Solar Hydrogen Production and Storage in Solid Form: Prospects for Materials and Methods
Abstract
:1. Introduction
2. Hydrogen as an Alternative Energy Source
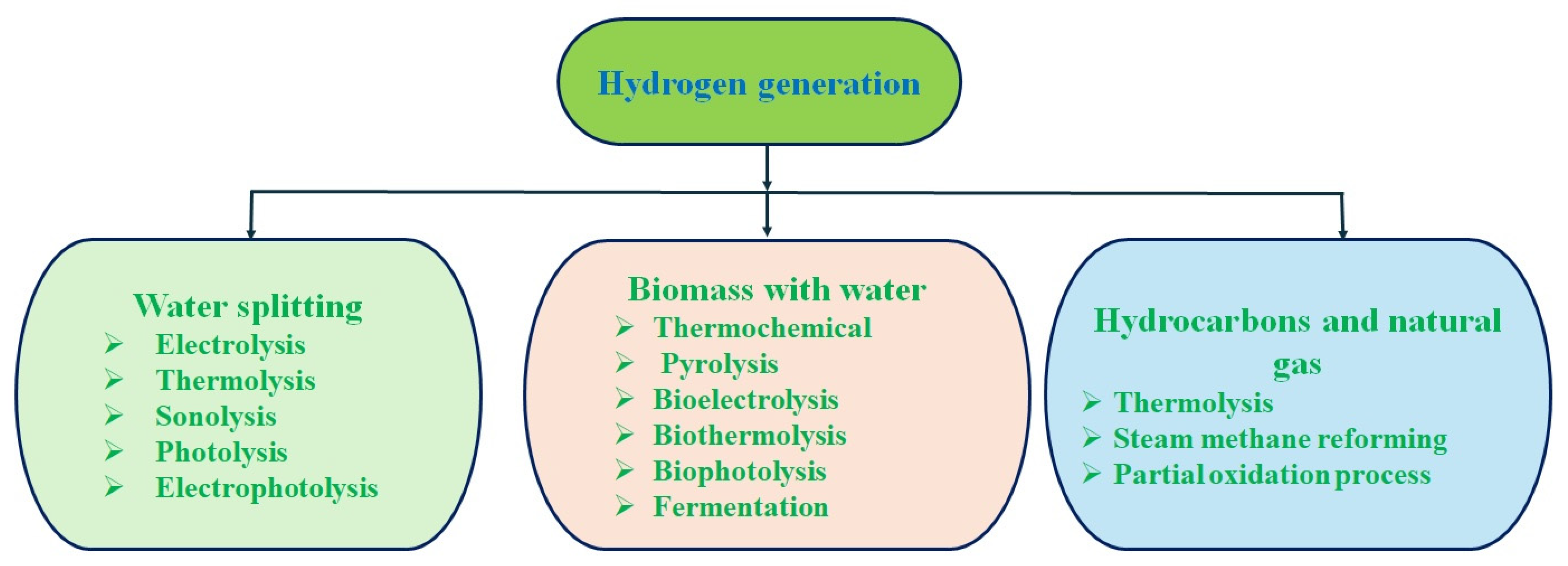
3. Hydrogen Production Techniques
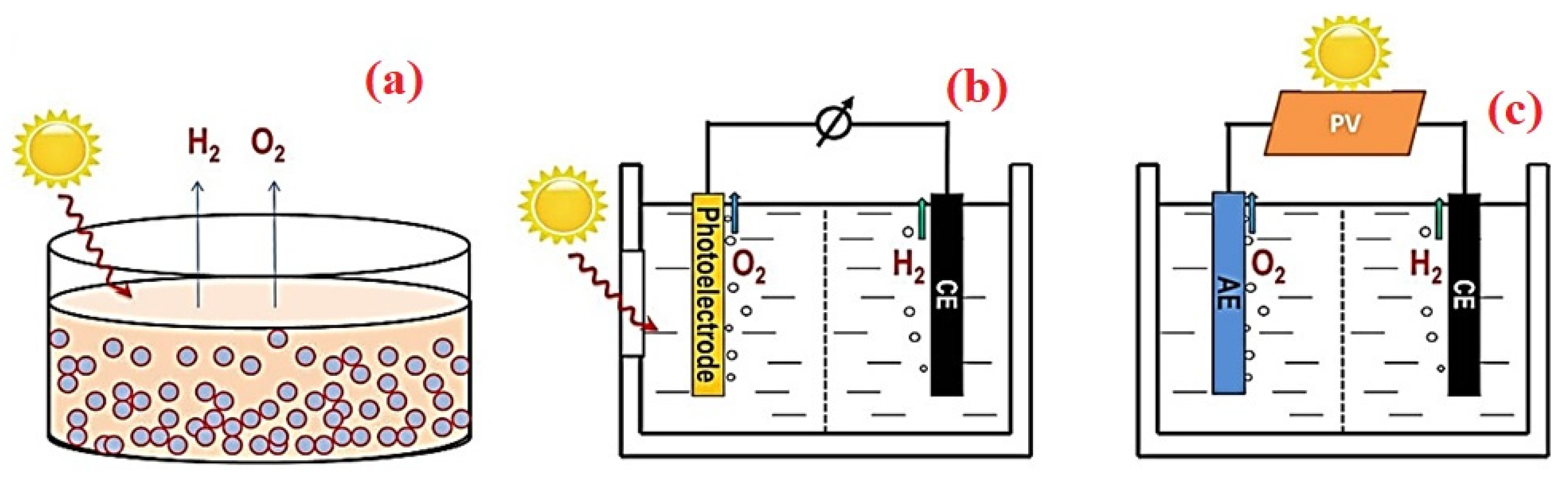
4. Solar Hydrogen from Water Splitting
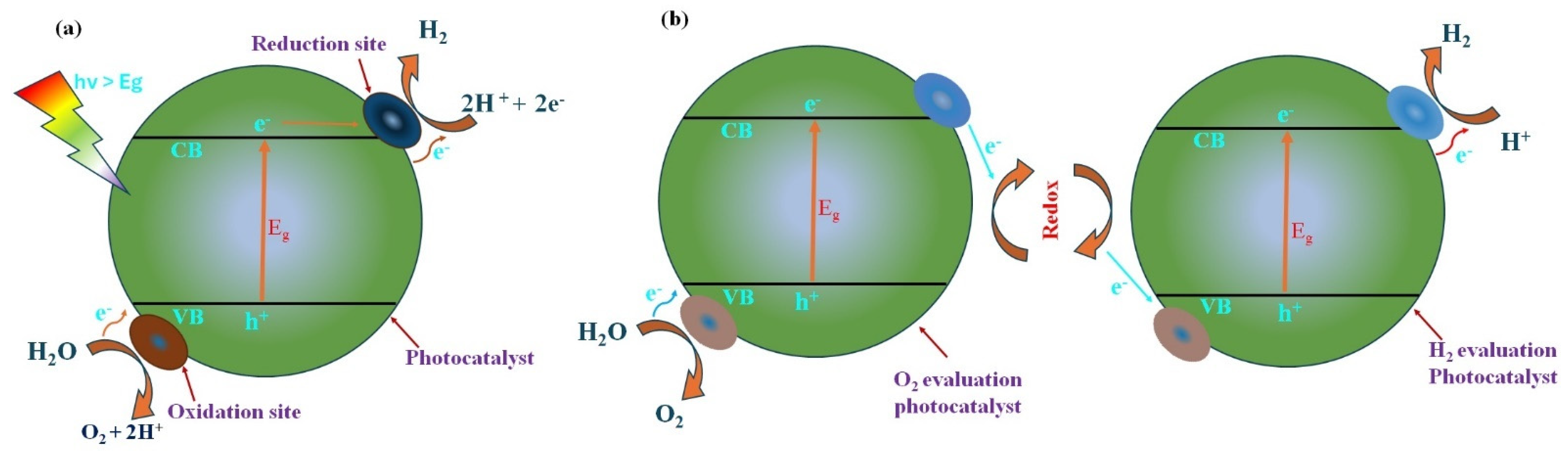
4.1. Mechanism of Solar Water Splitting
4.2. Advantages of Solar Hydrogen
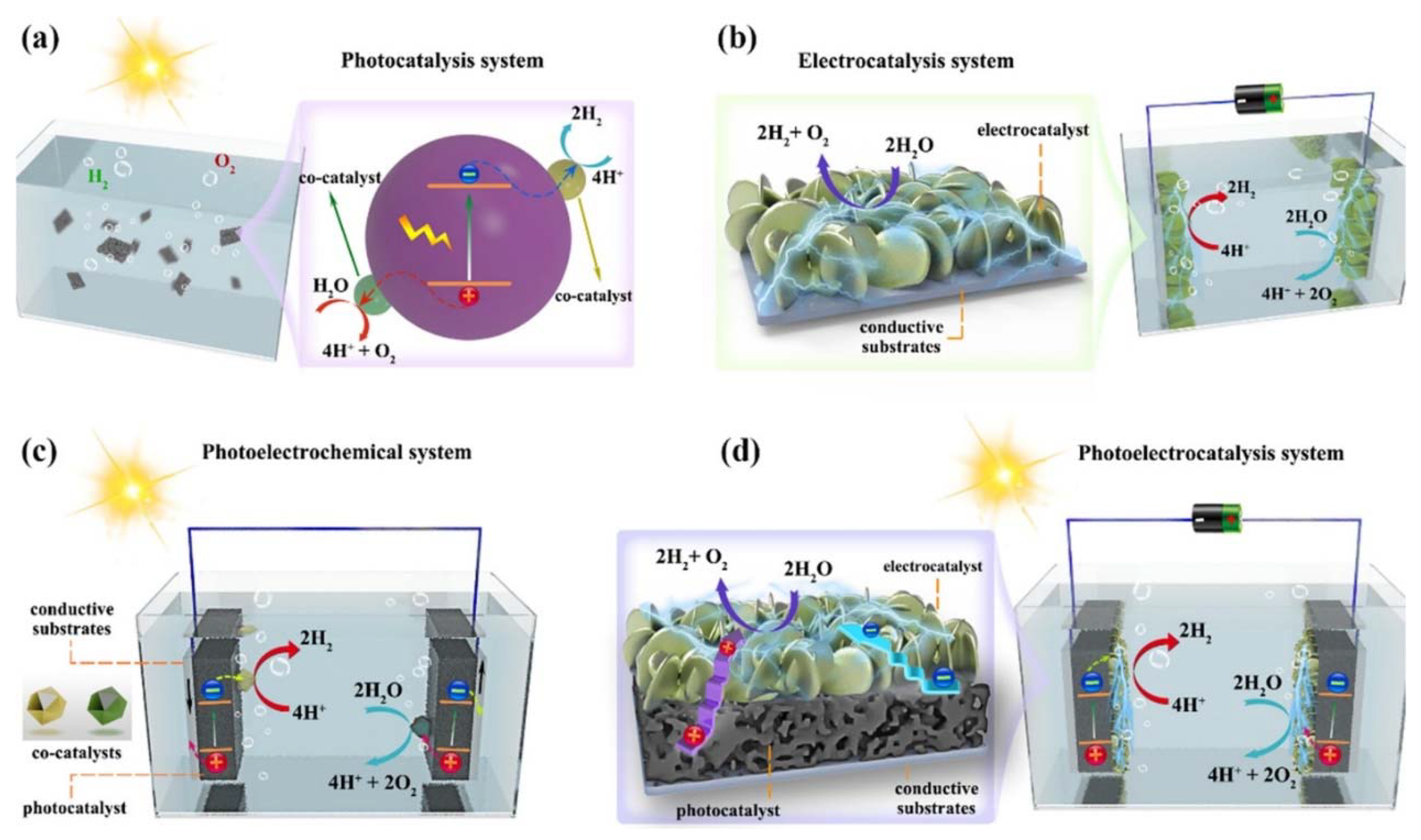
4.3. Materials and Methods for Solar Hydrogen Generation
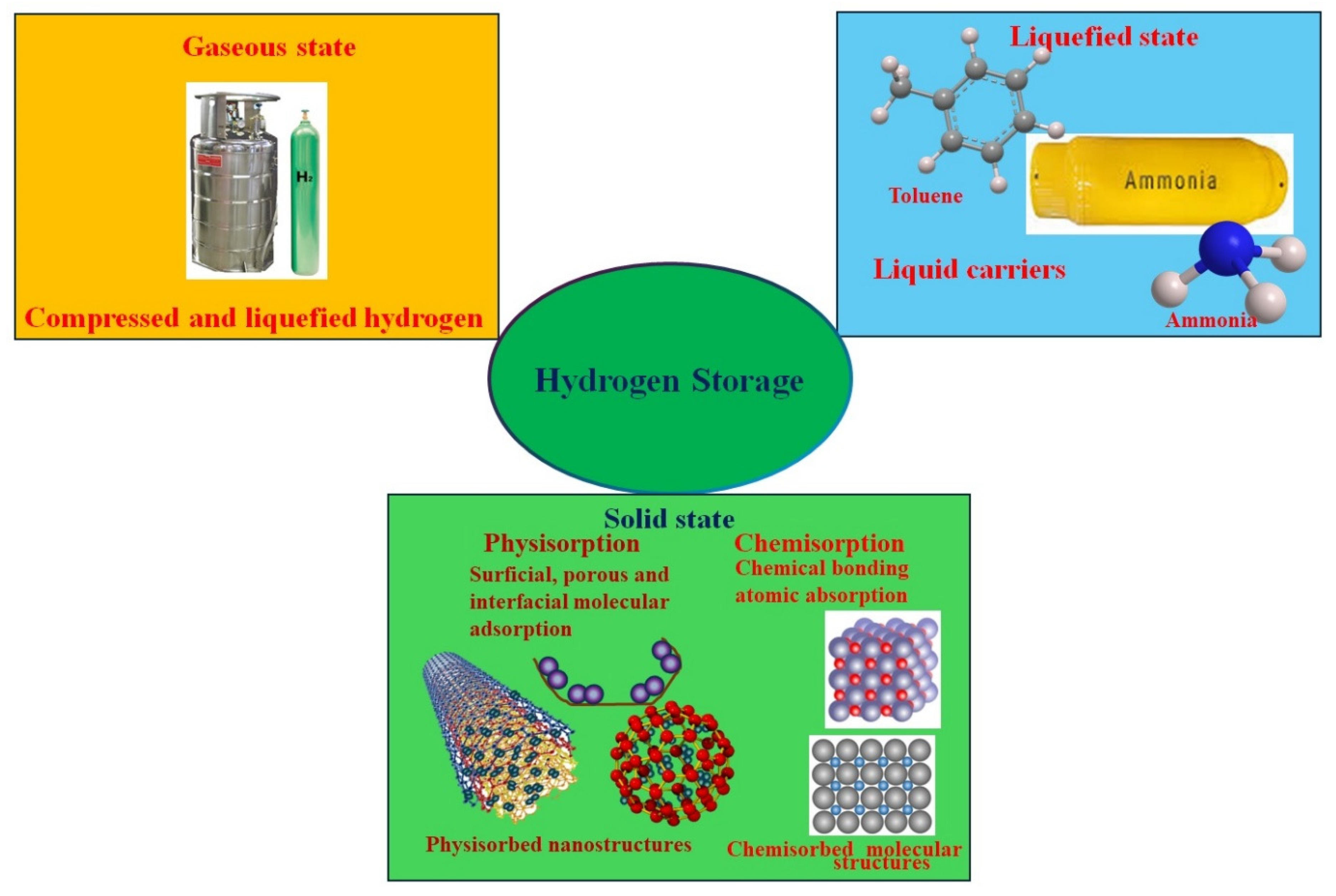
5. Hydrogen Storage Mechanisms
Solid Storage of Hydrogen
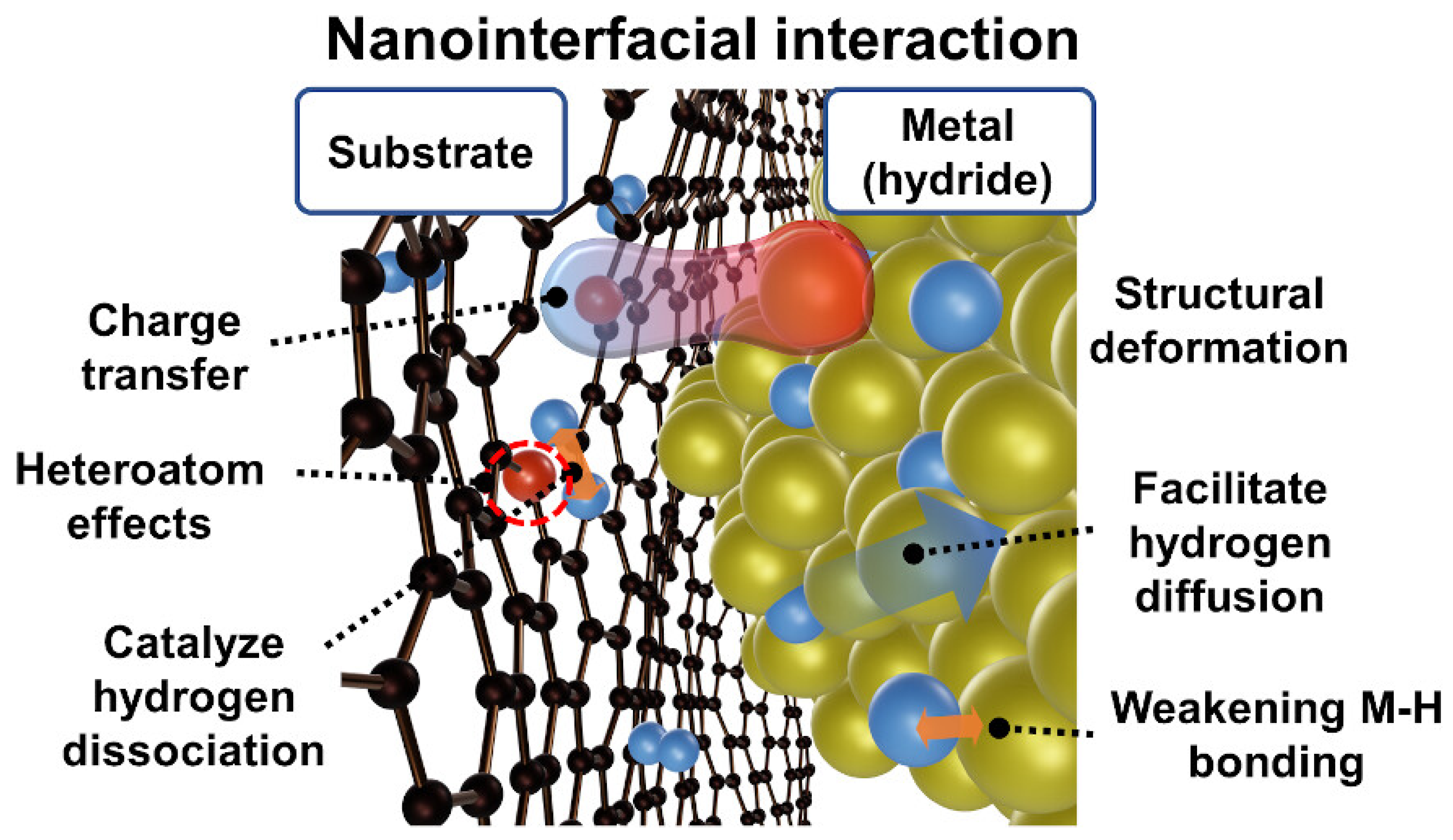
6. Materials for Solid Hydrogen Storage
6.1. Experimental Works on Solid Hydrogen Storage
6.2. Theoretical Works on Solid Hydrogen Storage
6.3. Utilization of Solid Stored Hydrogen
7. Current Challenges and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Mehr, A.S.; Phillips, A.D.; Brandon, M.P.; Pryce, M.T.; Carton, J.G. Recent challenges and development of technical and technoeconomic aspects for hydrogen storage, insights at different scales; A state of art review. Int. J. Hydrogen Energy 2024, 70, 786–815. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Kathalingam, A.; Jo, E.-B.; Sanmugam, A.; Jung, J.; Kim, H.-S. Engineering the active sites tuned MoS2 nanoarray structures by transition metal doping for hydrogen evolution and supercapacitor applications. J. Alloys Compd. 2022, 893, 162271. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Yang, O.; Yuan, W.; Liu, Y.; He, L.; Hu, Y.; Zhao, Z.; Zhou, L.; Wang, J.; et al. Self-Powered Seawater Electrolysis Based on a Triboelectric Nanogenerator for Hydrogen Production. ACS Nano 2022, 16, 15286–15296. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Y.; Zhang, J. A review of high density solid hydrogen storage materials by pyrolysis for promising mobile applications. Ind. Eng. Chem. Res. 2021, 60, 2737–2771. [Google Scholar] [CrossRef]
- Zacharia, R.; Rather, S.u. Review of Solid State Hydrogen Storage Methods Adopting Different Kinds of Novel Materials. J. Nanomater. 2015, 2015, 914845. [Google Scholar] [CrossRef]
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Ali Sheikh, Z.; Abbas, Z.; Aftab, S.; Nazir, G.; Kim, D.-K.; Kim, H.-S.; Jung, J. Experimental investigation on the electrodeposited nickel-based dichalcogenides for the efficient overall water splitting. Renew. Energy 2024, 228, 120645. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.a.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Rahim Malik, F.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar] [CrossRef]
- Habib, M.A.; Abdulrahman, G.A.Q.; Alquaity, A.B.S.; Qasem, N.A.A. Hydrogen combustion, production, and applications: A review. Alex. Eng. J. 2024, 100, 182–207. [Google Scholar] [CrossRef]
- Hassan, Q.; Tabar, V.S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. A review of green hydrogen production based on solar energy. Tech. Methods 2024, 11, 20220134. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review⁎. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Rohit, M.V.; Lee, J.-K. Recent Developments in Hydrogen Production, Storage, and Transportation: Challenges, Opportunities, and Perspectives. Fire 2024, 7, 233. [Google Scholar] [CrossRef]
