The Effect of Sulfur and Nitrogen Doping on the Oxygen Reduction Performance of Graphene/Iron Oxide Electrocatalysts Prepared by Using Microwave-Assisted Synthesis
Abstract
:1. Introduction
2. Materials and Methods
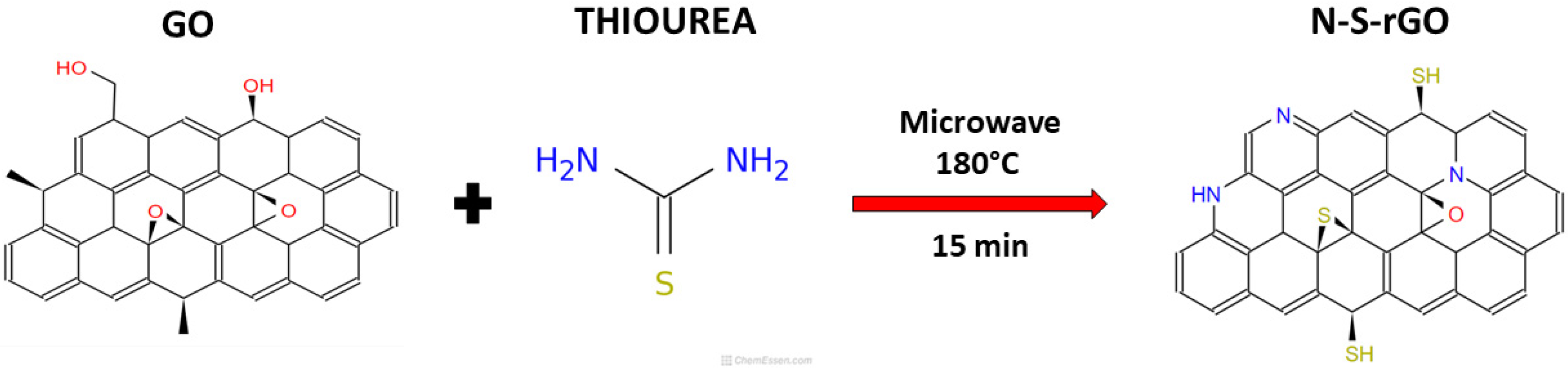
2.1. Catalyst Synthesis
2.2. Physical and Electrochemical Characterization
3. Results and Discussion
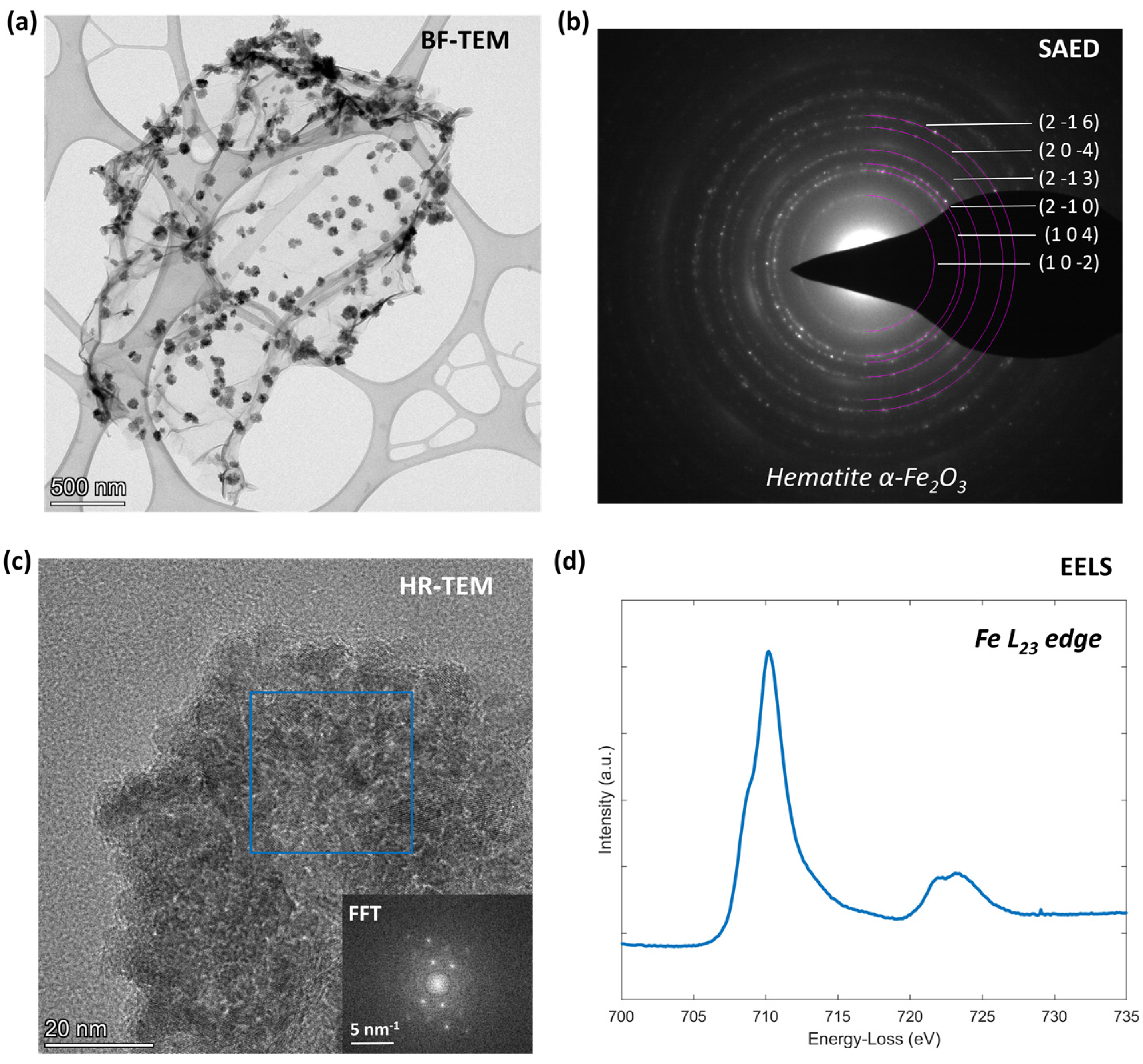
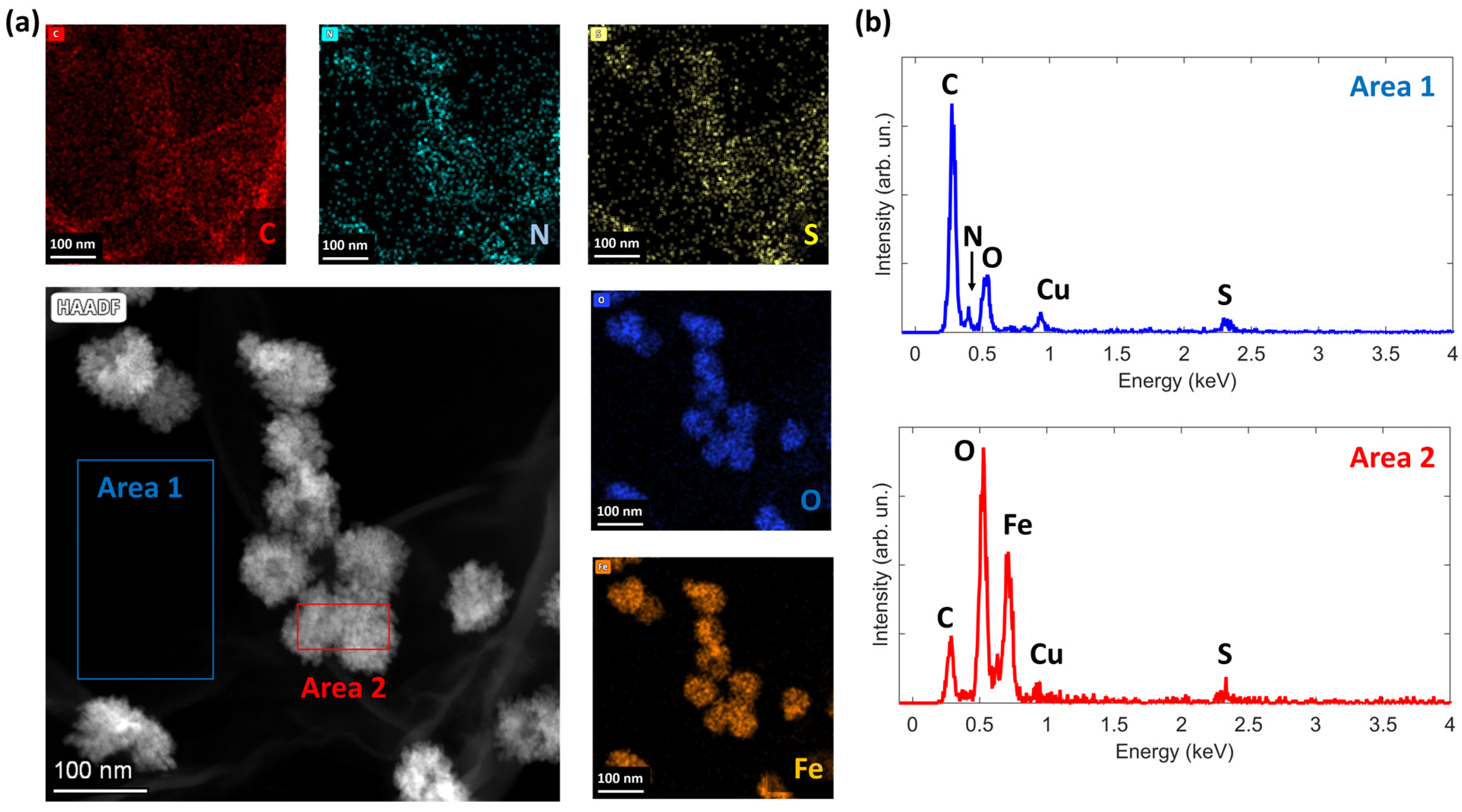
3.1. Morphological Analysis
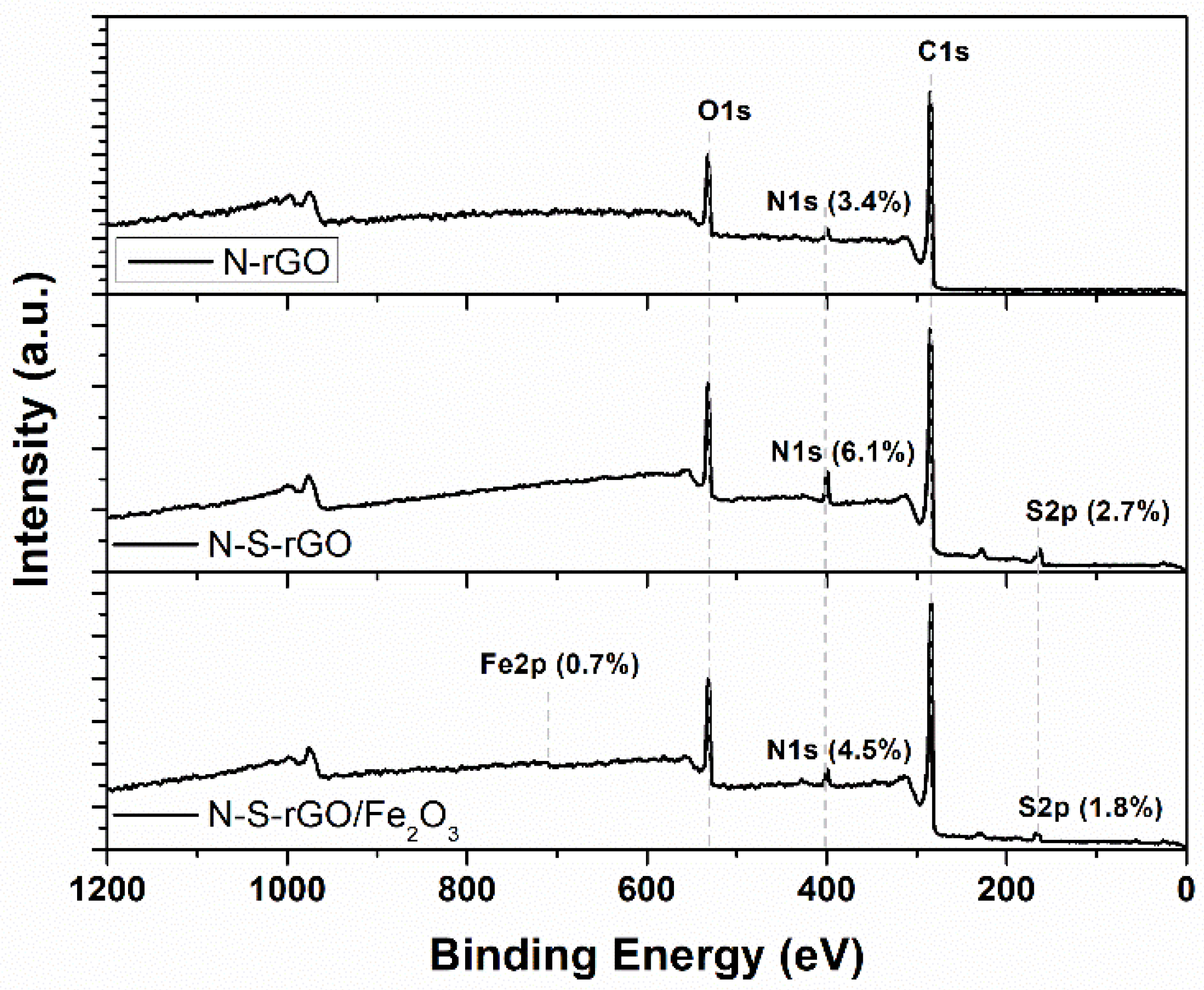
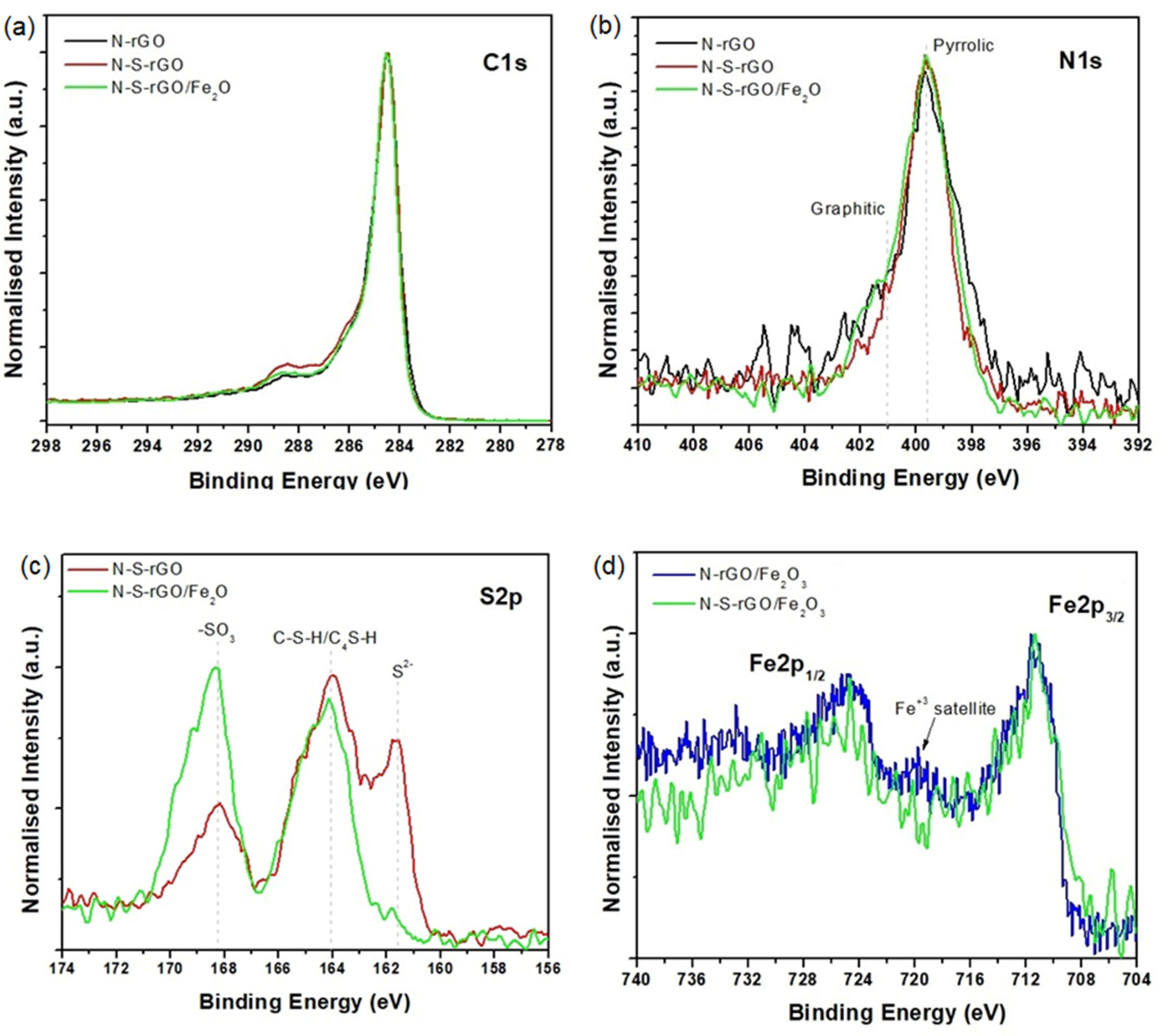
3.2. XPS and Raman Analysis
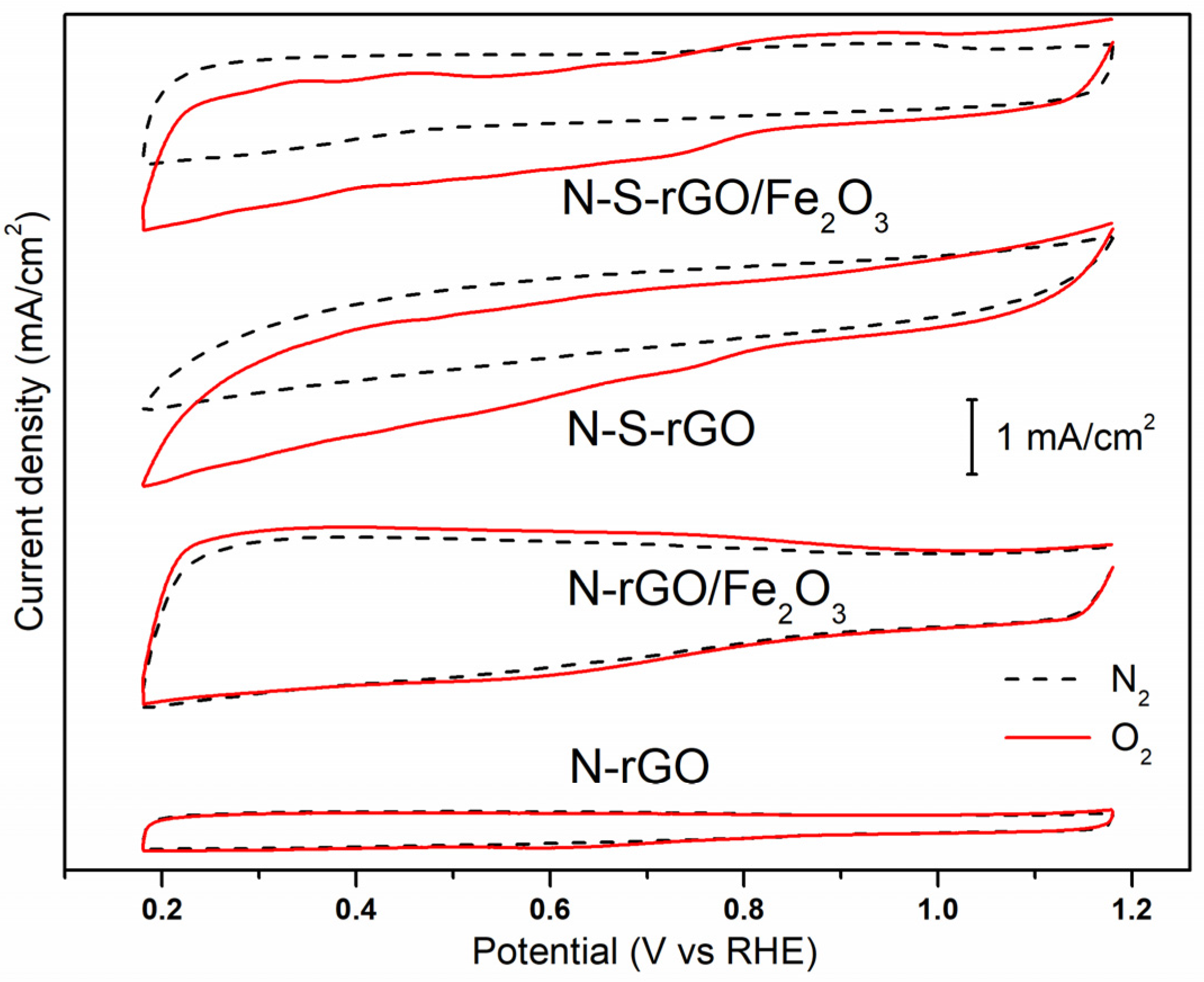
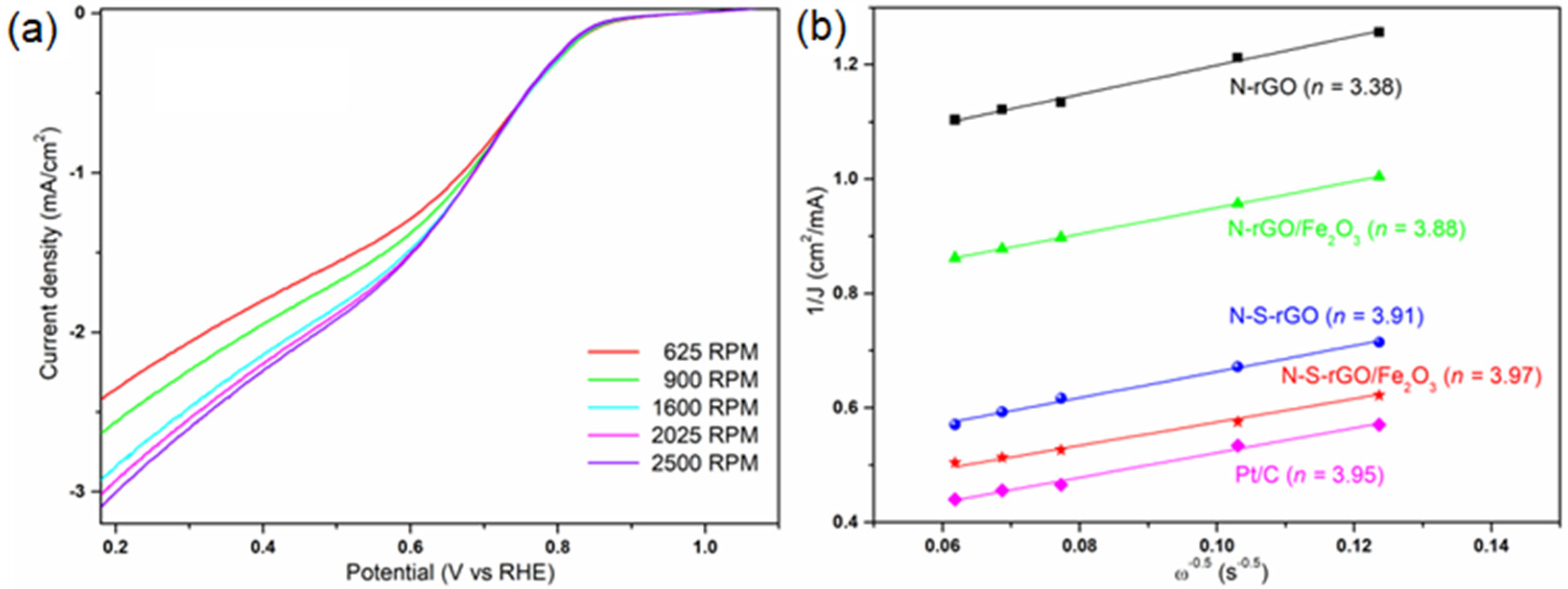
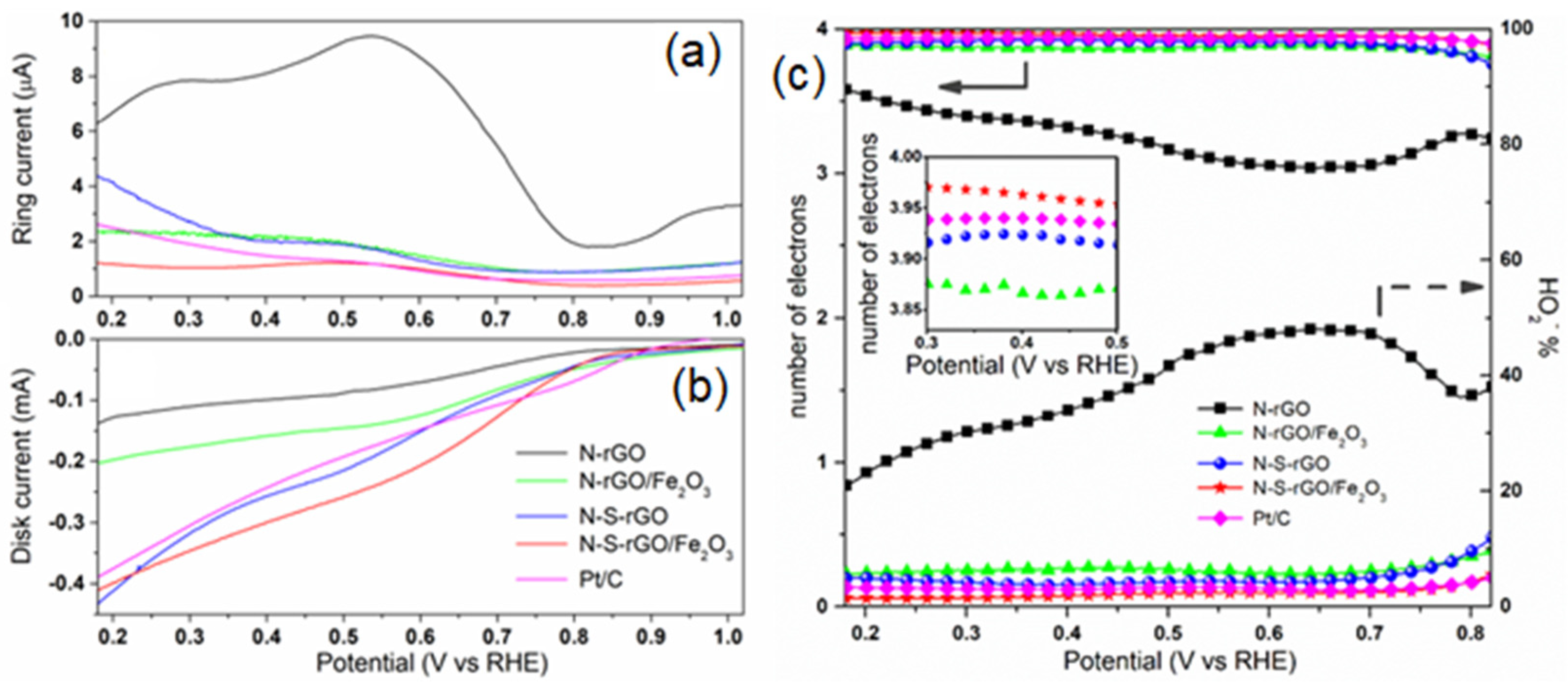
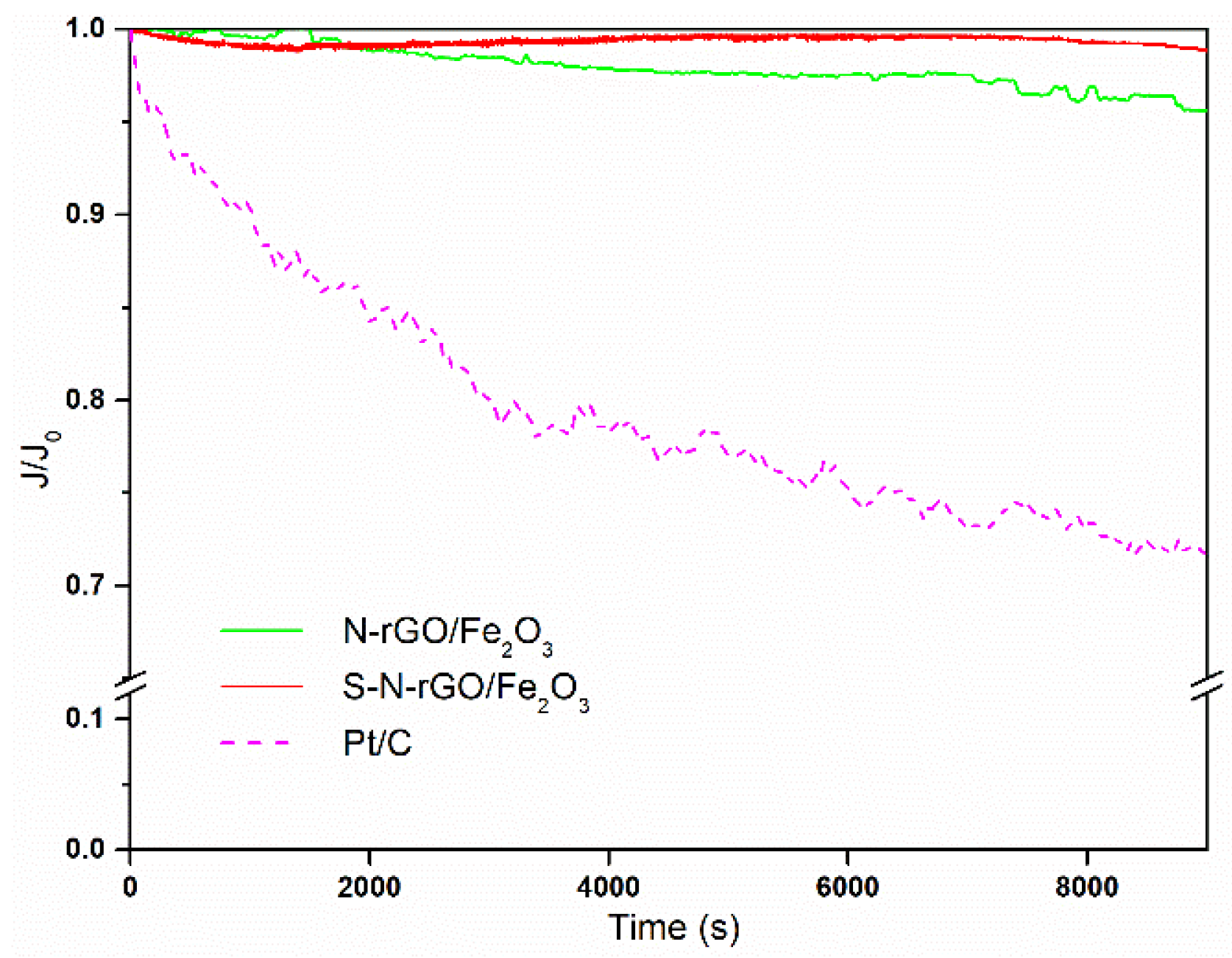
3.3. Electrochemical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitillo, J.G.; Eisaman, M.D.; Aradóttir, E.S.; Passarini, F.; Wang, T.; Sheehan, S.W. The role of carbon capture, utilization, and storage for economic pathways that limit global warming to below 1.5 °C. iScience 2022, 25, 104237. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Jain, S.; Nema, R. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Kjeang, E.; Djilali, N.; Sinton, D. Microfluidic fuel cells: A review. J. Power Sources 2009, 186, 353–369. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C.; Rani, J.V.; Kanakaiah, V.; Dadmal, T.; Rao, M.S.; Bhavanarushi, S. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Delmondo, L.; Salvador, G.P.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Gerosa, M.; Massaglia, G.; Chiodoni, A.; Quaglio, M. Nanostructured MnxOy for oxygen reduction reaction (ORR) catalysts. Appl. Surf. Sci. 2016, 388, 631–639. [Google Scholar] [CrossRef]
