Mechanical Properties of Ti3AlC2/Cu Composites Reinforced by MAX Phase Chemical Copper Plating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
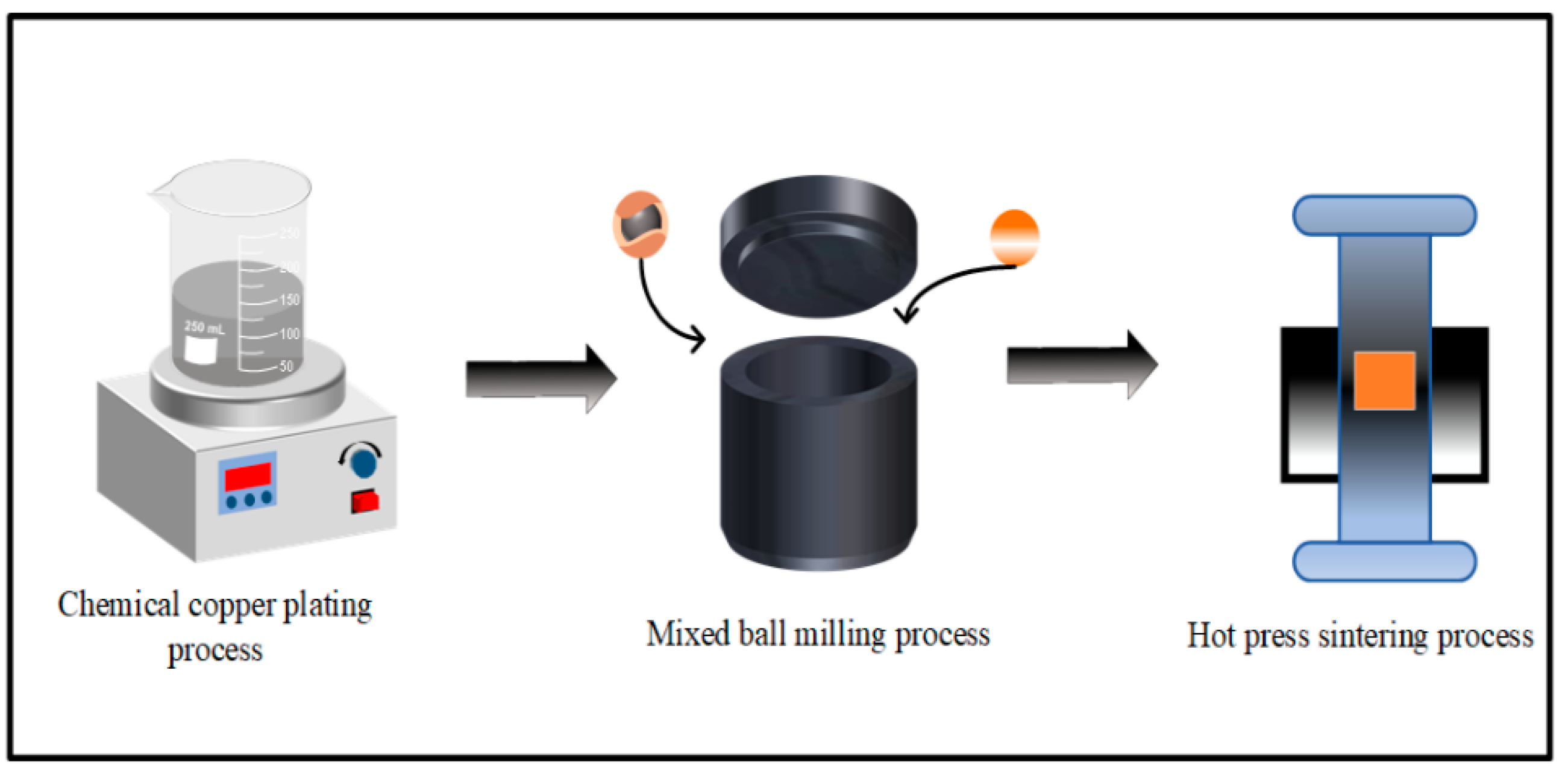
2.2. Preparation of Cu@Ti3AlC2 Powder
2.3. Preparation of Composite Material
2.4. Testing and Characterization
3. Results and Discussion
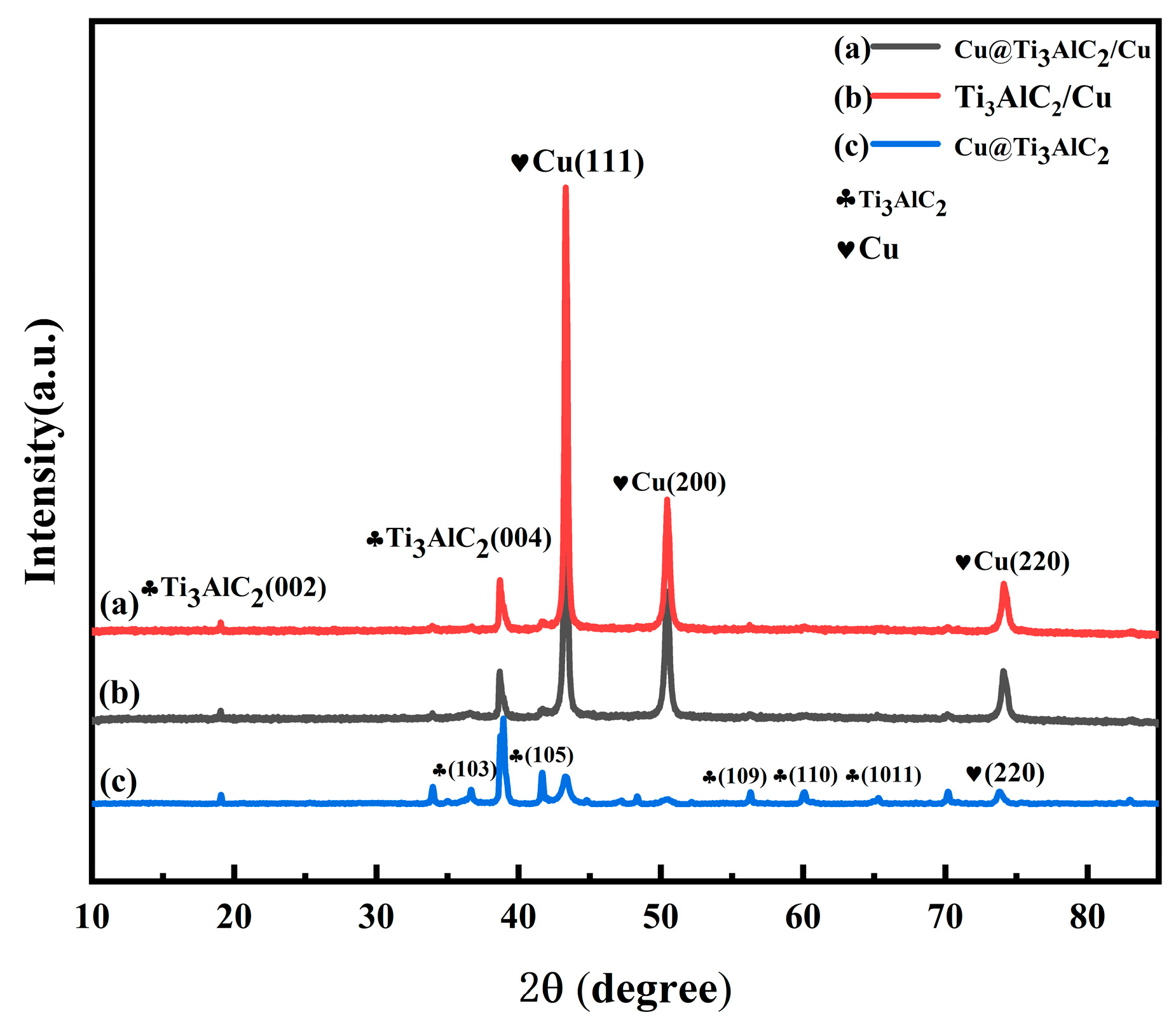
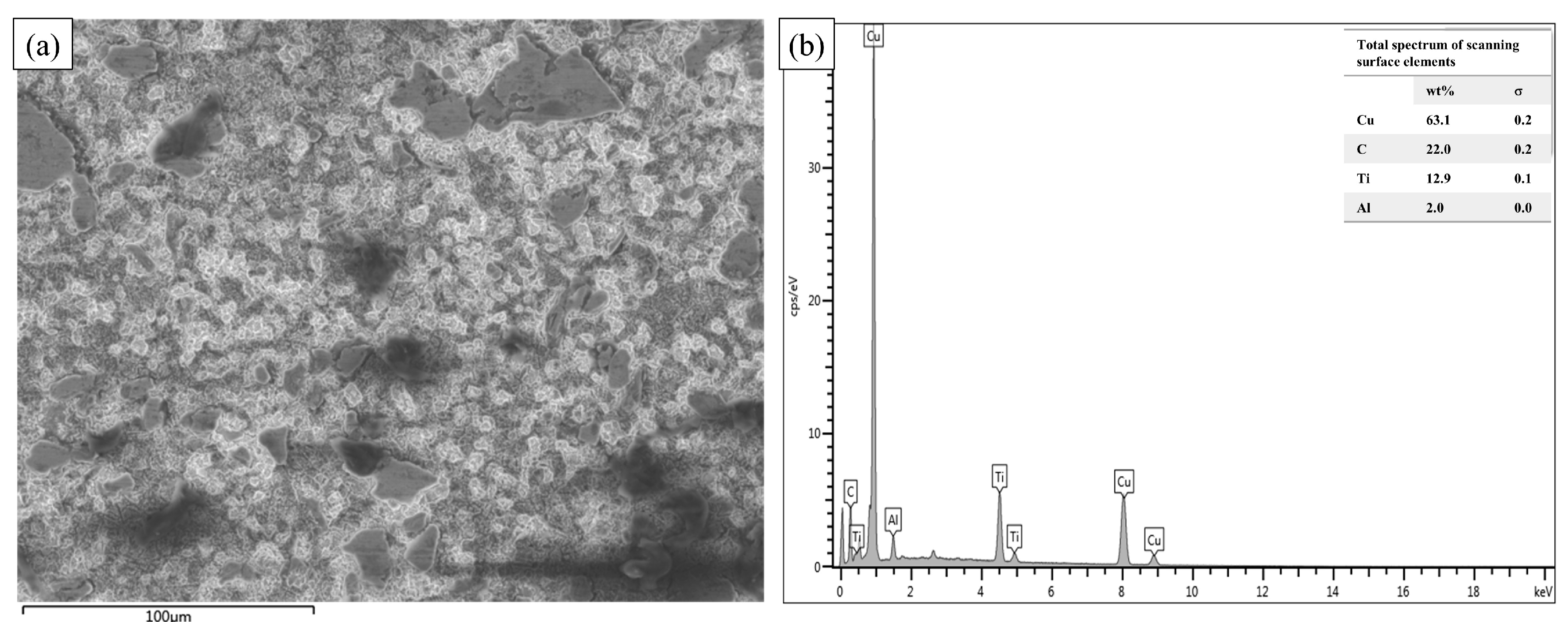
3.1. Physical Phase Analysis and Microstructure Characterization of Composite Powders
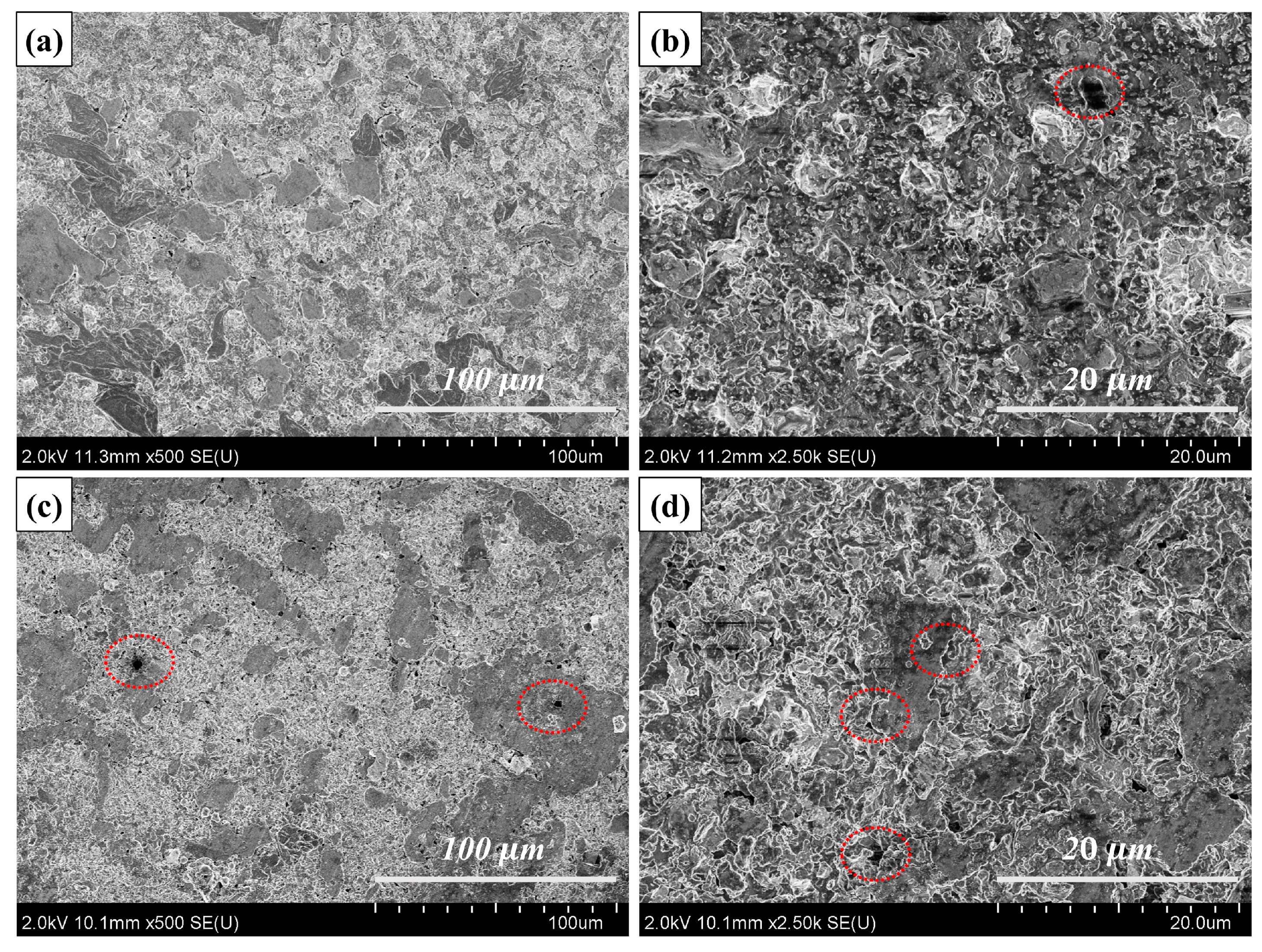
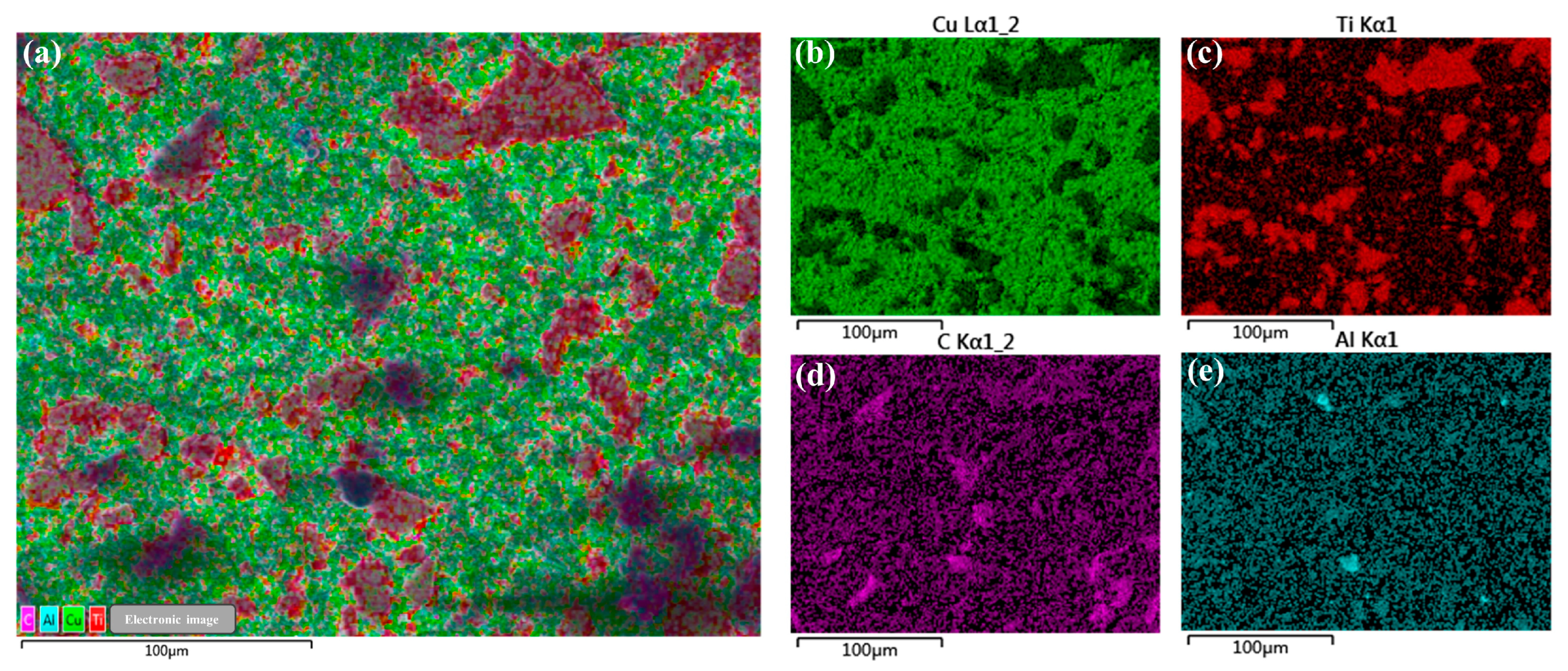
3.2. Physical Phase Analysis and Microcharacterization of Composites
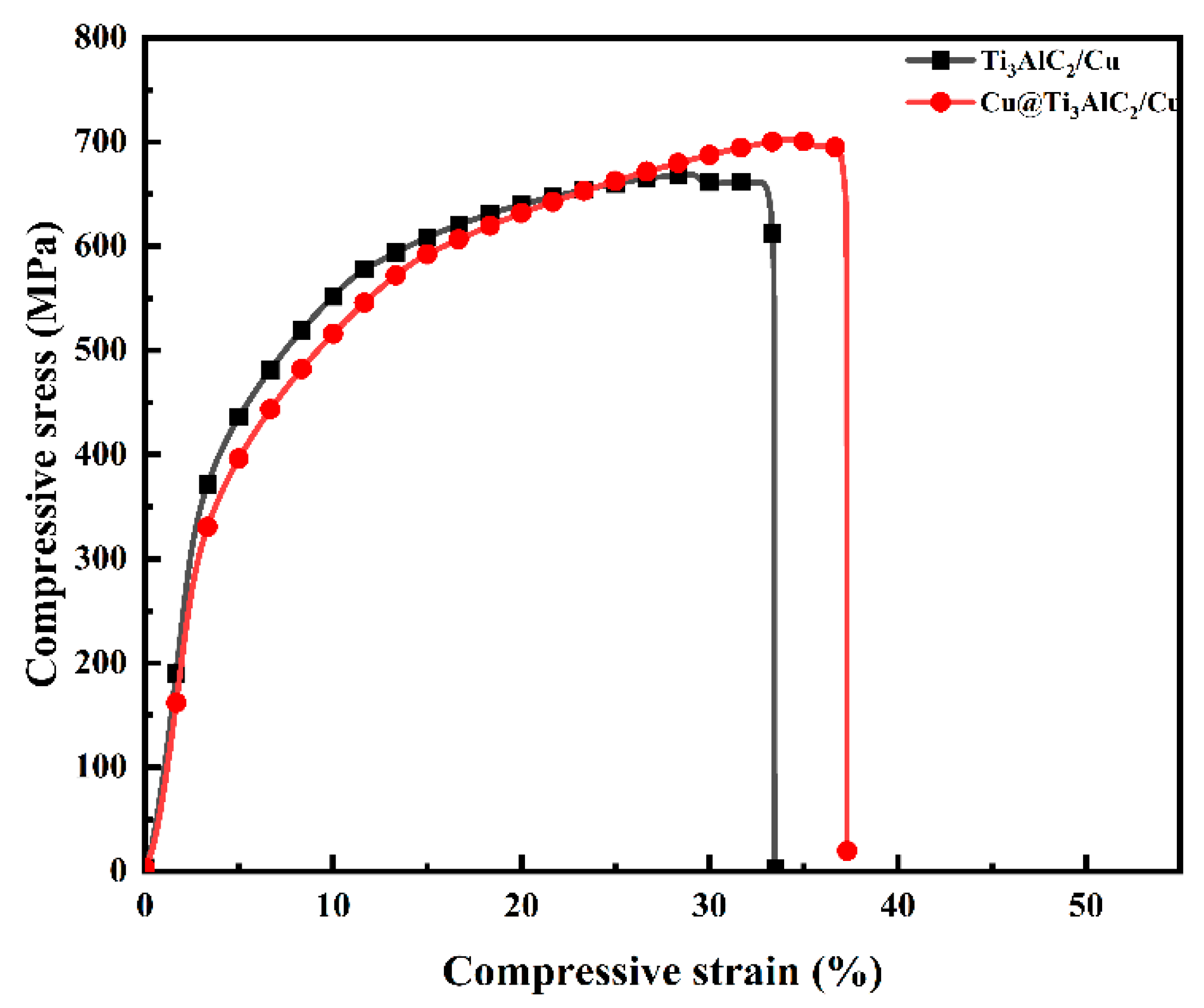
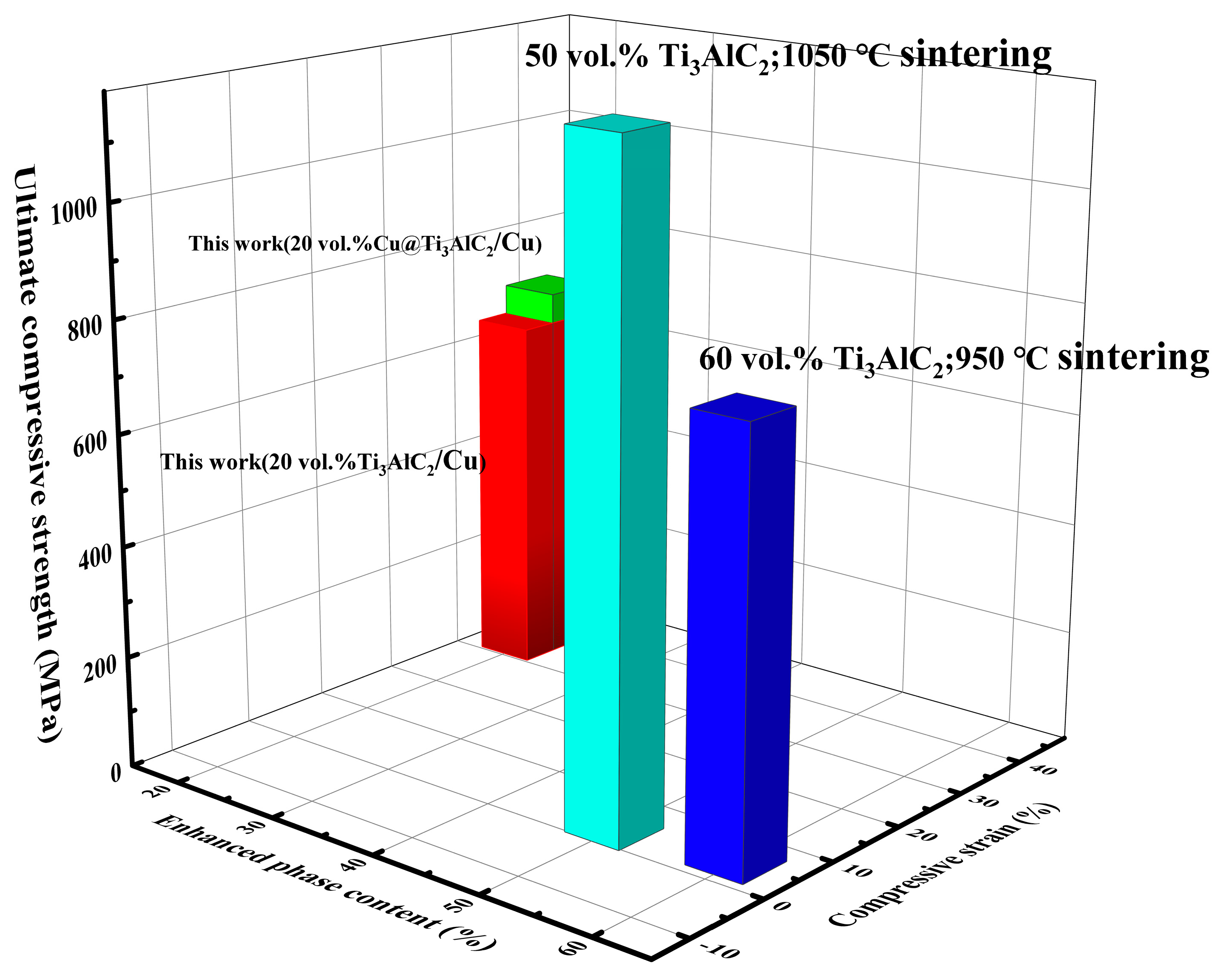
3.3. Mechanical Properties of Cu@Ti3AlC2/Cu Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Zhai, H.; Chen, L.; Zhou, Y.; Huang, Z.; Bei, G.; Greil, P. Sintering and properties of mechanical alloyed Ti3AlC2-Cu composites. Mater. Sci. Eng. A 2017, 685, 154–158. [Google Scholar] [CrossRef]
