Thermo- and Photoresponsive Smart Nanomaterial Based on Poly(diethyl vinyl phosphonate)-Capped Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Procedures
2.1.1. General Procedure for the Synthesis of Poly(diethyl vinyl phosphonate) (Referred to in Entry 1 of Table 1)
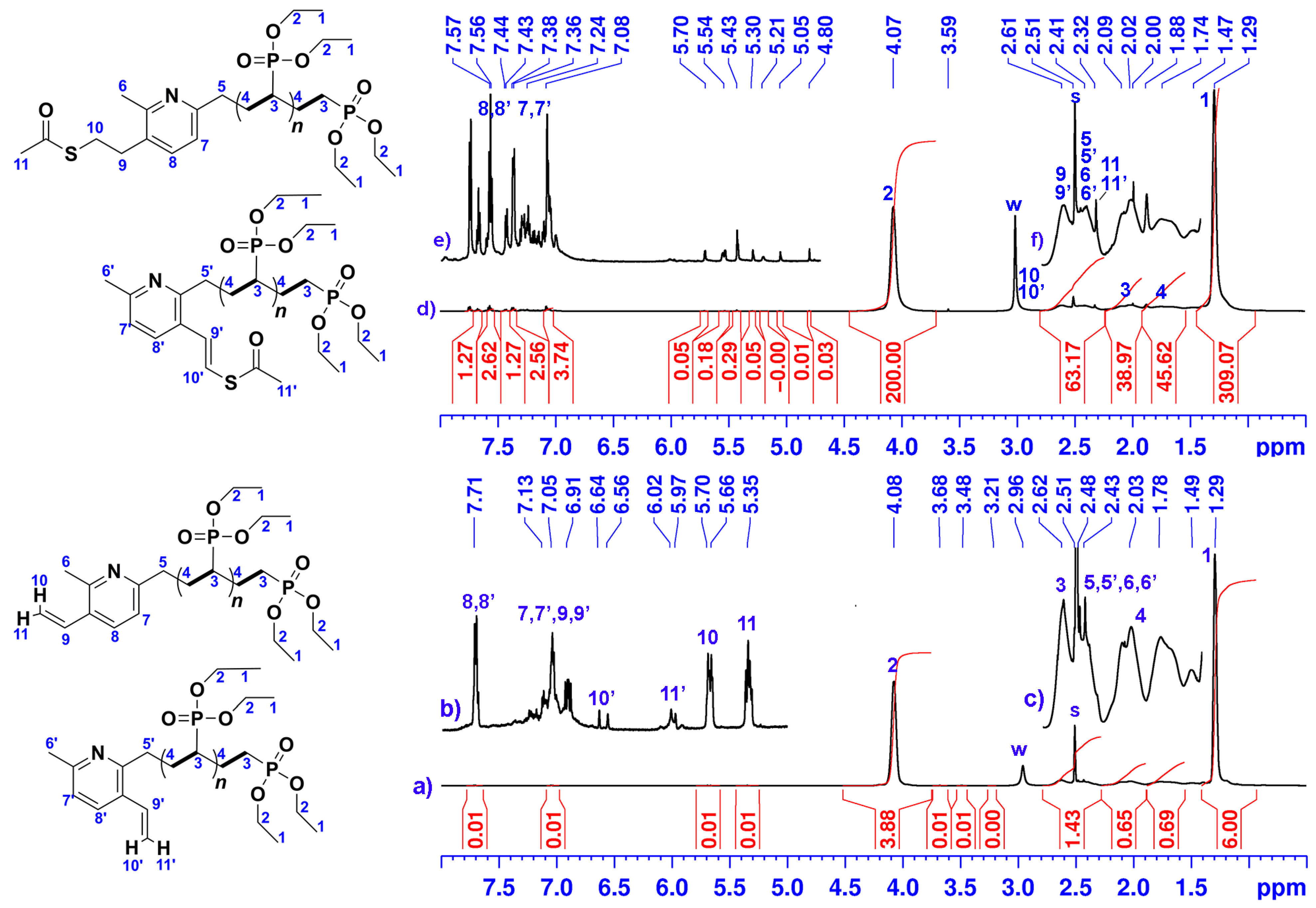
2.1.2. Thioacetylation of PDEVP
2.1.3. In Situ Synthesis of Thiolated-PDEVP and Formation of PDEVP-Coated Gold Nanoparticles
2.1.4. Cell Cultures
2.2. Instrumentation and Analytical Methods
2.3. Computational Details
2.4. Phototheral Effect
3. Results and Discussion
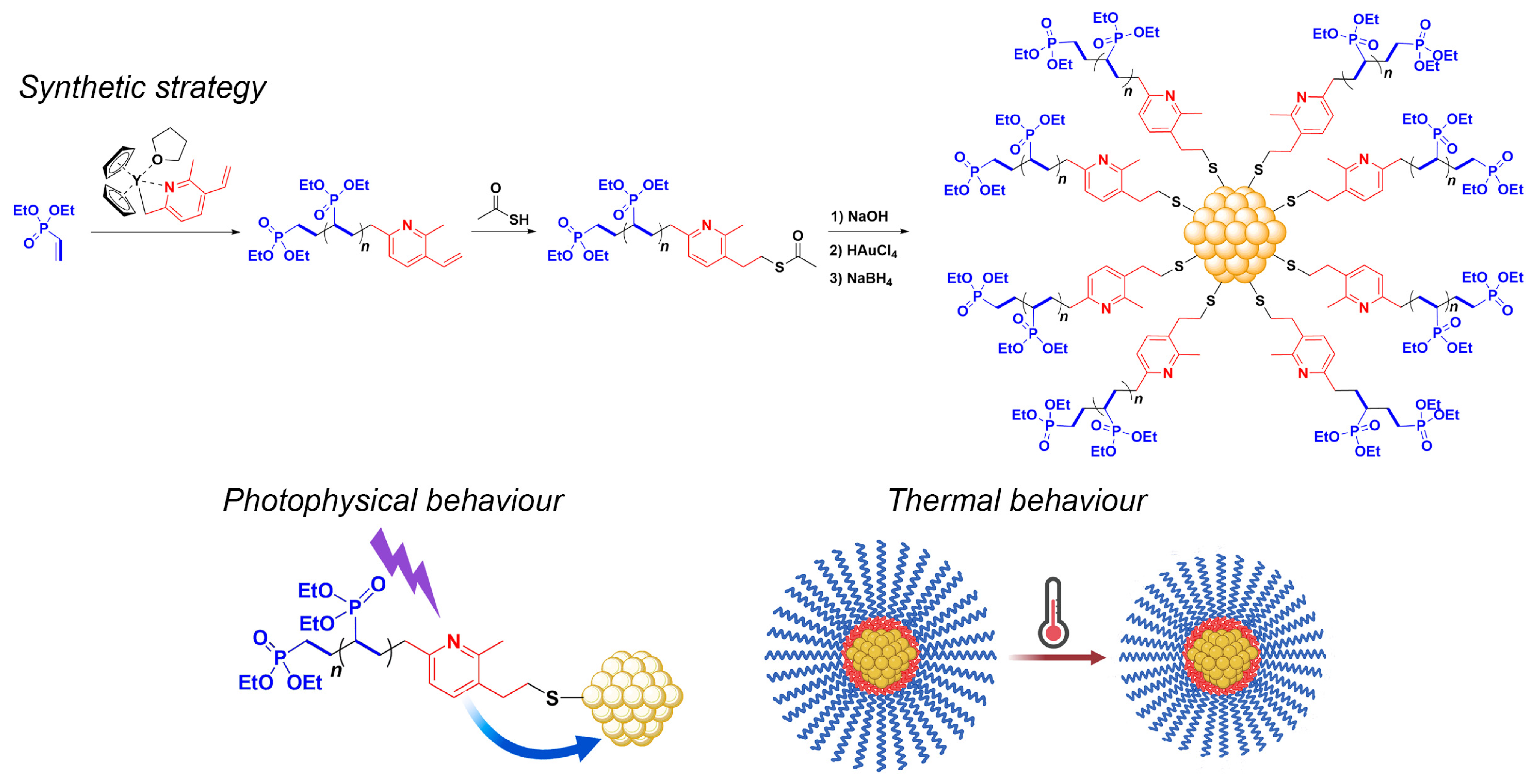
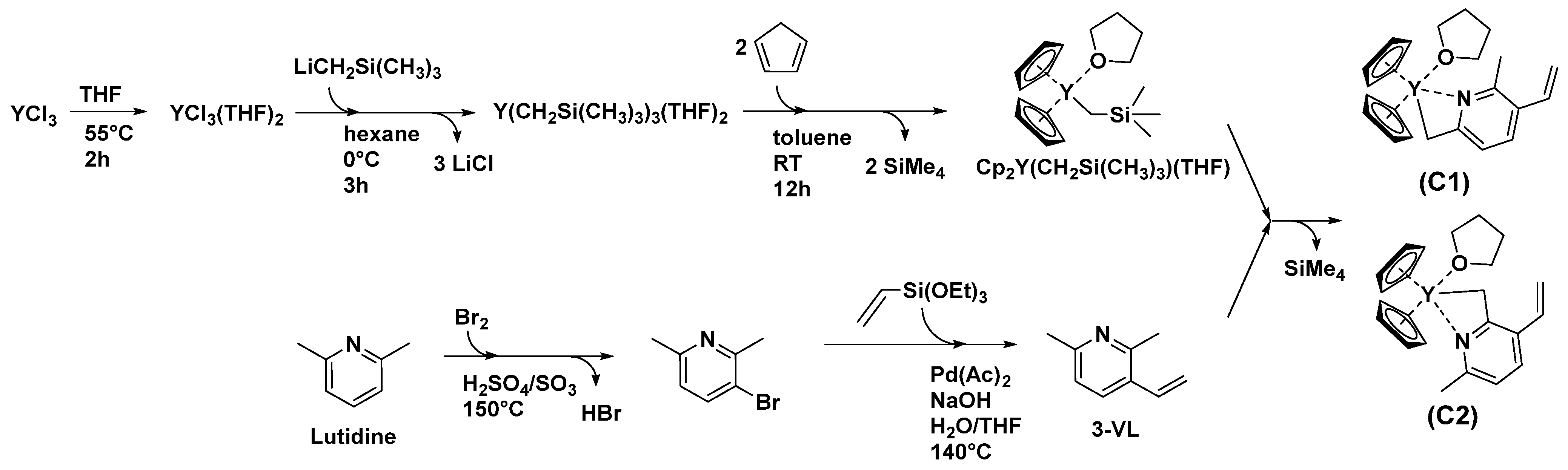
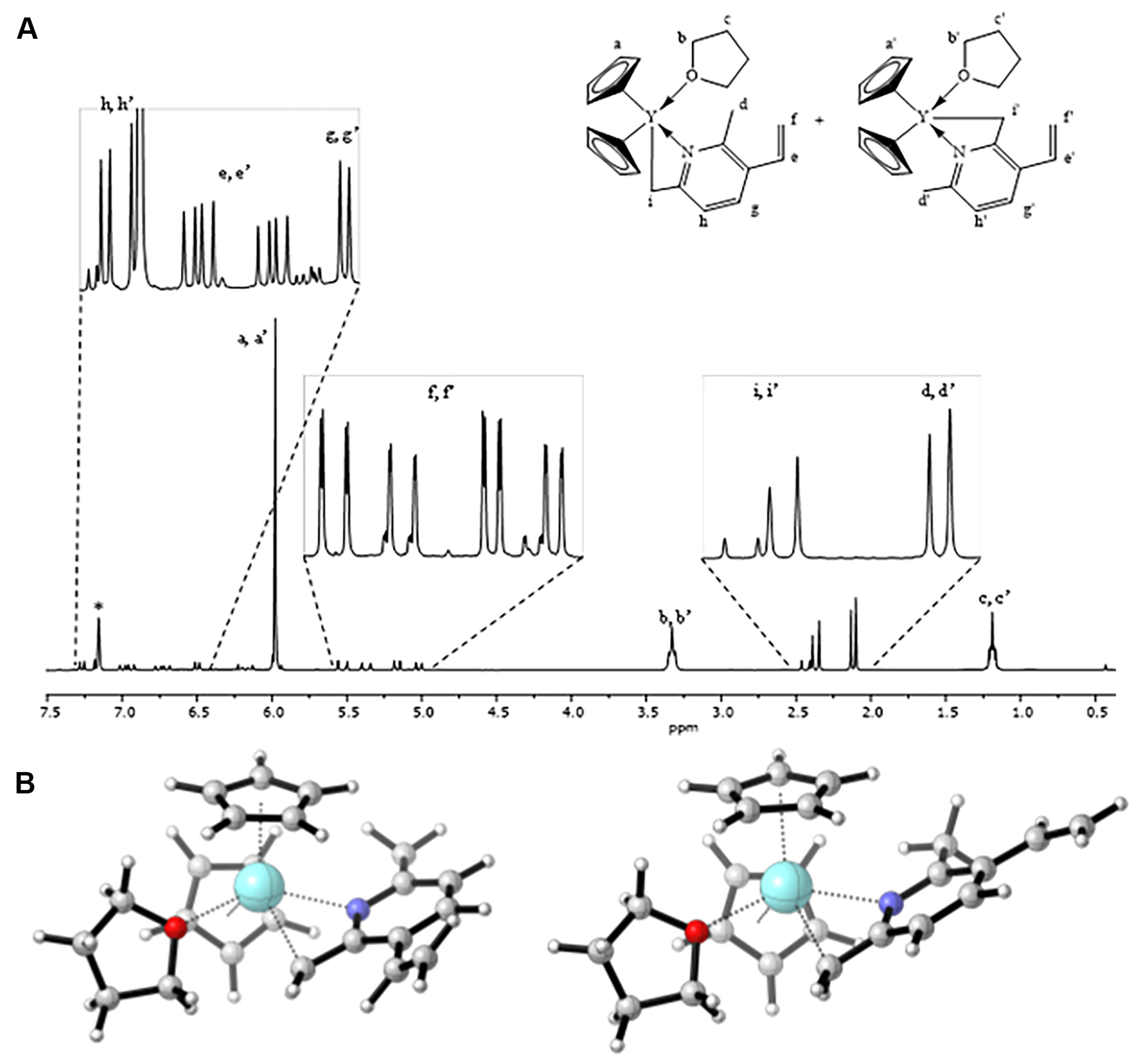
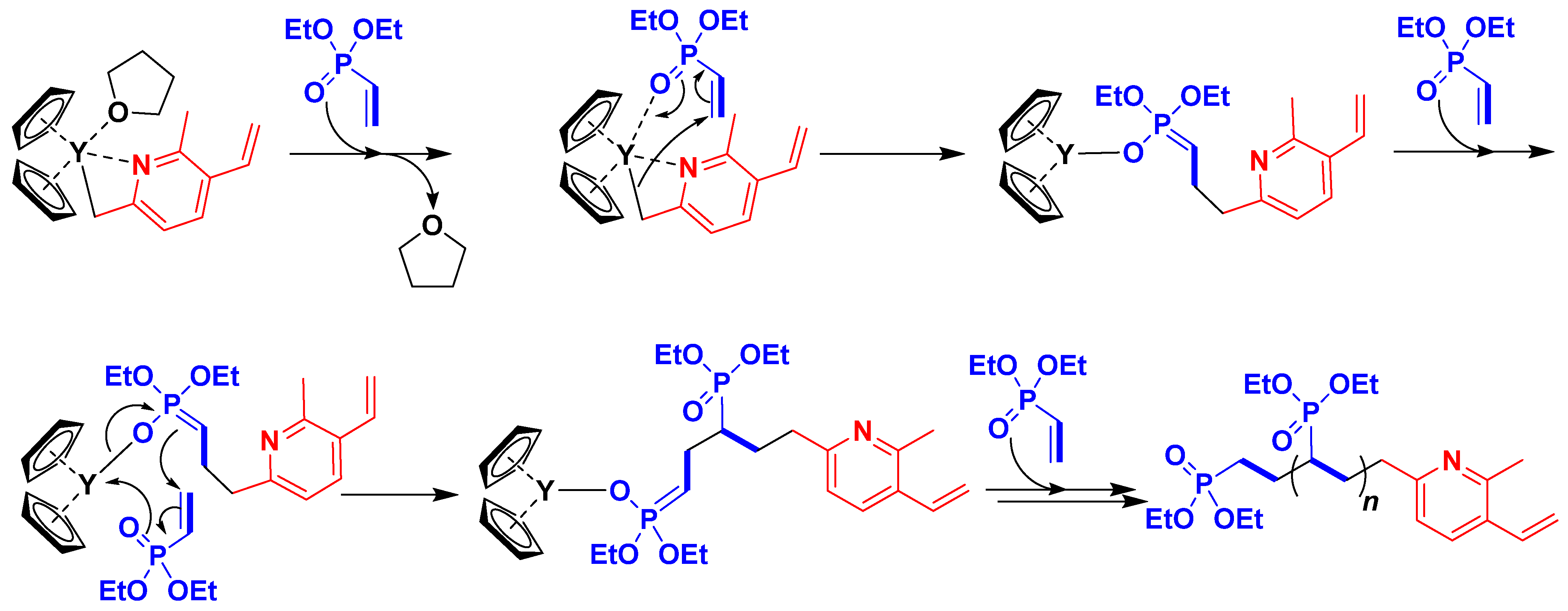
3.1. Synthesis of the Yttriocene Catalysts C1 and C2
3.2. Synthesis of PDEVPs End-Capped with Lutidine
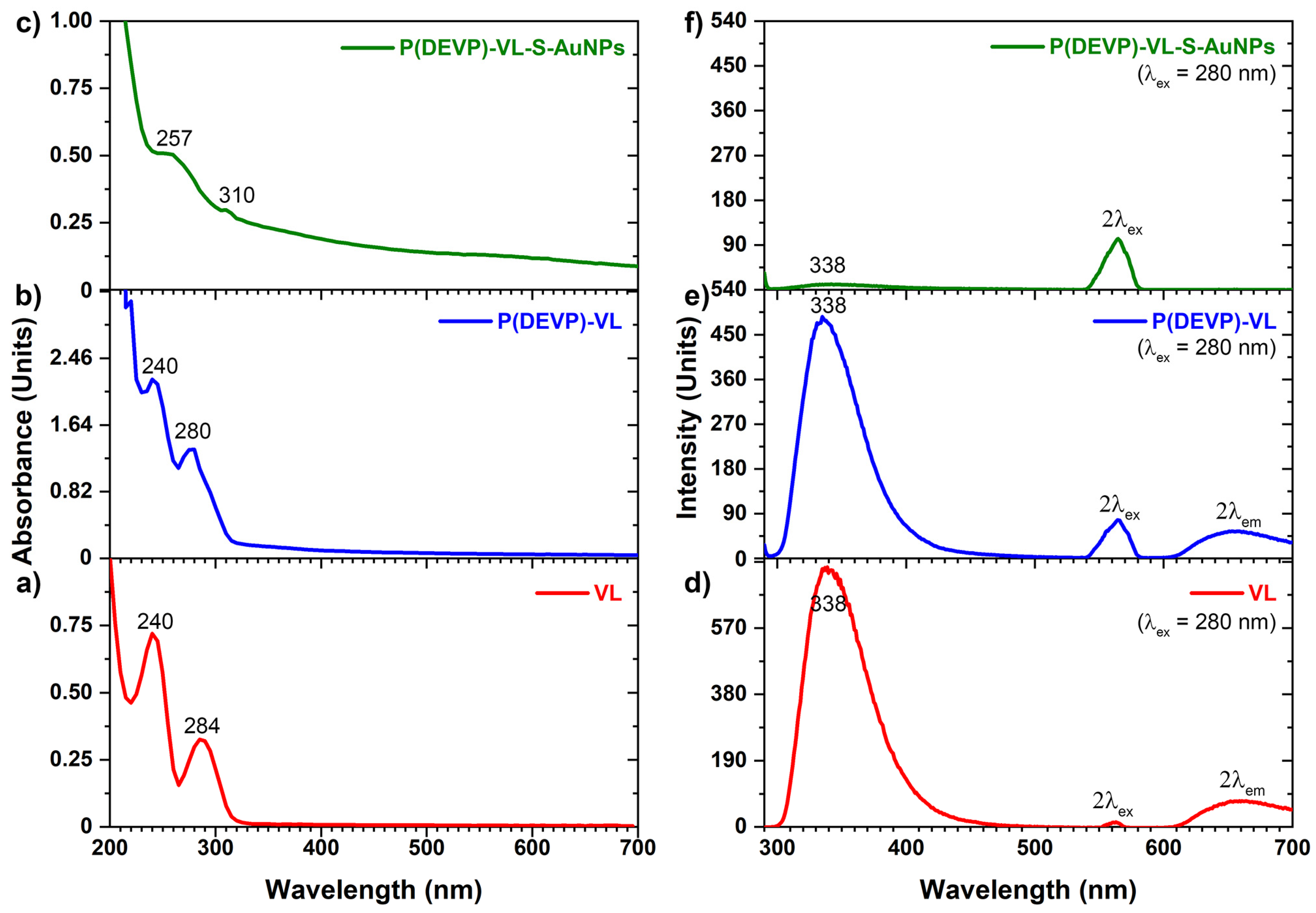
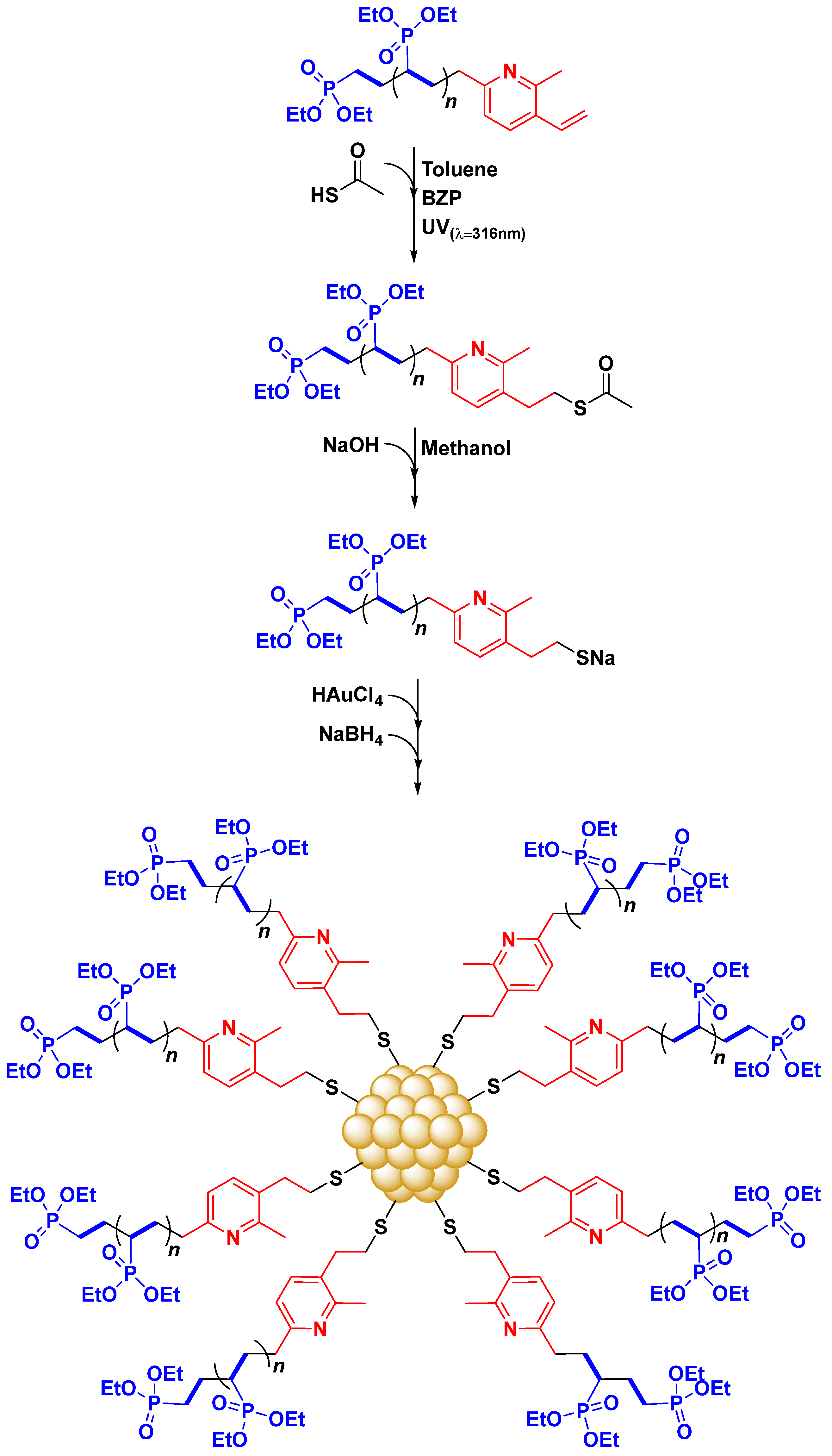
3.3. Synthesis of PVDEP-VL-Capped AuNPs
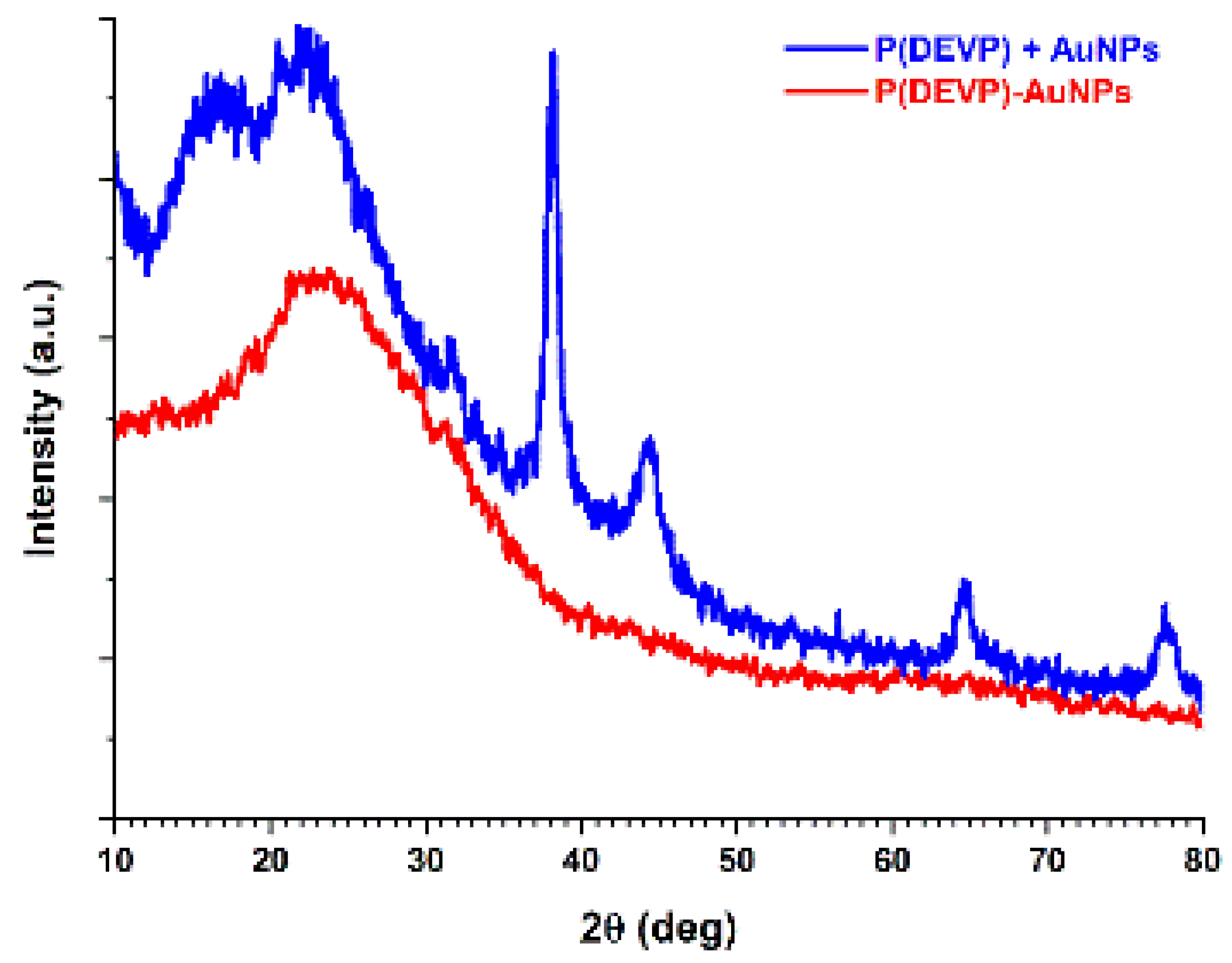
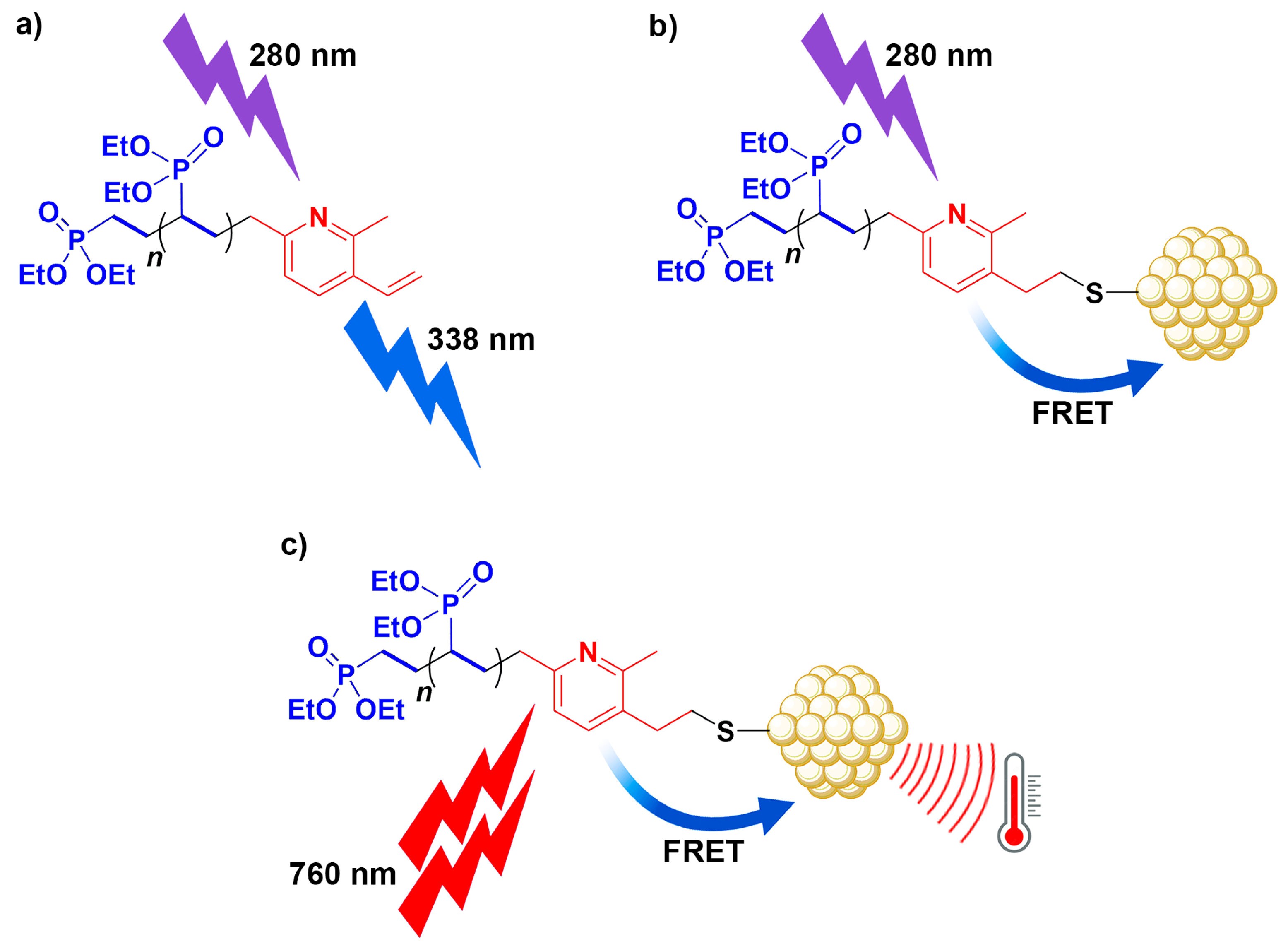
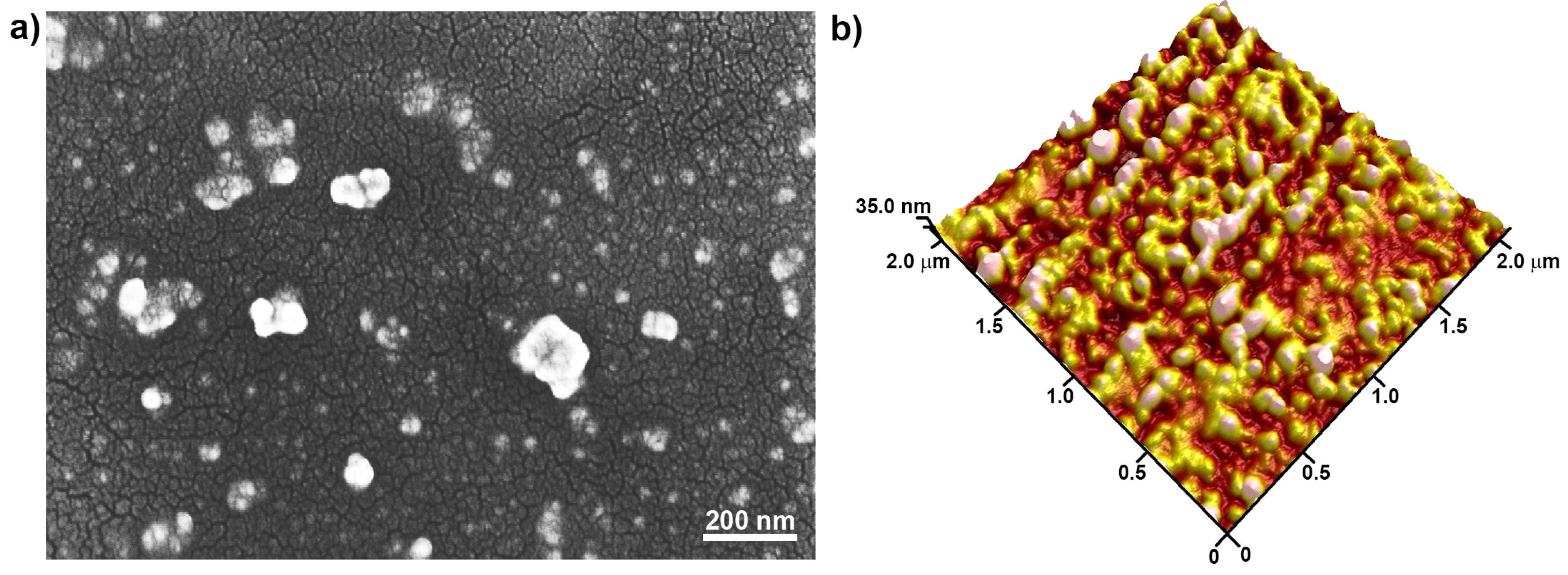
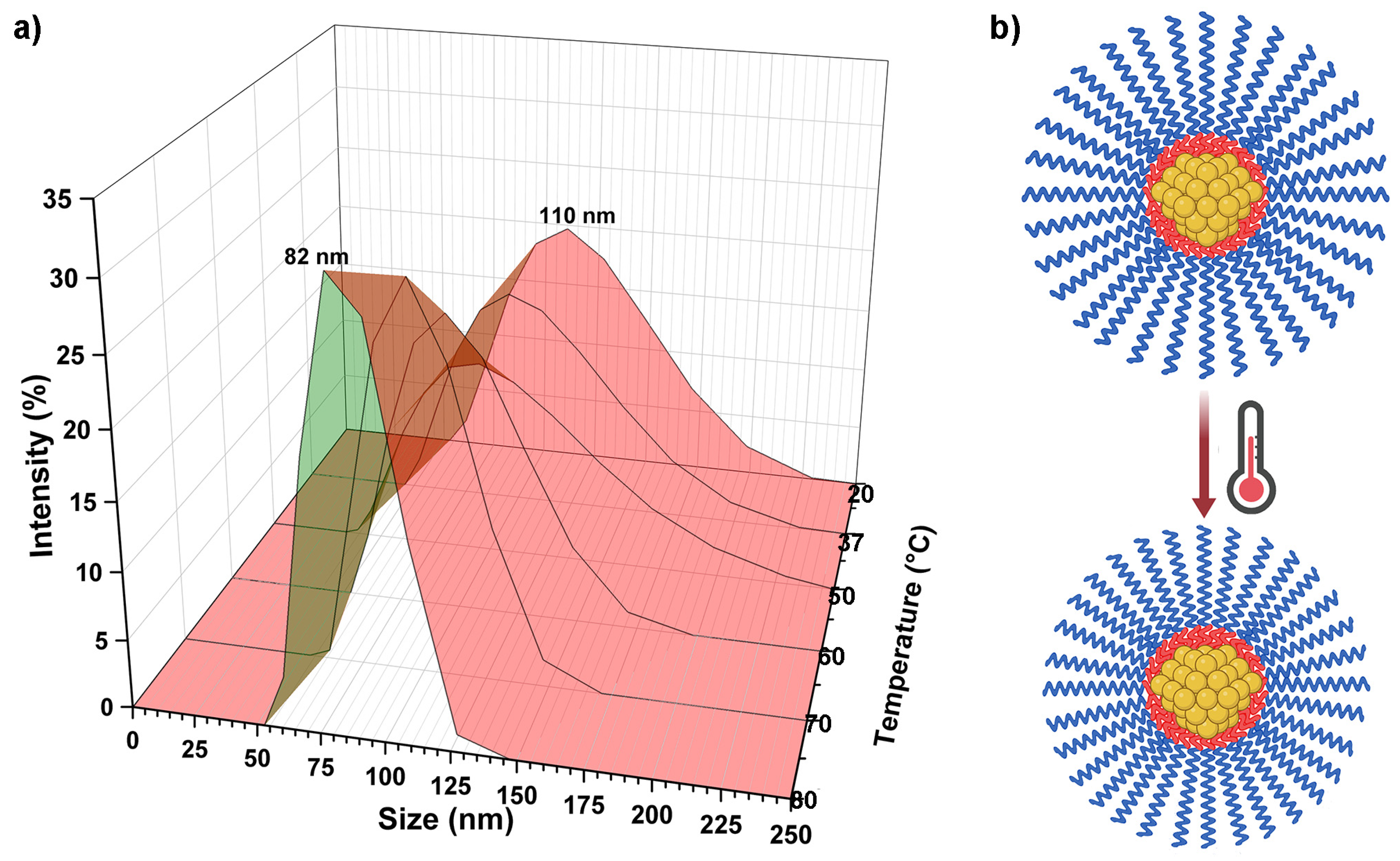
3.4. PVDEP-VL-Capped AuNPs: Morphology and Thermoresponsive Behavior
