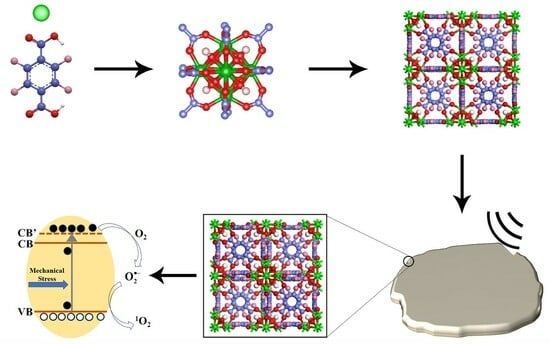
Mechanically Enhanced Detoxification of Chemical Warfare Agent Simulants by a Two-Dimensional Piezoresponsive Metal–Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterizations
2.3. Assembly of UiO-66-F4
2.4. Removal Experiments
2.5. Calculation Method
3. Results
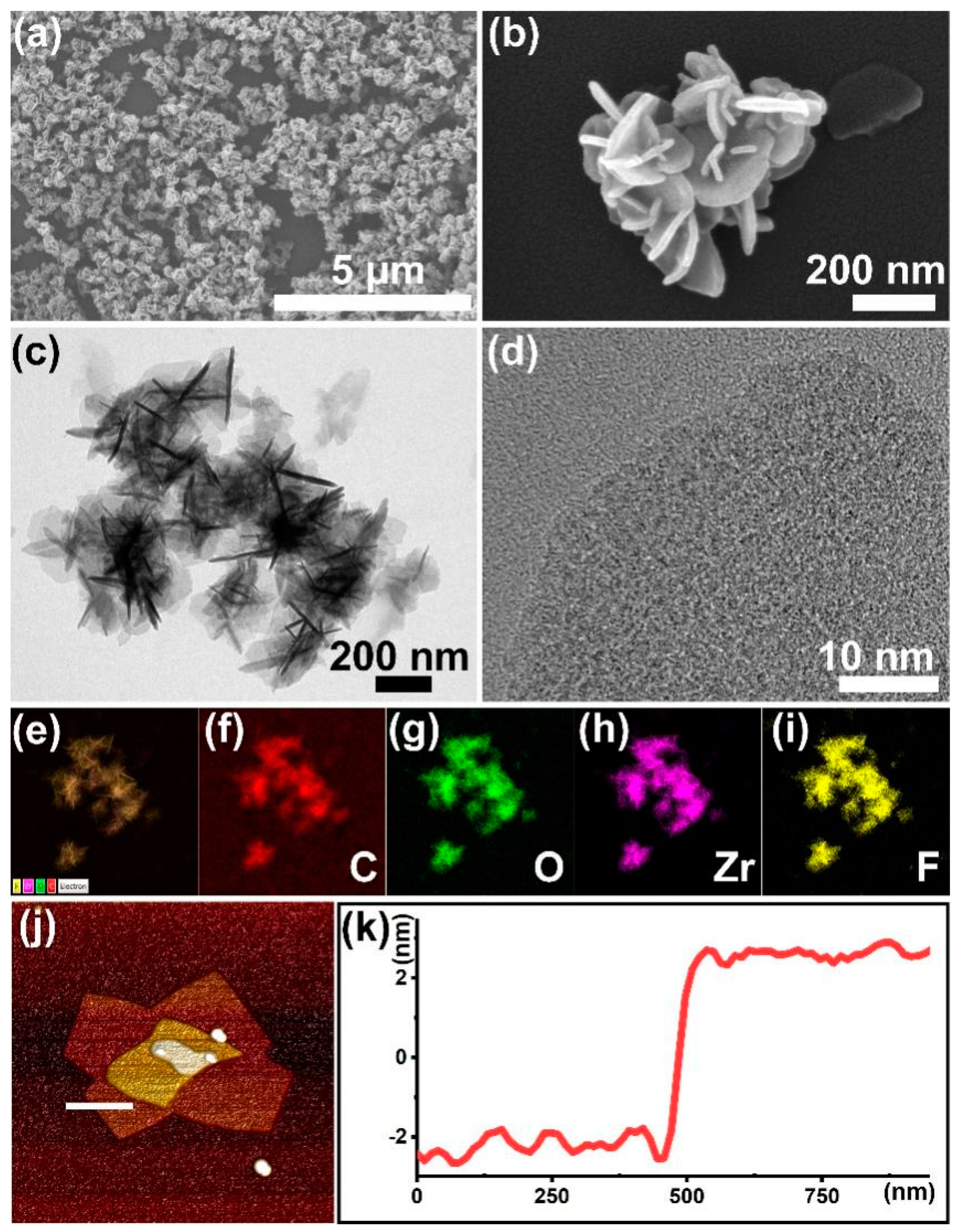
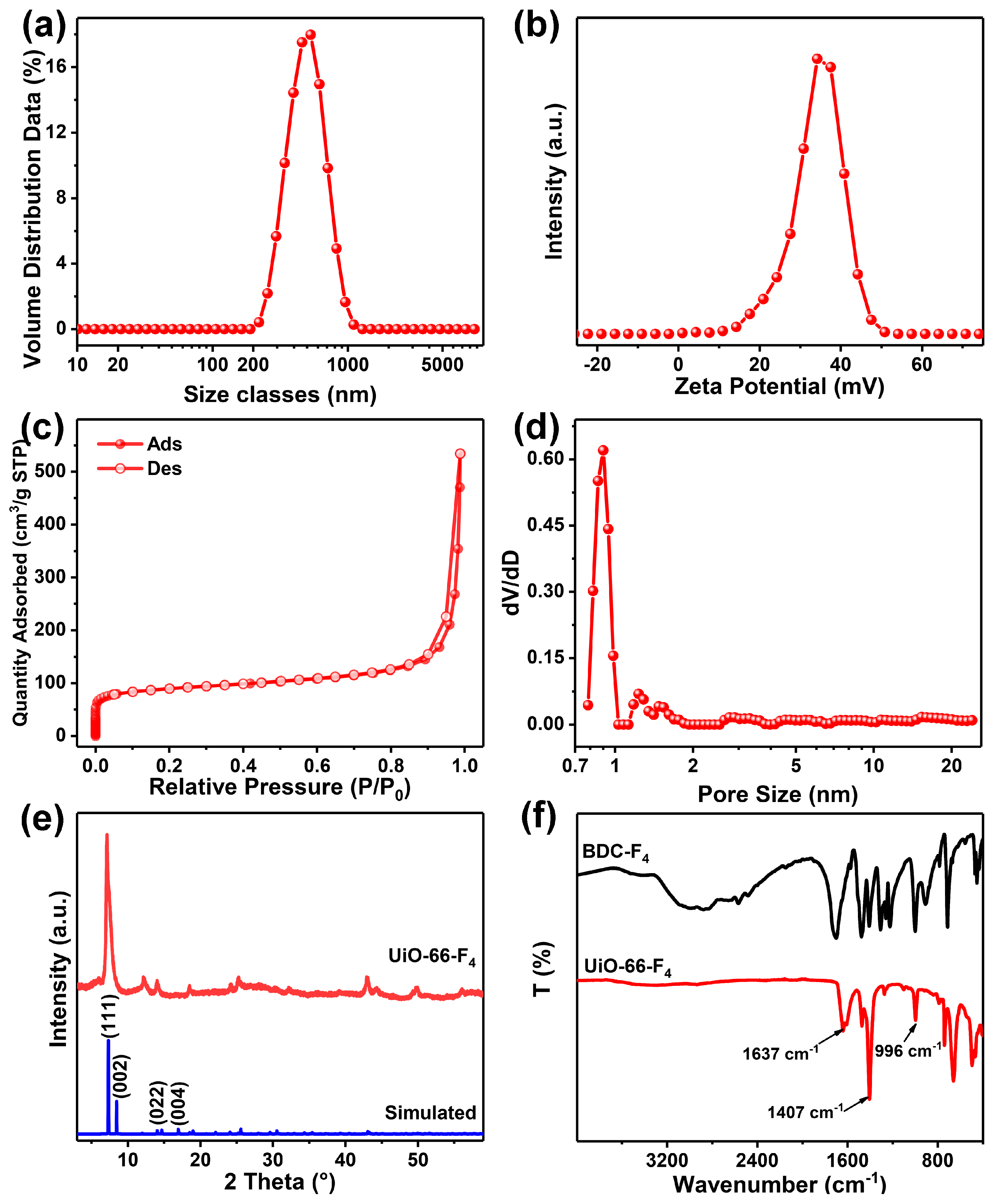
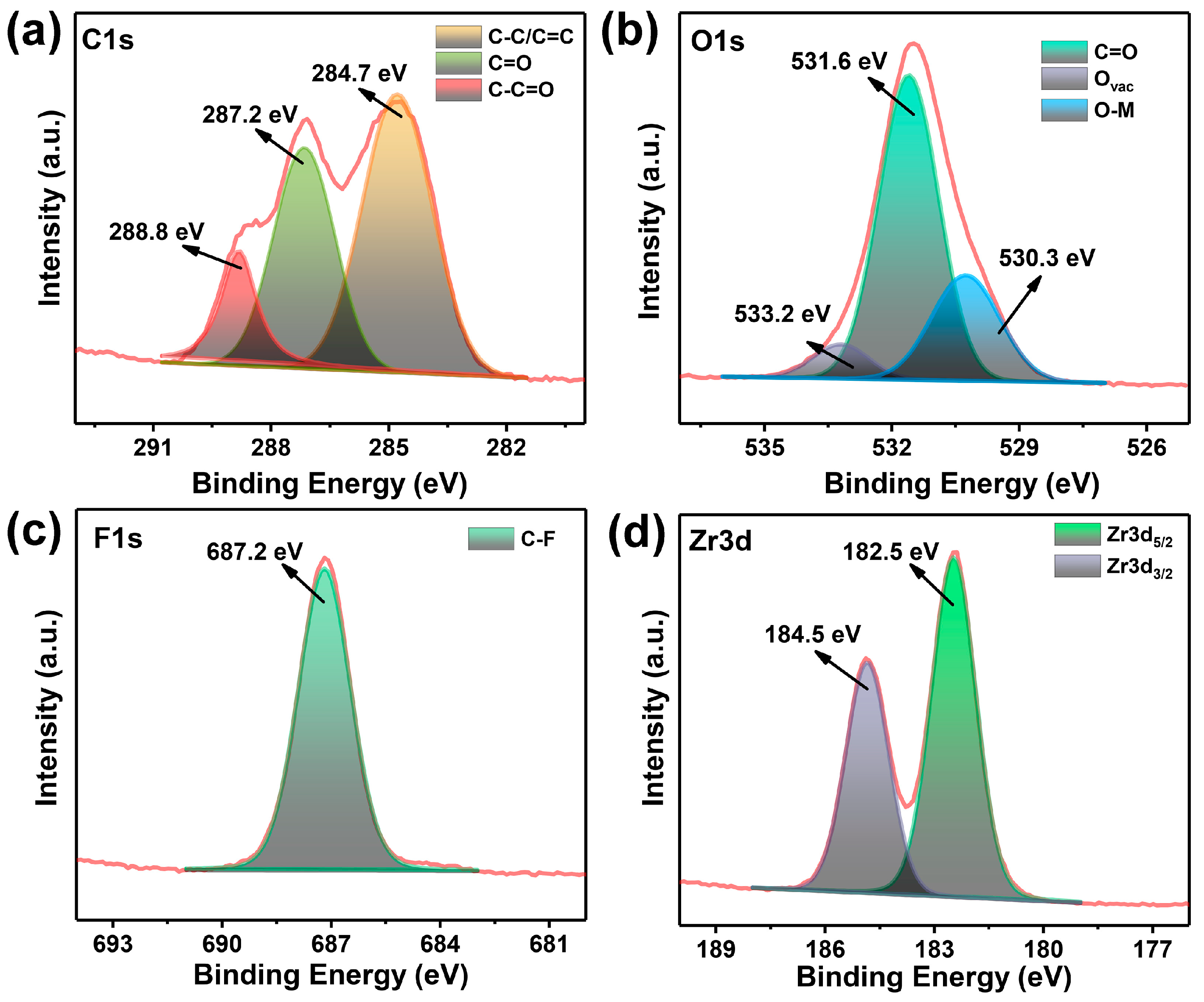
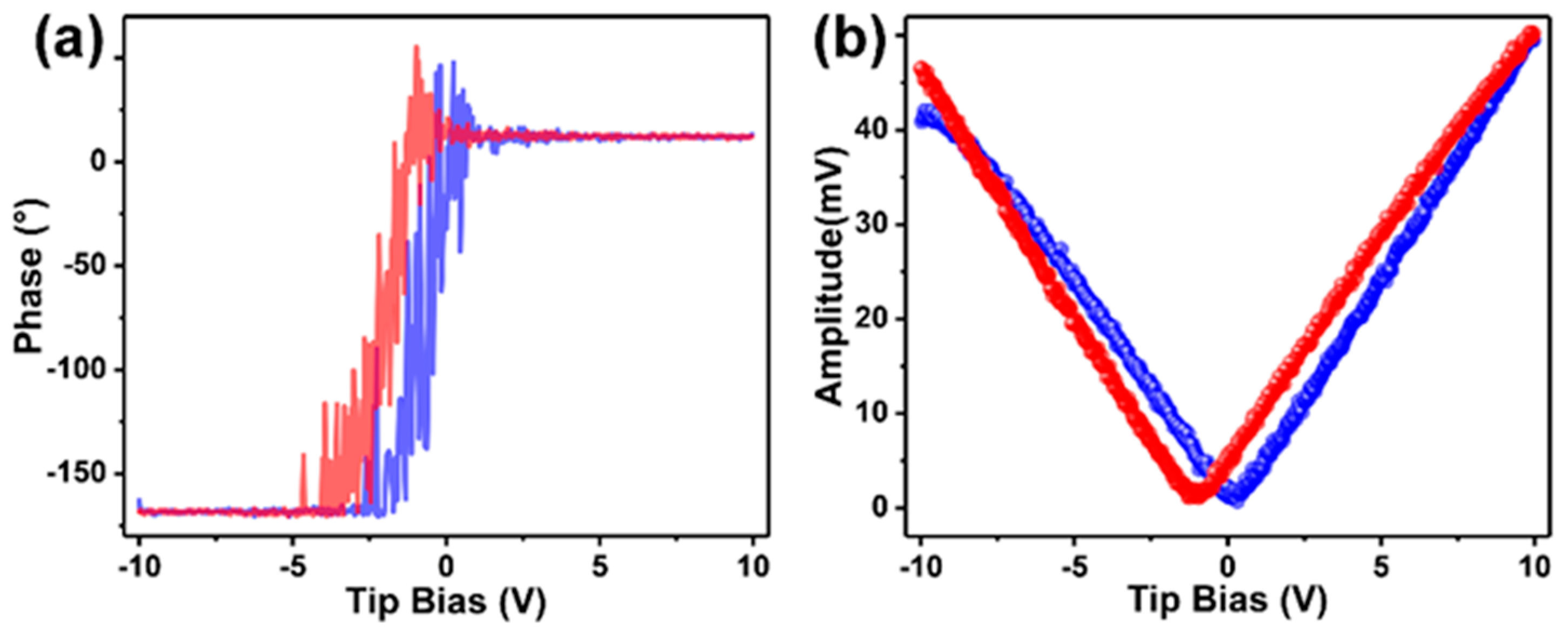
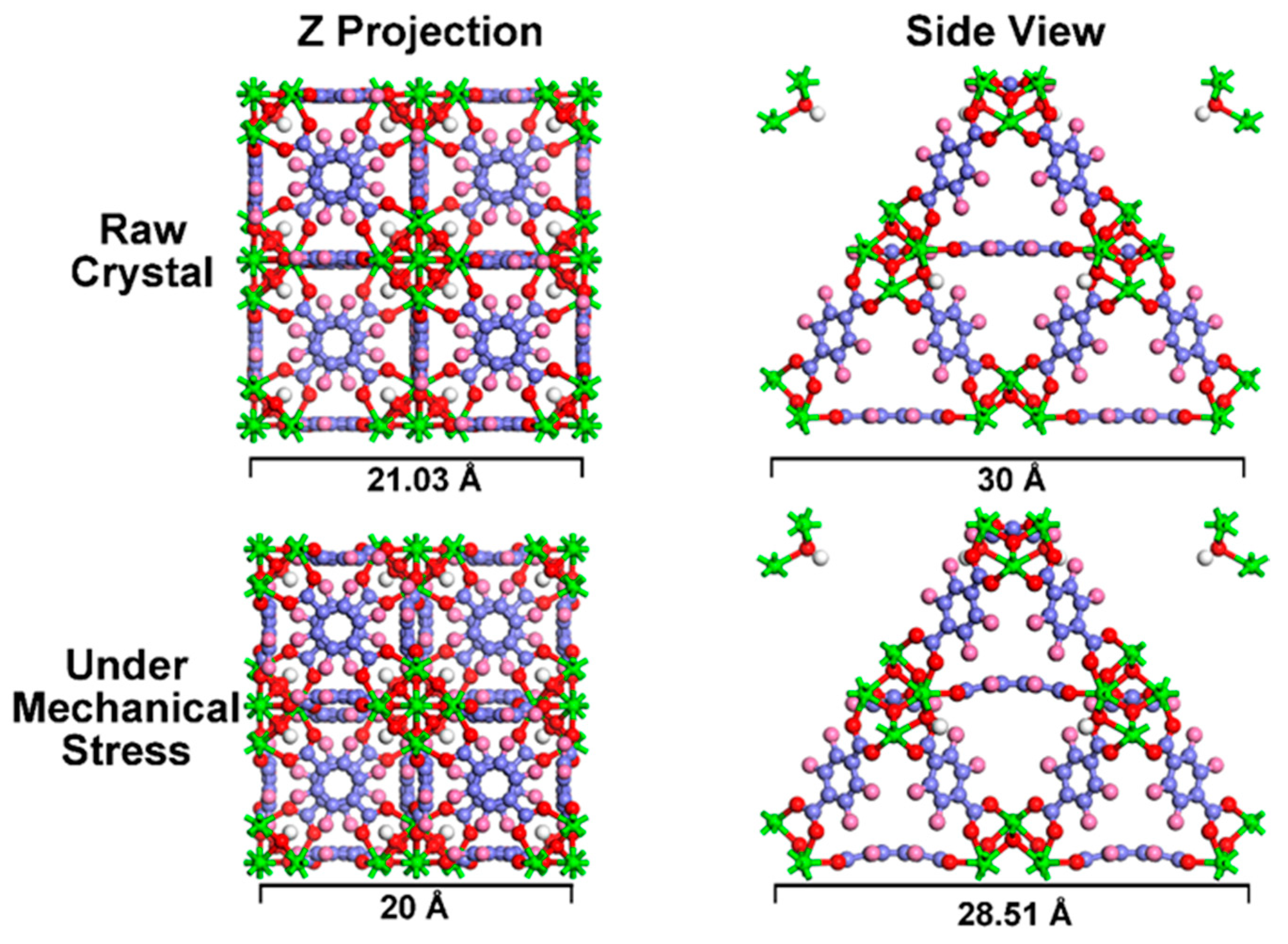
3.1. Assembly and Characterizations
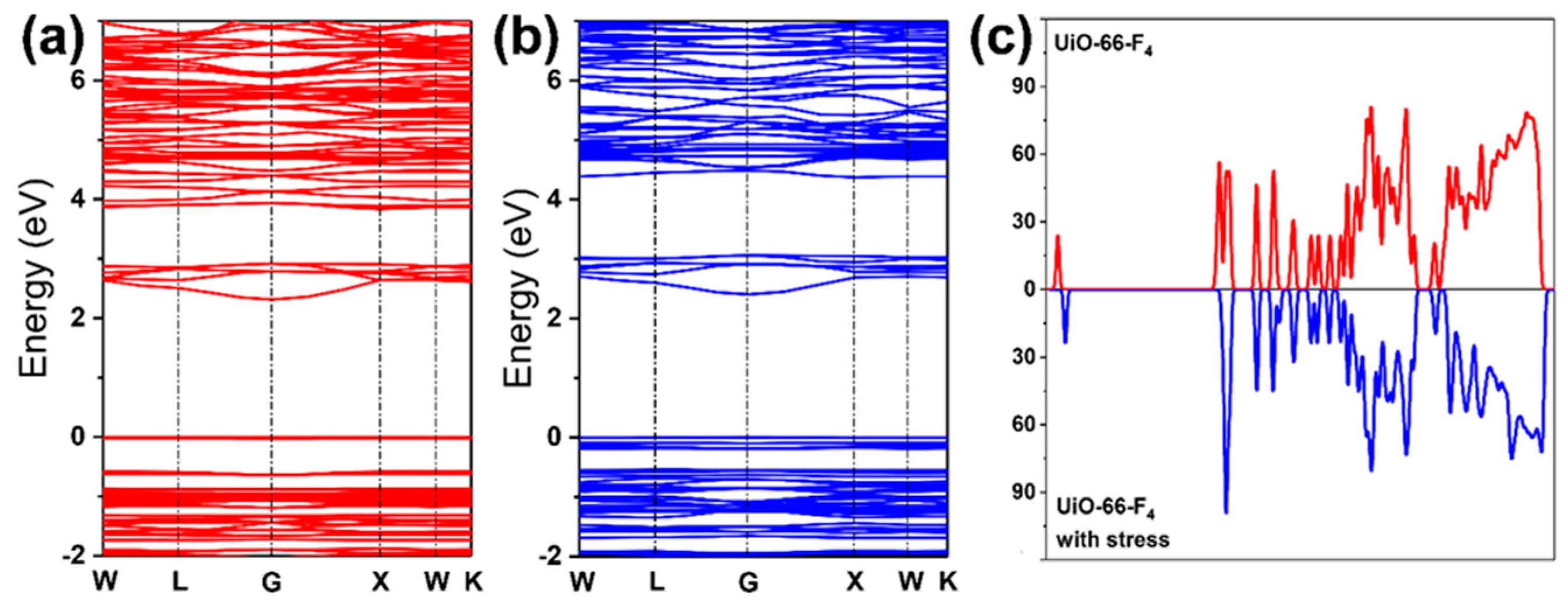
3.2. Mechanically Enhanced Detoxification Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Tao, C.A.; Zhao, S.; Zou, X.; Wang, F.; Wang, J. Porphyrin-Moiety-Functionalized Metal–Organic Layers Exhibiting Catalytic Capabilities for Detoxifying Nerve Agent and Blister Agent Simulants. ACS Appl. Mater. Interfaces 2023, 15, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.B.T.; Farha, O.K.; Hupp, J.T. Simple and Compelling Biomimetic Metal–Organic Framework Catalyst for the Degradation of Nerve Agent Simulants. Angew. Chem. Int. Ed. 2014, 126, 507–511. [Google Scholar] [CrossRef]
- Liao, Y.; Sheridan, T.; Liu, J.; Farha, O.; Hupp, J. Product Inhibition and the Catalytic Destruction of a Nerve Agent Simulant by Zirconium-Based Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 30565–30575. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tao, C.A.; Zhang, Z.; Chen, X.; Wang, J. Layer-by-Layer Fabrication of Core-Shell Fe3O4@UiO-66-NH2 with High Catalytic Reactivity toward the Hydrolysis of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2019, 11, 43156–43165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, R.; Huang, J.; Wang, F.; Wang, J. Facile Synthesis of Metal–Organic Layers with High Catalytic Performance toward Detoxification of a Chemical Warfare Agent Simulant. ACS Appl. Mater. Interfaces 2021, 13, 40863–40871. [Google Scholar] [CrossRef]
- Nawala, J.; Jóźwik, P.; Popiel, S. Thermal and Catalytic Methods Used for Destruction of Chemical Warfare Agents. Int. J. Environ. Sci. Technol. 2019, 16, 3899–3912. [Google Scholar] [CrossRef]
