Poly(Allylamine Hydrochloride) and ZnO Nanohybrid Coating for the Development of Hydrophobic, Antibacterial, and Biocompatible Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Nanoparticles
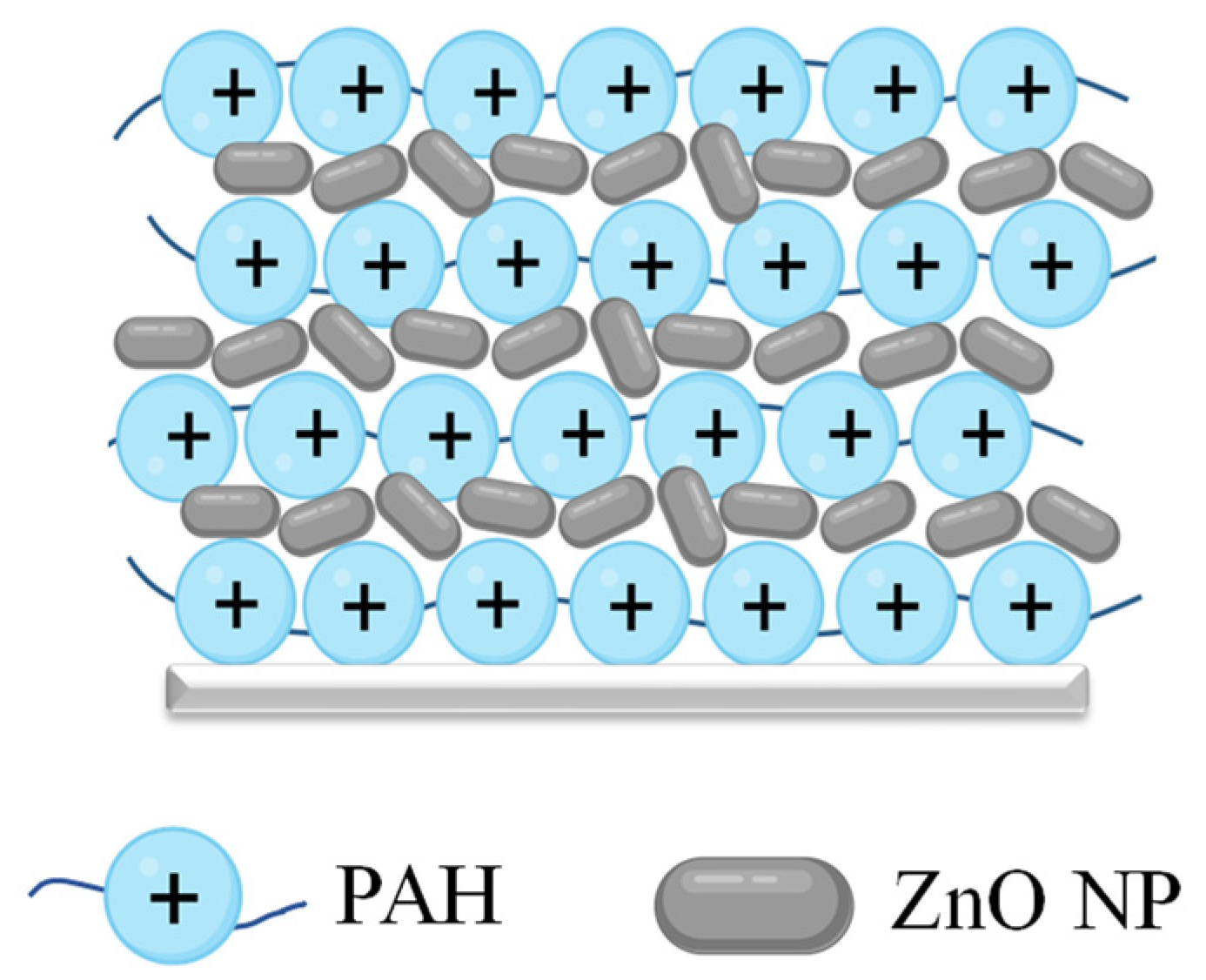
2.2. Fabrication of the Textile Coating
2.3. Nanoparticle Characterization
2.4. Characterisation of the Textile Coating
2.5. Antibacterial Studies
2.6. Biocompatibility Tests
2.7. Statistical Analysis
3. Results and Discussion
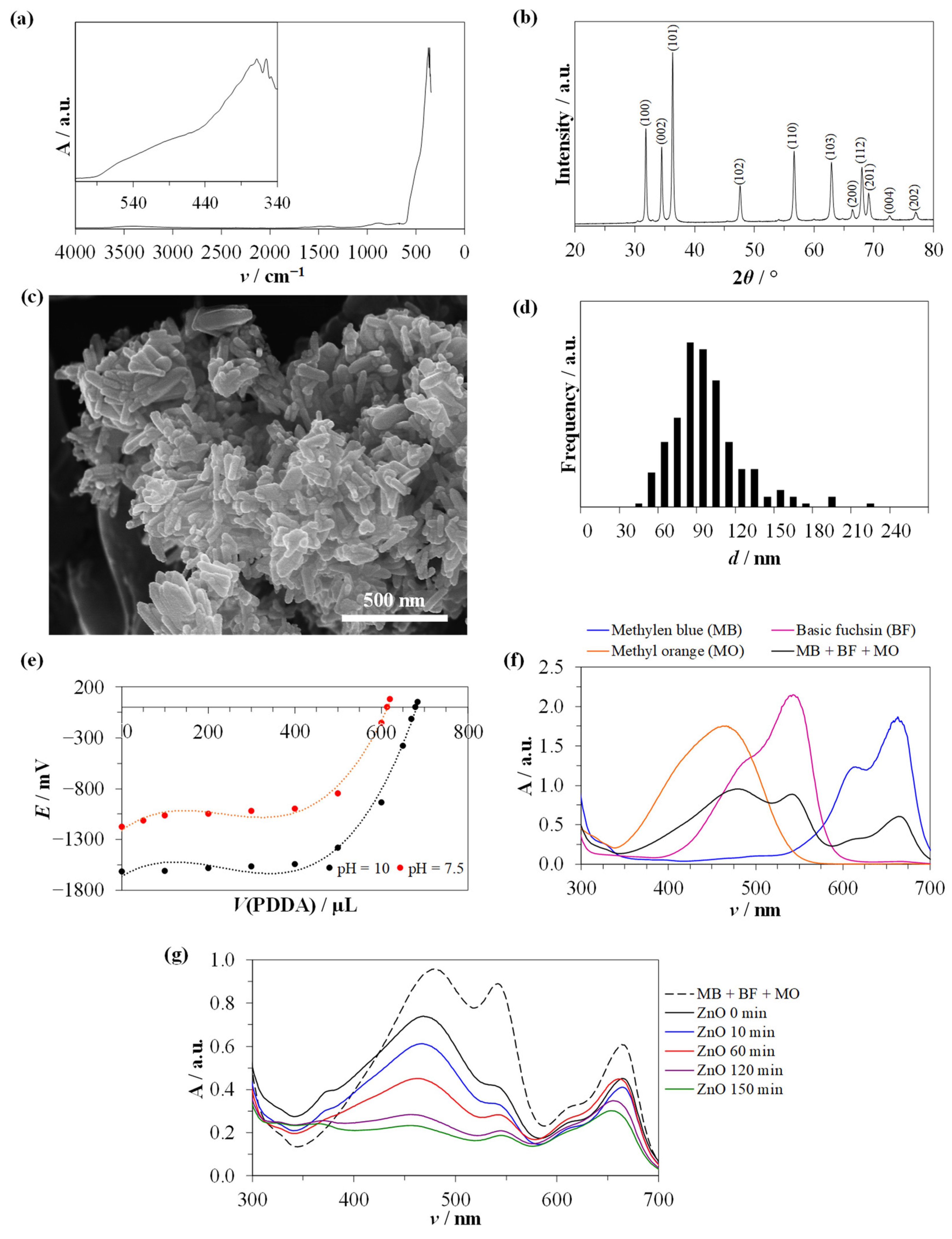
3.1. Nanoparticle Characterization
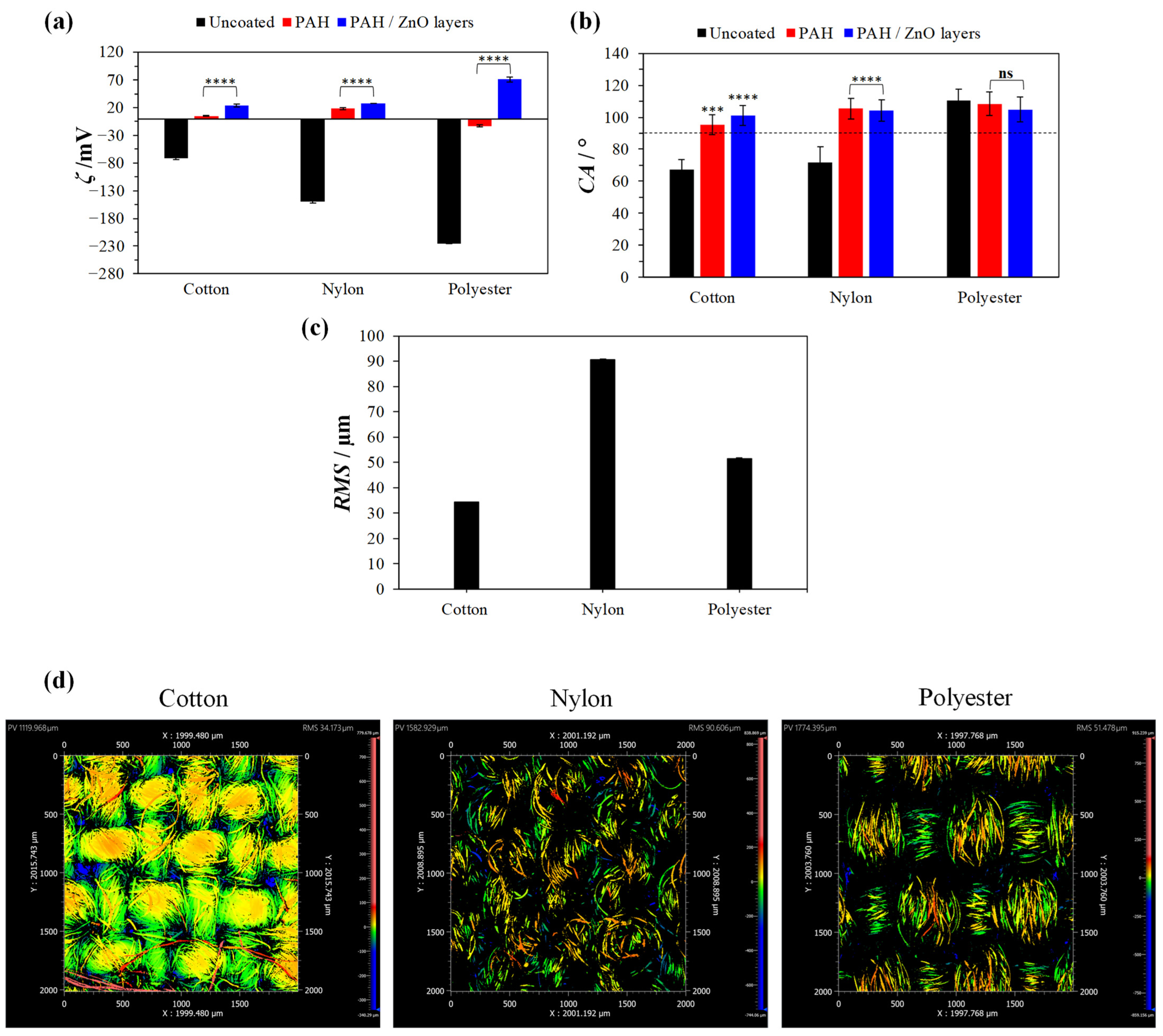
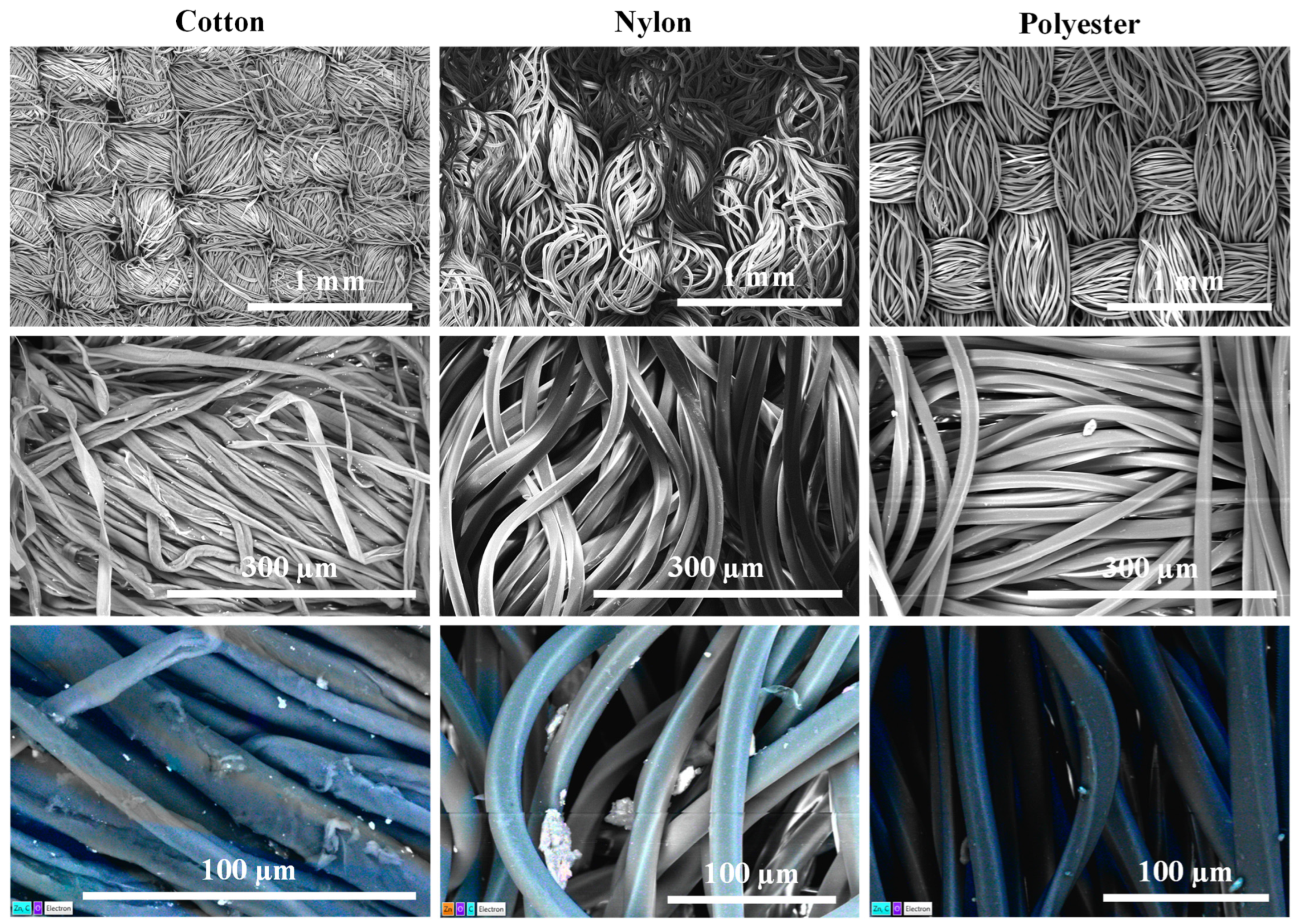
3.2. Textile Surface Characterization
3.3. Antibacterial Activity
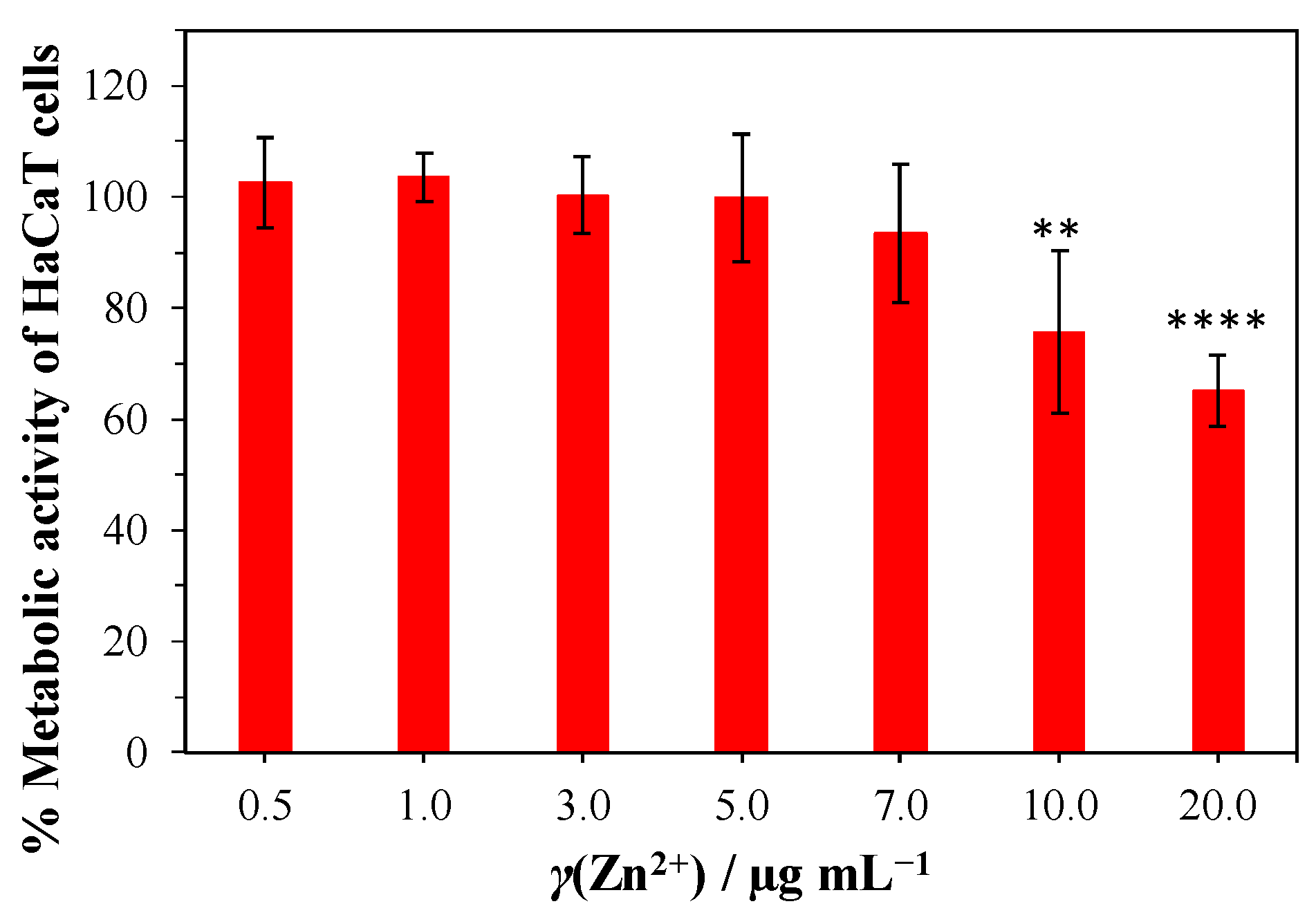
3.4. Biocompatibility Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nygren, E.; Gonzales Strömberg, L.; Logenius, J.; Husmark, U.; Löfström, C.; Bergström, B. Potential Sources of Contamination on Textiles and Hard Surfaces Identified as High-Touch Sites near the Patient Environment. PLoS ONE 2023, 18, e0287855. [Google Scholar] [CrossRef]
- Dixit, S.; Varshney, S.; Gupta, D.; Sharma, S. Textiles as Fomites in the Healthcare System. Appl. Microbiol. Biotechnol. 2023, 107, 3887–3897. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A Review on the Application of Inorganic Nano-Structured Materials in the Modification of Textiles: Focus on Anti-Microbial Properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Suellen Ferro de Oliveira, C.; Kekhasharú Tavaria, F. The Impact of Bioactive Textiles on Human Skin Microbiota. Eur. J. Pharm. Biopharm. 2023, 188, 66–77. [Google Scholar] [CrossRef]
- Morris, H.; Murray, R. Medical Textiles. Text. Prog. 2020, 52, 1–127. [Google Scholar] [CrossRef]
- Rajendran, S.; Anand, S.C.; Rigby, A.J. Textiles for Healthcare and Medical Applications. In Handbook of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–168. [Google Scholar]
- Ibrahim, N.A. Nanomaterials for Antibacterial Textiles; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128014714. [Google Scholar]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef]
- Zabielska, J.; Kunicka-Styczyńska, A.; Otlewska, A. Adhesive and Hydrophobic Properties of Pseudomonas Aeruginosa and Pseudomonas Cedrina Associated with Cosmetics. Ecol. Quest. 2018, 28, 41. [Google Scholar] [CrossRef]
- Kovačević, D.; Pratnekar, R.; Torkar, K.G.; Salopek, J.; Dražić, G.; Abram, A.; Bohinc, K. Influence of Polyelectrolyte Multilayer Properties on Bacterial Adhesion Capacity. Polymers 2016, 8, 345. [Google Scholar] [CrossRef]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Di Girolamo, M.; Sarret, G.; Bureau, S.; Fernandez-Martinez, A.; Lelong, C.; Eymard Vernain, E. In Situ Formation of Silver Nanoparticles (Ag-NPs) onto Textile Fibers. ACS Omega 2021, 6, 1316–1327. [Google Scholar] [CrossRef]
