Enhancing Maize Yield and Soil Health through the Residual Impact of Nanomaterials in Contaminated Soils to Sustain Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Area
2.2. Pot Experiment
2.3. Analysis of Soil Samples and Nanobiochar
2.4. Catalase Activity
2.5. Dehydrogenase Activity (DHA)
2.6. Microbial Biomass Carbon
2.7. Statistical Analysis
3. Results
3.1. Residual Effect of Nanomaterials on Remediation of Some Heavy Metals
3.2. Residual Effect of Nanomaterials on Some Soil Properties
3.3. Residual Effect of Nanomaterials on Soil Biological Activity
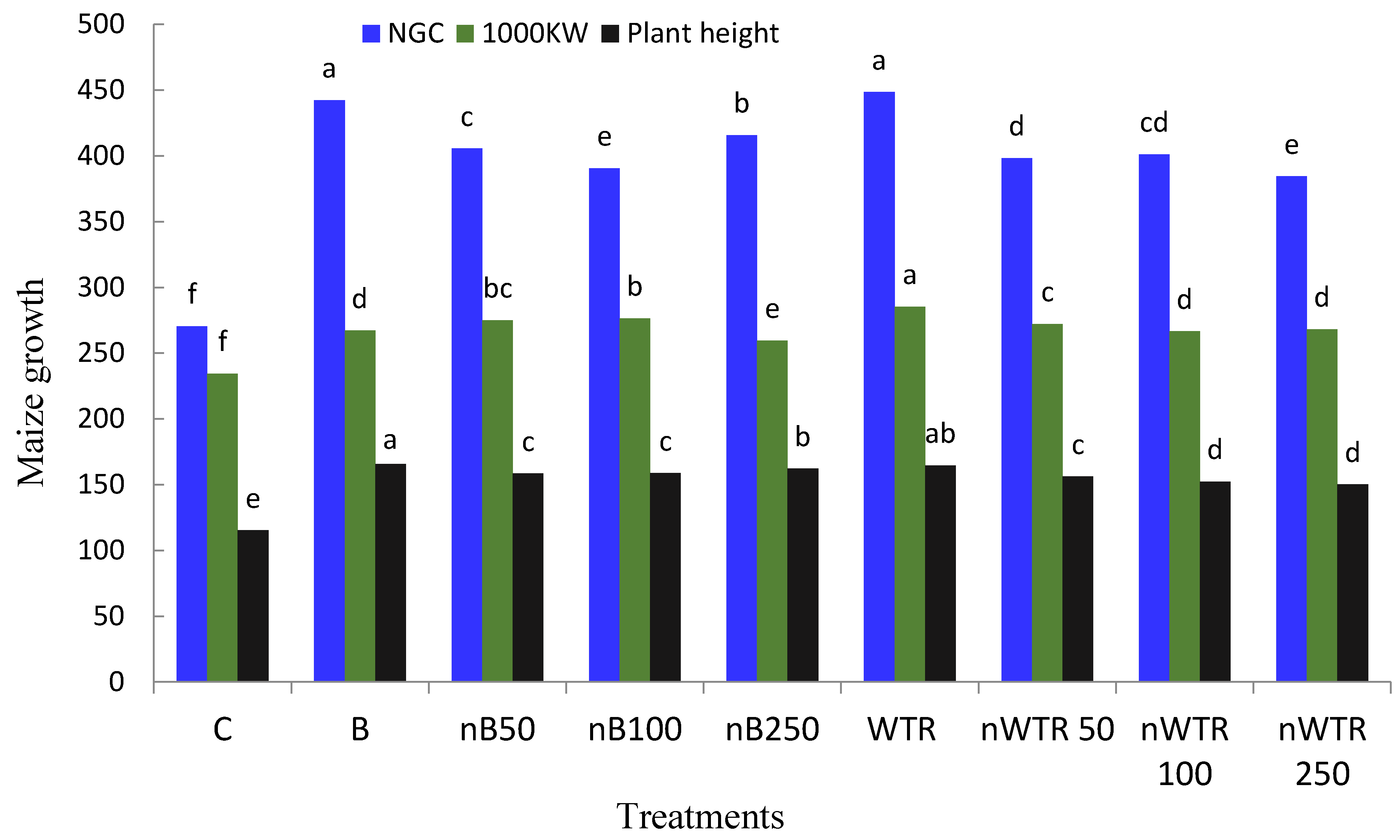
3.4. Residual Effect of nB and nWTR on Maize Growth
4. Discussion
4.1. Residual Effect of Nanomaterials on Immobilization of Some Heavy Metals
4.2. Residual Effect of nB and nWTR on Soil Properties
4.3. Residual Effect of nB and nWTR on Enzymatic Activity
4.4. Residual Effect of nB and nWTR on Maize Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoud, E.; El Baroudy, A.; Ali, N.; Sleem, M. Soil amendment with nanoresidues from water treatment increases P adsorption in saline soils. Environ. Chem. Lett. 2020, 18, 171–179. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander, E.L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Farooqui, A.R.; Tabassum, H.E.; Ahmad, A.S.; Mabood, A.B.; Ahmad, A.D.; Ahmad, I.Z. Role of nanoparticles in growth and development of plant. Int. J. Pharma Bio Sci. 2016, 7, 22–37. [Google Scholar] [CrossRef]
- Pratap, T.; Patel, M.; Pittman, C.U.; Nguyen, T.A.; Mohan, D. Chapter 23—Nanobiochar: A sustainable solution for agricultural and environmental applications. In Nanomaterials for Soil Remediation; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Elsawy, H.; El-shahawy, A.; Ibrahim, M.; AbdEl-Halim, A.; Talha, N.; Sedky, A.; Alfwuaires, M.; Alabbad, H.; Almeri, N.; Mahmoud, E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture 2022, 12, 2096. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Ahmed, B.; Singh, V.K.; Mandzhieva, S.; Sushkova, S.; Wang, B. Nano-biochar: A novel solution for sustainable agriculture and environmental remediation. Environ. Res. 2022, 210, 11289. [Google Scholar] [CrossRef]