- Jouypazadeh, H.; Farrokhpour, H. DFT and TD-DFT Study of the Adsorption and Detection of Sulfur Mustard Chemical Warfare Agent by the C24, C12Si12, Al12N12, Al12P12, Be12O12, B12N12 and Mg12O12 Nanocages. J. Mol. Struct. 2018, 1164, 227–238. [Google Scholar] [CrossRef]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T. Detection of Chemical Warfare Agents Using Nanostructured Metal Oxide Sensors. Sens. Actuators B Chem. 2005, 108, 41–55. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Vermeulen, N.A.; Moon, S.Y.; Hupp, J.T.; Farha, O.K. Catalytic Degradation of Chemical Warfare Agents and Their Simulants by Metal-organic Frameworks. Coord. Chem. Rev. 2016, 346, 101–111. [Google Scholar] [CrossRef]
- Kiani, S.; Farooq, A.; Ahmad, M.; Irfan, N.; Nawaz, M.; Irshad, M. Impregnation on Activated Carbon for Removal of Chemical Warfare Agents (CWAs) and Radioactive Content. Environ. Sci. Pollut. Res. 2021, 28, 60477–60494. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Moghadam, P.Z.; Li, A.; Colombo, V.; Navarro, J.A.R.; Calero, S.; Fa, D. Discovery of an Optimal Porous Crystalline Material for the Capture of Chemical Warfare Agents. Chem. Mater. 2018, 30, 4571–4579. [Google Scholar] [CrossRef]
- Li, J.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-Driven Micromotors for Rapid Photocatalytic Degradation of Biological and Chemical Warfare Agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef]
- Picard, B.; Chataigner, I.; Maddaluno, J.; Legros, J. Introduction to Chemical Warfare Agents, Relevant Simulants and Modern Neutralisation Methods. Org. Biomol. Chem. 2019, 17, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Bing, Y. Trace Detection of Organophosphorus Chemical Warfare Agents in Wastewater and Plants by Luminescent UIO-67(Hf) and Evaluating the Bioaccumulation of Organophosphorus Chemical Warfare Agents. ACS Appl. Mater. Interfaces 2018, 10, 14869–14876. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W. Detoxification of Chemical Warfare Agents by CuBTC. J. Porous Mater. 2014, 21, 121–126. [Google Scholar] [CrossRef]
- Couzon, N.; Dhainaut, J.; Campagne, C.; Royer, S.; Loiseau, T.; Volkringer, C. Porous Textile Composites (PTCs) for the Removal and the Decomposition of Chemical Warfare Agents (CWAs)-A Review. Coord. Chem. Rev. 2022, 467, 214598. [Google Scholar] [CrossRef]
