Effects of Substrate and Annealing Conditions on the Ferroelectric Properties of Non-Doped HfO2 Deposited by RF Plasma Sputter
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.-Y. A new ferroelectric memory device, metal-ferroelectric-semiconductor transistor. IEEE Trans. Electron Devices 1974, 21, 499–504. [Google Scholar] [CrossRef]
- Vorotilov, K.A.; Sigov, A.S. Ferroelectric memory. Phys. Solid State. 2012, 54, 894–899. [Google Scholar] [CrossRef]
- Lue, H.-T.; Wu, C.-J.; Tseng, T.-Y. Device modeling of ferroelectric memory field-effect transistor for the application of ferroelectric random access memory. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2003, 50, 5–14. [Google Scholar] [CrossRef]
- Müller, J.; Polakowski, P.; Mueller, S.; Mikolajick, T. Ferroelectric Hafnium Oxide Based Materials and Devices: Assessment of Current Status and Future Prospects. ECS J. Solid State Sci. Technol. 2015, 4, N30. [Google Scholar] [CrossRef]
- Mikolajick, T.; Schroeder, U.; Slesazeck, S. The Past, the Present, and the Future of Ferroelectric Memories. IEEE Trans. Electron Devices 2020, 67, 1434–1443. [Google Scholar] [CrossRef]
- Rørvik, P.M.; Grande, T.; Einarsrud, M.-A. One-Dimensional Nanostructures of Ferroelectric Perovskites. Adv. Mater. 2011, 23, 4007–4034. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Liu, Z.-B.; Tang, Y.-Y.; Li, P.-F.; Ma, R.-W.; Wei, R.-Y.; Zhang, Y.; You, Y.-M.; Ye, H.-Y.; Xiong, R.