wallenberg, L.R.; Samuelson, L. InAs1−xPx nanowires for device engineering. Nano Lett. 2006, 6, 403. [Google Scholar] [CrossRef] [PubMed]
- Mandl, B.; Keplinger, M.; Messing, M.E.; Kriegner, D.; Wallenberg, R.; Samuelson, L.; Bauer, G.; Stangl, J.; Holy, V.; Deppert, K. Self-seeded axio-radial InAs-InAs1-xPx nanowire heterostructures beyond “common” VLS growth. Nano Lett. 2018, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Himwas, C.; Collin, S.; Rale, P.; Chauvin, N.; Patriarche, G.; Oehler, F.; Julien, F.H.; Travers, L.; Harmand, J.C.; Tchernycheva, M. In situ passivation of GaAsP nanowires. Nanotechnology 2017, 28, 495707. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanchez, A.M.; Sun, Y.; Wu, J.; Aagesen, M.; Huo, S.; Kim, D.; Jurczak, P.; Xu, X.; Liu, H. Influence of droplet size on the growth of self-catalyzed ternary GaAsP nanowires. Nano Lett. 2016, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aagesen, M.; Holm, J.V.; Jørgensen, H.I.; Wu, J.; Liu, H. Self-catalyzed GaAsP nanowires grown on silicon substrates by solid-source molecular beam epitaxy. Nano Lett. 2013, 13, 3897. [Google Scholar] [CrossRef]
- Metaferia, W.; Persson, A.R.; Mergenthaler, K.; Yang, F.; Zhang, W.; Yartsev, A.; Wallenberg, R.; Pistol, M.E.; Deppert, K.; Samuelson, L.; et al. GaAsP nanowires grown by aerotaxy. Nano Lett. 2016, 16, 5701. [Google Scholar] [CrossRef] [PubMed]
- Dheeraj, D.L.; Patriarche, G.; Zhou, H.; Harmand, J.C.; Weman, H.; Fimland, B.O. Growth and structural characterization of GaAs/GaAsSb axial heterostructured nanowires. J. Cryst. Growth 2009, 311, 1847. [Google Scholar] [CrossRef]
- Plissard, S.; Dick, K.A.; Wallart, X.; Caroff, P. Gold-free GaAs/GaAsSb heterostructure nanowires grown on silicon. Appl. Phys. Lett. 2010, 96, 121901. [Google Scholar] [CrossRef]
- Tchernycheva, M.; Cirlin, G.E.; Patriarche, G.; Travers, L.; Zwiller, V.; Perinetti, U.; Harmand, J.C. Growth and characterization of InP nanowires with InAsP insertions. Nano Lett. 2007, 7, 1500. [Google Scholar] [CrossRef] [PubMed]
- Tateno, K.; Zhang, G.; Takiguchi, M.; Gotoh, H.; Sogawa, T. VLS growth of alternating InAsP/InP heterostructure nanowires for multiple-quantum-dot structures. Nano Lett. 2012, 12, 2888. [Google Scholar] [CrossRef] [PubMed]
- Bucci, G.; Zannier, V.; Rossi, F.; Musiał, A.; Boniecki, J.; Sęk, G.; Sorba, L. Zincblende InAsxP1–x/InP quantum dot nanowires for telecom wavelength emission. ACS Appl. Mater. Interfaces 2024, 16, 26491. [Google Scholar] [CrossRef] [PubMed]
- Zannier, V.; Rossi, F.; Ercolani, D.; Sorba, L. Growth dynamics of InAs/InP nanowire heterostructures by Au-assisted chemical beam epitaxy. Nanotechnology 2019, 30, 094003. [Google Scholar] [CrossRef] [PubMed]
- Priante, G.; Patriarche, G.; Oehler, F.; Glas, F.; Harmand, J.C. Abrupt GaP/GaAs interfaces in self-catalyzed nanowires. Nano Lett. 2015, 15, 6036. [Google Scholar] [CrossRef]
- Bolshakov, A.D.; Fedorov, V.V.; Sibirev, N.V.; Fetisova, M.V.; Moiseev, E.I.; Kryzhanovskaya, N.V.; Koval, O.Y.; Ubyivovk, E.V.; Mozharov, A.M.; Cirlin, G.E.; et al. Growth and characterization of GaP/GaPAs nanowire heterostructures with controllable composition. Phys. Stat. Sol. RRL 2019, 13, 1900350. [Google Scholar] [CrossRef]
- Zhang, Y.; Velichko, A.V.; Fonseka, H.A.; Parkinson, P.; Gott, J.A.; Davis, G.; Aagesen, M.; Sanchez, A.M.; Mowbray, D.; Liu, H. Defect-free axially-stacked GaAs/GaAsP nanowire quantum dots with strong carrier confinement. Nano Lett. 2021, 21, 5722. [Google Scholar] [CrossRef]
- Boulanger, J.P.; LaPierre, R.R. Unveiling transient GaAs/GaP nanowire growth behavior using group V oscillations. Cryst. Growth 2014, 388, 116. [Google Scholar] [CrossRef]
