Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO NPs
2.2. Physicochemical Characterization of ZnO NPs
2.2.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
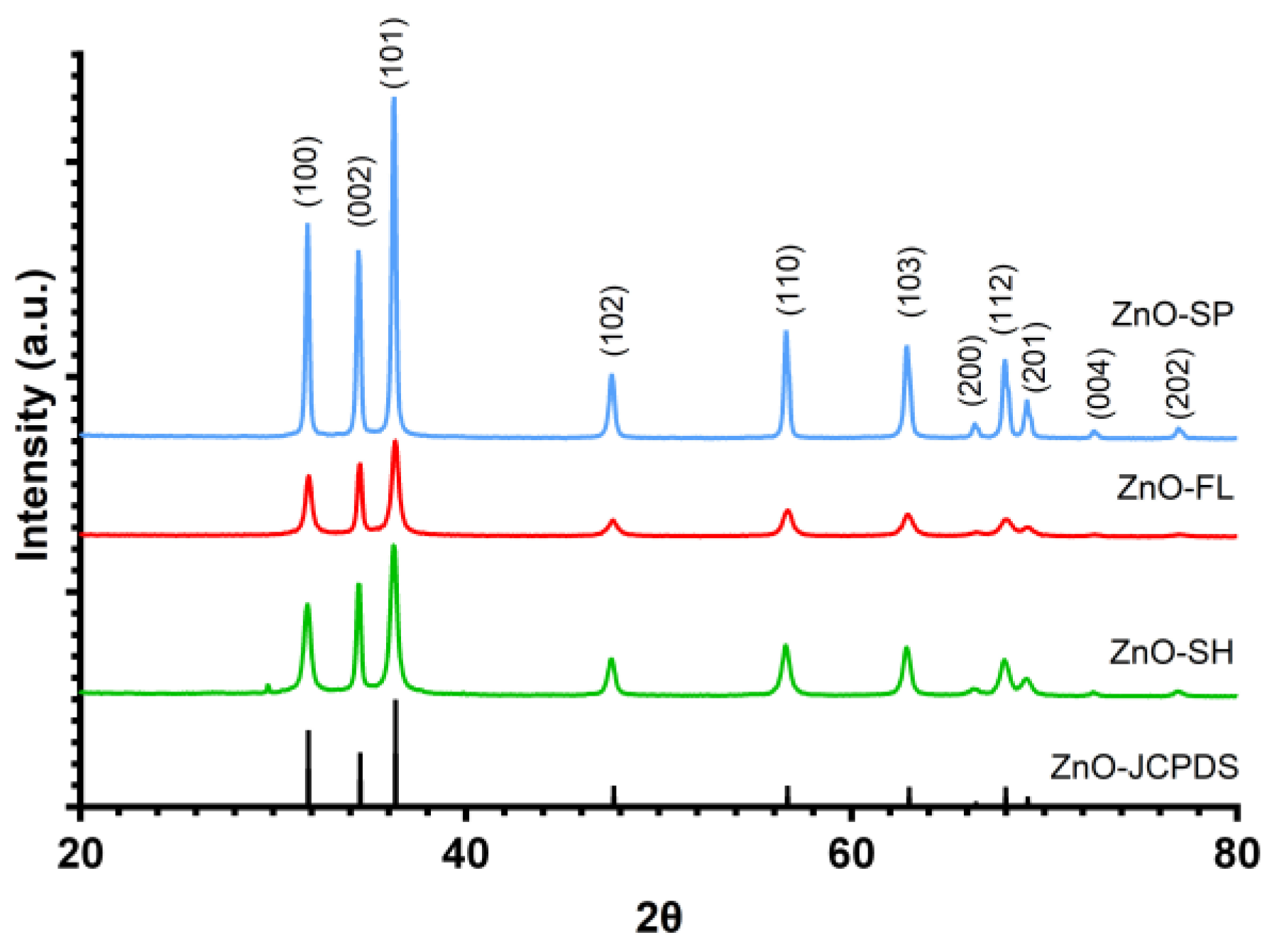
2.2.2. X-ray Powder Diffraction
2.2.3. Fourier-Transform Infrared Spectroscopy
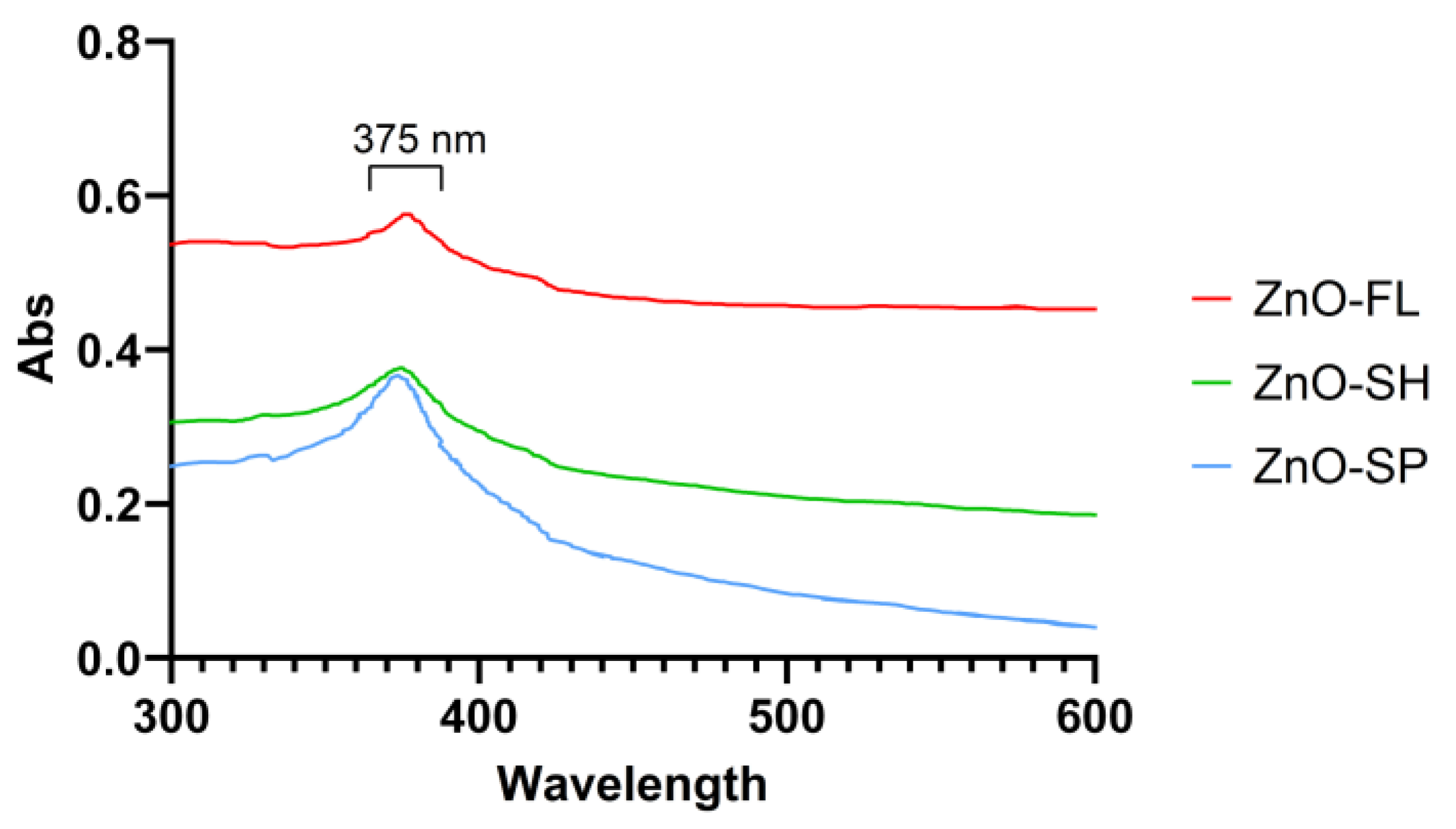
2.2.4. Ultraviolet–Visible Spectroscopy
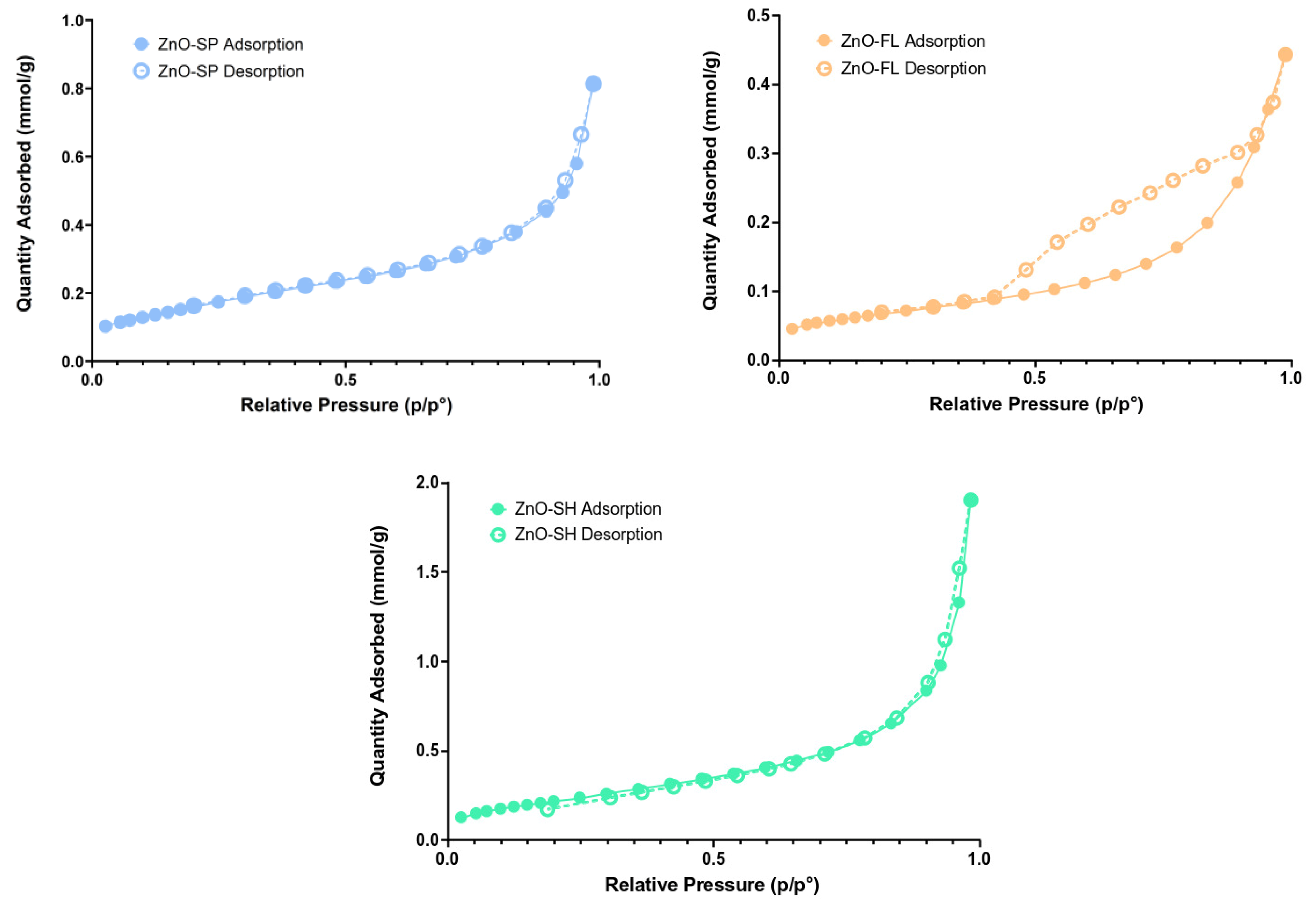
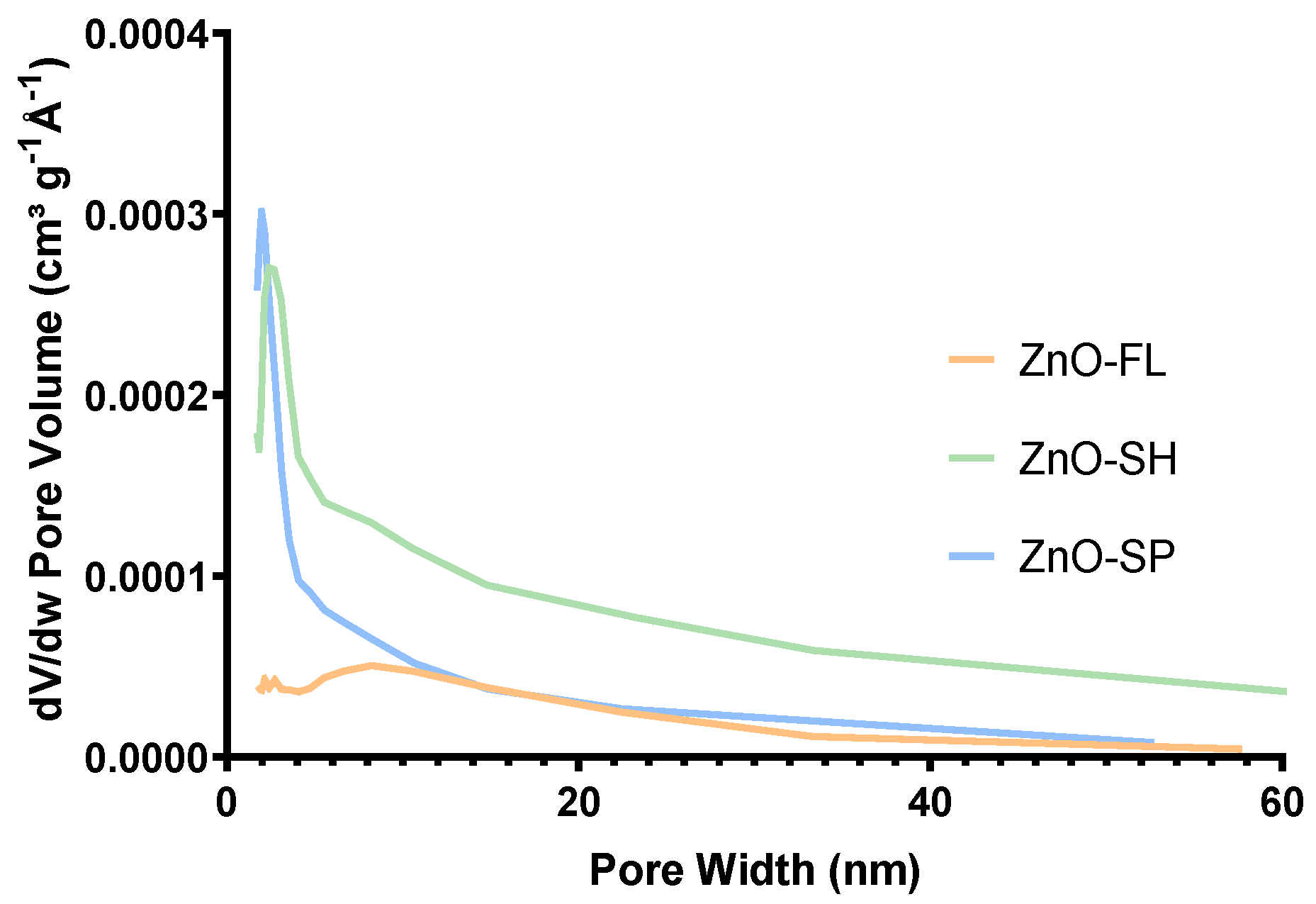
2.2.5. Surface Area and Porosity
2.2.6. Electron Paramagnetic Resonance
2.3. Antibacterial Activity of ZnO NPs
2.4. Data Handling and Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnO NPs
3.2. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of nanotechnology in food packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and Active Food Packaging: Insights in Novel Food Packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An untapped resource for food packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Espitia PJ, P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 425–431. [Google Scholar] [CrossRef]
- FDA. Part 182—Substances generally recognized as safe. In National Archives and Records Administration (NARA); FDA: Silver Spring, MD, USA, 2011. [Google Scholar]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2016, 14, 4408. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals 2021, 11, 2093. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A.; Reka, U. Hydrothermal synthesis and characterization of Zinc Oxide nanoparticles of various shapes under different reaction conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Wang, T.X.; Lou, T.J. Solvothermal synthesis and photoluminescence properties of ZnO nanorods and nanorod assemblies from ZnO2 nanoparticles. Mater. Lett. 2008, 62, 2329–2331. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.-S.; Seo, H.-K.; Shin, H.-S. Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl. Surf. Sci. 2007, 253, 7622–7626. [Google Scholar] [CrossRef]
- Kong, X.Y.; Ding, Y.; Yang, R.; Wang, Z.L. Single-Crystal Nanorings Formed by Epitaxial Self-Coiling of Polar Nanobelts. Science 2004, 303, 1348–1351. [Google Scholar] [CrossRef]
- Wu, J.-J.; Liu, S.-C.; Wu, C.-T.; Chen, K.-H.; Chen, L.-C. Heterostructures of ZnO–Zn coaxial nanocables and ZnO nanotubes. Appl. Phys. Lett. 2002, 81, 1312. [Google Scholar] [CrossRef]
- Li, X.; He, G.; Xiao, G.; Liu, H.; Wang, M. Synthesis and morphology control of ZnO nanostructures in microemulsions. J. Colloid Interface Sci. 2009, 333, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Z.L. Structures of planar defects in ZnO nanobelts and nanowires. Micron 2009, 40, 335–342. [Google Scholar] [CrossRef]
