Insights into the Mechanisms of Single-Photon and Two-Photon Excited Surface Enhanced Fluorescence by Submicrometer Silver Particles
Abstract
:1. Introduction
2. Materials and Methods
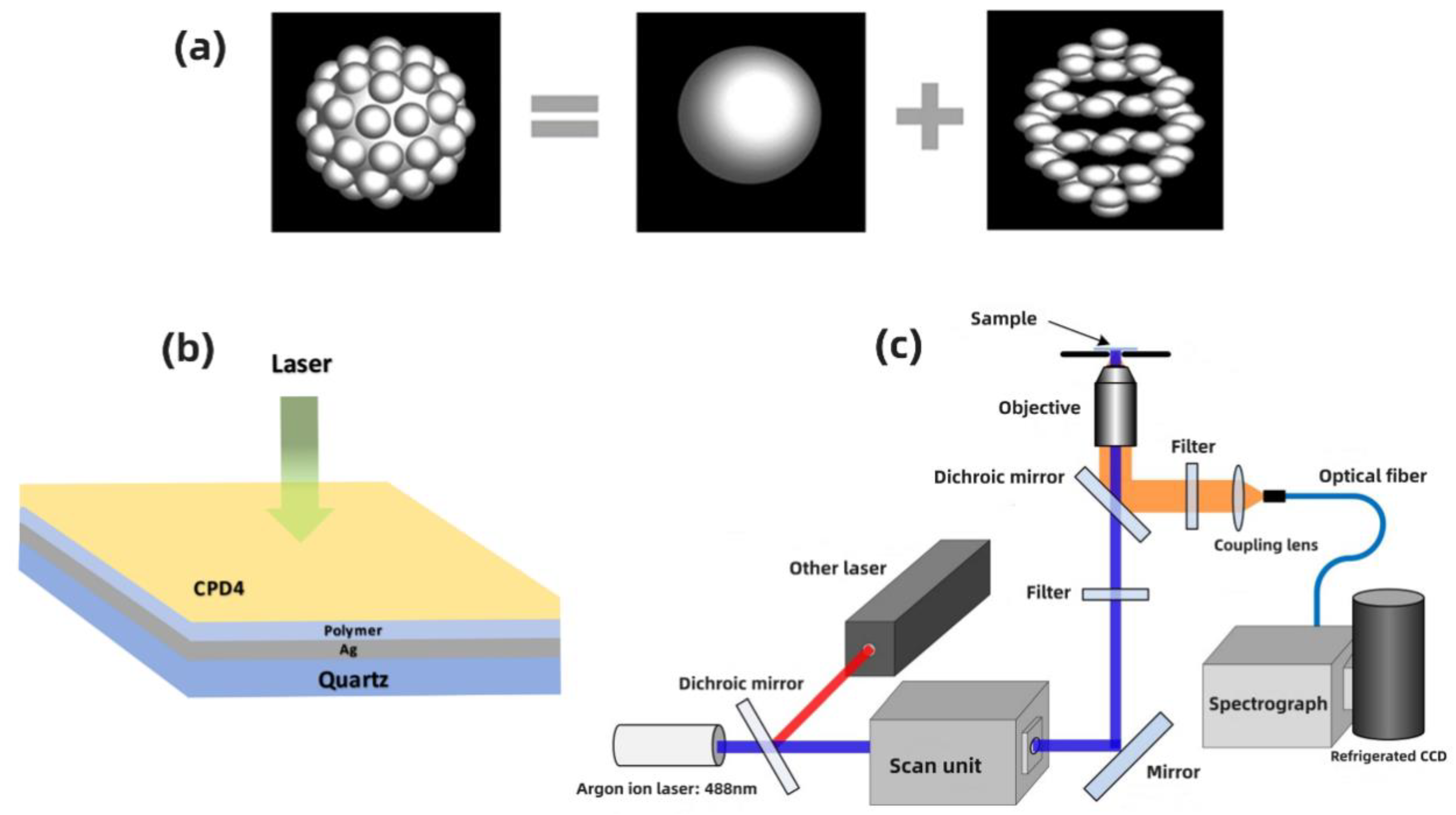
2.1. Synthesis of Submicrometer Silver Particles
2.2. Deposition of Submicrometer Silver Particles
2.3. Optical Modeling
2.4. Preparation of Samples
2.5. Instrumentation
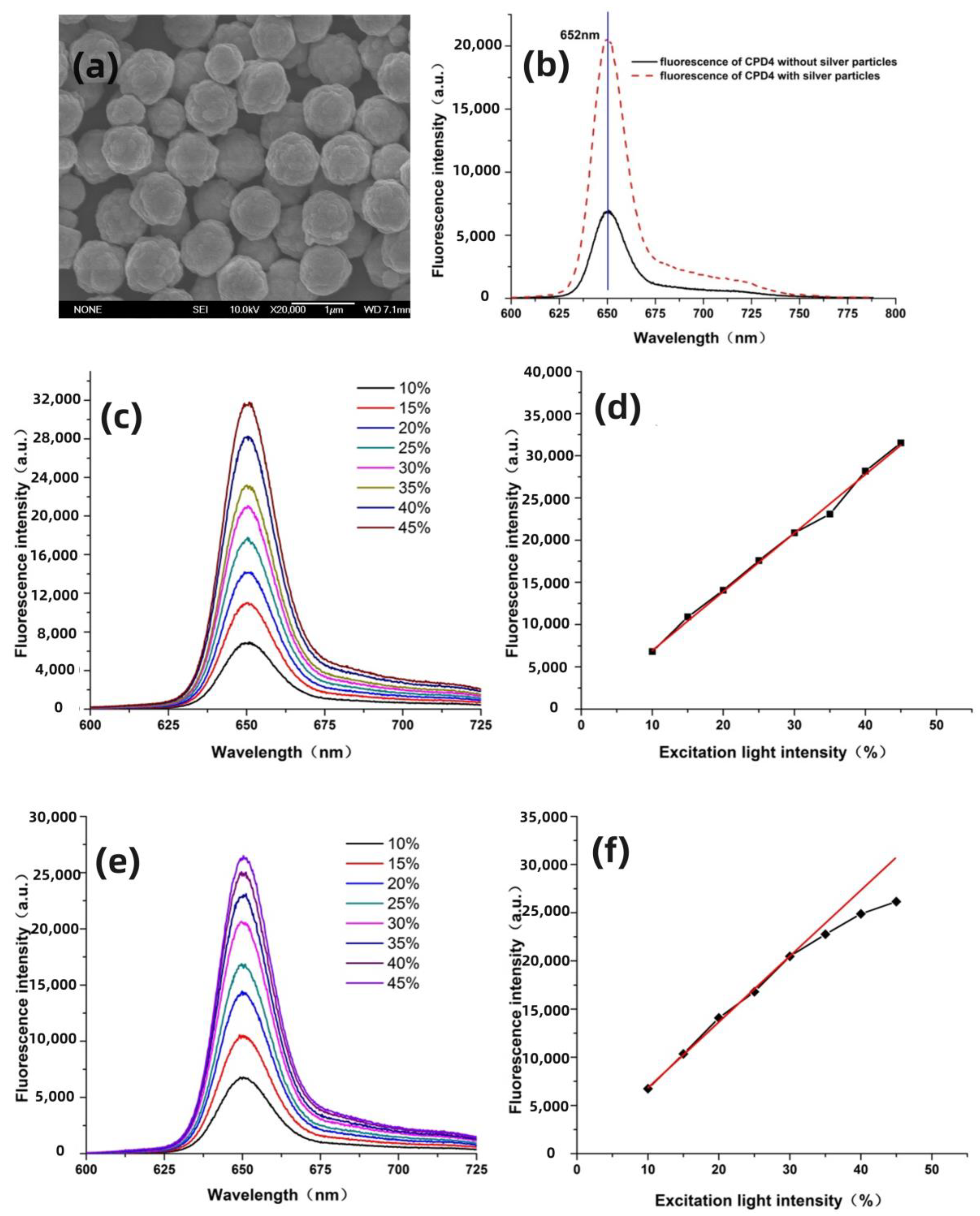
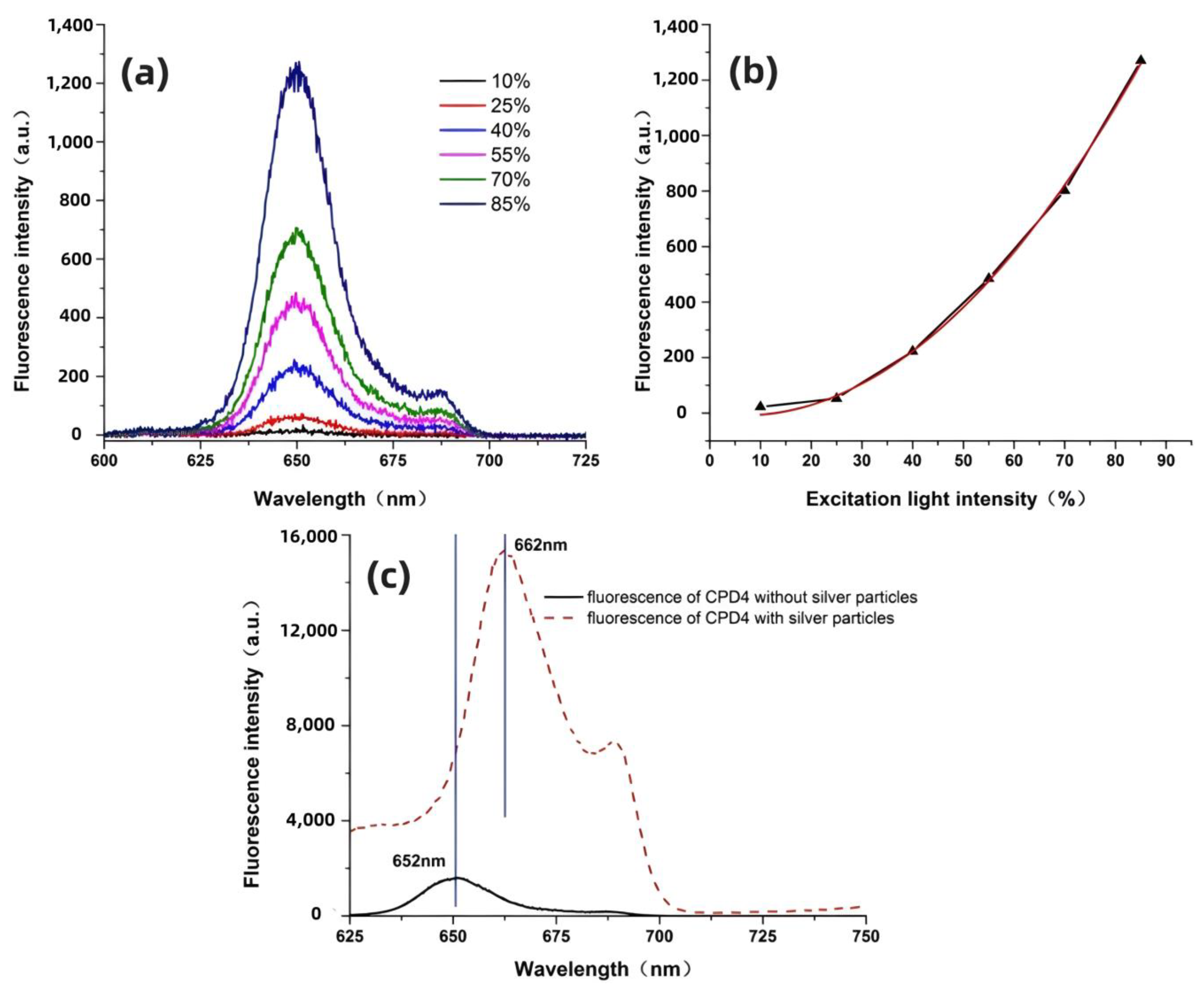
3. Results and Discussion
3.1. Enhancement Effect of Submicrometer Silver Particles under Both Single-Photon and Two-Photon Excitation
3.2. Theoretical Analysis and Molecular Excited State Dynamics Process Induced by Localized Surface Field
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, Z.; Huang, Y.; Zhang, Y.; Zhao, F.; Li, F.; Zhu, Q.; Jian, G. High selectivity and fluorescence reversible Eu3+ sensor based on GSH-capped AgZnInS QDs. Opt. Lett. 2023, 48, 944–947. [Google Scholar] [CrossRef]
- Nidheesh, V.R.; Mohapatra, A.K.; Nayak, R.; Unnikrishnan, V.K.; Kartha, V.B.; Chidangil, S. Bimodal UV photoacoustic and fluorescence sensor for breath analysis. Sens. Actuators B Chem. 2023, 379, 133242. [Google Scholar]
- Xiang, W.; Liu, B.; Qian, Y. Near-Infrared photosensitive dyes based on red fluorescence protein chromophore analogue for photodynamic therapy and two-photon fluorescence imaging. Dye. Pigment. 2022, 205, 110524. [Google Scholar] [CrossRef]
- Qin, F.; Shi, F.; Gao, X.; Yan, J.; Ye, Z.; Su, Y.; Fu, J.; Wang, Y. Self-Filtering illumination source and application in fluorescence imaging. Chin. Opt. Lett. 2023, 21, 011101. [Google Scholar] [CrossRef]
