High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
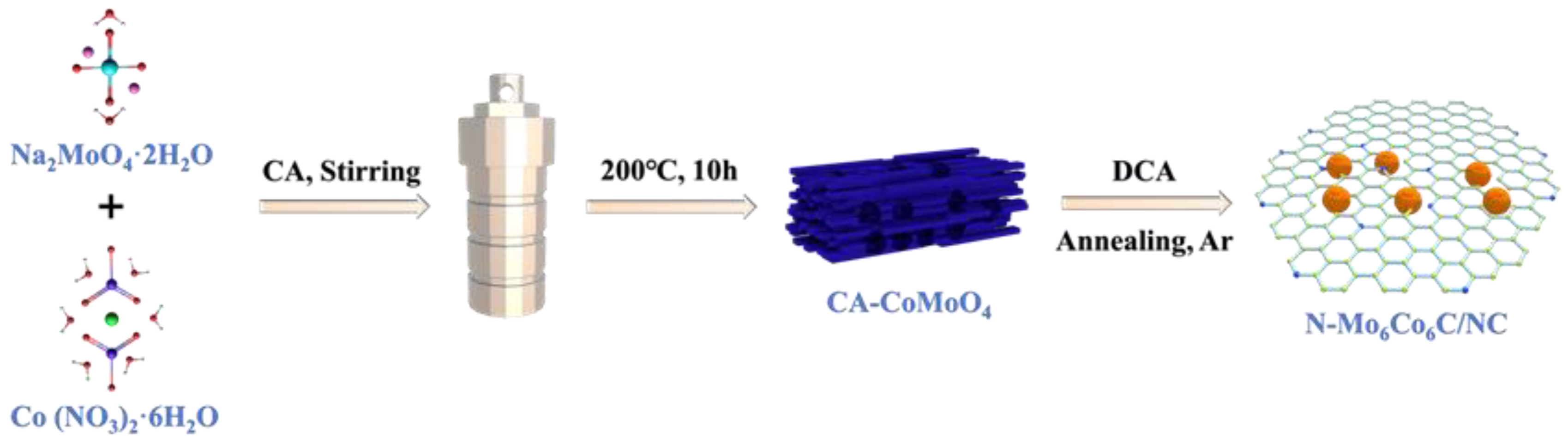
2.2. Synthesis of CA-CoMoO4
2.3. Synthesis of N-Co6Mo6C/NC
2.4. Synthesis of CoMoO4
2.5. Synthesis of CoMoxCy/C
2.6. Synthesis of N-CoMoxCy
2.7. Material Characterization
2.8. Electrochemical Measurements
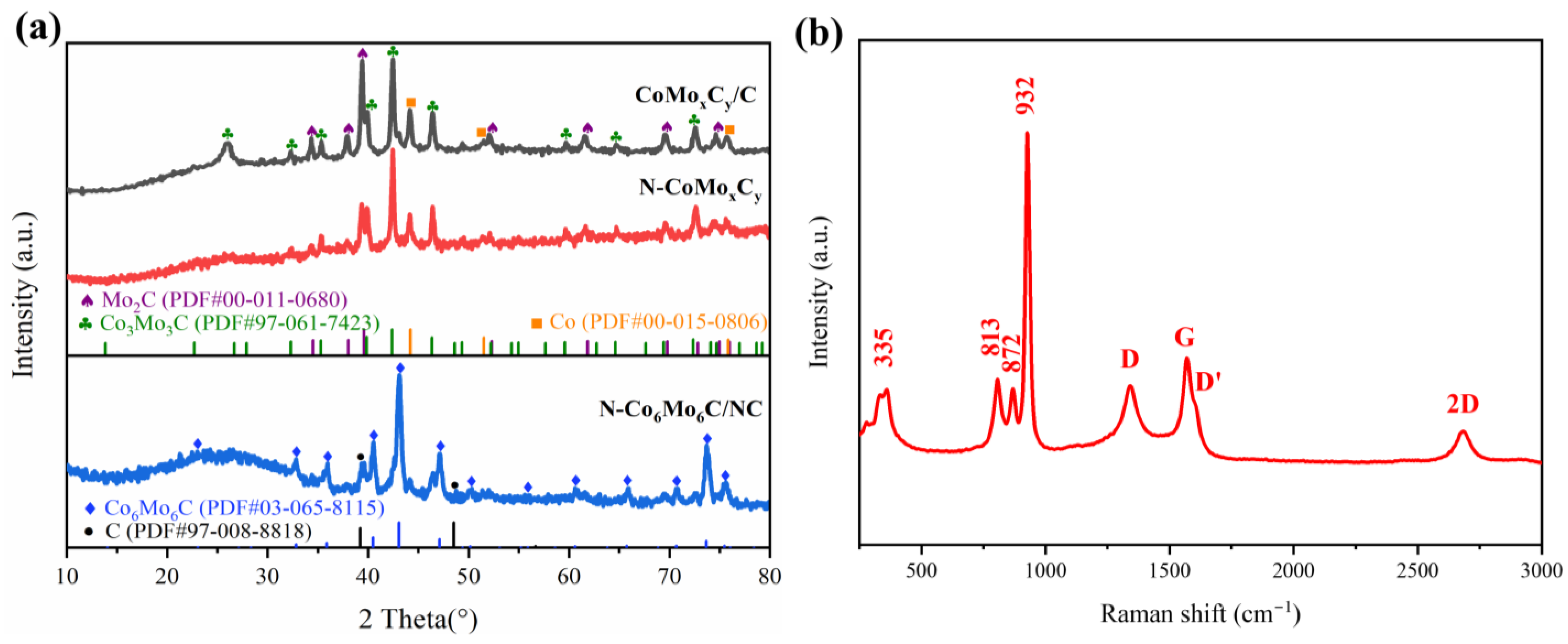
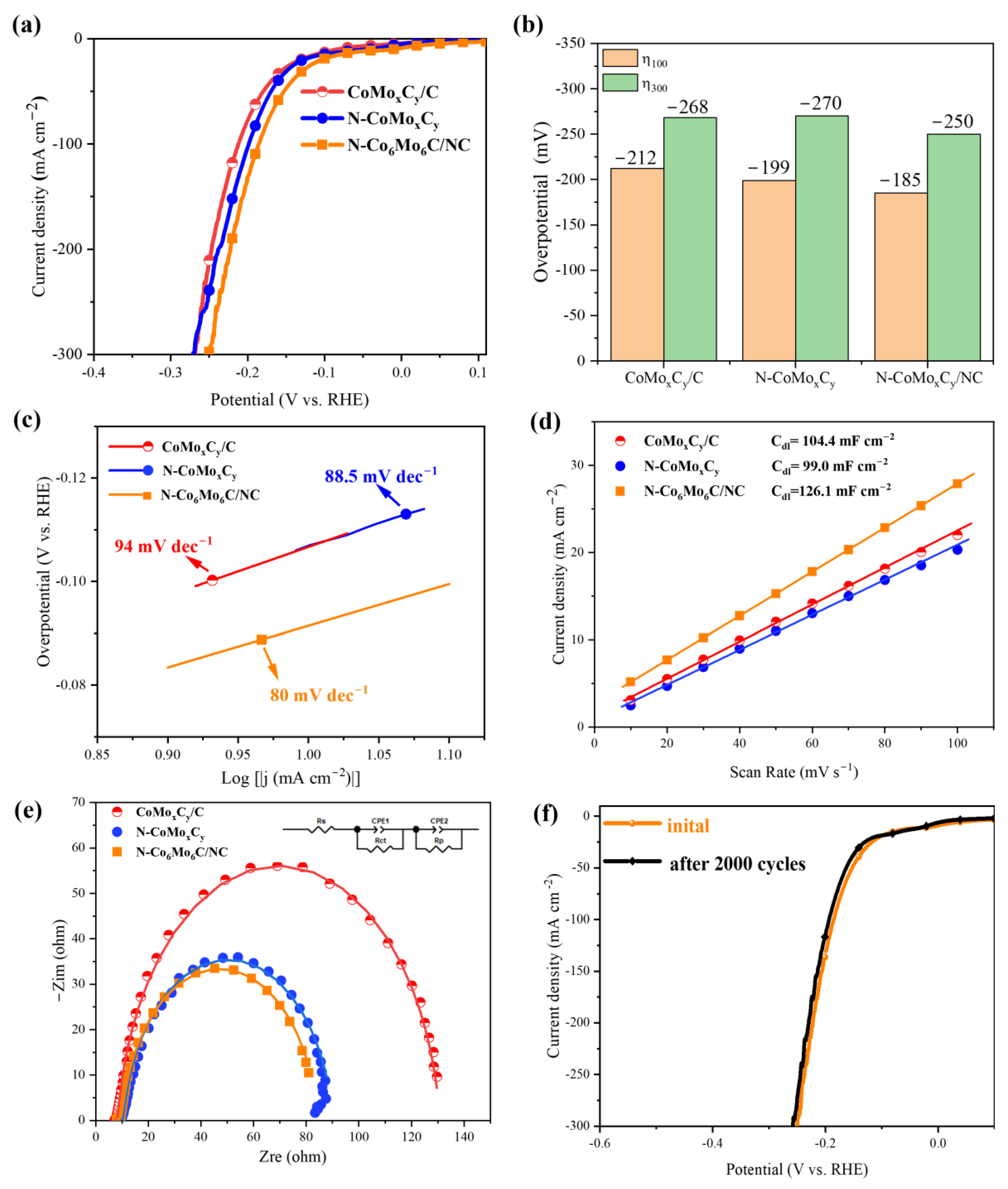
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B-Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, C.; Li, X.; Deng, Y.; Tao, B.; Xiao, W.; Li, L.; Li, N.; Luo, H. Self-Interconnected Porous Networks of NiCo Disulfide as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Acs Appl. Mater. Interfaces 2018, 10, 27723–27733. [Google Scholar] [CrossRef]
- Huang, J.; Han, J.; Wu, T.; Feng, K.; Yao, T.; Wang, X.; Liu, S.; Zhong, J.; Zhang, Z.; Zhang, Y.; et al. Boosting Hydrogen Transfer during Volmer Reaction at Oxides/Metal Nanocomposites for Efficient Alkaline Hydrogen Evolution. Acs Energy Lett. 2019, 4, 3002–3010. [Google Scholar] [CrossRef]
- Jiang, D.; Ma, W.; Yang, R.; Quan, B.; Li, D.; Meng, S.; Chen, M. Nickel-manganese bimetallic phosphides porous nanosheet arrays as highly active bifunctional hydrogen and oxygen evolution electrocatalysts for overall water splitting. Electrochim. Acta 2020, 329, 135121. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.-Z. Nitrogen and Oxygen Dual-Doped Carbon Hydrogel Film as a Substrate-Free Electrode for Highly Efficient Oxygen Evolution Reaction. Adv. Mater. 2014, 26, 2925–2930. [Google Scholar] [CrossRef]
- Fang, M.; Gao, W.; Dong, G.; Xia, Z.; Yip, S.; Qin, Y.; Qu, Y.; Ho, J.C. Hierarchical NiMo-based 3D electrocatalysts for highly-efficient hydrogen evolution in alkaline conditions. Nano Energy 2016, 27, 247–254. [Google Scholar] [CrossRef]
- Li, T.; Lv, Y.; Su, J.; Wang, Y.; Yang, Q.; Zhang, Y.; Zhou, J.; Xu, L.; Sun, D.; Tang, Y. Anchoring CoFe2O4 Nanoparticles on N-Doped Carbon Nanofibers for High-Performance Oxygen Evolution Reaction. Adv. Sci. 2017, 4, 1700226. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Z.; Lin, Y.; Wang, J.; Pan, H.; Xu, Z. Hierarchical heterostructure NiCo2O4@CoMoO4/NF as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 16950–16958. [Google Scholar] [CrossRef]
- Yan, X.; Tian, L.; He, M.; Chen, X. Three-Dimensional Crystalline/Amorphous Co/Co3O4 Core/Shell Nanosheets as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. Nano Lett. 2015, 15, 6015–6021. [Google Scholar] [CrossRef]
