Highly Sensitive Electrochemical Determination of Butylated Hydroxyanisole in Food Samples Using Electrochemical-Pretreated Three-Dimensional Graphene Electrode Modified with Silica Nanochannel Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Characteriaztions and Instrumentations
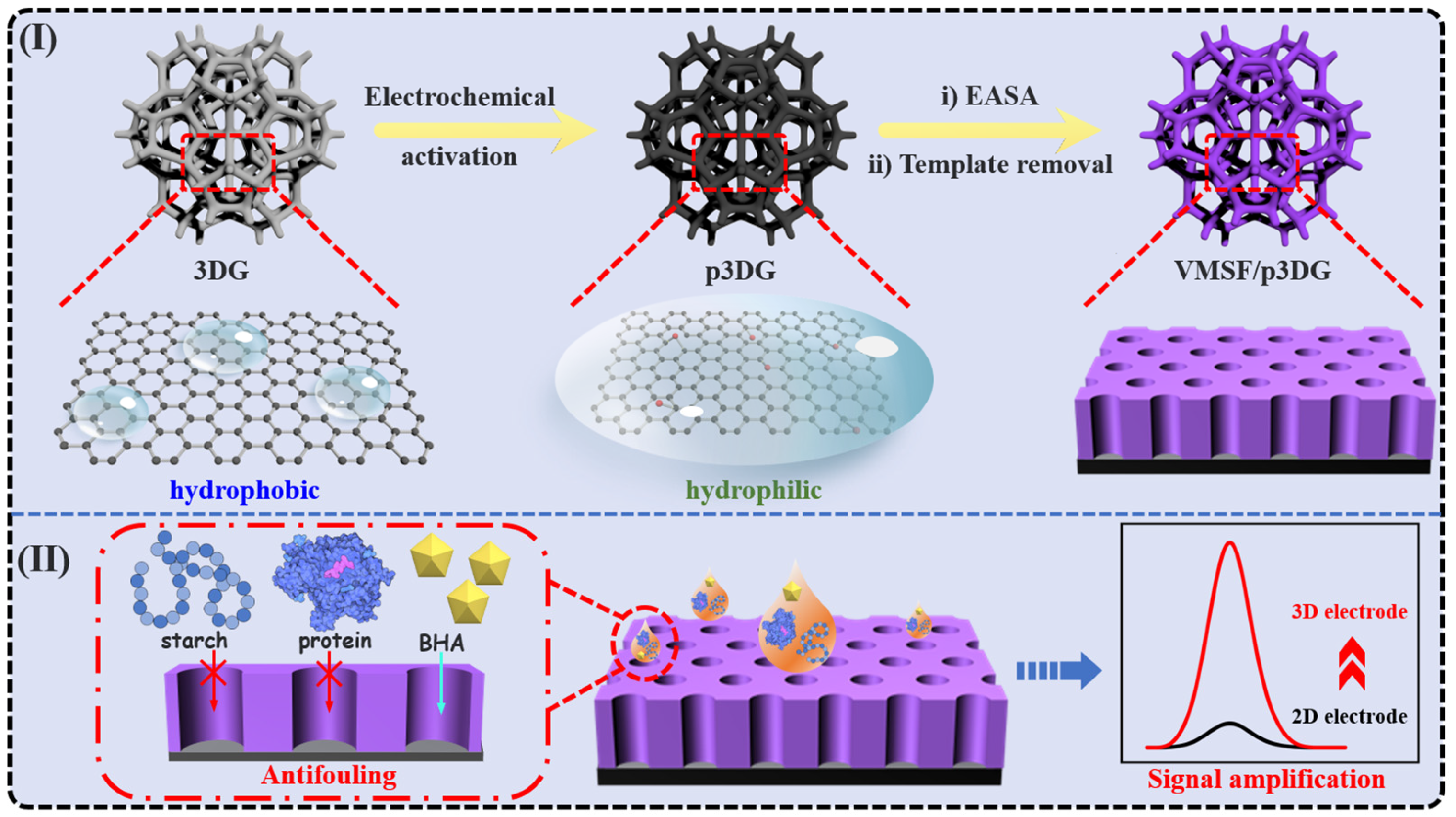
2.3. Preparation of p3DG
2.4. Preparation of VMSF/p3DG Electrode
2.5. Electrochemical Determination of BHA Using the VMSF/p3DG Electrode
3. Results and Discussion
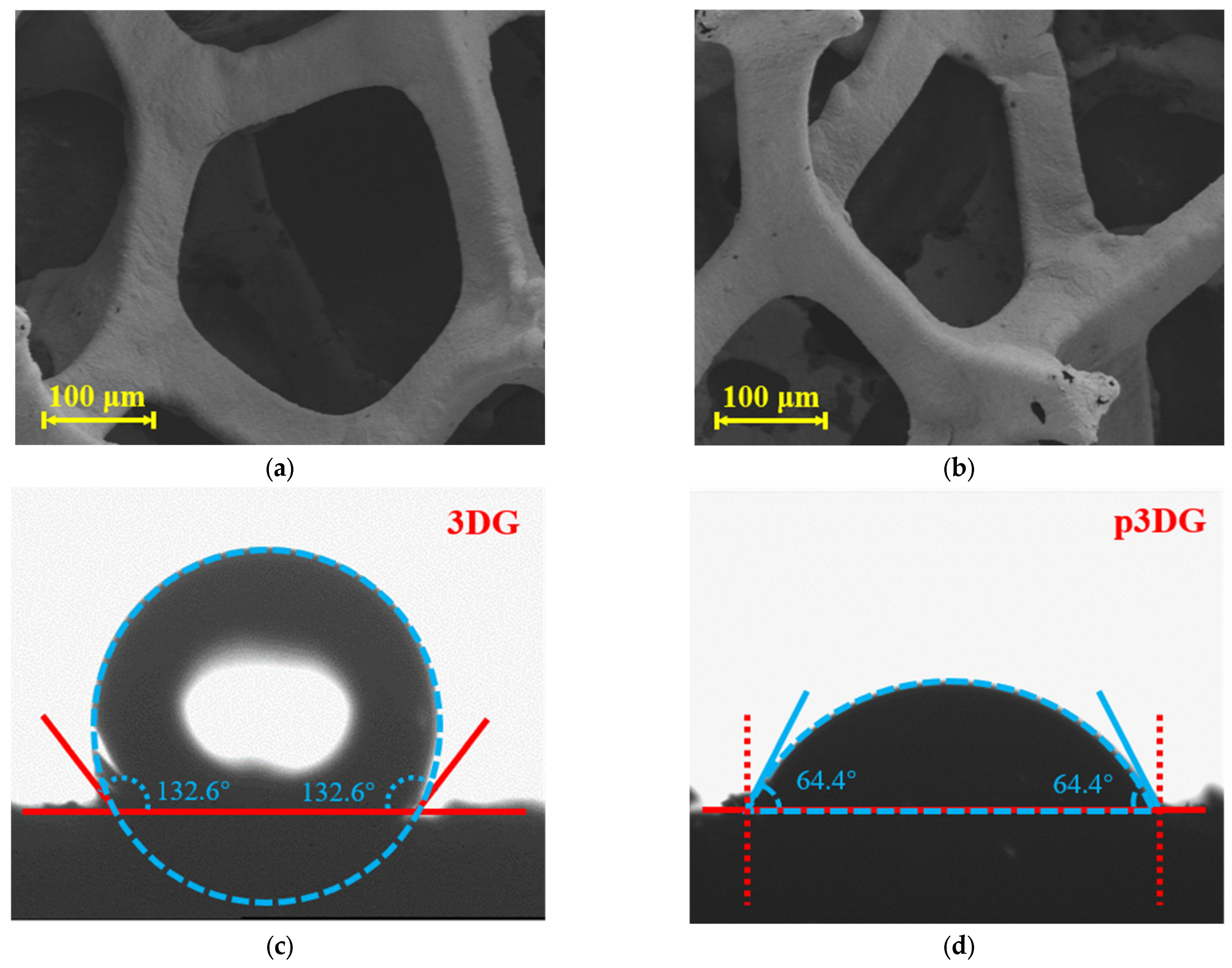
3.1. Characterization of 3DG and p3DG
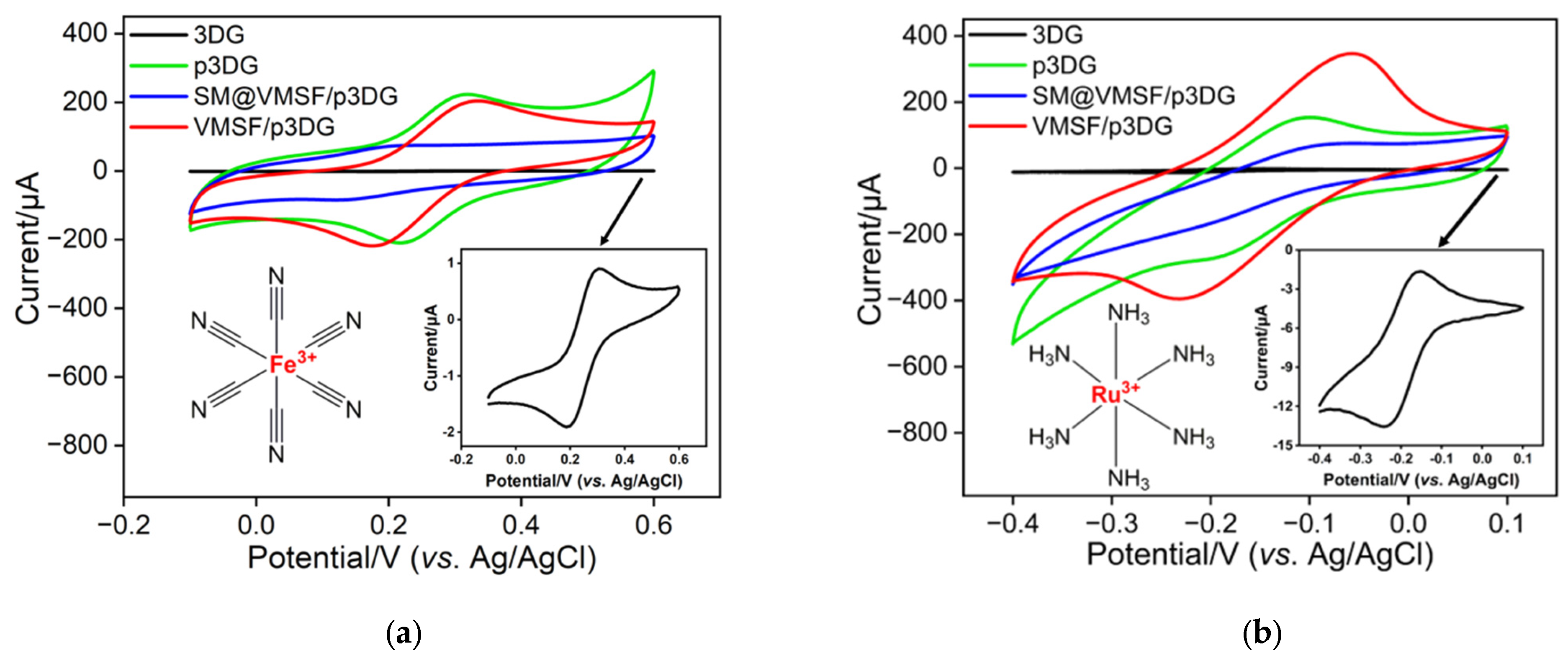
3.2. Charcterization of VMSF on p3DG
3.3. Enhanced Electrochemical Signal of BHA on VMSF/p3DG Electrode
3.4. Optimization of BHA Detection Conditions
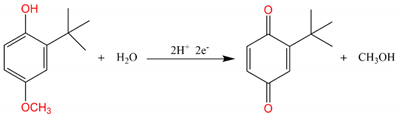
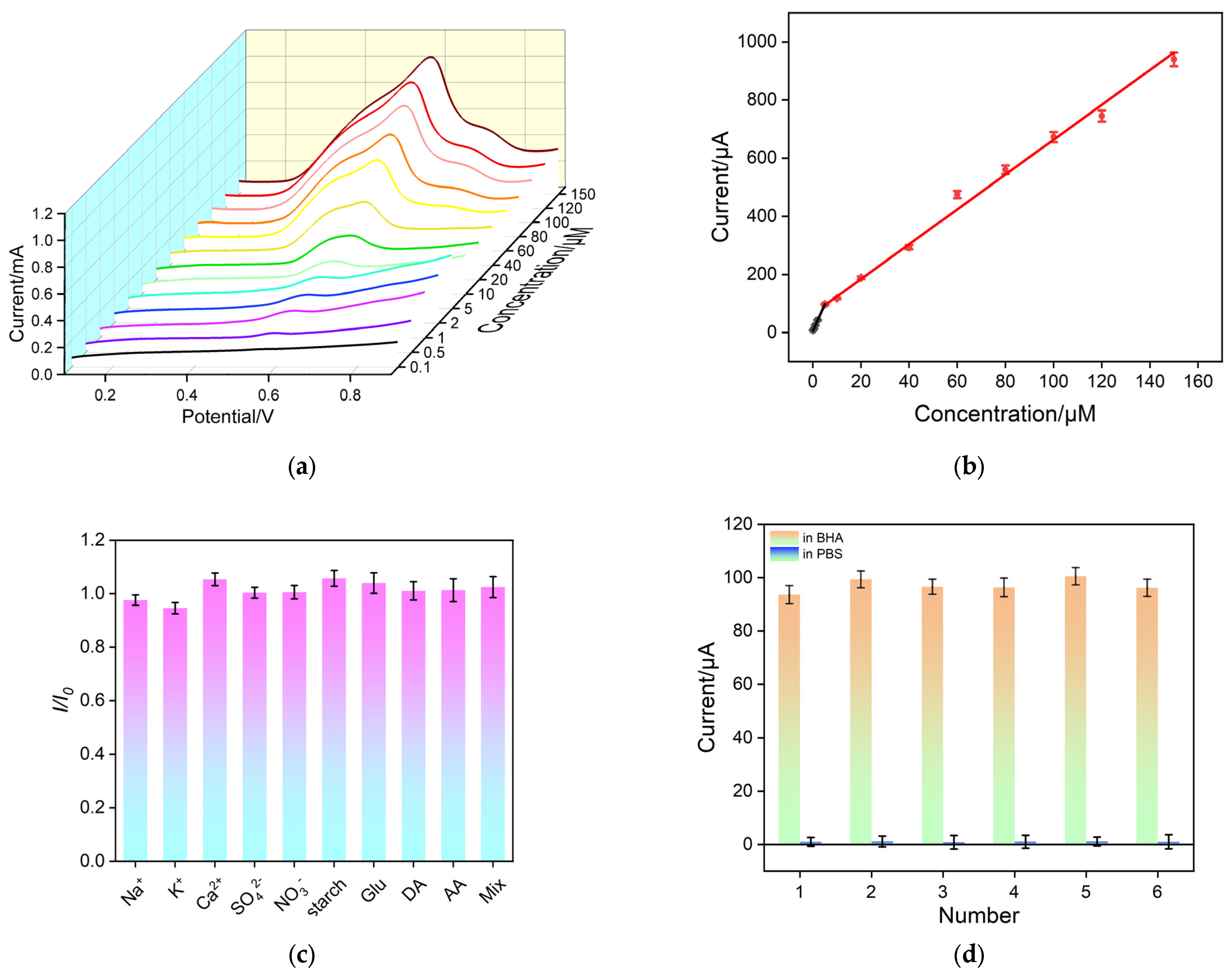
3.5. Electrochemical Detection of BHA
3.6. Anti-Interference/Anti-Fouling and Regeneration Characteristics of VMSF/p3DG Sensor
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, G.; Wang, Y.; Li, Y.; Zhang, L.; Gao, Y.; Li, Q.; Yu, X. Antioxidant properties of lipid concomitants in edible oils: A review. Food Chem. 2023, 422, 136219. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Castanheira, I.; Cruz, J.M.; Paseiro, P.; Sanches-Silva, A. Analytical strategies to evaluate antioxidants in food: A review. Trends Food Sci. Technol. 2010, 21, 229–246. [Google Scholar] [CrossRef]
- Motia, S.; Bouchikhi, B.; Bari, N.E. An electrochemical molecularly imprinted sensor based on chitosan capped with gold nanoparticles and its application for highly sensitive butylated hydroxyanisole analysis in foodstuff products. Talanta 2021, 223, 121689. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Sing, Y.; Nawi, M.; Hashim, N.; Mohamedali, A.; Saleh, M.; Sulaiman, S.; Talib, K.; Ahmad, K. Determination of synthetic phenolic antioxidants in food items using reversed-phase HPLC. Food Chem. 2007, 105, 389–394. [Google Scholar] [CrossRef]