-G. A Three-Dimensional Molecular Perovskite Ferroelectric: (3-Ammoniopyrrolidinium)RbBr3. J. Am. Chem. Soc. 2017, 139, 3954–3957. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Harris, D.T.; Keech, R.; Jones, J.L.; Maria, J.-P.; Trolier-McKinstry, S. Scaling Effects in Perovskite Ferroelectrics: Fundamental Limits and Process-Structure-Property Relations. J. Am. Ceram. Soc. 2016, 99, 2537–2557. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, Y.H.; Mikolajick, T.; Schroeder, U.; Hwang, C.S. Review and perspective on ferroelectric HfO2-based thin films for memory applications. MRS Commun. 2018, 8, 795–808. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, D.H.; Park, G.H.; Lee, J.; Lee, Y.; Park, M.H. A perspective on the physical scaling down of hafnia-based ferroelectrics. Nanotechnology 2023, 34, 202001. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Wang, J. Ferroelectric HfO2-based materials for next-generation ferroelectric memories. J. Adv. Dielect. 2016, 6, 1630003. [Google Scholar] [CrossRef]
- Polakowski, P.; Müller, J. Ferroelectricity in undoped hafnium oxide. Appl. Phys. Lett. 2015, 106, 232905. [Google Scholar] [CrossRef]
- Shimizu, T.; Katayama, K.; Kiguchi, T.; Akama, A.; Konno, T.J.; Funakubo, H. Growth of epitaxial orthorhombic YO1.5-substituted HfO2 thin film. Appl. Phys. Lett. 2015, 107, 032910. [Google Scholar] [CrossRef]