- Liu, Z.; Zhan, C.; Peng, L.; Cao, Y.; Chen, Y.; Ding, S.; Xiao, C.; Lai, X.; Li, J.; Wei, S.; et al. A CoMoO4-Co2Mo3O8 heterostructure with valence-rich molybdenum for a high-performance hydrogen evolution reaction in alkaline solution. J. Mater. Chem. A 2019, 7, 16761–16769. [Google Scholar] [CrossRef]
- Wang, X.; He, P.; Yang, Y.; Pan, Y.; Jin, Z.; Ling, R. Heterostructure Co3O4@NiWO4 nanocone arrays with enriched active area for efficient hydrogen evolution reaction. J. Alloys Compd. 2020, 844, 156095. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Arvand, M.; Daneshvar, S. Facile stepwise hydrothermal synthesis of hierarchical CoMoO4/CoMoO4 core/shell dandelion-like nanoarrays: A promising binder-free positive electrode for high-performance asymmetric supercapacitors. J. Electroanal. Chem. 2022, 904, 115934. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Zaman, S.F.; Podila, S.; Driss, H.; Al-Zahrani, A.A.; Daous, M.A.; Petrov, L. Ammonia decomposition over citric acid induced gamma-Mo2N and Co3Mo3N catalysts. Int. J. Hydrogen Energy 2018, 43, 4839–4844. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey Reduced Graphene Oxide Coupled with an Mo2N-Mo2C Heterojunction for Efficient Hydrogen Evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N Porous Nanowire Arrays Activated by Surface Oxidation as Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.; Zhang, L.; Sun, Y.; Xu, K.; Dai, Z.; Ma, F. Mace-like hierarchical MoS2/NiCo2S4 composites supported by carbon fiber paper: An efficient electrocatalyst for the hydrogen evolution reaction. J. Power Sources 2018, 377, 142–150. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Han, D.; Song, X.; Shi, L.; Song, Y.; Niu, S.; Xie, Y.; Cai, J.; Wu, S.; et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.; Dai, H.; Wang, Q.; Yang, R.; Yu, H.; Liao, M.; Zhang, J.; Chen, W.; Wei, Z.; et al. Boundary activated hydrogen evolution reaction on monolayer MoS2. Nat. Commun. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Tan, H.; Khan, S.U.; Ma, Y.; Zang, H.; Wang, Y.; Li, Y. MoP/Mo2C@C: A New Combination of Electrocatalysts for Highly Efficient Hydrogen Evolution over the Entire pH Range. Acs Appl. Mater. Interfaces 2017, 9, 16270–16279. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P Electrocatalyst for the Hydrogen Evolution Reaction and Direct Comparison with Morphologically Equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhao, Y.; Yu, W.; Jiang, X.; He, M.; Li, Z.; Ma, T.; Wu, Z.; Wang, L. Facile synthesis of MoP-Ru2P on porous N, P co-doped carbon for efficiently electrocatalytic hydrogen evolution reaction in full pH range. Appl. Catal. B Environ. 2022, 303, 120879. [Google Scholar] [CrossRef]
