Dispersion and Dosimetric Challenges of Hydrophobic Carbon-Based Nanoparticles in In Vitro Cellular Studies
Abstract
1. Introduction
2. Methods
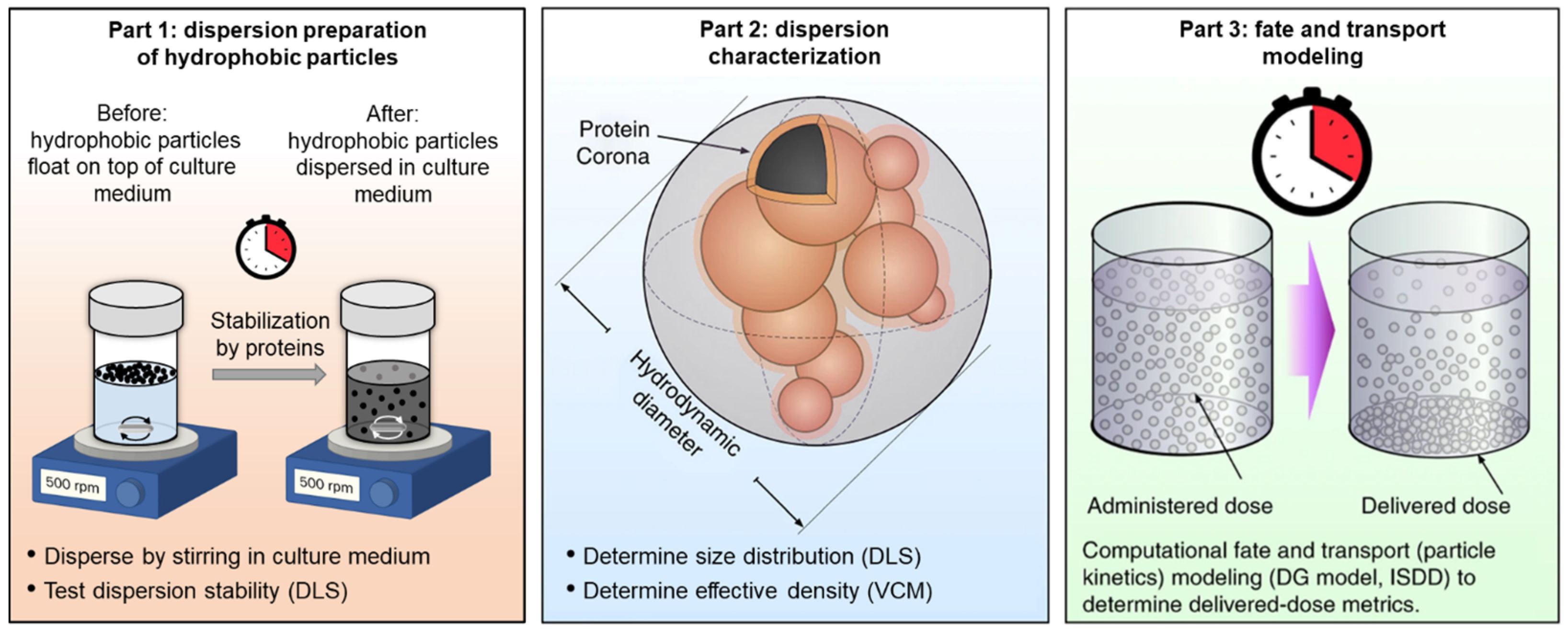
2.1. Summary of the Dispersion Preparation and Characterization Approach for Hydrophobic NPs
2.2. Synthesis and Contact Angle Characterization of Hydrophobic Black Carbon NPs
2.3. Model Cell Culture Media Used in the Case Study
2.4. Dispersion and Characterization of BC NPs in Cell Culture Media
2.5. Effective Density Measurements and Particle Dosimetry
3. Results and Discussion
3.1. Characterization of BC NPs
3.2. Hydrophobicity of BC NPs
3.3. Dispersion Preparation of BC NPs in Cell Culture Media as a Function of Stirring Time (Dispersion Energy)
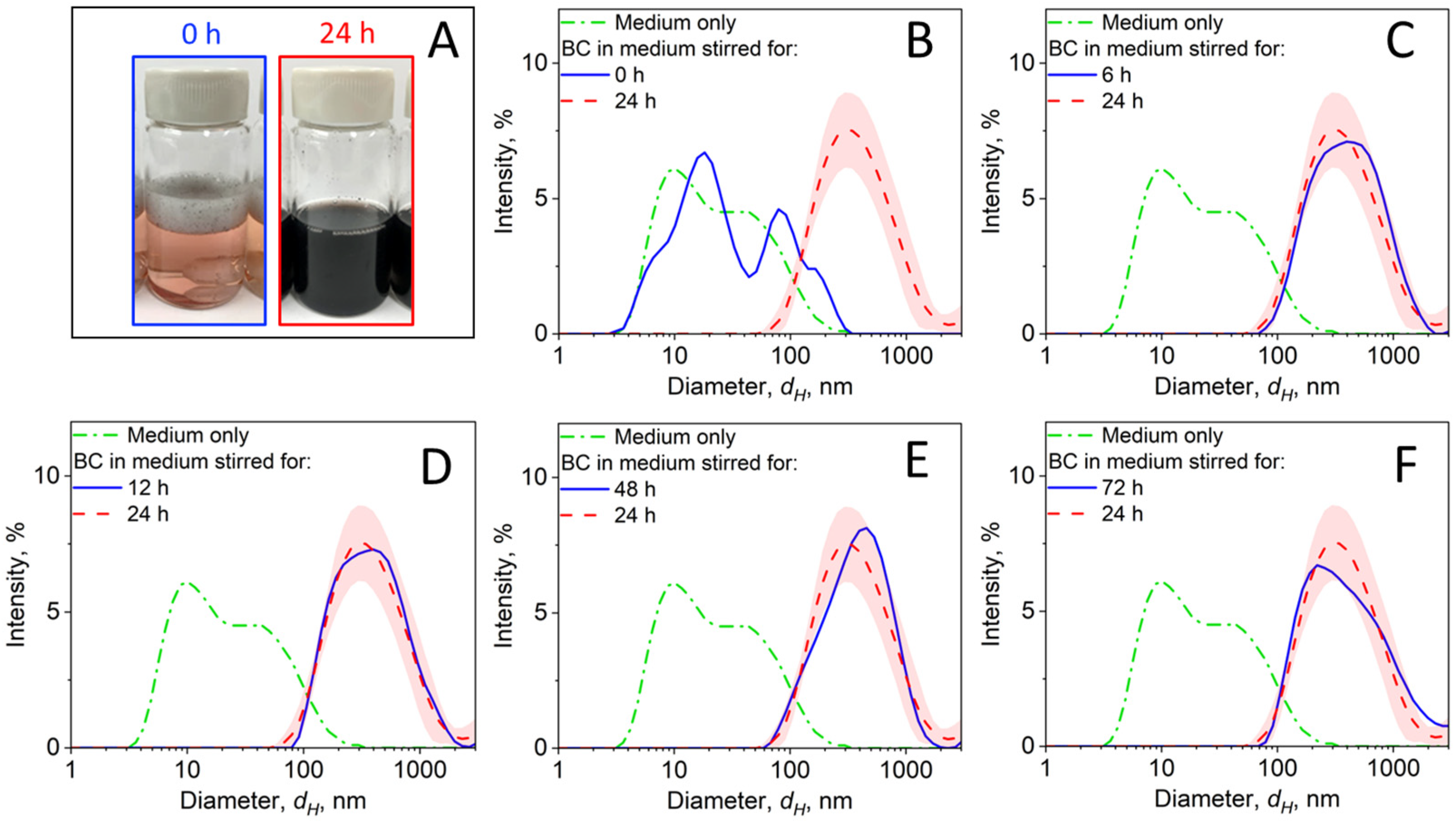
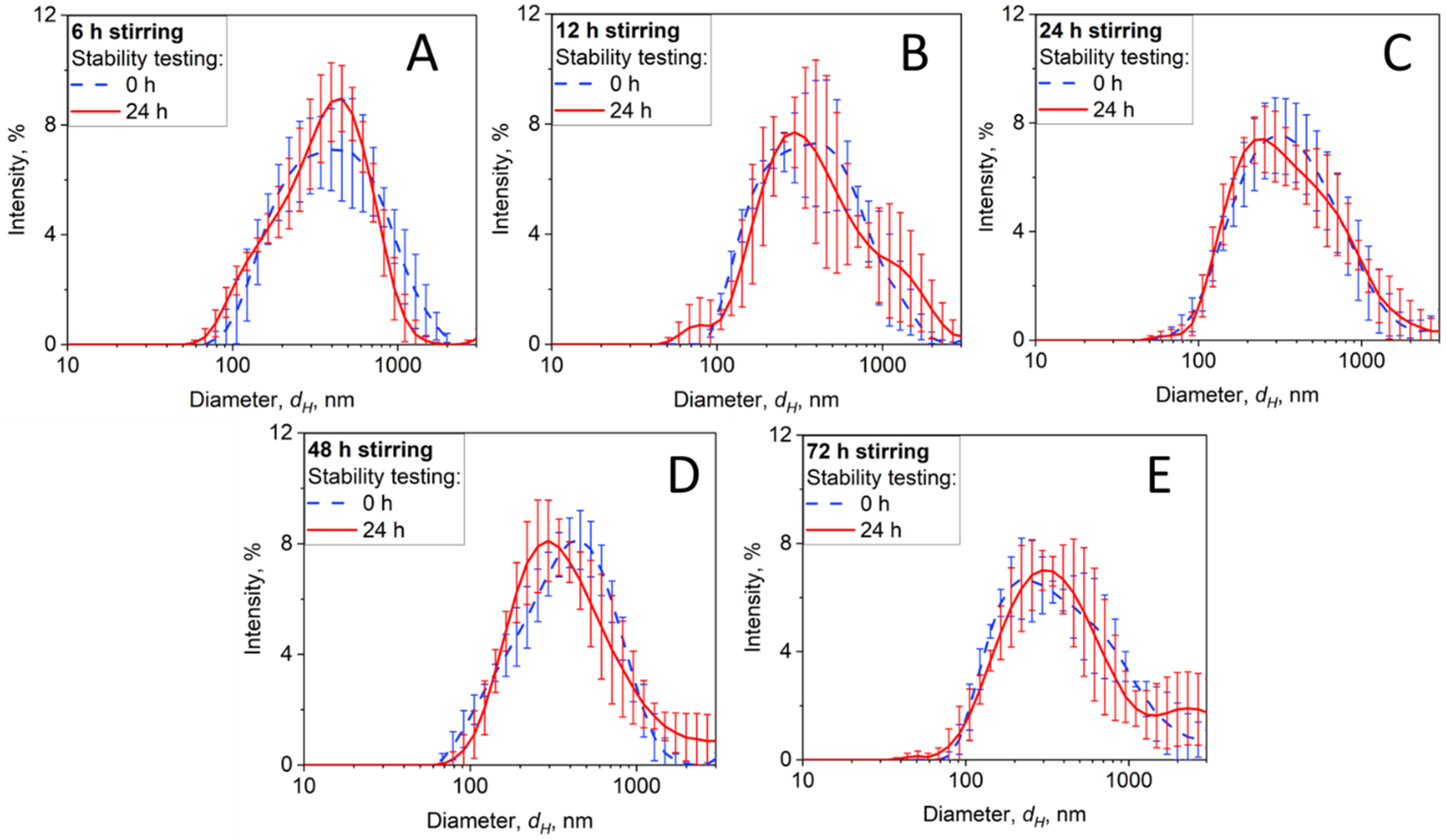
3.3.1. BC-RPMI Case Study
3.3.2. BC- SABM Case Study
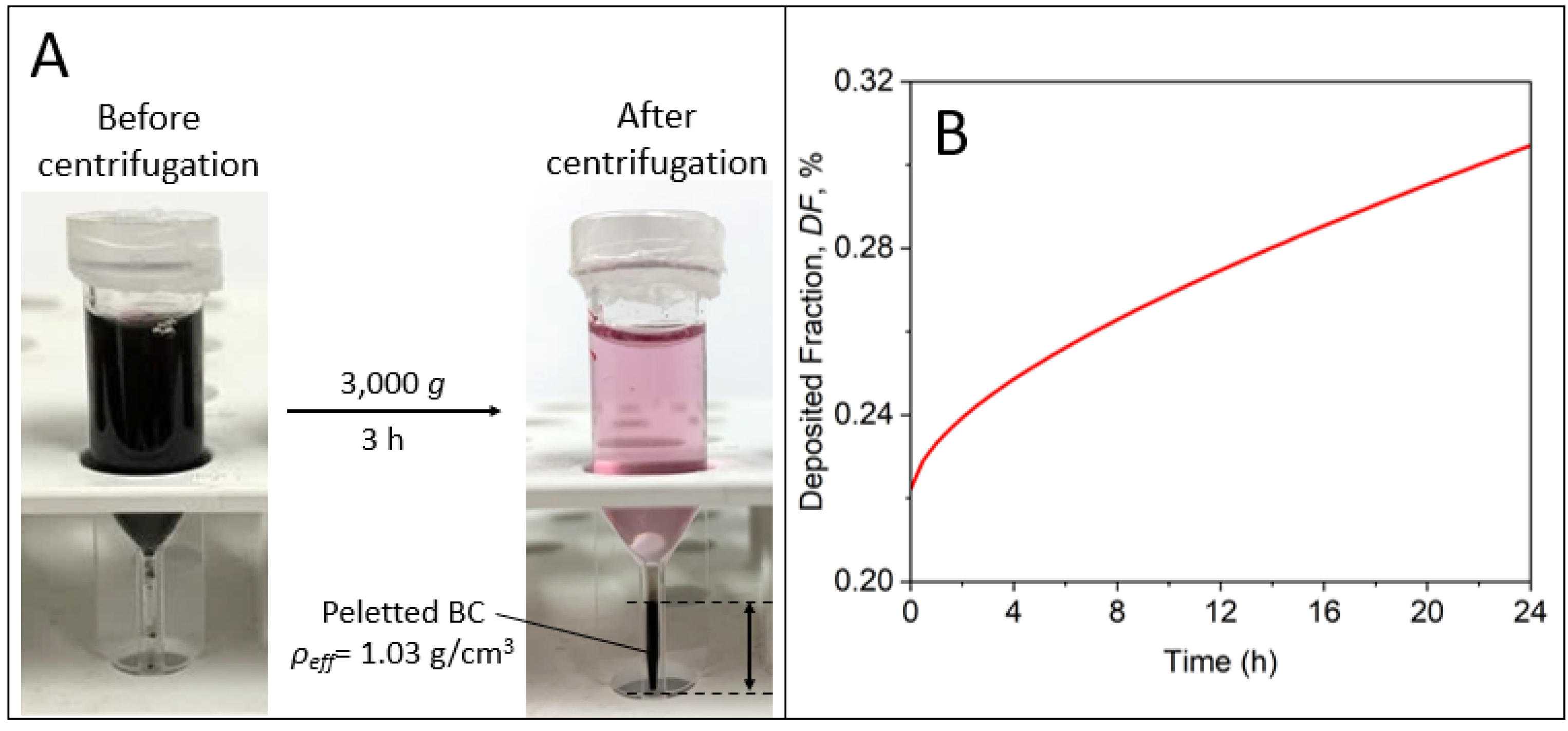
3.4. Effective NP Density and Dosimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4220. [Google Scholar] [CrossRef] [PubMed]
- Abegg, S.; Magro, L.; van den Broek, J.; Pratsinis, S.E.; Güntner, A.T. A pocket-sized device enables detection of methanol adulteration in alcoholic beverages. Nat. Food 2020, 1, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Servin, A.D.; White, J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. NanoImpact 2016, 1, 9–12. [Google Scholar] [CrossRef]
- Das, M.; Saxena, N.; Dwivedi, P.D. Emerging trends of nanoparticles application in food technology: Safety paradigms. Nanotoxicology 2009, 3, 10–18. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Madl, A.K.; Pinkerton, K.E. Health effects of inhaled engineered and incidental nanoparticles. Crit. Rev. Toxicol. 2009, 39, 629–658. [Google Scholar] [CrossRef]
