Hybrid-Mechanism Synergistic Flexible Nb2O5@WS2@C Carbon Nanofiber Anode for Superior Sodium Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
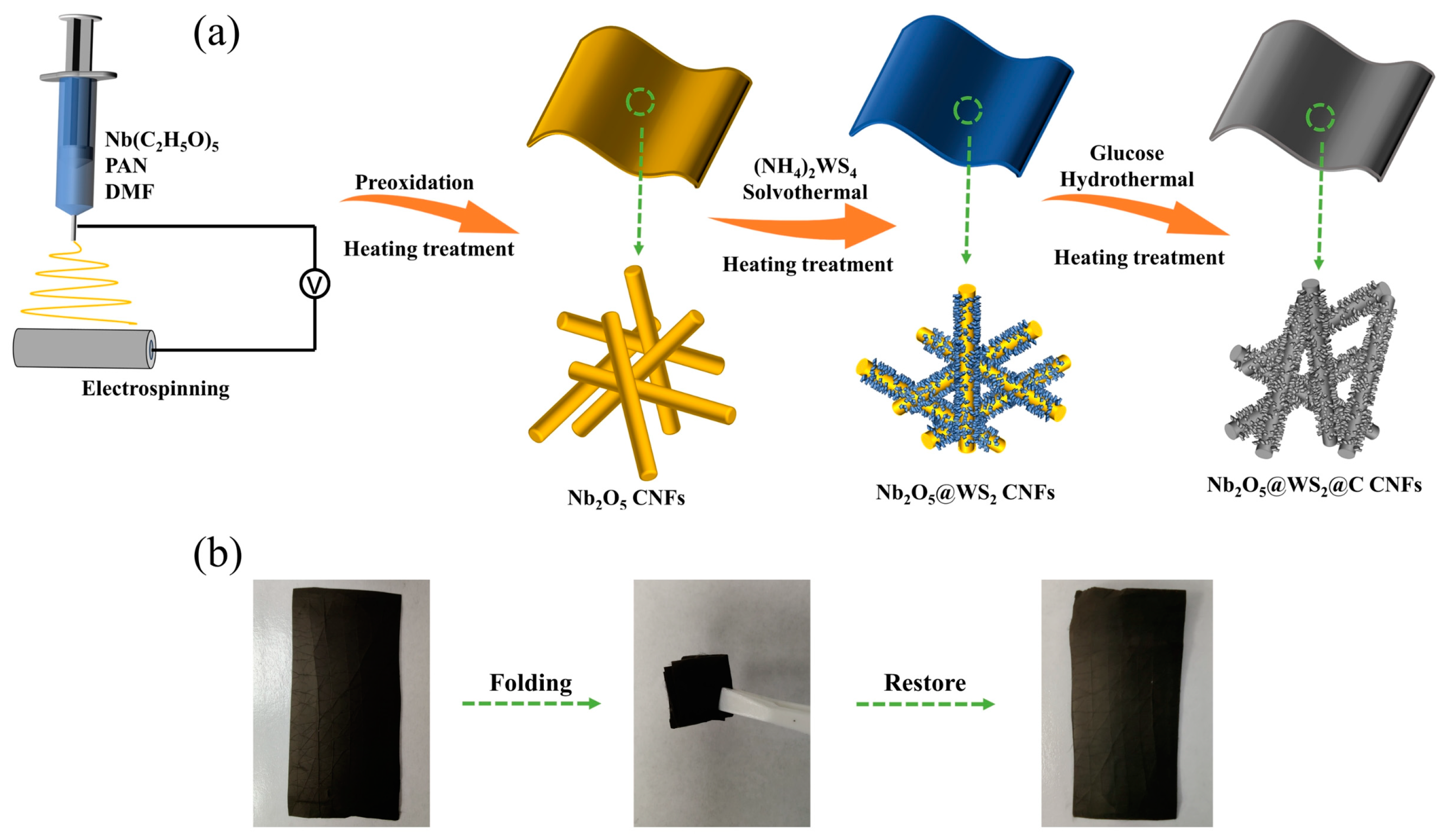
2.2. Synthesis of Nb2O5 CNFs
2.3. Synthesis of Nb2O5@WS2 CNFs
2.4. Synthesis of Nb2O5@WS2@C CNFs
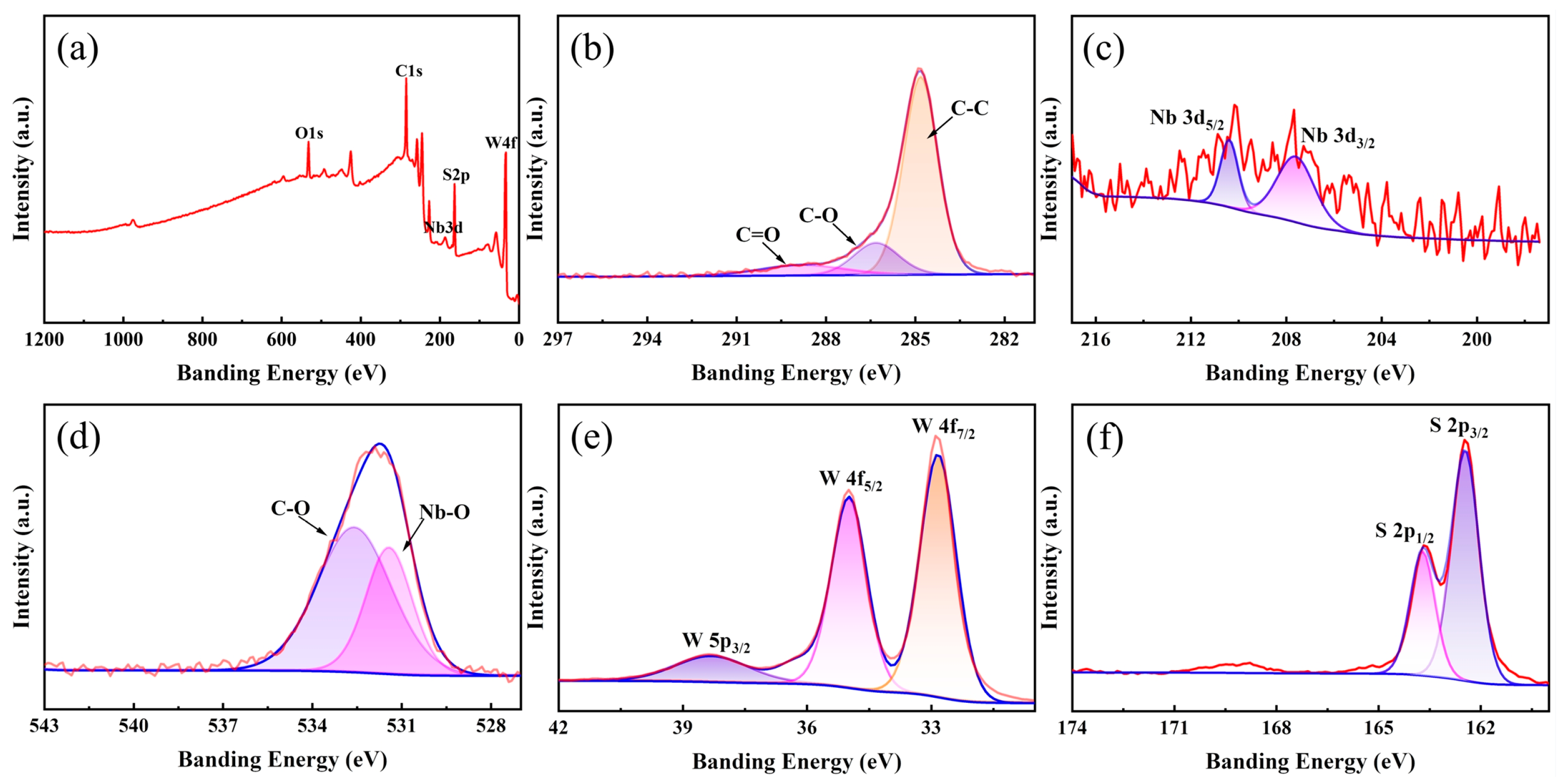
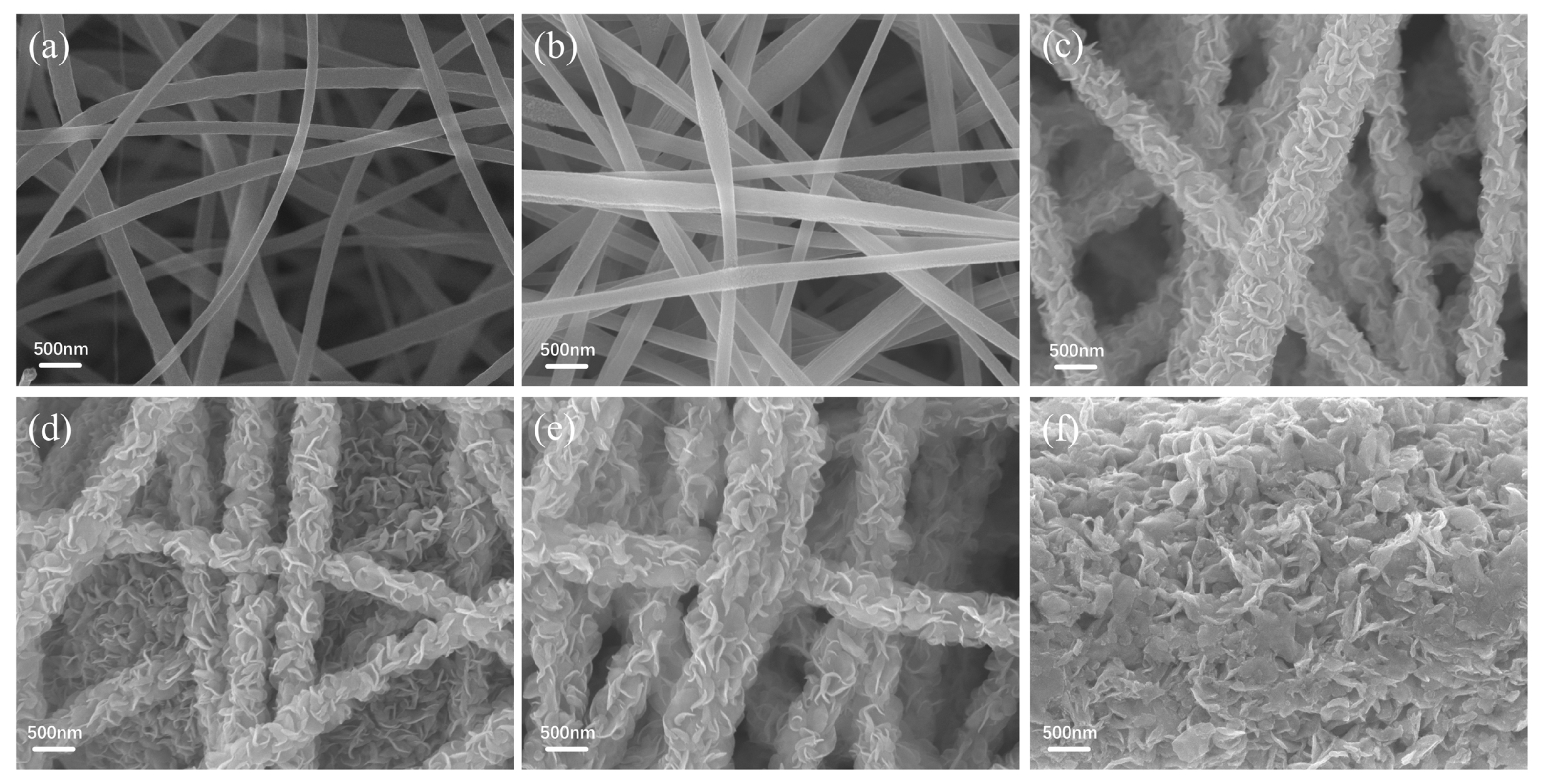
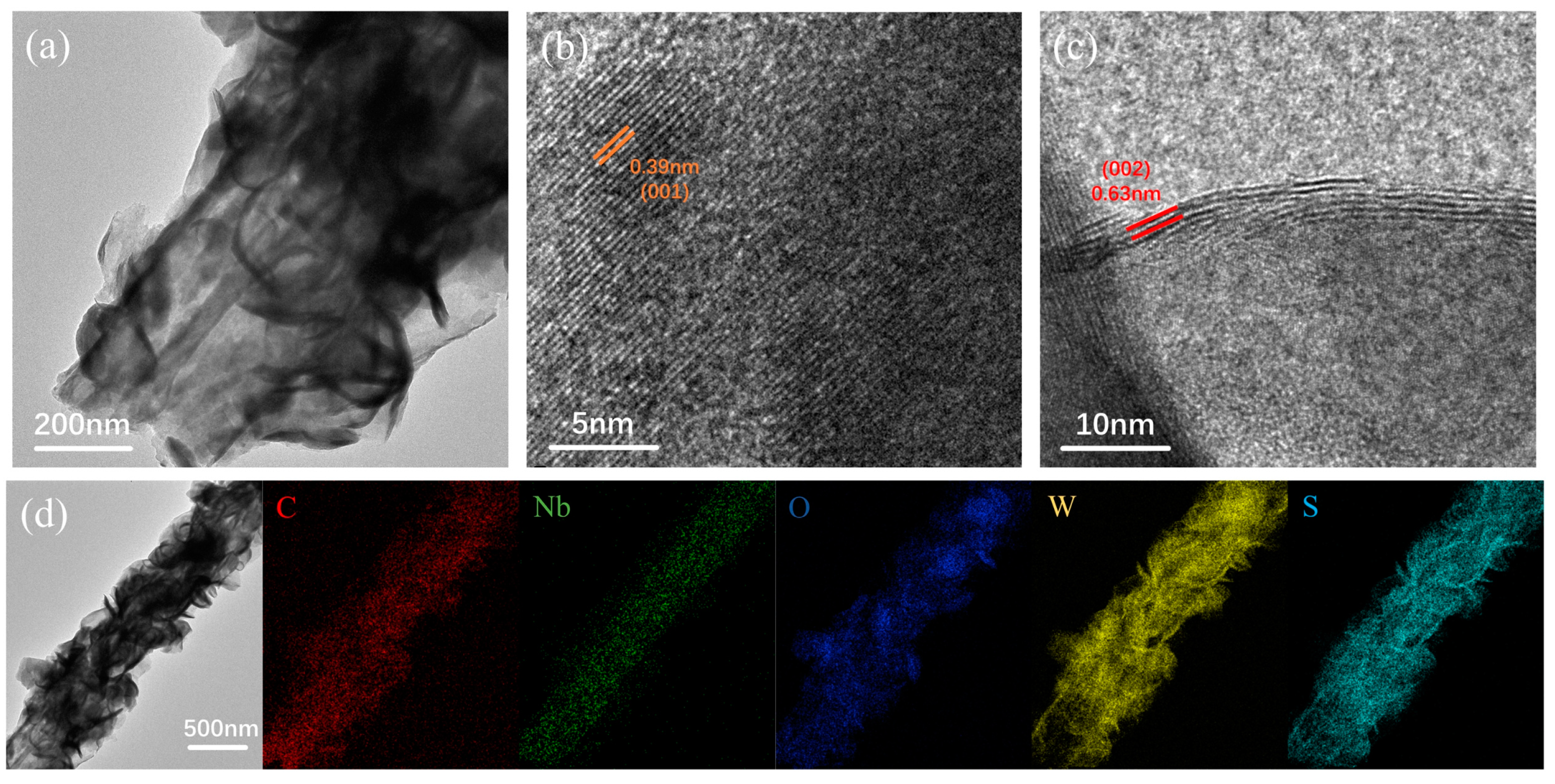
2.5. Characterizations
2.6. Electrochemical Tests
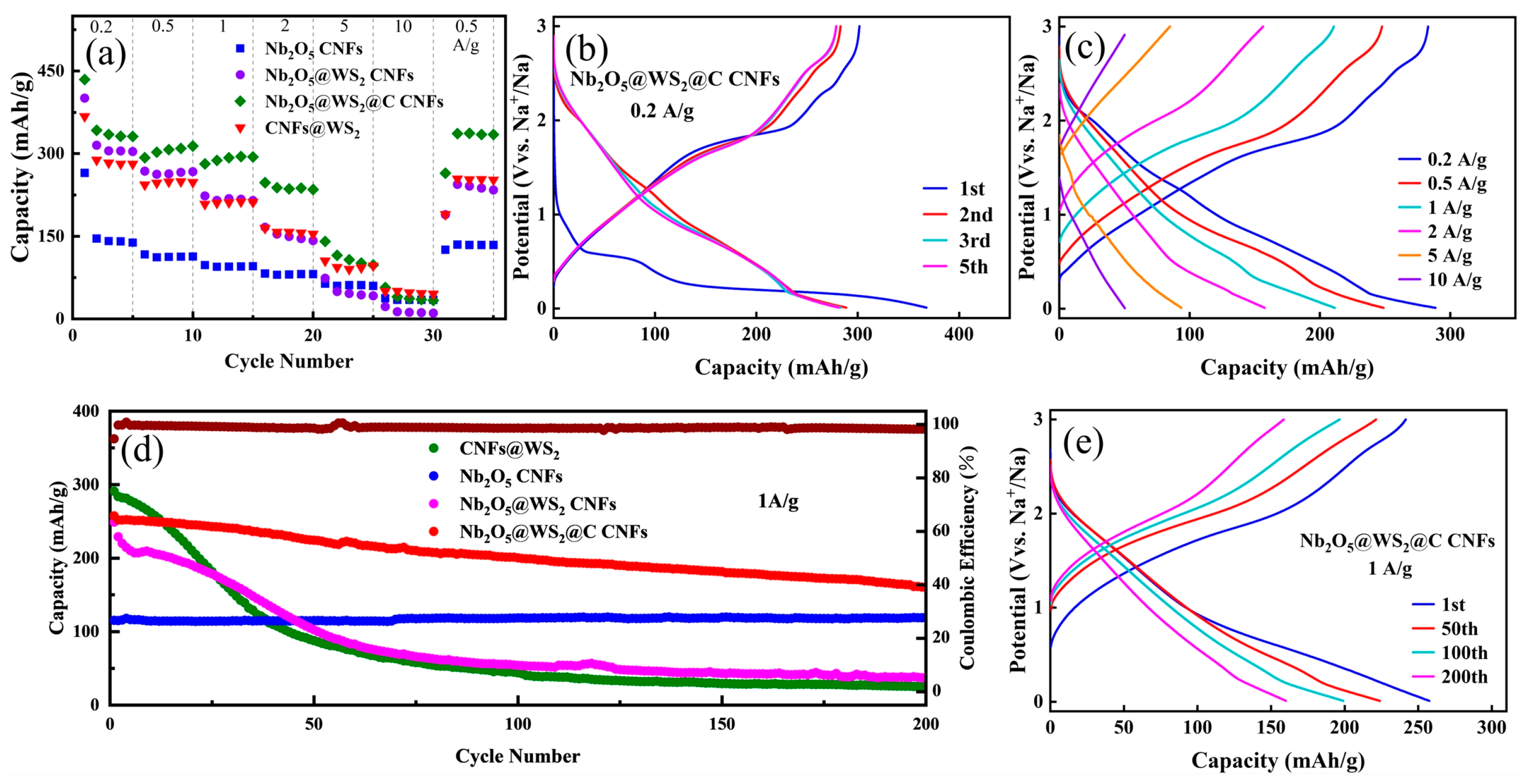
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.; Dai, Y.; Li, J.; Ye, C.; Zhou, W.; Yu, R.; Liao, X.; Li, J.; Zhang, W.; Zong, W.; et al. Cathodic Zn underpotential deposition: An evitable degradation mechanism in aqueous zinc-ion batteries. Sci. Bull. 2022, 67, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, M.; Meng, Y.; Lin, Z.; Cui, Y.; Chen, W. A High-Rate Lithium Manganese Oxide-Hydrogen Battery. Nano Lett. 2020, 20, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Regulating Mass Transport Behavior for High-Performance Lithium Metal Batteries and Fast-Charging Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002891. [Google Scholar] [CrossRef]