- Shcheslavskiy, V.I.; Yuzhakova, D.V.; Sachkova, D.A.; Shirmanova, M.V.; Becker, W. Macroscopic temporally and spectrally resolved fluorescence imaging enhanced by laser-wavelength multiplexin. Opt. Lett. 2023, 20, 48. [Google Scholar]
- Zhang, W.; Pan, P.; Wang, X.; Chen, Y.; Liu, X. Force-Controlled Mechanical Stimulation and Single-Neuron Fluorescence Imaging of Drosophila Larva. IEEE Robot. Autom. Lett. 2021, 6, 3736–3743. [Google Scholar] [CrossRef]
- Li, D.-H.; Gamage, R.S.; Oliver, A.; Patel, N.; Usama, S.; Kalen, J.; Schnermann, M.; Smith, B.D. Doubly Strapped Zwitterionic NIR-I and NIR-II Heptamethine Cyanine Dyes for Bioconjugation and Fluorescence Imaging. Angew. Chem. 2023, 135, e202305062. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Smalley, R.E. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Liu, G.L.; Kim, J.; Lu, Y.; Lee, L.P. Fluorescence enhancement of quantum dots enclosed in Au nanopockets with subwavelength aperture. Appl. Phys. Lett. 2006, 89, 241118. [Google Scholar] [CrossRef]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of surface plasmons and surface-plasmon polaritons. Rep. Prog. Phys. 2006, 70, 1. [Google Scholar] [CrossRef]
- Luo, X.; Ishihara, T. Surface plasmon resonant interference nanolithography technique. Appl. Phys. Lett. 2004, 84, 4780–4782. [Google Scholar] [CrossRef]
- Johansson, P.; Xu, H.; Käll, M. Surface-Enhanced Raman scattering and fluorescence near metal nanoparticles. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 72, 035427. [Google Scholar] [CrossRef]