- Yeganeh, B.; Kull, C.M.; Hull, M.S.; Marr, L.C. Characterization of airborne particles during production of carbonaceous nanomaterials. Environ. Sci. Technol. 2008, 42, 4600–4606. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W.; Meyer, J.; Muller, J.; Mueller, P.; Vilsmeier, K.; Stahlmecke, B.; Kuhlbusch, T.A. Release from nanomaterials during their use phase: Combined mechanical and chemical stresses applied to simple and multi-filler nanocomposites mimicking wear of nano-reinforced tires. Environ. Sci. Nano 2016, 3, 1036–1051. [Google Scholar] [CrossRef]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Park, K.; Kittelson, D.B.; McMurry, P.H. Structural properties of diesel exhaust particles measured by transmission electron microscopy (TEM): Relationships to particle mass and mobility. Aerosol Sci. Technol. 2004, 38, 881–889. [Google Scholar] [CrossRef]
- Corbin, J.C.; Mensah, A.A.; Pieber, S.M.; Orasche, J.; Michalke, B.; Zanatta, M.; Czech, H.; Massabo, D.; de Mongeot, F.B.; Mennucci, C.; et al. Trace Metals in Soot and PM2.5 from Heavy-Fuel-Oil Combustion in a Marine Engine. Environ. Sci. Technol. 2018, 52, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Lack, D.A.; Corbett, J.J. Black carbon from ships: A review of the effects of ship speed, fuel quality and exhaust gas scrubbing. Atmos. Chem. Phys. 2012, 12, 3985–4000. [Google Scholar] [CrossRef]
- Petzold, A.; Dopelheuer, A.; Brock, C.A.; Schroder, F. In situ observations and model calculations of black carbon emission by aircraft at cruise altitude. J. Geophys. Res.-Atmos. 1999, 104, 22171–22181. [Google Scholar]
- Stettler, M.E.J.; Boies, A.M.; Petzold, A.; Barrett, S.R.H. Global Civil Aviation Black Carbon Emissions. Environ. Sci. Technol. 2013, 47, 10397–10404. [Google Scholar] [CrossRef]
- Xu, R.; Ye, T.; Yue, X.; Yang, Z.; Yu, W.; Zhang, Y.; Bell, M.L.; Morawska, L.; Yu, P.; Zhang, Y. Global population exposure to landscape fire air pollution from 2000 to 2019. Nature 2023, 621, 521–529. [Google Scholar] [CrossRef]