- Bag, S.; Roy, K.; Gopinath, C.S.; Raj, C.R. Facile Single-Step Synthesis of Nitrogen-Doped Reduced Graphene Oxide-Mn3O4 Hybrid Functional Material for the Electrocatalytic Reduction of Oxygen. ACS Appl. Mater. Interfaces 2014, 6, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-Z.; Dai, Y.; Li, R.; Zou, J.-L.; Tian, G.-H.; Fu, H.-G. Low-temperature synthesized nitrogen-doped iron/iron carbide/partly-graphitized carbon as stable cathode catalysts for enhancing bioelectricity generation. Carbon NY 2015, 89, 8–19. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Muñoz-Tabares, J.A.; Chiodoni, A.; Agostino, V.; Margaria, V.; Gerosa, M.; Massaglia, G.; Quaglio, M. Microwave-Assisted Synthesis of Reduced Graphene Oxide/SnO2 Nanocomposite for Oxygen Reduction Reaction in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 4633–4643. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horizons 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Li, J.; Xue, H.; Xu, N.; Zhang, X.; Wang, Y.; He, R.; Huang, H.; Qiao, J. Co/Ni dual-metal embedded in heteroatom doped porous carbon core-shell bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Rep. Energy 2022, 2, 100090. [Google Scholar] [CrossRef]
- He, Y.; Tan, Q.; Lu, L.; Sokolowski, J.; Wu, G. Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction in PEM Fuel Cells: Self-Template Synthesis Approach to Enhancing Catalytic Activity and Stability. Electrochem. Energy Rev. 2019, 2, 231–251. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Y.; Cao, Y.; He, S.; Li, X.; Tong, S.; Wu, M. N-, O- and P-doped hollow carbons: Metal-free bifunctional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Appl. Catal. B Environ. 2019, 248, 239–248. [Google Scholar] [CrossRef]
- Camisasca, A.; Sacco, A.; Brescia, R.; Giordani, S. Boron/Nitrogen-Codoped Carbon Nano-Onion Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2018, 1, 5763–5773. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced graphene oxide-based materials for electrochemical energy conversion reactions. Carbon Energy 2019, 1, 85–108. [Google Scholar] [CrossRef]