- Chiu, W.S.; Khiew, P.S.; Cloke, M.; Isa, D.; Tan, T.K.; Radiman, S.; Abd-Shukor, R.; Hamid MA Abd Huang, N.M.; Lim, H.N. Photocatalytic study of two-dimensional ZnO nanopellets in the decomposition of methylene blue. Chem. Eng. J. 2010, 158, 345–352. [Google Scholar] [CrossRef]
- Jang, J.S.; Yu, C.-J.; Choi, S.H.; Ji, S.M.; Kim, E.S.; Lee, J.S. Topotactic synthesis of mesoporous ZnS and ZnO nanoplates and their photocatalytic activity. J. Catal. 2008, 254, 144–155. [Google Scholar] [CrossRef]
- Rao, M.D.; Gautam, P. Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Environ. Prog. Sustain. Energy 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polym. Lett. 2009, 3, 542–552. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles Loaded with Essential Oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: A green approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Shanmugam, M.; Umamaheswaran, P.D.; Venkattappan, A.; Shanmugam, A. Zinc Oxide Nanoparticles: Green Synthesis and Biomedical Applications. J. Clust. Sci. 2021, 32, 1441–1455. [Google Scholar] [CrossRef]
- Odeyemi, O.A. Public health implications of microbial food safety and foodborne diseases in developing countries. Food Nutr. Res. 2016, 60, 29819. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Poças, F.; Franz, R. Overview on European Regulatory Issues, Legislation, and EFSA Evaluations of Nanomaterials. In Nanomaterials for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–300. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; De Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess. Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. In Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A potential antibacterial agent. Prog. Nat. Sci. USA 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef]
- Preda, M.D.; Popa, M.L.; Neacșu, I.A.; Grumezescu, A.M.; Ginghină, O. Antimicrobial Clothing Based on Electrospun Fibers with ZnO Nanoparticles. Int. J. Mol. Sci. 2023, 24, 1629. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Amirkhanlou, S.; Ketabchi, M.; Parvin, N. Nanocrystalline/nanoparticle ZnO synthesized by high energy ball milling process. Mater. Lett. 2012, 86, 122–124. [Google Scholar] [CrossRef]
- Barreto, G.P.; Morales, G.; Quintanilla Ma, L.L. Microwave Assisted Synthesis of ZnO Nanoparticles: Effect of Precursor Reagents, Temperature, Irradiation Time, and Additives on Nano-ZnO Morphology Development. J. Mater. 2013, 2013, 478681. [Google Scholar] [CrossRef]
- El Faham, M.M.; Mostafa, A.M.; Mwafy, E.A. The effect of reaction temperature on structural, optical and electrical properties of tunable ZnO nanoparticles synthesized by hydrothermal method. J. Phys. Chem. Solids 2021, 154, 110089. [Google Scholar] [CrossRef]
- Ghoshal, T.; Biswas, S.; Paul, M.; De, S.K. Synthesis of ZnO Nanoparticles by Solvothermal Method and Their Ammonia Sensing Properties. J. Nanosci. Nanotechnol. 2009, 9, 5973–5980. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Pallon LK, H.; Andersson, R.L.; Martínez Abad, A.; Lagarón, J.-M.; Hedenqvist, M.S.; Ström, V.; Gedde, U.W.; Olsson, R.T. Water-based synthesis and cleaning methods for high purity ZnO nanoparticles—Comparing acetate, chloride, sulphate and nitrate zinc salt precursors. RSC Adv. 2014, 4, 35568–35577. [Google Scholar] [CrossRef]
- Alim, K.A.; Fonoberov, V.A.; Shamsa, M.; Balandin, A.A. Micro-Raman investigation of optical phonons in ZnO nanocrystals. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Mayekar, J.; Dhar, V.; Srinivasan, R. To Study the Role of Temperature and Sodium Hydroxide Concentration in the Synthesis of Zinc Oxide Nanoparticles. Int. J. Sci. Res. Publ. 2013, 3, 2250–3153. [Google Scholar]
- Agarwal, S.; Jangir, L.K.; Rathore, K.S.; Kumar, M.; Awasthi, K. Morphology-dependent structural and optical properties of ZnO nanostructures. Appl. Phys. A 2019, 125, 553. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. Int. Sch. Res. Not. Nanotechnol. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. Part A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- AL-Asady, Z.M.; AL-Hamdani, A.H.; Hussein, M.A. Study the optical and morphology properties of zinc oxide nanoparticles. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2020; p. 020061. [Google Scholar] [CrossRef]