- Yu, W.; Guénolé, J.; Ghanbaja, J.; Vallet, M.; Guitton, A. Frank partial dislocation in Ti2AlC-MAX phase induced by matrix-Cu diffusion. Scr. Mater. 2021, 191, 34–39. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.Y.; Zhou, Y.C. Structure stability of Ti3AlC2 in Cu and microstructure evolution of Cu–Ti3AlC2 composites. Acta Mater. 2007, 55, 4381–4390. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Magne, D.; Mauchamp, V.; Célérier, S.; Chartiera, P.; Cabioc’h, T. Site-projected electronic structure of two-dimensional Ti3C2MXene: The role of the surface functionalization groups. Phys. Chem. Chem. Phys. 2016, 18, 30946–30953. [Google Scholar] [CrossRef]
- Lin, Z.; Zhuo, M.; Zhou, Y.; Li, M.; Wang, J. Microstructural characterization of layered ternary Ti2AlC. Acta Mater. 2006, 54, 1009–1015. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, H.; Wang, W.; Li, S.; Huang, Z. A TiCx reinforced Fe (Al) matrix composite using in-situ reaction. Prog. Nat. Sci. Mater. Int. 2013, 23, 13–17. [Google Scholar] [CrossRef]
- Han, T.; Li, J.; Zhao, N.; Shi, C.; Liu, E.; He, F.; Ma, L.; Li, Q.; He, C. In-situ fabrication of nano-sized TiO2 reinforced Cu matrix composites with well-balanced mechanical properties and electrical conductivity. Powder Technol. 2017, 321, 66–73. [Google Scholar] [CrossRef]
- Efe, G.C.; Zeytin, S.; Bindal, C. The effect of SiC particle size on the properties of Cu–SiC composites. Mater. Des. 2012, 36, 633–639. [Google Scholar]
- Jahani, A.; Aval, H.J.; Rajabi, M.; Jamaati, R. Effects of Ti2SnC MAX phase reinforcement content on the properties of copper matrix composite produced by friction stir back extrusion process. Mater. Chem. Phys. 2023, 299, 127497. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.C. Microstructure, mechanical, and electrical properties of Cu–Ti3AlC2 and in situ Cu–TiCx composites. J. Mater. Res. 2008, 23, 924–932. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; He, Y.; Sun, Y.; He, X. Effect of joining temperature and holding time on microstructure and shear strength of Ti2AlC/Cu joints brazed using Ag-Cu filler alloy. Mater. Sci. Eng. A 2013, 567, 58–64. [Google Scholar] [CrossRef]
- Nechiche, M.; Gauthier-Brunet, V.; Mauchamp, V.; Joulain, A.; Cabioc’h, T.; Milhet, X.; Chartier, P.; Dubois, S. Synthesis and characterization of a new (Ti1-ε, Cuε) 3 (Al, Cu) C2 MAX phase solid solution. J. Eur. Ceram. Soc. 2017, 37, 459–466. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Lu, J.; Ma, B.; Wang, Z.; Cheong, L.Z.; Luo, K.; Zha, X.; Chen, K.; Persson, P.O.; et al. Single-atom-thick active layers realized in nanolaminated Ti3 (AlxCu1–x) C2 and its artificial enzyme behavior. ACS Nano 2019, 13, 9198–9205. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Lu, J.; Luo, K.; Chen, K.; Li, M.; Persson, P.O.; Hultman, L.; Eklund, P.; Du, S.; et al. Synthesis of MAX phases Nb2CuC and Ti2 (Al0.1Cu0.9) N by A-site replacement reaction in molten salts. Mater. Res. Lett. 2019, 7, 510–516. [Google Scholar] [CrossRef]
- Huang, Z.; Bonneville, J.; Zhai, H.; Gauthier-Brunet, V.; Dubois, S. Microstructural characterization and compression properties of TiC0.61/Cu (Al) composite synthesized from Cu and Ti3AlC2 powders. J. Alloys Compd. 2014, 602, 53–57. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Zhai, H.X.; Ai, M.X.; Wang, Y.; Zhang, H.B. Pressureless sintering and properties of Cu/Ti3AlC2 composites. Key Eng. Mater. 2008, 368, 998–1000. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, B.; Wang, X.; Yan, C. Mechanical properties of Ti3SiC2 particulate reinforced copper prepared by hot pressing of copper coated Ti3SiC2 and copper powder. Mater. Sci. Technol. 2004, 20, 661–665. [Google Scholar] [CrossRef]
- Yue, H.; Yao, L.; Gao, X.; Zhang, S.; Guo, E.; Zhang, H.; Lin, X.; Wang, B. Effect of ball-milling and graphene contents on the mechanical properties and fracture mechanisms of graphene nanosheets reinforced copper matrix composites. J. Alloys Compd. 2017, 691, 755–762. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Yan, F.; Lv, H.; Fu, Y.Q. Effect of reduced graphene oxides decorated by Ag and Ce on mechanical properties and electrical conductivity of copper matrix composites. Vacuum 2021, 183, 109861. [Google Scholar] [CrossRef]
- Kingery, W.D. Densification during sintering in the presence of a liquid phase. I. Theory. J. Appl. Phys. 1959, 30, 301–306. [Google Scholar] [CrossRef]
- German Randall, M.; Suri, P.; Park, S. Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Odoni, B.U. Mechanical properties, wear and corrosion behavior of copper matrix composites reinforced with steel machining chips. Eng. Sci. Technol. Int. J. 2016, 19, 1593–1599. [Google Scholar] [CrossRef]
- Chmielewski, M.; Pietrzak, K.; Strojny-Nędza, A.; Kaszyca, K.; Zybała, R.; Bazarnik, P.; Lewandowska, M.; Nosewicz, S. Microstructure and thermal properties of Cu-SiC composite materials depending on the sintering technique. Sci. Sinter. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Zhang, H.; Ma, L.; Fu, Y.-Q. In-situ generated graphene from wheat flour for enhancing mechanical and electrical properties of copper matrix composites. Mater. Sci. Eng. A 2022, 835, 142662. [Google Scholar] [CrossRef]




| Composite Material | Theoretical Density (g·cm−3) | Density (g·cm−3) | Compactness (%) | Porosity (%) |
|---|---|---|---|---|
| Cu@Ti3AlC2/Cu | 7.77 | 7.66 | 98.5 | 1.5 |
| Ti3AlC2/Cu | 7.77 | 7.53 | 96.9 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhai, Z.; Sun, C.; Wang, Z.; Li, D. Mechanical Properties of Ti3AlC2/Cu Composites Reinforced by MAX Phase Chemical Copper Plating. Nanomaterials 2024, 14, 418. https://doi.org/10.3390/nano14050418
Chen C, Zhai Z, Sun C, Wang Z, Li D. Mechanical Properties of Ti3AlC2/Cu Composites Reinforced by MAX Phase Chemical Copper Plating. Nanomaterials. 2024; 14(5):418. https://doi.org/10.3390/nano14050418
Chicago/Turabian StyleChen, Cong, Zhenjie Zhai, Changfei Sun, Zhe Wang, and Denghui Li. 2024. "Mechanical Properties of Ti3AlC2/Cu Composites Reinforced by MAX Phase Chemical Copper Plating" Nanomaterials 14, no. 5: 418. https://doi.org/10.3390/nano14050418