- Ly, A.; Murphy, E.; Wang, H.; Huang, Y.; Ferro, G.; Guo, S.; Asset, T.; Liu, Y.; Zenyuk, I.V.; Atanassov, P. Electrochemical trends of a hybrid platinum and metal–nitrogen–carbon catalyst library for the oxygen reduction reaction. EES Catal. 2023, 2, 624–637. [Google Scholar] [CrossRef]
- Bates, J.S.; Martinez, J.J.; Hall, M.N.; Al-Omari, A.A.; Murphy, E.; Zeng, Y.; Luo, F.; Primbs, M.; Menga, D.; Bibent, N.; et al. Chemical Kinetic Method for Active-Site Quantification in Fe-N-C Catalysts and Correlation with Molecular Probe and Spectroscopic Site-Counting Methods. J. Am. Chem. Soc. 2023, 145, 26222–26237. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, B.; Das, A.K.; Raj, C.R. Nitrogen and Sulfur Dual-Doped Reduced Graphene Oxide: Synergistic Effect of Dopants Towards Oxygen Reduction Reaction. Electrochim. Acta 2015, 163, 16–23. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, H.; Ding, J.; Liu, H.; Chen, P.; Lu, Y.; Lu, Y.; Zuo, W.; Han, L.; Guo, Y.; et al. Unraveling the Origin of Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced Oxygen Reduction Activity: Effect of Iron Spin-State Tuning. Angew. Chem. Int. Ed. 2021, 60, 25404–25410. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.; Xu, X.; Chen, Y.; Huang, S. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef]
- Du, L.; Zhang, G.; Liu, X.; Hassanpour, A.; Dubois, M.; Tavares, A.C.; Sun, S. Biomass-derived nonprecious metal catalysts for oxygen reduction reaction: The demand-oriented engineering of active sites and structures. Carbon Energy 2020, 2, 561–581. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.-X.; Wu, D.-L.; Xia, W.; Jia, D.-Z. Interaction between Nitrogen and Sulfur in Co-Doped Graphene and Synergetic Effect in Supercapacitor. Sci. Rep. 2015, 5, srep09591. [Google Scholar] [CrossRef]