- Burke, M.; Childs, M.L.; de la Cuesta, B.; Qiu, M.; Li, J.; Gould, C.F.; Heft-Neal, S.; Wara, M. The contribution of wildfire to PM2. 5 trends in the USA. Nature 2023, 622, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Nim, N.; Morris, J.; Tekasakul, P.; Dejchanchaiwong, R. Fine and ultrafine particle emission factors and new diagnostic ratios of PAHs for peat swamp forest fires. Environ. Pollut. 2023, 335, 122237. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, Z.; Yue, T.; Su, G.; Teng, C.; Yan, B. Crossing biological barriers by engineered nanoparticles. Chem. Res. Toxicol. 2020, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Campagnolo, L.; Fadeel, B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small 2013, 9, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Qu, J.; Zhang, H.; Cao, Y.; Ma, X.; Wang, S.; Wang, J.; Wu, J.; Zhang, T. Nose-to-brain translocation and nervous system injury in response to indium tin oxide nanoparticles of long-term low-dose exposures. Sci. Total Environ. 2023, 905, 167314. [Google Scholar] [CrossRef] [PubMed]
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro–in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359. [Google Scholar] [CrossRef]
- Garza, K.M.; Soto, K.F.; Murr, L.E. Cytotoxicity and reactive oxygen species generation from aggregated carbon and carbonaceous nanoparticulate materials. Int. J. Nanomed. 2008, 3, 83–94. [Google Scholar]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Cohen, J.M.; Teeguarden, J.G.; Demokritou, P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 2014, 11, 1–12. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part. Fibre Toxicol. 2015, 12, 1–20. [Google Scholar] [CrossRef]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 7, 36. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Miljevic, B.; Hedayat, F.; Stevanovic, S.; Fairfull-Smith, K.; Bottle, S.; Ristovski, Z. To sonicate or not to sonicate PM filters: Reactive oxygen species generation upon ultrasonic irradiation. Aerosol Sci. Technol. 2014, 48, 1276–1284. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Cai, Y.-X.; Liu, X.-C.; Ma, G.-W.; Lv, W.; Wang, M.