Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
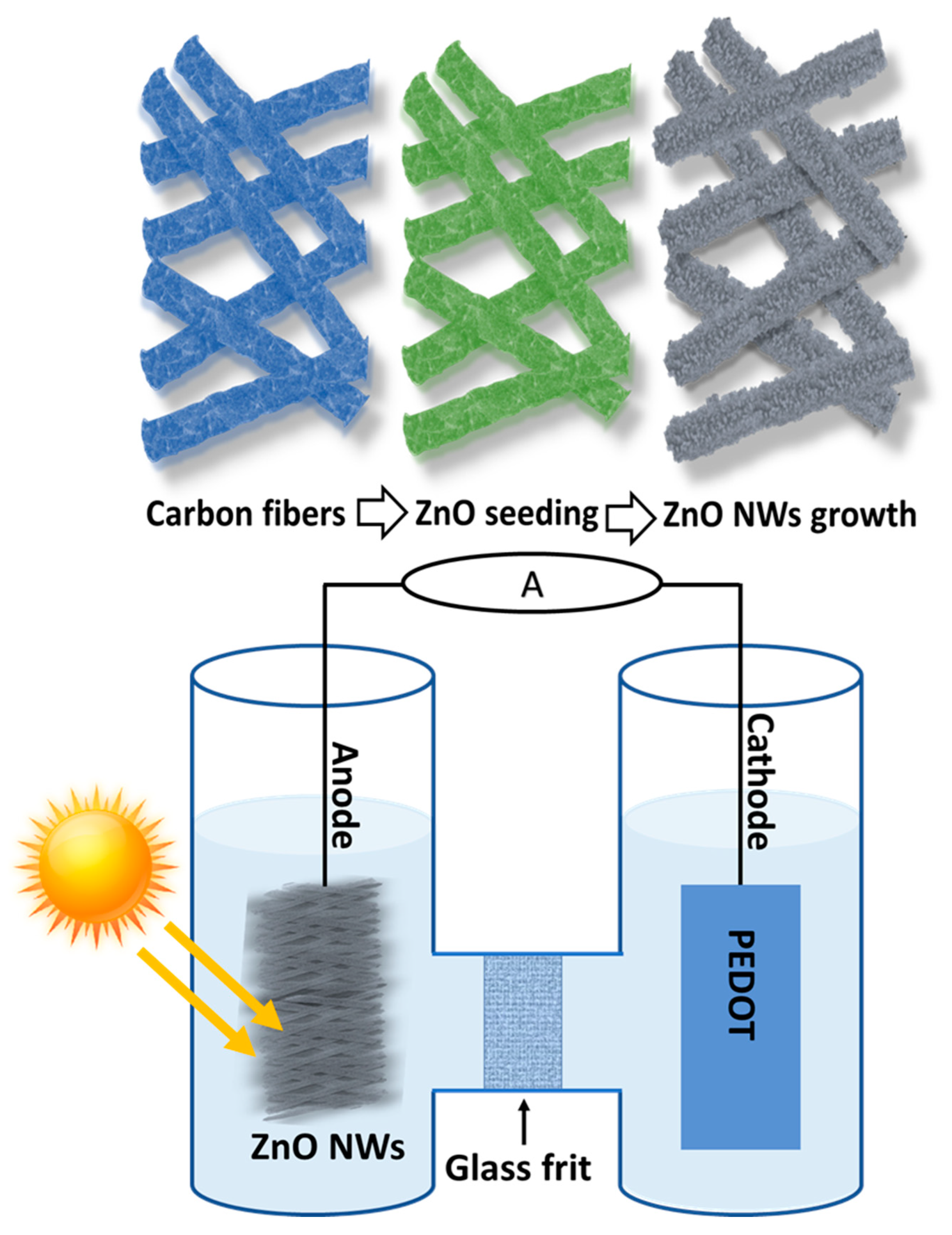
2.2. Growth of ZnO NWs on Carbon Fibers
2.3. Measurements
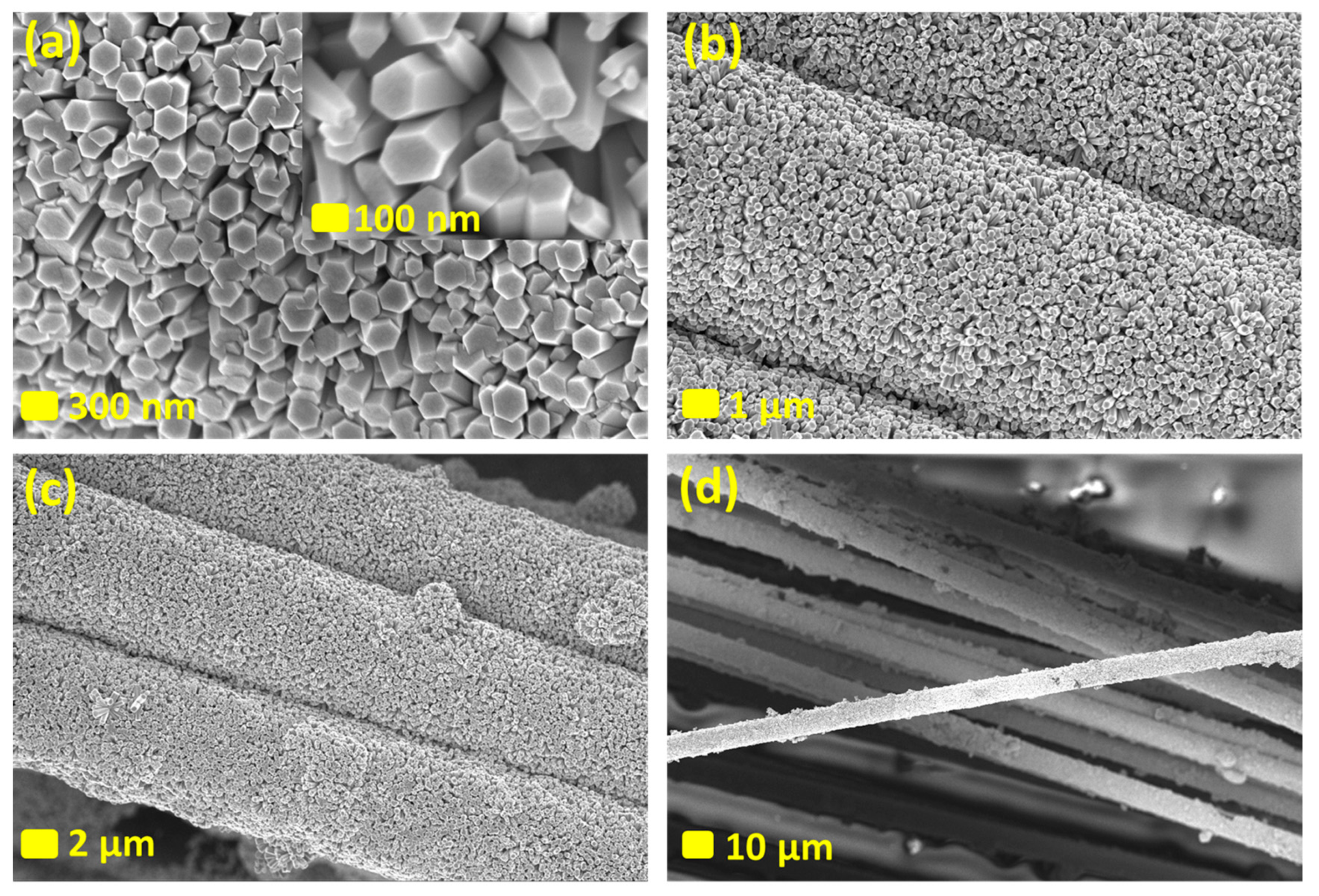
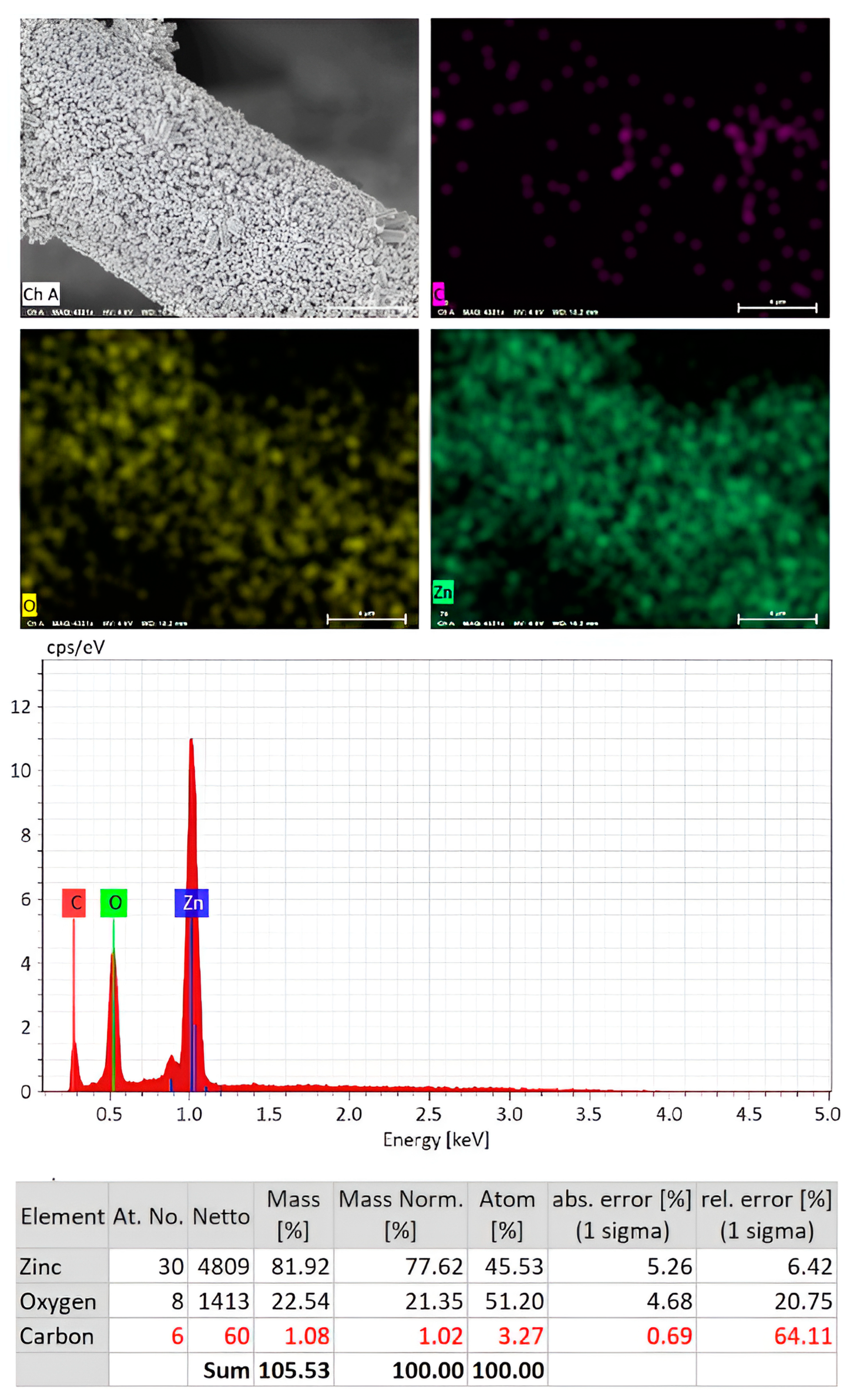
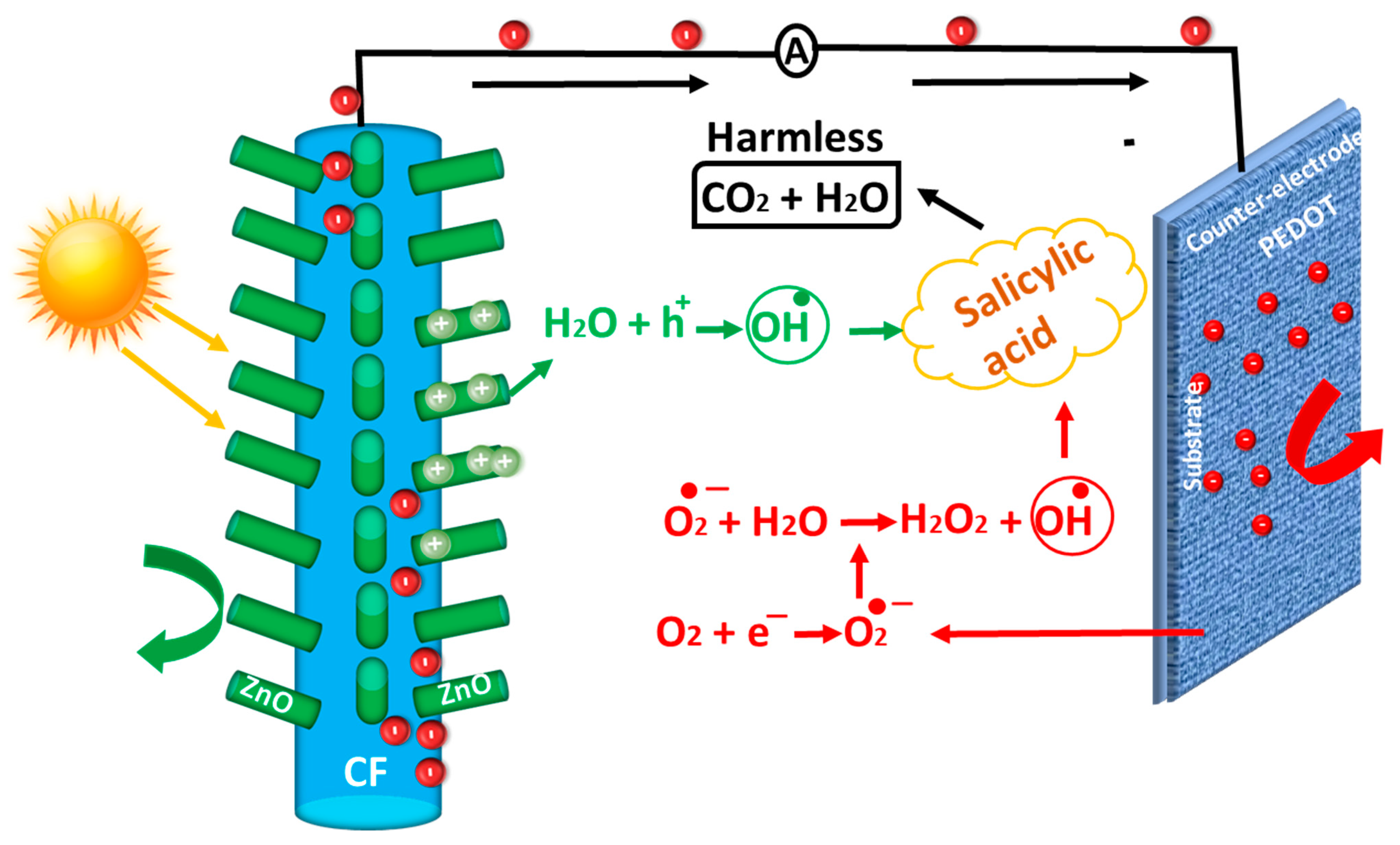
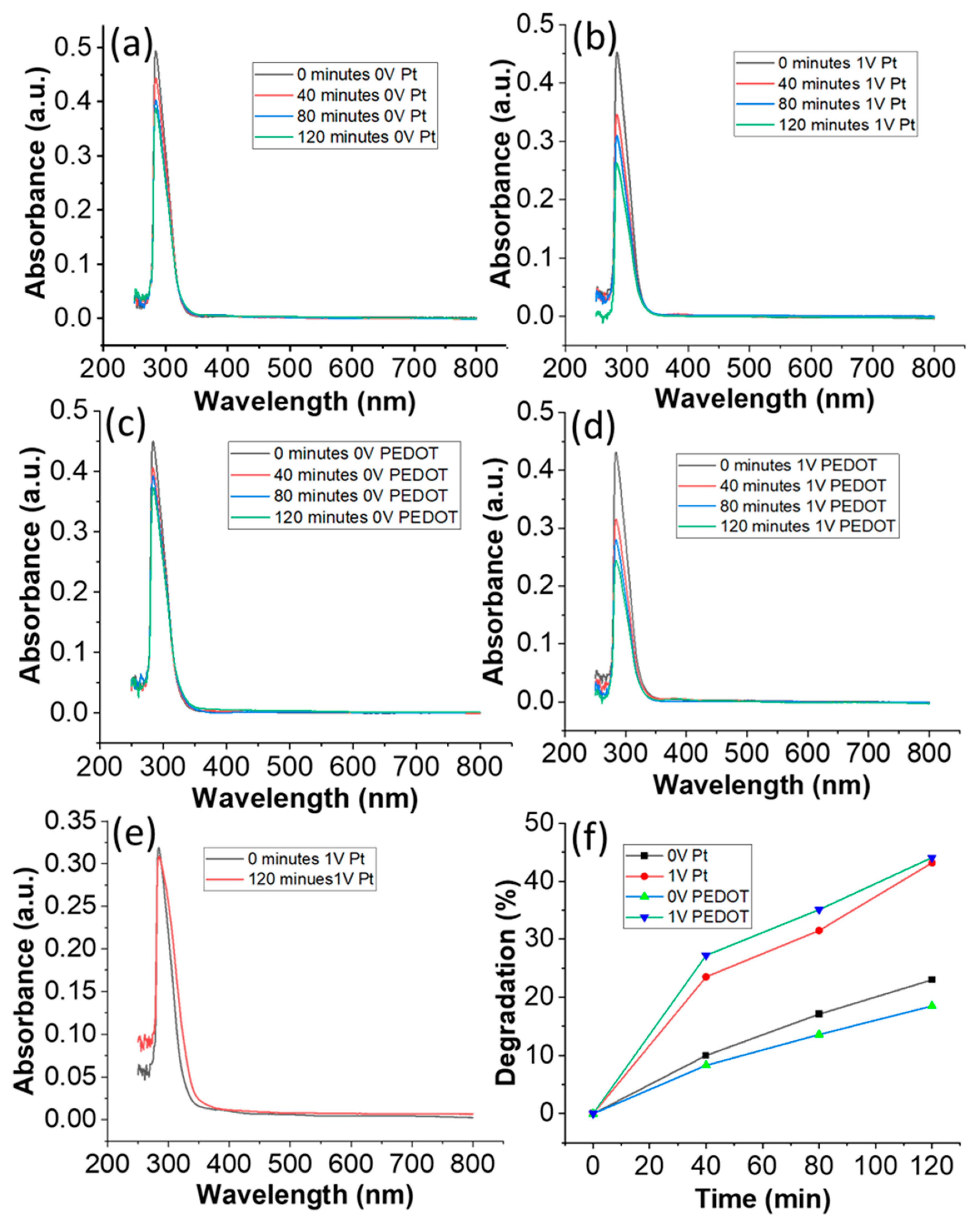
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arias, J.L.; Vergara, J.B.; Arias, E.L.; Gould, A.; Gazabon, D.O. Evaluation of low-cost alternatives for water purification in the stilt house villages of Santa Marta’s Cienaga Grande. Heliyon 2020, 6, e03062. [Google Scholar] [CrossRef] [PubMed]
- Pooi, C.K.; Ng, H.Y. Review of low-cost point-of-use water treatment systems for developing communities. NPJ Clean Water 2018, 1, 11. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the Internal Chemical Energy of Wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Santillan, A.L.; Ortiz, E.C.; Taviz’on, S.F.; Moggio, I.; Arias, E.; Gallardo-Vega, C.A.; Silva, J.A.M.; Barriga-Castro, E.D. Hollow ZnO microspheres functionalized with electrochemical graphene oxide for the photodegradation of salicylic acid. RSC Adv. 2019, 9, 6965. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, N.K.; Konstantinou, I.K. Occurrence and removal of emerging pharmaceutical, personal care compounds and caffeine tracer in municipal sewage treatment plant in Western Greece. J. Environ. Sci. Health Part B 2013, 48, 800. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Nanotechnology applications in pollution sensing and degradation in agriculture: A review. Environ. Chem. Lett. 2009, 7, 191. [Google Scholar] [CrossRef]
- Sugunan, A.; Dutta, J. Pollution Treatment, Remediation, and Sensing. In Nanotechnology; Harald, K., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Volume 2, ISBN 978-3-527-31735-6. [Google Scholar]
- Khan, M.M.; Pradhan, D.; Sohn, Y. Nanocomposites for Visible Light-Induced Photocatalysis; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2017; Volume 1, ISBN 978-3-319-62446-4. [Google Scholar] [CrossRef]
- Lee, S.L.; Ho, L.N.; Ong, S.A.; Wong, Y.S.; Voon, C.H.; Khalik, W.F.; Yusoff, N.A.; Nordin, N. Exploring the relationship between molecular structure of dyes and light sources for photodegradation and electricity generation in photocatalytic fuel cell. Chemosphere 2018, 209, 935. [Google Scholar] [CrossRef]
- He, Y.; Chen, K.; Leung, M.K.H.; Zhang, Y.; Li, L.; Li, G.; Xuan, J.; Li, J. Photocatalytic fuel cell—A review. Chem. Eng. J. 2021, 428, 131074. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Dong, L.; Shen, C.; Li, F.; Huang, M.; Ma, C.; Yang, B.; An, X.; Sand, W. Recent advances on photocatalytic fuel cell for environmental applications—The marriage of photocatalysis and fuel cells. Sci. Total Environ. 2019, 668, 966. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Khataee, A.; Dragoi, E.N.; Moradi, M.; Nabavifard, S.; Conti, G.O.; Khaneghah, A.M. Pollutants degradation and power generation by photocatalytic fuel cells: A comprehensive review. Arab. J. Chem. 2020, 13, 8458. [Google Scholar] [CrossRef]
- Prete, P.; Lovergine, N.; Tapfer, L. Nanostructure size evolution during Au-catalysed growth by carbo-thermal evaporation of well-aligned ZnO nanowires on (100)Si. Appl. Phys. A 2007, 88, 21. [Google Scholar] [CrossRef]
- Alvi, N.H. Luminescence Properties of ZnO Nanostructures and Their Implementation as White Light Emitting Diodes (LEDs). Ph.D. Thesis, Linköping University: Linköping, Sweden, 2011, Chapter 4; p. 43. [CrossRef]
- Faraz, S.M.; Jafri Syed, R.N.K.; Hashim, R.S.W.; Alvi, N.H.; Wahab, Q.; Nur, O. Effect of annealing temperature on the interface state density of n-ZnO nanorod/p-Si heterojunction diodes. Open Phys. 2021, 19, 467. [Google Scholar] [CrossRef]
