Thermal Stability and Crystallization Processes of Pd78Au4Si18 Thin Films Visualized via In Situ TEM
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Structural Characterization of As-Deposited Pd78Au4Si18 Film
3.2. Crystallization Processes of Pd78Au4Si18 Films via In Situ TEM
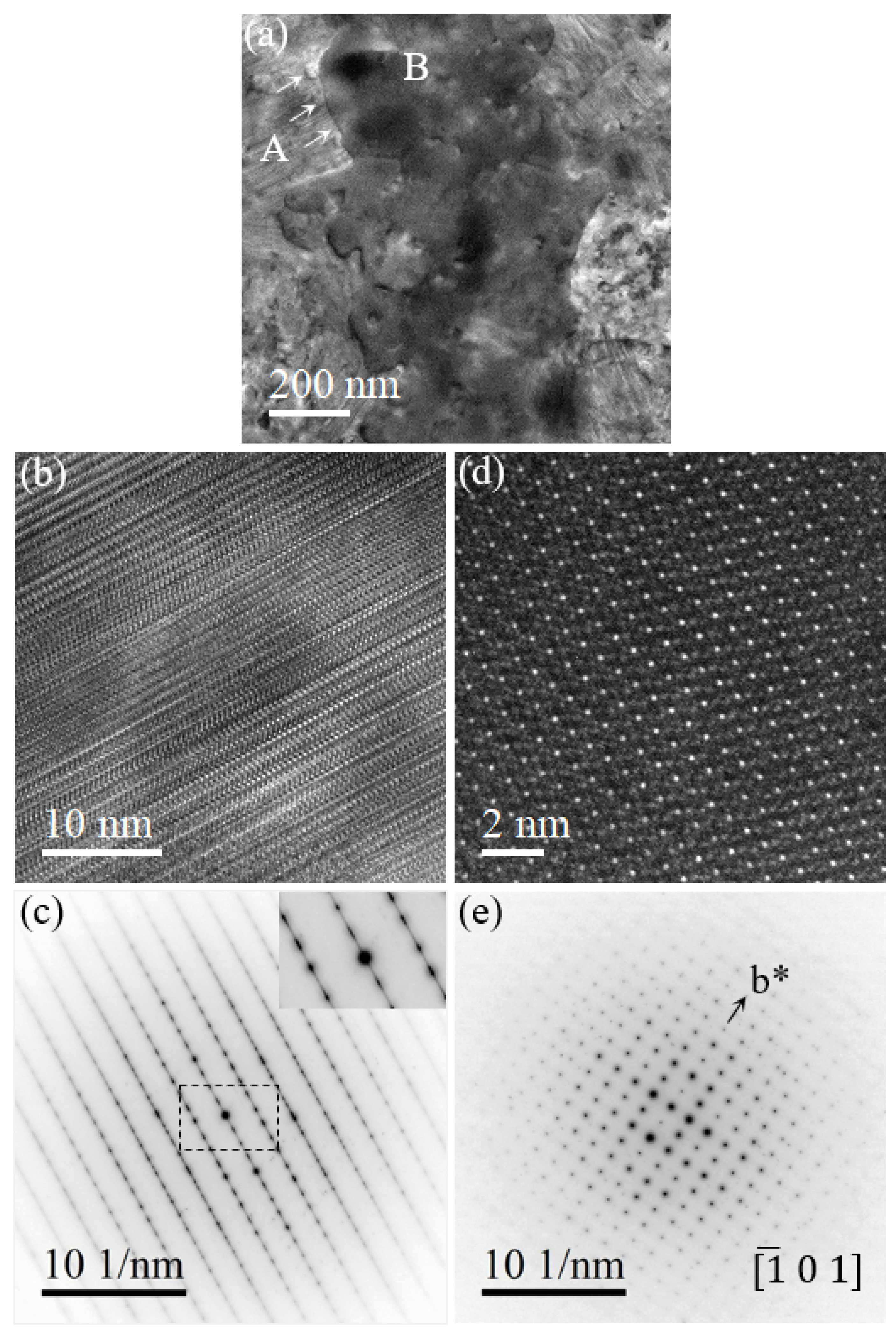
3.3. Transformation of Metastable Pd-Si to Stable Pd9Si2 and Pd3Si
3.4. Growth of Pd3Si at the Expense of Pd9Si2
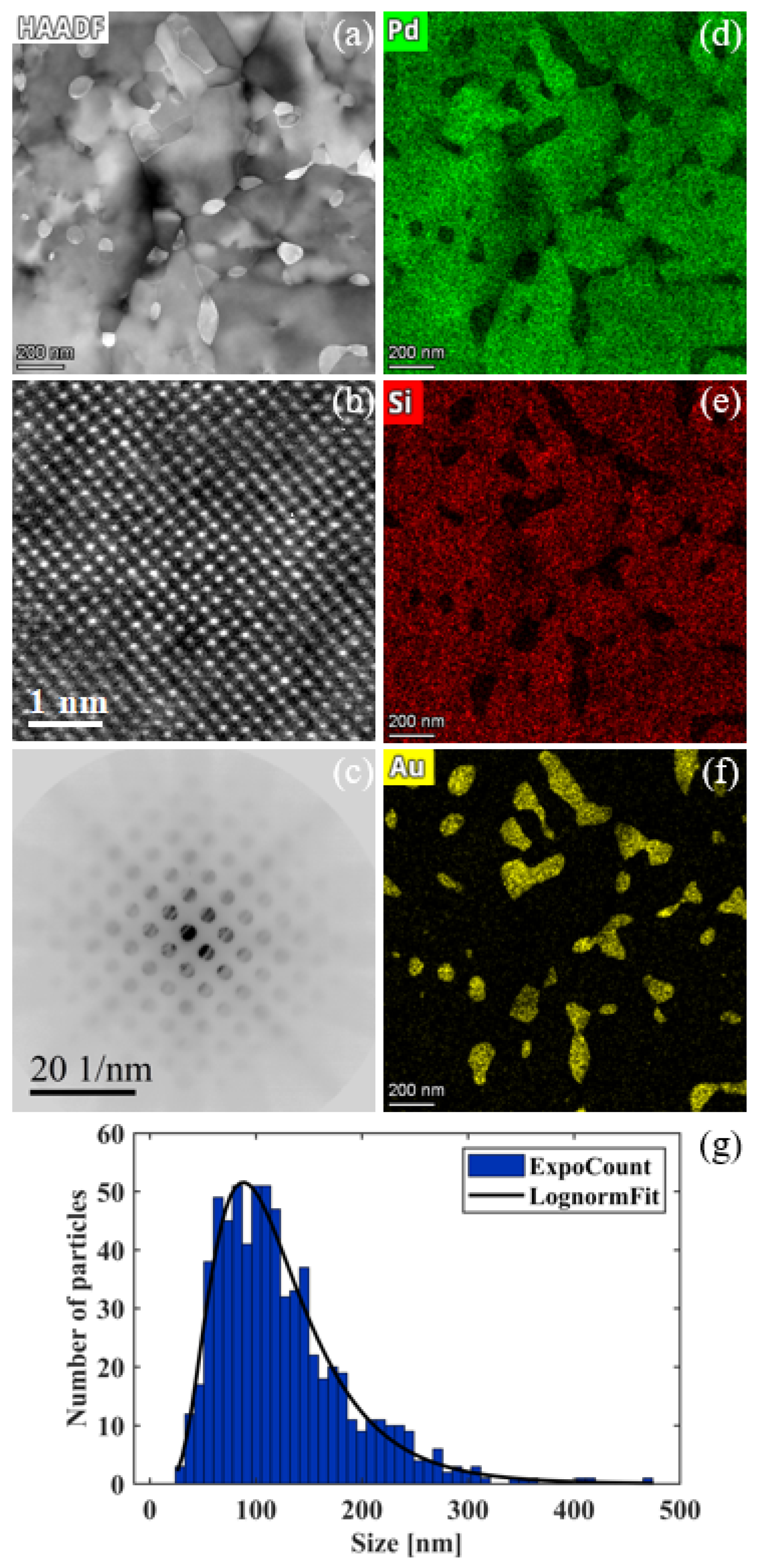
3.5. Precipitation of Au NPs at Elevated Temperatures
4. Discussion
4.1. Effects of Quenched-in Nuclei on Crystallization Processes of Pd-Au-Si Thin Films
4.2. Transformation of Pd9Si2 to Pd3Si at Elevated Temperatures
4.3. Precipitation of Au NPs from Crystallization of Amorphous Pd-Au-Si System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | transmission electron microscopy |
| SAED | selected area electron diffraction |
| HRTEM | high-resolution TEM |
| NBED | nanobeam electron diffraction |
| PLD | pulse laser deposition |
References
- Chen, M. A brief overview of bulk metallic glasses. Npg Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Lee, C.; Brechtl, J.; Liaw, P.K. Research on bulk-metallic glasses and high-entropy alloys in Peter K. Liaw’s group and with his colleagues. Metall. Mater. Trans. A 2021, 52, 2033–2093. [Google Scholar] [CrossRef]
- Sharma, A.; Zadorozhnyy, V. Review of the recent development in metallic glass and its composites. Metals 2021, 11, 1933. [Google Scholar] [CrossRef]
- Chu, J.P.; Jang, J.; Huang, J.; Chou, H.; Yang, Y.; Ye, J.; Wang, Y.; Lee, J.; Liu, F.; Liaw, P.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Film. 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Sohn, S.; Jung, Y.; Xie, Y.; Osuji, C.; Schroers, J.; Cha, J.J. Nanoscale size effects in crystallization of metallic glass nanorods. Nat. Commun. 2015, 6, 8157. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, K.; Shi, J.; Zheng, D.; Wang, W.; Gu, L.; Bai, H. Liquid-like behaviours of metallic glassy nanoparticles at room temperature. Nat. Commun. 