- Ren, J.-T.; Chen, L.; Yang, D.-D.; Yuan, Z.-Y. Molybdenum-based nanoparticles (Mo2C, MoP and MoS2) coupled heteroatoms-doped carbon nanosheets for efficient hydrogen evolution reaction. Appl. Catal. B Environ. 2020, 263, 118352. [Google Scholar] [CrossRef]
- Dong, J.; Wu, Q.; Huang, C.; Yao, W.; Xu, Q. Cost effective Mo rich Mo2C electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 10028–10035. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Liang, X.; Ma, F.; Zhang, J.; Tan, Y.; Pan, Z.; Bo, Y.; Wu, C.L. Encapsulating dual-phased Mo2C-WC nanocrystals into ultrathin carbon nanosheet assemblies for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2021, 408, 127270. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhang, H.; Ma, J.; Huang, Z.; Li, J.; Wang, Y. Transition-Metal Carbides as Hydrogen Evolution Reduction Electrocatalysts: Synthetic Methods and Optimization Strategies. Chem. A Eur. J. 2021, 27, 5074–5090. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Wang, X.; Li, S.; Pan, Y.; Wang, Y.; Song, X.; Tan, Z. Plant polyphenol-involved coordination assembly-derived Mo3Co3C/Mo2C/Co@NC with phase regulation and interface engineering for efficient hydrogen evolution reaction electrocatalysis. New J. Chem. 2022, 46, 13030–13036. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Zhao, L.; Sui, X.; Gu, D.; Wang, Z. Hierarchical Heterostructured Mo2C/Mo3Co3C Bouquet-like Nanowire Arrays: An Efficient Electrocatalyst for Hydrogen Evolution Reaction. Acs Sustain. Chem. Eng. 2019, 7, 7294–7303. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Wang, K.; Ao, M.; Qiu, J.; Zhang, X.; Wang, H.; Pham, G.H.; Liu, S. Co/Co6Mo6C@C nanoreactors derived from ZIF-67 composite for higher alcohols synthesis. Compos. Part B Eng. 2021, 209, 108608. [Google Scholar] [CrossRef]
- He, C.; Tao, J. Three-dimensional hollow porous Co6Mo6C nanoframe as an highly active and durable electrocatalyst for water splitting. J. Catal. 2017, 347, 63–71. [Google Scholar] [CrossRef]
- He, C.; Tao, J. 2D Co6Mo6C Nanosheets as Robust Hydrogen Evolution Reaction Electrocatalyst. Adv. Sustain. Syst. 2018, 2, 1700136. [Google Scholar] [CrossRef]