- Kuo, D.H.; Chen, S.Y.; Huang, M.H.; Wang, D.W.; Kuo, H.C. Epitaxial Growth of Well-Aligned ZnO Nanowires by Electron Beam Evaporation. Adv. Mater. 2007, 19, 670. [Google Scholar]
- Gong, J.; Liang, J.; Sumathy, K. Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials. Renew. Sustain. Energy Rev. 2012, 16, 5848. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Randorn, C.; Efstathiou, P.; Irvine, J.T.S. A red metallic oxide photocatalyst. Nat. Mater. 2012, 11, 595. [Google Scholar] [CrossRef]
- Teluri, A.S.; Bolouk, S.; Amini, M. Synthesis of ZnO microcrystals and their photocatalytic ability in the degradation of textile azo dyes. Res. Chem. Intermed. 2013, 39, 3345. [Google Scholar] [CrossRef]
- Hernández, S.; Cauda, V.; Hidalgo, D.; Rivera, V.F.; Manfredi, D.; Chiodoni, A.; Pirri, F.C. Fast and low-cost synthesis of 1D ZnO–TiO2 core–shell nanoarrays: Characterization and enhanced photo-electrochemical performance for water splitting. J. Alloy Compd. 2014, 615, S530. [Google Scholar] [CrossRef]
- Ekambaram, S.; Iikubo, Y.; Kudo, A. Combustion synthesis and photocatalytic properties of transition metal-incorporated ZnO. J. Alloy Compd. 2007, 433, 237. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Xie, Y.; Fan, Q.; Jimmy, C.Y.; Shu, Q.; Wang, C. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142. [Google Scholar] [CrossRef]