2019, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, C.R.; Shi, J.A.; Lu, Z.; Sun, Y.T.; Luo, P.; Gu, L.; Bai, H.Y.; Pan, M.X.; Wang, W.H. Fast Surface Dynamics of Metallic Glass Enable Superlatticelike Nanostructure Growth. Phys. Rev. Lett. 2017, 118, 016101. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zheng, H.; Smith, R.K.; wook Jun, Y.; Kisielowski, C.; Dahmen, U.; Alivisatos, A.P. Observation of Single Colloidal Platinum Nanocrystal Growth Trajectories. Science 2009, 324, 1309–1312. [Google Scholar] [CrossRef]
- Kodambaka, S.; Tersoff, J.; Reuter, M.C.; Ross, F.M. Germanium Nanowire Growth Below the Eutectic Temperature. Science 2007, 316, 729–732. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Sheng, H.; Zhang, Z.; Mao, S.X. Formation of monatomic metallic glasses through ultrafast liquid quenching. Nature 2014, 512, 177–180. [Google Scholar] [CrossRef]
- Salinga, M.; Carria, E.; Kaldenbach, A.; Bornhöfft, M.; Benke, J.; Mayer, J.; Wuttig, M. Measurement of crystal growth velocity in a melt-quenched phase-change material. Nat. Commun. 2013, 4, 2371. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, C.; Cheng, Y.Q.; Yue, Y.; Han, X.; Zhang, Z.; wei Shan, Z.; Mao, S.X.; Ye, M.; Yin, Y.; et al. Electron-beam-assisted superplastic shaping of nanoscale amorphous silica. Nat. Commun. 2010, 1, 24. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D.M. Formation, stability and structure of palladium-silicon based alloy glasses. Acta Metall. 1969, 17, 1021–1031. [Google Scholar] [CrossRef]
- Chou, C.P.; Turnbull, D. Transformation behavior of Pd Au Si metallic glasses. J. Non-Cryst. Solids 1975, 17, 169–188. [Google Scholar] [CrossRef]
- Mrafko, P.; Duhaj, P.; Švec, P. Crystallization of metastable phases in the Pd83Si17 amorphous alloy. J. Non-Cryst. Solids 2006, 352, 5284–5286. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Meylan, C.M.; Panagiotopoulos, N.T.; Georgarakis, K.; Greer, A. In-situ TEM study of the crystallization sequence in a gold-based metallic glass. Acta Mater. 2020, 196, 52–60. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, X.G.; Chen, L.; Li, W.H.; Xu, X.; Pan, B.T.; Li, W.R.; Zhou, W.H.; Li, L.; Huang, W.; et al. Recent development in the application of bulk metallic glasses. J. Mater. Sci. Technol. 2022, 131, 115–121. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hirata, A.; Guan, P.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2011, 10, 28–33. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, X.; Cheng, Y.; Fei, H.; Liu, F.; Liang, T.; Zeng, Z.; Ishii, T.; Wang, M.S.; Katsura, T.; et al. Synthesis of paracrystalline diamond. Nature 2021, 599, 605–610. [Google Scholar] [CrossRef]
- Yong, Q.; Zongquan, L. Phase transformation of gradient Pd-Si films. Chin. Phys. Lett. 1990, 7, 316. [Google Scholar] [CrossRef]
- Wysocki, J.A.; Duwez, P.E. Equilibrium silicides of palladium. Metall. Mater. Trans. A 1981, 12, 1455–1460. [Google Scholar] [CrossRef]
- Wu, L.J.; Guo, Y.X. Metastable phases in the crystallization of amorphous Pd80Si20 alloy. Philos. Mag. A 1997, 76, 151–162. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Altunin, R.R.; Yumashev, V.V.; Moiseenko, E.T.; Belousov, O.V.; Solovyov, L.A.; Volochaev, M.N.; Zeer, G.M. Kinetic study of a solid-state reaction in Ag/Al multilayer thin films by in situ electron diffraction and simultaneous thermal analysis. J. Alloys Compd. 2021, 871, 159474. [Google Scholar] [CrossRef]
- Moiseenko, E.T.; Yumashev, V.V.; Altunin, R.R.; Zeer, G.M.; Nikolaeva, N.S.; Belousov, O.V.; Zharkov, S.M. Solid-State Reaction in Cu/a-Si Nanolayers: A Comparative Study of STA and Electron Diffraction Data. Materials 2022, 15, 8457. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Yumashev, V.V.; Moiseenko, E.T.; Altunin, R.R.; Solovyov, L.A.; Volochaev, M.N.; Zeer, G.M.; Nikolaeva, N.S.; Belousov, O.V. Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness. Nanomaterials 2023, 13, 2925. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Y.; He, Y. Low-symmetry metastable phases formed during crystallization of the metallic glass Pd80Si20. Phys. Status Solidi A 1993, 138, 47–57. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Y.; He, Y. Metastable phases formed at the initial stage of crystallization for the metallic glass Pd80Si20. Phys. Status Solidi A 1995, 148, 351–362. [Google Scholar] [CrossRef]
