Recent Progress on Covalent Organic Frameworks Supporting Metal Nanoparticles as Promising Materials for Nitrophenol Reduction
Abstract
:1. Introduction
2. Nitrophenols
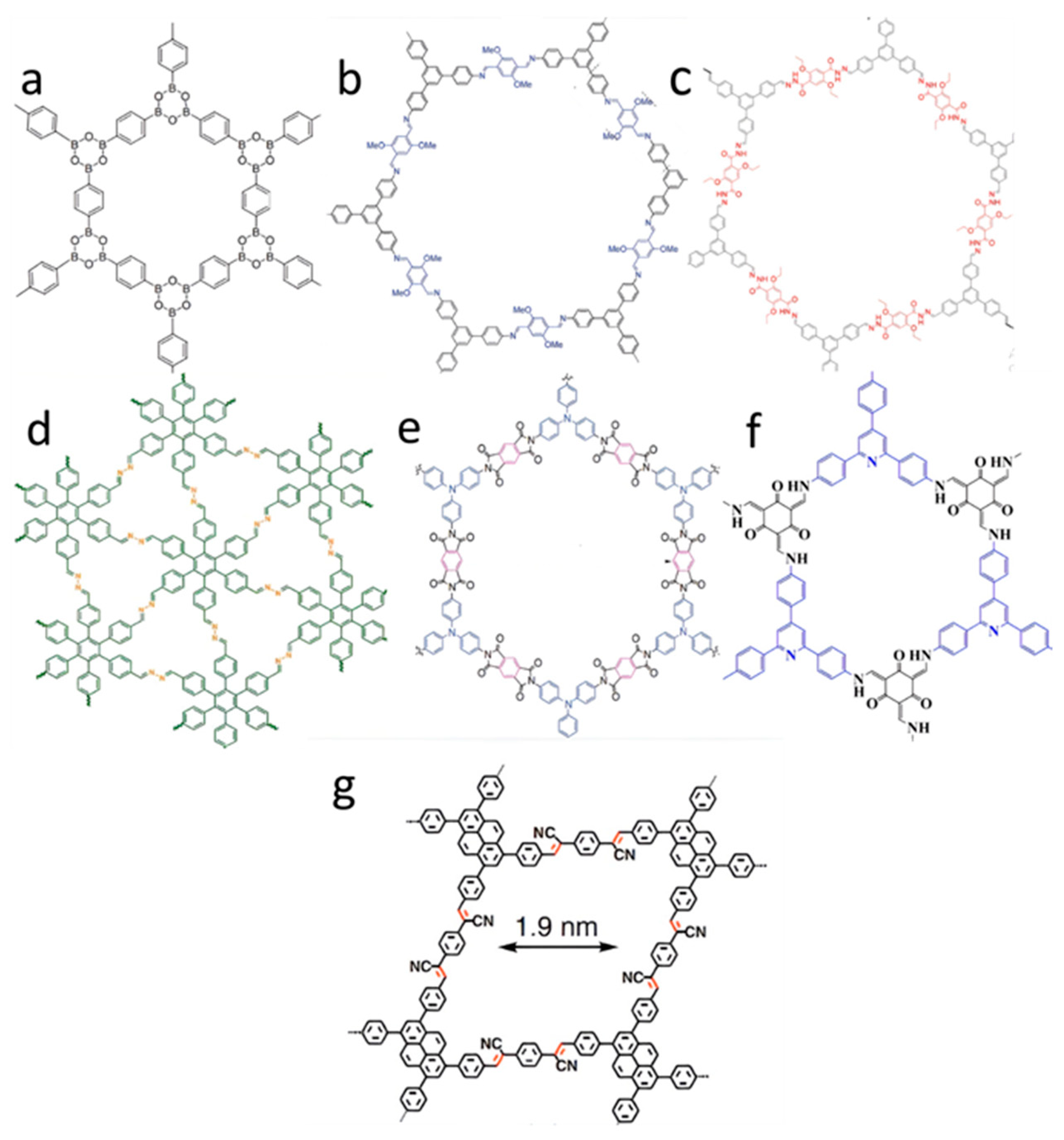
3. Synthesis Techniques of COFs
3.1. Solvothermal Method
3.2. Mechanochemical Method
3.3. Ionothermal Method
3.4. Microwave Method
3.5. Sonochemical Method
4. Reducing Nitrophenols Using COFs Containing Noble Metal Nanoparticles
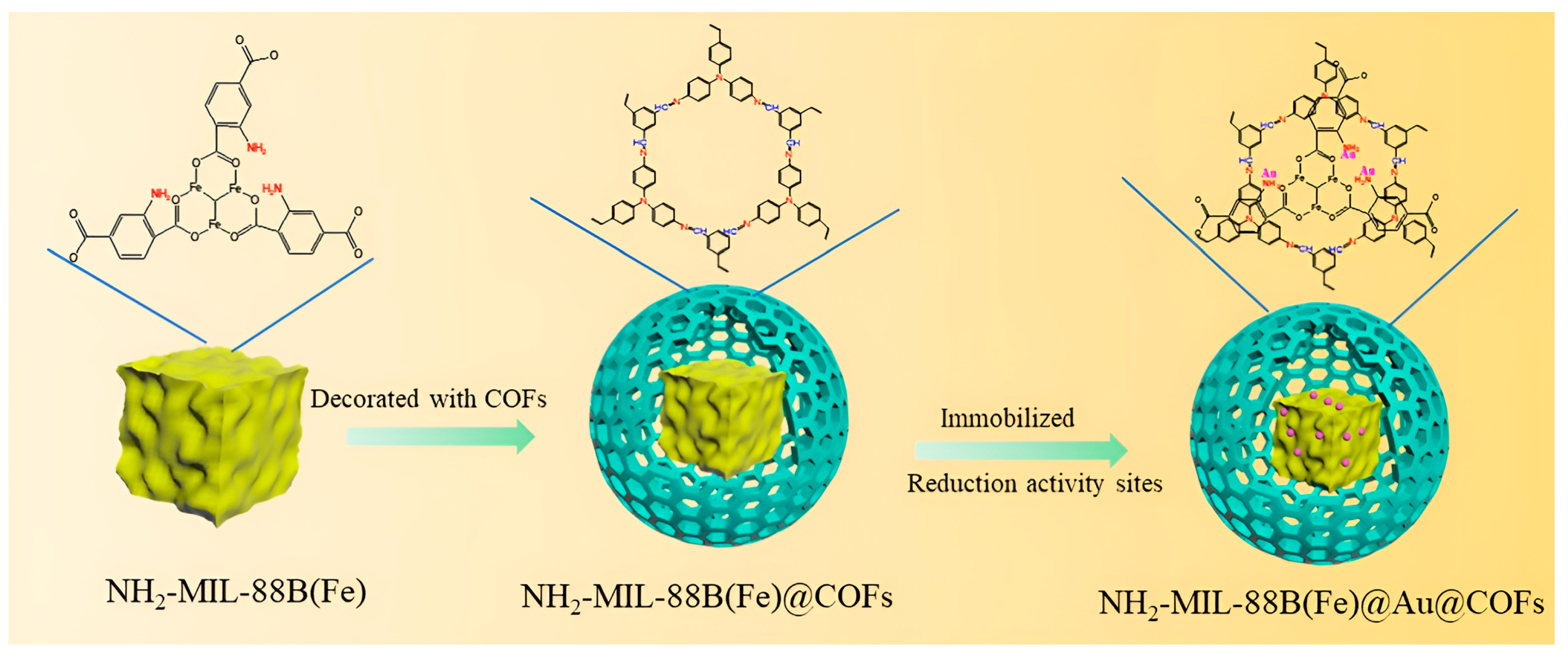
4.1. COFs Containing Gold (Au) Nanoparticle
4.2. COFs Containing Silver (Ag) Nanoparticles
4.3. COFs Containing Copper (Cu) Nanoparticles
4.4. COFs Containing Palladium (Pd) Nanoparticles
5. Conclusions, Challenges and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Mcadams, D.A.; Grunlan, J.C. Nano/micro-manufacturing of bioinspired materials: A review of methods to mimic natural structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef] [PubMed]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal organic frameworks mimicking natural enzymes: A structural and functional analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yaghi, O.M. Reticular chemistry: Molecular precision in infinite 2D and 3D. Mol. Front. J. 2019, 3, 66–83. [Google Scholar] [CrossRef]
- Azadi, E.; Dinari, M. Green and facile preparation of covalent organic frameworks based on reaction medium for advanced applications. Chem. A Eur. J. 2023, 29, e202301837. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Rejali, N.A.; Dinari, M.; Wang, Y. Post-synthetic modifications to covalent organic frameworks (COFs) for diverse applications. Chem. Commun. 2023, 59, 11631–11647. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Cheng, X.; Xie, Z.; Kuang, Q.; Zheng, L. The function of metal–organic frameworks in the application of MOF-based composites. Nanoscale Adv. 2020, 2, 2628–2647. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Dai, Z.; Sun, Y.; Liu, H.; Zheng, X.; Hu, Z. MOF-derived Fe based catalysts for efficiently Advanced Oxidation Processes: From single atoms to diatomic and nanoparticles. Prog. Nat. Sci. Mater. Int. 2023, 33, 534–554. [Google Scholar] [CrossRef]
- Taheri, N.; Dinari, M. Development of sulfonic acid-functionalized covalent organic polymer towards efficient adsorption of cationic dyes. Appl. Surf. Sci. Adv. 2023, 18, 100543. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, F.; Ren, H.; Jing, X.; Wang, W.; Ma, H.; Zhao, H.; Zhu, G. Targeted synthesis of a porous aromatic framework with a high adsorption capacity for organic molecules. J. Mater. Chem. 2011, 21, 13498–13502. [Google Scholar] [CrossRef]
- Zhu, Y.; Long, H.; Zhang, W. Imine-linked porous polymer frameworks with high small gas (H2, CO2, CH4, C2H2) uptake and CO2/N2 selectivity. Chem. Mater. 2013, 25, 1630–1635. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Mokhtari, N.; Khataei, M.M.; Dinari, M.; Monjezi, B.H.; Yamini, Y. Imine-based covalent triazine framework: Synthesis, characterization, and evaluation its adsorption. Mater. Lett. 2020, 263, 127221. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Dinari, M.; Hatami, M. Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J. Environ. Chem. Eng. 2019, 7, 102907. [Google Scholar] [CrossRef]
