Palladium-Functionalized Nanostructured Nickel–Cobalt Oxide as Alternative Catalyst for Hydrogen Sensing Using Pellistors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis Procedure
2.2. Characterization Methods
3. Results
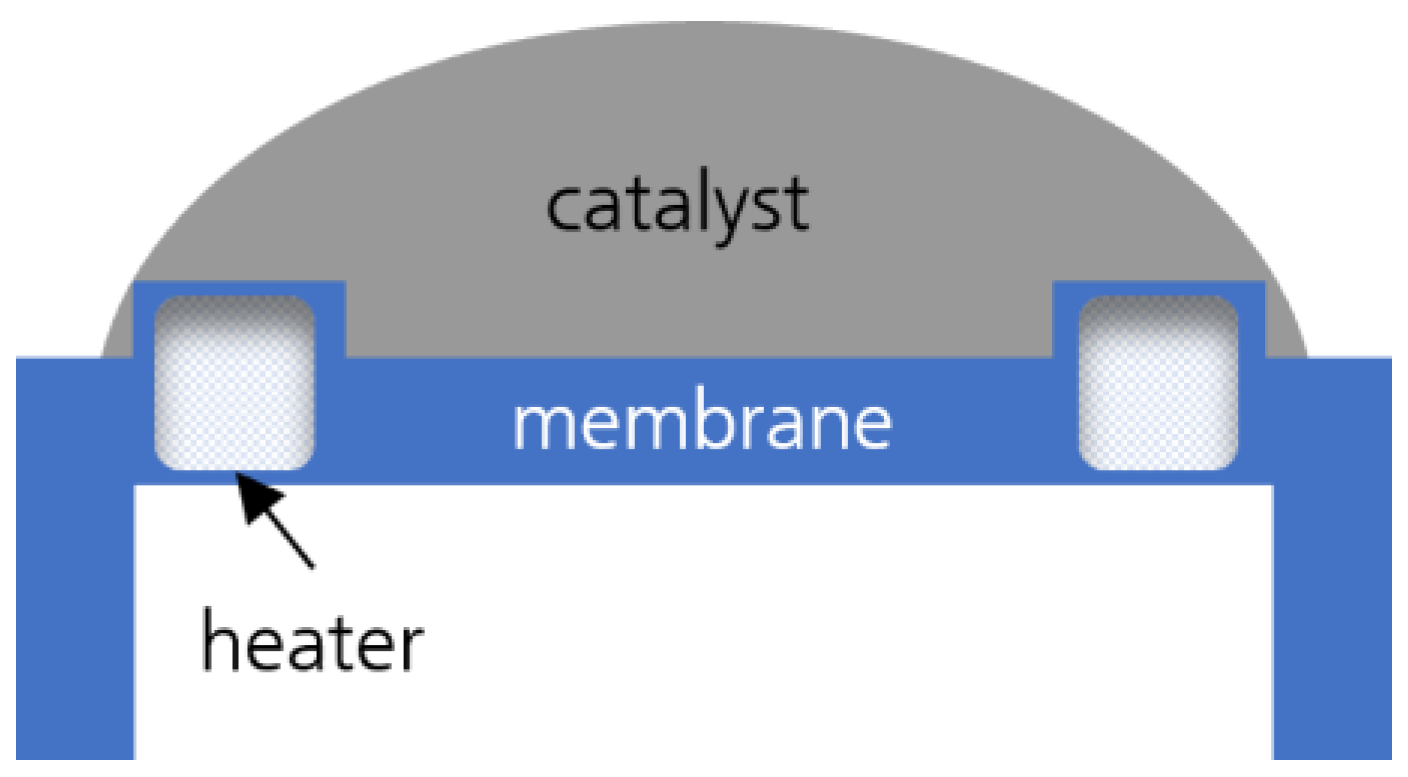
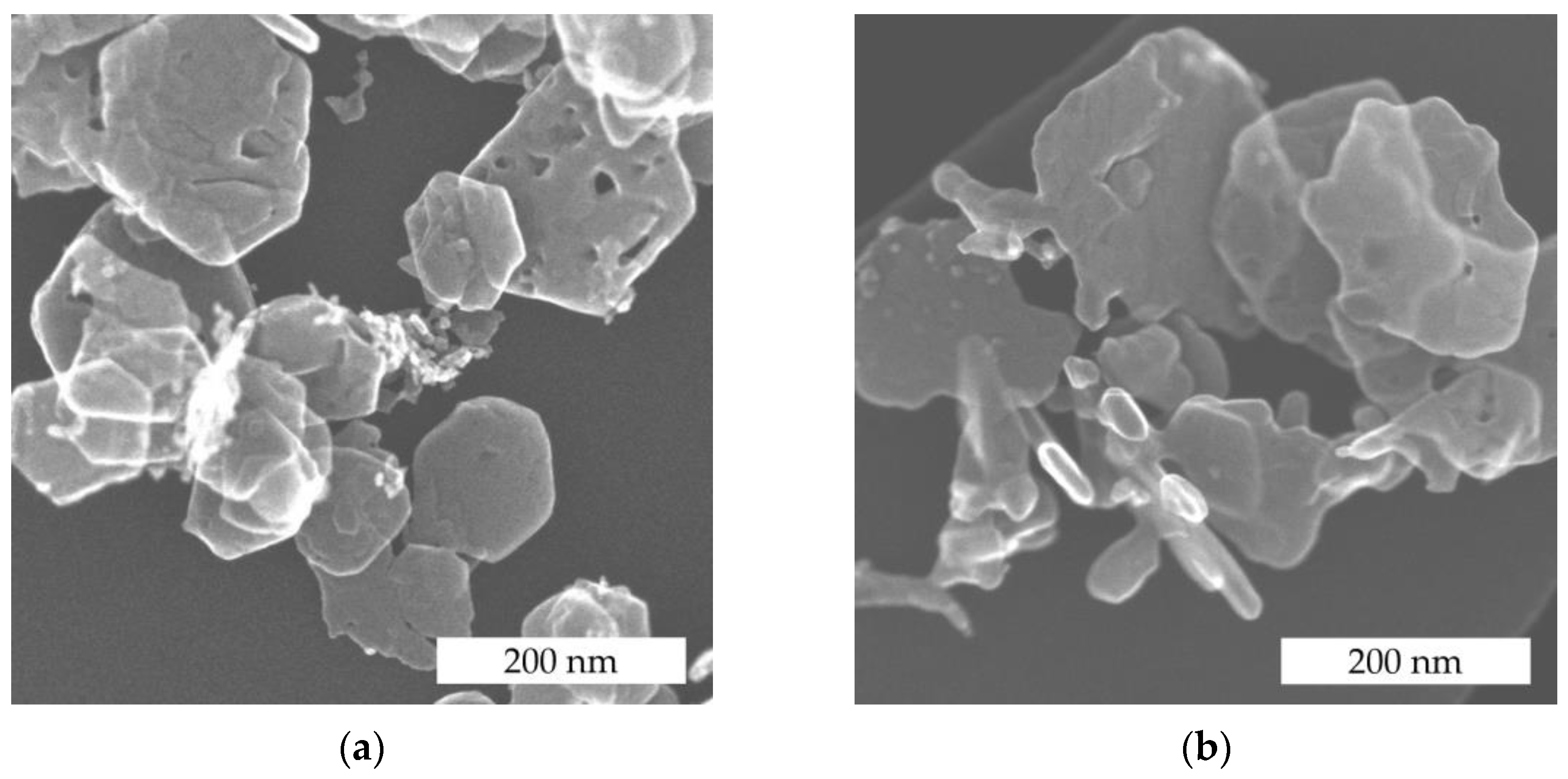
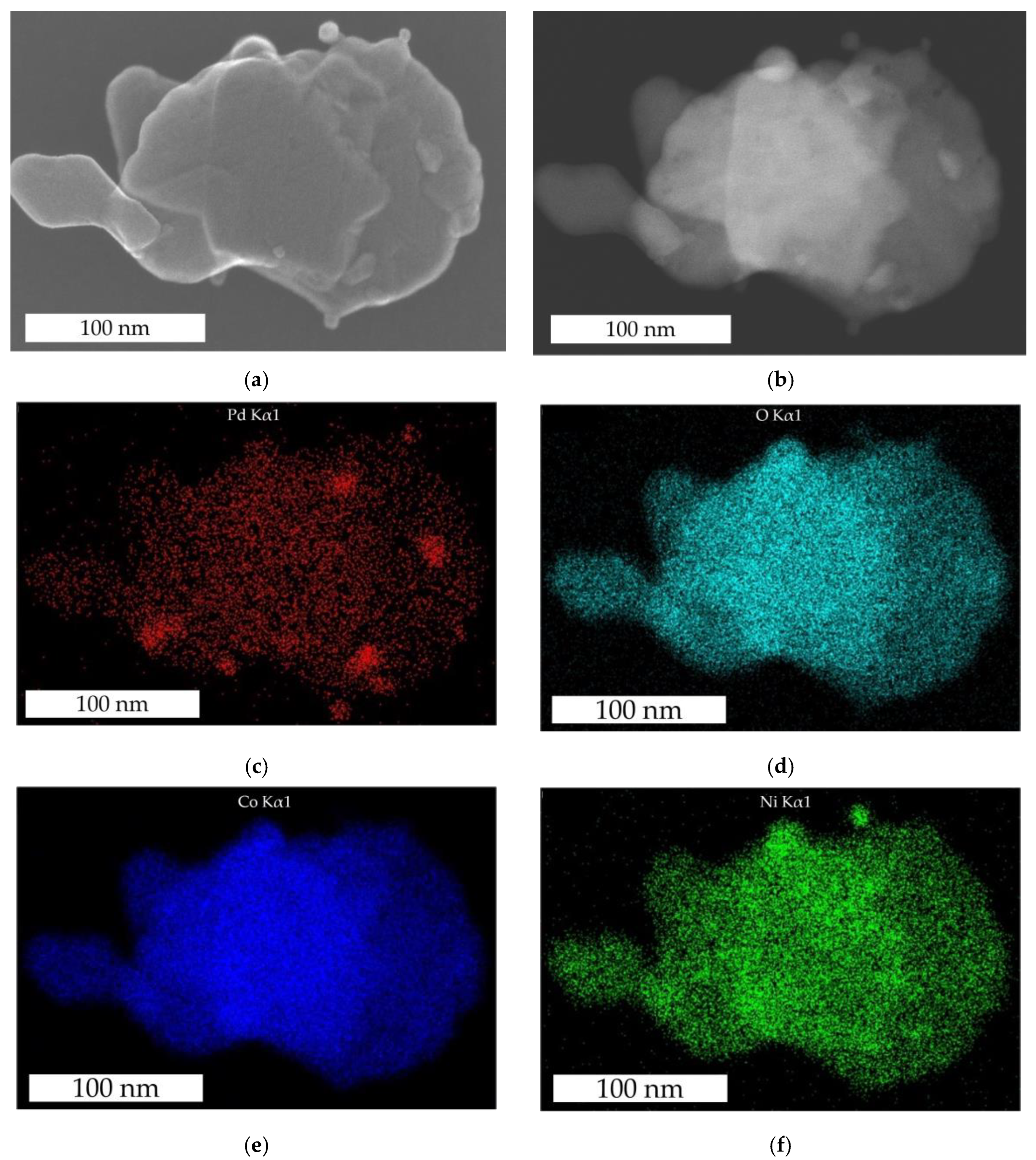
3.1. Particle Structure and Morphology of the Catalyst
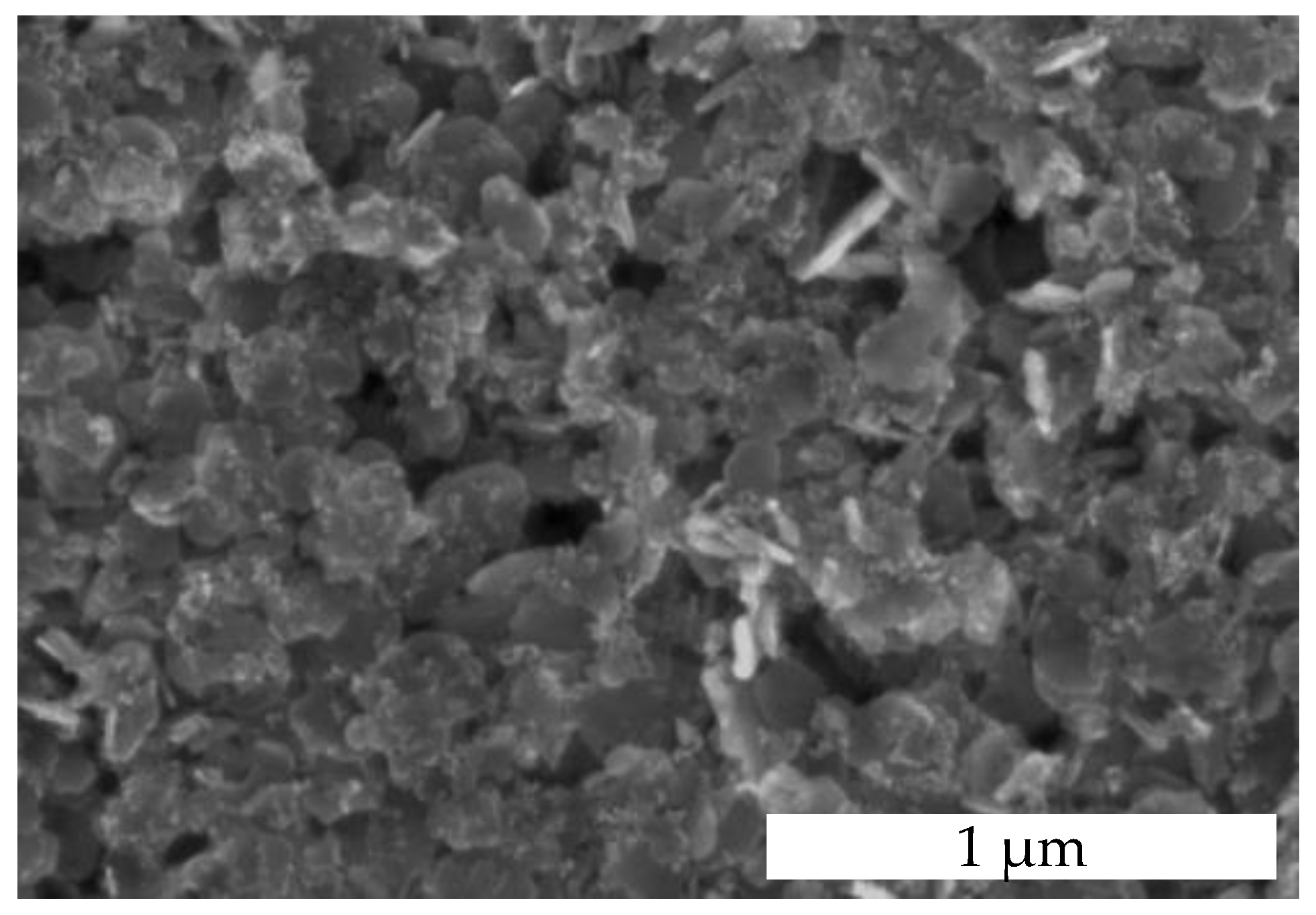
3.2. Morphology of the Catalytic Layer
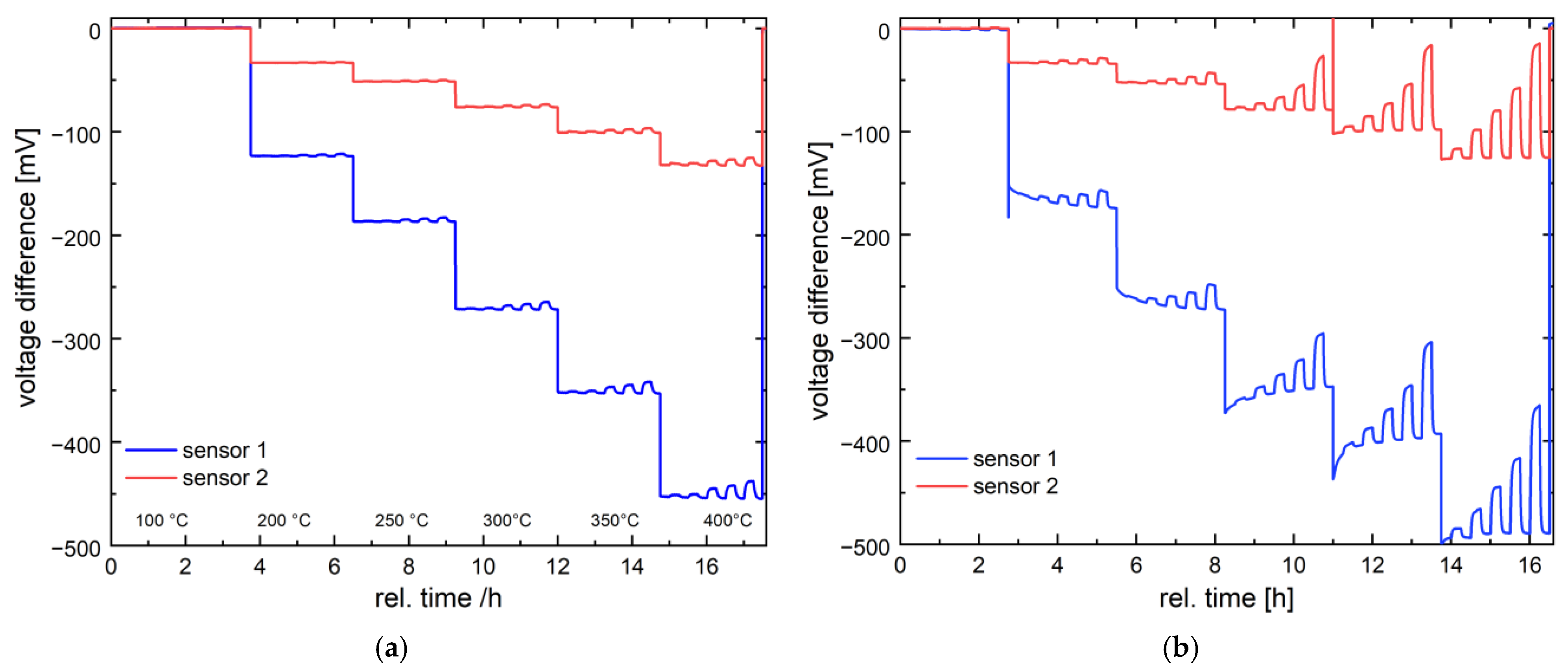
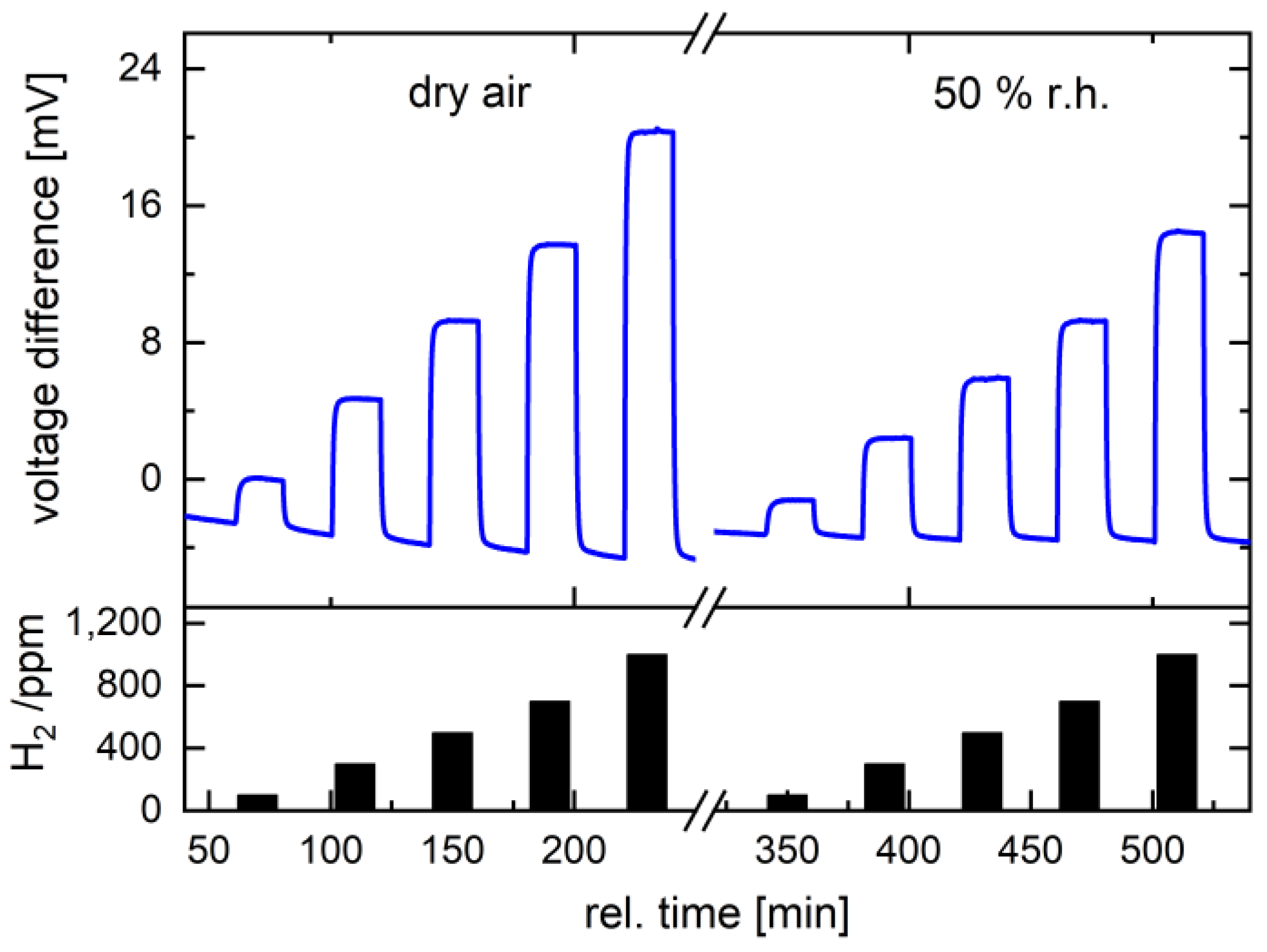
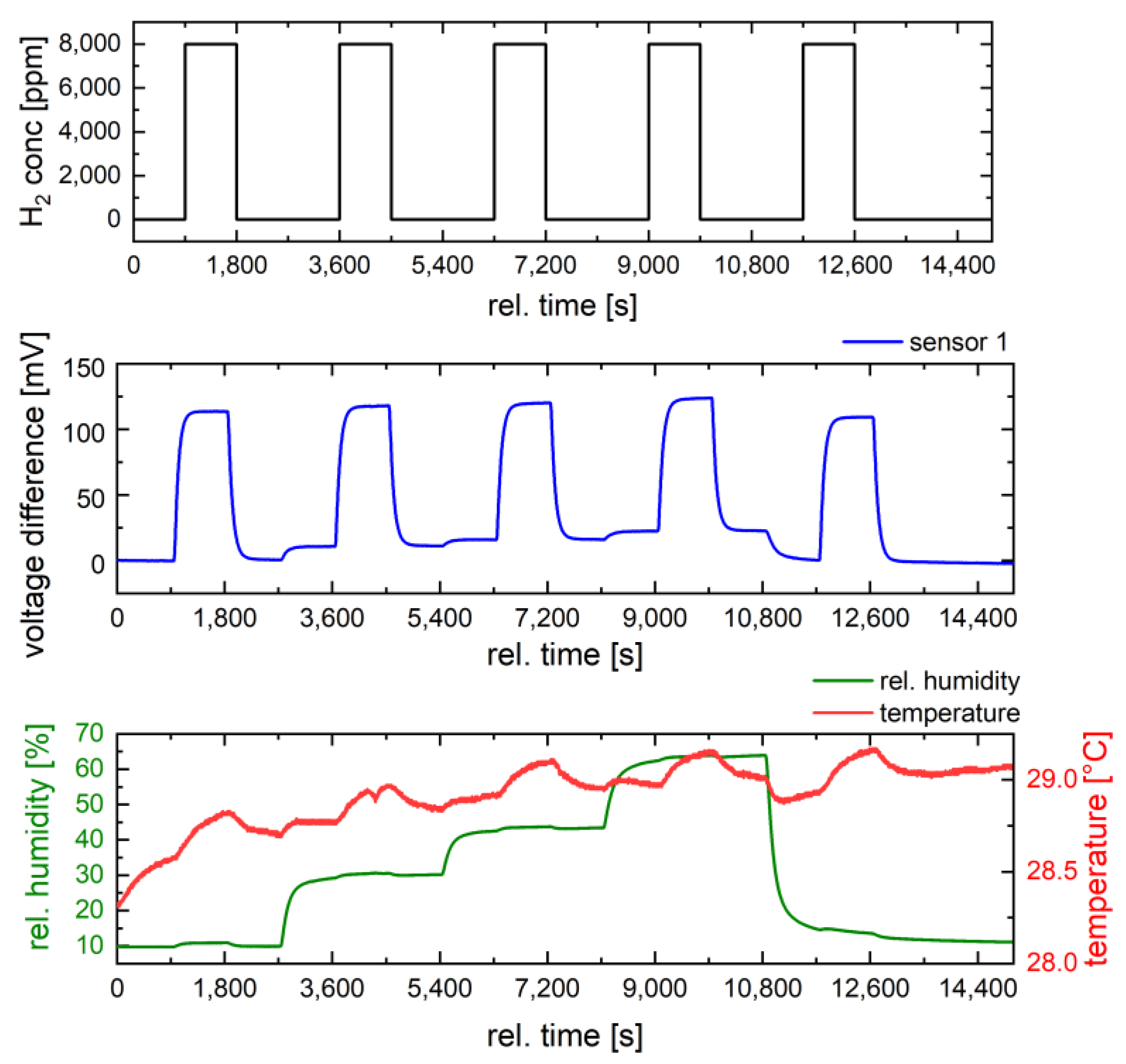
3.3. Gas Characterization of the Catalyst
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramaiyan, K.; Tsui, L.; Brosha, E.L.; Kreller, C.; Stetter, J.R.; Russ, T.; Du, W.; Peaslee, D.; Hunter, G.; Xu, J.; et al. Recent Developments in Sensor Technologies for Enabling the Hydrogen Economy. ECS Sens. Plus 2023, 2, 45601. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Kim, J.; Yu, J.; Lee, S.; Tahmasebi, A.; Jeon, C.