- El-shahawy, A.; Ibrahim, M.; AbdEl-Halim, A.H.; Talha, N.; Mahmoud, E. Spectroscopic and chemical properties of biochar and water treatment residual nanoparticles and their effects on canola growth and biological activity. Agriculture 2022, I. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, X.; Wang, Q.; Wei, W.; Zhang, T. Effects of nano carbon on soil erosion and nutrient loss in a semi-arid loess region of Northwestern China. Int. J. Agric. Biol. Eng. 2018, 11, 138–145. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Wang, X.; Sun, Q.; Dong, H.; Wang, G.; Chen, X.; Yin, C.; Han, Z.; Mao, Z. Effects of biochar on the growth of apple seedlings, soil enzyme activities and fungal communities in replant disease soil. Sci. Hortic. 2019, 256, 108641. [Google Scholar] [CrossRef]
- Mahdy, A.; Elkhatib, E.; Zhang, T.Q.; Fathi, N.; Lin, Z. Nano-Scale Drinking Water Treatment Residues Affect Arsenic Fractionation and Speciation in Biosolids-Amended Agricultural Soil. Appl. Sci. 2020, 10, 5633. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Mahdy, A.M.; Sherif, F.K.; Salama, K.A. Water treatment residual nanoparticles: A novel sorbent for enhanced phosphorus removal from aqueous medium. Curr. Nanosci. 2015, 11, 655–668. [Google Scholar] [CrossRef]
- Ginting, D.; Kessavalou, A.; Eghball, B.; Doran, J.W. Greenhouse gas emissions and soil indicators four years after manure and compost applications. J. Environ. Qual. 2003, 32, 23–32. [Google Scholar] [CrossRef]
- Eghball, B.; Power, J.F. Phosphorus and nitrogen based manure and compost applications corn production and soil phosphorus. Soil Sci. Soc. Am. J. 1999, 63, 895–901. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Shivashankar, K. Effect of organic matter and phosphorus on growth and yield of soybean (Glycine max). Indian J. Agron. 1999, 41, 65–68. [Google Scholar]
- Shehzadi, S.; Shah, Z.; Mohammad, W. Residual effect of organic wastes and chemical fertilizers on wheat yield under wheat-maize cropping sequence. Soil Environ. 2014, 33, 88–95. [Google Scholar]
- Rathore, A.L.; Chipde, S.J.; Pal, A.R. Direct and residual effects of bio-organic and inorganic fertilizers in rice (Oryza sativa)-wheat (Triticum aestivum) cropping system. Indian J. Agron. 1995, 40, 14–19. [Google Scholar] [CrossRef]
- Shah, Z.; Shah, T. Residual effect of biochar on soil properties and yield of maize (Zea mays L.) under different cropping systems. Open J. Soil Sci. 2018, 8, 16–35. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO); FAO: Rome, Italy, 2020. [Google Scholar]
- US EPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites, OSWER 9355.4-24; Office of Emergency and Remedial Response, United States Environmental Protection Agency: Washington DC, USA, 2002.