- Sarmah, M.K.; Singh, T.P.; Kalita, P.; Dewan, A. Sustainable hydrogen generation and storage—A review. RSC Adv. 2023, 13, 25253–25275. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Shakeel, M.R.; Harale, A.; Paglieri, S.; Mansour, R.B. Fuel reforming processes for hydrogen production. Fuel 2024, 359, 130427. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Silva, J.; Rocha, C.; Soria, M.A.; Madeira, L.M. Catalytic Steam Reforming of Biomass-Derived Oxygenates for H2 Production: A Review on Ni-Based Catalysts. ChemEngineering 2022, 6, 39. [Google Scholar] [CrossRef]
- Ozcan, H.; El-Emam, R.S.; Amini Horri, B. Thermochemical looping technologies for clean hydrogen production—Current status and recent advances. J. Clean. Prod. 2023, 382, 135295. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, L.; Fan, C.; Liu, H. Advances in Whole-Cell Photobiological Hydrogen Production. Adv. NanoBiomed Res. 2021, 1, 2000051. [Google Scholar] [CrossRef]
- Gwon, H.J.; Park, G.; Yun, J.; Ryu, W.; Ahn, H.S. Prolonged hydrogen production by engineered green algae photovoltaic power stations. Nat. Commun. 2023, 14, 6768. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Amar Dubrovin, I.; Ouaknin Hirsch, L.; Rozenfeld, S.; Gandu, B.; Menashe, O.; Schechter, A.; Cahan, R. Hydrogen Production in Microbial Electrolysis Cells Based on Bacterial Anodes Encapsulated in a Small Bioreactor Platform. Microorganisms 2022, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.E.; Qaisi, R.M.; Logan, B.E.; Hussain, M.M. Energy harvesting from organic liquids in micro-sized microbial fuel cells. NPG Asia Mater. 2014, 6, e89. [Google Scholar] [CrossRef]
- Pathak, P.; Yadav, A.; Padmanaban, S. Transition Toward Emission-Free Energy Systems by 2050: Potential Role of Hydrogen. Int. J. Hydrogen Energy 2023, 48, 9921–9927. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Baiju, S.; Masuda, U.; Datta, S.; Tarefder, K.; Chaturvedi, J.; Ramakrishna, S.; Tripathi, L.N. Photo-electrochemical green-hydrogen generation: Fundamentals and recent developments. Int. J. Hydrogen Energy 2024, 51, 779–808. [Google Scholar] [CrossRef]
- Al-Saeedi, S.I. Photoelectrochemical Green Hydrogen Production Utilizing ZnO Nanostructured Photoelectrodes. Micromachines 2023, 14, 1047. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Holmes-Gentle, I.; Tembhurne, S.; Suter, C.; Haussener, S. Kilowatt-scale solar hydrogen production system using a concentrated integrated photoelectrochemical device. Nat. Energy 2023, 8, 586–596. [Google Scholar] [CrossRef]
- Hisatomi, T.; Takanabe, K.; Domen, K. Photocatalytic Water-Splitting Reaction from Catalytic and Kinetic Perspectives. Catal. Lett. 2015, 145, 95–108. [Google Scholar] [CrossRef]
- Boretti, A. Which thermochemical water-splitting cycle is more suitable for high-temperature concentrated solar energy? Int. J. Hydrogen Energy 2022, 47, 20462–20474. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Penconi, M.; Rossi, F.; Ortica, F.; Elisei, F.; Gentili, P.L. Hydrogen Production from Water by Photolysis, Sonolysis and Sonophotolysis with Solid Solutions of Rare Earth, Gallium and Indium Oxides as Heterogeneous Catalysts. Sustainability 2015, 7, 9310–9325. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Khan, M.A.; Ziani, A.A.; Idriss, H. An Overview of the Photocatalytic Water Splitting over Suspended Particles. Catalysts 2021, 11, 60. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A review of hydrogen production processes by photocatalytic water splitting—From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic water splitting: Advantages and challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Li, B.; Tian, Z.; Li, L.; Wang, Y.-H.; Si, Y.; Wan, H.; Shi, J.; Huang, G.-F.; Hu, W.; Pan, A.; et al. Directional Charge Transfer Channels in a Monolithically Integrated Electrode for Photoassisted Overall Water Splitting. ACS Nano 2023, 17, 3465–3482. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, D.; Sun, Z.; Irfan, R.M.; Zhang, L.; Du, P. Cobalt nitride as an efficient cocatalyst on CdS nanorods for enhanced photocatalytic hydrogen production in water. Catal. Sci. Technol. 2017, 7, 1515–1522. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Zheng, L.; Hou, J.; Zheng, H.; Sun, S.; Wang, L. Machine Learning Guided Dopant Selection for Metal Oxide-Based Photoelectrochemical Water Splitting: The Case Study of Fe2O3 and CuO. Adv. Mater. 2022, 34, 2106776. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdel-wahab, M.S.; Elfayoumi, M.A.K.; Tawfik, W.Z. Highly efficient sputtered Ni-doped Cu2O photoelectrodes for solar hydrogen generation from water-splitting. Int. J. Hydrogen Energy 2023, 48, 1863–1876. [Google Scholar] [CrossRef]
- Marschall, R. 50 Years of Materials Research for Photocatalytic Water Splitting. Eur. J. Inorg. Chem. 2021, 2021, 2435–2441. [Google Scholar] [CrossRef]
- Guo, L. Advances in solar hydrogen technologies. Sol. Altern. Energy 2011. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J.-X.; Bai, F.-Y.; Shen, Y.-S.; Liu, K.; Liu, J.; Chen, L.-H.; Li, Y.; Su, B.-L. In Situ Conversion of Ti3C2 MXene to Sandwich Ti3C2/R-TiO2 for Promoted Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2023, 6, 5456–5463. [Google Scholar] [CrossRef]
- Patil, R.P.; Mahadik, M.A.; Chae, W.-S.; Choi, S.H.; Jang, J.S. Porous Zn1–xCdxSe/ZnO Nanorod Photoanode Fabricated from ZnO Building Blocks Grown on Zn Foil for Photoelectrochemical Solar Hydrogen Production. ACS Appl. Mater. Interfaces 2023, 15, 37361–37370. [Google Scholar] [CrossRef]
- Song, Z.; Li, C.; Chen, L.; Dolia, K.; Fu, S.; Sun, N.; Li, Y.; Wyatt, K.; Young, J.L.; Deutsch, T.G.; et al. All-Perovskite Tandem Photoelectrodes for Unassisted Solar Hydrogen Production. ACS Energy Lett. 2023, 8, 2611–2619. [Google Scholar] [CrossRef]
- Zhang, D.; De Santiago, H.A.; Xu, B.; Liu, C.; Trindell, J.A.; Li, W.; Park, J.; Rodriguez, M.A.; Coker, E.N.; Sugar, J.D.; et al. Compositionally Complex Perovskite Oxides for Solar Thermochemical Water Splitting. Chem. Mater. 2023, 35, 1901–1915. [Google Scholar] [CrossRef]
- Fehr, A.M.K.; Deutsch, T.G.; Toma, F.M.; Wong, M.S.; Mohite, A.D. Technoeconomic Model and Pathway to <$2/kg Green Hydrogen Using Integrated Halide Perovskite Photoelectrochemical Cells. ACS Energy Lett. 2023, 8, 4976–4983. [Google Scholar] [CrossRef]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and Photoelectrocatalytic Water Splitting by Porous g-C3N4 Nanosheets for Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 12656–12665. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Nath, S.; Bapat, R.D.; Sharma, R.K.; Jagannath; Dutta, B.; Hassan, P.A.; Tripathi, A.K. Superior Interfacial Contact Yields Efficient Electron Transfer Rate and Enhanced Solar Photocatalytic Hydrogen Generation in M/C3N4 Schottky Junctions. ACS Appl. Mater. Interfaces 2023, 15, 39926–39945. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Liu, S.; Qi, W.; Liu, J.; Meng, X.; Adimi, S.; Attfield, J.P.; Yang, M. Modulating Electronic Structure to Improve the Solar to Hydrogen Efficiency of Cobalt Nitride with Lattice Doping. ACS Catal. 2023, 13, 2214–2222. [Google Scholar] [CrossRef]