- Periyasamy, G.; Annamalai, K.; Patil, I.M.; Kakade, B. Sulfur and nitrogen co-doped rGO sheets as efficient electrocatalyst for oxygen reduction reaction in alkaline medium. Diam. Relat. Mater. 2021, 114, 108338. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon NY 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Shan, H.; Li, X.; Cui, Y.; Xiong, D.; Yan, B.; Li, D.; Lushington, A.; Sun, X. Sulfur/Nitrogen Dual-doped Porous Graphene Aerogels Enhancing Anode Performance of Lithium Ion Batteries. Electrochim. Acta 2016, 205, 188–197. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Ly, A.; Gili, A.; Murphy, E.; Asset, T.; Liu, Y.; De Andrade, V.; Segre, C.U.; et al. Catalysts by pyrolysis: Transforming metal-organic frameworks (MOFs) precursors into metal-nitrogen-carbon (M-N-C) materials. Mater. Today 2023, 69, 66–78. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Muñoz-Tabares, J.A.; Armandi, M.; Chiodoni, A.; Pirri, C.F. One-Pot Microwave-Assisted Synthesis of Reduced Graphene Oxide/Iron Oxide Nanocomposite Catalyst for the Oxygen Reduction Reaction. ChemistrySelect 2016, 1, 3640–3646. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Huang, W.-H.; Chen, C.-L.; Jia, Y.; Dai, S.; Li, T.; Zhao, Y.; Qiu, Y.; Waterhouse, G.I.; et al. Toward More Efficient Carbon-Based Electrocatalysts for Hydrogen Peroxide Synthesis: Roles of Cobalt and Carbon Defects in Two-Electron ORR Catalysis. Nano Lett. 2023, 23, 1100–1108. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, H.; Gao, F.; Jiang, G. 2D transitional-metal nickel compounds monolayer: Highly efficient multifunctional electrocatalysts for the HER, OER and ORR. Int. J. Hydrog. Energy 2023, 48, 4242–4252. [Google Scholar] [CrossRef]