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis. In Total Carbon, Organic Carbon and Organic Matter; Page, A.L., Miller, R.H., Kenny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Olsen, S.R.; Watanabe, F.S.; Cosper, H.R.; Larson, W.E.; Nelson, L.B. Residual phosphorus availability in long-time rotations on calcareous soils. Soil Sci. 1954, 78, 141–152. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture: Washington, DC, USA, 2014.
- Grossman, R.B.; Reinsch, T.G. Bulk Density and Linear Extensibility. In Methods of Soil Analysis: Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 201–228. [Google Scholar]
- Graber, E.R.; Singh, B.; Lehmann, K.H. Determination of Cation Exchange Capacity in Biochar. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; Csiro Publishing: Clayton, Australia, 2017; pp. 74–84. [Google Scholar]
- Lindsay, W.L.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Johnson, I.; Temple, K.L. Some variables affecting the measurement of catalase activity in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–216. [Google Scholar] [CrossRef]
- Minczewski, J.; Marczenko, Z. Separation and Spectrophotometric Determination of Elements, 2nd ed.; Horwood: Chichester, UK, 1986. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der DehydrogenaseaktivitAt im Boden mittels triphenytetrazoliumchlorid (TTC). Landwirtsch. Forsch 1968, 21, 249–258. [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction, an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Hu, X.; Cao, C.; Zhiping, A. Size and activity of the soil microbial biomass and chemical and biological properties. Commun. Soil Sci. Plant Anal. 2007, 40, 2072–2086. [Google Scholar]