- Evans, A.M.; Ryder, M.R.; Ji, W.; Strauss, M.J.; Corcos, A.R.; Vitaku, E.; Flanders, N.C.; Bisbey, R.P.; Dichtel, W.R. Trends in the thermal stability of two-dimensional covalent organic frameworks. Faraday Discuss 2021, 225, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Hao, W.; Li, Y.; Chen, L. Covalent organic frameworks constructed from flexible building blocks with high adsorption capacity for pollutants. ACS Appl. Nano Mater. 2018, 1, 4756–4761. [Google Scholar] [CrossRef]
- Mokhtari, N.; Dinari, M. Developing novel amine-linked covalent organic frameworks towards reversible iodine capture. Sep. Purif. Technol. 2022, 301, 121948. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 16068. [Google Scholar] [CrossRef]
- Ma, X.; Scott, T.F. Approaches and challenges in the synthesis of three-dimensional covalent-organic frameworks. Commun. Chem. 2018, 1, 98. [Google Scholar] [CrossRef]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef]
- Mokhtari, N.; Afshari, M.; Dinari, M. Synthesis and characterization of a novel fluorene-based covalent triazine framework as a chemical adsorbent for highly efficient dye removal. Polymer 2020, 195, 122430. [Google Scholar] [CrossRef]
- Mokhtari, N.; Dinari, M.; Khosravi Esmaeiltarkhani, F. Imine-linked covalent organic frameworks: A biocompatible and pH-dependent carrier for in vitro sustained release of doxorubicin. ACS Omega 2023, 8, 25565–25573. [Google Scholar] [CrossRef]
- Fang, Q.; Gu, S.; Zheng, J.; Zhuang, Z.; Qiu, S.; Yan, Y. 3D microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew. Chem. 2014, 126, 2922–2926. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, J.; Ma, D.; Li, P.; Li, S.; Li, H.; Zhou, J.; Ma, X.; Feng, X.; Wang, B. Three-dimensional anionic cyclodextrin-based covalent organic frameworks. Angew. Chem. Int. Ed. 2017, 56, 16313–16317. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly conjugated three-dimensional covalent organic frameworks based on spirobifluorene for perovskite solar cell enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Xu, Y.X.; Pang, H. Two-dimensional MOF and COF nanosheets: Synthesis and applications in electrochemistry. Chem. A Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor–acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, H.R.; EL-Mahdy, A.F.; Kuo, S.W. Covalent organic frameworks: Design principles, synthetic strategies, and diverse applications. Giant 2021, 6, 100054. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Thompson, C.M.; Nguyen, A.X.; Occhialini, G.; McCandless, G.T.; Smaldone, R.A. An azine-linked hexaphenylbenzene based covalent organic framework. Chem. Commun. 2016, 52, 2843–2845. [Google Scholar] [CrossRef]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.; Hung, Y.-H.; Mansoure, T.H.; Yu, H.-H.; Hsu, Y.-S.; Wu, K.C.; Kuo, S.-W. Synthesis of [3 + 3] β-ketoenamine-tethered covalent organic frameworks (COFs) for high-performance supercapacitance and CO2 storage. J. Taiwan Inst. Chem. Eng. 2019, 103, 199–208. [Google Scholar] [CrossRef]
- Jin, E.; Li, J.; Geng, K.; Jiang, Q.; Xu, H.; Xu, Q.; Jiang, D. Designed synthesis of stable light-emitting two-dimensional sp2 carbon-conjugated covalent organic frameworks. Nat. Commun. 2018, 9, 4143. [Google Scholar] [CrossRef]