- Guo, L.; Xie, M.; Yan, A.; Wan, Y.; Wu, Y. Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC-MS. Anal. Bioanal. Chem. 2006, 386, 1881–1887. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A Review on electrochemical sensors and biosensors used in assessing antioxidant activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Lu, H.; Shi, L.; Wang, P.; Ali, Z.; Li, J. Electrochemical detection of bisphenols in food: A review. Food Chem. 2021, 346, 128895. [Google Scholar] [CrossRef]
- Wang, K.; Lin, X.; Zhang, M.; Li, Y.; Luo, C.; Wu, J. Review of electrochemical biosensors for food safety detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent advances in perovskite oxides for non-enzymatic electrochemical sensors: A review. Anal. Chim. Acta 2023, 1251, 341007. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, X.; Sun, H.; Miao, T.; Li, M.; Zhou, S.; Zhou, W. Participation of lattice oxygen in perovskite oxide as a highly sensitive sensor for p-phenylenediamine detection. Molecules 2023, 28, 1122. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Tan, G.H.; Khor, S.M. Graphite nanocomposites sensor for multiplex detection of antioxidants in food. Food Chem. 2017, 237, 912–920. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Johnson, I.; Chen, M. 3D Continuously porous graphene for energy applications. Adv. Mater. 2022, 34, 2108750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef]
- Zheng, W.; Su, R.; Lin, X.; Liu, J. Nanochannel array modified three-dimensional graphene electrode for sensitive electrochemical detection of 2,4,6-trichlorophenol and prochloraz. Front. Chem. 2022, 10, 954802. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, M.; Ta, H.Q.; Zhao, L.; Shi, Q.; Fu, L.; Choi, J.; Yang, R.; Liu, Z.; Rümmeli, M.H. Synthesis of doped porous 3D graphenestructures by chemical vapor deposition and its applications. Adv. Funct. Mater. 2019, 29, 1904457. [Google Scholar] [CrossRef]