- Yu, X.; Amrhein, C.; Deshusses, M.A.; Matsumoto, M.R. perchlorate reduction by autotrophic bacteria in the presence of zero-valent iron. Environ. Sci. Technol. 2006, 40, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Quinones, K.D.; Hovsepyan, A.; Oppong-Anane, A.; Bonzongo, J.C.J. Insights into the mechanisms of mercury sorption onto aluminum-based drinking water treatment residuals. J. Hazard. Mater. 2016, 307, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical fow constructed wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B.; Hu, D. Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ. Sci. Technol. 2013, 47, 2737–2745. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochar. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef]
- Mahmoud, A.H.; Saleh, M.E.; Abdel-Salam, A.A. Effect of Rice Husk Biochar on Cadmium Immobilization in Soil and Uptake by Wheat Plant Grown on Lacustrine Soil. Alex. J. Agric. Res. 2011, 56, 117–125. [Google Scholar]
- Gunarathne, V.; Senadheera, J.A.I.; Gunarathne, U.; Almaroai, Y.A.; Vithanage, M. Reclamation of Salt-Affected Soils. In Soil and Groundwater Remediation Technologies; CRC Press: Boca Raton, FL, USA, 2020; pp. 183–199. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving salt leaching in a simulated saline soil column by three biochars derived from rice straw (Oryza sativa L.), sunflower straw (Helianthus annuus), and cow manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Wang, Z.; Li, Y.; Wang, L.; Ding, L.; Gao, X.; Ma, Y.; Guo, Y. Application studies of activated carbon derived from rice husks produced by chemical-thermal process—A review. Adv. Colloid Interface Sci. 2011, 163, 39–52. [Google Scholar] [CrossRef]
- Apori, S.O.; Byalebeka, J. Contribution of corncob biochar to the chemical properties of a ferralsol in Uganda. Arab. J. Geosci. 2021, 14, 1290. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zhang, S.; Huang, L.; Wang, H.; Wang, W.; Ye, Q. Starch-based hydrogel loading with carbendazim for controlled-release and water absorption. Carbohydr. Polym. 2015, 125, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.; Leifeld, J.; Knicker, H.; Dufey, J.E.; Deforce, K.; Cornélis, J.T. Long term change in chemical properties of preindustrial charcoal particles aged in forest and agricultural temperate soil. Org. Geochem. 2017, 107, 33–45. [Google Scholar] [CrossRef]
