Advances in Nanodynamic Therapy for Cancer Treatment
Abstract
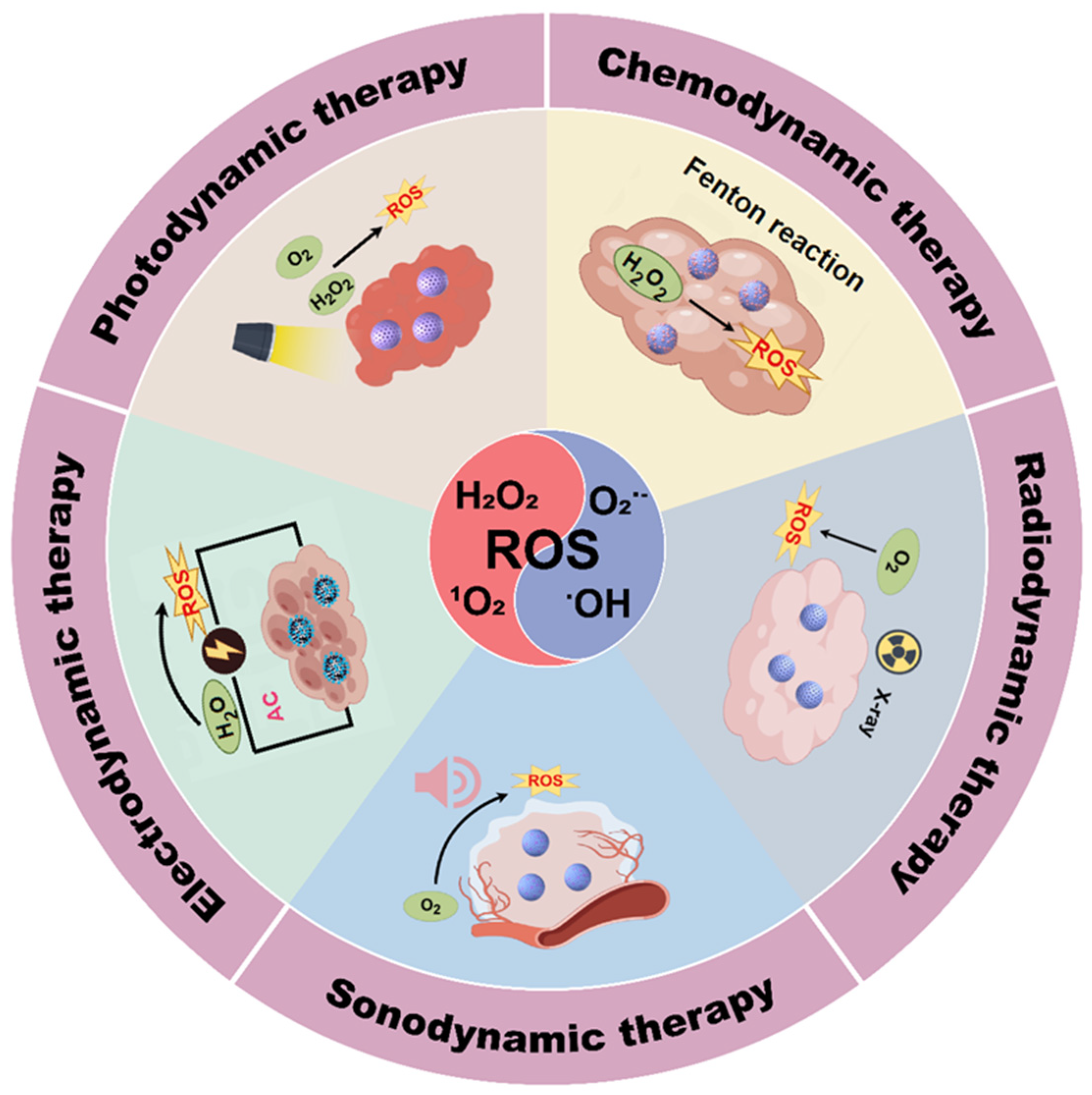
1. Introduction
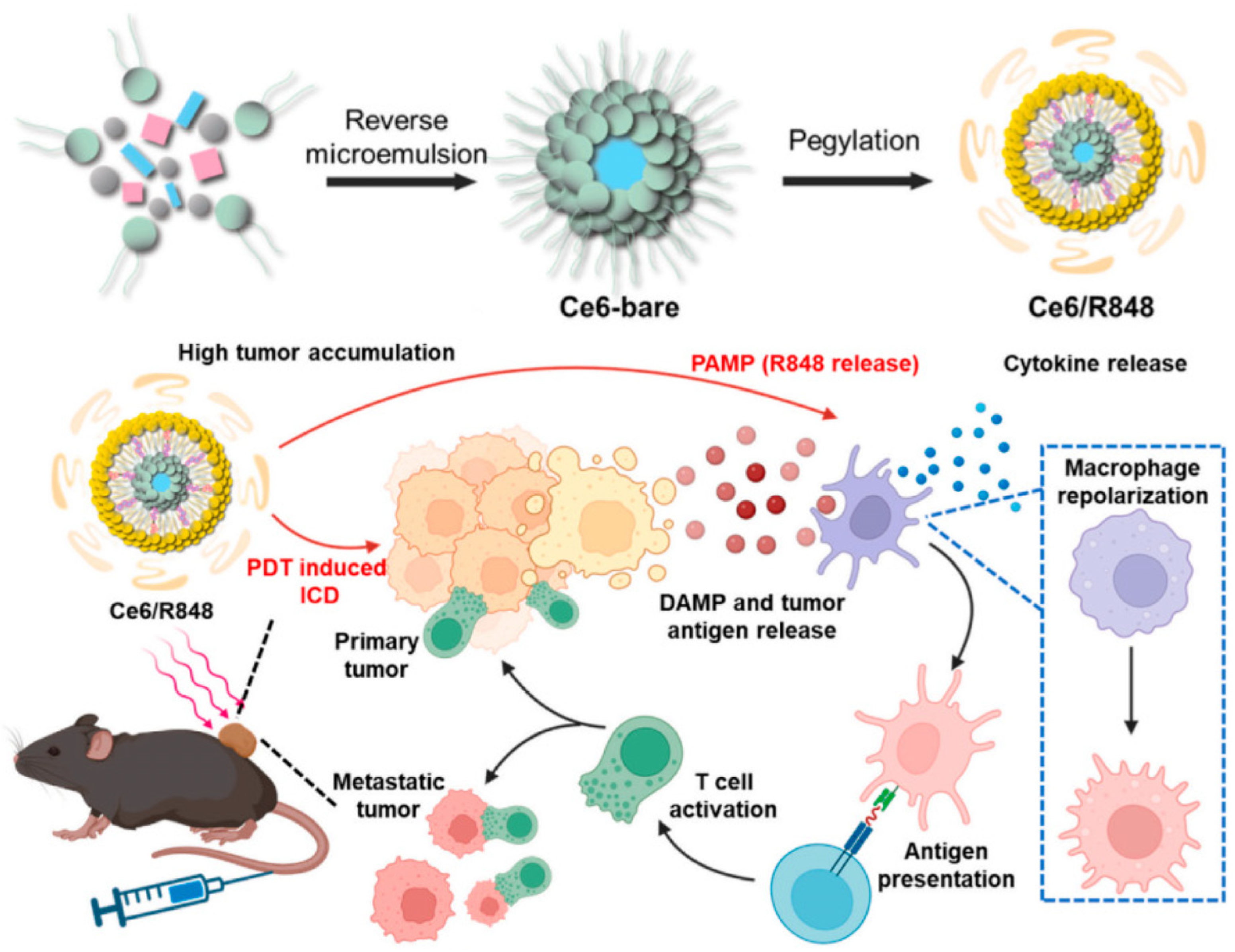
2. Photodynamic Therapy
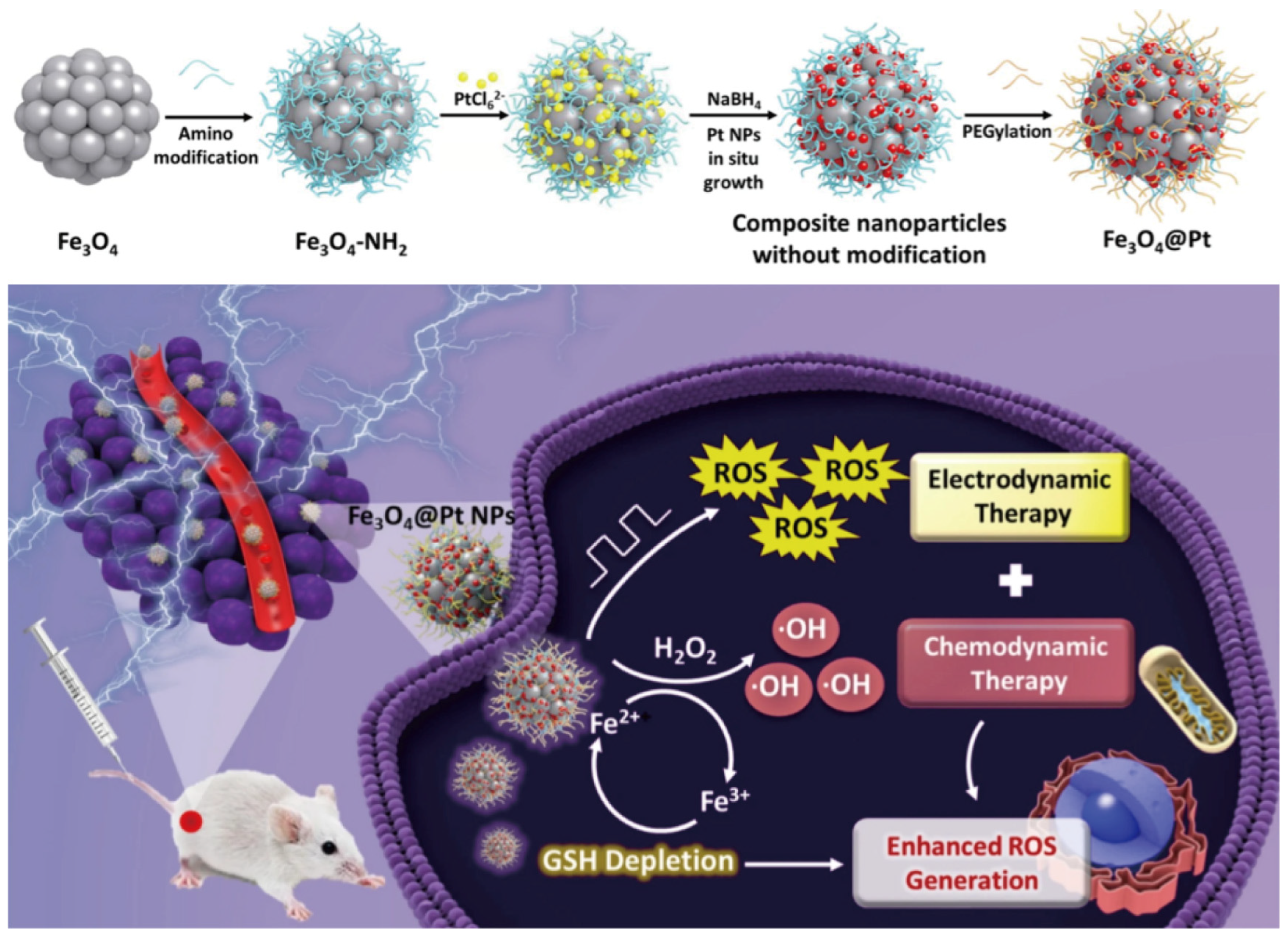
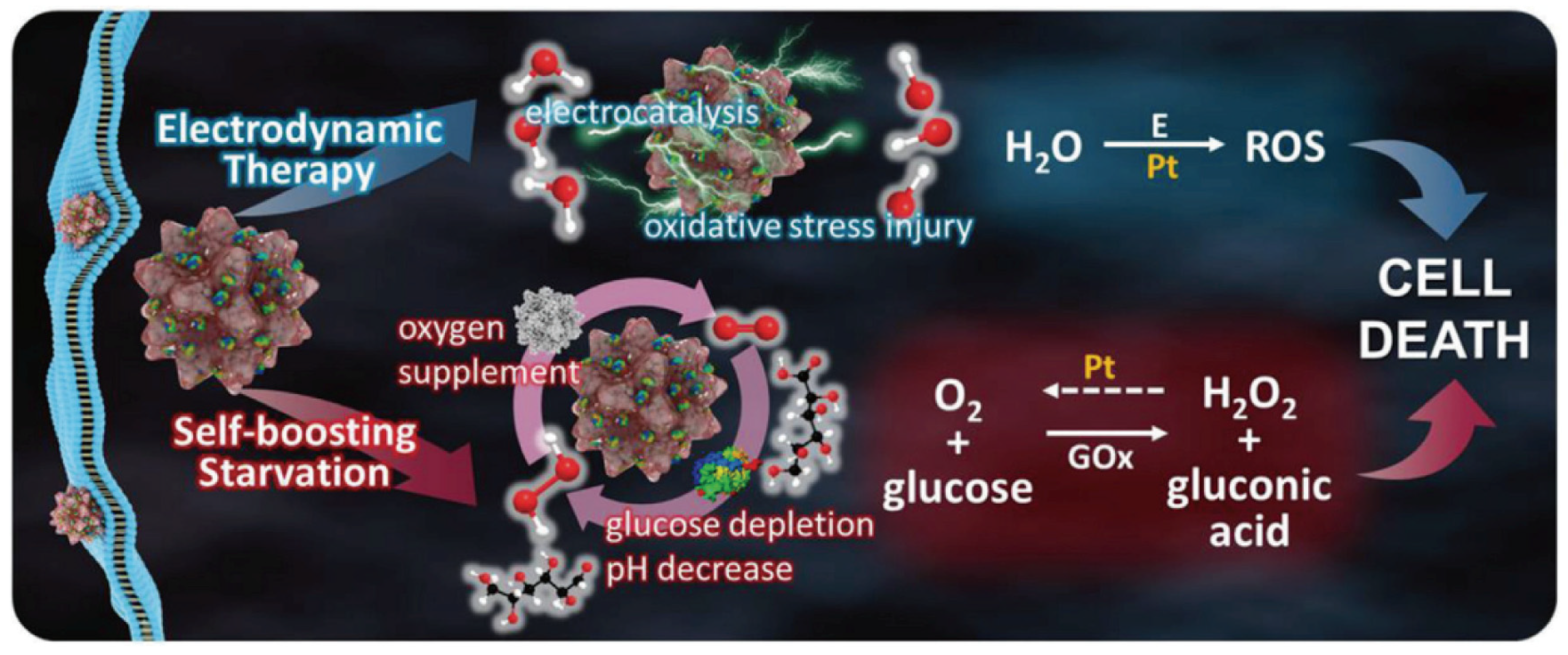
3. Electrodynamic Therapy
4. Sonodynamic Therapy
5. Radiodynamic Therapy
6. Chemodynamic Therapy
7. Role of Nanomedicine in NDT
8. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Jiang, Y.; Wang, Q.; Lai, Y.; Holloway, A. The status and challenges of HPV vaccine programme in China: An exploration of the related policy obstacles. BMJ Glob. Health 2023, 8, e012554. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Naghavi, S.M.H.; Mozafari, M.; Afshari, E. Nanoscale biomaterials for terahertz imaging: A non-invasive approach for early cancer detection. Transl. Oncol. 2023, 27, 101565. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.; Bhupathi, D.; Chen, J.; Huang, T.; Li, W.; Ma, Y.; Sotirovska, N.; Steggerda, S.; Zhang, W.; Parlati, F. 705 Anti-tumor activity of CB-668, a potent, selective and orally bioavailable small-molecule inhibitor of the immuno-suppressive enzyme Interleukin 4 (IL-4)-Induced Gene 1 (IL4I1). J. Immunother. Cancer 2020, 8 (Suppl. S3), A423–A424. [Google Scholar]
- Isaguliants, M.G.; Trotsenko, I.; Buonaguro, F.M. An overview of “Chronic viral infection and cancer, openings for vaccines” virtual symposium of the TechVac Network—December 16–17, 2021. Infect. Agent Cancer 2022, 17 (Suppl. S2), 28. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshimatsu, G.; Faustman, D.L. The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells 2022, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, J.; He, H.; Ma, C.; Wang, K. Contributing to liquid biopsy: Optical and electrochemical methods in cancer biomarker analysis. Coord. Chem. Rev. 2020, 415, 213317. [Google Scholar] [CrossRef]
- Youness, R.A.; Mohamed, A.H.; Efthimiadou, E.K.; Mekky, R.Y.; Braoudaki, M.; Fahmy, S.A. A Snapshot of Photoresponsive Liposomes in Cancer Chemotherapy and Immunotherapy: Opportunities and Challenges. ACS Omega 2023, 8, 44424–44436. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yan, M.; Hong, M. Sound energy enhancement via impedance-matched anisotropic metamaterial. Mater. Des. 2021, 197, 109254. [Google Scholar] [CrossRef]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J.; et al. Antitumor Effect of Photodynamic Therapy/Sonodynamic Therapy/Sono-Photodynamic Therapy of Chlorin e6 and Other Applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Feng, W.; Qian, X.; Yu, L.; Chen, Y.; Li, Y. Emerging Nanomedicine-Enabled/Enhanced Nanodynamic Therapies beyond Traditional Photodynamics. Adv. Mater. 2021, 33, e2005062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gao, Y.; Yang, Z.; Wang, N.; Ge, J.; Cao, X.; Kou, D.; Gu, Y.; Li, C.; Afshari, M.J.; et al. Biomimetic Upconversion Nanoplatform Synergizes Photodynamic Therapy and Enhanced Radiotherapy against Tumor Metastasis. ACS Appl. Mater. Interfaces 2023, 15, 26431–26441. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, Z.; Liu, H.; Chen, L.; Bai, Y.; Wu, Q.; Yu, X.; Wei, W.; Wang, M. Multifunctional nanomedicines-enabled chemodynamic-synergized multimodal tumor therapy via Fenton and Fenton-like reactions. Theranostics 2023, 13, 1974–2014. [Google Scholar] [CrossRef] [PubMed]
- Florido, J.; Rodriguez-Santana, C.; Martinez-Ruiz, L.; López-Rodríguez, A.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G. Understanding the Mechanism of Action of Melatonin, Which Induces ROS Production in Cancer Cells. Antioxidants 2022, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Du, J.; Wang, Q.; Chen, G.; Li, X.; Zhang, Z.; Wang, S.; Jing, W.; Miao, Q.; Li, Y.; et al. Dye-augmented bandgap engineering of a degradable cascade nanoreactor for tumor immune microenvironment-enhanced dynamic phototherapy of breast cancer. Acta Biomater. 2024, 176, 390–404. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Wang, H.; Lin, F.; Huang, H.; Chen, Z.; Yang, Z.; Chi, Z.; Zhou, X. Type-I organic photosensitizers with two complementary reactive oxygen species based on donor-acceptor (D-A) molecules. Dye. Pigment. 2023, 218, 111444. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Li, K.; Muhammad, S.; Ju, M.; Liu, L.; Huang, Y.; Wang, B.; Ding, W.; Shen, B.; et al. Fluorogenic toolbox for facile detecting of hydroxyl radicals: From designing principles to diagnostics applications. TrAC Trends Anal. Chem. 2022, 157, 116734. [Google Scholar] [CrossRef]
