Carbon-Nanotube-Based Superhydrophobic Magnetic Nanomaterial as Absorbent for Rapid and Efficient Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Synthesis of Magnetic Nickel Ferrite Nanoparticles
2.3. Synthesis of Nickel Ferrite Supported over Alumina for CNT Growth Using CVD
2.4. Synthesis of CNTs
2.5. Preparation of Superhydrophobic Magnetic PU Sponges
2.6. Study of Superhydrophobic and Oleophilic Properties
2.7. Study of Hydrophobic Properties, Oil/Water Separation Efficiency, and Oil Absorption Capacity
3. Results and Discussion
3.1. Structural, Morphological, and Physicochemical Characterization of CNT and NiFe2O4 Nanoparticles
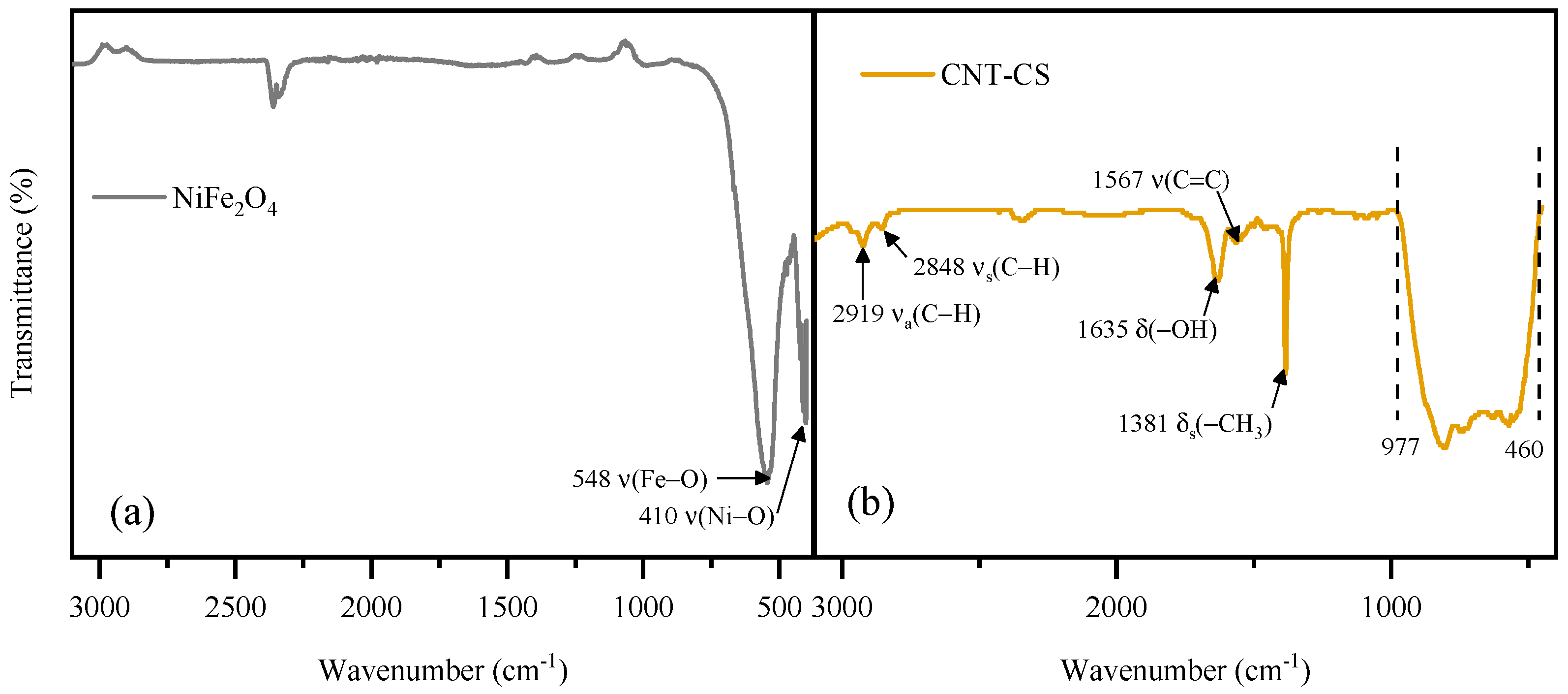
3.1.1. FTIR
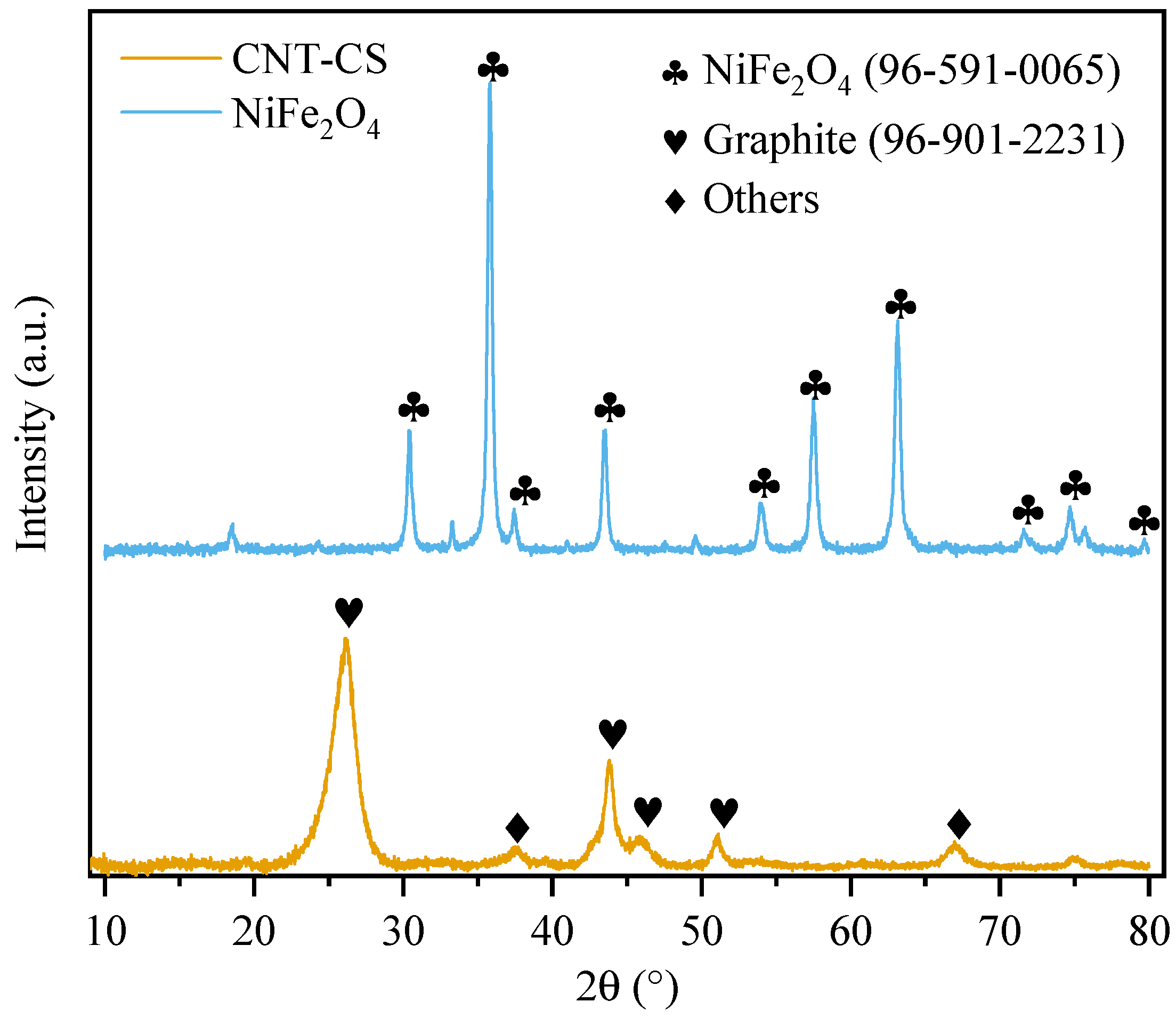
3.1.2. XRD
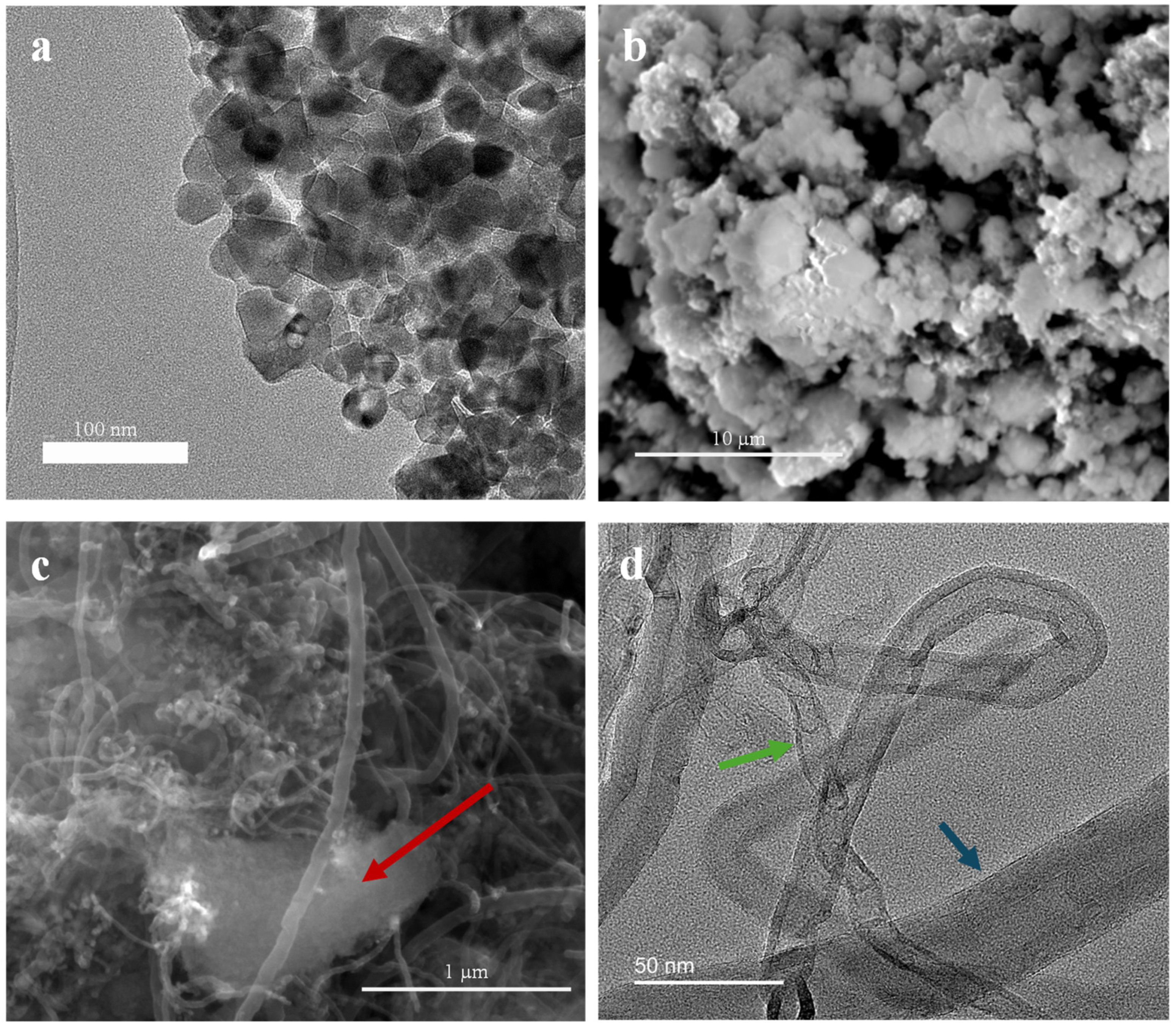
3.1.3. SEM and TEM Images
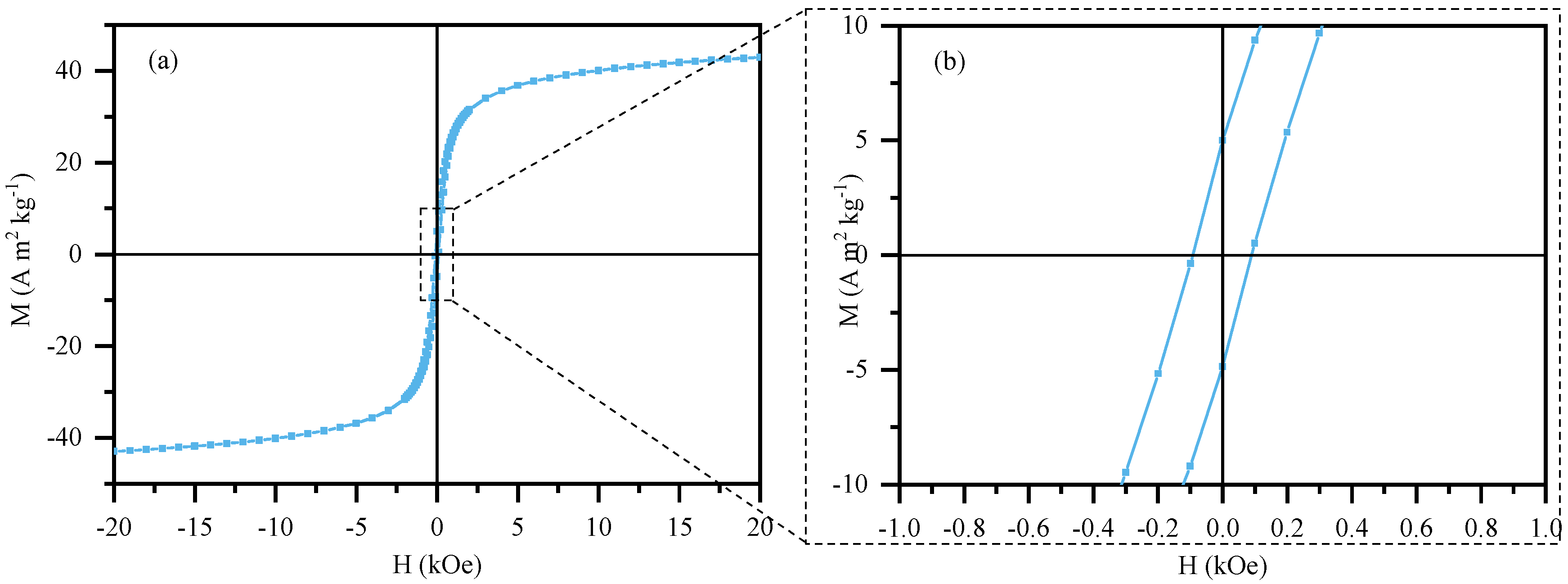
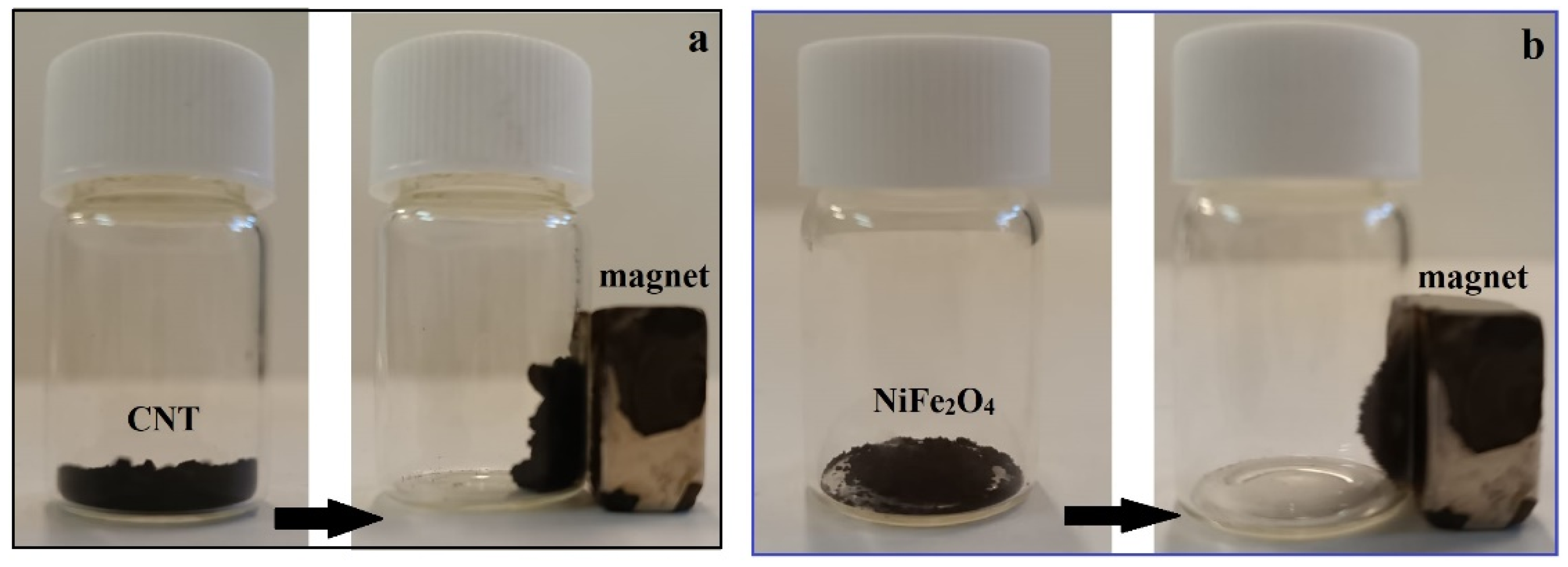
3.1.4. Magnetic Characterization
3.2. Characterization of Newly Prepared PU/CNT/NiFe2O4/PDMS Sponge
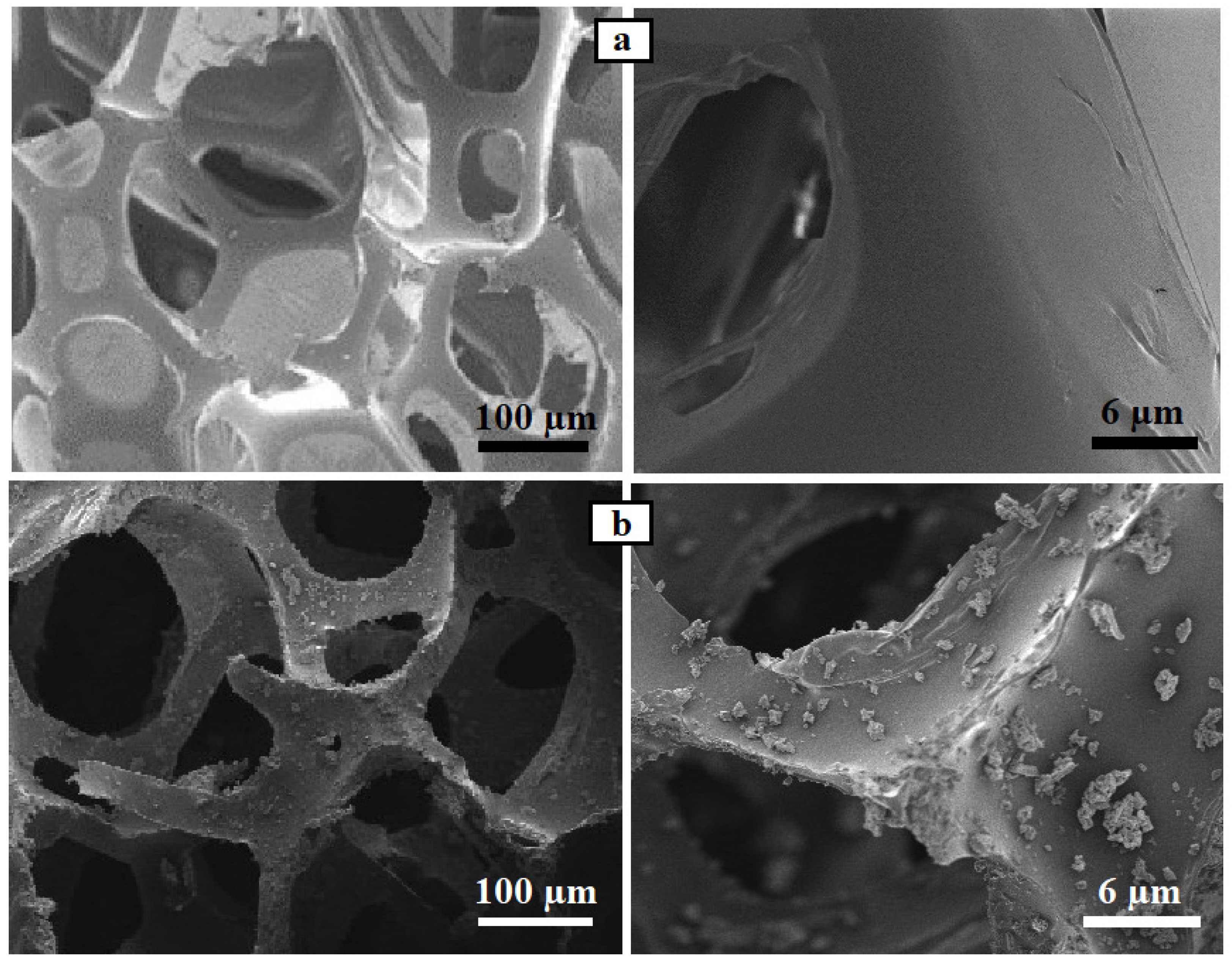
3.2.1. SEM Images of Newly Prepared PU/CNT/NiFe2O4/PDMS Sponge
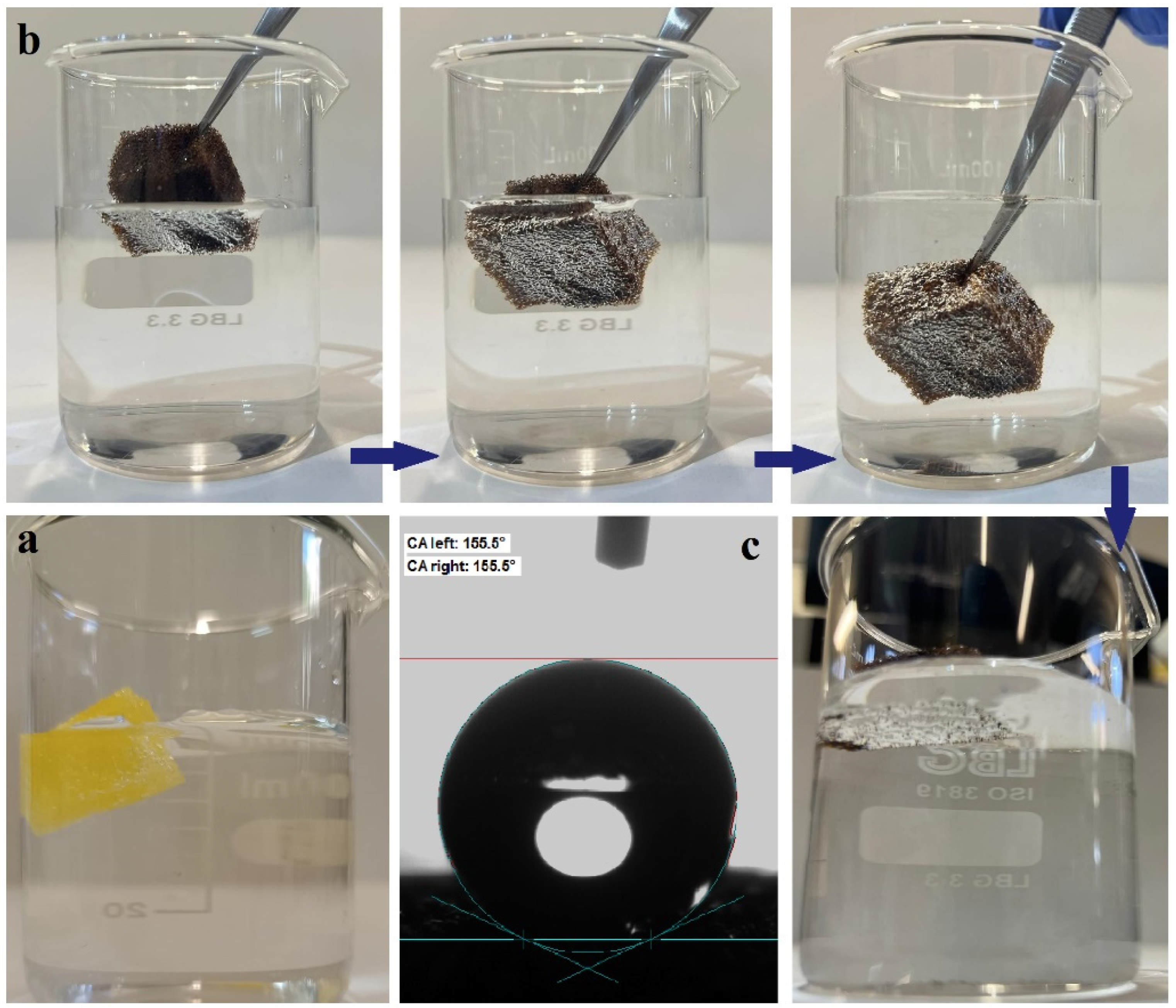
3.2.2. Hydrophobic and Oleophilic Properties of Newly Prepared PU/CNT/NiFe2O4/PDMS Sponge
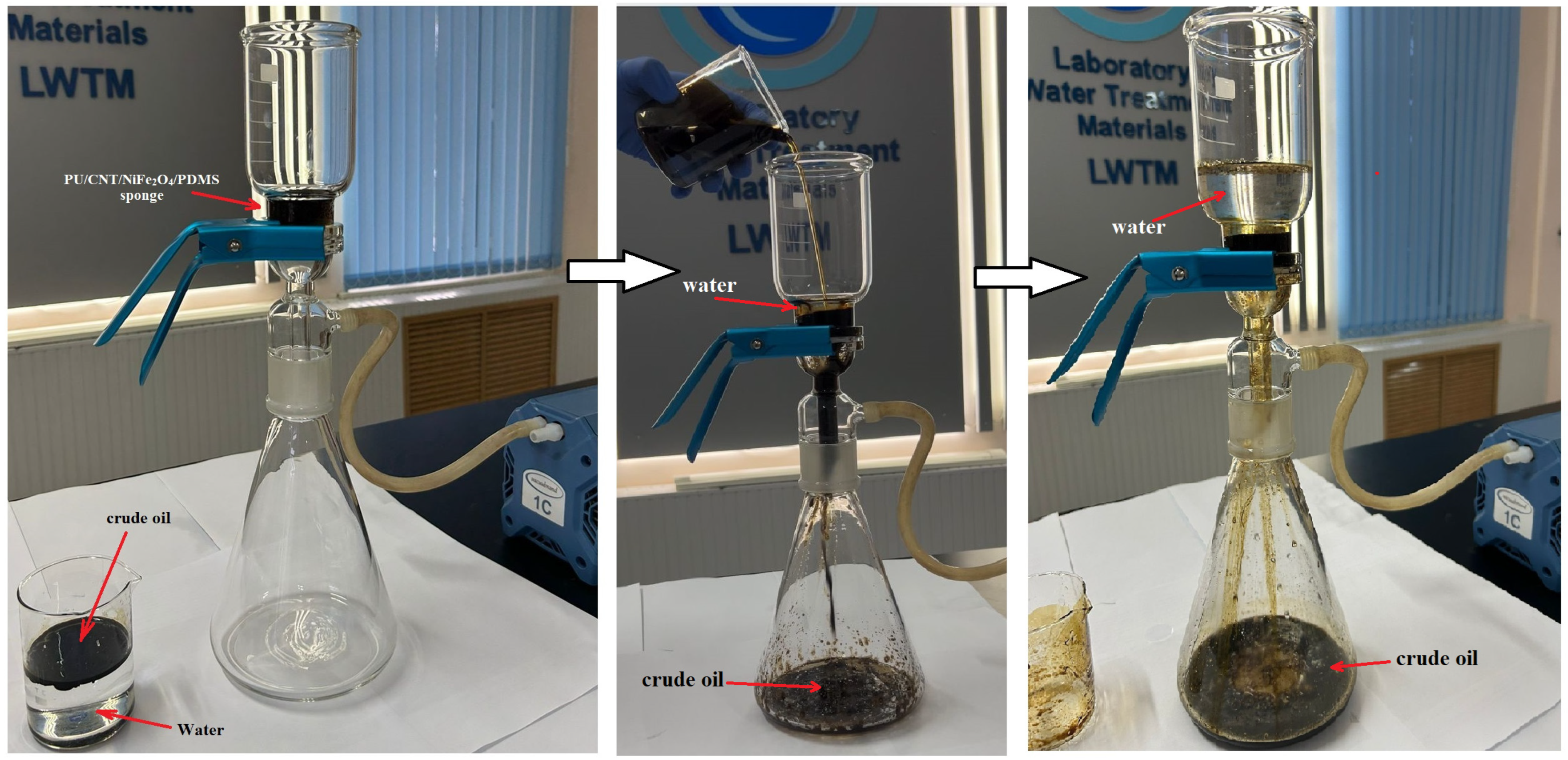
3.2.3. Oil/Organic Solvent–Water Separation Efficiency and Oil/Organic Solvent Absorption Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, P.; Huang, L.; Pan, W.; Wu, S.; Feng, X.; Song, J.; Xing, Y. Facile Preparation of Durable Superhydrophobic-Superoleophilic Mesh Using Simple Chemical Oxidation for Oil-Water Separation under Harsh Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126777. [Google Scholar] [CrossRef]
- Kudaibergenov, K.; Ongarbayev, Y.; Zulkhair, M.; Tulepov, M.; Tileuberdi, Y. Rice Husk Ash for Oil Spill Cleanup. Appl. Mech. Mater. 2013, 446–447, 1508–1511. [Google Scholar] [CrossRef]