- Dong, J.; Han, X.; Liu, Y.; Li, H.; Cui, Y. Metal–covalent organic frameworks (MCOFs): A bridge between metal–organic frameworks and covalent organic frameworks. Angew. Chem. Int. Ed. 2020, 59, 13722–13733. [Google Scholar] [CrossRef]
- Kelishadi, R. Environmental Pollution: Health Effects and Operational Implications for Pollutants Removal; Hindawi: London, UK, 2012. [Google Scholar]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury (II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Cai, Y.; Wang, S.; Hu, B.; Li, B.; Ding, X.; Zhuang, L.; Wang, X. Application of covalent organic frameworks and metal–organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies. Carbon Res. 2023, 2, 8. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, S.; Zhuang, L.; Hu, B.; Wang, S.; Chen, J.; Wang, X. Application of biochar-based photocatalysts for adsorption-(photo) degradation/reduction of environmental contaminants: Mechanism, challenges and perspective. Biochar 2022, 4, 45. [Google Scholar] [CrossRef]
- Nouruzi, N.; Dinari, M.; Mokhtari, N.; Farajzadeh, M.; Gholipour, B.; Rostamnia, S. Selective catalytic generation of hydrogen over covalent organic polymer supported Pd nanoparticles (COP-Pd). Mol. Catal. 2020, 493, 111057. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Xu, H.; Jiang, D. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. J. Am. Chem. Soc. 2017, 139, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Y.; Zhu, X.; Chen, B. Hollow multi-shelled Co3O4 as nanoreactors to activate peroxymonosulfate for highly effective degradation of Carbamazepine: A novel strategy to reduce nano-catalyst agglomeration. J. Hazard. Mater. 2022, 427, 127890. [Google Scholar] [CrossRef]
- Gates, B.C. Supported metal cluster catalysts. J. Mol. Catal. A Chem. 2000, 163, 55–65. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef]
- Ju, K.-S.; Parales, R.E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial degradation of nitrophenols and their derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Dere, E.; Ozdikicioglu, F.; Tosunoglu, H. Hepatotoxicity of Dinitro-o-cresol in rats (Rattus norvegicus). Acta Vet. 2007, 57, 497–507. [Google Scholar]
- Arora, P.K.; Sharma, A.; Mehta, R.; Shenoy, B.D.; Srivastava, A.; Singh, V.P. Metabolism of 4-chloro-2-nitrophenol in a Gram-positive bacterium, Exiguobacterium sp. PMA. Microb. Cell Factories 2012, 11, 150. [Google Scholar] [CrossRef]
- Harris, M.O.; Corcoran, J. Toxicological Profile for Dinitrophenols; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1995.
- Preiss, A.; Elend, M.; Gerling, S.; Berger-Preiss, E.; Steinbach, K. Identification of highly polar nitroaromatic compounds in leachate and ground water samples from a TNT-contaminated waste site by LC-MS, LC-NMR, and off-line NMR and MS investigations. Anal. Bioanal. Chem. 2007, 389, 1979–1988. [Google Scholar] [CrossRef]
- Asman, W.A.; Jørgensen, A.; Bossi, R.; Vejrup, K.V.; Mogensen, B.B.; Glasius, M. Wet deposition of pesticides and nitrophenols at two sites in Denmark: Measurements and contributions from regional sources. Chemosphere 2005, 59, 1023–1031. [Google Scholar] [CrossRef]