- Khan, A.; Habib, M.R.; Kumar, R.R.; Islam, S.M.; Arivazhagan, V.; Salman, M.; Yang, D.; Yu, X. Wetting behaviors and applications of metal-catalyzed CVD grown graphene. J. Mater. Chem. A 2018, 6, 22437–22464. [Google Scholar] [CrossRef]
- Xiao, W.; Li, B.; Yan, J.; Wang, L.; Huang, X.; Gao, J. Three dimensional graphene composites: Preparation, morphology and their multi-functional applications. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107335. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef]
- Rezvani, E.; Hatamie, A.; Berahman, M.; Simchi, M.; Angizi, S.; Rahmati, R.; Kennedy, J.; Simchi, A. Synthesis, first-principle simulation, and application of three-dimensional ceria nanoparticles/graphene nanocomposite for non-enzymatic hydrogen peroxide detection. J. Electrochem. Soc. 2019, 166, H3167–H3174. [Google Scholar] [CrossRef]
- Xi, F.; Zhao, D.; Wang, X.; Chen, P. Non-enzymatic detection of hydrogen peroxide using a functionalized three-dimensional graphene electrode. Electrochem. Commun. 2013, 26, 81–84. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Zhou, J.; Li, Y.; Chen, S.; Zhang, L.; Ma, Y.; Wang, L.; Yan, X. Three-dimensional nitrogen-doped graphene as an ultrasensitive electrochemical sensor for the detection of dopamine. Nanoscale 2015, 7, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Three-dimensional electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on monolithic and macroporous graphene foam. Biosens. Bioelectron. 2015, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, K.; Zhang, J.; Jie, X.; Chen, Z.; Lai, Y. A polyester-silica anti-condensation surface with anti-fouling property. Chem. Eng. J. 2022, 440, 135934. [Google Scholar] [CrossRef]
- Qi, L.; Liang, R.; Jiang, T.; Qin, W. Anti-fouling polymeric membrane ion-selective electrodes. Trends Anal. Chem. 2022, 150, 116572. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.; Huang, H.; Lv, N.; Liu, J.; Liu, Y. Nanochannel array on electrochemically polarized screen printed carbon electrode for rapid and sensitive electrochemical determination of clozapine in human whole blood. Molecules 2022, 27, 2739. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Yan, F.; Lin, J. N-Doped Graphene quantum dots confined within silica nanochannels for enhanced electrochemical detection of doxorubicin. Molecules 2023, 28, 6443. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wu, Z.; Zhuang, Y.; Sun, L.; Fan, X.; Zhao, T.; Yi, L.; Gu, Y. Biomimetic functional material-based sensors for food safety analysis: A review. Food Chem. 2023, 405, 134974. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, S.; Lin, X.; Liu, J.; Wang, K. Label-free electrochemical aptasensor based on the vertically-aligned mesoporous silica films for determination of aflatoxin B1. Biosensors 2023, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, S.; Wu, W.; Liu, J. Electrochemiluminescence aptasensor with dual signal amplification by silica nanochannel-based confinement effect on nanocatalyst and efficient emitter enrichment for highly sensitive detection of C-reactive protein. Molecules 2023, 28, 7664. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, S.; Yan, F.; Liu, J. Electrochemiluminescence enzyme biosensors for ultrasensitive determination of glucose using glucose dehydrogenase immobilized on vertical silica nanochannels. Sens. Actuators B Chem. 2024, 402, 135119. [Google Scholar] [CrossRef]
- Villa, C.C.; Galus, S.; Nowacka, M.; Magri, A.; Petriccione, M.; Gutiérrez, T.J. Molecular sieves for food applications: A review. Trends Food Sci. Technol. 2020, 102, 102–122. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Chang, Q.; Gu, X.; He, L.; Xi, F. A highly sensitive immunosensor based on nanochannel-confined nano-gold enhanced electrochemiluminescence for procalcitonin detection. Front. Chem. 2023, 11, 1274424. [Google Scholar] [CrossRef]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, G.; Dou, Y.; Li, W.; Mou, C.Y.; Zhang, X.; Asiri, A.M.; Zhao, D. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple stöber-solution growth approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Qu, H.; Xi, F. Immunosensor with enhanced electrochemiluminescence signal using platinum nanoparticles confined within nanochannels for highly sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 6559. [Google Scholar] [CrossRef]