- Gupta, U.; Naidu, B.S.; Maitra, U.; Singh, A.; Shirodkar, S.N.; Waghmare, U.V.; Rao, C.N.R. Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater. 2014, 2, 092802. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, X.; Zhang, M.; Wu, X.; Chen, R.; Zhang, C.; Sun, C.; Du, Y.; Xu, Q.-H.; Hu, B. Construction of Covalent Organic Frameworks with Partly Condensed Networks for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2023, 6, 9427–9433. [Google Scholar] [CrossRef]
- Li, L.; Deng, Z.; Yu, L.; Lin, Z.; Wang, W.; Yang, G. Amorphous transitional metal borides as substitutes for Pt cocatalysts for photocatalytic water splitting. Nano Energy 2016, 27, 103–113. [Google Scholar] [CrossRef]
- Ramírez, A.M.R.; Heidari, S.; Vergara, A.; Aguilera, M.V.; Preuss, P.; Camarada, M.B.; Fischer, A. Rhenium-Based Electrocatalysts for Water Splitting. ACS Mater. Au 2023, 3, 177–200. [Google Scholar] [CrossRef]
- Zhu, T.; Han, J.; Sun, T.; Chen, J.; Wang, S.; Ren, S.; Pi, X.; Xu, J.; Chen, K. Interface-Enhanced SiOx/Ru Heterocatalysts for Efficient Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 8200–8207. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Lee, J.; Kim, Y.S.; Song, J.; Oh, J.; Lee, S.M.; Jeong, M.; Kim, Y.; Kwak, J.H.; Cho, S.; et al. High-performance and stable photoelectrochemical water splitting cell with organic-photoactive-layer-based photoanode. Nat. Commun. 2020, 11, 5509. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Chen, W.T.; Hsu, W.-L.; Chen, C.K.; Cheng, H.W.H.; Chan, T.-S.; Huang, D.-W.; Liu, R.-S.; Tsai, D.P. Plasmonic zinc oxide/silver photoelectrode for green hydrogen production. SPIE Int. Soc. Opt. Photonics 2013, 20. [Google Scholar] [CrossRef]
- Kathalingam, A.; Kim, H.-S. Coulomb blockade and plasmonic nanoantenna effect in back gated ZnO nanorod FET. Optik 2016, 127, 5226–5229. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Yang, Y.; Li, Y.; Wang, C.-G.; Gu, C.; Wu, L.; Hu, J.; Zhu, J.; Yu, S.-H. Near-Infrared-Active Periodic Plasmonic Heterostructures Enable High-Efficiency Photoelectrochemical Hydrogen Production. Chem. Mater. 2023, 35, 5822–5831. [Google Scholar] [CrossRef]
- Kotkondawar, A.V.; Moinuddin, A.A.; Rayalu, S.S. Plasmon-Induced and Multichannel Electron Transfer-Improved Photocatalytic Hydrogen Production by CdS–Au–Pt Heterostructure. ACS Appl. Energy Mater. 2023, 6, 7692–7701. [Google Scholar] [CrossRef]
- Joshi, K.; Mistry, K.; Tripathi, B.; Chandra, P.; Shinde, S.M.; Kumar, M.; Santola, D.; Chokshi, H.; Gurrala, P. MoS2 Nanostructures for Solar Hydrogen Generation via Membraneless Electrochemical Water Splitting. ACS Appl. Electron. Mater. 2023, 5, 1461–1470. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. PtTe2/Sb2S3 Nanoscale Heterostructures for the Photocatalytic Direct Z-Scheme with High Solar-to-Hydrogen Efficiency: A Theoretical Investigation. ACS Appl. Nano Mater. 2023, 6, 5591–5601. [Google Scholar] [CrossRef]