- Minh, T.D.; Ncibi, M.C.; Srivastava, V.; Doshi, B.; Sillanpää, M. Micro/Nano-Machines for Spilled-Oil Cleanup and Recovery: A Review. Chemosphere 2021, 271, 129516. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Q.; Xu, J.; Sun, S.; Cheng, Y.; Qiu, F.; Zhang, T. Easily Fabricated Low-Energy Consumption Joule-Heated Superhydrophobic Foam for Fast Cleanup of Viscous Crude Oil Spills. ACS Appl. Mater. Interfaces 2021, 13, 51652–51660. [Google Scholar] [CrossRef]
- Kussainova, B.; Tazhkenova, G.; Kazarinov, I.; Nurlybayeva, A.; Lamichova, A.; Kusepova, L.; Mashan, T.; Tantybayeva, B.; Saurbayeva, B.; Seitbekova, G.; et al. Comparative Study of the Physico-Chemical Properties of Sorbents Based on Natural Bentonites Modified with Iron (III) and Aluminium (III) Polyhydroxocations. Coatings 2023, 13, 1974. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, Z.; Chen, H.; Xiang, Q.; Wang, Q.; Xie, C.; Liu, Y. Superhydrophobic polyurethane sponge based on sepiolite for efficient oil/water separation. J. Hazard. Mater. 2022, 434, 128833. [Google Scholar] [CrossRef]
- Kang, L.; Shi, L.; Song, L.; Guo, X. Facile fabrication of superhydrophobic porous materials using the water-based aza-Michael reaction for high-efficiency oil-water separation. Sep. Purif. Technol. 2023, 308, 122880. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Waheed, A. A review on super-wettable porous membranes and materials based on bio-polymeric chitosan for oil-water separation. Adv. Colloid Interface Sci. 2022, 303, 102635. [Google Scholar] [CrossRef]
- Yang, S.D.; Li, H.Y.; Liu, S.; Wang, S.S.; Li, H.M.; Li, H.M.; Qi, W.S.; Xu, Q.; Zhang, Q.; Zhu, J.; et al. Wodyetia bifurcate structured carbon fabrics with durable superhydrophobicity for high-efficiency oil-water separation. J. Hazard. Mater. 2022, 439, 129688. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Su, X.; Liang, T.; Zeng, X. Thiolated graphene-based superhydrophobic sponges for oil-water separation. Chem. Eng. J. 2017, 316, 736–743. [Google Scholar] [CrossRef]