- Wang, M.; Tang, Y. A Review on the Features and Progress of Dual-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703320. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Liu, Y.; Xu, K.; Zhao, N.; Lao, C.; Shen, J.; Chen, Z. Novel 3D grid porous Li4Ti5O12 thick electrodes fabricated by 3D printing for high performance lithium-ion batteries. J. Adv. Ceram. 2022, 11, 295–307. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Liao, J.; Ni, W.; Wang, C.; Ma, J. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MoS2. J. Energy Chem. 2019, 33, 100–124. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, C.; He, J.; Tu, X.; Sun, W.; Pan, H.; Zhou, Y.; Rui, X.; Zhang, B.; Huang, K. Structural Engineering in Graphite-Based Metal-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2107277. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Peng, S.; Wang, J.; Yan, W.; Ramakrishna, S. One-dimensional nanomaterials toward electrochemical sodium-ion storage applications via electrospinning. Energy Storage Mater. 2020, 25, 443–476. [Google Scholar] [CrossRef]
- Yu, F.; Huang, T.; Zhang, P.; Tao, Y.; Cui, F.-Z.; Xie, Q.; Yao, S.; Wang, F. Design and synthesis of electrode materials with both battery-type and capacitive charge storage. Energy Storage Mater. 2019, 22, 235–255. [Google Scholar] [CrossRef]