- Walcarius, A. Electroinduced surfactant self-assembly driven to vertical growth of oriented mesoporous films. Acc. Chem. Res. 2021, 54, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gu, X.; Zhang, S.; Zou, Y.; Yan, F. Magnetic graphene oxide and vertically-ordered mesoporous silica film for universal and sensitive homogeneous electrochemiluminescence aptasensor platform. Microchem. J. 2024, 200, 110315. [Google Scholar] [CrossRef]
- Li, F.; Han, Q.; Xi, F. The fabrication of a probe-integrated electrochemiluminescence aptasensor based on double-layered nanochannel array with opposite charges for the sensitive determination of C-reactive protein. Molecules 2023, 28, 7867. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhao, Y.; Liu, J. Solid electrochemiluminescence sensor by immobilization of emitter ruthenium(ii)tris(bipyridine) in bipolar silica nanochannel film for sensitive detection of oxalate in serum and urine. Nanomaterials 2024, 14, 390. [Google Scholar] [CrossRef]
- Xing, J.; Han, Q.; Liu, J.; Yan, Z. Electrochemical aptasensor fabricated by anchoring recognition aptamers and immobilizing redox probes on bipolar silica nanochannel array for reagentless detection of carbohydrate antigen 15-3. Front. Chem. 2023, 11, 1324469. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Su, B. Adsorption of microperoxidase-11 in vertical silica mesochannels and electrochemical investigation of its electron transfer properties. Electrochim. Acta 2015, 161, 290–296. [Google Scholar] [CrossRef]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ding, H.; Yan, F.; Guo, W.; Su, B. Electrochemical detection of Alzheimer’s disease related substances in biofluids by silica nanochannel membrane modified glassy carbon