- Singh, D.K.; Pandey, D.K.; Yadav, R.R.; Singh, D. A study of nanosized zinc oxide and its nanofluid. Pramana 2012, 78, 759–766. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Alothman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Sulciute, A.; Nishimura, K.; Gilshtein, E.; Cesano, F.; Viscardi, G.; Nasibulin, A.G.; Ohno, Y.; Rackauskas, S. ZnO Nanostructures Application in Electrochemistry: Influence of Morphology. J. Phys. Chem. C 2021, 125, 1472–1482. [Google Scholar] [CrossRef]
- Erdem, E. Microwave power, temperature, atmospheric and light dependence of intrinsic defects in ZnO nanoparticles: A study of electron paramagnetic resonance (EPR) spectroscopy. J. Alloys Compd. 2014, 605, 34–44. [Google Scholar] [CrossRef]
- Nadupalli, S.; Repp, S.; Weber, S.; Erdem, E. About defect phenomena in ZnO nanocrystals. Nanoscale 2021, 13, 9160–9171. [Google Scholar] [CrossRef] [PubMed]
- Parashar, S.K.S.; Murty, B.S.; Repp, S.; Weber, S.; Erdem, E. Investigation of intrinsic defects in core-shell structured ZnO nanocrystals. J. Appl. Phys. 2012, 111, 113712. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Tuc Altaf, C.; Colak, T.O.; Rostas, A.M.; Popa, A.; Toloman, D.; Suciu, M.; Demirci Sankir, N.; Sankir, M. Impact on the Photocatalytic Dye Degradation of Morphology and Annealing-Induced Defects in Zinc Oxide Nanostructures. ACS Omega 2023, 8, 14952–14964. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Rahul, A.B.; Thara, G.M.; Nair, A.S. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Acacia caesia Bark Extract and Its Photocatalytic and Antimicrobial Activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Soares Silva, F.; Carvalho, M.; Bento de Carvalho, T.; Gama, M.; Poças, F.; Teixeira, P. Antimicrobial activity of in-situ bacterial nanocellulose-zinc oxide composites for food packaging. Food Packag. Shelf Life 2023, 40, 101201. [Google Scholar] [CrossRef]
- Elkady, M.F.; Shokry Hassan, H.; Hafez, E.E.; Fouad, A. Construction of Zinc Oxide into Different Morphological Structures to Be Utilized as Antimicrobial Agent against Multidrug Resistant Bacteria. Bioinorg. Chem. Appl. 2015, 2015, 536854. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]


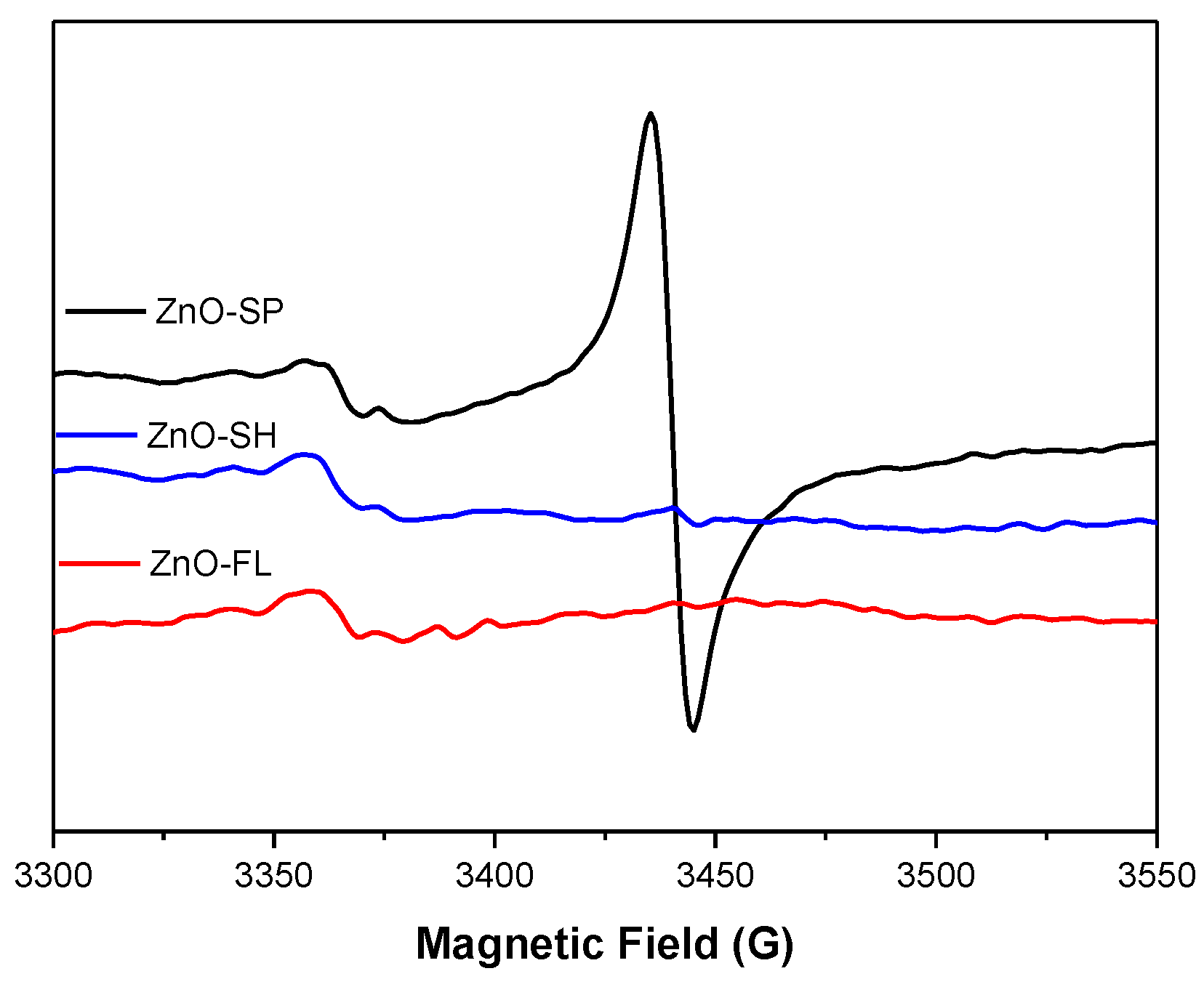
 ), ZnO-FL (
), ZnO-FL ( ), ZnO-SH (
), ZnO-SH ( ) and microorganism control (
) and microorganism control ( ) for (a) E. coli 22 °C and (b) S. aureus 22 °C.
) for (a) E. coli 22 °C and (b) S. aureus 22 °C.
 ), ZnO-FL (
), ZnO-FL ( ), ZnO-SH (
), ZnO-SH ( ) and microorganism control (
) and microorganism control ( ) for (a) E. coli 22 °C and (b) S. aureus 22 °C.