- Chasara, R.S.; Ajayi, T.O.; Leshilo, D.M.; Poka, M.S.; Witika, B.A. Exploring novel strategies to improve anti-tumour efficiency: The potential for targeting reactive oxygen species. Heliyon 2023, 9, e19896. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, Y.; Lu, J.; Lin, Y.; Feng, S.; Luo, X.; Di, D.; Wang, S.; Zhao, Q. Recent trends of mesoporous silica-based nanoplatforms for nanodynamic therapies. Coord. Chem. Rev. 2022, 469, 214687. [Google Scholar] [CrossRef]
- Song, A.; Wang, Y.; Xu, J.; Wang, X.; Wu, Y.; Wang, H.; Yao, C.; Dai, H.; Zhang, Y.; Wang, Q.; et al. Yeast Nanoparticle-powered Tumor Photodynamic-Immunotherapy. Nano Today 2024, 54, 102109. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Q.; Jin, Q.; Chao, Y.; Sun, L.; Han, X.; Xu, J.; Tian, L.; Zhang, J.; Liu, T.; et al. Sonodynamic therapy with immune modulatable two-dimensional coordination nanosheets for enhanced anti-tumor immunotherapy. Nano Res. 2021, 14, 212–221. [Google Scholar] [CrossRef]
- Li, Y.; Gong, T.; Gao, H.; Chen, Y.; Li, H.; Zhao, P.; Jiang, Y.; Wang, K.; Wu, Y.; Zheng, X.; et al. ZIF-Based Nanoparticles Combine X-Ray-Induced Nitrosative Stress with Autophagy Management for Hypoxic Prostate Cancer Therapy. Angew. Chem. Int. Ed. 2021, 60, 15472–15481. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Meng, Q.; Liu, Z.; Liu, S.; Tong, W.; An, B.; Ding, B.; Ma, P.; Lin, J. Iron oxide-EDTA nanoparticles for chelation-enhanced chemodynamic therapy and ion interference therapy. Biomater. Sci. 2023, 11, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Suo, F.; Haslinger, K.; Quax, W.J. Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery. Pharmaceutics 2022, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Z.; Zhang, R.; Du, J.; Zhu, L.; Li, X.; Yang, H.; Miao, Y.; Li, Y. Carbon monoxide-based immunogenic cell death amplifier remodels the hypoxic microenvironment for tumor sono-immunotherapy. Chem. Eng. J. 2024, 480, 148269. [Google Scholar] [CrossRef]
- Tian, M.; Xin, X.; Wu, R.; Guan, W.; Zhou, W. Advances in intelligent-responsive nanocarriers for cancer therapy. Pharmacol. Res. 2022, 178, 106184. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lv, H.; Duan, X.; Du, Y.; Tang, Y.; Xu, W. Advancements in Macrophage-Targeted Drug Delivery for Effective Disease Management. Int. J. Nanomed. 2023, 18, 6915–6940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, J.; Lee, M.J.; Peng, C.; Luo, T.; Tillman, L.; Weichselbaum, R.R.; Lin, W. Nanoscale coordination polymer synergizes photodynamic therapy and toll-like receptor activation for enhanced antigen presentation and antitumor immunity. Biomaterials 2023, 302, 122334. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Chumakov, S.P.; Shipunova, V.O.; Ryabova, A.V.; Telegin, G.B.; Kabashin, A.V.; Deyev, S.M.; Proshkina, G.M. Genetically encoded BRET-activated photodynamic therapy for the treatment of deep-seated tumors. Light Sci. Appl. 2022, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Lu, Y.; Zhu, X.; Zheng, W.; Chen, K.; Liu, S.; Wu, J.; Guan, W. Improved Photodynamic Therapy Based on Glutaminase Blockage via Tumor Membrane Coated CB-839/IR-780 Nanoparticles. Small 2023, 20, e2305174. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Y.; Chen, K.; Huang, Y.; Liu, Y.; Xu, S.; Wang, W. CDK4/6 nano-PROTAC enhances mitochondria-dependent photodynamic therapy and anti-tumor immunity. Nano Today 2023, 50, 101890. [Google Scholar] [CrossRef]
- Chen, T.; Fu, Y.; Zhang, R.; Han, G.; Li, X. KCl-CaCO3 nanoclusters armoured with Pt nanocrystals for enhanced electro-driven tumor inhibition. Biomater. Sci. 2022, 10, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Q.; Feng, Z.; Xu, Q.; Zhang, X.; Yang, Y.; Zhang, Y.; Liang, X.-J.; Yu, Z.; Yu, M. Glutamine Antagonist Synergizes with Electrodynamic Therapy to Induce Tumor Regression and Systemic Antitumor Immunity. ACS Nano 2022, 16, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ha, E.; Zhou, Z.; Zhang, J.; Zhu, Y.; Ai, F.; Yan, L.; He, S.; Li, L.; Hu, J. “Spark” PtMnIr Nanozymes for Electrodynamic-Boosted Multienzymatic Tumor Immunotherapy. Adv. Mater. 2023, 36, e2308747. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gu, T.; Cheng, L.; Li, X.; Han, G.; Liu, Z. Porous Pt nanoparticles loaded with doxorubicin to enable synergistic Chemo-/Electrodynamic Therapy. Biomaterials 2020, 255, 120202. [Google Scholar] [CrossRef]