3.5. Photothermal Effect of PVDEP-VL-Capped AuNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Yuan, Y.; Raheja, K.; Milbrandt, N.B.; Beilharz, S.; Tene, S.; Oshabaheebwa, S.; Gurkan, U.A.; Samia, A.C.S.; Karayilan, M. Thermoresponsive polymers with LCST transition: Synthesis, characterization, and their impact on biomedical frontiers. RSC Appl. Polym. 2023, 1, 158–189. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; De Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Phys.-Triggered Nanosyst. Ther. Diagn. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Tomlins, P.; Sahota, T.S. Thermoresponsive Gels. Gels 2017, 3, 4. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- He, F.; Tang, G.; Min, X.; Hu, M.; Shao, L.; Bi, Y. Living/controlled free radical polymerization of N-vinyl caprolactam. Prog. Chem. 2016, 28, 328–336. [Google Scholar] [CrossRef]
- Ramos, J.; Imaz, A.; Forcada, J. Temperature-sensitive nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide). Polym. Chem. 2012, 3, 852–856. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Shu, X. A review of thermoresponsive drug delivery systems based on LCST/UCST polymer nanofibers. J. Phys. Conf. Ser. 2023, 2539, 012032. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Cheng, F.; Liu, Y.; Li, W.-G.; Chen, Y.; Pan, H.; Liu, H.-J. Thermoresponsive gold nanoparticles with adjustable lower critical solution temperature as colorimetric sensors for temperature, pH and salt concentration. J. Mater. Chem. 2010, 20, 278–284. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Top. Issue Polym. Chem. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Soller, B.S.; Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Precision Polymerization of Vinylphosphonates and Conjugated Nitrogen-Containing Vinyl Monomers. Chem. Rev. 2016, 116, 1993–2022. [Google Scholar] [CrossRef]
- Vidal, F.; Gowda, R.R.; Chen, E.Y.-X. Chemoselective, Stereospecific, and Living Polymerization of Polar Divinyl Monomers by Chiral Zirconocenium Catalysts. J. Am. Chem. Soc. 2015, 137, 9469–9480. [Google Scholar] [CrossRef]
- Mariott, W.R.; Chen, E.Y.-X. Stereospecific, Coordination Polymerization of Acrylamides by Chiral ansa-Metallocenium Alkyl and Ester Enolate Cations. Macromolecules 2004, 37, 4741–4743. [Google Scholar] [CrossRef]