- Doering, W.E.; Piotti, M.E.; Natan, M.J. SERS as a Foundation for Nanoscale, Optically Detected Biological Labels. Adv. Mater. 2007, 19, 3100–3108. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.; Ma, X.; Huang, R.; Xu, J.; Cai, L.; Xu, L. Surface enhanced Raman scattering active substrate based on hydrogel microspheres for pretreatment-free detection of glucose in biological samples. Talanta 2023, 260, 124657. [Google Scholar] [CrossRef]
- An, R.; Zheng, H.; Dong, Y. Ti-Si-Zr-Zn Nanometallic Glass Substrate with a Tunable Zinc Composition for Surface-Enhanced Raman Scattering of Cytochrome c. ACS Appl. Mater. Interfaces 2023, 15, 25275–25284. [Google Scholar] [CrossRef]
- Fang, Y.; Seong, N.H.; Dlot, D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef]
- Zhang, W.; Ganesh, N.; Mathias, P.C.; Cunninghan, B.T. Enhanced Fluorescence on a Photonic Crystal Surface Incorporating Nanorod Structures. Small 2008, 4, 2199–2203. [Google Scholar] [CrossRef]
- Cocilovo, B.; Herrera, O.D. Surface-Enhanced Two-Photon Excitation Fluorescence of Various Fluorophores Evaluated Using a Multiphoton Microscope. J. Light. Technol. 2015, 33, 3446–3452. [Google Scholar] [CrossRef]
- Yue, Y.; Nan, J.; Che, Y.; Xu, H.; Sun, W.; Zhang, F.; Wang, L.; Xu, W.; Niu, J.; Zhu, S. Thermal-Annealing-Regulated plasmonic enhanced fluorescence platform enables accurate detection of antigen/antibody against infectious diseases. Nano Res. 2023, 16, 3215–3223. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; CMH, M. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2023, 35, 2107986. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.; Grésillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2008, 41, 013001. [Google Scholar] [CrossRef]