- Snider, V.G.; Hill, C.L. Functionalized Reactive Polymers for the Removal of Chemical Warfare Agents: A Review. J. Hazard. Mater. 2023, 442, 130015. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical Warfare Agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Black, R.M. History and Perspectives of Bioanalytical Methods for Chemical Warfare Agent Detection. J. Chromatogr. B 2010, 878, 1207–1215. [Google Scholar] [CrossRef]
- Eubanks, L.M.; Dickerson, T.J.; Janda, K.D. Technological Advancements for the Detection of and Protection Against Biological and Chemical Warfare Agents. Chem. Soc. Rev. 2007, 36, 458–470. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kulandaisamy, A.J.; Babu, K.J.; Das, A.; Balaguru Rayappan, J.B. Metal Organic Framework Functionalized Textiles as Protective Clothing for the Detection and Detoxification of Chemical Warfare Agents—A Review. Ind. Eng. Chem. Res. 2021, 60, 4218–4239. [Google Scholar] [CrossRef]
- Hong-Cai, Z.; Long, J.R.; Omar, M.Y. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Zhou, H.C.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed]
- Užarević, K.; Wang, T.C.; Moon, S.-Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF Composites: Preparations and Applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Isley Iii, W.C.; Ghosh, P.; Liao, P.; Bury, W.; Wagner, G.W.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W.; et al. Destruction of chemical warfare agents using metal–organic frameworks. Nat. Mater. 2015, 14, 512–516. [Google Scholar] [CrossRef]
- De Koning, M.C.; Van Grol, M.; Breijaert, T. Degradation of Paraoxon and the Chemical Warfare Agents VX, Tabun, and Soman by the Metal–Organic Frameworks UiO-66-NH2, MOF-808, NU-1000, and PCN-777. Inorg. Chem. 2017, 56, 11804–11809. [Google Scholar] [CrossRef]
- Barton, H.F.; Jamir, J.D.; Davis, A.K.; Peterson, G.W.; Parsons, G.N. Doubly Protective MOF-Photo-Fabrics: Facile Template-Free Synthesis of PCN-222-Textiles Enables Rapid Hydrolysis, Photo-Hydrolysis and Selective Oxidation of Multiple Chemical Warfare Agents and Simulants. Chem.—Eur. J. 2021, 27, 1465–1472. [Google Scholar] [CrossRef]
- Chen, H.; Snurr, R.Q. Insights into Catalytic Gas-Phase Hydrolysis of Organophosphate Chemical Warfare Agents by MOF-Supported Bimetallic Metal-Oxo Clusters. ACS Appl. Mater. Interfaces 2020, 12, 14631–14640. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Du, J.; Han, F.; Shi, T.; Zhang, H.; Lu, Y.; Long, S.; Sun, W.; Fan, J.; Peng, X. Piezoelectric Metal–Organic Frameworks Based Sonosensitizer for Enhanced Nanozyme Catalytic and Sonodynamic Therapies. ACS Nano 2023, 17, 7901–7910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, H.; Hu, J.; Tian, Q.; He, D.; Lu, G.; Zhu, M. Fe-Metal Organic Framework Converts Mechanical Energy with Piezoelectric Polarization to Remove Carbamazepine in Water: Efficiency, Pathway and Mechanism. Chem. Eng. J. 2023, 460, 141839. [Google Scholar] [CrossRef]
- He, J.; Yi, Z.; Chen, Q.; Li, Z.; Hu, J.; Zhu, M. Harvesting Mechanical Energy Induces Piezoelectric Polarization of MIL-100(Fe) for Cocatalyst-free Hydrogen Production. Chem. Commun. 2022, 58, 10723–10726. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, D.; Xie, C.; Hang, X.; He, C.; Jiang, H.-L. Piezo-Photocatalysis over Metal–Organic Frameworks: Promoting Photocatalytic Activity by Piezoelectric Effect. Adv. Mater. 2021, 33, 2106308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, M.; Zhang, Y.; Zhao, Z.; Zhang, Q.; Mu, Z.; Long, Y.; Jiang, Y.; Liu, Y.; Zhang, J.; et al. Harvesting Mechanical Energy for Hydrogen Generation by Piezoelectric Metal–Organic Frameworks. Mater. Horiz. 2022, 9, 1978–1983. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, H.; Kang, D.W.; Shin, J.; Kang, M.; Singh, N.; Jeong, J.-E.; Hong, C.S.; Kim, J.S. Isomeric sp2-C-conjugated porous organic polymer-mediated photo-and sono-catalytic detoxification of sulfur mustard simulant under ambient conditions. Matter 2021, 4, 3774–3785. [Google Scholar] [CrossRef]
- Milman, V.; Winkler, B.; White, J.A.; Pickard, C.J.; Payne, M.C.; Akhmatskaya, E.V.; Nobes, R.H. Electronic Structure, Properties, and Phase Stability of Inorganic Crystals: A Pseudopotential Plane-wave Study. Int. J. Quantum Chem. 2000, 77, 895–910. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1998, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P. Restoring the Density-Gradient Expansion for Exchange in a GGA for Solid and Surfaces. In Proceedings of the 2008 APS March Meeting, New Orleans, LA, USA, 10–14 March 2008. [Google Scholar]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Zhao, S.; Long, Y.; Shen, X.; Wang, S.; Su, Y.; Zhang, X.; Zhang, Z. Regulation of Electronic Structures of MOF-derived Carbon via Ligand Adjustment for Enhanced Fenton-like Reactions. Sci. Total Environ. 2021, 799, 149497. [Google Scholar] [CrossRef]