-X. Hydrophobic modification on the surface of SiO2 nanoparticle: Wettability control. Langmuir 2020, 36, 14924–14932. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Ji, X.; Lu, M.; Deng, L.; Liu, Z.; Yin, B.; Ni, D. A general method for endowing hydrophobic nanoparticles with water dispersion abilities. J. Mater. Chem. B 2023, 11, 8464–8470. [Google Scholar] [CrossRef] [PubMed]
- Dederichs, T.; Moller, M.; Weichold, O. Colloidal stability of hydrophobic nanoparticles in ionic surfactant solutions: Definition of the critical dispersion concentration. Langmuir 2009, 25, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Don Porto Carero, A.; Hoet, P.; Verschaeve, L.; Schoeters, G.; Nemery, B. Genotoxic effects of carbon black particles, diesel exhaust particles, and urban air particulates and their extracts on a human alveolar epithelial cell line (A549) and a human monocytic cell line (THP-1). Environ. Mol. Mutagen. 2001, 37, 155–163. [Google Scholar] [CrossRef]
- Coreas, R.; Castillo, C.; Li, Z.; Yan, D.; Gao, Z.; Chen, J.; Bitounis, D.; Parviz, D.; Strano, M.S.; Demokritou, P.; et al. Biological Impacts of Reduced Graphene Oxide Affected by Protein Corona Formation. Chem. Res. Toxicol. 2022, 35, 1244–1256. [Google Scholar] [CrossRef]
- Stone, V.; Shaw, J.; Brown, D.; MacNee, W.; Faux, S.; Donaldson, K. The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol. Vitr. 1998, 12, 649–659. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.M.; Rumfeldt, J.A.; Lepock, J.R.; Meiering, E.M. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004, 13, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Wong Shi Kam, N.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef]
- Deguchi, S.; Yamazaki, T.; Mukai, S.-A.; Usami, R.; Horikoshi, K. Stabilization of C60 nanoparticles by protein adsorption and its implications for toxicity studies. Chem. Res. Toxicol. 2007, 20, 854–858. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Simulation study of the interaction of some albumin subdomains with a flat graphite surface. Langmuir 2003, 19, 3403–3412. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Kopac, T. Protein corona, understanding the nanoparticle–protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, M.; Saudrais, F.; Devineau, S.; Chédin, S.; Jamme, F.; Leroy, J.; Rakotozandriny, K.; Taché, O.; Brotons, G.; Pin, S.; et al. Role of the Protein Corona in the Colloidal Behavior of Microplastics. Langmuir 2023, 39, 4291–4303. [Google Scholar] [CrossRef]
- Trivanovic, U.; Kelesidis, G.A.; Pratsinis, S.E. High-throughput generation of aircraft-like soot. Aerosol Sci. Technol. 2022, 56, 732–743. [Google Scholar] [CrossRef]
- Trivanovic, U.; Pereira Martins, M.; Benz, S.; Kelesidis, G.A.; Pratsinis, S.E. Dynamics of soot surface growth and agglomeration by enclosed spray combustion of jet fuel. Fuel 2023, 342, 127864. [Google Scholar] [CrossRef]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact angle measurements using a simplified experimental setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yon, J.; Bescond, A.; Liu, F. On the radiative properties of soot aggregates part 1: Necking and overlapping. J. Quant. Spectrosc. Radiat. Transf. 2015, 162, 197–206. [Google Scholar] [CrossRef]
- DeLoid, G.; Cohen, J.M.; Darrah, T.; Derk, R.; Rojanasakul, L.; Pyrgiotakis, G.; Wohlleben, W.; Demokritou, P. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 2014, 5, 3514. [Google Scholar] [CrossRef]
- Song, C.; Wang, P.; Makse, H.A. A phase diagram for jammed matter. Nature 2008, 453, 629–632. [Google Scholar] [CrossRef]
- Cheimarios, N.; Pem, B.; Tsoumanis, A.; Ilić, K.; Vrček, I.V.; Melagraki, G.; Bitounis, D.; Isigonis, P.; Dusinska, M.; Lynch, I.; et al. An In Vitro Dosimetry Tool for the Numerical Transport Modeling of Engineered Nanomaterials Powered by the Enalos RiskGONE Cloud Platform. Nanomaterials 2022, 12, 3935. [Google Scholar] [CrossRef]