- Plewa, A.; Kulka, A.; Hanc, E.; Sun, J.; Nowak, M.; Redel, K.; Lu, L.; Molenda, J. Abnormal Phenomena of Multi-Way Sodium Storage in Selenide Electrode. Adv. Funct. Mater. 2021, 31, 2102406. [Google Scholar] [CrossRef]
- Liu, T.; He, Q.; Li, L.; Wang, H. Integrating antimony/amorphous vanadium oxide composite structure into electrospun carbon nanofibers for synergistically enhanced lithium/sodium-ion storage. J. Colloid Interface Sci. 2022, 624, 362–369. [Google Scholar] [CrossRef]
- Yang, L.; Yang, B.; Chen, X.; Wang, H.; Dang, J.; Liu, X. Bimetallic alloy SbSn nanodots filled in electrospun N-doped carbon fibers for high performance Na-ion battery anode. Electrochim. Acta 2021, 389, 138246. [Google Scholar] [CrossRef]
- Jia, G.; Chao, D.; Tiep, N.H.; Zhang, Z.; Fan, H.J. Intercalation Na-ion storage in two-dimensional MoS2-xSex and capacity enhancement by selenium substitution. Energy Storage Mater. 2018, 14, 136–142. [Google Scholar] [CrossRef]
- Kulka, A.; Hanc, A.; Walczak, K.; Płotek, J.; Sun, J.; Lu, L.; Borca, C.; Huthwelker, T. Direct evidence of an unanticipated crystalline phase responsible for the high performance of few-layered-MoS2 anodes for Na-ion batteries. Energy Storage Mater. 2022, 48, 314–324. [Google Scholar] [CrossRef]