- Lai, Y.; Lu, N.; Ouyang, A.; Zhang, Q.; Zhang, P. Ferroptosis promotes sonodynamic therapy: A platinum(ii)–indocyanine sonosensitizer. Chem. Sci. 2022, 13, 9921–9926. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, J.; Lin, W.; Fu, Z.; Ji, X.; Yu, B.; Lu, M.; Cui, W.; Deng, L.; Engle, J.W.; et al. Open-Shell Nanosensitizers for Glutathione Responsive Cancer Sonodynamic Therapy. Adv. Mater. 2022, 34, e2110283. [Google Scholar] [CrossRef]
- Fan, C.-H.; Wu, N.; Yeh, C.-K. Enhanced sonodynamic therapy by carbon dots-shelled microbubbles with focused ultrasound. Ultrason. Sonochem. 2023, 94, 106342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Yu, H.; Zhao, Y.; Xu, Z.; Kang, Y.; Xue, P. Zirconia-Platinum Nanohybrids for Ultrasound-Activated Sonodynamic-Thermodynamic Bimodal Therapy by Inducing Intense Intracellular Oxidative Stress. Small 2022, 18, e2203080. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ni, K.; Luo, T.; Lan, G.; Arina, A.; Xu, Z.; Mao, J.; Weichselbaum, R.R.; Spiotto, M.; Lin, W. Reprogramming of Neutrophils as Non-canonical Antigen Presenting Cells by Radiotherapy–Radiodynamic Therapy to Facilitate Immune-Mediated Tumor Regression. ACS Nano 2021, 15, 17515–17527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yan, F.; Yang, L.; Li, B.; Xue, R.; Yu, W.; Wang, Y.; Huang, L.; Wang, L.; Han, R.; et al. Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response. Theranostics 2023, 13, 1042–1058. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Mei, Z.; Yang, H.; Wang, B.; Xie, C.; Xu, Y.; Tian, J. Oxygen-Enriched MOF-Hemoglobin X-ray Nanosensitizer for Enhanced Cancer Radio–Radiodynamic Therapy. ACS Mater. Lett. 2023, 5, 3237–3247. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, B.; Liang, S.; Zhao, Y.; Cheng, Z.; Xing, B.; Ma, P.; Lin, J. Intelligent MoS2–CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/immunotherapy. Biomaterials 2021, 268, 120545. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, M.; Sheng, D.; Luo, Y.; Wang, D.; Yu, X.; Wang, Z.; Ran, H.; Li, P. Low-intensity focused ultrasound-augmented Cascade chemodynamic therapy via boosting ROS generation. Biomaterials 2021, 271, 120710. [Google Scholar] [CrossRef] [PubMed]
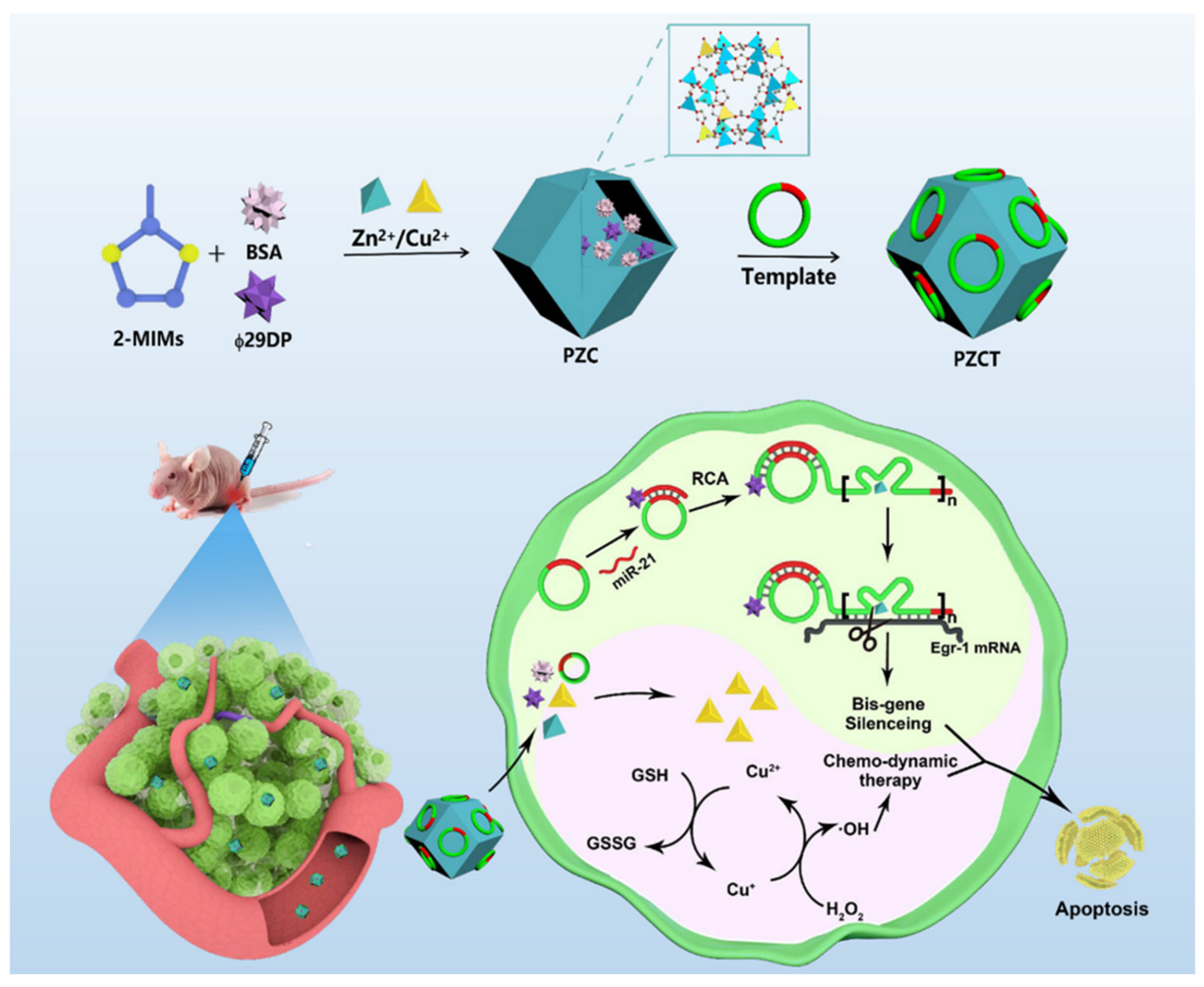
- Huang, T.; Pan, W.-L.; Li, M.-S.; Zou, Y.-M.; Meng, W.; Li, M.-M.; Zhang, W.-H.; Dai, Z.; Chen, J.-X.; Chen, J. Bioorthogonal Activation of RCA-Based Amplifiable DNAzymes in Bimetallic NMOF for Precise Gene/Chemo-Dynamic Combination Therapy. ACS Mater. Lett. 2024, 6, 498–507. [Google Scholar] [CrossRef]
- Jain, R.; Mohanty, S.; Sarode, I.; Biswas, S.; Singhvi, G.; Dubey, S.K. Multifunctional Photoactive Nanomaterials for Photodynamic Therapy against Tumor: Recent Advancements and Perspectives. Pharmaceutics 2022, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Digby, E.M.; Ayan, S.; Shrestha, P.; Gehrmann, E.J.; Winter, A.H.; Beharry, A.A. Photocaged DNA-Binding Photosensitizer Enables Photocontrol of Nuclear Entry for Dual-Targeted Photodynamic Therapy. J. Med. Chem. 2022, 65, 16679–16694. [Google Scholar] [CrossRef]
- Li, W.-P.; Yen, C.-J.; Wu, B.-S.; Wong, T.-W. Recent Advances in Photodynamic Therapy for Deep-Seated Tumors with the Aid of Nanomedicine. Biomedicines 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.; Sun, T.; Peng, N.; Wang, Z.; Chen, D.; Qiu, H.; Zhao, H. Photodynamic Therapy-Induced Anti-Tumor Immunity: Influence Factors and Synergistic Enhancement Strategies. Pharmaceutics 2023, 15, 2617. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-D.; Li, S.-L.; Huang, Z.-L.; Zhang, L.; Liu, H.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D. A non-aggregated zinc(II) phthalocyanine with hexadeca cations for antitumor and antibacterial photodynamic therapies. J. Photochem. Photobiol. B Biol. 2020, 213, 112086. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Minute, L.; Teijeira, A.; Sanchez-Paulete, A.R.; Ochoa, M.C.; Alvarez, M.; Otano, I.; Etxeberrria, I.; Bolaños, E.; Azpilikueta, A.; Garasa, S.; et al. Cellular cytotoxicity is a form of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, W.; Jia, L.; Shi, F.; Cao, M.; Sun, J.; Xu, C.; Li, Z.; Cheng, Z.; Zhao, S.-C.; et al. A novel autophagy activator ginsenoside Rh2 enhances the efficacy of immunogenic chemotherapy. Clin. Transl. Med. 2023, 13, e1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kin, T. Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers 2021, 13, 4756. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulos, I.V.; Kakalis, A.; Birmpilis, A.; Angelis, N.; Orologas-Stavrou, N.; Rousakis, P.; Panteli, C.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.-A.; et al. Belantamab mafodotin induces immunogenic cell death within 24 h post-administration in newly diagnosed multiple myeloma patients. Am. J. Hematol. 2023, 98, E65–E67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Jiang, Y.-P.; Zhang, J.-Y.; Tang, X.-Q.; Lou, J.-S.; Huang, X.-Y. Roles of Fascin in Dendritic Cells. Cancers 2023, 15, 3691. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G. Advancing our understanding of monocyte HLA-DR, S100A9, and the potential for individualized therapies in sepsis. Mil. Med. Res. 2023, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, X.; Jin, L.; Lin, L.; Lin, H.; Yang, Z.; Huang, W. Strategic Design of Conquering Hypoxia in Tumor for Advanced Photodynamic Therapy. Adv. Healthc. Mater. 2023, 12, e2300530. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, M.; Sun, S.; Yu, L.; Wen, S.; Liu, Y.; Peng, Z.; Hao, H.; Wang, T.; Wu, M. Mitochondrial-Targeting Nanotrapper Captured Copper Ions to Alleviate Tumor Hypoxia for Amplified Photoimmunotherapy in Breast Cancer. ACS Appl. Mater. Interfaces 2024, 16, 2166–2179. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Chen, G.; Chen, J.-F.; Wang, J.; Deng, S.-H.; Cheng, D. Glutathione-depleting polymer delivering chlorin e6 for enhancing photodynamic therapy. RSC Adv. 2022, 12, 21609–21620. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, K.; Si, Y.; Shan, C.; Guo, H.; Chen, M.; Wu, L. Dendritic organosilica nanospheres with large mesopores as multi-guests vehicle for photoacoustic/ultrasound imaging-guided photodynamic therapy. J. Colloid Interface Sci. 2021, 583, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Zhang, Z.; Xiong, L.; Wang, Y.; Wen, Y. Photodynamic Therapy Combined with Ferroptosis Is a Synergistic Antitumor Therapy Strategy. Cancers 2023, 15, 5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Guo, Y.; Zai, W.; Li, X.; Xiong, W.; Zhao, X.; Yao, Y.; Hu, Y.; Zou, Z.; et al. Photosynthetic microorganisms coupled photodynamic therapy for enhanced antitumor immune effect. Bioact. Mater. 2022, 12, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.; Mao, K.; Wu, C.; Chen, H.; Wang, J.; Wang, X.; Cong, X.; Li, Y.; Meng, X.; et al. Photodynamic therapy produces enhanced efficacy of antitumor immunotherapy by simultaneously inducing intratumoral release of sorafenib. Biomaterials 2020, 240, 119845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ba, J.; Kang, Y.; Gong, Z.; Liang, T.; Zhang, Y.; Qi, J.; Wang, J. Recent Progress in CDK4/6 Inhibitors and PROTACs. Molecules 2023, 28, 8060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Jiao, Y.; Wen, X.; Yang, S.; Zhu, Y. Noninvasive remote-controlled nanomedicine by using electric field stimulation for in vivo effective cancer therapy. J. Biomater. Appl. 2022, 37, 249–258. [Google Scholar] [CrossRef]
- Gu, T.; Chen, T.; Cheng, L.; Li, X.; Han, G.; Liu, Z. Mesoporous silica decorated with platinum nanoparticles for drug delivery and synergistic electrodynamic-chemotherapy. Nano Res. 2020, 13, 2209–2215. [Google Scholar] [CrossRef]