- Lv, J.Q.; Lang, Z.; Fu, J.; Lan, Q.; Liu, R.; Zang, H.; Li, Y.; Ye, D.; Streb, C. Molecular Iron Oxide Clusters Boost the Oxygen Reduction Reaction of Platinum Electrocatalysts at Near-Neutral pH. Angew. Chem. Int. Ed. 2022, 61, e202202650. [Google Scholar] [CrossRef]
- Wang, K.; Chen, H.; Zhang, X.; Tong, Y.; Song, S.; Tsiakaras, P.; Wang, Y. Iron oxide@graphitic carbon core-shell nanoparticles embedded in ordered mesoporous N-doped carbon matrix as an efficient cathode catalyst for PEMFC. Appl. Catal. B Environ. 2019, 264, 118468. [Google Scholar] [CrossRef]
- Li, G.-L.; Liu, C.-D.; Chen, S.-M.; Hao, C.; Cheng, G.-C.; Xie, Y.-Y. Promotion of oxygen reduction performance by Fe3O4 nanoparticles support nitrogen-doped three dimensional meso/macroporous carbon based electrocatalyst. Int. J. Hydrog. Energy 2017, 42, 4133–4145. [Google Scholar] [CrossRef]
- Hof, F.; Liu, M.; Valenti, G.; Picheau, E.; Paolucci, F.; Pénicaud, A. Size Control of Nanographene Supported Iron Oxide Nanoparticles Enhances Their Electrocatalytic Performance for the Oxygen Reduction and Oxygen Evolution Reactions. J. Phys. Chem. C 2019, 123, 20774–20780. [Google Scholar] [CrossRef]
- Bhange, S.N.; Unni, S.M.; Kurungot, S. Graphene with Fe and S Coordinated Active Centers: An Active Competitor for the Fe–N–C Active Center for Oxygen Reduction Reaction in Acidic and Basic pH Conditions. ACS Appl. Energy Mater. 2018, 1, 368–376. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wu, Z.; Wang, S.; Wu, Y.; Liu, X. Heteroatom (Nitrogen/Sulfur)-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions. Catalysts 2018, 8, 475. [Google Scholar] [CrossRef]
- Van Aken, P.A.; Liebscher, B. Quantification of ferrous/ferric ratios in minerals: New evaluation schemes of Fe L 23 electron energy-loss near-edge spectra. Phys. Chem. Miner. 2002, 29, 188–200. [Google Scholar] [CrossRef]