- Deng, Z.; Lv, K.; Zhu, G.; Zhao, X.; Feng, Y. Superhydrophobic Sponge for Highly Efficient Separation of Both Stratified and Emulsified Oil–Water Mixtures. Ind. Eng. Chem. Res. 2023, 62, 9714–9725. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Zheng, S.; Jia, K.; Yu, L.; Xin, J.; Ning, J.; Wen, W.; Huang, L.; Xie, J. Environment-Friendly superhydrophobic sponge for highly efficient Oil/Water separation and microplastic removal. Sep. Purif. Technol. 2023, 319, 124060. [Google Scholar] [CrossRef]
- Rather, A.M.; Jana, N.; Hazarikaa, P.; Manna, U. Sustainable polymeric material for the facile and repetitive removal of oil-spills through the complementary use of both selective-absorption and active-filtration processes. J. Mater. Chem. A 2017, 5, 23339–23348. [Google Scholar] [CrossRef]
- Shome, A.; Maji, K.; Rather, A.M.; Yashwanth, A.; Patel, D.K.; Manna, U. A Scalable Chemical Approach for the Synthesis of a Highly Tolerant and Efficient Oil Absorbent. Chem. Asian J. 2019, 14, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Shome, A.; Rather, A.M.; Manna, U. Chemically reactive protein nanoparticles for synthesis of a durable and deformable superhydrophobic material. Nanoscale Adv. 2019, 1, 1746–1753. [Google Scholar] [CrossRef]
- Veisi, H.; Ozturk, T.; Karmakar, B.; Tamoradi, T.; Hemmati, S. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst in Suzuki-Miyaura coupling and 4-nitrophenol reduction. Carbohydr. Polym. 2020, 235, 115966. [Google Scholar] [CrossRef]
- Aslani, E.; Abri, A.; Pazhang, M. Immobilization of trypsin onto Fe3O4@SiO2–NH2 and study of its activity and stability. Colloids Surf. B Biointerfaces 2018, 170, 553–562. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Z.; Wang, Z.; Lu, J.; Li, Y.; Cao, Y.; Cheng, H. Facile fabrication of acid-resistant and hydrophobic Fe3O4@SiO2@C magnetic particles for valid oil-water separation application. Surf. Interfaces 2020, 21, 100651. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Wang, F.; Wang, Y. Adsorption Performance of Amino Functionalized Magnetic Molecular Sieve Adsorbent for Effective Removal of Lead Ion from Aqueous Solution. Nanomaterials 2021, 11, 2353. [Google Scholar] [CrossRef]