- Drexhage, K.H. Interaction of light with monomolecular dye lasers. In Progress in Optics; Wolfe, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 161–232. [Google Scholar]
- Lakowicz, J.; Geddes, C.; Gryczynski, I. Advances in Surface-Enhanced fluorescence. J. Fluoresc. 2004, 14, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Radiative decay engineering 4. Experimental studies of surface plasmon-coupled directional emission. Anal. Biochem. 2004, 324, 170–182. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-Enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Shen, Y.B.; D’Auria, S. Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes and resonance energy transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Malicka, J.; Gryczynski, I.; Gryczynski, Z.; Geddes, C.D. Radiative decay engineering: The role of photonic mode density in biotechnology. J. Phys. D Appl. Phys. 2003, 36, R240. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.M.; Falci, R.F.; Andrade, G.F.S.; Mzaria, J.V.S.; Mauricio, A.P. Surface-Enhanced Fluorescence of Erbium Ions on Copper Nanoparticles Containing Tellurite Glasses. Plasmonics 2021, 16, 139–145. [Google Scholar] [CrossRef]
- Chen, S.H.; Hsieh, W.J.; Hong, Y.W. Gold nanohole arrays with ring-shaped silver nanoparticles for highly efficient plasmon-enhanced fluorescence. Results Phys. 2023, 51, 106740. [Google Scholar] [CrossRef]
- Zhao, W.; Wen, Z.; Xu, Q.; Zhou, Z.; Hao, J. Remarkable photoluminescence enhancement of CsPbBr3 perovskite quantum dots assisted by metallic thin films. Nanophotonics 2021, 10, 2257–2264. [Google Scholar] [CrossRef]
