The Development of Metal-Free Porous Organic Polymers for Sustainable Carbon Dioxide Photoreduction
Abstract
:1. Introduction
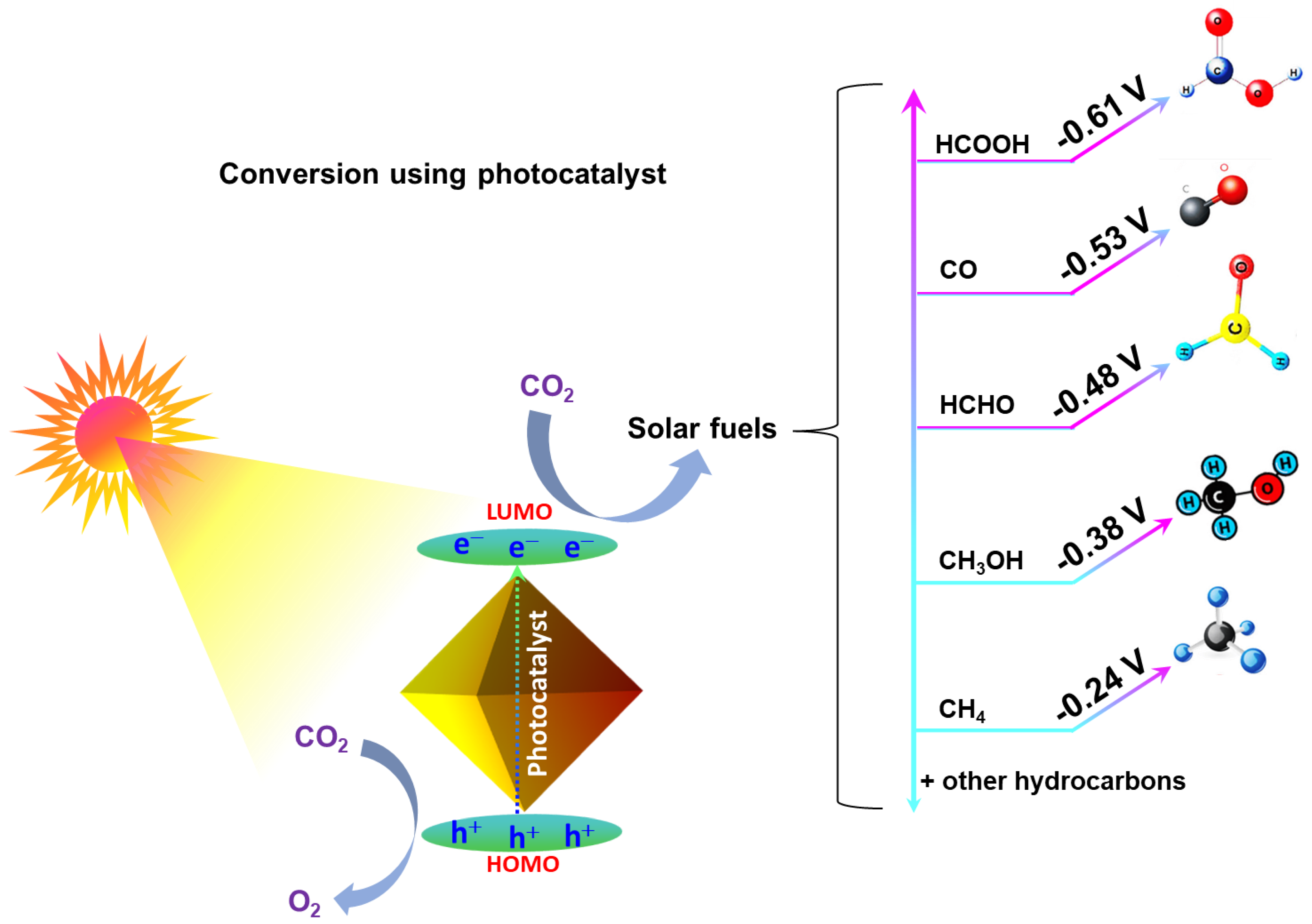
2. Fundamentals of Photocatalytic CO2 Reduction

- Light excitation characteristics: This encompasses properties of incident light, including wavelength, intensity, and polarization. These factors influence the generation of photogenerated electrons;
- Band structure: The electronic band structure of the catalyst material dictates the energetics of electron-transfer processes, impacting the reactions that can occur;
- Effectiveness of separating photogenerated charge carriers: Efficient separation of photogenerated electrons and holes is essential to prevent recombination, maximizing the utilization of photogenerated carriers for catalysis.
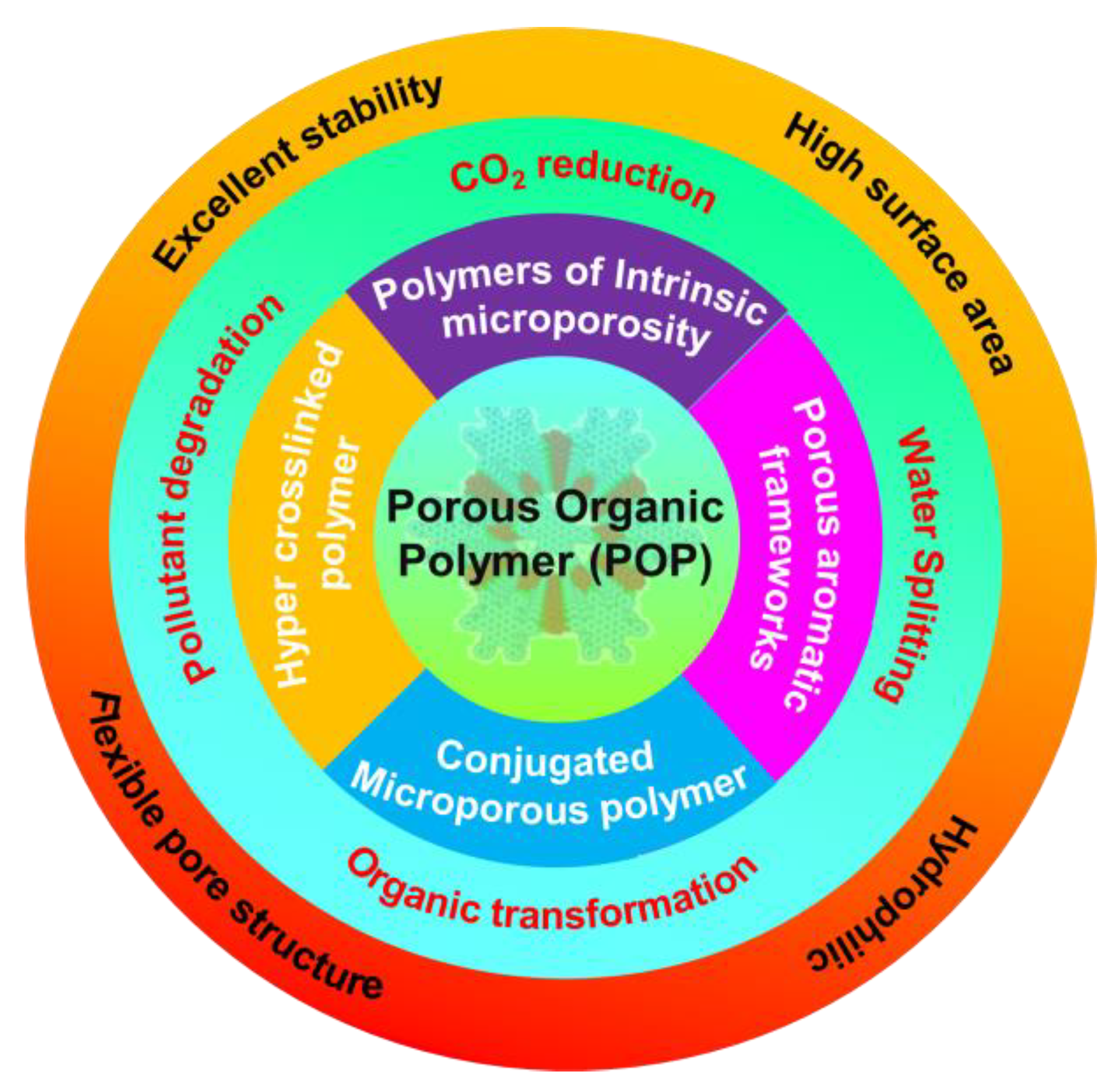
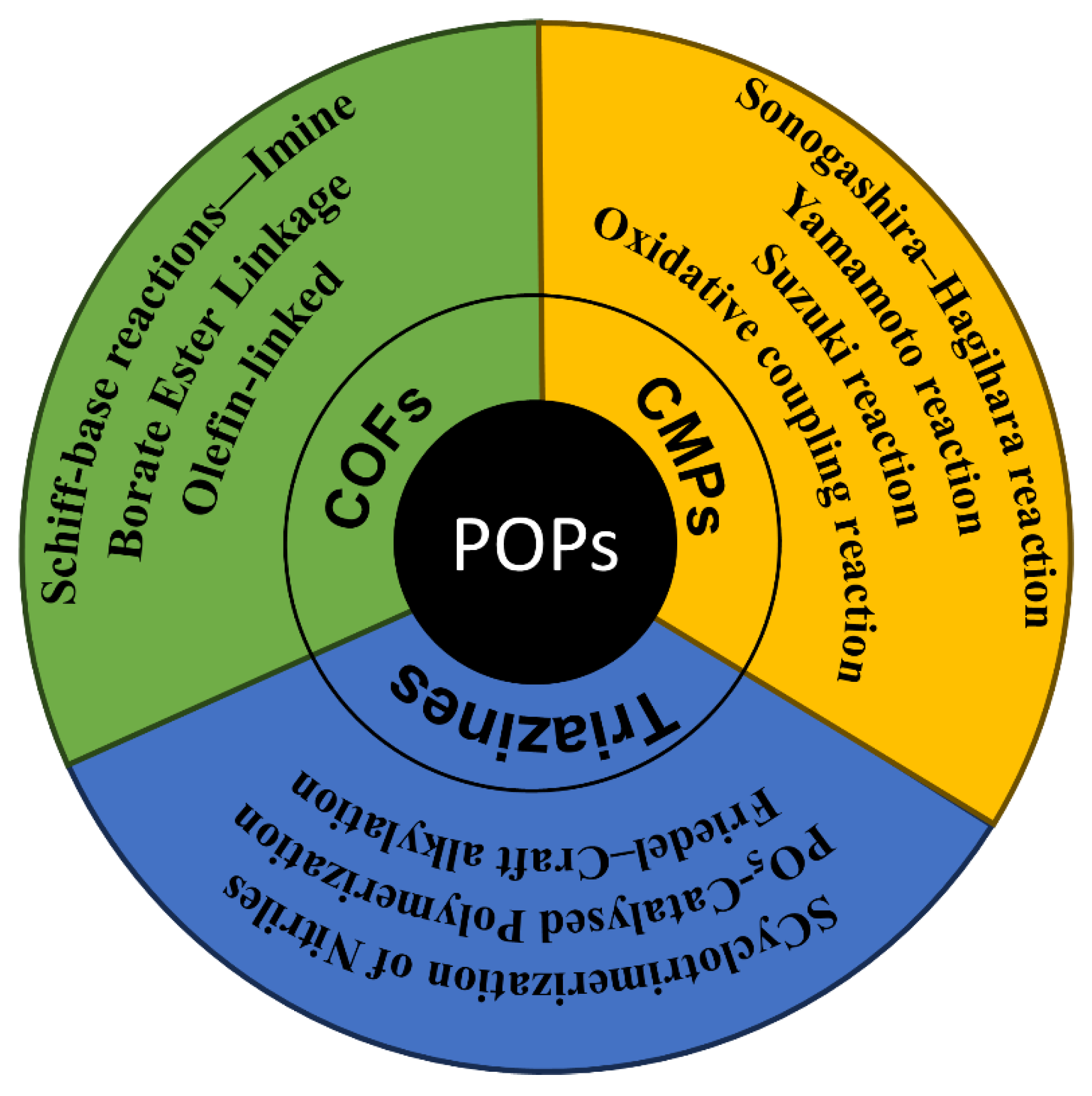
3. Structure and Properties of POPs
4. POPs in Photocatalysis
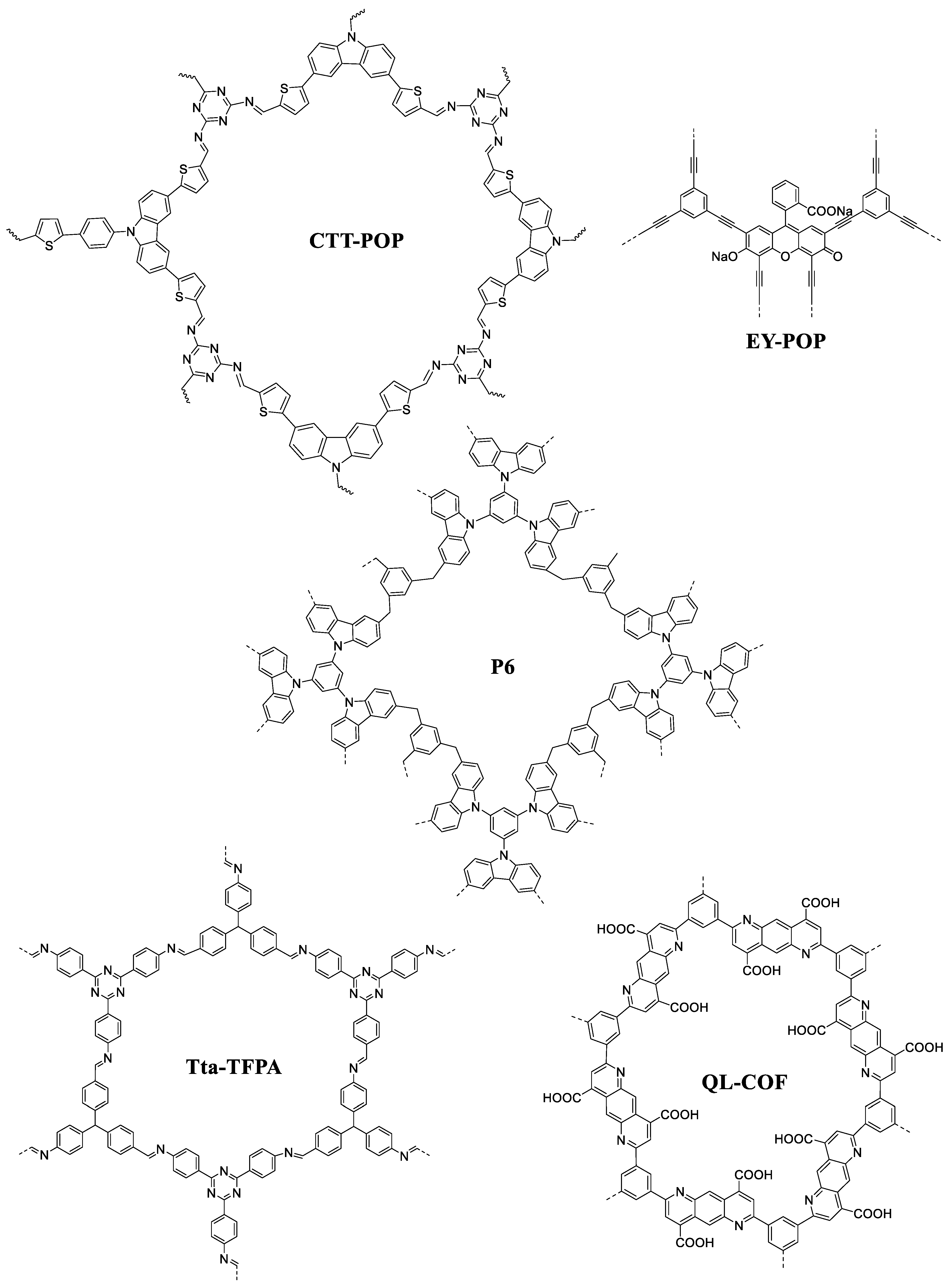
4.1. Molecular Architectures of POPs for Photocatalysis
4.1.1. Carbazole-Based Amorphous POPs
4.1.2. Porphyrin-Based Amorphous POPs
4.1.3. Pyrene-Based Amorphous POPs
4.1.4. Organic Dyes-Based Amorphous POPs
4.2. POPs in CO2 Photoreduction
5. Metal-Free POPs for CO2 Photoreduction
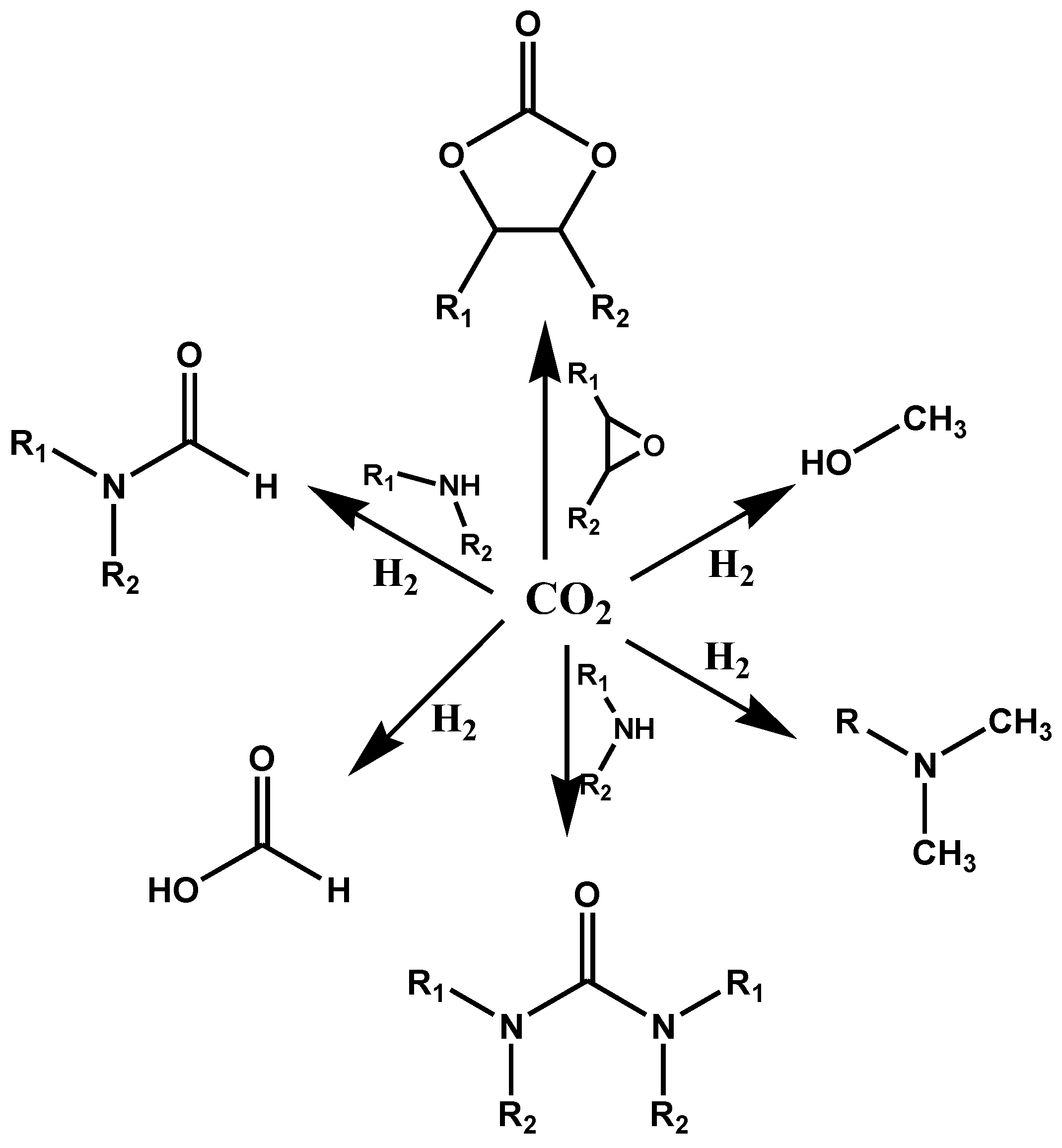
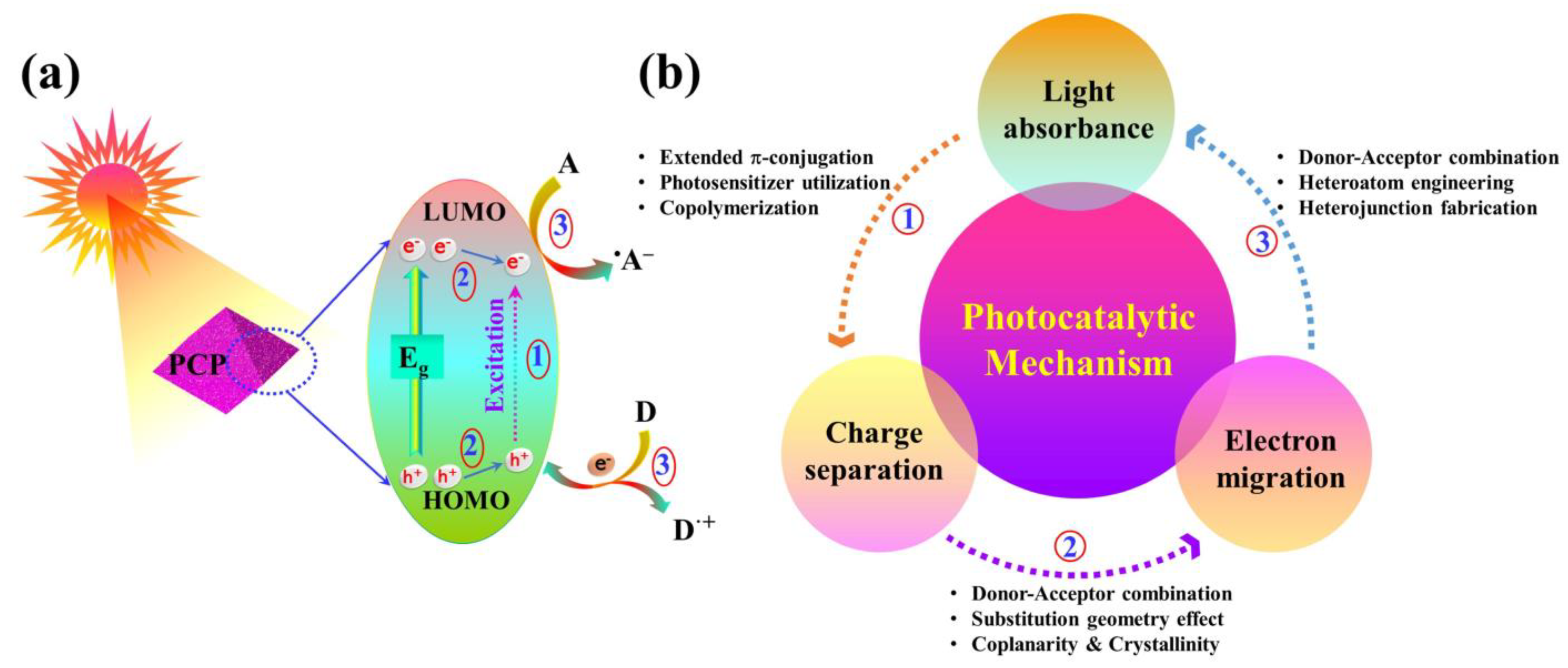
5.1. Photoreduction Mechanisms Specific to Metal-Free POPs
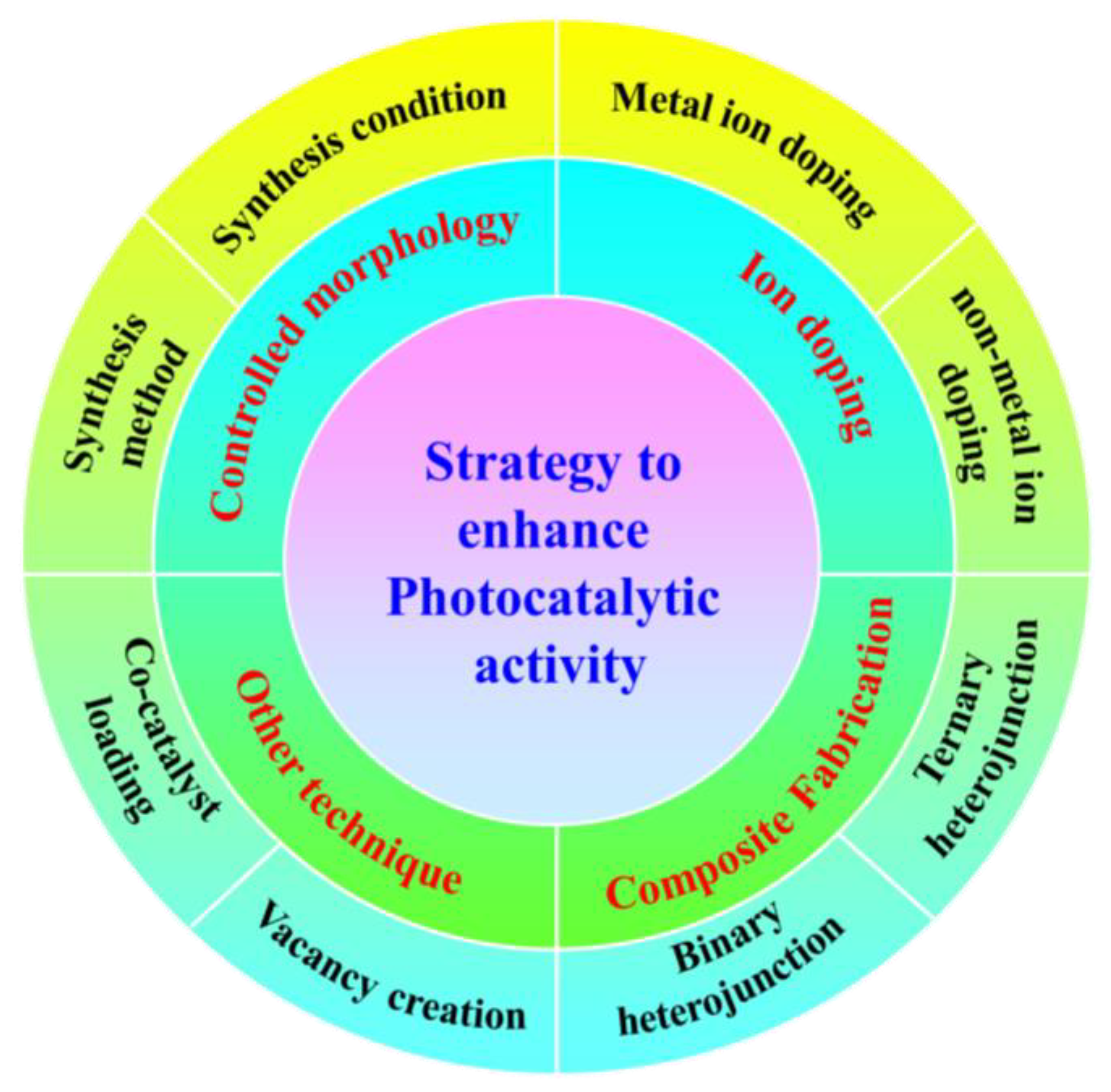
5.2. Structural Features to Enhance the Photocatalytic Efficiency of Metal-Free POPs
5.3. Recent Breakthroughs in Metal-Free POPs for CO2 Reduction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPY | Bipyridine |
| C2H4 | Ethene |
| C2H5OH | Ethanol |
| C2H6 | Ethane |
| CB | Conduction Band |
| CH2O | Formal aldehyde |
| CH3OH | Methanol |
| CH4 | Methane |
| CMP | Conjugated Microporous Polymers |
| CO2 | Carbon dioxide |
| COF | Covalent Organic Framework |
| CTF | Covalent triazine frameworks |
| D-A system | Donor–Acceptor system |
| DFT | Density Functional Theory |
| EY dye | Eosin Y Dye |
| H2O | Water |
| HCO2H | Formic acid |
| HOF | Hydrogen-Bonded Organic Framework |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| PCP | Porous Conjugated Polymers |
| POP | Porous Organic polymers |
| PPI | Porous Polyimide |
| PT-CMPS | Pyrene-4,5,9,10-tetraone-linked Conjugated Microporous Polymers |
| VB | Valence Band |
References
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Xiong, X.; Mao, C.; Yang, Z.; Zhang, Q.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. Photocatalytic CO2 Reduction to CO over Ni Single Atoms Supported on Defect-Rich Zirconia. Adv. Energy Mater. 2020, 10, 2002928. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Liu, Y.; Cho, K.Y.; Jung, C.H.; Oh, W.C. Novel ternary composite of LaYAgO4 and TiO2 united with graphene and its complement: Photocatalytic performance of CO2 reduction into methanol. Mater. Sci. Semicond Process 2021, 121, 105456. [Google Scholar] [CrossRef]