- Kudaibergenova, R.; Ualibek, O.; Sugurbekov, E.; Demeuova, G.; Frochot, C.; Acherar, S.; Sugurbekova, G. Reduced Graphene Oxide-Based Superhydrophobic Magnetic Nanomaterial as High Selective and Recyclable Sorbent for Oil/Organic Solvent Wastewater Treatment. Int. J. Environ. Sci. Technol. 2022, 19, 8491–8506. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Abdul Rahim, S.H.; Proverbio, E.; Milone, C. Assessment of Sorption Kinetics of Carbon Nanotube-based Composite Foams for Oil Recovery Application. J. Appl. Polym. Sci. 2019, 136, 47374. [Google Scholar] [CrossRef]
- Zhu, Y.; Miao, J.; Zhang, Y.; Li, C.; Wang, Y.; Cheng, Y.; Long, M.; Wang, J.; Wu, C. Carbon nanotubes production from real-world waste plastics and the pyrolysis behaviour. Waste Manag. 2023, 166, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Moretto, S.; Silva, A.S.; de Tuesta, J.L.D.; Roman, F.F.; Cortesi, R.; Bertão, A.R.; Bañobre-López, M.; Pedrosa, M.; Silva, A.M.; Gomes, H.T. Comprehensive Characterization and Development of Multi-Core Shell Superparamagnetic Nanoparticles for Controlled Delivery of Drugs and Their Kinetic Release Modelling. Mater. Today Chem. 2023, 33, 101748. [Google Scholar] [CrossRef]
- Roman, F.F.; Diaz de Tuesta, J.L.; Sanches, F.K.K.; Silva, A.S.; Marin, P.; Machado, B.F.; Serp, P.; Pedrosa, M.; Silva, A.M.T.; Faria, J.L.; et al. Selective Denitrification of Simulated Oily Wastewater by Oxidation Using Janus-Structured Carbon Nanotubes. Catal. Today 2023, 420, 114001. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Kompotiatis, L.; Kontogeorgakos, A.; Kordas, G. Microwave Behavior of Ferrites Prepared via Sol–Gel Method. J. Magn. Magn. Mater. 2002, 246, 360–365. [Google Scholar] [CrossRef]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Production and Application of Carbon Nanotubes, as a Co-Product of Hydrogen from the Pyrolysis-Catalytic Reforming of Waste Plastic. Process Saf. Environ. Prot. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Silva, A.S.; Roman, F.F.; Sanches, L.F.; da Silva, F.A.; Pereira, A.I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Polyolefin-Derived Carbon Nanotubes as Magnetic Catalysts for Wet Peroxide Oxidation of Paracetamol in Aqueous Solutions. Catal. Today 2023, 419, 114162. [Google Scholar] [CrossRef]