- Torruella, P.; Arenal, R.; de la Peña, F.; Saghi, Z.; Yedra, L.; Eljarrat, A.; López-Conesa, L.; Estrader, M.; López-Ortega, A.; Salazar-Alvarez, G.; et al. 3D Visualization of the Iron Oxidation State in FeO/Fe3O4Core–Shell Nanocubes from Electron Energy Loss Tomography. Nano Lett. 2016, 16, 5068–5073. [Google Scholar] [CrossRef]
- Golla-Schindler, U.; Benner, G.; Putnis, A. Laterally resolved EELS for ELNES mapping of the Fe L2,3- and O K-edge. Ultramicroscopy 2003, 96, 573–582. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. Primary and secondary oxygen-induced C1s binding energy shifts in x-ray photoelectron spectroscopy of polymers. Anal. Chem. 1992, 64, 1729–1736. [Google Scholar] [CrossRef]
- Garino, N.; Castellino, M.; Sacco, A.; Risplendi, F.; Muñoz-Tabares, J.A.; Armandi, M.; Chiodoni, A.; Salomon, D.; Quaglio, M.; Pirri, C.F.; et al. Proving the existence of Mn porphyrin-like complexes hosted in reduced graphene oxide with outstanding performance as oxygen reduction reaction catalysts. 2d Mater. 2019, 6, 045001. [Google Scholar] [CrossRef]
- Ishida, T.; Choi, N.; Mizutani, W.; Tokumoto, H.; Kojima, I.; Azehara, H.; Hokari, H.; Akiba, U.; Fujihira, M. High-Resolution X-ray Photoelectron Spectra of Organosulfur Monolayers on Au(111): S(2p) Spectral Dependence on Molecular Species. Langmuir 1999, 15, 6799–6806. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Yadav, R.; Joshi, P.; Hara, M.; Yoshimura, M. In situ electrochemical Raman investigation of charge storage in rGO and N-doped rGO. Phys. Chem. Chem. Phys. 2021, 23, 11789–11796. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Chakravadhanula, V.S.K.; Schwarzburger, N.I.; Cambaz, M.A.; Behm, R.J.; Kübel, C.; Fichtner, M. Performance study of magnesium–sulfur battery using a graphene based sulfur composite cathode electrode and a non-nucleophilic Mg electrolyte. Nanoscale 2016, 8, 3296–3306. [Google Scholar] [CrossRef]