- Harrison, M.A.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Lu, Z.; Pan, J.; Li, M. A novel biosensor-based method for the detection of p-nitrophenol in agricultural soil. Chemosphere 2023, 313, 137306. [Google Scholar] [CrossRef]
- Qiu, C.; Yuan, S.; Li, X.; Wang, H.; Bakheet, B.; Komarneni, S.; Wang, Y. Investigation of the synergistic effects for p-nitrophenol mineralization by a combined process of ozonation and electrolysis using a boron-doped diamond anode. J. Hazard. Mater. 2014, 280, 644–653. [Google Scholar] [CrossRef]
- Bhatti, Z.I.; Toda, H.; Furukawa, K. p-Nitrophenol degradation by activated sludge attached on nonwovens. Water Res. 2002, 36, 1135–1142. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, J.; Shen, Z.; Li, J.; Zhang, H.; Cao, X.; Ye, Z.; Ji, G.; Liu, Q.; Hu, Y.; et al. Sequentially modified carbon felt for enhanced p-nitrophenol biodegradation through direct interspecific electron transfer. J. Hazard. Mater. 2023, 451, 131055. [Google Scholar] [CrossRef]
- Moond, M.; Singh, S.; Sangwan, S.; Devi, R.; Rani, J.; Beniwal, A.; Beniwal, R. Synthesis, optimization and characterization of Silver nanoparticles using aqueous seed extract of Trigonella foenum-graceum L. for catalytic reduction of p-nitrophenol. ChemistrySelect 2023, 8, e202301006. [Google Scholar] [CrossRef]
- Abdollahi, M.; Mohammadirad, A. 4-Nitrophenol. In Encyclopedia of Toxicology, 3rd ed.; Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 575–577. [Google Scholar]
- Hassan, S.S.; Carlson, K.; Mohanty, S.K.; Sirajuddin; Canlier, A. Ultra-rapid catalytic degradation of 4-nitrophenol with ionic liquid recoverable and reusable ibuprofen derived silver nanoparticles. Environ. Pollut. 2018, 237, 731–739. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Zhang, A.; Song, Z.; Sun, Q.; Huo, B.; Yang, W.; Barrow, C.J.; Liu, J. Insight into catalytic mechanisms for the reduction of nitrophenol via heterojunctions of gold nanoclusters on 2D boron nitride nanosheets. ChemNanoMat 2019, 5, 784–791. [Google Scholar] [CrossRef]
- Balooch, M.D.; Naeimi, A.; Iranmanesh, P.; Saeednia, S. A Green Silver Nanoparticles using Pistachio Wastes for Dyes Degradation Exposing Visible Light and Reduction of 4-Nitrophenol. ChemistrySelect 2023, 8, e202300652. [Google Scholar]
- Mitchell, S.; Waring, R. Aminophenols. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Weideman, R.A.; Kelly, K.C.; Kazi, S.; Cung, A.; Roberts, K.W.; Smith, H.J.; Sarosi, G.A.; Little, B.B.; Cryer, B. Risks of clinically significant upper gastrointestinal events with etodolac and naproxen: A historical cohort analysis. Gastroenterology 2004, 127, 1322–1328. [Google Scholar] [CrossRef]
- Tucceri, R. Practical applications of poly (o-aminophenol) film electrodes. Open Phys. Chem. J. 2010, 4, 45–61. [Google Scholar]
- Evangelista, V.; Acosta, B.; Miridonov, S.; Smolentseva, E.; Fuentes, S.; Simakov, A. Highly active Au-CeO2@ ZrO2 yolk–shell nanoreactors for the reduction of 4-nitrophenol to 4-aminophenol. Appl. Catal. B Environ. 2015, 166, 518–528. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Chen, J.; Ge, R.; Leng, W.; Dong, B.; Huang, J.; Gao, Y. A novel 3D covalent organic framework membrane grown on a porous α-Al2O3 substrate under solvothermal conditions. Chem. Commun. 2015, 51, 15562–15565. [Google Scholar] [CrossRef]