- Kudaibergenova, R.; Sugurbekov, Y.; Demeuova, G.; Sugurbekova, G. Facile Fabrication of High-Performance Superhydrophobic Reusable Oil-Absorbing Sponges. Coatings 2023, 13, 1777. [Google Scholar] [CrossRef]
- Silva, A.S.; Roman, F.F.; Dias, A.V.; Diaz de Tuesta, J.L.; Narcizo, A.; da Silva, A.P.F.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Ferrari, A.M.C.; et al. Hybrid Multi-Core Shell Magnetic Nanoparticles for Wet Peroxide Oxidation of Paracetamol: Application in Synthetic and Real Matrices. J. Environ. Chem. Eng. 2023, 11, 110806. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Shrivastava, V.S. Facile Synthesis of Nickel Oxide Nanoparticles for the Degradation of Methylene Blue and Rhodamine B Dye: A Comparative Study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and Reduction of Multiwalled Carbon Nanotubes—Preparation and Characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wu, J.; Fan, J.; Wu, Y. Qualitatively and quantitatively characterizing water adsorption of a cellulose nanofiber film using micro-FTIR spectroscopy. RSC Adv. 2018, 8, 4214–4220. [Google Scholar] [CrossRef]
- Cherpin, C.; Lister, D.; Dacquait, F.; Liu, L. Study of the Solid-State Synthesis of Nickel Ferrite (NiFe2O4) by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Raman Spectroscopy. Materials 2021, 14, 2557. [Google Scholar] [CrossRef]
- Sugurbekova, G.; Sugurbekov, E.; Demeuova, G.; Gabdullina, A.; Kudaibergenova, R. Magnetic Behavior of MFe2O4 Nanopowders for Water Treatment. Key Eng. Mater. 2024, 974, 83–90. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles—A comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Ngoma, M.M.; Mathaba, M.; Moothi, K. Effect of Carbon Nanotubes Loading and Pressure on the Performance of a Polyethersulfone (PES)/Carbon Nanotubes (CNT) Membrane. Sci. Rep. 2021, 11, 23805. [Google Scholar] [CrossRef]