- Qi, J.; Pei, H.; Yu, C.; Peng, W.; Zhao, J.; Wei, Y. Tip-Enhanced two-photon excited fluorescence spectroscopy of monolayer black phosphorus. Results Phys. 2023, 53, 106997. [Google Scholar] [CrossRef]
- Purcell, E.M. Spontaneous emission probabilities at radio frequencies. Phys. Rev. 1946, 69, 681. [Google Scholar]
- Koyama, K.; Yoshita, M.; Baba, M.; Suemoto, T.; Akiyama, H. High collection efficiency in fluorescence microscopy with a solid immersion lens. Appl. Phys. Lett. 1999, 75, 1667–1669. [Google Scholar] [CrossRef]
- Chou, R.Y.; Lu, G.; Shen, H.; He, Y.; Chen, Y.; Perrian, P.; Martini, M.; Tillement, O.; Gong, Q. A hybrid nanoantenna for highly enhanced directional spontaneous emission. J. Appl. Phys. 2014, 115, 244310. [Google Scholar] [CrossRef]
- Barron, L.D.; Gadegaard, N.; Kadodwala, M.; Hendry, E.; Carpy, T.; Johnston, J. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 2010, 5, 783–787. [Google Scholar]
- Ji, B.; Giovanelli, E.; Habert, B.; Spinicelli, P.; Nasilowski, M. Non-Blinking quantum dot with a plasmonic nanoshell resonator. Nat. Nanotechnol. 2015, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, Z.; Wang, W.; Wu, Y. Highly surface-roughened “flower-like” silver nanoparticles for extremely sensitive substrates of surface-enhanced Raman scattering. Adv. Mater. 2009, 21, 4614–4618. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.; Feld, M.S. Surface-Enhanced Raman scattering and biophysics. J. Phys. Condens. Matter 2002, 14, 597–624. [Google Scholar] [CrossRef]