-H.; Lucas, J. Advances in catalytic hydrogen combustion research: Catalysts, mechanism, kinetics, and reactor designs. Int. J. Hydrogen Energy 2021, 46, 40073–40104. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Hydrogen Infrastructure for Energy Applications: Production, Storage, Distribution and Safety; Hydrogen Logistics: Safety and Risks Issues; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-812036-1. [Google Scholar]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Baranov, A.M.; Talipov, V.A.; Mironov, S.M.; Akbari, S.; Kolesnik, I.V.; Orlova, E.D.; Napolskii, K.S. Investigation of catalytic hydrogen sensors with platinum group catalysts. Sens. Actuators B 2021, 346, 130515. [Google Scholar] [CrossRef]
- Kalinin, I.A.; Roslyakov, I.V.; Bograchev, D.; Kushnir, S.E.; Ivanov, I.I.; Dyakov, A.V.; Napolskii, K.S. High performance microheater-based catalytic hydrogen sensors fabricated on porous anodic alumina substrates. Sens. Actuators B 2024, 404, 135270. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrogen Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, T.; Wang, H.; Tang, C.; Zhang, L. A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology. Micromachines 2019, 10, 71. [Google Scholar] [CrossRef]
- Lee, E.-B.; Hwang, I.-S.; Cha, J.-H.; Lee, H.-J.; Lee, W.-B.; Pak, J.J.; Lee, J.-H.; Ju, B.-K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Brauns, E.; Morsbach, E.; Schnurpfeil, G.; Bäumer, M.; Lang, W. A miniaturized catalytic gas sensor for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B 2013, 187, 420–425. [Google Scholar] [CrossRef]
- Choi, Y.; Tajima, K.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Combustor of ceramic Pt/alumina catalyst and its application for micro-thermoelectric hydrogen sensor. Appl. Catal. A-Gen. 2005, 287, 19–24. [Google Scholar] [CrossRef]
- Jones, M.G.; Nevell, T.G. The detection of hydrogen using catalytic flammable gas sensors. Sens. Actuators B 1989, 16, 215–224. [Google Scholar] [CrossRef]
- Singh, S.A.; Vishwanath, K.; Madras, G. Role of Hydrogen and Oxygen Activation over Pt and Pd-Doped Composites for Catalytic Hydrogen Combustion. ACS Appl. Mater. Interfaces 2017, 9, 19380–19388. [Google Scholar] [CrossRef]