- Boro, B.; Kim, N.; Kim, J.S.; Paul, R.; Nailwal, Y.; Choi, Y.; Seo, D.H.; Mondal, J.; Ryu, J. Photocatalytic H2O2 production from water and air using porous organic polymers. J. Colloid Interface Sci. 2023, 652, 1784–1792. [Google Scholar] [CrossRef]
- Skorjanc, T.; Kamal, K.M.; Alkhoori, A.; Mali, G.; Mohammed, A.K.; Asfari, Z.; Polychronopoulou, K.; Likozar, B.; Trabolsi, A.; Shetty, D. Polythiacalixarene-Embedded Gold Nanoparticles for Visible-Light-Driven Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2022, 14, 30796–30801. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous Organic Polymers for CO2 Storage and Conversion Reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, Y.; Liu, Z. Design of porous organic polymer catalysts for transformation of carbon dioxide. Green Chem. Eng. 2022, 3, 96–110. [Google Scholar] [CrossRef]
- Guo, Y.; Mei, S.; Yuan, K.; Wang, D.J.; Liu, H.C.; Yan, C.H.; Zhang, Y.W. Low-Temperature CO2 Methanation over CeO2-Supported Ru Single Atoms, Nanoclusters, and Nanoparticles Competitively Tuned by Strong Metal-Support Interactions and H-Spillover Effect. ACS Catal. 2018, 8, 6203–6215. [Google Scholar] [CrossRef]
- Chen, L.; Unocic, R.R.; Hoffman, A.S.; Hong, J.; Braga, A.H.; Bao, Z.; Bare, S.R.; Szanyi, J. Unlocking the Catalytic Potential of TiO2-Supported Pt Single Atoms for the Reverse Water-Gas Shift Reaction by Altering Their Chemical Environment. JACS Au 2021, 1, 977–986. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar] [CrossRef]
- Dostagir, N.H.M.; Rattanawan, R.; Gao, M.; Ota, J.; Hasegawa, J.Y.; Asakura, K.; Fukouka, A.; Shrotri, A. Co Single Atoms in ZrO2 with Inherent Oxygen Vacancies for Selective Hydrogenation of CO2 to CO. ACS Catal. 2021, 11, 9450–9461. [Google Scholar] [CrossRef]
- Millet, M.M.; Algara-Sille, G.; Wrabetz, S.; Mazheika, A.; Girgsdies, F.; Teschner, D.; Seitz, F.; Tarasov, A.; Levchenko, S.V.; Schlögl, R.; et al. Ni Single Atom Catalysts for CO2 Activation. J. Am. Chem. Soc. 2019, 141, 2451–2461. [Google Scholar] [CrossRef]
- Rivera-Cárcamo, C.; Scarfiello, C.; García, A.B.; Tison, Y.; Martinez, H.; Baaziz, W.; Ersen, O.; Le Berre, C.; Serp, P. Stabilization of Metal Single Atoms on Carbon and TiO2 Supports for CO2 Hydrogenation: The Importance of Regulating Charge Transfer. Adv. Mater. Interfaces 2021, 8, 2001777. [Google Scholar] [CrossRef]
- Caparrós, F.J.; Soler, L.; Rossell, M.D.; Angurell, I.; Piccolo, L.; Rossell, O.; Llorca, J. Remarkable Carbon Dioxide Hydrogenation to Ethanol on a Palladium/Iron Oxide Single-Atom Catalyst. ChemCatChem 2018, 10, 2365–2369. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; He, Q.; Zhang, H.; Xu, S.; He, X.; Ji, H. A facile route to fabricate double atom catalysts with controllable atomic spacing for the r-WGS reaction. J. Mater. Chem. A Mater. 2020, 8, 2364–2368. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yao, S.; Song, X.; Huang, W.; Hülsey, M.J.; Yan, N. Support-dependent rate-determining step of CO2 hydrogenation to formic acid on metal oxide supported Pd catalysts. J. Catal. 2019, 367, 57–67. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, H.; Liu, K.; Cullen, D.; More, K.; Wang, M.; Feng, Z.; Wang, G.; Wu, G.; Li, Y. Unveiling Active Sites of CO2 Reduction on Nitrogen-Coordinated and Atomically Dispersed Iron and Cobalt Catalysts. ACS Catal. 