- Arunkumar, T.; Karthikeyan, R.; Ram Subramani, R.; Viswanathan, K.; Anish, M. Synthesis and Characterisation of Multi-Walled Carbon Nanotubes (MWCNTs). Int. J. Ambient Energy 2020, 41, 452–456. [Google Scholar] [CrossRef]
- Silva, A.S.; Diaz de Tuesta, J.L.; Sayuri Berberich, T.; Delezuk Inglez, S.; Bertão, A.R.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Gomes, H.T. Doxorubicin Delivery Performance of Superparamagnetic Carbon Multi-Core Shell Nanoparticles: PH Dependence, Stability and Kinetic Insight. Nanoscale 2022, 14, 7220–7232. [Google Scholar] [CrossRef]
- Veksha, A.; Chen, W.; Liang, L.; Lisak, G. Converting Polyolefin Plastics into Few-Walled Carbon Nanotubes via a Tandem Catalytic Process: Importance of Gas Composition and System Configuration. J. Hazard. Mater. 2022, 435, 128949. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tan, S.; Song, R. Universal Ni-Mo-Mg Catalysts Combined with Carbon Blacks for the Preparation of Carbon Nanotubes from Polyolefins. J. Appl. Polym. Sci. 2017, 134, 44647. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Hong, R. Solvothermal Reflux Synthesis of NiFe2O4 Nanocrystals Dielectric and Magnetic Hyperthermia Properties. J. Mater. Sci. Mater. Electron. 2020, 31, 799–806. [Google Scholar] [CrossRef]
- Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, C.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Development of Novel Magnetoliposomes Containing Nickel Ferrite Nanoparticles Covered with Gold for Applications in Thermotherapy. Materials 2020, 13, 815. [Google Scholar] [CrossRef]
- Lipert, K.; Ritschel, M.; Leonhardt, A.; Krupskaya, Y.; Büchner, B.; Klingeler, R. Magnetic Properties of Carbon Nanotubes with and without Catalyst. J. Phys. Conf. Ser. 2010, 200, 072061. [Google Scholar] [CrossRef]