- You, J.; Liu, Z.; Guo, Z.; Ruan, M.; Yan, W. Doping of W Ions to Modulate the Polarization Intensity of Bi2WO6 for Efficient Piezoelectric–Photoelechochemical Water Splitting. ACS Appl. Energy Mater. 2022, 5, 11472–11482. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, G.; Bae, S.Y.; Kim, H.J.; Lee, D.H.; Ko, S.; Kim, S.-K.; Lee, G.; You, H.R.; Choi, H.; et al. Ecofriendly AgBiS2 Nanocrystal Photoanode for Highly Efficient Visible-Light-Driven Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2023, 6, 3872–3880. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Dong, L.; Mu, H.; Li, D.; Zhang, F.; Xu, H.; Xie, H. Enhancement Mechanism of Photocatalytic Hydrogen Production Activity of CeO2/CdS by Morphology Regulation. ACS Appl. Energy Mater. 2023, 6, 7722–7736. [Google Scholar] [CrossRef]
- Pihosh, Y.; Nandal, V.; Shoji, R.; Bekarevich, R.; Higashi, T.; Nicolosi, V.; Matsuzaki, H.; Seki, K.; Domen, K. Nanostructured Tantalum Nitride for Enhanced Solar Water Splitting. ACS Energy Lett. 2023, 8, 2106–2112. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Nann, T.; Voelcker, N.H. Nanostructured silicon photoelectrodes for solar water electrolysis. Nano Energy 2015, 17, 308–322. [Google Scholar] [CrossRef]
- Gil-Rostra, J.; Castillo-Seoane, J.; Guo, Q.; Jorge Sobrido, A.B.; González-Elipe, A.R.; Borrás, A. Photoelectrochemical Water Splitting with ITO/WO(3)/BiVO(4)/CoPi Multishell Nanotubes Enabled by a Vacuum and Plasma Soft-Template Synthesis. ACS Appl. Mater. Interfaces 2023, 15, 9250–9262. [Google Scholar] [CrossRef]
- Wang, H.; Gao, R.-T.; Nguyen, N.T.; Bai, J.; Ren, S.; Liu, X.; Zhang, X.; Wang, L. Superhydrophilic CoFe Dispersion of Hydrogel Electrocatalysts for Quasi-Solid-State Photoelectrochemical Water Splitting. ACS Nano 2023, 17, 22071–22081. [Google Scholar] [CrossRef]
- Zhang, P.; Huo, Y.; Wang, F.; Fang, F.; Sun, D. Direct Electrochemical Seawater Splitting for Green Hydrogen and Artificial Reefs. ACS Appl. Energy Mater. 2023, 6, 7636–7642. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, Y.; He, X.; Fu, J.; Liu, J. Hierarchical Waterweed-like Photoanodes for Sustainable Photoelectrochemical Hydrogen Production. ACS Appl. Energy Mater. 2023, 6, 3460–3467. [Google Scholar] [CrossRef]
- Jia, X.; Kang, H.; Hou, G.; Lu, S.; Yao, Y.; Wang, Q.; Qin, W.; Wu, X. Coupling Ferrocyanide-Assisted PW/PB Redox with Efficient Direct Seawater Electrolysis for Hydrogen Production. ACS Catal. 2023, 13, 3692–3701. [Google Scholar] [CrossRef]
- Wang, G.; Yang, X.; Qian, F.; Zhang, J.Z.; Li, Y. Double-Sided CdS and CdSe Quantum Dot Co-Sensitized ZnO Nanowire Arrays for Photoelectrochemical Hydrogen Generation. Nano Lett. 2010, 10, 1088–1092. [Google Scholar] [CrossRef]
- Brinkman, L.; Bulfin, B.; Steinfeld, A. Thermochemical Hydrogen Storage via the Reversible Reduction and Oxidation of Metal Oxides. Energy Fuels 2021, 35, 18756–18767. [Google Scholar] [CrossRef]
- Chu, C.; Wu, K.; Luo, B.; Cao, Q.; Zhang, H. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology—A review. Carbon Resour. Convers. 2023, 6, 334–351. [Google Scholar] [CrossRef]
- Carvalho, M.C.S.; Marques, J.; Matos, H.A.; Granjo, J.F.O. Long-distance hydrogen delivery using a toluene-based liquid organic hydrogen carrier system. In Computer Aided Chemical Engineering; Türkay, M., Gani, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 50, pp. 1647–1652. [Google Scholar]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Lo Russo, S. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Dun, C.; Jeong, S.; Kwon, D.-H.; Kang, S.; Stavila, V.; Zhang, Z.; Lee, J.-W.; Mattox, T.M.; Heo, T.W.; Wood, B.C.; et al. Hydrogen Storage Performance of Preferentially Oriented Mg/rGO Hybrids. Chem. Mater. 2022, 34, 2963–2971. [Google Scholar] [CrossRef]