) for (a) E. coli 22 °C and (b) S. aureus 22 °C.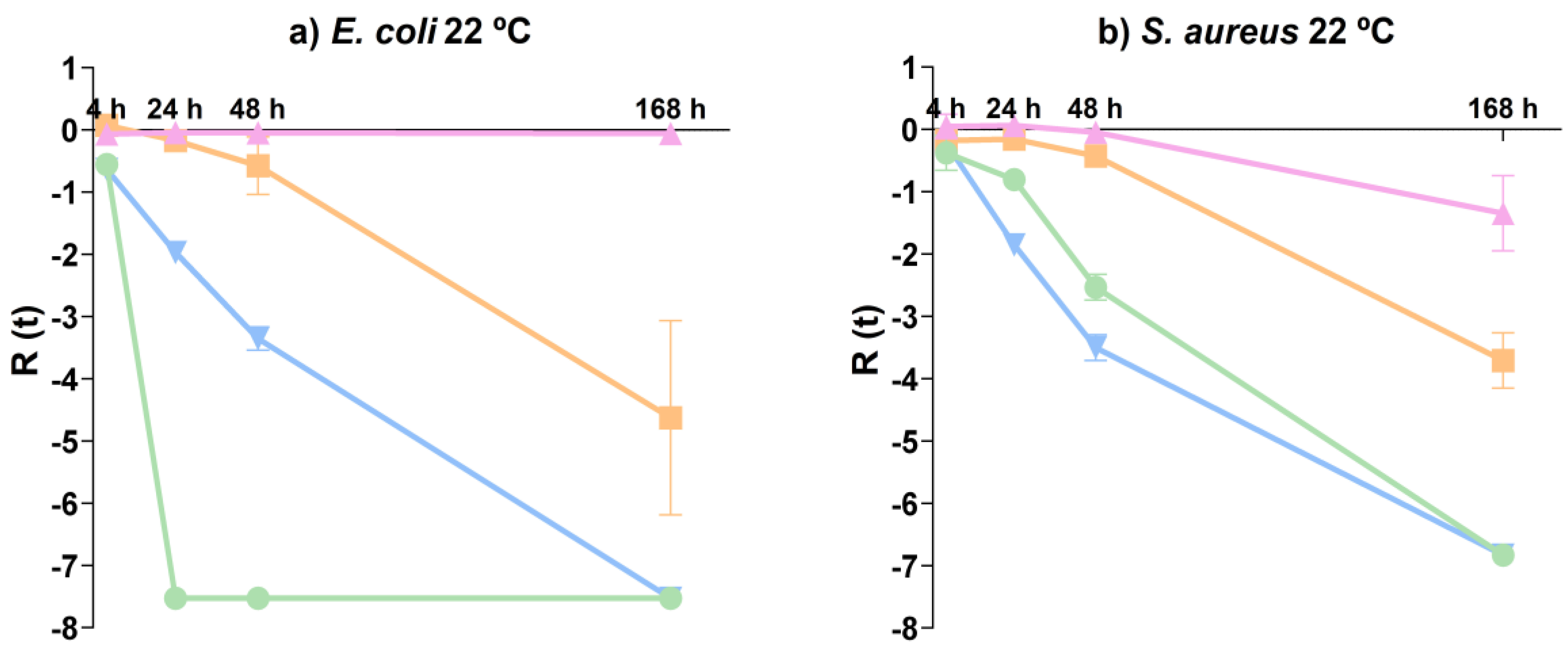
| Parameter | ZnO-SP | ZnO-FL | ZnO-SH |
|---|---|---|---|
| Specific surface area (m2 g−1) | 13.4 | 5.3 | 18.5 |
| Pore width (nm) | 9.8 | 7.0 | 11.9 |
| Pore volume (cm3 g−1) | 0.03 | 0.02 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Pereira, E.; Teixeira, P.; Poças, F. Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials 2024, 14, 638. https://doi.org/10.3390/nano14070638
Mendes AR, Granadeiro CM, Leite A, Pereira E, Teixeira P, Poças F. Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials. 2024; 14(7):638. https://doi.org/10.3390/nano14070638
Chicago/Turabian StyleMendes, Ana Rita, Carlos M. Granadeiro, Andreia Leite, Eulália Pereira, Paula Teixeira, and Fátima Poças. 2024. "Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size" Nanomaterials 14, no. 7: 638. https://doi.org/10.3390/nano14070638
APA StyleMendes, A. R., Granadeiro, C. M., Leite, A., Pereira, E., Teixeira, P., & Poças, F. (2024). Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials, 14(7), 638. https://doi.org/10.3390/nano14070638