- Ek, M.; Borg, B.M.; Johansson, J.; Dick, K.A. Diameter limitation in growth of III-Sb-containing nanowire heterostructures. ACS Nano 2013, 7, 3668. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, M.; Zou, J.; Guo, Y.-N.; Zhang, X.; Kim, Y.; Joyce, H.J.; Gao, Q.; Tan, H.H.; Jagadish, C. Nature of heterointerfaces in GaAs/InAs and InAs/GaAs axial nanowire heterostructures. Appl. Phys. Lett. 2008, 93, 101911. [Google Scholar] [CrossRef]
- Heiß, M.; Gustafsson, A.; Conesa-Boj, S.; Peiró, F.; Morante, J.R.; Abstreiter, G.; Arbiol, J.G.; Samuelson, L.; Fontcuberta i Morral, A. Catalyst-free nanowires with axial InxGa1-xAs/GaAs heterostructures. Nanotechnology 2009, 20, 075603. [Google Scholar] [CrossRef] [PubMed]
- Priante, G.; Glas, F.; Patriarche, G.; Pantzas, K.; Oehler, F.; Harmand, J.C. Sharpening the interfaces of axial heterostructures in self-catalyzed AlGaAs nanowires: Experiment and theory. Nano Lett. 2016, 16, 1917. [Google Scholar] [CrossRef] [PubMed]
- Sjokvist, R.; Jacobsson, D.; Tornberg, M.; Wallenberg, R.; Leshchenko, E.D.; Johansson, J.; Dick, K.A. Compositional correlation between the nanoparticle and the growing Au-assisted InxGa1-xAs nanowire. J. Phys. Chem. Lett. 2021, 12, 7590. [Google Scholar] [CrossRef] [PubMed]
- Leshchenko, E.D.; Dubrovskii, V.G. An overview of modeling approaches for compositional control in III–V ternary nan-owires. Nanomaterials 2023, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, V.G. Liquid-solid and vapor-solid distributions of vapor-liquid-solid III–V ternary nanowires. Phys. Rev. Mater. 2023, 7, 096001. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Circumventing the uncertainties of the liquid phase in the compositional control of VLS III–V ternary nanowires based on group V intermix. Nanomaterials 2024, 14, 207. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Leshchenko, E.D. Composition of III–V ternary materials under arbitrary material fluxes: The general approach unifying kinetics and thermodynamics. Phys. Rev. Mater. 2023, 7, 074603. [Google Scholar] [CrossRef]
- Glas, F. Comparison of modeling strategies for the growth of heterostructures in III–V nanowires. Cryst. Growth Des. 2017, 17, 4785. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Koryakin, A.A.; Sibirev, N.V. Understanding the composition of ternary III–V nanowires and axial nanowire heterostructures in nucleation-limited regime. Mater. Des. 2017, 132, 400. [Google Scholar] [CrossRef]
- Ghasemi, M.; Johansson, J. The composition of gold alloy seeded InGaAs nanowires in the nucleation limited regime. Cryst. Growth Des. 2017, 17, 1630. [Google Scholar]
- Johansson, J.; Ghasemi, M. Kinetically limited composition of ternary III–V nanowires. Phys. Rev. Mater. 2017, 1, 040401. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Composition of vapor-liquid-solid III–V ternary nanowires based on group III intermix. Nanomaterials 2023, 13, 2532. [Google Scholar] [CrossRef] [PubMed]
- McLean, D. Grain Boundaries in Metals; Oxford University Press: New York, NY, USA, 1957. [Google Scholar]
- Biefeld, R.M. The preparation of InSb and InAs1−xSbx by metalorganic chemical vapor deposition. J. Cryst. Growth 1986, 75, 255. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Refinement of nucleation theory for vapor-liquid-solid nanowires. Cryst. Growth Des. 2017, 17, 2589. [Google Scholar] [CrossRef]
- Harmand, J.C.; Patriarche, G.; Glas, F.; Panciera, F.; Florea, I.; Maurice, J.-L.; Travers, L.; Ollivier, Y. Atomic step flow on a nanofacet. Phys. Rev. Lett. 2018, 121, 166101. [Google Scholar] [CrossRef] [PubMed]
- Glas, F.; Panciera, F.; Harmand, J.C. Statistics of nucleation and growth of single monolayers in nanowires: Towards a deterministic regime. Phys. Stat. Solidi RRL 2022, 16, 2100647. [Google Scholar] [CrossRef]
- Glas, F. Incomplete monolayer regime and mixed regime of nanowire growth. Phys. Rev. Mater. 2024, 8, 043401. [Google Scholar] [CrossRef]
- Glas, F.; Ramdani, M.R.; Patriarche, G.; Harmand, J.C. Predictive modeling of self-catalyzed III–V nanowire growth. Phys. Rev. B 2013, 88, 195304. [Google Scholar] [CrossRef]