- Nyakuchena, J.; Ostresh, S.; Streater, D.; Pattengale, B.; Neu, J.; Fiankor, C.; Hu, W.; Kinigstein, E.D.; Zhang, J.; Zhang, X.; et al. Direct Evidence of Photoinduced Charge Transport Mechanism in 2D Conductive Metal Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 21050–21058. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.-K.; Loh, K.P. Coordination-Assisted Assembly of 1-D Nanostructured Light-Harvesting Antenna. J. Am. Chem. Soc. 2009, 131, 7210–7211. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships Between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption Behaviors of Organic Micropollutants on Zirconium Metal–Organic Framework UiO-66: Analysis of Surface Interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef]
- Min, X.; Wu, X.; Shao, P.; Ren, Z.; Ding, L.; Luo, X. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019, 358, 321–330. [Google Scholar] [CrossRef]
- Qiu, H.; Ye, M.; Zeng, Q.; Li, W.; Fortner, J.; Liu, L.; Yang, L. Fabrication of Agricultural Waste Supported UiO-66 Nanoparticles With High Utilization in Phosphate Removal from Water. Chem. Eng. J. 2019, 360, 621–630. [Google Scholar] [CrossRef]
- Peng, M.; You, D.; Shi, H.; Shao, P.; Ren, W.; Yang, L.; Sheng, X.; Shao, J.; Ding, X.; Ding, L.; et al. Disclosing the Role of Defective UiO-66 Over Sb(V) Removal: A Joint Experimental and Theoretical Study. Chem. Eng. J. 2022, 448, 137612. [Google Scholar] [CrossRef]
- Banerjee, D.; Xu, W.; Nie, Z.; Johnson, L.E.; Coghlan, C.; Sushko, M.L.; Kim, D.; Schweiger, M.J.; Kruger, A.A.; Doonan, C.J. Zirconium-based metal–organic framework for removal of perrhenate from water. Inorg. Chem. 2016, 55, 8241–8243. [Google Scholar] [CrossRef]
- Wang, J.; Ye, G.; Wang, H.; Chen, W.; Chu, H.; Wei, J.; Wang, D.; Li, Y. In Situ Implanting Single Tungsten Site Into Defective UiO-66(Zr) by Solvent-Free Route for Efficient Oxidative Desulfurization at Room Temperature. Angew. Chem. Int. Ed. 2021, 133, 20481–20487. [Google Scholar]
- Geravand, E.; Farzaneh, F.; Ghiasi, M. Metalation and DFT Studies of Metal Organic Frameworks UiO-66(Zr) with Vanadium Chloride as Allyl Alcohol Epoxidation Catalyst. J. Mol. Struct. 2019, 1198, 126940. [Google Scholar] [CrossRef]
- Driscoll, D.M.; Troya, D.; Usov, P.M.; Maynes, A.J.; Morris, A.J.; Morris, J.R. Characterization of Undercoordinated Zr Defect Sites in UiO-66 with Vibrational Spectroscopy of Adsorbed CO. J. Phys. Chem. C 2018, 122, 14582–14589. [Google Scholar] [CrossRef]