- Golshadi, Z.; Dinari, M.; Knebel, A.; Lützenkirchen, J.; Monjezi, B.H. Metal organic and covalent organic framework-based QCM sensors for environmental pollutant detection and beyond. Coord. Chem. Rev. 2024, 521, 216163. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Zheng, Q.; Brown, N.; Behera, R.; Yang, C.; Uddin, M.H.; Saintlima, A.; Middlebrooks, L.; Li, J.; Ingram, C.; et al. Microwave-assisted synthesis of mixed-linker covalent organic frameworks enabling tunable and ultrahigh iodine capture. Chem. Eng. J. 2024, 485, 149135. [Google Scholar] [CrossRef]
- Wei, X.; Bi, M.; Lou, Q.; Di, D.; Liu, B.; Pei, D. Fast and facile sonochemical fabrication of covalent organic frameworks in water for the adsorption of flavonoids: Adsorption behaviors and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134731. [Google Scholar] [CrossRef]
- Liu, J.; He, K.; Wu, W.; Song, T.-B.; Kanatzidis, M.G. In situ synthesis of highly dispersed and ultrafine metal nanoparticles from chalcogels. J. Am. Chem. Soc. 2017, 139, 2900–2903. [Google Scholar] [CrossRef]
- Van Santen, R.A.; Neurock, M. Molecular Heterogeneous Catalysis: A Conceptual and Computational Approach; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pachfule, P.; Panda, M.K.; Kandambeth, S.; Shivaprasad, S.M.; Díaz, D.D.; Banerjee, R. Multifunctional and robust covalent organic framework–nanoparticle hybrids. J. Mater. Chem. A 2014, 2, 7944–7952. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Pachfule, P.; Kandambeth, S.; Díaz, D.D.; Banerjee, R. Highly stable covalent organic framework–Au nanoparticles hybrids for enhanced activity for nitrophenol reduction. Chem. Commun. 2014, 50, 3169–3172. [Google Scholar] [CrossRef]
- Shi, X.; Yao, Y.; Xu, Y.; Liu, K.; Zhu, G.; Chi, L.; Lu, G. Imparting catalytic activity to a covalent organic framework material by nanoparticle encapsulation. ACS Appl. Mater. Interfaces 2017, 9, 7481–7488. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, X.; Hua, R.; Zhang, R.; Yao, Y.; Zhao, B.; Liu, T.; Zheng, J.; Lu, G. Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@ COF supported noble metal nanoparticles. Appl. Catal. B Environ. 2020, 260, 118142. [Google Scholar] [CrossRef]
- Zhang, Q.-P.; Sun, Y.-L.; Cheng, G.; Wang, Z.; Ma, H.; Ding, S.-Y.; Tan, B.; Bu, J.-H.; Zhang, C. Highly dispersed gold nanoparticles anchoring on post-modified covalent organic framework for catalytic application. Chem. Eng. J. 2020, 391, 123471. [Google Scholar] [CrossRef]
- Tan, X.-P.; Zeng, W.; Fan, Y.; Yan, J.; Zhao, G. Covalent organic frameworks bearing pillar[6]arene-reduced Au nanoparticles for the catalytic reduction of nitroaromatics. Nanotechnology 2020, 31, 135705. [Google Scholar] [CrossRef]
- Bhadra, M.; Kandambeth, S.; Sahoo, M.K.; Addicoat, M.; Balaraman, E.; Banerjee, R. Triazine functionalized porous covalent organic framework for photo-organocatalytic E–Z isomerization of olefins. J. Am. Chem. Soc. 2019, 141, 6152–6156. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Niu, L.; Zhao, X.; Tang, Z.; Wu, F.; Lei, Q.; Wang, J.; Wang, X.; Liang, W.; Wang, X. Solid-solid synthesis of covalent organic framework as a support for growth of controllable ultrafine Au nanoparticles. Sci. Total Environ. 2022, 835, 155423. [Google Scholar] [CrossRef]