- Krogstrup, P.; Jørgensen, H.I.; Johnson, E.; Madsen, M.H.; Sørensen, C.B.; Fontcuberta i Morral, A.; Aagesen, M.; Nygård, J.; Glas, F. Advances in the theory of III–V nanowire growth dynamics. J. Phys. D Appl. Phys. 2013, 46, 313001. [Google Scholar] [CrossRef]
- Pishchagin, A.; Glas, F.; Patriarche, G.; Cattoni, A.; Harmand, J.C.; Oehler, F. Dynamics of droplet consumption in vapor–liquid–solid III–V nanowire growth. Cryst. Growth Des. 2021, 21, 4647. [Google Scholar] [CrossRef]
- Mosiiets, D.; Genuist, Y.; Cibert, J.; Bellet-Amalric, E.; Hocevar, M. Dual-adatom diffusion-limited growth model for compound nanowires: Application to InAs nanowires. Cryst. Growth Des. 2024, 24, 3888. [Google Scholar] [CrossRef]
- Ansara, I.; Chatillon, C.; Lukas, H.L.; Nishizawa, T.; Ohtani, H.; Ishida, K.; Hillert, M.; Sundman, B.; Argent, B.B.; Watson, A.; et al. A binary database for III–V compound semiconductor systems. Calphad 1994, 2, 177. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE unary database ver. 4.4. Calphad 1991, 15, 317. [Google Scholar] [CrossRef]
- Ramdani, M.R.; Harmand, J.C.; Glas, F.; Patriarche, G.; Travers, L. Arsenic pathways in self-catalyzed growth of GaAs nanowires. Cryst. Growth Des. 2013, 13, 91. [Google Scholar] [CrossRef]
- Li, L.; Pan, D.; Xue, Y.; Wang, X.; Lin, M.; Su, D.; Zhang, Q.; Yu, X.; So, H.; Wei, D.; et al. Near full-composition-range high-quality GaAs1−xSbx nanowires grown by molecular-beam epitaxy. Nano Lett. 2017, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, T.; Tomeda, A.; Watanabe, K.; Kamakura, Y.; Mori, N.; Naruse, N.; Mera, Y.; Yamashita, Y.; Nakamura, Y. Methodology of thermoelectric power factor enhancement by controlling nanowire interface. ACS Appl. Mater. Interfaces 2018, 10, 37709. [Google Scholar] [CrossRef]
- Bae, S.-H.; Lee, S.; Koo, H.; Lin, L.; Jo, B.H.; Park, C.; Wang, Z.L. The memristive properties of a single VO2 nanowire with switching controlled by self-heating. Adv. Mater. 2013, 25, 5098. [Google Scholar] [CrossRef]
- Bansal, S.; Prakash, K.; Sharma, K.; Sardana, N.; Kumar, S.; Gupta, N.; Singh, A.K. A highly efficient bilayer graphene/ZnO/silicon nanowire based heterojunction photodetector with broadband spectral response. Nanotechnology 2020, 31, 405205. [Google Scholar] [CrossRef]

| Parameter | Role in NW Composition | Temperature Dependence | Group III Flux Dependence | V/III Ratio Dependence |
|---|---|---|---|---|
| Vapor-related supersaturation parameter | Eliminates interactions in liquid and solid at | Increases with | Decreases with | Decreases with V/III flux ratio in vapor |
| Effective V/III flux ratio for the droplet | Eliminates non-linearity of the vapor–solid distribution at | Contains temperature-dependent diffusion length of group III adatoms | Independent | Increases with V/III flux ratio in vapor |
| Desorption/transport parameter | Leads to non-linear vapor–solid distribution at large | Contains unknown ratio of diffusion coefficients in liquid. Increases with for IIIPxAs1−x NWs according to our results | Independent | Independent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrovskii, V.G.; Leshchenko, E.D. Interplay of Kinetic and Thermodynamic Factors in the Stationary Composition of Vapor–Liquid–Solid IIIVxV1−x Nanowires. Nanomaterials 2024, 14, 1333. https://doi.org/10.3390/nano14161333
Dubrovskii VG, Leshchenko ED. Interplay of Kinetic and Thermodynamic Factors in the Stationary Composition of Vapor–Liquid–Solid IIIVxV1−x Nanowires. Nanomaterials. 2024; 14(16):1333. https://doi.org/10.3390/nano14161333
Chicago/Turabian StyleDubrovskii, Vladimir G., and Egor D. Leshchenko. 2024. "Interplay of Kinetic and Thermodynamic Factors in the Stationary Composition of Vapor–Liquid–Solid IIIVxV1−x Nanowires" Nanomaterials 14, no. 16: 1333. https://doi.org/10.3390/nano14161333