- Sharma, P.; Xiang, F.-X.; Shao, D.-F.; Zhang, D.; Tsymbal, E.Y.; Hamilton, A.R.; Seidel, J. A room-temperature ferroelectric semimetal. Sci. Adv. 2019, 5, eaax5080. [Google Scholar] [CrossRef]
- Li, L.; Wondraczek, L.; Peng, M.; Ma, Z.; Zou, B. Force-induced 1540 nm luminescence: Role of piezotronic effect in energy transfer process for mechanoluminescence. Nano Energy 2020, 69, 104413. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, J.; Cheng, Y.; Zhang, Y.-W.; Zeng, K. Design of the Hybrid Metal–Organic Frameworks as Potential Supramolecular Piezo-/Ferroelectrics. J. Phys. Chem. C 2019, 123, 3122–3129. [Google Scholar] [CrossRef]
- Thomas, G.; Spitzer, D. Double-side microcantilevers as a key to understand the adsorption mechanisms and kinetics of chemical warfare agents on vertically-aligned TiO2 nanotubes. J. Hazard. Mater. 2021, 406, 124672. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, X.; Zhang, L.; Gao, F.; Lei, R.; Jiang, C.; Feng, W.; Liu, P. Tuning piezoelectric field for optimizing the coupling effect of piezo-photocatalysis. Appl. Catal. B Environ. 2020, 278, 119291. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, J.; Gao, X.; Che, H.; Wang, P.; Liu, B.; Ao, Y. Piezo-enhanced photocatalytic performance of ZnO nanorod array for pollutants degradation in dynamic water: Insight into the effect of velocity and inner flow field. Nano Energy 2022, 101, 107614. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Oldham, C.J.; Peterson, G.W.; Parsons, G.N. UiO-66-NH2 Metal–Organic Framework (MOF) Nucleation on TiO2, ZnO, and Al2O3 Atomic Layer Deposition-Treated Polymer Fibers: Role of Metal Oxide on MOF Growth and Catalytic Hydrolysis of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2017, 9, 44847–44855. [Google Scholar] [CrossRef]
- Aina, P.O.; Mondal, S.K.; Rownaghi, A.A.; Rezaei, F. Assessing Detoxification Performance of Metal Hydroxide@Polymer Composites Prepared via Matrix-Incorporation and Spray-Coating Techniques. ACS Appl. Eng. Mater. 2024, 2, 454–466. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D.; Li, C. Photothermally Enhanced Detoxification of Chemical Warfare Agent Simulants Using Bioinspired Core–Shell Dopamine–Melanin@Metal–Organic Frameworks and Their Fabrics. ACS Appl. Mater. Interfaces 2019, 11, 7927–7935. [Google Scholar] [CrossRef]