2018, 8, 3116–3122. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Wang, Z.; Xu, X.; Sun, M.; Kang, L. Controllable dispersion of cobalt phthalocyanine molecules on graphene oxide for enhanced photocatalytic CO2 reduction. Mol. Catal. 2023, 546, 113253. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Hung, S.F.; Xu, A.; Wang, X.; Li, F.; Hsu, S.H.; Li, Y.; Wicks, J.; Cervantes, E.G.; Rasouli, A.S.; Li, Y.C.; et al. A metal-supported single-atom catalytic site enables carbon dioxide hydrogenation. Nat. Commun. 2022, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Liu, W.; Wang, Y.; Huang, J.; Chen, Y.; Zhou, Y.; Chen, Y.; Ma, M.; Lei, K.; Xie, H.; et al. Photocatalytic CO2 conversion over single-atom MoN2 sites of covalent organic framework. Appl. Catal. B 2021, 291, 120146. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal–Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, K.; Qiu, L.Q.; Yang, Z.W.; He, L.N. Ferric Porphyrin-Based Porous Organic Polymers for CO2 Photocatalytic Reduction to Syngas with Selectivity Control. Chem. Mater. 2021, 33, 8863–8872. [Google Scholar] [CrossRef]
- Das, R.; Paul, R.; Parui, A.; Shrotri, A.; Atzori, C.; Lomachenko, K.A.; Singh, A.K.; Mondal, J.; Peter, S.C. Engineering the Charge Density on an In2.77S4/Porous Organic Polymer Hybrid Photocatalyst for CO2-to-Ethylene Conversion Reaction. J. Am. Chem. Soc. 2023, 145, 422–435. [Google Scholar] [CrossRef]
- Wang, T.X.; Liang, H.P.; Anito, D.A.; Ding, X.; Han, B.H. Emerging applications of porous organic polymers in visible-light photocatalysis. J. Mater. Chem. A Mater. 2020, 8, 7003–7034. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Li, Z.; Han, B.; Bai, W.; Wei, G.; Li, X.; Qi, J.; Liu, D.; Zheng, Y.; Zhu, L. Photocatalytic CO2 RR for gas fuel production: Opportunities and challenges. Sep. Purif. Technol. 2023, 324, 124528. [Google Scholar] [CrossRef]
- Masdeu-Bultó, A.M.; Reguero, M.; Claver, C. Mechanistic Insights of Photocatalytic CO2 Reduction: Experimental versus Computational Studies. Eur. J. Inorg. Chem. 2022, 2022, e202100975. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. Catalytic conversion of CO2 to chemicals and fuels: The collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Mao, G.; Yin, S.; Long, B.; Deng, G.J.; Ali, A.; Song, T. Polarization engineering of porous organic polymers for superior photocatalytic synthesis of disulfides and CO2 reduction. J. Mater. Chem. A Mater. 2022, 13, 24147–24155. [Google Scholar] [CrossRef]
- Gemeda, T.N.; Chang, L.H.; Liang, Y.T.; Phan, V.H.K.; Fadhilah, G.; Prasetyo, F.; Ahmed, M.T. A Recent Review on Photocatalytic CO2 Reduction in Generating Sustainable Carbon-Based Fuels. In Green Energy and Technology; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2023; pp. 205–261. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, I.H.; Chakraborty, A.; Naskar, M.K.; Sarkar, M.; Islam, S.K.M. Porous organic polymer (POP) nanosheets: An efficient photo-catalyst for visible-light assisted CO2 reduction. Mater. Adv. 2022, 3, 3165–3173. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Rittiruam, M.; Saelee, T.; Márquez, V.; Shaikh, N.S.; Khajondetchairit, P.; Pathan, S.C.; Jiraborvornpongsa, N.; Praserthdam, S.; Praserthdam, P. High entropy materials frontier and theoretical insights for logistics CO2 reduction and hydrogenation: Electrocatalysis, photocatalysis and thermo-catalysis. J. Alloys. Compd. 2023, 969, 172232. [Google Scholar] [CrossRef]
- Hong, X.; Fang, Y.; Chao, D. A metal–free porous organic polymer with high CO2 adsorption for robust aqueous CO2 photoreduction via molecular regulation. Mol. Catal. 2023, 549, 113476. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, N.; Zhang, C.; Wang, X.; Zeng, J.; Chen, Y.; Wang, F.; Jiang, J.X. Rational design of donor-Π-acceptor conjugated microporous polymers for photocatalytic hydrogen production. Appl. Catal. B 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Ding, X.; Han, B.H. Porous Organic Polymers for Photocatalytic Carbon Dioxide Reduction. ChemPhotoChem 2021, 5, 406–417. [Google Scholar] [CrossRef]
- Giri, A.; Khakre, Y.; Shreeraj, G.; Dutta, T.K.; Kundu, S.; Patra, A. The order-disorder conundrum: A trade-off between crystalline and amorphous porous organic polymers for task-specific applications. J. Mater. Chem. A Mater. 2022, 10, 17077–17121. [Google Scholar] [CrossRef]
- Qiao, S.; Di, M.; Jiang, J.X.; Han, B.H. Conjugated porous polymers for photocatalysis: The road from catalytic mechanism, molecular structure to advanced applications. Energy Chem. 2022, 4, 100094. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Deng, Q.; Chen, W.; Zhang, Y.; Zhou, Y.; Zou, Z. Recent Progress of Amorphous Porous Organic Polymers as Heterogeneous Photocatalysts for Organic Synthesis. Adv. Funct. Mater. 2023, 33, 2307179. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nath, I.; Song, S.; Mohamed, S.; Khan, A.; Heynderickx, P.M.; Verpoort, F. Porous organic polymer composites as surging catalysts for visible-light-driven chemical transformations and pollutant degradation. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100319. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.X.; Yan, X.P. A versatile covalent organic framework-based platform for sensing biomolecules. Chem. Commun. 2017, 53, 11469–11471. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828. [Google Scholar] [CrossRef] [PubMed]
- Pavliuk, M.V.; Wrede, S.; Liu, A.; Brnovic, A.; Wang, S.; Axelsson, M.; Tian, H. Preparation, characterization, evaluation and mechanistic study of organic polymer nano-photocatalysts for solar fuel production. Chem. Soc. Rev. 2022, 51, 6909–6935. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Anil, A.G.; James, A.; Patra, A. Multifunctional Porous Organic Polymers: Tuning of Porosity, CO2, and H2 Storage and Visible-Light-Driven Photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 27669–27678. [Google Scholar] [CrossRef]
- Machado, T.F.; Valente, A.J.M.; Serra, M.E.S.; Murtinho, D. Triazene-Linked Porous Organic Polymers for Heterogeneous Catalysis and Pollutant Adsorption. ACS Appl. Polym. Mater. 2024, 6, 4171–4185. [Google Scholar] [CrossRef]