- Hsain, H.A.; Lee, Y.; Materano, M.; Mittmann, T.; Payne, A.; Mikolajick, T.; Schroeder, U.; Parsons, G.N.; Jones, J.L. Many routes to ferroelectric HfO2: A review of current deposition methods. J. Vac. Sci. Technol. A 2022, 40, 010803. [Google Scholar] [CrossRef]
- Mittmann, T.; Materano, M.; Lomenzo, P.D.; Park, M.H.; Stolichnov, I.; Cavalieri, M.; Zhou, C.; Chung, C.-C.; Jones, J.L.; Szyjka, T.; et al. Origin of Ferroelectric Phase in Undoped HfO2 Films Deposited by Sputtering. Adv. Mater. Interfaces 2019, 6, 1900042. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Z.; Yin, X.; Chen, C.; Fan, Z.; Qin, M.; Zeng, M.; Zhou, G.; Gao, X.; Lu, X. Ferroelectricity in dopant-free HfO2 thin films prepared by pulsed laser deposition. J. Mater. 2022, 8, 311–318. [Google Scholar] [CrossRef]
- Kumar, M.; Seo, H. High-Performing Self-Powered Photosensing and Reconfigurable Pyro-photoelectric Memory with Ferroelectric Hafnium Oxide. Adv. Mater. 2022, 34, 2106881. [Google Scholar] [CrossRef]
- Li, W.; Sun, Z.; Tian, D.; Nevirkovets, I.P.; Dou, S.-X. Platinum dendritic nanoparticles with magnetic behavior. J. Appl. Phys. 2014, 116, 033911. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Takai, O.; Saito, N. Epitaxial growth of (111)-oriented BaTiO3/SrTiO3 perovskite superlattices on Pt(111)/Ti/Al2O3(0001) substrates. Appl. Phys. Lett. 2013, 103, 112902. [Google Scholar] [CrossRef]