- Islamoglu, T.; Atilgan, A.; Moon, S.-Y.; Peterson, G.W.; DeCoste, J.B.; Hall, M.; Hupp, J.T.; Farha, O.K. Cerium(IV) vs Zirconium(IV) Based Metal–Organic Frameworks for Detoxification of a Nerve Agent. Chem. Mater. 2017, 29, 2672–2675. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, M.; Hu, Q.; Li, B.-X.; Qu, J.; Yu, Z.-Z.; Yang, D. Spontaneously super-hygroscopic MOF-gel microreactors for efficient detoxification of nerve agent simulant in atmospheric environments. Appl. Catal. B Environ. 2023, 328, 122516. [Google Scholar] [CrossRef]
- Jiang, P.; Niu, Y.; Cao, J.; Xie, D.; Li, J.; Guo, T. A MOF-doped molecularly imprinted polymer/MOF hybrid gel incorporating with pH-buffering sodium acrylate for practical detoxification of organophosphorus nerve agents. Chem. Eng. J. 2024, 481, 148377. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Ji, Z.; Henderson, B.L.; Xia, T.; Li, L.; Zink, J.I.; Nel, A.E.; Mädler, L. Role of Fe Doping in Tuning the Band Gap of TiO2 for the Photo-Oxidation-Induced Cytotoxicity Paradigm. J. Am. Chem. Soc. 2011, 133, 11270–11278. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, Y.; Liu, X.; Cui, Z.; Zheng, Y.; Liang, Y.; Li, Z.; Zhu, S.; Lei, J.; Feng, X.; et al. The Enhanced Photocatalytic Sterilization of MOF-Based Nanohybrid for Rapid and Portable Therapy of Bacteria-Infected Open Wounds. Bioact. Mater. 2022, 13, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.; Xue, F.; Wu, Y.; Xu, H.; Yi, T.; Li, Q. An Imine-Linked Metal–Organic Framework as a Reactive Oxygen Species Generator. Angew. Chem. Int. Ed. 2021, 60, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, S.; Zhao, Z.; Li, S.; Wang, F. Piezocatalytic Effect Induced Hydrogen Production from Water over Non-noble Metal Ni Deposited Ultralong GaN Nanowires. ACS Appl. Mater. Interfaces 2021, 13, 10916–10924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, Y.; Zhou, W.; Wang, X.; Chang, K.; Liu, G.; Liu, H.; Kako, T.; Ye, J. Superior Photocatalytic H2 Production with Cocatalytic Co/Ni Species Anchored on Sulfide Semiconductor. Adv. Mater. 2017, 29, 1703258. [Google Scholar] [CrossRef]