- Eno, E.A.; Louis, H.; Akpainyang, P.S.; Ikenyirimba, O.J.; Unimuke, T.O.; Offiong, O.E.; Adeyinka, A.S. Molecular Modeling of Cu-, Ag-, and Au-Decorated Aluminum Nitride Nanotubes for Hydrogen Storage Application. ACS Appl. Energy Mater. 2023, 6, 4437–4452. [Google Scholar] [CrossRef]
- Peng, Q.; Dearden, A.K.; Crean, J.; Han, L.; Liu, S.; Wen, X.; De, S. New materials graphyne, graphdiyne, graphone, and graphane: Review of properties, synthesis, and application in nanotechnology. Nanotechnol. Sci. Appl. 2014, 7, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, M.; Oh, H.; Lee, K. Electrochemical anodic formation of VO 2 nanotubes and hydrogen sorption property. J. Electrochem. Sci. Technol. 2020, 12, 212–216. [Google Scholar] [CrossRef]
- Cho, Y.; Cho, H.; Cho, E.S. Nanointerface engineering of metal hydrides for advanced hydrogen storage. Chem. Mater. 2023, 35, 366–385. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Zheng, J.; Li, X.; Akiba, E.; Li, H.-W. Perspectives and challenges of hydrogen storage in solid-state hydrides. Chin. J. Chem. Eng. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Salman, M.S.; Zubair, M.; Yang, Y.; Bedford, N.M.; Aguey-Zinsou, K.-F. Doping and Structure-Promoted Destabilization of NaBH4 Nanocubes for Hydrogen Storage. ACS Appl. Nano Mater. 2023, 6, 4178–4189. [Google Scholar] [CrossRef]
- Berlouis, L.E.; Jubin, C.; McMillan, B.G.; Morrow, J.; Spicer, M.D.; Tang, L.P.; Bordelanne, O.; Weston, M. Enhanced hydrogen storage in Ni/Ce composite oxides. Phys. Chem. Chem. Phys. 2007, 9, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Li, Y.; Liang, F.; Liu, B.; Liu, W.; Wang, Q.; Wang, L. Highly efficient hydrogen storage capacity of 2.5 wt% above 0.1 MPa using Y and Cr codoped V-based alloys. ACS Appl. Energy Mater. 2022, 5, 3282–3289. [Google Scholar] [CrossRef]
- Shashikala, K.; Kumar, A.; Betty, C.A.; Banerjee, S.; Sengupta, P.; Pillai, C.G.S. Improvement of the hydrogen storage properties and electrochemical characteristics of Ti0.85VFe0.15 alloy by Ce substitution. J. Alloys Compd. 2011, 509, 9079–9083. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Stock, S.; Kostoglou, N.; Selinger, J.; Spirk, S.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; Rebholz, C.; Mitterer, C.; Paris, O. Coffee waste-derived nanoporous carbons for hydrogen storage. ACS Appl. Energy Mater. 2022, 5, 10915–10926. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, Y.-H.; Dillon, A.; Heben, M.; Zhang, S. Hydrogen storage in novel organometallic buckyballs. Phys. Rev. Lett. 2005, 94, 155504. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jena, P.; Wang, Q.; Marquez, M. First-principles study of hydrogen storage on Li12C60. J. Am. Chem. Soc. 2006, 128, 9741–9745. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Aminorroaya-Yamini, S.; Nevirkovets, I.; Konstantinov, K.; Liu, H.K. Enhanced hydrogen storage in graphene oxide-MWCNTs composite at room temperature. Adv. Energy Mater. 2012, 2, 1439–1446. [Google Scholar] [CrossRef]
- Balderas-Xicohtencatl, R.; Villajos, J.A.; Casabán, J.; Wong, D.; Maiwald, M.; Hirscher, M. ZIF-8 pellets as a robust material for hydrogen cryo-adsorption tanks. ACS Appl. Energy Mater. 2023, 6, 9145–9152. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Zhang, X.; Zhu, L. Hydrogen Storage on Metal-Coated B80 Buckyballs with Density Functional Theory. J. Phys. Chem. C 2009, 113, 7052–7057. [Google Scholar] [CrossRef]