- Subramanyan, K.; Akshay, M.; Lee, Y.-S.; Aravindan, V. Fabrication of Na-Ion Full-Cells using Carbon-Coated Na3V2(PO4)2O2F Cathode with Conversion Type CuO Nanoparticles from Spent Li-Ion Batteries. Small Methods 2022, 6, 2200257. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Chen, F.; Liu, S.; Bayaguud, A.; Feng, Y.; Zhang, Z.; Fu, Y.; Yu, Y.; Zhu, C. Advantageous Functional Integration of Adsorption-Intercalation-Conversion Hybrid Mechanisms in 3D Flexible Nb2O5@Hard Carbon@MoS2@Soft Carbon Fiber Paper Anodes for Ultrafast and Super-Stable Sodium Storage. Adv. Funct. Mater. 2020, 30, 1908665. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Huang, X.; Lan, T.; Wei, M.; Ma, T. Metal–organic framework derived hierarchical porous TiO2 nanopills as a super stable anode for Na-ion batteries. J. Energy Chem. 2017, 26, 667–672. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q.-H. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- She, L.; Zhang, F.; Jia, C.; Kang, L.; Li, Q.; He, X.; Sun, J.; Lei, Z.; Liu, Z.-H. Electrospun Nb2O5 nanorods/microporous multichannel carbon nanofiber film anode for Na+ ion capacitors with good performance. J. Colloid Interface Sci. 2020, 573, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, J.