- Song, T.; Tan, H.; Dix, N.; Moalla, R.; Lyu, J.; Saint-Girons, G.; Bachelet, R.; Sánchez, F.; Fina, I. Stabilization of the Ferroelectric Phase in Epitaxial Hf1–xZrxO2 Enabling Coexistence of Ferroelectric and Enhanced Piezoelectric Properties. ACS Appl. Electron. Mater. 2021, 3, 5, 2106–2113. [Google Scholar] [CrossRef]
- Ryu, H.; Xu, K.; Kim, J.; Kang, S.; Guo, J.; Zhu, W. Exploring New Metal Electrodes for Ferroelectric Aluminum-Doped Hafnium Oxide. IEEE Trans. Electron Devices 2019, 66, 2359–2364. [Google Scholar] [CrossRef]
- Weeks, S.L.; Pal, A.; Narasimhan, V.K.; Littau, K.A.; Chiang, T. Engineering of Ferroelectric HfO2–ZrO2 Nanolaminates. ACS Appl. Mater. Interfaces 2017, 9, 13440–13447. [Google Scholar] [CrossRef]
- Lowther, J.E.; Dewhurst, J.K.; Leger, J.M.; Haines, J. Relative stability of ZrO2 and HfO2 structural phases. Phys. Rev. B 1999, 60, 14485–14488. [Google Scholar] [CrossRef]
- Ma, C.; Rossman, G.R. Tistarite, Ti2O3, a new refractory mineral from the Allende meteorite. Am. Mineral. 2009, 94, 841–844. [Google Scholar] [CrossRef]
- Robertson, A.L.; Solá, F.; Zhu, D.; Salem, J.; White, K.W. White, Microscale fracture mechanisms of HfO2-Si environmental barrier coatings. J. Eur. Ceram. Soc. 2019, 39, 2409–2418. [Google Scholar] [CrossRef]
- He, G.; Liu, M.; Zhu, L.Q.; Chang, M.; Fang, Q.; Zhang, L.D. Effect of postdeposition annealing on the thermal stability and structural characteristics of sputtered HfO2 films on Si (100). Surf. Sci. 2005, 576, 67–75. [Google Scholar] [CrossRef]