- Jaiswal, A.; Chakraborty, B.; Sahu, S. A Computational Insight on the Effect of Encapsulation and Li Functionalization on Si12C12 Heterofullerene for H2 Adsorption: A Strategy for Effective Hydrogen Storage. ACS Appl. Energy Mater. 2023, 6, 3374–3389. [Google Scholar] [CrossRef]
- Jiang, Q.; Bai, X.; Jia, Z.; Lu, S.; Song, P.; Chen, Y.; Shan, P.; Cui, H.; Feng, R.; Kang, Q.; et al. Density Functional Theory Study of Superalkali NLi4-Decorated Graphdiyne Nanosheets as Hydrogen Storage Materials. ACS Appl. Nano Mater. 2023, 6, 14063–14075. [Google Scholar] [CrossRef]
- Morita, K.; Tsuchiya, B. A mechanism for water splitting and hydrogen absorbing functions of metal–oxide layered hydrogen storage materials studied by means of ion beam analysis. Surf. Interface Anal. 2014, 46, 113–127. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Islam, N.; Pandith, A.H. Theoretical investigations on C2H4Nb complex as a potential hydrogen storage system, using moller–plesset (MP2) and density functional theory. Int. J. Quantum Chem. 2014, 114, 449–457. [Google Scholar] [CrossRef]
- Bai, H.; Bai, B.; Zhang, L.; Huang, W.; Mu, Y.-W.; Zhai, H.-J.; Li, S.-D. Lithium-Decorated Borospherene B40: A Promising Hydrogen Storage Medium. Sci. Rep. 2016, 6, 35518. [Google Scholar] [CrossRef]
- German, E.; Sandoval, J.; Recio, A.; Seif, A.; Alonso, J.A.; López, M.J. Supported Metal Nanohydrides for Hydrogen Storage. Chem. Mater. 2023, 35, 1134–1147. [Google Scholar] [CrossRef]
- Desales-Guzmán, L.A.; Vazquez-Rivas, E.; Pacheco Sánchez, J.H. Ab Initio Study on the Potential of Metal-Decorated Pristine and Activated Carbyne for Hydrogen Storage Applications. Energy Fuels 2023, 37, 12400–12415. [Google Scholar] [CrossRef]
- Arzac, G.M.; Calvo, M.E.; Fernández, A. Understanding the Problem of Hydrogen Storage Using a Demonstration: Coupling a Hydrogen Generator Based on the Hydrolysis of Sodium Borohydride to a Fuel-Cell Kit. J. Chem. Educ. 2023, 100, 4554–4558. [Google Scholar] [CrossRef]
- Karim, S.; Faissal, A.; Noureddine, E.B. Chapter 27—National Renewable Energy Laboratory. In Handbook of Algal Biofuels; El-Sheekh, M., Abomohra, A.E.-F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 599–613. [Google Scholar]
- Flores, F.; Feijoo, F.; DeStephano, P.; Herc, L.; Pfeifer, A.; Duić, N. Assessment of the impacts of renewable energy variability in long-term decarbonization strategies. Appl. Energy 2024, 368, 123464. [Google Scholar] [CrossRef]
- Carrington, G.; Stephenson, J. The politics of energy scenarios: Are International Energy Agency and other conservative projections hampering the renewable energy transition? Energy Res. Soc. Sci. 2018, 46, 103–113. [Google Scholar] [CrossRef]
- Liu, B.; Lai, M.; Wang, Y.; Wang, Y.; Chen, J.; Song, C. Assessment of green hydrogen production by volatile renewable energy under different SSPs scenarios in China. Renew. Energy 2024, 235, 121296. [Google Scholar] [CrossRef]


| S. No | Material/ Method | Light Source | STH Conversion Efficiency (AQE)/ H2 Yield/Photocurrent Density | Ref |
|---|---|---|---|---|
| 1 | FACsPb(IBr)3 Perovskite | AM1.5G | photocurrent density = 12.5 mA cm−2 AQE = 15% | [60] |
| 2 | NiCoB/CdS | 300 W Xe lamp | AQE = 97.42% H2 yield = 144.8 mmol h−1 g−1 | [69] |
| 3 | Co3N onto CdS NRs | 300 W Xe lamp | AQE = ∼14.9% H2 yield = 137.33 μmol h−1 mg−1 | [51] |