- Fan, S.; Tang, L.; Zhao, X.; Xu, G.; Fan, W. Facile Preparation of Durable Superhydrophobic Coating by Liquid-Phase Deposition for Versatile Oil/Water Separation. Coatings 2023, 13, 925. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, S.W.; Kim, J.H.; Seo, H.O.; Kim, Y.D. Oil Absorption Capacity of Bare and PDMS-Coated PET Non-Woven Fabric; Dependency of Fiber Strand Thickness and Oil Viscosity. Curr. Appl. Phys. 2018, 18, 369–376. [Google Scholar] [CrossRef]
- Xiang, W.; Gong, S.; Zhu, J. Eco-Friendly Fluorine Functionalized Superhydrophobic/Superoleophilic Zeolitic Imidazolate Frameworks–Based Composite for Continuous Oil–Water Separation. Molecules 2023, 28, 2843. [Google Scholar] [CrossRef]
- Belachew, G.B.; Hu, C.-C.; Chang, Y.-Y.; Wang, C.-F.; Hung, W.-S.; Chen, J.-K.; Lai, J.-Y. An Eco-Friendly Manner to Prepare Superwetting Melamine Sponges with Switchable Wettability for the Separation of Oil/Water Mixtures and Emulsions. Polymer 2024, 16, 693. [Google Scholar] [CrossRef]
- Wang, C.-F.; Lin, S.-J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef]
- Turco, A.; Malitesta, C.; Barillaro, G.; Greco, A.; Maffezzoli, A.; Mazzotta, E. A Magnetic and Highly Reusable Macroporous Superhydrophobic/Superoleophilic PDMS/MWNT Nanocomposite for Oil Sorption from Water. J. Mater. Chem. A Mater. 2015, 3, 17685–17696. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Liu, Q.; Tong, Q.; Wang, B. Fabrication of PDMS@Fe3O4/MS Composite Materials and Its Application for Oil-Water Separation. Materials 2021, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, R.; Ge, W.; Xie, A.; Chang, Z.; Tian, S.; Zhou, Z.; Yan, Y. 3D Macroscopic Superhydrophobic Magnetic Porous Carbon Aerogel Converted from Biorenewable Popcorn for Selective Oil-Water Separation. Mater. Des. 2018, 139, 122–131. [Google Scholar] [CrossRef]
- Kim, D.W.; Eum, K.; Kim, H.; Kim, D.; de Mello, M.D.; Park, K.; Tsapatsis, M. Continuous ZIF-8/Reduced Graphene Oxide Nanocoating for Ultrafast Oil/Water Separation. Chem. Eng. J. 2019, 372, 509–515. [Google Scholar] [CrossRef]



| Absorbents | Organics | Q, g/g | References |
|---|---|---|---|