- Hernández-Arriaga, H.; López-Luna, E.; Martínez-Guerra, E.; Turrubiartes, M.M.; Rodríguez, A.G.; Vidal, M.A. Growth of HfO2/TiO2 nanolaminates by atomic layer deposition and HfO2-TiO2 by atomic partial layer deposition. J. Appl. Phys. 2017, 121, 064302. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, X.; Chen, P.; Xiao, M.; Monny, S.A.; Wang, S.; Konarova, M.; Du, A.; Wang, L. Understanding the Roles of Oxygen Vacancies in Hematite-Based Photoelectrochemical Processes. Angew. Chem. 2019, 131, 1042–1046. [Google Scholar] [CrossRef]
- Kumar, M.; Mookerjee, S.; Som, T. Field-induced doping-mediated tunability in work function of Al-doped ZnO: Kelvin probe force microscopy and first-principle theory. Nanotechnology 2016, 27, 375702. [Google Scholar] [CrossRef]
- Basu, T.; Kumar, M.; Nandy, S.; Satpati, B.; Saini, C.P.; Kanjilal, A.; Som, T. Thickness-dependent blue shift in the excitonic peak of conformally grown ZnO: Al on ion-beam fabricated self-organized Si ripples. J. Appl. Phys. 2015, 118, 04903. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, H.; Xia, Q.; Ye, C.; Wei, X.; Wang, J.; Zhang, L.; Zhu, L.Q. Role of Oxygen Vacancies at the TiO2/HfO2 Interface in Flexible Oxide-Based Resistive Switching Memory. Adv. Electron. Mater. 2019, 5, 1800833. [Google Scholar] [CrossRef]
- Aliev, V.S.; Gerasimova, A.K.; Kruchinin, V.N.; Gritsenko, V.A.; Prosvirin, I.P.; Badmaeva, I.A. The atomic structure and chemical composition of HfOx (x < 2) films prepared by ion-beam sputtering deposition. Mater. Res. Express. 2016, 3, 085008. [Google Scholar] [CrossRef]