- Das, R.; Kishan, R.; Muthukumar, D.; Pillai, R.S.; Nagaraja, C.M. Engineering the functionality of porous organic polymers (POPs) for metal/cocatalyst-free CO2 fixation at atmospheric conditions. J. Environ. Chem. Eng. 2024, 12, 113777. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Zhang, P.; Wang, Y.; Lu, W. Synthesis of porphyrin porous organic polymers and their application of water pollution treatment: A review. Environ. Technol. Innov. 2023, 29, 102972. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Wu, M.; Zhou, Z. Design, preparation and application of conjugated microporous polymers. Polym. Int. 2014, 63, 381–392. [Google Scholar] [CrossRef]
- Sen, S.; Diab, R.; Al-Sayah, M.H.; Jabbour, R.; Equbal, A.; El-Kadri, O.M. Selective Carbon Dioxide Capture and Ultrahigh Iodine Uptake by Tetraphenylethylene-Functionalized Nitrogen-Rich Porous Organic Polymers. ACS Appl. Polym. Mater. 2024, 6, 1314–1324. [Google Scholar] [CrossRef]
- Mohan, A.; Al-Sayah, M.H.; Ahmed, A.; El-Kadri, O.M. Triazine-based porous organic polymers for reversible capture of iodine and utilization in antibacterial application. Sci. Rep. 2022, 12, 2638. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.A.; Al-Sayah, M.H.; Sen, S.; Ibrahim, T.H.; El-Kadri, O.M. Fluorescent aminal linked porous organic polymer for reversible iodine capture and sensing. Sci. Rep. 2020, 10, 15943. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.A.; Sara, Z.; Al-Sayah, M.H.; Ibrahim, T.H.; Khamis, M.I.; El-Kadri, O.M. Simultaneous adsorption and reduction of cr(Vi) to cr(iii) in aqueous solution using nitrogen-rich aminal linked porous organic polymers. Sustainability 2021, 13, 923. [Google Scholar] [CrossRef]
- Sen, S.; Al-Sayah, M.H.; Mohammed, M.S.; Abu-Abdoun, I.I.; El-Kadri, O.M. Multifunctional nitrogen-rich aminal-linked luminescent porous organic polymers for iodine enrichment and selective detection of Fe3+ ions. J. Mater. Sci. 2020, 55, 10896–10909. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Xue, R.; Ma, B.; Li, Q.; Wu, N.; Liu, H.; Yao, W.; Guo, H.; Yang, W. Ultrastable Triazine-Based Covalent Organic Framework with an Interlayer Hydrogen Bonding for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2019, 11, 26355–26363. [Google Scholar] [CrossRef]
- Hunt, J.R.; Doonan, C.J.; LeVangie, J.D.; Côté, A.P.; Yaghi, O.M. Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 2008, 130, 11872–11873. [Google Scholar] [CrossRef]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A Mater. 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Wang, C.A.; Li, Y.W.; Cheng, X.L.; Zhang, J.P.; Han, Y.F. Eosin Y dye-based porous organic polymers for highly efficient heterogeneous photocatalytic dehydrogenative coupling reaction. RSC Adv. 2017, 7, 408–414. [Google Scholar] [CrossRef]