- Miyake, G.; Caporaso, L.; Cavallo, L.; Chen, E.Y.-X. Coordination−Addition Polymerization and Kinetic Resolution of Methacrylamides by Chiral Metallocene Catalysts. Macromolecules 2009, 42, 1462–1471. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamamoto, H.; Yokota, K.; Miyake, S.; Nakamura, A. Synthesis of monodispersed high molecular weight polymers and isolation of an organolanthanide(III) intermediate coordinated by a penultimate poly(MMA) unit. J. Am. Chem. Soc. 1992, 114, 4908–4910. [Google Scholar] [CrossRef]
- Yasuda, H. Organo-rare-earth-metal initiated living polymerizations of polar and nonpolar monomers. J. Organomet. Chem. 2002, 647, 128–138. [Google Scholar] [CrossRef]
- Salzinger, S.; Soller, B.S.; Plikhta, A.; Seemann, U.B.; Herdtweck, E.; Rieger, B. Mechanistic Studies on Initiation and Propagation of Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates. J. Am. Chem. Soc. 2013, 135, 13030–13040. [Google Scholar] [CrossRef]
- Weingarten, P.; Thomas, S.R.; Luiza de Andrade Querino, A.; Halama, K.; Kränzlein, M.; Casini, A.; Rieger, B. A graft-to strategy of poly(vinylphosphonates) on dopazide-coated gold nanoparticles using in situ catalyst activation. RSC Adv. 2024, 14, 8145–8149. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, A.; Speranza, V.; Canton, P.; Capacchione, C.; Milione, S.; Grassi, A. Novel nanostructured semicrystalline ionomers by chemoselective sulfonation of multiblock copolymers of syndiotactic polystyrene with polybutadiene. RSC Adv. 2014, 4, 60158–60167. [Google Scholar] [CrossRef]
- Buonerba, A.; Speranza, V.; Grassi, A. Novel synthetic strategy for the sulfonation of polybutadiene and styrene-butadiene copolymers. Macromolecules 2013, 46, 778–784. [Google Scholar] [CrossRef]
- Leute, M. Macromolecules with Phosphorus Functionalities. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2007. [Google Scholar]
- Zimmermann, N.; Meggers, E.; Schultz, P.G. A second-generation copper(II)-mediated metallo-DNA-base pair. Bioorg. Chem. 2004, 32, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Alacid, E.; Nájera, C. Aqueous Sodium Hydroxide Promoted Cross-Coupling Reactions of Alkenyltrialkoxysilanes under Ligand-Free Conditions. J. Org. Chem. 2008, 73, 2315–2322. [Google Scholar] [CrossRef]
- Gordillo, Á.; de Jesús, E.; López-Mardomingo, C. Consecutive palladium-catalyzed Hiyama–Heck reactions in aqueous media under ligand-free conditions. Chem. Commun. 2007, 39, 4056–4058. [Google Scholar] [CrossRef]
- Hultzsch, K.C.; Voth, P.; Beckerle, K.; Spaniol, T.P.; Okuda, J. Single-Component Polymerization Catalysts for Ethylene and Styrene: Synthesis, Characterization, and Reactivity of Alkyl and Hydrido Yttrium Complexes Containing a Linked Amido−Cyclopentadienyl Ligand. Organometallics 2000, 19, 228–243. [Google Scholar] [CrossRef]
- Buonerba, A.; Lapenta, R.; Donniacuo, A.; Licasale, M.; Vezzoli, E.; Milione, S.; Capacchione, C.; Tecce, M.F.; Falqui, A.; Piacentini, R.; et al. NIR multiphoton ablation of cancer cells, fluorescence quenching and cellular uptake of dansyl-glutathione-coated gold nanoparticles. Sci. Rep. 2020, 10, 11380. [Google Scholar] [CrossRef]
- Vitiello, R.; Taddeo, F.; Russo, V.; Turco, R.; Buonerba, A.; Grassi, A.; Di Serio, M.; Tesser, R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering 2021, 5, 46. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824, Erratum in: Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Ipe, B.I.; Thomas, K.G. Investigations on Nanoparticle−Chromophore and Interchromophore Interactions in Pyrene-Capped Gold Nanoparticles. J. Phys. Chem. B 2004, 108, 13265–13272. [Google Scholar] [CrossRef]