- Pakulski, D.; Montes-García, V.; Gorczyński, A.; Czepa, W.; Chudziak, T.; Samorì, P.; Ciesielski, A. Thiol-decorated covalent organic frameworks as multifunctional materials for high-performance supercapacitors and heterogeneous catalysis. J. Mater. Chem. A 2022, 10, 16685–16696. [Google Scholar] [CrossRef]
- Liu, S.; Mao, C.; Yang, C.; Hong, P.; Zhu, M.; Zhou, Y.; Zhang, Y. Rational design of a MOF@ Au@ COF catalyst with electron synergy for the reduction of 4-nitrophenol. New J. Chem. 2023, 47, 8161–8169. [Google Scholar] [CrossRef]
- Le Hoang, T.T.T.; Insin, N.; Sukpirom, N. Catalytic activity of silver nanoparticles anchored on layered double hydroxides and hydroxyapatite. Inorg. Chem. Commun. 2020, 121, 108199. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Wang, R.-L.; Li, D.P.; Wang, L.J.; Zhang, X.; Zhou, Z.Y.; Mu, J.L.; Su, Z.M. The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalton Trans. 2019, 48, 1051–1059. [Google Scholar] [CrossRef]
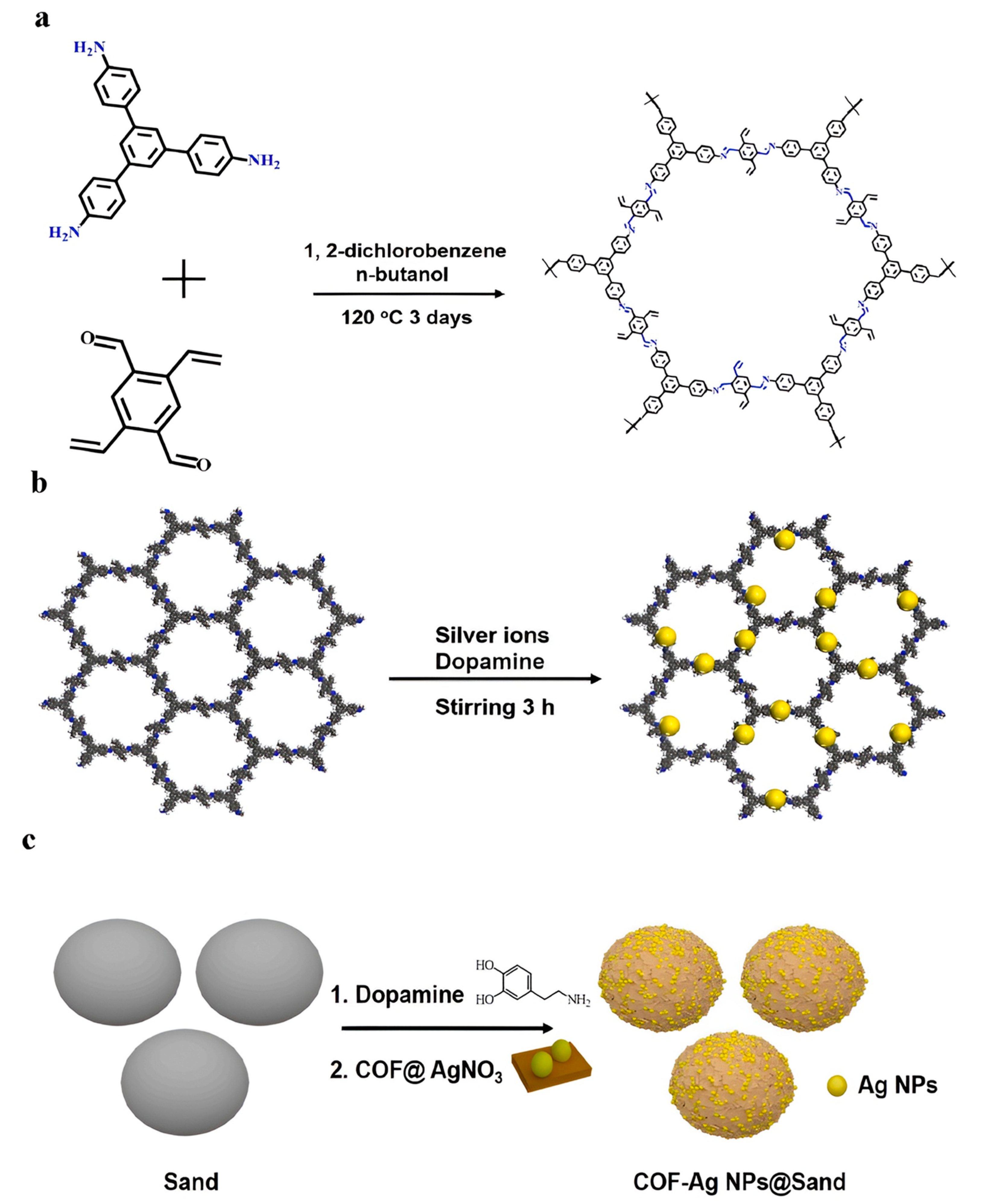
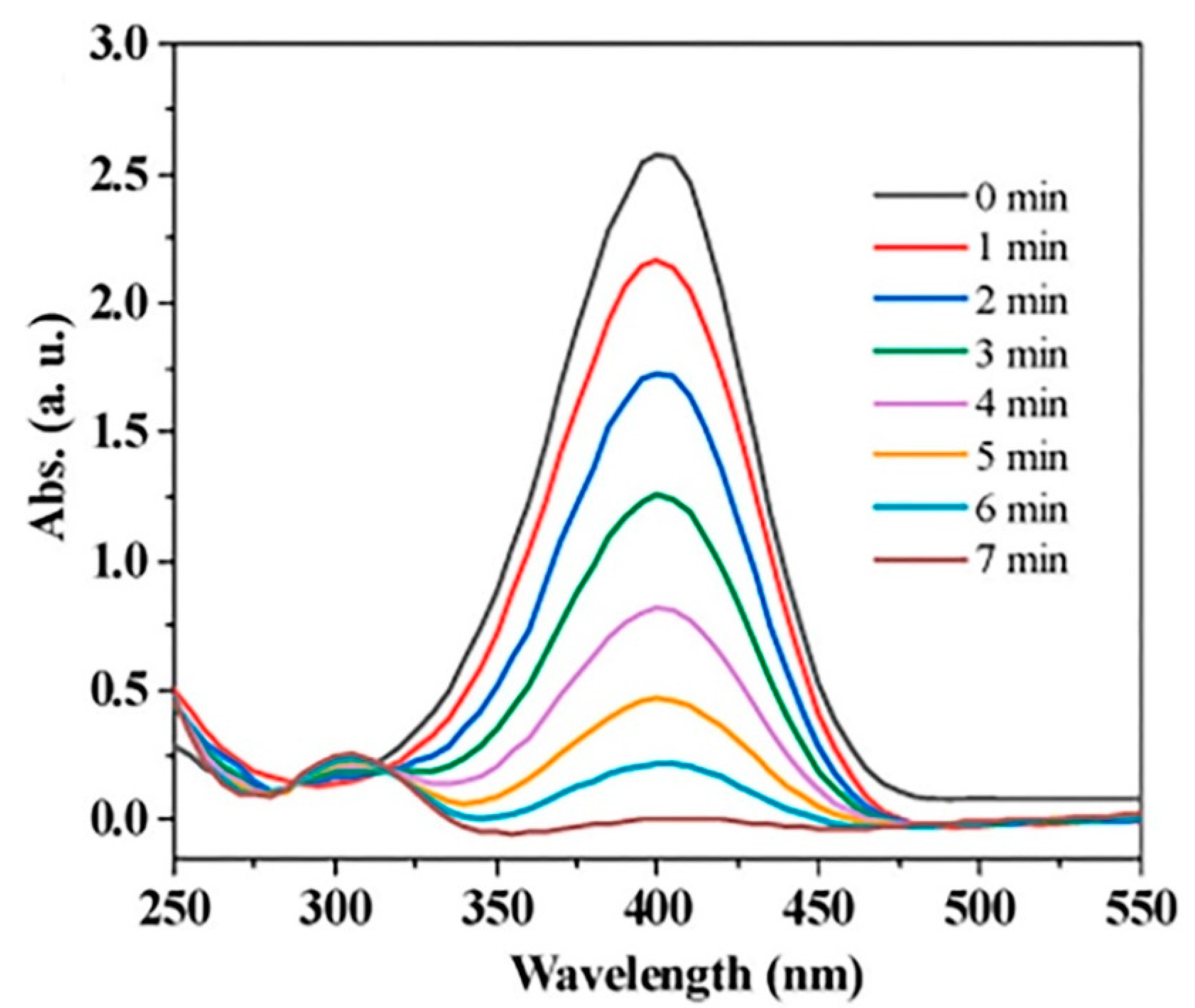
- Wang, N.; Wang, F.; Pan, F.; Yu, S.; Pan, D. Highly efficient silver catalyst supported by a spherical covalent organic framework for the continuous reduction of 4-nitrophenol. ACS Appl. Mater. Interfaces 2021, 13, 3209–3220. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, F.; Wang, N. Towards application of a covalent organic framework-silver nanoparticles@ sand heterostructure as a high-efficiency catalyst for flow-through reduction of organic pollutants. Appl. Surf. Sci. 2021, 565, 150580. [Google Scholar] [CrossRef]
- Chen, R.; Hu, T.; Zhang, W.; He, C.; Li, Y. Synthesis of nitrogen-containing covalent organic framework with reversible iodine capture capability. Microporous Mesoporous Mater. 2021, 312, 110739. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wang, Q.; Zhang, K.; Luo, Y.; Liu, Y.; Lyu, Y.; Huang, B. Porphyrin-based covalent triazine framework and its carbonized derivative as catalyst scaffold of Au and Ag nanoparticles for 4-nitrophenol reduction. Microporous Mesoporous Mater. 2022, 330, 111611. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, J.; Liu, Y.; Lyu, Y. Porphyrin-based covalent triazine frameworks: Porosity, adsorption performance, and drug delivery. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2594–2600. [Google Scholar] [CrossRef]
- Huang, J.; Tao, Y.; Ran, S.; Yang, Y.; Li, C.; Luo, P.; Chen, Z.; Tan, X. A hydroxy-containing three dimensional covalent organic framework bearing silver nanoparticles for reduction of 4-nitrophenol and degradation of organic dyes. New J. Chem. 2022, 46, 17153–17160. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, Y.; Li, H.; Guan, X.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Qiu, S.; Valtchev, V. Postsynthetic functionalization of three-dimensional covalent organic frameworks for selective extraction of lanthanide ions. Angew. Chem. 2018, 130, 6150–6156. [Google Scholar] [CrossRef]
- Rezaei, F.; Dinari, M. Novel covalent organic polymer-supported Ag nanoparticles as a catalyst for nitroaromatics reduction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126441. [Google Scholar] [CrossRef]