- Román, L.E.; Villalva, C.; Uribe, C.; Paraguay-Delgado, F.; Sousa, J.; Vigo, J.; Vera, C.M.; Gómez, M.M.; Solís, J.L. Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers 2022, 14, 3066. [Google Scholar] [CrossRef] [PubMed]
- Casalinuovo, S.; Caschera, D.; Quaranta, S.; Genova, V.; Buzzin, A.; Federici, F.; de Cesare, G.; Puglisi, D.; Caputo, D. Gold Nanoparticles-Functionalized Cotton as Promising Flexible and Green Substrate for Impedometric VOC Detection. Materials 2023, 16, 5826. [Google Scholar] [CrossRef] [PubMed]
- Glažar, D.; Jerman, I.; Tomšič, B.; Chouhan, R.S.; Simončič, B. Emerging and Promising Multifunctional Nanomaterial for Textile Application Based on Graphitic Carbon Nitride Heterostructure Nanocomposites. Nanomaterials 2023, 13, 408. [Google Scholar] [CrossRef]
- Cao, C.; Wang, F.; Lu, M.; Zhou, Y. Facile Preparation of Cotton Fabric Modified with Iron Oxide Nanoparticles with Durable Superhydrophobicity for Oil/Water Separation. Mater. Chem. Phys. 2023, 307, 128127. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent Advances in TiO2-Functionalized Textile Surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Shim, K.; Abdellatif, M.; Choi, E.; Kim, D. Nanostructured ZnO Films on Stainless Steel Are Highly Safe and Effective for Antimicrobial Applications. Appl. Microbiol. Biotechnol. 2017, 101, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, M.-J.; Lee, J.; Pyun, J.-C.; Khang, D.-Y. Recyclable, Antibacterial, Isoporous Through-Hole Membrane Air Filters with Hydrothermally Grown ZnO Nanorods. Nanomaterials 2021, 11, 3381. [Google Scholar] [CrossRef]
- Manna, J.; Begum, G.; Kumar, K.P.; Misra, S.; Rana, R.K. Enabling Antibacterial Coating via Bioinspired Mineralization of Nanostructured ZnO on Fabrics under Mild Conditions. ACS Appl. Mater. Interfaces 2013, 5, 4457–4463. [Google Scholar] [CrossRef]
- Tănase, M.A.; Soare, A.C.; Oancea, P.; Răducan, A.; Mihăescu, C.I.; Alexandrescu, E.; Petcu, C.; Diţu, L.M.; Ferbinteanu, M.; Cojocaru, B.; et al. Facile In Situ Synthesis of ZnO Flower-like Hierarchical Nanostructures by the Microwave Irradiation Method for Multifunctional Textile Coatings. Nanomaterials 2021, 11, 2574. [Google Scholar] [CrossRef]
- Irfan, M.; Hussain, H.; Saleem, B.; Saleem, M.; Shukrullah, S.; Legutko, S.; Petrů, J.; Naz, M.Y.; Pagáč, M.; Rahman, S.; et al. Evaluation of Ultrasonically ZnO Loading Effect on Photocatalytic Self-Cleaning, UV Protection and Antibacterial Activity of Plasma/Citric Acid-Activated