- Wu, R.-J.; Tian, X.-M.; Hua, Z.-Q.; Lu, N.; Wang, P. A low temperature catalytic combustible gas sensor based on Ru supported zeolite catalyst films. Chin. J. Anal. Chem. 2021, 49, 63–68. [Google Scholar] [CrossRef]
- Haruta, M.; Sano, H. Catalytic combustion of hydrogen I—Its role in hydrogen utilization system and screening of catalysts materials. Int. J. Hydrogen Energy 1981, 6, 601–608. [Google Scholar] [CrossRef]
- Baranov, A.; Ivanov, I.; Karpova, E. Effects of Flameless Catalytic Combustion of Hydrogen on the Parameters of Catalytic Sensors with Platinum-group Catalysts. Sens. Mater. 2023, 35, 3585–3594. [Google Scholar] [CrossRef]
- Shin, W.; Tajima, K.; Choi, Y.; Izu, N.; Matsubara, I.; Murayama, N. Planar catalytic combustor film for thermoelectric hydrogen sensor. Sens. Actuators B 2005, 108, 455–460. [Google Scholar] [CrossRef]
- Han, C.-H.; Hong, D.-W.; Han, S.-D.; Gwak, J.; Singh, K.C. Catalytic combustion type hydrogen gas sensor. Sens. Actuators B 2007, 125, 224–228. [Google Scholar] [CrossRef]
- Beck, A.; Rzepka, P.; Marshall, K.P.; Stoian, D.; van Bokhoven, J.A. Hydrogen Interaction with Oxide Supports in the Presence and Absence of Platinum. J. Phys. Chem. C 2022, 126, 17589–17597. [Google Scholar] [CrossRef]