- Yu, C.; Cao, F.; Li, X.; Li, G.; Xie, Y.; Jimmy, C.Y.; Shu, Q.; Fan, Q.; Chen, J. Hydrothermal synthesis and characterization of novel PbWO4 microspheres with hierarchical nanostructures and enhanced photocatalytic performance in dye degradation. Chem. Eng. J. 2013, 219, 86. [Google Scholar] [CrossRef]
- Gu, C.; Xiong, S.; Zhong, Z.; Wang, Y.; Xing, W. A promising carbon fiber-based photocatalyst with hierarchical structure for dye degradation. RSC Adv. 2017, 7, 22234. [Google Scholar] [CrossRef]
- Albiss, B.; Abu-Dalo, M. Photocatalytic Degradation of Methylene Blue Using Zinc Oxide Nanorods Grown on Activated Carbon Fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Du, Y.; Fu, C.; Gao, Y.; Liu, L.; Liu, Y.; Xing, L.; Zhao, F. Carbon fibers/ZnO nanowires hybrid nanogenerator based on an insulating interface barrier. RSC Adv. 2017, 7, 21452. [Google Scholar] [CrossRef]
- Meng, H.; Hou, W.; Xu, X.; Xu, J.; Zhang, X. TiO2-loaded activated carbon fiber: Hydrothermal synthesis, adsorption properties and photo catalytic activity under visible light irradiation. Particuology 2014, 14, 38. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, F.; Lin, C.; Wang, Z.L. Direct growth of TiO2 nanosheet arrays on carbon fibers for highly efficient photocatalytic degradation of methyl orange. Adv. Mater. 