| PU/CNT/NiFe2O4/PDMS | Crude oil, olive oil, chloroform, toluene, acetone, ethanol, gasoline, hexane | 21.38–44.83 | In this work |
| CNT/PDMS-coated PU sponge | Soybean oil, used motor oil, diesel oil, n-hexadecane, gasoline, n-hexane | 15–25 | [50] |
| Fe3O4-PDMS/MWNTs sponge | Dichloromethane, petroleum ether, hexane, chloroform, tetrahydrofuran, toluene, gasoline | 8.5–20 | [51] |
| PDMS@Fe3O4/MS sponge | kerosene, diesel, corn germ oil | 14.68–17.99 | [52] |
| Fe3O4@carbon aerogel | Engine oil, chloroethane, corn oil | 10–11 | [53] |
| PU/MgFe2O4/RGO/SO sponge | Crude oil, olive oil, chloroform, toluene, ethanol, acetone, hexane | 16.61–44.86 | [28] |
| PU/MgFe2O4/SO sponge | Olive oil, chloroform, toluene, ethanol, acetone, hexane | 3.5–19 | [28] |
| ZIF-8/rGO/PU foam | Chloroform, hexane, acetonitrile, toluene, acetone, methanol, ethanol, isopropyl alcohol, butanol, octanol, ethylene glycol | 15–35 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudaibergenova, R.M.; Roman, F.F.; Silva, A.S.; Sugurbekova, G.K. Carbon-Nanotube-Based Superhydrophobic Magnetic Nanomaterial as Absorbent for Rapid and Efficient Oil/Water Separation. Nanomaterials 2024, 14, 1942. https://doi.org/10.3390/nano14231942
Kudaibergenova RM, Roman FF, Silva AS, Sugurbekova GK. Carbon-Nanotube-Based Superhydrophobic Magnetic Nanomaterial as Absorbent for Rapid and Efficient Oil/Water Separation. Nanomaterials. 2024; 14(23):1942. https://doi.org/10.3390/nano14231942
Chicago/Turabian StyleKudaibergenova, Rabiga M., Fernanda F. Roman, Adriano S. Silva, and Gulnar K. Sugurbekova. 2024. "Carbon-Nanotube-Based Superhydrophobic Magnetic Nanomaterial as Absorbent for Rapid and Efficient Oil/Water Separation" Nanomaterials 14, no. 23: 1942. https://doi.org/10.3390/nano14231942
APA StyleKudaibergenova, R. M., Roman, F. F., Silva, A. S., & Sugurbekova, G. K. (2024). Carbon-Nanotube-Based Superhydrophobic Magnetic Nanomaterial as Absorbent for Rapid and Efficient Oil/Water Separation. Nanomaterials, 14(23), 1942. https://doi.org/10.3390/nano14231942