- Thomas, K.G.; Kamat, P.V. Chromophore-Functionalized Gold Nanoparticles. Acc. Chem. Res. 2003, 36, 888–898. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Jiang, J.; Li, J. Electroactive gold nanoparticles protected by 4-ferrocene thiophenol monolayer. J. Colloid Interface Sci. 2003, 264, 109–113. [Google Scholar] [CrossRef]
- Ohyama, J.; Hitomi, Y.; Higuchi, Y.; Shinagawa, M.; Mukai, H.; Kodera, M.; Teramura, K.; Shishido, T.; Tanaka, T. One-phase synthesis of small gold nanoparticles coated by a horizontal porphyrin monolayer. Chem. Commun. 2008, 47, 6300–6302. [Google Scholar] [CrossRef]
- Zhang, N.; Salzinger, S.; Rieger, B. Poly(vinylphosphonate)s with Widely Tunable LCST: A Promising Alternative to Conventional Thermoresponsive Polymers. Macromolecules 2012, 45, 9751–9758. [Google Scholar] [CrossRef]
| Entry | [DEVP]/[Y] | Yield | MW | DEVP |
|---|---|---|---|---|
| (Molar Ratio) | (g) | (kDa) | (Units in Polymer Chain) | |
| 1 | 40 | 1.39 | 17.6 | 107 |
| 2 | 60 | 1.48 | 18.2 | 111 |