- Karousis, N.; Tasis, D. Porous covalent organic frameworks in photocatalytic ROS-mediated processes. Energy Adv. 2024, 3, 712–740. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 1979 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Sebati, W.; Ray, S.S. Advances in nanostructured metal-encapsulated porous organic-polymer composites for catalyzed organic chemical synthesis. Catalysts 2018, 8, 492. [Google Scholar] [CrossRef]
- Barman, S.; Singh, A.; Rahimi, F.A.; Maji, T.K. Metal-Free Catalysis: A Redox-Active Donor-Acceptor Conjugated Microporous Polymer for Selective Visible-Light-Driven CO2 Reduction to CH4. J. Am. Chem. Soc. 2021, 143, 16284–16292. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, E.; Gao, J.; Zhang, S.; Liu, P.; Wang, J.C.; Zhang, Y.; Cui, C.X.; Jiang, J. Metal-free azo-bridged porphyrin porous organic polymers for visible-light-driven CO2 reduction to CO with high selectivity. Dalton Trans. 2020, 43, 7592–7597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, Z.; Ma, X.; Xiang, Z. Ultrastable and Efficient Visible-Light-Driven Hydrogen Production Based on Donor-Acceptor Copolymerized Covalent Organic Polymer. ACS Appl. Mater. Interfaces 2018, 10, 30698–30705. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, N.; Kong, X.; Hu, Z.; Zhong, F.; Li, Y.; Tan, H. Novel Carbazole-Based Porous Organic Polymer for Efficient Iodine Capture and Rhodamine B Adsorption. ACS Appl. Mater. Interfaces 2023, 15, 14846–14853. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Zhang, Y.; Chen, W.; Yu, S.; Chen, Y. Porphyrin/phthalocyanine-based porous organic polymers for pollutant removal and detection: Synthesis, mechanisms, and challenges. Environ. Res. 2023, 239, 117406. [Google Scholar] [CrossRef]
- Ji, W.; Wang, T.X.; Ding, X.; Lei, S.; Han, B.H. Porphyrin- and phthalocyanine-based porous organic polymers: From synthesis to application. Coord. Chem. Rev. 2021, 439, 213875. [Google Scholar] [CrossRef]
- Zhong, Z.; Xia, X.; Yang, X.; Li, N.; He, J.; Chen, D.; Gu, P.; Xu, Q.; Lu, J. Metal-free modification of porphyrin-based porous organic polymers for effective photocatalytic degradation of bisphenol A in water. Sep. Purif. Technol. 2022, 301, 121981. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Kotp, M.G.; Elewa, A.M.; El-Mahdy, A.F.M.; Chou, H.H.; Kuo, S.W. Tunable Pyridyl-Based Conjugated Microporous Polymers for Visible Light-Driven Hydrogen Evolution. ACS Appl. Energy Mater. 2021, 4, 13140–13151. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chaganti, S.V.; Sharma, S.U.; Samy, M.M.; Ejaz, M.; Lee, J.T.; Zhang, K.; Kuo, S.W. Constructing Conjugated Microporous Polymers Containing the Pyrene-4,5,9,10-Tetraone Unit for Energy Storage. ACS Appl. Energy Mater. 2022, 5, 10130–10140. [Google Scholar] [CrossRef]
- Mohata, S.; Das, R.; Koner, K.; Riyaz, M.; Das, K.; Chakraborty, S.; Ogaeri, Y.; Nishiyama, Y.; Peter, S.C.; Banerjee, R. Selective Metal-Free CO2 Photoreduction in Water Using Porous Nanostructures with Internal Molecular Free Volume. J. Am. Chem. Soc. 2023, 145, 23802–23813. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Zhang, K.A.I. Designing conjugated porous polymers for visible light-driven photocatalytic chemical transformations. Mater. Horiz. 2020, 7, 15–31. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Wang, X.; Li, Z.; Zhou, W. Halogen modified organic porous semiconductors in photocatalysis: Mechanism, synthesis, and application. Adv. Powder Mater. 2024, 3, 100178. [Google Scholar] [CrossRef]