- Thomas, D.G.; Smith, J.N.; Thrall, B.D.; Baer, D.R.; Jolley, H.; Munusamy, P.; Kodali, V.; Demokritou, P.; Cohen, J.; Teeguarden, J.G. ISD3: A particokinetic model for predicting the combined effects of particle sedimentation, diffusion and dissolution on cellular dosimetry for in vitro systems. Part. Fibre Toxicol. 2018, 15, 1–22. [Google Scholar] [CrossRef]
- Kelesidis, G.A.; Rossi, N.; Pratsinis, S.E. Porosity and crystallinity dynamics of carbon black during internal and surface oxidation. Carbon 2022, 197, 334–340. [Google Scholar] [CrossRef]
- Parent, P.; Laffon, C.; Marhaba, I.; Ferry, D.; Regier, T.; Ortega, I.; Chazallon, B.; Carpentier, Y.; Focsa, C. Nanoscale characterization of aircraft soot: A high-resolution transmission electron microscopy, Raman spectroscopy, X-ray photoelectron and near-edge X-ray absorption spectroscopy study. Carbon 2016, 101, 86–100. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Shieh, H.-Y.; Lee, W.-J.; Lai, S.-O. Health-risk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in a carbon black manufacturing industry. Sci. Total Environ. 2001, 278, 137–150. [Google Scholar] [CrossRef]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1565–1618. [Google Scholar] [CrossRef]
- Scheepers, P.; Bos, R. Combustion of diesel fuel from a toxicological perspective: I. Origin of incomplete combustion products. Int. Arch. Occup. Environ. Health 1992, 64, 149–161. [Google Scholar] [CrossRef]
- Jeannet, N.; Fierz, M.; Kalberer, M.; Burtscher, H.; Geiser, M. Nano aerosol chamber for in-vitro toxicity (NACIVT) studies. Nanotoxicology 2015, 9, 34–42. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Delaval, M.; Leni, Z.; Keller, A.; Brem, B.T.; Siegerist, F.; Schönenberger, D.; Durdina, L.; Elser, M.; Burtscher, H. Non-volatile particle emissions from aircraft turbine engines at ground-idle induce oxidative stress in bronchial cells. Commun. Biol. 2019, 2, 90. [Google Scholar] [CrossRef]