| 3 | 90 | 1.69 | 25.6 | 156 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonerba, A.; Lapenta, R.; Della Monica, F.; Piacentini, R.; Baldino, L.; Scognamiglio, M.R.; Speranza, V.; Milione, S.; Capacchione, C.; Rieger, B.; et al. Thermo- and Photoresponsive Smart Nanomaterial Based on Poly(diethyl vinyl phosphonate)-Capped Gold Nanoparticles. Nanomaterials 2024, 14, 1589. https://doi.org/10.3390/nano14191589
Buonerba A, Lapenta R, Della Monica F, Piacentini R, Baldino L, Scognamiglio MR, Speranza V, Milione S, Capacchione C, Rieger B, et al. Thermo- and Photoresponsive Smart Nanomaterial Based on Poly(diethyl vinyl phosphonate)-Capped Gold Nanoparticles. Nanomaterials. 2024; 14(19):1589. https://doi.org/10.3390/nano14191589
Chicago/Turabian StyleBuonerba, Antonio, Rosita Lapenta, Francesco Della Monica, Roberto Piacentini, Lucia Baldino, Maria Rosa Scognamiglio, Vito Speranza, Stefano Milione, Carmine Capacchione, Bernhard Rieger, and et al. 2024. "Thermo- and Photoresponsive Smart Nanomaterial Based on Poly(diethyl vinyl phosphonate)-Capped Gold Nanoparticles" Nanomaterials 14, no. 19: 1589. https://doi.org/10.3390/nano14191589