- Wong, Y.L.; Tobin, J.M.; Xu, Z.; Vilela, F. Conjugated porous polymers for photocatalytic applications. J. Mater. Chem. A Mater. 2016, 4, 18677–18686. [Google Scholar] [CrossRef]
- McCormick, T.M.; Bridges, C.R.; Carrera, E.I.; Dicarmine, P.M.; Gibson, G.L.; Hollinger, J.; Kozycz, L.M.; Seferos, D.S. Conjugated polymers: Evaluating DFT methods for more accurate orbital energy modelling. Macromolecules 2013, 46, 3879–3886. [Google Scholar] [CrossRef]
- Liu, P.; Xing, L.; Lin, H.; Wang, H.; Zhou, Z.; Su, Z. Construction of porous covalent organic polymer as photocatalysts for RhB degradation under visible light. Sci. Bull. 2017, 62, 931–937. [Google Scholar] [CrossRef]
- Das, N.; Paul, R.; Biswas, S.; Das, R.; Chatterjee, R.; Bhaumik, A.; Peter, S.C.; Wong, B.M.; Mondal, J. Photo-Responsive Signatures in a Porous Organic Polymer Enable Visible Light-Driven CO2 Photofixation. ACS Sustain. Chem. Eng. 2023, 11, 2066–2078. [Google Scholar] [CrossRef]
- Song, C.K.; Eckstein, B.J.; Tam, T.L.D.; Trahey, L.; Marks, T.J. Conjugated polymer energy level shifts in lithium-ion battery electrolytes. ACS Appl. Mater. Interfaces 2014, 6, 19347–19354. [Google Scholar] [CrossRef]
- Shit, S.C.; Powar, N.S.; Kalita, P.; Paul, R.; Xu, S.; Jung, J.W.; Cho, C.H.; In, S.I.; Mondal, J. Selective photocatalytic CO2 reduction to CH4 over metal-free porous polyimide in the solid-gas mode. Chem. Commun. 2022, 58, 13716–13719. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chowdhury, I.H.; Chowdhury, A.H.; Singh, N.; Sarkar, M.; Islam, S.M. Metal-Free Covalent Organic Framework for Facile Production of Solar Fuel via CO2 Reduction. Ind. Eng. Chem. Res. 2022, 61, 17044–17056. [Google Scholar] [CrossRef]
- Narzary, B.B.; Karatayeva, U.; Mintah, J.; Villeda-Hernandez, M.; Faul, C.F.J. Bifunctional metal-free porous polyimide networks for CO2 capture and conversion. Mater. Chem. Front 2023, 7, 4473–4481. [Google Scholar] [CrossRef]
- Mondal, S.; Powar, N.S.; Paul, R.; Kwon, H.; Das, N.; Wong, B.M.; In, S.I.; Mondal, J. Nanoarchitectonics of Metal-Free Porous Polyketone as Photocatalytic Assemblies for Artificial Photosynthesis. ACS Appl. Mater. Interfaces 2022, 14, 771–783. [Google Scholar] [CrossRef]
- Ravi, S.; Kim, J.; Choi, Y.; Han, H.H.; Wu, S.; Xiao, R.; Bae, Y.S. Metal-Free Amine-Anchored Triazine-Based Covalent Organic Polymers for Selective CO2 Adsorption and Conversion to Cyclic Carbonates Under Mild Conditions. ACS Sustain. Chem. Eng. 2023, 11, 1190–1199. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Shang, J.; Peng, J.; Zhu, T. Construction of a novel metal-free heterostructure photocatalyst PRGO/TP-COF for enhanced photocatalytic CO2 reduction. Appl. Catal. B 2024, 350, 123937. [Google Scholar] [CrossRef]
- Cui, J.; Li, C.X.; Zhou, J.; Hua, Y.; Zhou, Z.Y.; Su, Z.M. A covalent organic framework integrating photocatalytic center and electron reservoir for photocatalytic CO2 reduction to HCOOH. Sep. Purif. Technol. 2024, 346, 127466. [Google Scholar] [CrossRef]