- Huang, K.; Yao, H.; Yan, M.; Zhang, H.; Yuan, G.; Wang, Q.; Xue, J.; Li, J.; Chen, J. A MCL-1-targeted photosensitizer to combat triple-negative breast cancer with enhanced photodynamic efficacy, sensitization to ROS-induced damage, and immune response. J. Inorg. Biochem. 2022, 237, 111997. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chu, Q.; Li, M.; Han, G.; Li, X. Fe3O4@Pt nanoparticles to enable combinational electrodynamic/chemodynamic therapy. J. Nanobio-Technol. 2021, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Zámocká, M.; Majtán, J.; Bauerová-Hlinková, V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12, 472. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Gao, X.; Liu, J.; Zheng, Q.; Li, Y.; Yu, P.; Wang, M.; Mao, L. Biodegradable Metal–Organic-Frameworks-Mediated Protein Delivery Enables Intracellular Cascade Biocatalysis and Pyroptosis In Vivo. ACS Appl. Mater. Interfaces 2022, 14, 47472–47481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Wang, H.; Meng, Z.; Wang, Y.; Miao, W.; Li, X.; Ren, H. Starvation-amplified CO generation for enhanced cancer therapy via an erythrocyte membrane-biomimetic gas nanofactory. Acta Biomater. 2019, 92, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gao, J.; Fang, C.; Zhou, Y.; Li, X.; Han, G. Porous Pt Nanospheres Incorporated with GOx to Enable Synergistic Oxygen-Inductive Starvation/Electrodynamic Tumor Therapy. Adv. Sci. 2020, 7, 2001223. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Huang, J.; Jiang, C.; Zhou, L.; Zheng, M.; Nezamzadeh-Ejhieh, A.; Qi, N.; Lu, C.; Liu, J. A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies. Pharmaceutics 2023, 15, 2071. [Google Scholar] [CrossRef] [PubMed]
- Truong Hoang, Q.; Nguyen Cao, T.G.; Kang, S.J.; Lee, M.; Kang, J.H.; Park, H.S.; Kim, J.-E.; Bhang, S.H.; Ko, Y.T.; Rhee, W.J.; et al. Exosome membrane-sheathed and multi-stimuli-responsive MnO2 nanoparticles with self-oxygenation and energy depletion abilities potentiate the sonodynamic therapy of hypoxic tumors. Chem. Eng. J. 2023, 472, 144871. [Google Scholar] [CrossRef]
- Dai, Z.; Exner, A.A. Sonotheranostics and Sonogenetics Special Issue. Bioconjugate Chem. 2022, 33, 991–992. [Google Scholar] [CrossRef]
- Li, Y.; Lin, L.; Xie, J.; Wei, L.; Xiong, S.; Yu, K.; Zhang, B.; Wang, S.; Li, Z.; Tang, Y.; et al. ROS-Triggered Self-Assembled Nanoparticles Based on a Chemo-Sonodynamic Combinational Therapy Strategy for the Noninvasive Elimination of Hypoxic Tumors. ACS Appl. Mater. Interfaces 2023, 15, 15893–15906. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Hu, D.; Yuan, L.; Yu, Y.; Li, Y.; Qian, Z. Newly developed gas-assisted sonodynamic therapy in cancer treatment. Acta Pharm. Sin. B 2023, 13, 2926–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Yang, H.; Yu, L.; Xu, Y.; Sharma, A.; Yin, P.; Li, X.; Kim, J.S.; Sun, Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021, 50, 11227–11248. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zeng, W.; Tie, C.; Yu, M.; Hao, H.; Deng, Y.; Li, Q.; Zheng, H.; Wu, M.; Mei, L. Engineered gold/black phosphorus nanoplatforms with remodeling tumor microenvironment for sonoactivated catalytic tumor theranostics. Bioact. Mater. 2022, 10, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, L.; Ma, Y.; Liu, Y.; Zhang, Q.; Qin, H.; Chen, Y.; Tian, B.; Dong, J. Peptide-Appended Nanosonosensitizers Targeting Tumor Glycolysis for Synergistic Sonodynamic–Immunometabolic Therapy of Spinal-Metastasized Tumors. Adv. Mater. 2023, 35, e2304246. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qiao, X.; Qiu, L.; Xu, H.; Xiang, H.; Ding, H.; Chen, Y. Engineering Versatile Nanomedicines for Ultrasonic Tumor Immunotherapy. Adv. Sci. 2023, 11, e2305392. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, R.; Huang, Y.; Wang, K.; Li, H.; Bao, Y.; Wu, C.; Zhang, Y.; Tian, X.; Wang, X. Nanosonosensitizer-Augmented Sonodynamic Therapy Combined with Checkpoint Blockade for Cancer Immunotherapy. Int. J. Nanomed. 2021, 16, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Xu, Q.; Zhang, Z.; Wei, Z.; Yong, T.; Bie, N.; Zhang, X.; Li, X.; Li, J.; Gan, L.; et al. Biomimetic sonodynamic therapy-nanovaccine integration platform potentiates Anti-PD-1 therapy in hypoxic tumors. Nano Today 2021, 38, 101195. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Y.; Fang, N.; Jiao, Q.; Zhu, H.-l.; Li, Z. In situ imaging of signaling molecule carbon monoxide in plants with a fluorescent probe. Plant Physiol. 2023, 193, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Luo, Q.; He, Y.; He, Y.; Zhang, X.; Wang, J.; Ni, S.; Gao, D.; Wang, D. Endogenous Nitric Oxide Releases in Situ for RNS/ROS Synergistic Cancer Therapy. Adv. Funct. Mater. 2024, 2314536. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, M.; Liu, J.; Sun, X.; Yang, P.; Qu, F.; Lin, H. Porous Molybdenum Nitride Nanosphere as Carrier-Free and Efficient Nitric Oxide Donor for Synergistic Nitric Oxide and Chemo/Sonodynamic Therapy. ACS Nano 2023, 17, 20098–20111. [Google Scholar] [CrossRef]