-H.; Chou, T.-F.; Zhao, B.; El-Sayed, M.A.; Liu, M. Unraveling the Nature of Anomalously Fast Energy Storage in T-Nb2O5. J. Am. Chem. Soc. 2017, 139, 7071–7081. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Ma, C.; Hou, J.; Zhang, Z.; Feng, R.; Yang, L.; Zhang, X.; Lu, H.; Liu, J.; Li, Y.; et al. Integrating Nanoreactor with O–Nb–C Heterointerface Design and Defects Engineering Toward High-Efficiency and Longevous Sodium Ion Battery. Adv. Energy Mater. 2022, 12, 2103716. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Song, Z.; Xiao, X.; Cao, Z.; Xiong, D.; Deng, W.; Hou, H.; Yang, Y.; Zou, G.; et al. Enabling Electron Delocalization by Conductor Heterostructure for Highly Reversible Sodium Storage. Adv. Funct. Mater. 2023, 2312905. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, J.; Liang, P.; Zheng, H.; Wu, M.; Chen, J.; Shi, F.; Yan, K.; Liu, J.; Bian, K.; et al. Heterostructured WS2/MoS2@carbon hollow microspheres anchored on graphene for high-performance Li/Na storage. Chem. Eng. J. 2022, 443, 136080. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, X.; Chen, M.; Chen, H.; Huang, K.-J.; Wang, L.; Xu, J. Self-supporting 1T-MoS2@WS2@CC composite materials for potential high-capacity sodium storage system. J. Colloid Interface Sci. 2023, 630, 426–435. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, K.; Yao, Z.; Kang, J.; Lam, D.; Yang, D.; Ai, W.; Wolverton, C.; Hersam, M.C.; Huang, Y.; et al. Lithium/Sodium-Ion Batteries: In Situ, Atomic-Resolution Observation of Lithiation and Sodiation of WS2 Nanoflakes: Implications for Lithium-Ion and Sodium-Ion Batteries (Small 24/2021). Small 2021, 17, 2170120. [Google Scholar] [CrossRef]
- Wu, H.; Xu, N.; Jiang, Z.; Zheng, A.; Shi, Q.; Lv, R.; Ni, L.; Diao, G.; Chen, M. Space and interface confinement effect of necklace-box structural FeS2/WS2 carbon nanofibers to enhance Na+ storage performance and electrochemical kinetics. Chem. Eng. J. 2022, 427, 131002. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xu, X.; Wang, Y.-X.; Chou, S.-L.; Cao, A.; Chen, L.; Dou, S.-X. Lotus rhizome-like S/N–C with embedded WS2 for superior sodium storage. J. Mater. Chem. A 2019, 7, 25932–25943. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, H.E.; Ren, Q.S.; Deng, H.W.; Li, Z.; Du, B.; Zhang, T. Improved corrosion resistance of TiAlCrNbMo alloy to lead-bismuth eutectic by pre-oxidation. J. Mater. Res. Technol. 2024, 28, 707–718. [Google Scholar] [CrossRef]