2012, 24, 4761. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Yang, F. High flux carbon fiber cloth membrane with thin catalyst coating integrates bio-electricity generation in wastewater treatment. J. Membr. Sci. 2016, 505, 130. [Google Scholar] [CrossRef]
- Son, H.K.; Sivakumar, S.; Rood, M.J.; Kim, B.J. Electrothermal adsorption and desorption of volatile organic compounds on activated carbon fiber cloth. J. Hazard. Mater. 2016, 301, 27. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 Nanoparticles Grown on Carbon Fiber Paper: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 4897. [Google Scholar] [CrossRef]
- Lin, X.; Li, M.; Li, Y.J.; Chen, W. Enhancement of the catalytic activity of ordered mesoporous TiO2 by using carbon fiber support and appropriate evaluation of synergy between surface adsorption and photocatalysis by Langmuir–Hinshelwood (L–H) integration equation. RSC Adv. 2015, 5, 105227. [Google Scholar] [CrossRef]
- Gu, X.; Yu, N.; Zhang, L.; Yang, J.; Hu, J.; Chen, Z. Growth of TiO2 nanorod bundles on carbon fibers as flexible and weaveable photocatalyst/photoelectrode. RSC Adv. 2015, 5, 102868. [Google Scholar] [CrossRef]
- Weng, B.; Xu, F.; Yu, F. Fabrication of hierarchical Bi4Ti3O12 nanosheets on carbon fibers with improved photocatalytic activity. Mater. Lett. 2015, 145, 70. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. MoS2/CdS/TiO2 ternary composite incorporated into carbon nanofibers for the removal of organic pollutants from water. Inorg. Chem. Commun. 2019, 102, 113. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO−Carbon Nanofiber Hetero-architectures. ACS Appl. Mater. Interfaces 2011, 3, 590. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, L.; Kies, K.; Lindquist, S.E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350. [Google Scholar] [CrossRef]
- Womelsdorf, H.; Hoheisel, W.; Passing, G. Nanoparticulate, Redispersible Zinc Oxide Gels. In U.S. Patent; US 6,710,091 B1, 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-6710091-B1 (accessed on 4 January 2024).
- Wang, M.; Ye, C.H.; Zhang, Y.; Hua, G.M.; Wang, H.X.; Kong, M.G.; Zhang, L.D. Synthesis of well-aligned ZnO nanorod arrays with high optical property via a low-temperature solution method. J. Cryst. Growth. 2006, 291, 334. [Google Scholar] [CrossRef]

| (a) 0V | (b) 1V | ||||
| Time (min) | Current (μA) | Dark current (μA) | Time (min) | Current (μA) | Dark current (μA) |
| 0 | 21.6 ± 0.1 | 4.0 ± 0.1 | 0 | 74.09 ± 0.1 | 32.01 ± 0.1 |