- Jiang, F.; Lee, C.; Zhang, W.; Jiang, W.; Cao, Z.; Chong, H.B.; Yang, W.; Zhan, S.; Li, J.; Teng, Y.; et al. Radiodynamic therapy with CsI(na)@MgO nanoparticles and 5-aminolevulinic acid. J. Nanobiotechnol. 2022, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Chan, C.; Duan, X.; Guo, N.; Veroneau, S.S.; Weichselbaum, R.R.; Lin, W. Ultrathin Metal-Organic-Layer Mediated Radiotherapy-Radiodynamic Therapy. Matter 2019, 1, 1331–1353. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, D.; Wilson, B.C. The use of nanomaterials in advancing photodynamic therapy (PDT) for deep-seated tumors and synergy with radiotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1250804. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Krogman, E.; Chawla, Y.; Ting, A. Sensitization of Tumor Cells to IFNy-Mediated Cell Death. J. Immunol. 2023, 210 (Suppl. S1), 245.22. [Google Scholar] [CrossRef]
- Liu, J.; Hu, F.; Wu, M.; Tian, L.; Gong, F.; Zhong, X.; Chen, M.; Liu, Z.; Liu, B. Bioorthogonal Coordination Polymer Nanoparticles with Aggregation-Induced Emission for Deep Tumor-Penetrating Radio- and Radiodynamic Therapy. Adv. Mater. 2021, 33, e2007888. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yang, M.; Liu, P.; Tang, Z.; Yang, Y.; Zhan, M.; Chen, T.; Li, X.; Lu, L. Designing Bimetallic Metal-Organic Framework-Based Heterojunction Radiosensitizer for Enhanced Radiodynamic Therapy and Immunotherapy. Adv. Funct. Mater. 2023, 34, 2312919. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, Y.; Zhou, Y.; Lv, K.; Fu, X.; Gong, J.; Yao, S.; Wang, X.; Feng, J.; Zhang, H. Utilizing dual-pathway energy transfer in upconversion nanoconjugates for reinforced photodynamic therapy. Nano Res. 2024, 17, 2941–2948. [Google Scholar] [CrossRef]
- Li, J.; Lv, Z.; Guo, Y.; Fang, J.; Wang, A.; Feng, Y.; Zhang, Y.; Zhu, J.; Zhao, Z.; Cheng, X.; et al. Hafnium (Hf)-Chelating Porphyrin-Decorated Gold Nanosensitizers for Enhanced Radio–Radiodynamic Therapy of Colon Carcinoma. ACS Nano 2023, 17, 25147–25156. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Guller, A.; Mahbub, S.B.; Goldys, E.M. Oxygen-Carrying Polymer Nanoconstructs for Radiodynamic Therapy of Deep Hypoxic Malignant Tumors. Biomedicines 2021, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lu, N.; Shen, Z.; Tang, W.; Shen, B.; Cui, Z.; Shan, L.; Yang, Z.; Wang, Z.; Jacobson, O.; et al. Generic synthesis of small-sized hollow mesoporous organosilica nanoparticles for oxygen-independent X-ray-activated synergistic therapy. Nat. Commun. 2019, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wu, Y.; Wu, M.; Zhao, Q.; Li, H.; Liu, J.; Liu, X.; Zhang, X.; Zeng, Y. Engineered Red Blood Cell Biomimetic Nanovesicle with Oxygen Self-Supply for Near-Infrared-II Fluorescence-Guided Synergetic Chemo-Photodynamic Therapy against Hypoxic Tumors. ACS Appl. Mater. Interfaces 2021, 13, 52435–52449. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Xie, L.; Wang, G.; Li, J.; Zhang, Z.; Li, B.; Guo, S.; Deng, C.X.; Dai, Y. Oxygen-Enriched Metal-Phenolic X-Ray Nanoprocessor for Cancer Radio-Radiodynamic Therapy in Combination with Checkpoint Blockade Immunotherapy. Adv. Sci. 2020, 8, 2003338. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Jiang, J.; Wan, C.; Pan, G.; Kong, F.; Zhai, R.; Hu, C.; Ying, H. AntiPD-L1 antibody conjugated Au-SPIOs nanoplatform for enhancing radiosensitivity and triggering anti-tumor immune response. Sci. Rep. 2022, 12, 19542. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, J.; Zheng, C. Research Progress on Improving the Efficiency of CDT by Exacerbating Tumor Acidification. Int. J. Nanomed. 2022, 17, 2611–2628. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Duan, X.; Zhang, F.; Ban, X.; Mao, J.; Cao, M.; Han, S.; Shuai, X.; Shen, J. Theranostic Nanomedicine for Synergistic Chemodynamic Therapy and Chemotherapy of Orthotopic Glioma. Adv. Sci. 2020, 7, 2003036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, S.; Zheng, H.; Yang, S.; Zhou, L.; Liu, K.; Zhang, Q.; Zhang, H. Cu-doped cerium oxide-based nanomedicine for tumor microenvironment-stimulative chemo-chemodynamic therapy with minimal side effects. Colloids Surf. B Biointerfaces 2021, 205, 111878. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, Y.; Wu, F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2021, 18, e2103868. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ge, K.; Duan, J.; Du, X.; Zhou, G.; Ma, L.; Gao, S.; Zhang, J. A double-gain theranostic nanoplatform based on self-supplying H2O2 nanocomposites for synergistic chemodynamic/gas therapy. J. Colloid Interface Sci. 2024, 654, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Zhou, S.; Sun, M.; Chen, L.; Wang, J.; Xu, M.; Liu, S.; Liang, K.; Zhang, Q.; et al. Tailored Chemodynamic Nanomedicine Improves Pancreatic Cancer Treatment via Controllable Damaging Neoplastic Cells and Reprogramming Tumor Microenvironment. Nano Lett. 2020, 20, 6780–6790. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An Ultrasound Activated Vesicle of Janus Au-MnO Nanoparticles for Promoted Tumor Penetration and Sono-Chemodynamic Therapy of Orthotopic Liver Cancer. Angew. Chem. Int. Ed. 2019, 59, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Fan, T.; Zhou, L.; Fang, K.; Sun, Y.; Hu, X.; Wang, A.; Li, R.; Zhu, Z.; Dong, C.; et al. A positive self-amplified H2O2 and acidity circulation for boosting CDT-PTT-starvation therapy. J. Control. Release 2023, 354, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Younis, M.R.; Zhang, J.; Qu, J.; Lin, J.; Huang, P. Programmable NIR-II Photothermal-Enhanced Starvation-Primed Chemodynamic Therapy using Glucose Oxidase-Functionalized Ancient Pigment Nanosheets. Small 2020, 16, e2001518. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal–Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2019, 32, e1906024. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent advances in Ti-based MOFs in biomedical applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Pei, Z.; Pei, Y.; James, T.D. Tumor microenvironment-oriented MOFs for chemodynamic therapy. Coord. Chem. Rev. 2023, 484, 215098. [Google Scholar] [CrossRef]
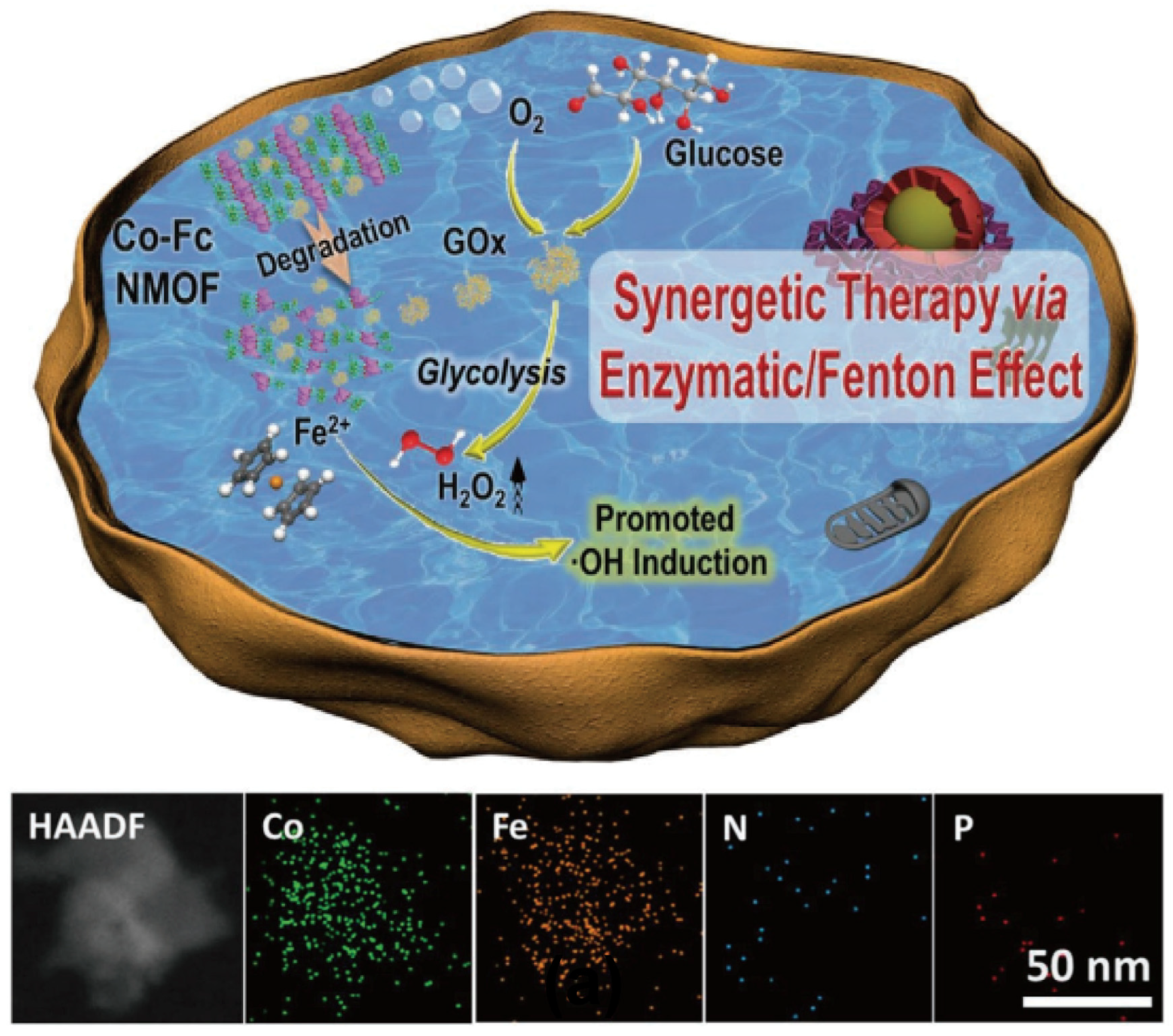
- Fang, C.; Deng, Z.; Cao, G.; Chu, Q.; Wu, Y.; Li, X.; Peng, X.; Han, G. Co–Ferrocene MOF/Glucose Oxidase as Cascade Nanozyme for Effective Tumor Therapy. Adv. Funct. Mater. 2020, 30, 1910085. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Shi, L.; Tang, Q.; Li, B.; Wang, X.; Jin, Y. Nanotrains of DNA Copper Nanoclusters That Triggered a Cascade Fenton-Like Reaction and Glutathione Depletion to Doubly Enhance Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 37280–37290. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Y.; Du, W.; Liu, Z.; Zhang, S.; Yang, J.; Yao, H.; Liu, T.; Ma, M.; Chen, H. Clearable Theranostic Platform with a pH-Independent Chemodynamic Therapy Enhancement Strategy for Synergetic Photothermal Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18133–18144. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M.; Nel, A.E.; Brinker, C.J.; Chan, W.C.W.; Chen, C.; Chen, X.; Ho, D.; Hu, T.; Kataoka, K.; Kotov, N.A. What Do We Mean When We Say Nanomedicine? ACS Nano 2022, 16, 13257–13259. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- Piranfar, A.; Souri, M.; Rahmim, A.; Soltani, M. Localized radiotherapy of solid tumors using radiopharmaceutical loaded implantable system: Insights from a mathematical model. Front. Oncol. 2024, 14, 1320371. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.B.; Banerjee, R. Special Issue: Nanomedicine Advances in Infectious Disease. ACS Biomater. Sci. Eng. 2021, 7, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Collin, B.; Auffan, M.; Doelsch, E.; Proux, O.; Kieffer, I.; Ortet, P.; Santaella, C. Bacterial Metabolites and Particle Size Determine Cerium Oxide Nanomaterial Biotransformation. Environ. Sci. Technol. 