- Tan, G.; Wang, S.; Zhu, Y.; Lei, Z.; Peng, Y.; Wang, X.; He, T.; Chen, J.; Mao, C.; Ning, C. Surface-Selective Preferential Production of Reactive Oxygen Species on Piezoelectric Ceramics for Bacterial Killing. ACS Appl. Mater. Interfaces 2016, 8, 24306–24309. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, W.; Chen, Y.; He, D.; Isaev, A.B.; Zhu, M. Dissolved Oxygen in Aeration-Driven Piezo-catalytic for Antibiotics Pollutants Removal in Water. Chin. Chem. Lett. 2023, 34, 108229. [Google Scholar] [CrossRef]
- Nie, G.; Xiao, L.; Bi, J.; Wang, S.; Duan, X. New Insight to Piezocatalytic Peroxymonosulfate Activation: The Critical Role of Dissolved Oxygen in Mediating Radical and Nonradical Pathways. Appl. Catal. B Environ. 2022, 315, 121584. [Google Scholar] [CrossRef]
- Dai, J.; Shao, N.; Zhang, S.; Zhao, Z.; Long, Y.; Zhao, S.; Li, S.; Zhao, C.; Zhang, Z.; Liu, W. Enhanced Piezocatalytic Activity of Sr0.5Ba0.5Nb2O6 Nanostructures by Engineering Surface Oxygen Vacancies and Self-Generated Heterojunctions. ACS Appl. Mater. Interfaces 2021, 13, 7259–7267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Dong, S.; Wang, P.; Gao, G. Ultrasonic Activation of Inert Poly(tetrafluoroethylene) Enables Piezocatalytic Generation of Reactive Oxygen Species. Nat. Commun. 2021, 12, 3508. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, S.; Li, Y.; Huang, J.; Yang, X.; Wang, J.; Tao, C.-a. Mechanically Enhanced Detoxification of Chemical Warfare Agent Simulants by a Two-Dimensional Piezoresponsive Metal–Organic Framework. Nanomaterials 2024, 14, 559. https://doi.org/10.3390/nano14070559
Liu Y, Zhao S, Li Y, Huang J, Yang X, Wang J, Tao C-a. Mechanically Enhanced Detoxification of Chemical Warfare Agent Simulants by a Two-Dimensional Piezoresponsive Metal–Organic Framework. Nanomaterials. 2024; 14(7):559. https://doi.org/10.3390/nano14070559
Chicago/Turabian StyleLiu, Yuyang, Shiyin Zhao, Yujiao Li, Jian Huang, Xuheng Yang, Jianfang Wang, and Cheng-an Tao. 2024. "Mechanically Enhanced Detoxification of Chemical Warfare Agent Simulants by a Two-Dimensional Piezoresponsive Metal–Organic Framework" Nanomaterials 14, no. 7: 559. https://doi.org/10.3390/nano14070559
APA StyleLiu, Y., Zhao, S., Li, Y., Huang, J., Yang, X., Wang, J., & Tao, C.-a. (2024). Mechanically Enhanced Detoxification of Chemical Warfare Agent Simulants by a Two-Dimensional Piezoresponsive Metal–Organic Framework. Nanomaterials, 14(7), 559. https://doi.org/10.3390/nano14070559