- Sarkar, M.; Chakrabortty, P.; Sengupta, M.; Kothari, A.C.; Islam, M.S.; Islam, S.M. Light-Mediated Sustainable Conversion of Carbon Dioxide to Valuable Methanol by Highly Efficient Covalent Organic Framework g-C3N4 Composites as a Reusable Photocatalyst. Ind. Eng. Chem. Res. 2024, 63, 5573–5590. [Google Scholar] [CrossRef]
- Yu, X.; Gong, K.; Tian, S.; Gao, G.; Xie, J.; Jin, X.H. A hydrophilic fully conjugated covalent organic framework for photocatalytic CO2 reduction to CO nearly 100% using pure water. J. Mater. Chem. A Mater. 2023, 11, 5627–5635. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Omr, H.A.E.; Al Othman, Z.A.; Lee, H. Design and synthesis of metal-free ethene-based covalent organic framework photocatalysts for efficient, selective, and long-term stable CO2 conversion into methane. J. Colloid. Interface Sci. 2023, 633, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Li, Z.; Hu, Y.; Luo, X.; Li, J. A novel metal-free porous covalent organic polymer for efficient room-temperature photocatalytic CO2 reduction via dry-reforming of methane. Green Energy Environ. 2023, 9, 1407–1418. [Google Scholar] [CrossRef]

| Type of Catalyst | Catalyst | Product(s) | Conversion % | Ref. |
|---|---|---|---|---|
| Thermocatalyst | Ru- CeO2 | CH4 | 100% | [9] |
| Pt-TiO2 | CO | 100% | [10] | |
| Pd-In2O3 | CH3OH | 78% | [11] | |
| Co-doped ZrO2 | CO | 95% | [12] | |
| Ni-MgO | CO | [13] | ||
| Ni-CNT/TiO2 | CO | 95.5% | [14] | |
| Fe3O4 with Pd | C2H5OH | 0.3% | [15] | |
| CNT, Pd and La2O3 | CO | 36% | [16] | |
| N-doped C with Ni | CO | 51% | [17] | |
| ZnO with Pd | HCOOH | [18] | ||
| Electrocatalyst | Fe–N–C | CO | 93% | [19] |
| Fe/Pc graphene composites | CO | >94% | [20] | |
| Co–N2 on ZIF | CO | 94% | [21] | |
| Cu-FeSA | CH4 | 64% | [22] | |
| Photocatalyst | Mo-COF | CO | 6.19 µmol g−1 h−1 | [23] |
| CH4 | 1.08 µmol g−1 h−1 | |||
| Co-MOF 525 | CO | 200.6 µmol g−1 h−1 | [24] | |
| CH4 | 36.67 µmol g−1 h−1 | |||
| Ni-SA-5/ZrO2 | CO | 11.8 µmol g−1 h−1 | [2] | |
| POPnFe | CO/H2 | [25] | ||
| (POP)-embedded In2.77S4 | C2H5OH | 67% | [26] | |
| S-rich thiacalixarene-based POP with AuNP | CO | 6.74 μmol g−1 over 4 h | [5] | |
| LaYAgO4-Graphene-TiO2 | CH3OH | 12.27% | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bariki, R.; Joseph, R.G.; El-Kadri, O.M.; Al-Sayah, M.H. The Development of Metal-Free Porous Organic Polymers for Sustainable Carbon Dioxide Photoreduction. Nanomaterials 2024, 14, 1432. https://doi.org/10.3390/nano14171432
Bariki R, Joseph RG, El-Kadri OM, Al-Sayah MH. The Development of Metal-Free Porous Organic Polymers for Sustainable Carbon Dioxide Photoreduction. Nanomaterials. 2024; 14(17):1432. https://doi.org/10.3390/nano14171432
Chicago/Turabian StyleBariki, Ranjit, Reshma G. Joseph, Oussama M. El-Kadri, and Mohammad H. Al-Sayah. 2024. "The Development of Metal-Free Porous Organic Polymers for Sustainable Carbon Dioxide Photoreduction" Nanomaterials 14, no. 17: 1432. https://doi.org/10.3390/nano14171432