- Petersen, S.O.; Frohne, P.S.; Kennedy, A.C. Dynamics of a soil microbial community under spring wheat. Soil Sci. Soc. Am. J. 2002, 66, 826–833. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Pinna, M.V.; Castaldi, P.; Deiana, P.; Pusino, A.; Garau, G. Sorption behavior of sulfamethazine on unamended and manure-amended soils and short-term impact on soil microbial community. Ecotoxicol. Environ. Saf. 2012, 84, 234–242. [Google Scholar] [CrossRef]
- Bergkvist, P.; Jarvis, N.; Berggren, D.; Carlgren, K. Long-term effects of sewage sludge applications on soil properties, cadmium availability and distribution in arable soil. Agric. Ecosyst. Environ. 2003, 97, 167–179. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631, 1175–1191. [Google Scholar] [CrossRef]
- Mapfumo, P.; Mtambanengwe, F.; Vanlauwe, B. Organic matter quality and management effects on enrichment of soil organic matter fractions in contrasting soils in Zimbabwe. Plant Soil 2007, 296, 137–150. [Google Scholar] [CrossRef]
- Ali, M.; Ghaloo, S.H.; Ahmad, M. Influence of various water stress and potassium levels on the quality parameters of canola. Artech J. Res. Stud. Agric. Sci. 2018, 1, 1–6. [Google Scholar]
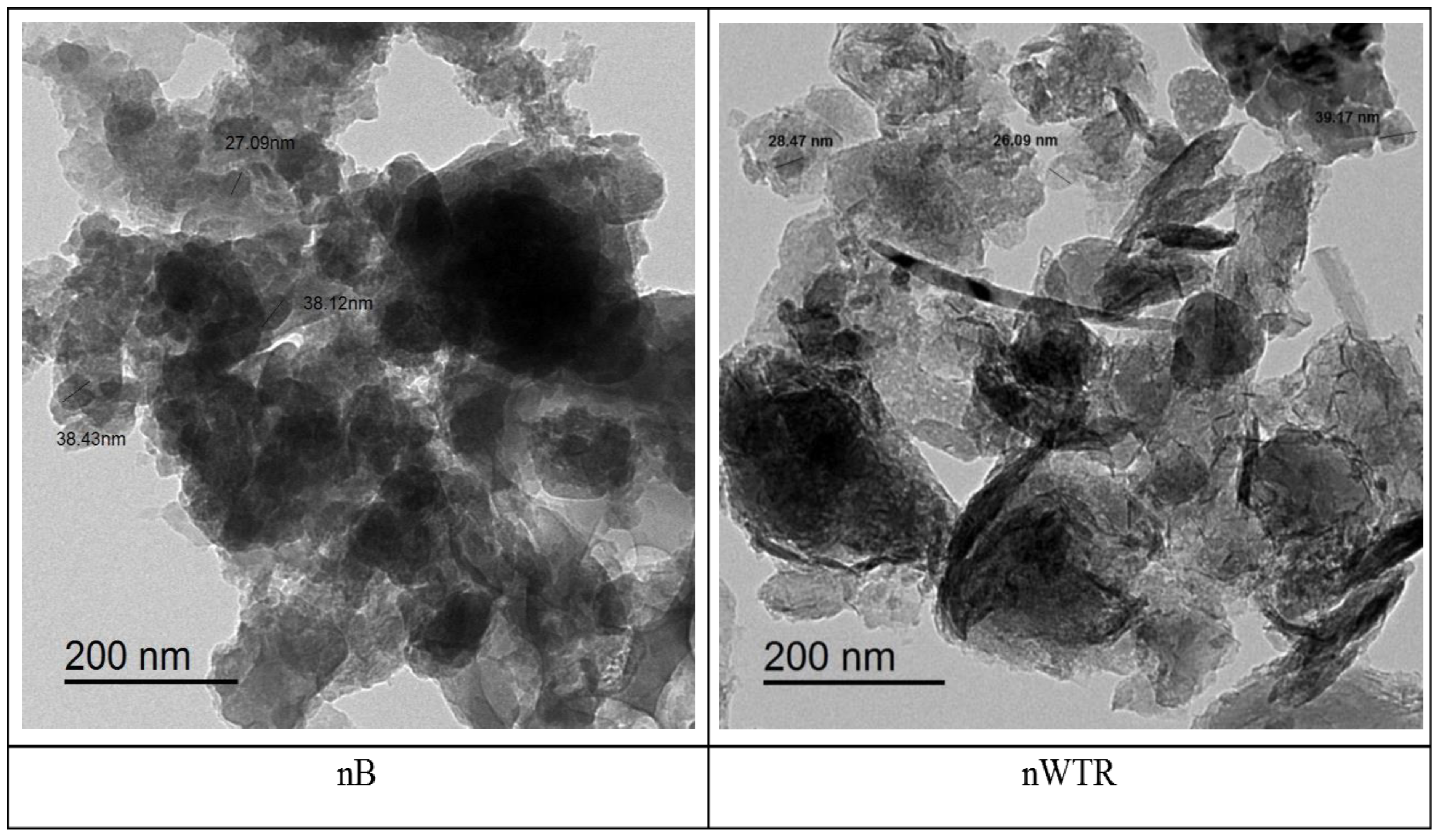
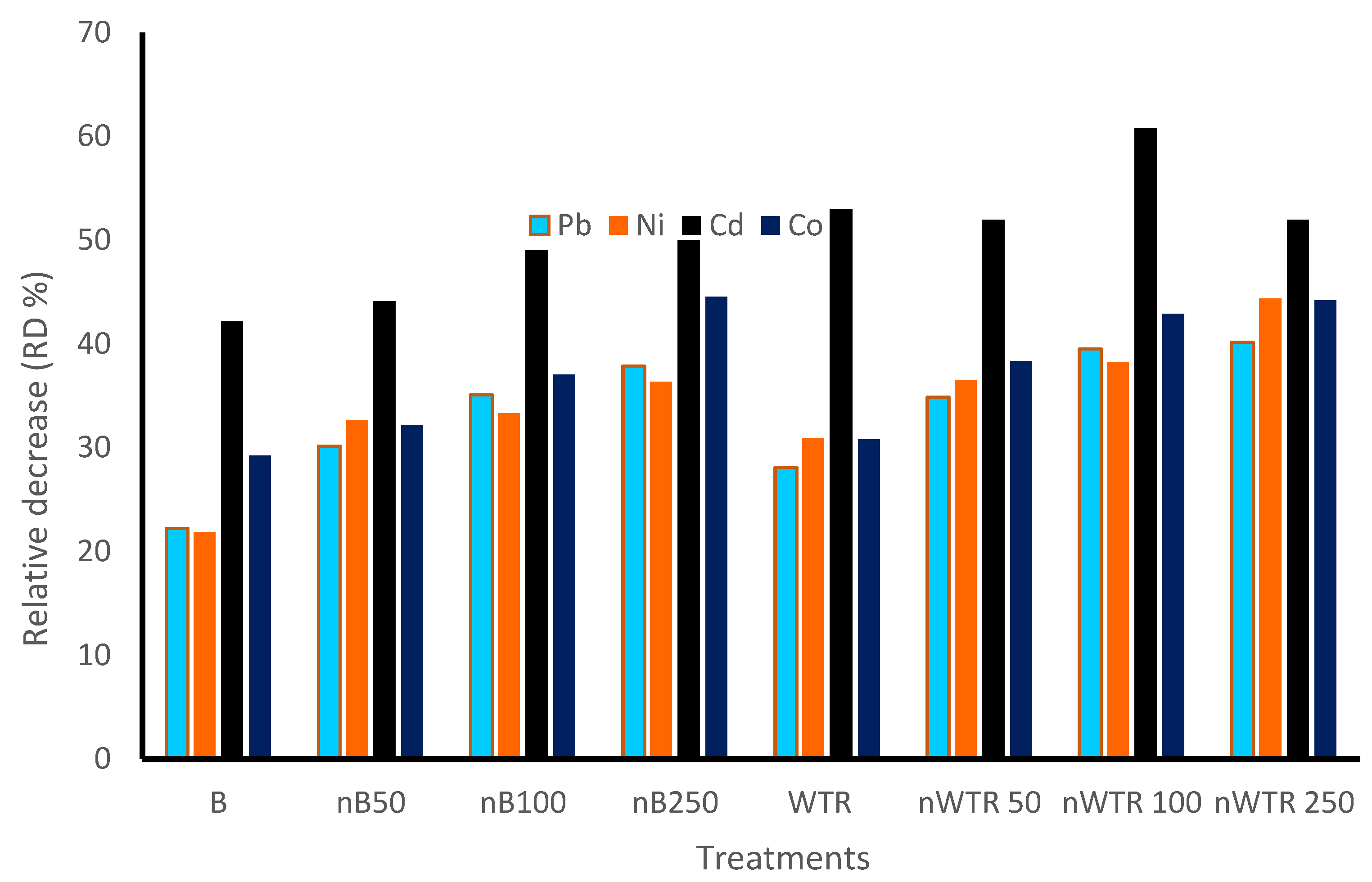
| Characteristics | Units | Soil | nB | nWTR |
|---|---|---|---|---|
| Sand | % | 12.56 | - | - |
| Silt | 28.26 | - | - | |
| Clay | 59.18 | - | 68.6 | |
| Soil texture | clay | - | - | |
| pH | 7.84 | 8.24 | 7.49 | |
| EC | dSm−1 | 3.78 | 2.45 | 1.12 |
| Ca++ | meqL−1 | 7.9 | 55.11 | 5.56 |
| Mg++ | 4.5 | 24.30 | 5.50 | |
| K+ | 0.57 | - | - | |
| Na+ | 25.7 | - | - | |
| Cl− | 18 | - | - | |
| HCO3− | 2.5 | - | - | |
| SO4−− | 18.3 | - | - | |
| SAR | 10.29 | - | - | |
| OM | % | 1.51 | 49.8 | 4.82 |
| CEC | cmol(+) kg−1 | 31.3 | 38.85 | |