- Sacco, A.; Garino, N.; Lamberti, A.; Pirri, C.F.; Quaglio, M. Anodically-grown TiO 2 nanotubes: Effect of the crystallization on the catalytic activity toward the oxygen reduction reaction. Appl. Surf. Sci. 2017, 412, 447–454. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Nitrogen-Doped Ordered Mesoporous Graphitic Arrays with High Electrocatalytic Activity for Oxygen Reduction. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef]
- Ai, W.; Luo, Z.; Jiang, J.; Zhu, J.; Du, Z.; Fan, Z.; Xie, L.; Zhang, H.; Huang, W.; Yu, T. Nitrogen and Sulfur Codoped Graphene: Multifunctional Electrode Materials for High-Performance Li-Ion Batteries and Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 6186–6192. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; He, G.; Zhang, X.; Bao, S.; Hu, W. Bioinspired synthesis of nitrogen/sulfur co-doped graphene as an efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 279, 252–258. [Google Scholar] [CrossRef]
- Delmondo, L.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Massaglia, G.; Castellino, M.; Salvador, G.P.; Pirri, C.F.; Quaglio, M.; Chiodoni, A. Thermal evolution of MnxOy nanofibres as catalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2017, 19, 28781–28787. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef]
- Liu, X.; Hu, W. Iron oxide/oxyhydroxide decorated graphene oxides for oxygen reduction reaction catalysis: A comparison study. RSC Adv. 2016, 6, 29848–29854. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy as a tool to investigate the electroreduction of carbon dioxide: A short review. J. CO2 Util. 2018, 27, 22–31. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Jiang, K.; Li, K.; Liu, Y.-Q.; Wang, D.; Ye, Y. The Role of Sulfur Doping in Determining the Performance of Fe/N-C Based Electrocatalysts for Oxygen Reduction Reactions. J. Electrochem. Soc. 2021, 168, 054523. [Google Scholar] [CrossRef]
- Pan, F.; Duan, Y.; Zhang, X.; Zhang, J. A Facile Synthesis of Nitrogen/Sulfur Co-Doped Graphene for the Oxygen Reduction Reaction. ChemCatChem 2016, 8, 163–170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellino, M.; Sacco, A.; Fontana, M.; Chiodoni, A.; Pirri, C.F.; Garino, N. The Effect of Sulfur and Nitrogen Doping on the Oxygen Reduction Performance of Graphene/Iron Oxide Electrocatalysts Prepared by Using Microwave-Assisted Synthesis. Nanomaterials 2024, 14, 560. https://doi.org/10.3390/nano14070560
Castellino M, Sacco A, Fontana M, Chiodoni A, Pirri CF, Garino N. The Effect of Sulfur and Nitrogen Doping on the Oxygen Reduction Performance of Graphene/Iron Oxide Electrocatalysts Prepared by Using Microwave-Assisted Synthesis. Nanomaterials. 2024; 14(7):560. https://doi.org/10.3390/nano14070560
Chicago/Turabian StyleCastellino, Micaela, Adriano Sacco, Marco Fontana, Angelica Chiodoni, Candido Fabrizio Pirri, and Nadia Garino. 2024. "The Effect of Sulfur and Nitrogen Doping on the Oxygen Reduction Performance of Graphene/Iron Oxide Electrocatalysts Prepared by Using Microwave-Assisted Synthesis" Nanomaterials 14, no. 7: 560. https://doi.org/10.3390/nano14070560
APA StyleCastellino, M., Sacco, A., Fontana, M., Chiodoni, A., Pirri, C. F., & Garino, N. (2024). The Effect of Sulfur and Nitrogen Doping on the Oxygen Reduction Performance of Graphene/Iron Oxide Electrocatalysts Prepared by Using Microwave-Assisted Synthesis. Nanomaterials, 14(7), 560. https://doi.org/10.3390/nano14070560