- Li, Y.; Fei, N.; Li, W.; Cao, Y.; Ge, X.; Dai, S.; Yan, K.; Yuwen, Q.; Zhou, X.; Yuan, W.; et al. H2 activation on metal oxides promoted by highly dispersed Pd. Catal. Commun. 2023, 177, 106645. [Google Scholar] [CrossRef]
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-Temperature Methane Oxidation for Efficient Emission Control in Natural Gas Vehicles: Pd and Beyond. ACS Catal. 2020, 10, 14304–14314. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Yurchenko, O.; Pernau, H.-F.; Engel, L.; Wöllenstein, J. Differential thermal analysis techniques as a tool for preliminary examination of catalyst for combustion. Sci. Rep. 2023, 13, 9792. [Google Scholar] [CrossRef] [PubMed]
- Bierer, B.; Grgić, D.; Yurchenko, O.; Engel, L.; Pernau, H.-F.; Jägle, M.; Reindl, L.; Wöllenstein, J. Low-power sensor node for the detection of methane and propane. J. Sens. Sens. Syst. 2021, 10, 185–191. [Google Scholar] [CrossRef]
- Tao, F.F.; Shan, J.; Nguyen, L.; Wang, Z.; Zhang, S.; Zhang, L.; Wu, Z.; Huang, W.; Zeng, S.; Hu, P. Understanding complete oxidation of methane on spinel oxides at a molecular level. Nat. Commun. 2015, 6, 7798. [Google Scholar] [CrossRef]
- Yurchenko, O.; Diehle, P.; Altmann, F.; Schmitt, K.; Wollenstein, J. Co3O4-Based Materials as Potential Catalysts for Methane Detection in Catalytic Gas Sensors. Sensors 2024, 24, 2599. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Batkin, A.M.; Teleguina, N.S.; Bragina, G.O.; Zaikovsky, V.I.; Prosvirin, I.P.; Khudorozhkov, A.K.; Bukhtiyarov, V.I. Particle Size Effect on CH4 Oxidation Over Noble Metals: Comparison of Pt and Pd Catalysts. Top. Catal. 2013, 56, 306–310. [Google Scholar] [CrossRef]
- Du, J.; Zhao, D.; Wang, C.; Zhao, Y.; Li, H.; Luo, Y. Size effects of Pd nanoparticles supported over CeZrPAl for methane oxidation. Catal. Sci. Technol. 2020, 10, 7875–7882. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Low-temperature CH4 Catalytic Combustion over Pd Catalyst Supported on Co3O4 Nanocrystals with Well-Defined Crystal Planes. ChemCatChem 2011, 3, 868–874. [Google Scholar] [CrossRef]