| 40 | 19.1 ± 0.1 | 3.8 ± 0.1 | 40 | 64.0 ± 0.1 | 25.6 ± 0.1 |
| 80 | 18.53 ± 0.1 | 3.7 ± 0.1 | 80 | 57.03 ± 0.1 | 20.3 ± 0.1 |
| 120 | 17.31 ± 0.1 | 3.6 ± 0.1 | 120 | 53.01 ± 0.1 | 16.21 ± 0.1 |
| (c) 0V | (d) 1V | ||||
| Time (min) | Current (μA) | Dark current (μA) | Time (min) | Current (μA) | Dark current (μA) |
| 0 | 9.1 ± 0.1 | 1.6 ± 0.1 | 0 | 39.56 ± 0.1 | 17.6 ± 0.1 |
| 40 | 8.3 ± 0.1 | 1.5 ± 0.1 | 40 | 35.93 ± 0.1 | 14.5 ± 0.1 |
| 80 | 8.02 ± 0.1 | 1.5 ± 0.1 | 80 | 32.59 ± 0.1 | 12.7 ± 0.1 |
| 120 | 7.95 ± 0.1 | 1.4 ± 0.1 | 120 | 31.05 ± 0.1 | 12.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvi, N.u.H.; Sandberg, M. Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells. Nanomaterials 2024, 14, 636. https://doi.org/10.3390/nano14070636
Alvi NuH, Sandberg M. Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells. Nanomaterials. 2024; 14(7):636. https://doi.org/10.3390/nano14070636
Chicago/Turabian StyleAlvi, Naveed ul Hassan, and Mats Sandberg. 2024. "Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells" Nanomaterials 14, no. 7: 636. https://doi.org/10.3390/nano14070636
APA StyleAlvi, N. u. H., & Sandberg, M. (2024). Sustainable and Low-Cost Electrodes for Photocatalytic Fuel Cells. Nanomaterials, 14(7), 636. https://doi.org/10.3390/nano14070636