| Total N | % | - | 1.42 | 0.98 |
| Total P | % | - | 0.96 | 0.06 |
| Total K | % | - | 1.42 | 0.65 |
| Total Al | % | - | - | 0.25 |
| Available P | mg kg−1 | 45.6 | 856.2 | 14.46 |
| Treatment | Pb | Relative Decrease (RD %) of Pb | Ni | Relative Decrease (RD %) of Ni | Cd | Relative Decrease (RD %) of Cd | Co | Relative Decrease (RD %) of Co |
|---|---|---|---|---|---|---|---|---|
| C | 62.12 a | 12.48 a | 0.204 a | 14.63 a | ||||
| B | 48.32 b | 22.21 | 9.75 b | 21.87 | 0.118 b | 42.15 | 10.35 b | 29.25 |
| nB50 | 43.38 cd | 30.16 | 8.4 bc | 32.69 | 0.114 b | 44.11 | 9.92 bc | 32.19 |
| nB100 | 40.32 def | 35.09 | 8.32 bc | 33.33 | 0.104 b | 49.01 | 9.21 bcd | 37.04 |
| nB250 | 38.59 ef | 37.87 | 7.94 bc | 36.37 | 0.102 b | 50 | 8.11 d | 44.56 |
| WTR | 44.64 c | 28.13 | 8.62 bc | 30.92 | 0.096 b | 52.94 | 10.12 b | 30.82 |
| nWTR50 | 40.46 de | 34.86 | 7.92 c | 36.53 | 0.098 b | 51.96 | 9.02 bcd | 38.34 |
| nWTR100 | 37.57 ef | 39.52 | 7.71 c | 38.22 | 0.08 b | 60.78 | 8.35 cd | 42.92 |
| nWTR250 | 37.17 f | 40.16 | 6.94 c | 44.39 | 0.098 b | 51.96 | 8.16 d | 44.22 |
| F-test | ** | ** | ** | ** | ||||
| LSD(0.05) | 2.387 | 1.35 | 0.032 | 1.182 | ||||
| LSD(0.01) | 3.271 | 1.815 | 0.044 | 1.621 |
| Treatment | EC | pH | SAR | Anions | Cations | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | HCO3− meq L−1 | SO4−− | Ca++ | Mg++ | Na+ meq L−1 | K+ | ||||
| C | 3.92 a | 7.89 a | 10.48 a | 18.70 a | 4.50 ab | 16.90 a | 8.20 a | 4.70 a | 26.70 a | 0.53 b |
| B | 3.08 ab | 7.68 a | 9.51 ab | 14.70 b | 2.50 c | 14.06 b | 6.50 b | 3.70 ab | 20.90 b | 0.82 a |
| nB50 | 2.17 bc | 7.74 a | 7.80 bc | 10.30 c | 2.50 c | 9.60 c | 4.60 c | 2.60 bc | 14.80 c | 0.53 b |
| nB100 | 2.03 c | 7.79 a | 7.43 c | 9.40 c | 2.50 c | 8.70 cd | 4.10 c | 2.40 c | 13.40 c | 0.66 ab |
| nB250 | 2.18 bc | 7.65 a | 7.63 c | 9.90 c | 2.50 c | 9.20 c | 4.40 c | 2.50 c | 14.10 c | 0.62 ab |
| WTR | 2.65 bc | 7.67 a | 6.86 c | 9.40 c | 3.00 bc | 8.50 cd | 4.10 c | 2.40 c | 13.40 c | 0.49 b |