- Xiao, X.; Cai, H.; Huang, Q.; Wang, B.; Wang, X.; Luo, Q.; Li, Y.; Zhang, H.; Gong, Q.; Ma, X. Polymeric dual-modal imaging nanoprobe with two-photon aggregation-induced emission for fluorescence imaging and gadolinium-chelation for magnetic resonance imaging. Bioact. Mater. 2023, 19, 538–549. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, P.; Zhang, L.; Mao, S.C.; Lin, L. Enhancement of Raman scattering by field superposition of rough submicrometer silver particles. Appl. Phys. Lett. 2012, 100, 173103. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Fan, X.M.; Lu, Y.B.; Wang, C.Y.; Huang, X.D.; Zhang, L.J. Broadband Enhancement in the Spectral Response of Photovoltaic Modules with Flower-like Silver Particles. Photonics 2023, 10, 1102. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, P.; Li, X.; Liu, J.T.; Lin, L.; Fan, Z.W. Further localization of optical field for flower-like silver particles under laser radiation. Laser Phys. Lett. 2013, 10, 045901. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Shen, Y. Multiphoton Excitation of Fluorescence near Metallic Particles: Enhanced and Localized Excitation. J. Phys. Chem. B 2002, 106, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, J.; Gryczynski, I.; Malicka, J.; Makowiec, S.; Lakowicz, J.R.; Gryczynski, Z. Two-Photon induced fluorescence of Cy5-DNA in buffer solution and on silver island films. Biochem. Biophys. Res. Commun. 2005, 328, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Mrozek, I.; Grabhorn, H. Surface-Enhanced Raman scattering. J. Phys. Condens. Matter 1992, 4, 1143. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Zhang, Z.; Zhao, J.S.; Guo, Y.; Fang, Y.Z.; Ning, Y.J. In Situ Growth of Ultrasmall Cesium Lead Bromine Quantum Dots in a Mesoporous Silica Matrix and Their Application in Flexible Light-Emitting Diodes. Nanoscale 2019, 11, 16499–16507. [Google Scholar] [CrossRef]