- Baumgarten, L.; Szyjka, T.; Mittmann, T.; Materano, M.; Matveyev, Y.; Schlueter, C.; Thomas, M.; Uwe, S.; Müller, M. Impact of vacancies and impurities on ferroelectricity in PVD- and ALD-grown HfO2 films. Appl. Phys. Lett. 2021, 118, 032903. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hong, K.; Na, K.; Yang, J.; Lee, T.H.; Kim, B.; Bark, C.W.; Kim, J.Y.; Lee, S.; Jang, H.W. Nonvolatile Control of Metal–Insulator Transition in VO2 by Ferroelectric Gating. Adv. Mater. 2022, 34, 2203097. [Google Scholar] [CrossRef]

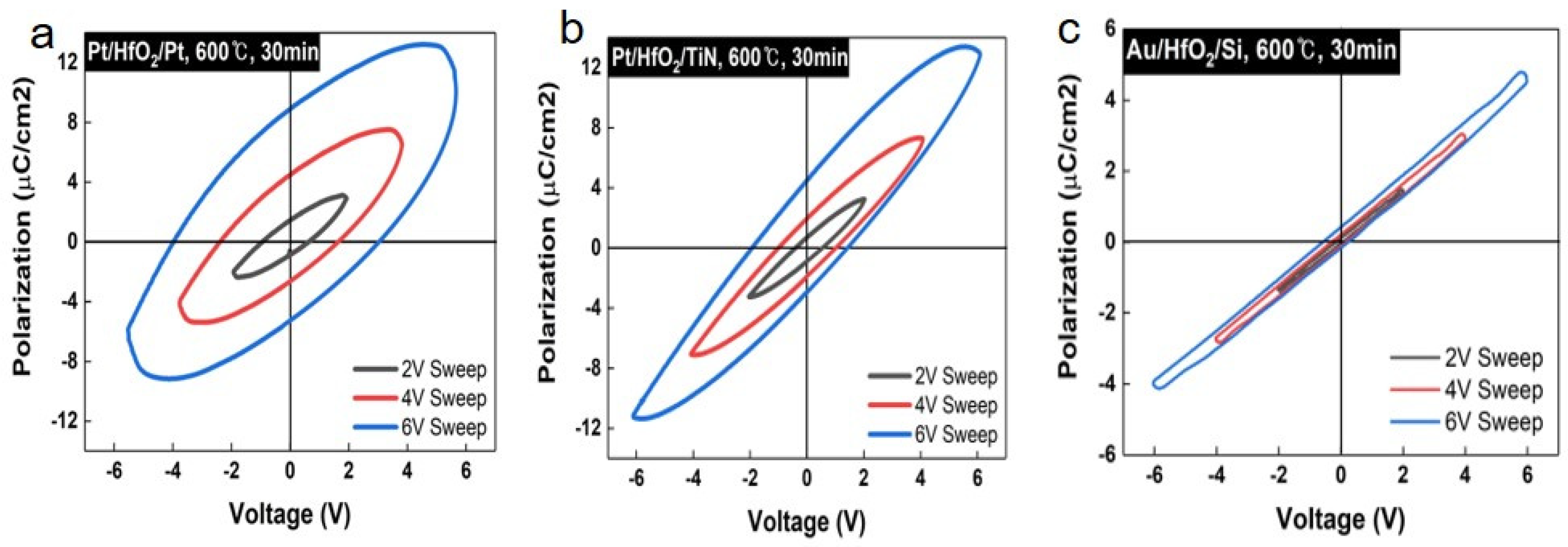
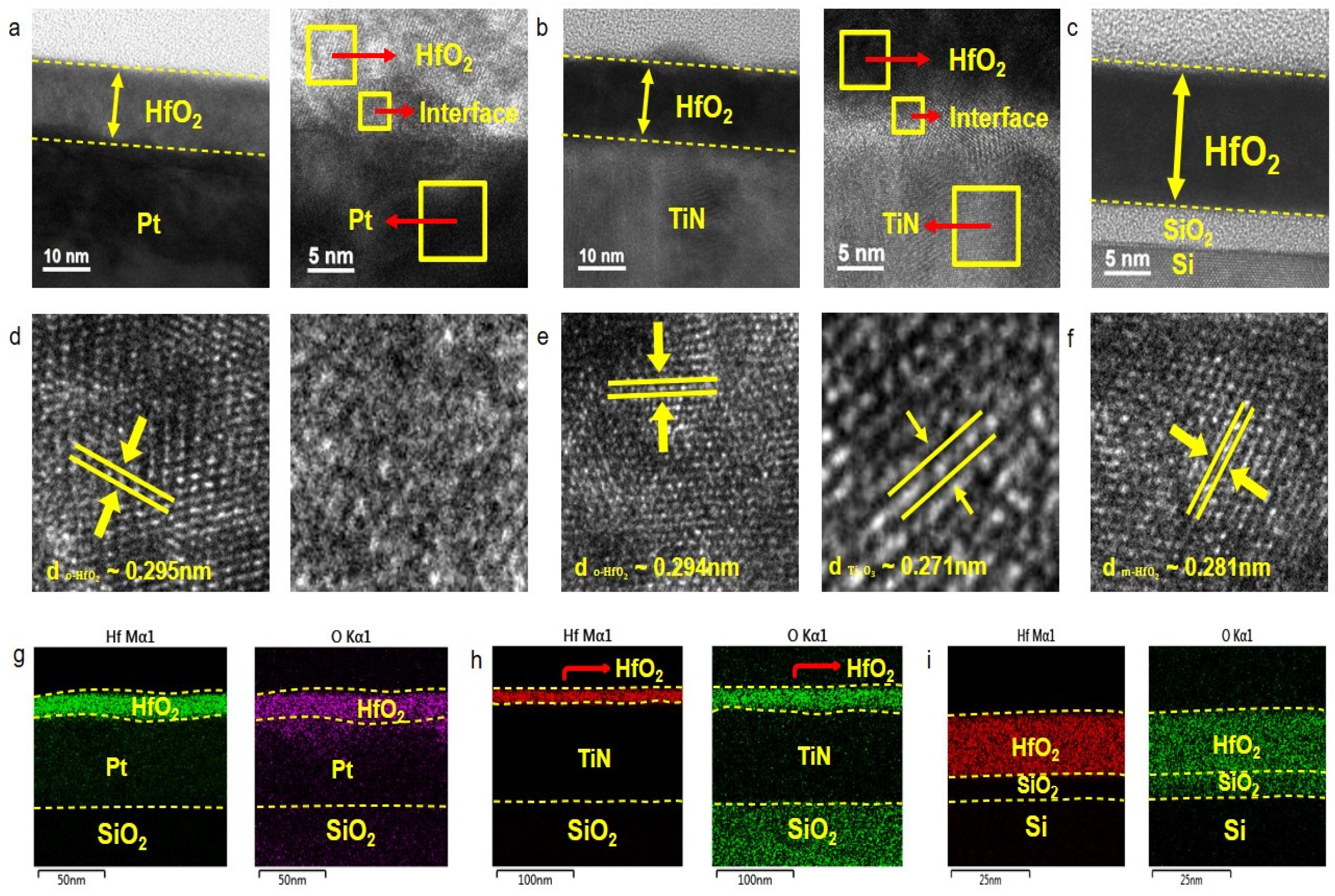
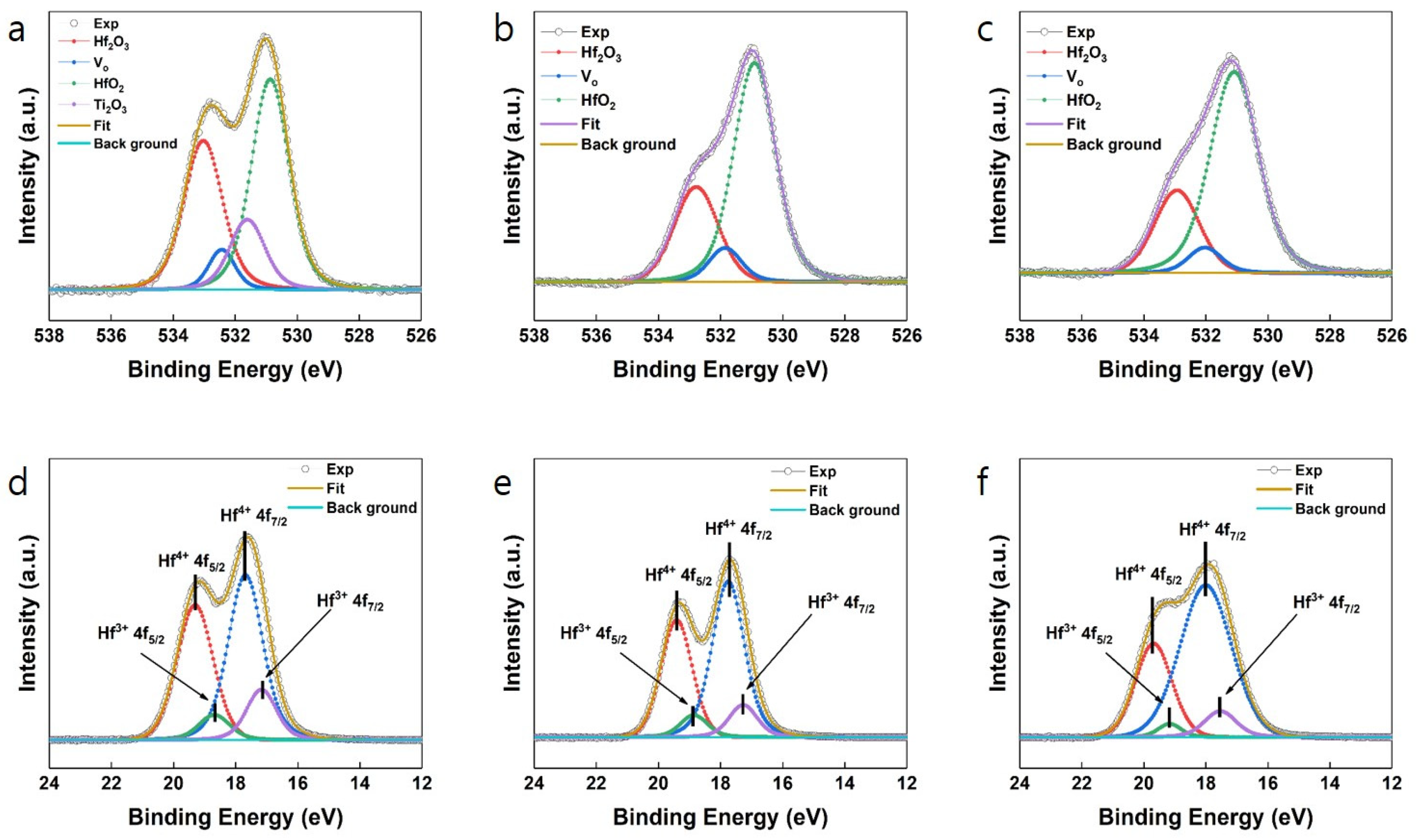
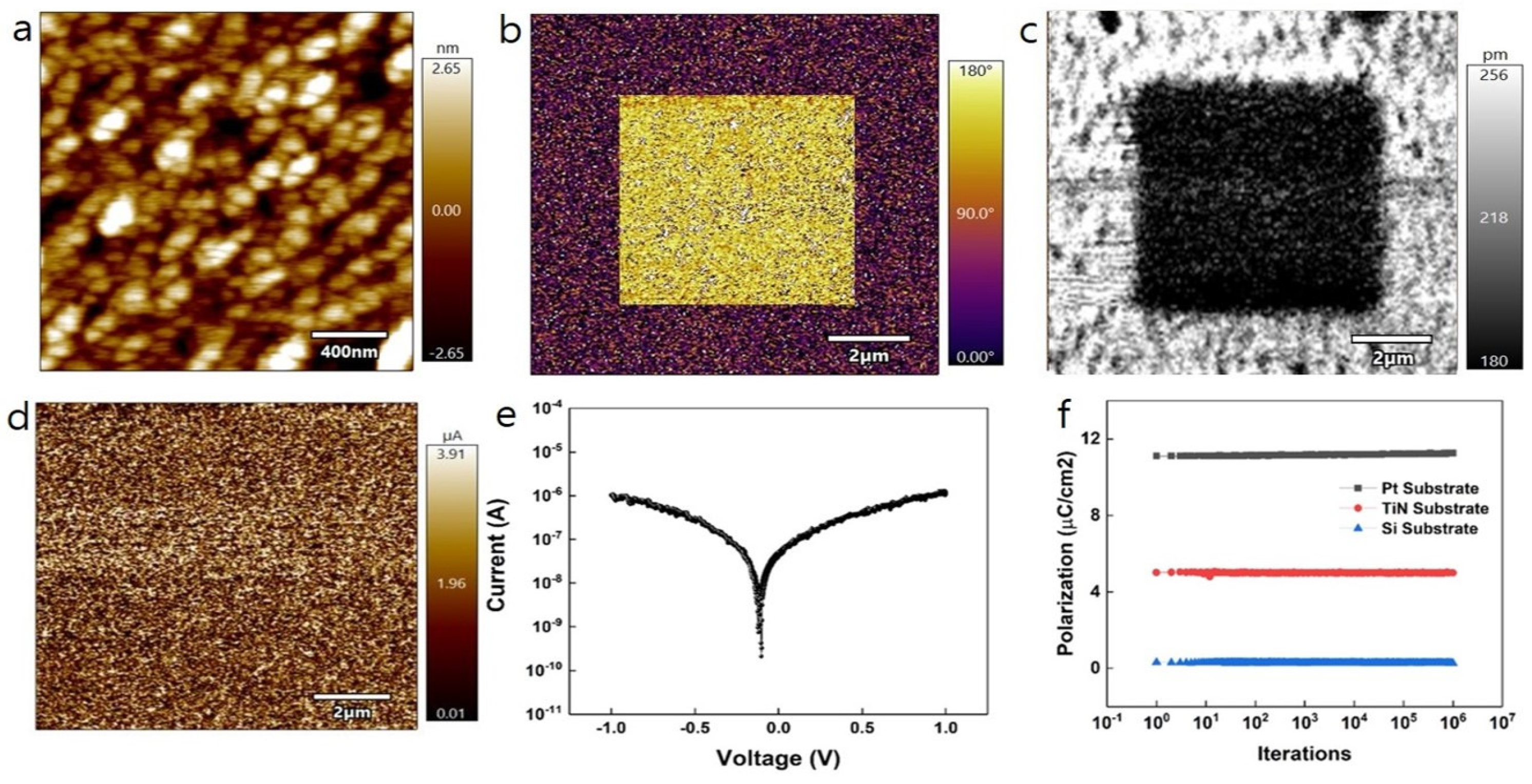
| Samples | Remanent Polarization (2Pr) (μC/cm2) | Coercive Field (V) |
|---|---|---|
| Pt | 14.24 (±0.01) | 4.03 |
| TiN | 7.43 (±0.01) | 1.89 |
| Si | 0.88 (±0.01) | 0.58 |
| Samples | Binding Energy (eV) | |||
|---|---|---|---|---|
| Hf2O3 | Oxygen Vacancy | HfO2 | Ti2O3 | |
| Pt | 532.78 | 531.85 | 530.9 | - |
| TiN | 533.02 | 532.43 | 530.87 | 531.61 |
| Si | 532.93 | 532.03 | 531.08 | - |
| Samples | Relative binding ratio (%) | |||
| Hf2O3 | Oxygen vacancy | HfO2 | Ti2O3 | |
| Pt | 27.71 | 7.76 | 64.53 | - |
| TiN | 33.51 | 6.1 | 45.99 | 14.4 |
| Si | 24.9 | 6 | 69.1 | - |
| Sample | Binding Energy (eV) | |||
|---|---|---|---|---|
| Hf4+ 4f5/2 | Hf4+ 4f7/2 | Hf3+ 4f5/2 | Hf3+ 4f7/2 | |