- Zivcová, Z.; Gregorová, E.; Pabst, W.; Smith, D.S.; Michot, A.; Poulier, C. Thermal conductivity of porous alumina ceramics prepared using starch as a pore-forming agent. J. Eur. Ceram. Soc. 2009, 29, 347–353. [Google Scholar] [CrossRef]
- Lyu, X.; Yurchenko, O.; Diehle, P.; Altmann, F.; Wöllenstein, J.; Schmitt, K. Accelerated Deactivation of Mesoporous Co3O4-Supported Au–Pd Catalyst through Gas Sensor Operation. Chemosensors 2023, 11, 271. [Google Scholar] [CrossRef]
- Sahoo, P.; Djieutedjeu, H.; Poudeu, P.F.P. Co3O4 nanostructures: The effect of synthesis conditions on particles size, magnetism and transport properties. J. Mater. Chem. A 2013, 1, 15022–15030. [Google Scholar] [CrossRef]
- Hadano, H.; Miyagi, A.; Okuno, T.; Nagawa, Y.; Ishiguro, Y. Development of a Catalytic Combustion Type Gas Sensor with Low Power Consumption. ECS Trans. 2016, 75, 195–198. [Google Scholar] [CrossRef]
- Jang, W.; Park, J.-S.; Lee, K.-W.; Roh, Y. Methane and hydrogen sensing properties of catalytic combustion type single-chip micro gas sensors with two different Pt film thicknesses for heaters. Micro Nano Syst. Lett. 2018, 6, 733. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchenko, O.; Benkendorf, M.; Diehle, P.; Schmitt, K.; Wöllenstein, J. Palladium-Functionalized Nanostructured Nickel–Cobalt Oxide as Alternative Catalyst for Hydrogen Sensing Using Pellistors. Nanomaterials 2024, 14, 1619. https://doi.org/10.3390/nano14201619
Yurchenko O, Benkendorf M, Diehle P, Schmitt K, Wöllenstein J. Palladium-Functionalized Nanostructured Nickel–Cobalt Oxide as Alternative Catalyst for Hydrogen Sensing Using Pellistors. Nanomaterials. 2024; 14(20):1619. https://doi.org/10.3390/nano14201619
Chicago/Turabian StyleYurchenko, Olena, Mike Benkendorf, Patrick Diehle, Katrin Schmitt, and Jürgen Wöllenstein. 2024. "Palladium-Functionalized Nanostructured Nickel–Cobalt Oxide as Alternative Catalyst for Hydrogen Sensing Using Pellistors" Nanomaterials 14, no. 20: 1619. https://doi.org/10.3390/nano14201619
APA StyleYurchenko, O., Benkendorf, M., Diehle, P., Schmitt, K., & Wöllenstein, J. (2024). Palladium-Functionalized Nanostructured Nickel–Cobalt Oxide as Alternative Catalyst for Hydrogen Sensing Using Pellistors. Nanomaterials, 14(20), 1619. https://doi.org/10.3390/nano14201619