electrodes. Analyst 2018, 143, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tang, H.; Luo, X.; Zhou, H.; Lin, X.; Wang, K.; Fei, Y.; Xi, F.; Liu, J. Vertically ordered mesoporous silica-nanochannel film-equipped three-dimensional macroporous graphene as sensitive electrochemiluminescence platform. Front. Chem. 2021, 9, 770512. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.; Xuan, L.; Lu, L.; Huang, J.; Yan, F.; Liu, J.; Dong, X.; Chen, P. Improved adhesion and performance of vertically-aligned mesoporous silica-nanochannel film on reduced graphene oxide for direct electrochemical analysis of human serum. Sens. Actuators B Chem. 2019, 288, 133–140. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.; Lim, K.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their Application for Fe3+ Sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Wang, H.; Li, J.; Qu, L.; Harrington, P.d.B. High-selective and sensitive voltammetric sensor for butylated hydroxyanisole based on AuNPs-PVP-graphene nanocomposites. Talanta 2015, 138, 169–175. [Google Scholar] [CrossRef]
- Sousa Carvalho, R.M.; Yotsumoto Neto, S.; Carvalho Silva, F.; Santos Damos, F.; de Cássia Silva Luz, R. A sensitive sensor based on CuTSPc and reduced graphene oxide for simultaneous determination of the BHA and TBHQ antioxidants in biodiesel samples. Electroanalysis 2016, 28, 2930–2938. [Google Scholar] [CrossRef]
- Yong, Y.; Dong, X.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Guan, Y.; Luo, P.; Wang, Y. Recent advance in fabricating monolithic 3D porous graphene and their applications in biosensing and biofuel cells. Biosens. Bioelectron. 2017, 89, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Kan, X. Sensitive detection of butylated hydroxyanisole based on free-standing paper decorated with gold and NiO nanoparticles. Microchem. J. 2020, 159, 105511. [Google Scholar] [CrossRef]