| 4 | Rh/RGO | Visible light | AQE = 79.3% H2 yield = 98.1 mmol h−1 g−1 | [68] |
| 5 | Thiol ligand-AgBiS2 NC | AM1.5G | AQE = 67% | [80] |
| 6 | CeO2/CdS | 300 W Xe lamp | 444 μmol g−1 h−1 | [81] |
| 7 | Nano-g-C3N4/ Cu dendrites | AM1.5G | H2 yield = 59.2 μmol/cm2 | [88] |
| 8 | Ni-doped Cu2O | 400 W Xe lamp | photocurrent density = 5.72 mA cm2 | [55] |
| 9 | Ti3C2/R-TiO2 | 300 W Xe lamp | H2 yield = 1.62 mmol g−1 h−1 | [58] |
| 10 | V−CoN-Eosin-Y | - | AQE = 38% H2 yield = 21.21 μmol mg−1 h−1 | [66] |
| 11 | Ta3N5 NRs | AM1.5G | photocurrent density = 10.96 mA cm−2 | [82] |
| 12 | Bi3(SenTe1−n)2 | - | AQE = 22% photocurrent density = 13.8 mA cm−2 | [75] |
| 13 | Pt/Au/CdS | Mercury lamp | AQE = 4.20% H2 yield = 15 mmol h−1 g−1 | [76] |
| 14 | Zn1−xCdxSe/ ZnO NR | 300 W XE lamp | H2 yield = 199 μmol cm−2/3 h photocurrent density = 7.8 mA cm−2 | [59] |
| 15 | Bi2YO4Cl | AM 1.5G | AQE = 2.52% photocurrent density = ∼1.57 mA cm−2 | [58] |
| 16 | LaSrMn/ FCoAlO3 | - | H2 yield = 89.97 mmol h−1 g−1 | [61] |
| 17 | [Fe(CN)6]3 | - | photocurrent density = 320 mA cm−2 | [89] |
| 18 | CdS/CdSe (QD)-ZnO NWs | - | AQE = ∼45% photocurrent density = ∼12 mA cm−2 | [90] |
| 19 | NiFe-LDHs | AM1.5 G | AQE = 4.33% photocurrent density = 15.1 mA cm−2 | [72] |
| 20 | Bi3(SenTe1−n)2 ternary alloy | - | AQE = 22% photocurrent density = 13.8 mA cm−2 | [75] |
| 21 | CoFe-PAM/ BiVO4 | AM 1.5G | photocurrent density = 5.7 mA cm−2 | [85] |
| S. No | Materials/Method | Storage Capacity | H2-Releasing Temperature | Ref |
|---|---|---|---|---|
| 1 | Pt/Li2ZrO3/Pt/ | 5 wt% | 573 K | [111] |
| 2 | Vanadium doped-Sodium borohydride (NaBH4)/experimental | Releasing 5.3 mass% H2 | 355 °C | [102] |
| 3 | Aligned structures of GO–MWCNT composite | Up to 2.6 wt% | Room temperature | [110] |
| 4 | Ti−V−Mn−Cr−Y alloys | 2.53 wt% hydrogen capacity | 423 K | [104] |
| 5 | Ni/Ce composite | 0.24 wt% | 327 C | [103] |
| S. No | Materials/Method | Storage Capacity | H2-Releasing Temperature | Ref |
|---|---|---|---|---|
| 1 | Li atom-decorated Ar@Si12C12 cages/DFT Modeling | Gravimetric density of 9.7 wt% | 100−120 K | [113] |
| 2 | Activated carbyne/ DFT Modeling | 9 to 15 wt% | Near ambient conditions | [116] |
| 3 | NLi4-decorated graphdiyne nanosheets/ DFT Modeling | 8.91 wt%, | - | [114] |
| 4 | Preferentially oriented Mg/rGO Hybrids | 6.2 wt% | - | [96] |
| 5 | Lithium-decorated Borosphere Ti-B40-nH2 | 13.8 wt% | - | [117] |
| 6 | Cu-, Ag-, and Au-decorated aluminum nanotubes | 5.8 wt% | - | [97] |
| 7 | Metal-coated B80 Buckyballs (Ca12B80) | 9.0 wt% | - | [112] |
| 8 | Chemically activated carbyne (YC12-7H2) | 9 to 15 wt% | Room temperature | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaikalam, K.; Vikraman, D.; Karuppasamy, K.; Kim, H.-S. Solar Hydrogen Production and Storage in Solid Form: Prospects for Materials and Methods. Nanomaterials 2024, 14, 1560. https://doi.org/10.3390/nano14191560
Adaikalam K, Vikraman D, Karuppasamy K, Kim H-S. Solar Hydrogen Production and Storage in Solid Form: Prospects for Materials and Methods. Nanomaterials. 2024; 14(19):1560. https://doi.org/10.3390/nano14191560
Chicago/Turabian StyleAdaikalam, Kathalingam, Dhanasekaran Vikraman, K. Karuppasamy, and Hyun-Seok Kim. 2024. "Solar Hydrogen Production and Storage in Solid Form: Prospects for Materials and Methods" Nanomaterials 14, no. 19: 1560. https://doi.org/10.3390/nano14191560