- Rajesh, K.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 2018, 154, 593–600. [Google Scholar] [CrossRef]
- Jia, W.; Tian, F.; Zhang, M.; Li, X.; Ye, S.; Ma, Y.; Wang, W.; Zhang, Y.; Meng, C.; Zeng, G.; et al. Nitrogen-doped porous carbon-encapsulated copper composite for efficient reduction of 4-nitrophenol. J. Colloid Interface Sci. 2021, 594, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, M.; Lang, Z.L.; Liu, J.; Liu, M.; Chang, J.N.; Li, L.Y.; Shang, L.J.; Wang, M.; Li, S.L.; et al. Semiconductor/covalent-organic-framework Z-scheme heterojunctions for artificial photosynthesis. Angew. Chem. 2020, 132, 6562–6568. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Dai, L.; Qin, G.; Guo, J.; Zhang, Q.; Li, S.; Sherazi, T.A.; Zhang, S. High-efficiency Pd nanoparticles loaded porous organic polymers membrane catalytic reactors. Chem. Commun. 2021, 57, 3131–3134. [Google Scholar] [CrossRef]
- Jin, D.; Wang, B.; Wu, X.; Li, J.; Mu, M.; Chen, L. Construction and catalytic applications of an amino-functionalized covalent organic framework. Transit. Met. Chem. 2019, 44, 689–697. [Google Scholar] [CrossRef]
- Wang, F.; Pan, F.; Li, G.; Zhang, P.; Wang, N. Construction of spherical montmorillonite supported Cu-based catalyst doping with a covalent organic framework for 4-nitrophenol removal. Appl. Clay Sci. 2021, 214, 106278. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, D.; Chen, W.; Li, H.; Zhang, S.; Liang, C. Covalent organic framework-functionalized magnetic CuFe2O4/Ag nanoparticles for the reduction of 4-nitrophenol. Nanomaterials 2020, 10, 426. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, J.; Wen, W.; Zhang, X.; Wang, S. Spherical covalent organic framework supported Cu/Ag bimetallic nanoparticles with highly catalytic activity for reduction of 4-nitrophenol. J. Solid State Chem. 2022, 311, 123116. [Google Scholar] [CrossRef]
- Ma, W.; Zheng, Q.; He, Y.; Li, G.; Guo, W.; Lin, Z.; Zhang, L. Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides. J. Am. Chem. Soc. 2019, 141, 18271–18277. [Google Scholar] [CrossRef]
- Liu, Y.; He, Q.; Li, Y.; Li, Y.; He, C. Synthesis of copper-based nanoparticles confined in a pyridine N-containing COF and their catalytic applications. Microporous Mesoporous Mater. 2024, 364, 112868. [Google Scholar] [CrossRef]
- Rezaei, F.; Dinari, M. Cu nanoparticles embedded in the porous organic polymer as highly effective catalysts for nitroaromatics reduction. Microporous Mesoporous Mater. 2021, 325, 111339. [Google Scholar] [CrossRef]
- Kidambi, S.; Dai, J.; Li, J.; Bruening, M.L. Selective hydrogenation by Pd nanoparticles embedded in polyelectrolyte multilayers. J. Am. Chem. Soc. 2004, 126, 2658–2659. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.Y.; Bi, Y.Y.; Wei, J.F.; Chen, Z.G. Pd nanoparticles in ionic liquid brush: A highly active and reusable heterogeneous catalytic assembly for solvent-free or on-water hydrogenation of nitroarene under mild conditions. ACS Catal. 2011, 1, 657–664. [Google Scholar] [CrossRef]
- Lu, S.; Hu, Y.; Wan, S.; McCaffrey, R.; Jin, Y.; Gu, H.; Zhang, W. Synthesis of ultrafine and highly dispersed metal nanoparticles confined in a thioether-containing covalent organic framework and their catalytic applications. J. Am. Chem. Soc. 2017, 139, 17082–17088. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Jia, Z. Tunable synthesis of Pd/COF-LZU1 for efficient catalysis in nitrophenol reduction. J. Nanopart. Res. 2020, 22, 270. [Google Scholar] [CrossRef]