- Delaval, M.N.; Jonsdottir, H.R.; Leni, Z.; Keller, A.; Brem, B.T.; Siegerist, F.; Schönenberger, D.; Durdina, L.; Elser, M.; Salathe, M. Responses of reconstituted human bronchial epithelia from normal and health-compromised donors to non-volatile particulate matter emissions from an aircraft turbofan engine. Environ. Pollut. 2022, 307, 119521. [Google Scholar] [CrossRef] [PubMed]
- Pierdominici, M.; Maselli, A.; Cecchetti, S.; Tinari, A.; Mastrofrancesco, A.; Alfè, M.; Gargiulo, V.; Beatrice, C.; Di Blasio, G.; Carpinelli, G. Diesel exhaust particle exposure in vitro impacts T lymphocyte phenotype and function. Part. Fibre Toxicol. 2014, 11, 74. [Google Scholar] [CrossRef]
- Kanungo, M.; Mettu, S.; Law, K.-Y.; Daniel, S. Effect of Roughness Geometry on Wetting and Dewetting of Rough PDMS Surfaces. Langmuir 2014, 30, 7358–7368. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Q.; Thompson, J.E. The wetting behavior of fresh and aged soot studied through contact angle measurements. Atmos. Clim. Sci. 2017, 7, 11. [Google Scholar] [CrossRef]
- Xu, L.; Karunakaran, R.G.; Guo, J.; Yang, S. Transparent, superhydrophobic surfaces from one-step spin coating of hydrophobic nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 1118–1125. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Loth, E. Superhydrophobic and electroconductive carbon nanotube-fluorinated acrylic copolymer nanocomposites from emulsions. Chem. Eng. J. 2013, 221, 522–530. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Hu, S.; Sun, N.; Huo, C.; Liu, R. The culprits of superoxide dismutase inactivation under size-dependent stress of ultrafine carbon black: Superoxide anion, genotoxicity and protein corona. Sci. Total Environ. 2023, 862, 160770. [Google Scholar] [CrossRef]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Watson, C.; DeLoid, G.; Pal, A.; Demokritou, P. Buoyant nanoparticles: Implications for nano-biointeractions in cellular studies. Small 2016, 12, 3172–3180. [Google Scholar] [CrossRef]
- Rader, C.P.; Sterner, T.; Jakob, F.; Schütze, N.; Eulert, J. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J. Arthroplast. 1999, 14, 840–848. [Google Scholar] [CrossRef]