- Brearley, W.H.; Shieh, P.C.; Howe, J.M. Atomic structure of the crystalline/amorphous interface in a directionally crystallized Pd80Si20 alloy. Metall. Trans. A 1991, 22, 1287–1298. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Georgarakis, K.; Zadorozhnyy, V.; Chen, N.; Nakayama, K.; Vaughan, G.; Yavari, A.R.; Inoue, A. Atomic structure changes and phase transformation behavior in Pd–Si bulk glass-forming alloy. Intermetallics 2012, 20, 135–140. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Calculations of Mixing Enthalpy and Mismatch Entropy for Ternary Amorphous Alloys. Mater. Trans. JIM 2000, 41, 1372–1378. [Google Scholar] [CrossRef]
- Matsushita, M.; Hirotsu, Y.; Ohkubo, T.; Oikawa, T.; Makino, A. Structure analysis of amorphous Pd75Si25 alloy by electron diffraction and high-resolution electron microscopy. Mater. Sci. Eng. A 1996, 217–218, 392–396. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kai, H.; Hirotsu, Y. Structural modeling of Pd–Si and Fe–Zr–B amorphous alloys based on the microphase separation model. Mater. Sci. Eng. A 2001, 304, 300–304. [Google Scholar] [CrossRef]
- Fanfoni, M.; Tomellini, M. The Johnson-Mehl- Avrami-Kohnogorov model: A brief review. Il Nuovo C. D 1998, 20, 1171–1182. [Google Scholar] [CrossRef]
- German, E.I.; Tsydypov, S.B.; Ojovan, M.I.; Darmaev, M.V. Structure of Argon Solid Phases Formed from the Liquid State at Different Isobaric Cooling Rates. Appl. Sci. 2024, 14, 1295. [Google Scholar] [CrossRef]
- Masumoto, T.; Maddin, R. The mechanical properties of palladium 20 a/o silicon alloy quenched from the liquid state. Acta Metall. 1971, 19, 725–741. [Google Scholar] [CrossRef]
- Duhaj, P.; Sládek, V.; Mrafko, P. Electron microscopic study of the crystallization kinetics of the amorphous alloy Pd80Si20. J. Non-Cryst. Solids 1974, 13, 341–354. [Google Scholar] [CrossRef]