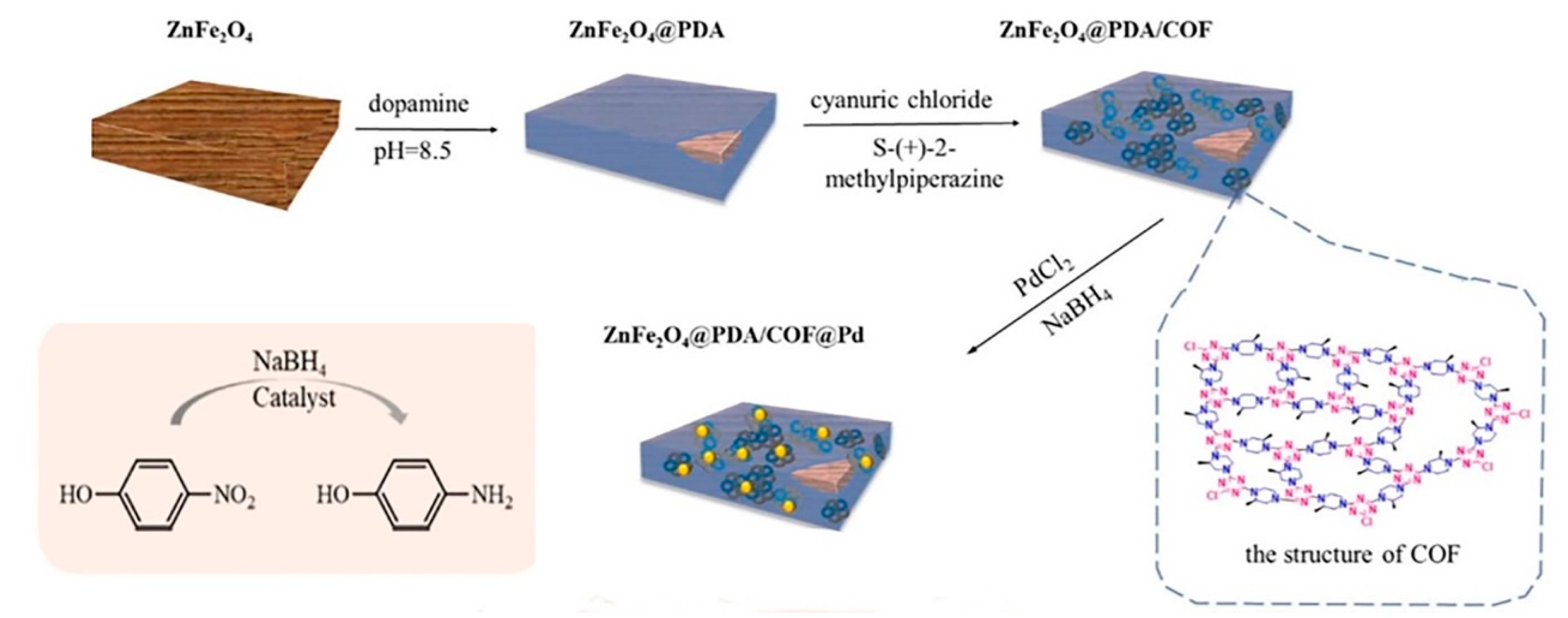
- Lv, K.; Yan, G.; Wang, G.; Chen, Z.; Hu, J. Palladium-Decorated Covalent Organic Framework Supported on Zinc Ferrite as Magnetic Catalyst for Suzuki Reaction and p-Nitrophenol Reduction. Catal. Lett. 2023, 153, 2959–2974. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zhao, Q.; Chen, G. ZnFe2O4 multi-porous microbricks/graphene hybrid photocatalyst: Facile synthesis, improved activity and photocatalytic mechanism. Appl. Catal. B. Environ. 2013, 142, 80–88. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Chen, Q.; Chen, Z.; Hu, J. The synthesis of size-controlled hollow spherical covalent organic frameworks and its application in photocatalysis and Suzuki coupling reactions. J. Catal. 2022, 416, 29–38. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Yan, G.; Hu, J. Palladium nanoparticles immobilized on COF-modified honeycomb chitosan microcapsules as catalysts for the Suzuki reaction and p-nitrophenol reduction. New J. Chem. 2023, 47, 297–306. [Google Scholar] [CrossRef]
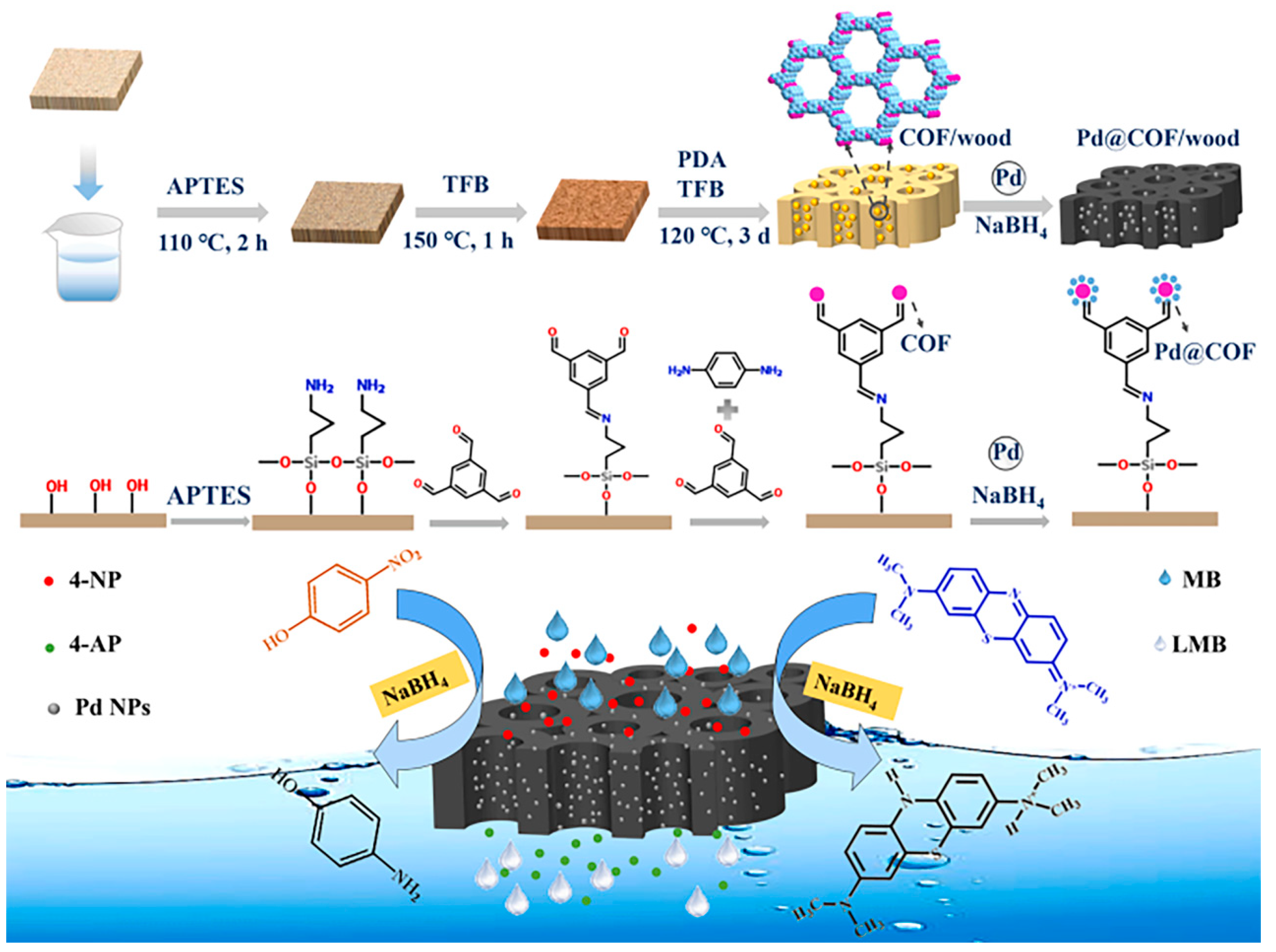
- Cui, Z.; Wu, J.; Wu, T.; Xu, Y.; Li, H.; Yu, Y.; Kang, L.; Cai, Y.; Li, J.; Tian, D. Novel wood membrane decorated with covalent organic frameworks and palladium nanoparticles for reduction of aromatic organic contaminants. Sep. Purif. Technol. 2023, 319, 124112. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Z.; Chen, Z.; Wang, J.; Hu, J.; Xu, X. Pd NPs encapsulated by COF in nitrogen-doped macroporous chitosan carbon microspheres act as an efficient and recyclable multifunctional catalyst. Appl. Surf. Sci. 2023, 631, 157538. [Google Scholar] [CrossRef]
- Hao, M.; Xie, Y.; Liu, X.; Chen, Z.; Yang, H.; Waterhouse, G.I.N.; Ma, S.; Wang, X. Modulating uranium extraction performance of multivariate covalent organic frameworks through donor–acceptor linkers and amidoxime nanotraps. JACS Au 2023, 3, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Altınışık, S.; Koyuncu, S. A Novel Viologen-Derived Covalent Organic Framework Based Metal Free Catalyst for Nitrophenol Reduction. ChemCatChem 2023, 15, e202201418. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinari, M.; Golshadi, Z.; Asadi, P.; Norton, A.E.; Reid, K.R.; Karimi, B. Recent Progress on Covalent Organic Frameworks Supporting Metal Nanoparticles as Promising Materials for Nitrophenol Reduction. Nanomaterials 2024, 14, 1458. https://doi.org/10.3390/nano14171458
Dinari M, Golshadi Z, Asadi P, Norton AE, Reid KR, Karimi B. Recent Progress on Covalent Organic Frameworks Supporting Metal Nanoparticles as Promising Materials for Nitrophenol Reduction. Nanomaterials. 2024; 14(17):1458. https://doi.org/10.3390/nano14171458
Chicago/Turabian StyleDinari, Mohammad, Zaynab Golshadi, Parvin Asadi, Amie E. Norton, Katelyn R. Reid, and Benson Karimi. 2024. "Recent Progress on Covalent Organic Frameworks Supporting Metal Nanoparticles as Promising Materials for Nitrophenol Reduction" Nanomaterials 14, no. 17: 1458. https://doi.org/10.3390/nano14171458