- Wang, P.; Han, C.; Zhou, F.; Lu, J.; Han, X.; Wang, Z. Electrochemical determination of tert-butylhydroquinone and butylated hydroxyanisole at choline functionalized film supported graphene interface. Sens. Actuators B Chem. 2016, 224, 885–891. [Google Scholar] [CrossRef]
- Yue, X.; Song, W.; Zhu, W.; Wang, J.; Wang, Y. In situ surface electrochemical co-reduction route towards controllable construction of AuNPs/ERGO electrochemical sensing platform for simultaneous determination of BHA and TBHQ. Electrochim. Acta 2015, 182, 847–855. [Google Scholar] [CrossRef]
- Caramit, R.P.; de Freitas Andrade, A.G.; Gomes de Souza, J.B.; de Araujo, T.A.; Viana, L.H.; Trindade, M.A.G.; Ferreira, V.S. A new voltammetric method for the simultaneous determination of the antioxidants TBHQ and BHA in biodiesel using multi-walled carbon nanotube screen-printed electrodes. Fuel 2013, 105, 306–313. [Google Scholar] [CrossRef]
- Manoranjitham, J.J.; Narayanan, S.S. Electrochemical sensor for determination of butylated hydroxyanisole (BHA) in food products using poly O-cresolphthalein complexone coated multiwalled carbon nanotubes electrode. Food Chem. 2021, 342, 128246. [Google Scholar] [CrossRef]
- Freitas, K.; Fatibello-Filho, O. Simultaneous determination of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) in food samples using a carbon composite electrode modified with Cu3(PO4)2 immobilized in polyester resin. Talanta 2010, 81, 1102–1108. [Google Scholar] [CrossRef]
- Gan, T.; Zhao, A.; Wang, S.; Lv, Z.; Sun, J. Hierarchical triple-shelled porous hollow zinc oxide spheres wrapped in graphene oxide as efficient sensor material for simultaneous electrochemical determination of synthetic antioxidants in vegetable oil. Sens. Actuators B Chem. 2016, 235, 707–716. [Google Scholar] [CrossRef]
- Monteiro, T.; Yotsumoto Neto, S.; Damos, F.; Luz, R. Development of a photoelectrochemical sensor for detection of TBHQ antioxidant based on LiTCNE-TiO2 composite under visible LED light. J. Electroanal. Chem. 2016, 774, 36–41. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Molecularly imprinted electrochemical sensor based on 3D-flower-like MoS2 decorated with silver nanoparticles for highly selective detection of butylated hydroxyanisole. Food Chem. 2022, 387, 132899. [Google Scholar] [CrossRef]