- Matsusaki, T.; Kawanabe, K.; Ise, K.; Nakayama, T.; Toguchida, J.; Nakamura, T. Gene expression profile of macrophage-like U937 cells in response to polyethylene particles: A novel cell-particle culture system. J. Arthroplast. 2007, 22, 960–965. [Google Scholar] [CrossRef]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, J.; Zhang, Y.; Gaurav, J.; Deloid, G.; Spyrogianni, A.; Sarosiek, K.A.; Bello, D.; Demokritou, P. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]

| BC in RPMI Stirred for | Immediately after Stirring | Stability Testing (24 h) | ||
|---|---|---|---|---|
| Z-Average (nm) | PDI | Z-Average (nm) | PDI | |
| 6 h | 347 ± 10 | 0.573 ± 0.011 | 378 ± 22 | 0.489 ± 0.047 |
| 12 h | 345 ± 25 | 0.484 ± 0.041 | 357 ± 6 | 0.474 ± 0.045 |
| 24 h | 329 ± 12 | 0.430 ± 0.031 | 326 ± 9 | 0.487 ± 0.066 |
| 48 h | 347 ± 5 | 0.425 ± 0.031 | 332 ± 6 | 0.413 ± 0.010 |
| 72 h | 331 ± 2 | 0.426 ± 0.016 | 338 ± 2 | 0.433 ± 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizonova, D.; Trivanovic, U.; Demokritou, P.; Kelesidis, G.A. Dispersion and Dosimetric Challenges of Hydrophobic Carbon-Based Nanoparticles in In Vitro Cellular Studies. Nanomaterials 2024, 14, 589. https://doi.org/10.3390/nano14070589
Lizonova D, Trivanovic U, Demokritou P, Kelesidis GA. Dispersion and Dosimetric Challenges of Hydrophobic Carbon-Based Nanoparticles in In Vitro Cellular Studies. Nanomaterials. 2024; 14(7):589. https://doi.org/10.3390/nano14070589
Chicago/Turabian StyleLizonova, Denisa, Una Trivanovic, Philip Demokritou, and Georgios A. Kelesidis. 2024. "Dispersion and Dosimetric Challenges of Hydrophobic Carbon-Based Nanoparticles in In Vitro Cellular Studies" Nanomaterials 14, no. 7: 589. https://doi.org/10.3390/nano14070589
APA StyleLizonova, D., Trivanovic, U., Demokritou, P., & Kelesidis, G. A. (2024). Dispersion and Dosimetric Challenges of Hydrophobic Carbon-Based Nanoparticles in In Vitro Cellular Studies. Nanomaterials, 14(7), 589. https://doi.org/10.3390/nano14070589