- He, C.; Zhang, S.; Tao, J.; Qiu, Y. Ultrafine Co6Mo6C nanocrystals on reduced graphene oxide as efficient and highly stable electrocatalyst for hydrogen generation. Int. J. Hydrogen Energy 2018, 43, 20323–20331. [Google Scholar] [CrossRef]
- Geng, S.; Xu, S.; Yu, Y.S.; Yang, W.; Feng, M.; Li, H. N-doped Co6Mo6C nanorods as highly active and durable bifunctional electrocatalysts for water splitting. J. Electroanal. Chem. 2020, 871, 114271. [Google Scholar] [CrossRef]
- Rameez Ahmad, M.; Sanjay, U.; Pandey, O.P. A review on recent advances and progress in Mo2C@C: A suitable and stable electrocatalyst for HER. Int. J. Hydrogen Energy 2023, 48, 13044–13067. [Google Scholar] [CrossRef]
- Aitbekova, A.; Zhou, C.; Stone, M.L.; Lezama-Pacheco, J.S.; Yang, A.-C.; Hoffman, A.S.; Goodman, E.D.; Huber, P.; Stebbins, J.F.; Bustillo, K.C.; et al. Templated encapsulation of platinum-based catalysts promotes high-temperature stability to 1,100 °C. Nat. Mater. 2022, 21, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, H.; Luo, Y.; Mei, L.; Guo, D.; Li, Q.; Wang, T. Enhanced electrochemical performance of CoMoO4 nanorods/reduced graphene oxide as anode material for lithium-ion batteries. Electrochim. Acta 2015, 158, 327–332. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Krishnamoorthy, K.; Kim, S.J. Improved electrochemical performances of binder-free CoMoO4 nanoplate arrays@Ni foam electrode using redox additive electrolyte. J. Power Sources 2016, 306, 378–386. [Google Scholar] [CrossRef]
- Min, F.; Jingle, H.; Yang, P.; Churong, H.; Xin, Y.; Shaoming, H. Tuning the Electronic Structures of Cobalt-Molybdenum Bimetallic Carbides to Boost the Hydrogen Oxidation Reaction in Alkaline Medium. Chem. Eng. J. 2021, 428, 131206. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Chen, S.; Ren, W.; Chen, X.; Zhao, C. Synergistic bimetallic CoFe2O4 clusters supported on graphene for ambient electrocatalytic reduction of nitrogen to ammonia. Chem. Commun. 2019, 56, 11477. [Google Scholar] [CrossRef]
- Padmanathan, N.; Razeeb, K.M.; Selladurai, S. Hydrothermal synthesis of carbon- and reduced graphene oxide-supported CoMoO4 nanorods for supercapacitor. Ionics 2014, 20, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, H.; Pan, Y.; Lin, W.; Xu, S.; Sun, Q.; Liu, E.; He, F.; Diao, L.; He, C.; et al. Graphite Carbon Nanosheet-Coated Cobalt-Doped Molybdenum Carbide Nanoparticles for Efficient Alkaline Hydrogen Evolution Reaction. Acs Appl. Nano Mater. 