| Pt | 19.34 | 17.74 | 18.76 | 17.14 |
| TiN | 19.55 | 17.87 | 18.98 | 17.34 |
| Si | 19.57 | 17.94 | 18.78 | 17.18 |
| Samples | Relative binding ratio (%) | |||
| Hf4+ | Hf3+ | |||
| Pt | 69.67 (±0.01) | 30.33 (±0.01) | ||
| TiN | 71.79 (±0.01) | 28.21 (±0.01) | ||
| Si | 84.09 (±0.01) | 15.91 (±0.01) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Ahn, Y.; Won, B.; Lee, S.; Park, H.; Kumar, M.; Seo, H. Effects of Substrate and Annealing Conditions on the Ferroelectric Properties of Non-Doped HfO2 Deposited by RF Plasma Sputter. Nanomaterials 2024, 14, 1386. https://doi.org/10.3390/nano14171386
Lim S, Ahn Y, Won B, Lee S, Park H, Kumar M, Seo H. Effects of Substrate and Annealing Conditions on the Ferroelectric Properties of Non-Doped HfO2 Deposited by RF Plasma Sputter. Nanomaterials. 2024; 14(17):1386. https://doi.org/10.3390/nano14171386
Chicago/Turabian StyleLim, Seokwon, Yeonghwan Ahn, Beomho Won, Suwan Lee, Hayoung Park, Mohit Kumar, and Hyungtak Seo. 2024. "Effects of Substrate and Annealing Conditions on the Ferroelectric Properties of Non-Doped HfO2 Deposited by RF Plasma Sputter" Nanomaterials 14, no. 17: 1386. https://doi.org/10.3390/nano14171386
APA StyleLim, S., Ahn, Y., Won, B., Lee, S., Park, H., Kumar, M., & Seo, H. (2024). Effects of Substrate and Annealing Conditions on the Ferroelectric Properties of Non-Doped HfO2 Deposited by RF Plasma Sputter. Nanomaterials, 14(17), 1386. https://doi.org/10.3390/nano14171386