| nWTR 50 | 2.49 bc | 7.71 a | 8.60 bc | 10.60 c | 3.00 bc | 9.40 c | 4.30 c | 2.10 c | 15.40 c | 0.55 b |
| nWTR 100 | 2.18 bc | 7.68 a | 7.82 bc | 10.40 c | 5.00 a | 7.20 d | 4.60 c | 2.60 bc | 14.80 c | 0.59 b |
| nWTR 250 | 2.01 c | 7.69 a | 6.94 c | 8.90c | 2.50 c | 7.10 d | 3.60 c | 2.10 c | 11.70 c | 0.63 ab |
| F-test | ** | NS | ** | ** | ** | ** | ** | ** | ** | * |
| LSD(0.05) | 0.678 | 1.821 | 1.541 | 3.039 | 1.357 | 1.715 | 1.443 | 0.958 | 3.470 | 0.156 |
| LSD(0.01) | 0.93 | 2.49 | 2.11 | 4.16 | 1.86 | 2.35 | 1.97 | 1.31 | 4.75 | 0.21 |
| Treatments | Exchangeable Cations (cmol kg−1) | CEC cmol kg−1 | ESP | |||
|---|---|---|---|---|---|---|
| Ca++ | Mg++ | Na+ | K+ | |||
| C | 20.88 d | 10.91 de | 9.52 a | 1.14 cd | 43.09 f | 22.09 a |
| B | 26.52 bc | 11.37 de | 9.05 b | 1.08 d | 48.92 cde | 18.49 b |
| nB50 | 25.16 c | 11.71 bcd | 8.76 c | 1.43 ab | 49.14 cde | 17.82 bc |
| nB100 | 25.78 c | 13.33 a | 8.12 d | 1.35 ab | 59.65 a | 16.35 c |
| nB250 | 30.84 a | 11.28 de | 8.95 bc | 1.46 a | 53.21 b | 16.82 bc |
| WTR | 28.61 ab | 10.63 e | 6.14 f | 1.34 ab | 47.03 e | 13.05 d |
| nWTR50 | 27.13 bc | 12.46 b | 6.83 e | 1.26 bc | 48.62 de | 14.04 d |
| nWTR100 | 29.42 a | 11.62 cd | 7.06 e | 1.37 ab | 49.83 cd | 14.16 d |
| nWTR250 | 30.73 a | 12.28 bc | 6.87 e | 1.41 ab | 51.46 bc | 13.35 d |
| F-test | ** | ** | ** | ** | ** | ** |
| LSD(0.05) | 1.632 | 0.816 | 0.284 | 0.171 | 2.018 | 1.295 |
| LSD(0.01) | 2.236 | 1.118 | 0.390 | 0.235 | 2.765 | 1.774 |
| Treatment | O.M % | Available Nutrients | ||
|---|---|---|---|---|
| N | P mg kg−1 | K | ||
| C | 1.23 c | 47.57 d | 41.7 a | 273 e |
| B | 1.56 a | 59.5 a | 45.6 a | 358.8 abcd |
| nB50 | 1.49 ab | 52.5 bcd | 44.4 a | 304.2 de |
| nB100 | 1.47 ab | 57.75 ab | 43.3 a | 421.2 ab |
| nB250 | 1.31 bc | 56 abc | 43.8 a | 390 abc |
| WTR | 1.49 ab | 54.25 abc | 41.6 a | 343.2 bcde |
| nWTR50 | 1.42 abc | 52.5 bcd | 45.6 a | 327.6 cde |