2021, 4, 372–380. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; et al. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloys Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Liu, X.; Amiinu, I.S.; Liu, S.; Pu, Z.; Li, W.; Ye, B.; Tan, D.; Mu, S. H2O2-Assisted Synthesis of Porous N-Doped Graphene/Molybdenum Nitride Composites with Boosted Oxygen Reduction Reaction. Adv. Mater. Interfaces 2017, 4, 1601227. [Google Scholar] [CrossRef]
- Lin, X.; Zhenduo, C.; Shengli, Z.; Yanqin, L.; Zhaoyang, L.; Shuilin, W.; Chuntao, C.; Shuiyuan, L. Self-supporting CoMoC nanoporous catalysts for N2 reduction reaction under ambient conditions. Appl. Surf. Sci. 2020, 521, 272–279. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Rao, T.N. MoO2/Multiwalled Carbon Nanotubes (MWCNT) Hybrid for Use as a Li-Ion Battery Anode. Acs Appl. Mater. Interfaces 2013, 5, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hu, X.; Ning, S.; Kang, X.; Chen, S. Supported Heterostructured MoC/Mo2C Nanoribbons and Nanoflowers as Highly Active Electrocatalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8458–8465. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Wang, Z.; Lei, Y.; Wang, B.; Wu, N.; Han, C.; Xie, S.; Gou, Y. Three-dimensional (3D) interconnected networks fabricated via in-situ growth of N-doped graphene/carbon nanotubes on Co-containing carbon nanofibers for enhanced oxygen reduction. Nano Res. 2016, 9, 317–328. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Chen, X.; He, X.; Wang, X.; Dong, S.; Gu, L.; Liu, Z.; Huang, C.; Cui, G. Molybdenum Nitride/N-Doped Carbon Nanospheres for Lithium-O2 Battery Cathode Electrocatalyst. Acs Appl. Mater. Interfaces 2013, 5, 3677–3682. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, Y.; Jia, W.; Lu, Z.; Jia, D. A cage-confinement strategy to fabricate Pt-Mo6Co6C heterojunction for highly efficient PH-universal hydrogen evolution. Appl. Catal. B Environ. 2021, 298, 120579. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Zang, M.; Xu, N.; Cao, G.; Chen, Z.; Cui, J.; Gan, L.; Dai, H.; Yang, X.; Wang, P. Cobalt Molybdenum Oxide Derived High-Performance Electrocatalyst for the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 5062–5069. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.; Kang, Z.H.; Wang, E.B.; Li, Y.G. Highly Efficient Hydrogen Evolution from Seawater by a Low-Cost and Stable CoMoP@C Electrocatalyst Superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Liu, Y.R.; Shang, X.; Gao, W.K.; Dong, B.; Li, X.; Li, X.H.; Zhao, J.C.