Cotton Fabric. Nanomaterials 2022, 12, 2122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Song, Y. Electrospun Gelatin Fibers Surface Loaded ZnO Particles as a Potential Biodegradable Antibacterial Wound Dressing. Nanomaterials 2019, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. Hybrid Poly(Allylamine Hydrochloride)–Graphene Oxide Microcapsules: Preparation, Characterization and Application in Textiles with Controlled Release Behavior. Mater. Adv. 2020, 1, 804–813. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. A Poly(Allylamine Hydrochloride)/Poly(Styrene Sulfonate) Microcapsule-Coated Cotton Fabric for Stimulus-Responsive Textiles. RSC Adv. 2020, 10, 17731–17738. [Google Scholar] [CrossRef] [PubMed]
- Manna, J.; Goswami, S.; Shilpa, N.; Sahu, N.; Rana, R.K. Biomimetic Method To Assemble Nanostructured Ag@ZnO on Cotton Fabrics: Application as Self-Cleaning Flexible Materials with Visible-Light Photocatalysis and Antibacterial Activities. ACS Appl. Mater. Interfaces 2015, 7, 8076–8082. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M. Toxicity of Copper Oxide Nanoparticles: A Review Study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Najmi, Z.; Matijaković Mlinarić, N.; Calogero Scalia, A.; Cochis, A.; Selmani, A.; Učakar, A.; Abram, A.; Zore, A.; Delač, I.; Jerman, I.; et al. Antibacterial Evaluation of Different Prosthetic Liner Textiles Coated by CuO Nanoparticles. Heliyon 2024, 10, e23849. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; Scott Vanepps, J. Unexpected Insights into Antibacterial Activity of Zinc Oxide Nanoparticles against Methicillin Resistant: Staphylococcus Aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.H.; Shim, K.D.; Kim, D.J.; Jhee, K.H.; Lee, H.W.; Heo, C.H.; Kim, H.M.; Jang, E.S. Antibacterial Mechanism of ZnO Nanoparticles under Dark Conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar] [CrossRef]
- Ahmed, D.; Osman, M.; Mustafa, M.A.; Abbas Mustafa, M. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Zinc Acetate Dihydrate and Sodium Hydroxide. J. Nanosci. Nanoeng. 2015, 1, 248–251. [Google Scholar]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef] [PubMed]
- Bujak, I.T.; Kralj, M.B.; Kosyakov, D.S.; Ul’yanovskii, N.V.; Lebedev, A.T.; Trebše, P. Photolytic and Photocatalytic Degradation of Doxazosin in Aqueous Solution. Sci. Total Environ. 2020, 740, 140131. [Google Scholar] [CrossRef] [PubMed]
- ISO 20743:2021; Determination of Antibacterial Activity of Textile Products. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/79819.html (accessed on 15 May 2023).
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 1 July 2023).