- Mo, X.M.; Li, T.; Huang, F.C.; Li, Z.X.; Zhou, Y.L.; Lin, T.; Ouyang, Y.F.; Tao, X.M.; Pan, C.F. High-Efficiency and Air-Stable Perovskite Quantum Dots Light-Emitting Diodes with an All-Inorganic Heterostructure. Nano Lett. 2017, 17, 313–321. [Google Scholar]

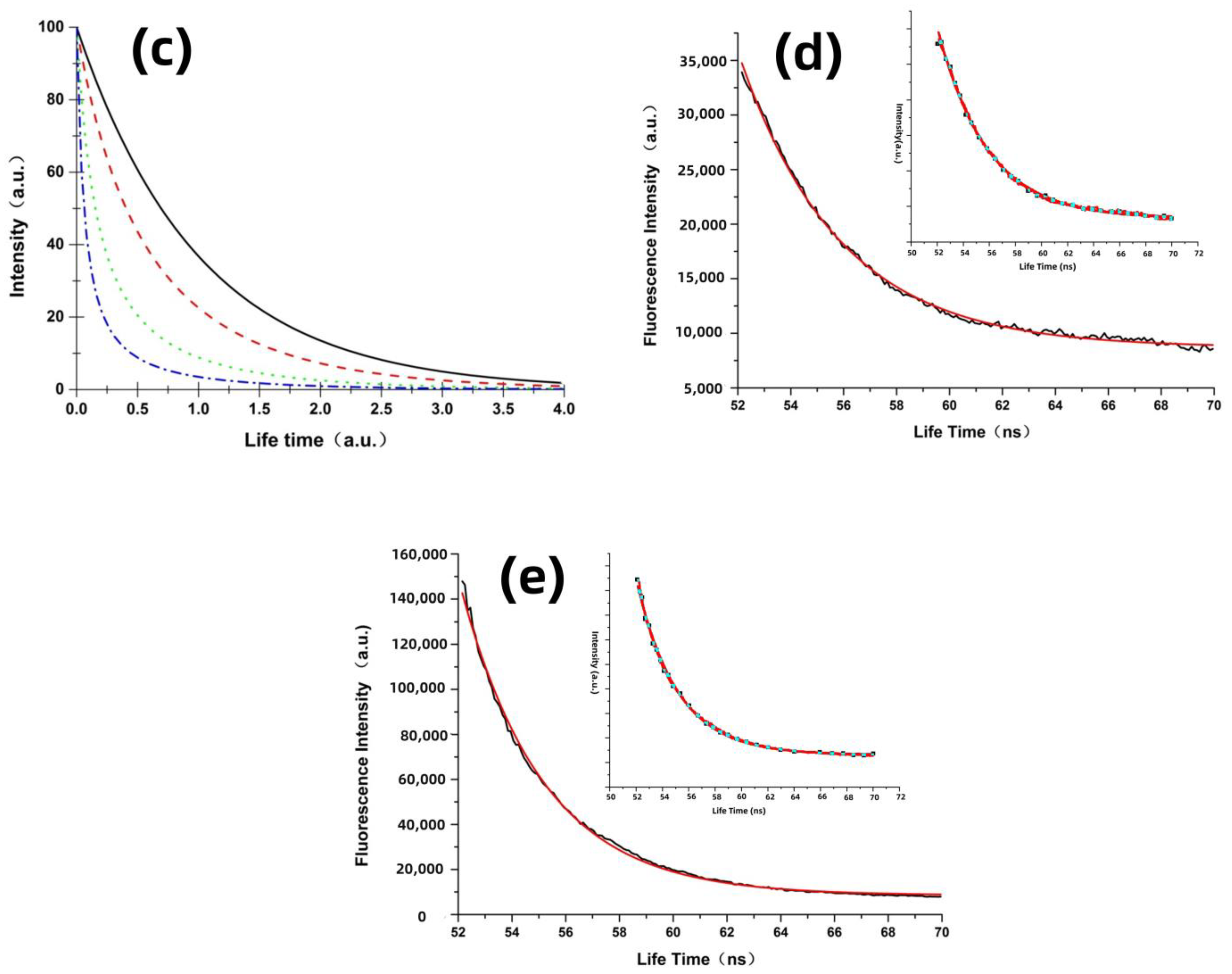
| Samples | A1 | (ns) | Standard Error | (ns) | Standard Error | A2 | R-Square | Confidence Level | |
|---|---|---|---|---|---|---|---|---|---|
| CPD4 | 0.53 | 3.46 | 0.017 | 4.19 | 0.014 | 0.47 | 3.8 | 0.991 | 95% |
| CPD4 with the silver particles | 0.56 | 3.32 | 0.019 | 2.59 | 0.016 | 0.44 | 3 | 0.993 | 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, F.; Du, Z.; Fan, X.; Huang, X.; Zhang, L.; Li, S.; Liu, Z.; Wang, C. Insights into the Mechanisms of Single-Photon and Two-Photon Excited Surface Enhanced Fluorescence by Submicrometer Silver Particles. Nanomaterials 2024, 14, 1451. https://doi.org/10.3390/nano14171451
Wang Y, Zhang F, Du Z, Fan X, Huang X, Zhang L, Li S, Liu Z, Wang C. Insights into the Mechanisms of Single-Photon and Two-Photon Excited Surface Enhanced Fluorescence by Submicrometer Silver Particles. Nanomaterials. 2024; 14(17):1451. https://doi.org/10.3390/nano14171451
Chicago/Turabian StyleWang, Yan, Feng Zhang, Zaifa Du, Xinmin Fan, Xiaodong Huang, Lujun Zhang, Sensen Li, Zhaohong Liu, and Chunyan Wang. 2024. "Insights into the Mechanisms of Single-Photon and Two-Photon Excited Surface Enhanced Fluorescence by Submicrometer Silver Particles" Nanomaterials 14, no. 17: 1451. https://doi.org/10.3390/nano14171451