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. In Situ Sulfurized CoMoS/CoMoO4 Shell-Core Nanorods Supported on N-Doped Reduced Graphene Oxide (NRGO) as Efficient Electrocatalyst for Hydrogen Evolution Reaction. J. Mater. Chem. A 2017, 5, 2885–2896. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, H.; Wang, Z.; Xiong, Y.; Yuan, S.; Zeng, X.; He, D.; Mu, S. Metal-Organic Frameworks Derived Reverse-Encapsulation Co-NC@Mo2C Complex for Efficient Overall Water Splitting. Nano Energy 2019, 57, 746–752. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.-Q.; Wu, C.-Y.; Xiao, K.; Liu, Z.-Q. Heterostructures Composed of N-Doped Carbon Nanotubes Encapsulating Cobalt and β-Mo2C Nanoparticles as Bifunctional Electrodes for Water Splitting. Angew. Chemie Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Peng, S.; Fan, L.; Li, L.; Qin, X.; Ramakrishna, S. Thin MoS2 Nanosheets Grafted MOFs-Derived Porous Co-N-C Flakes Grown on Electrospun Carbon Nanofibers as Self-Supported Bifunctional Catalysts for Overall Water Splitting. J. Mater. Chem. A 2017, 5, 23898–23908. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhou, J.; Han, L.; Sun, C.; Wang, Q.; Su, Z. Ternary Hybrids as Efficient Bifunctional Electrocatalysts Derived from Bimetallic Metal-Organic-Frameworks for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 5789–5796. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Su, S.; Li, J.; Ping, D.; Li, Y.; He, M.; Yu, X.; Wei, Z.; Liu, Y.; Li, S.; et al. High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C. Nanomaterials 2024, 14, 1422. https://doi.org/10.3390/nano14171422
Jin R, Su S, Li J, Ping D, Li Y, He M, Yu X, Wei Z, Liu Y, Li S, et al. High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C. Nanomaterials. 2024; 14(17):1422. https://doi.org/10.3390/nano14171422
Chicago/Turabian StyleJin, Renzhe, Shilong Su, Ju Li, Dehai Ping, Yuanyuan Li, Mengyuan He, Xiaomei Yu, Zhengyu Wei, Yong Liu, Songjie Li, and et al. 2024. "High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C" Nanomaterials 14, no. 17: 1422. https://doi.org/10.3390/nano14171422
APA StyleJin, R., Su, S., Li, J., Ping, D., Li, Y., He, M., Yu, X., Wei, Z., Liu, Y., Li, S., & Zheng, J. (2024). High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C. Nanomaterials, 14(17), 1422. https://doi.org/10.3390/nano14171422