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Rice as Soft Biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Nageswara Rao, B.; Tirupathi Rao, P.; Vasudha, K.; Esub Basha, S.; Prasanna, D.S.L.; Bhushana Rao, T.; Samatha, K.; Ramachandra, R.K. Physiochemical Characterization of Sodium Doped Zinc Oxide Nano Powder for Antimicrobial Applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122297. [Google Scholar] [CrossRef]
- Yurkinskii, V.P.; Firsova, E.G.; Proskura, S.A. Thermal dissociation of sodium hydroxide upon evacuation. Russ. J. Appl. Chem. 2005, 78, 360–362. [Google Scholar] [CrossRef]
- Kumar, R.; Miyaoka, H.; Shinzato, K.; Ichikawa, T. Analysis of Sodium Generation by Sodium Oxide Decomposition on Corrosion Resistance Materials: A New Approach towards Sodium Redox Water-Splitting Cycle. RSC Adv. 2021, 11, 21017–21022. [Google Scholar] [CrossRef]
- Dumbrava, A.; Matei, C.; Diacon, A.; Moscalu, F.; Berger, D. Novel ZnO-Biochar Nanocomposites Obtained by Hydrothermal Method in Extracts of Ulva Lactuca Collected from Black Sea. Ceram. Int. 2023, 49, 10003–10013. [Google Scholar] [CrossRef]
- Sun, L.; Rippon, J.A.; Cookson, P.G.; Koulaeva, O.; Wang, X. Effects of Undoped and Manganese-Doped Zinc Oxide Nanoparticles on the Colour Fading of Dyed Polyester Fabrics. Chem. Eng. J. 2009, 147, 391–398. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [PubMed]
- Willdigg, J.R.; Helmann, J.D. Mini Review: Bacterial Membrane Composition and Its Modulation in Response to Stress. Front. Mol. Biosci. 2021, 8, 634438. [Google Scholar] [CrossRef] [PubMed]
- Møllebjerg, A.; Palmén, L.G.; Gori, K.; Meyer, R.L. The Bacterial Life Cycle in Textiles Is Governed by Fiber Hydrophobicity. Microbiol. Spectr. 2021, 9, e01185-21. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Zore, A.; Bohinc, K. Bacterial Adhesion of Streptococcus Mutans to Dental Material Surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface Roughness Mediated Adhesion Forces between Borosilicate Glass and Gram-Positive Bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef]
- Barbera, L.; De Plano, L.M.; Franco, D.; Gattuso, G.; Guglielmino, S.P.P.; Lando, G.; Notti, A.; Parisi, M.F.; Pisagatti, I. Antiadhesive and Antibacterial Properties of Pillar[5]Arene-Based Multilayers. Chem. Commun. 2018, 54, 10203–10206. [Google Scholar] [CrossRef]
- Cao, W.; Gao, C. A Hydrogel Adhesive Fabricated from Poly(Ethylene Glycol) Diacrylate and Poly(Allylamine Hydrochloride) with Fast and Spontaneous Degradability and Anti-Bacterial Property. Polymer 2020, 186, 122082. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Abugharbieh, A.; Yasmeen, F.; Buabeng, E.; Mathew, S.; Samaroo, D.; Cheng, H.P. The Crosslinking of Polysaccharides with Polyamines and Dextran-Polyallylamine Antibacterial Hydrogels. Int. J. Biol. Macromol. 2015, 72, 88–93. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Pettolino, F.; Flanagan, B.; Gidley, M.J.; Gilbert, E.P. Structure of Cellulose Microfibrils in Mature Cotton Fibres. Carbohydr. Polym. 2017, 175, 450–463. [Google Scholar] [CrossRef]