- Nylund, A. Some notes on the palladium-silicon system. Acta Chem. Scand. 1966, 20, 2381–2386. [Google Scholar] [CrossRef]
- Massara, R.; Feschotte, P. Le systeme binaire Pd-Si. J. Alloys Compd. 1993, 190, 249–254. [Google Scholar] [CrossRef]
- Okamoto, H. Pd-Si (palladium-silicon). J. Phase Equilibria Diffus. 2007, 28, 231–232. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, D. Behaviour of TEM metal grids during in-situ heating experiments. Ultramicroscopy 2009, 109, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Cesaria, M.; Taurino, A.; Catalano, M.; Caricato, A.P.; Martino, M. Edge-melting: Nanoscale key-mechanism to explain nanoparticle formation from heated TEM grids. Appl. Surf. Sci. 2016, 365, 191–201. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Shibue, K.; Osamura, K.; Shingu, H.P.; Murakami, Y. DSC study of Pd76Au6Si18 amorphous alloy. J. Mater. Sci. Lett. 1982, 1, 127–130. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Osamura, K. SAXS study on crystallization of an amorphous Pd76Au6Si18 alloy. J. Mater. Sci. 1984, 19, 1476–1485. [Google Scholar] [CrossRef]




| a (Å) | b (Å) | c (Å) | V (Å3) | (eV) | (eV) | (/Å3) | |
|---|---|---|---|---|---|---|---|
| 5.934 | 7.639 | 5.350 | 242.529 | −93.6 | −9.8 | −40.2 | |
| 9.263 | 7.616 | 9.659 | 681.417 | −249.8 | −20.0 | −29.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Zhao, R.; Lu, Z.; Su, H.; Liang, B.; Liu, B.; Ma, C.; Zhu, Y.; Li, Z. Thermal Stability and Crystallization Processes of Pd78Au4Si18 Thin Films Visualized via In Situ TEM. Nanomaterials 2024, 14, 635. https://doi.org/10.3390/nano14070635
Yu B, Zhao R, Lu Z, Su H, Liang B, Liu B, Ma C, Zhu Y, Li Z. Thermal Stability and Crystallization Processes of Pd78Au4Si18 Thin Films Visualized via In Situ TEM. Nanomaterials. 2024; 14(7):635. https://doi.org/10.3390/nano14070635
Chicago/Turabian StyleYu, Bingjiao, Rui Zhao, Zhen Lu, Hangbo Su, Binye Liang, Bingjie Liu, Chunlan Ma, Yan Zhu, and Zian Li. 2024. "Thermal Stability and Crystallization Processes of Pd78Au4Si18 Thin Films Visualized via In Situ TEM" Nanomaterials 14, no. 7: 635. https://doi.org/10.3390/nano14070635