| nWTR100 | 1.46 abc | 54.25 abc | 43.4 a | 436.8 a |
| nWTR250 | 1.32 bc | 50.75 cd | 41.8 a | 374.4 abcd |
| F-test | * | ** | NS | ** |
| LSD(0.05) | 0.242 | 4.502 | 4.401 | 58.267 |
| LSD(0.01) | 0.332 | 6.168 | 6.03 | 79.831 |
| Treatments | Grain Yield t ha−1 | MBC mg kg−1 | DHA mg TPF/g Dry Soil | CLA (mL of 0.02 mol/L KMnO4 g−1) |
|---|---|---|---|---|
| C | 1.89 c | 165.7 f | 0.56 d | 0.04 g |
| B | 3.54 ab | 265.8 b | 0.66 c | 0.06 fg |
| nB50 | 3.05 b | 218.3 cd | 0.71 bc | 0.14 bc |
| nB100 | 2.92 b | 267.4 b | 0.69 bc | 0.08 efg |
| nB250 | 3.01 b | 271.6 b | 0.74 b | 0.13 bcd |
| WTR | 3.81 a | 224.9 c | 0.68 c | 0.11 cde |
| nWTR50 | 3.24 ab | 295.1 a | 0.78 a | 0.18 a |
| nWTR100 | 3.18 ab | 203.5 e | 0.69 bc | 0.16 b |
| nWTR250 | 3.06 b | 204.9 de | 0.67 c | 0.09 def |
| F-test | ** | ** | ** | ** |
| LSD(0.05) | 0.520 | 10.758 | 0.042 | 0.031 |
| LSD(0.01) | 0.712 | 14.739 | 0.057 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E.; El-shahawy, A.; Ibrahim, M.; Abd El-Halim, A.E.-H.A.; Abo-Ogiala, A.; Shokr, M.S.; Mohamed, E.S.; Rebouh, N.Y.; Ismail, S.M. Enhancing Maize Yield and Soil Health through the Residual Impact of Nanomaterials in Contaminated Soils to Sustain Food. Nanomaterials 2024, 14, 369. https://doi.org/10.3390/nano14040369
Mahmoud E, El-shahawy A, Ibrahim M, Abd El-Halim AE-HA, Abo-Ogiala A, Shokr MS, Mohamed ES, Rebouh NY, Ismail SM. Enhancing Maize Yield and Soil Health through the Residual Impact of Nanomaterials in Contaminated Soils to Sustain Food. Nanomaterials. 2024; 14(4):369. https://doi.org/10.3390/nano14040369
Chicago/Turabian StyleMahmoud, Esawy, Asmaa El-shahawy, Mahmoud Ibrahim, Abd El-Halim A. Abd El-Halim, Atef Abo-Ogiala, Mohamed. S. Shokr, Elsayed Said Mohamed, Nazih Y. Rebouh, and Sahar Mohamed Ismail. 2024. "Enhancing Maize Yield and Soil Health through the Residual Impact of Nanomaterials in Contaminated Soils to Sustain Food" Nanomaterials 14, no. 4: 369. https://doi.org/10.3390/nano14040369