- Hoff, G.P.; Du Pont, E.I. Nylon as a Textile Fiber. Ind. Eng. Chem. 1940, 32, 1560–1564. [Google Scholar] [CrossRef]
- Grishanov, S. Structure and Properties of Textile Materials. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 28–63. [Google Scholar]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How Do We Determine the Efficacy of an Antibacterial Surface? A Review of Standardised Antibacterial Material Testing Methods. Antibiotics 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Dincă, V.; Mocanu, A.; Isopencu, G.; Busuioc, C.; Brajnicov, S.; Vlad, A.; Icriverzi, M.; Roseanu, A.; Dinescu, M.; Stroescu, M.; et al. Biocompatible Pure ZnO Nanoparticles-3D Bacterial Cellulose Biointerfaces with Antibacterial Properties. Arab. J. Chem. 2020, 13, 3521–3533. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, M.; Balani, K. Effect of ZnO Morphology on Affecting Bactericidal Property of Ultra High Molecular Weight Polyethylene Biocomposite. Mater. Sci. Eng. C 2016, 62, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of Physical and Chemical Interactions in the Antibacterial Behavior of ZnO Nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]

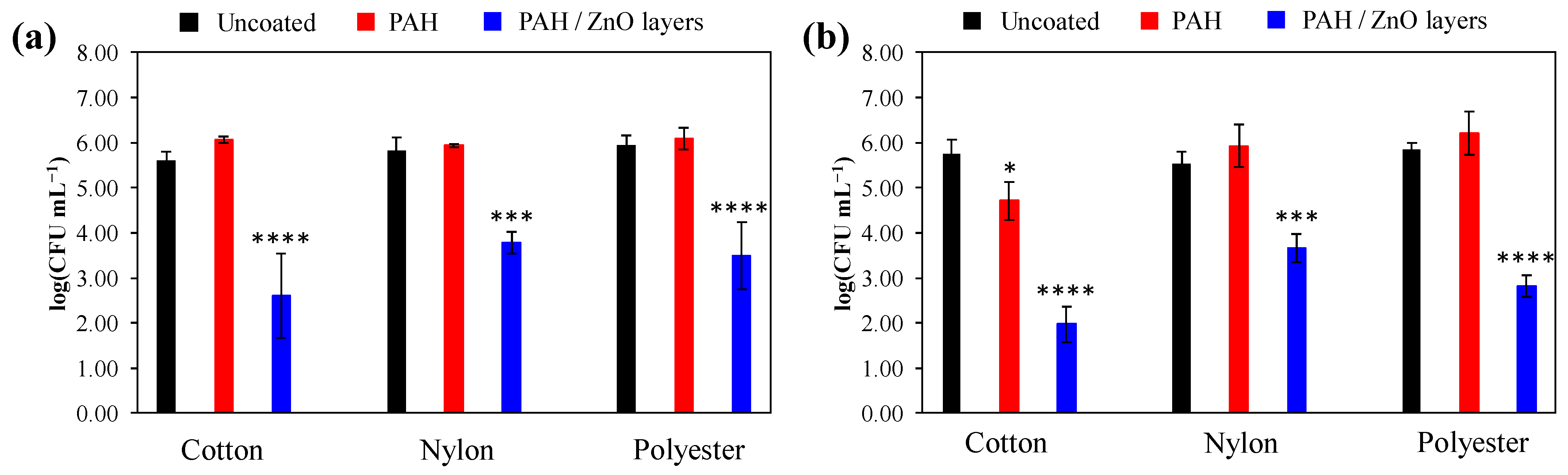
| Planktonic S. aureus | Adhered S. aureus | |||
|---|---|---|---|---|
| Logarithmic Reduction | Percentage Reduction/% | Logarithmic Reduction | Percentage Reduction/% | |
| Cotton PAH | ** | ** | 1.11 ± 0.42 | 90.12 ± 6.81 |
| Cotton PAH/ZnO | 3.02 ± 0.95 | 99.77 ± 0.21 | 3.85 ± 0.39 | 99.98 ± 0.01 |
| Nylon PAH | ** | ** | ** | ** |
| Nylon PAH/ZnO | 2.10 ± 0.24 | 99.12 ± 0.44 | 2.22 ± 0.31 | 99.27 ± 0.55 |
| Polyester PAH | ** | ** | ** | ** |
| Polyester PAH/ZnO | 2.49 ± 0.74 | 99.21 ± 1.10 | 3.16 ± 0.24 | 99.92 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matijaković Mlinarić, N.; Wawrzaszek, B.; Kowalska, K.; Selmani, A.; Učakar, A.; Vidmar, J.; Kušter, M.; Van de Velde, N.; Trebše, P.; Sever Škapin, A.; et al. Poly(Allylamine Hydrochloride) and ZnO Nanohybrid Coating for the Development of Hydrophobic, Antibacterial, and Biocompatible Textiles. Nanomaterials 2024, 14, 570. https://doi.org/10.3390/nano14070570
Matijaković Mlinarić N, Wawrzaszek B, Kowalska K, Selmani A, Učakar A, Vidmar J, Kušter M, Van de Velde N, Trebše P, Sever Škapin A, et al. Poly(Allylamine Hydrochloride) and ZnO Nanohybrid Coating for the Development of Hydrophobic, Antibacterial, and Biocompatible Textiles. Nanomaterials. 2024; 14(7):570. https://doi.org/10.3390/nano14070570
Chicago/Turabian StyleMatijaković Mlinarić, Nives, Barbara Wawrzaszek, Klaudia Kowalska, Atiđa Selmani, Aleksander Učakar, Janja Vidmar, Monika Kušter, Nigel Van de Velde, Polonca Trebše, Andrijana Sever Škapin, and et al. 2024. "Poly(Allylamine Hydrochloride) and ZnO Nanohybrid Coating for the Development of Hydrophobic, Antibacterial, and Biocompatible Textiles" Nanomaterials 14, no. 7: 570. https://doi.org/10.3390/nano14070570