- Ding, M.; Zou, J. Rapid micropreparation procedure for the gas chromatographic-mass spectrometric determination of BHT, BHA and TBHQ in edible oils. Food Chem. 2012, 131, 1051–1055. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Y.; Ni, B.; Sun, J.; Wu, X.; Lin, X. Covalent organic framework-based monolithic column with hydrophilic and π-π stacking interaction for efficient in-tube solid-phase microextraction of synthetic phenolic antioxidants. Microchem. J. 2023, 186, 108311. [Google Scholar] [CrossRef]


| Sample a | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Edible oil | 0.00 | 5.49 | - | 3.8 |
| 5.00 | 10.62 | 103 | 3.2 | |
| 10.0 | 15.38 | 98.9 | 1.8 | |
| 50.0 | 56.02 | 101 | 2.1 | |
| Coffee b | 5.00 | 5.06 | 101 | 3.7 |
| 10.0 | 9.79 | 97.9 | 3.0 | |
| 50.0 | 50.3 | 101 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhang, S.; Ma, X.; Yan, F.; Tang, W. Highly Sensitive Electrochemical Determination of Butylated Hydroxyanisole in Food Samples Using Electrochemical-Pretreated Three-Dimensional Graphene Electrode Modified with Silica Nanochannel Film. Nanomaterials 2024, 14, 569. https://doi.org/10.3390/nano14070569
Huang C, Zhang S, Ma X, Yan F, Tang W. Highly Sensitive Electrochemical Determination of Butylated Hydroxyanisole in Food Samples Using Electrochemical-Pretreated Three-Dimensional Graphene Electrode Modified with Silica Nanochannel Film. Nanomaterials. 2024; 14(7):569. https://doi.org/10.3390/nano14070569
Chicago/Turabian StyleHuang, Chengqing, Shiyue Zhang, Xinying Ma, Fei Yan, and Weizhong Tang. 2024. "Highly Sensitive Electrochemical Determination of Butylated Hydroxyanisole in Food Samples Using Electrochemical-Pretreated Three-Dimensional Graphene Electrode Modified with Silica Nanochannel Film" Nanomaterials 14, no. 7: 569. https://doi.org/10.3390/nano14070569