- Geng, S.; Zhou, T.; Jia, M.; Shen, X.; Gao, P.; Tian, S.; Zhou, P.; Liu, B.; Zhou, J.; Zhuo, S.; et al. Carbon-coated WS2 nanosheets supported on carbon nanofibers for high-rate potassium-ion capacitors. Energy Environ. Sci. 2021, 14, 3184–3193. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials 2021, 11, 580. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, L.; Mao, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Mesopore-Induced Ultrafast Na+-Storage in T-Nb2O5/Carbon Nanofiber Films toward Flexible High-Power Na-Ion Capacitors. Small 2019, 15, 1804539. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Feng, Z.; Tan, Y.; Deng, Q.; Yao, L. Hybrid-Mechanism Synergistic Flexible Nb2O5@WS2@C Carbon Nanofiber Anode for Superior Sodium Storage. Nanomaterials 2024, 14, 631. https://doi.org/10.3390/nano14070631
Zhao Y, Feng Z, Tan Y, Deng Q, Yao L. Hybrid-Mechanism Synergistic Flexible Nb2O5@WS2@C Carbon Nanofiber Anode for Superior Sodium Storage. Nanomaterials. 2024; 14(7):631. https://doi.org/10.3390/nano14070631
Chicago/Turabian StyleZhao, Yang, Ziwen Feng, Yipeng Tan, Qinglin Deng, and Lingmin Yao. 2024. "Hybrid-Mechanism Synergistic Flexible Nb2O5@WS2@C Carbon Nanofiber Anode for Superior Sodium Storage" Nanomaterials 14, no. 7: 631. https://doi.org/10.3390/nano14070631