2022, 56, 16838–16847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, C.; Lin, X.; Sun, R.; Li, Z.; Chen, S.; Liu, Y.; Wu, J.; Yu, Z.; Liu, X. Tunable Nanoparticles with Aggregation-Induced Emission Heater for Precise Synergistic Photothermal and Thermodynamic Oral Cancer Therapy of Patient-Derived Tumor Xenograft. Adv. Sci. 2023, 10, 2205780. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Lee, M.; Mohapatra, A.; Lee, H.; Vasukutty, A.; Baek, S.; Lee, J.Y.; Park, I.-K. “Three-in-one”: A Photoactivable Nanoplatform Evokes Anti-Immune Response by Inhibiting BRD4-cMYC-PDL1 Axis to Intensify Photo-Immunotherapy. Adv. Healthc. Mater. 2024, e2304293. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, H.; Wu, J.; Guo, H.; Chen, X. Dissecting the pleiotropic roles of reactive oxygen species (ROS) in lung cancer: From carcinogenesis toward therapy. Med. Res. Rev. 2024, 1–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Fang, X.; Miao, B.; Wang, Y.; Liu, J.; Nie, G.; Zhang, B. Copper decorated Ti3C2 nanosystem with NIR-II-induced GSH-depletion and reactive oxygen species generation for efficient nanodynamic therapy. Nanophotonics 2022, 11, 5189–5204. [Google Scholar] [CrossRef]
- Chen, H.; Yao, Y.; Zhao, X.; Tan, N. Programmable site-specific delivery of an alkaline phosphatase-activatable prodrug and a mitochondria-targeted cyclopeptide for combination therapy in colon cancer. Biomater. Sci. 2023, 11, 7114–7123. [Google Scholar] [CrossRef] [PubMed]





| Nanosensitizers | Nanodynamic Therapy (NDT) | Therapeutic Properties | Ref. |
|---|---|---|---|
| Ce6/R848 | PDT | Ce6-mediated PDT releases tumor antigens, delivers R848-activated TLR7/8 into tumors, and enhances anti-tumor immune responses. | [30] |
| NanoLuc-miniSOG | PDT | Gene encoding activates PDT to generate ROS for treating deep-seated tumors. | [31] |
| IRCB@M | PDT | Reducing oxygen consumption, blocking GSH, preventing the depletion of ROS, and enhancing the therapeutic efficacy of PDT. | [32] |
| PROTAC/Ce6 | PDT | The ROS generated by PDT induce ICD, triggering an anti-tumor immune response. | [33] |
| KCCP | EDT | In the presence of chlorine, the electric field activates Pt NPs and Ca2+ to produce ROS, enhancing tumor suppression. | [34] |
| Pt-Pd@DON | EDT | EDT combined with immunotherapy synergistically suppresses tumor growth. | [35] |
| PtMnIr | EDT | Nanocatalysts in combination with EDT continuously generate ROS while depleting GSH, promoting ferroptosis and apoptosis in tumor cells. | [36] |
| DOX@pPt-PEG | EDT | ROS produced by the combination of EDT and chemotherapy can induce the inhibition of P-gp, thereby synergistically suppressing tumor growth. | [37] |
| Pt-Cy | SDT | Under US irradiation, 1O2 is generated, inducing ferroptosis and inhibiting tumor growth. | [38] |
| Cu(II)NS | SDT | Overexpressed GSH in the TME reduces Cu2+ to Cu+, enabling the production of ROS in SDT. | [39] |
| C-dots MBs | SDT | Following US irradiation, the generation of ROS enhances tumor therapy. | [40] |
| ZrO2−x@Pt | SDT | With the aid of immune checkpoint blockade, sonodynamic-thermotherapy is combined for the treatment of tumors. | [41] |
| Hf-DBP-Pt | RDT | ROS generation via RT-RDT remodels the TME, facilitating neutrophil-mediated tumor regression. | [42] |
| AuNC@DHLA | RDT | Without the need for additional scintillators or photosensitizers, excessive ROS production under hypoxic conditions leads to significant therapeutic effects on solid tumors in vivo. | [43] |
| Hb@HP(Hf) | RDT | Massive oxygen delivery effectively alleviates hypoxia in the TME, thereby greatly enhancing the tumor treatment efficiency of RT-RDT. | [44] |
| MCBR | CDT | The Fenton-like reaction generates •OH, synergizing photothermal therapy, CDT, and immunotherapy for anti-tumor activity. | [45] |
| PLGA-SPIO& Vc | CDT | CDT combined with SDT, where Vc decomposes into H2O2, creates favorable conditions for the Fenton-like reaction, enabling continuous generation of ROS, thereby enhancing the anti-tumor effect. | [46] |
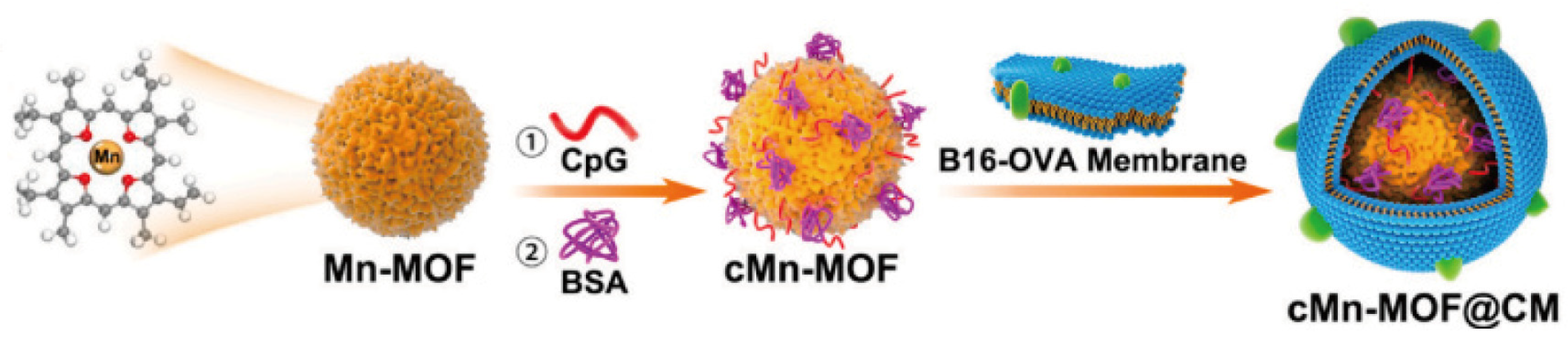
| NMOF ZIF-8 | CDT | The precise and efficient combination of gene and CDT significantly demonstrates tumor eradication effects. | [47] |
| Nanomedicines | Chemical Formula | Chemical Formula | Ref. |
|---|---|---|---|
| Chlorin e6 | C33H36N4O6 | 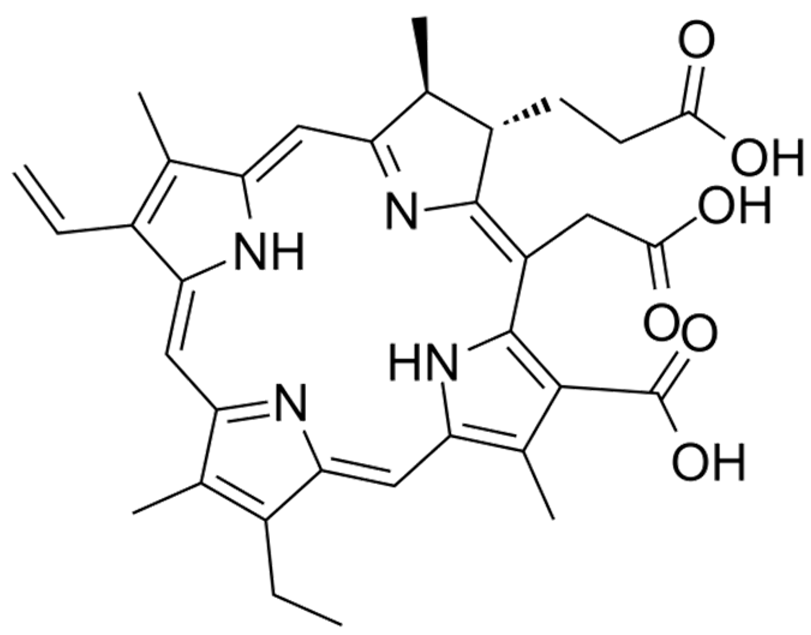 | [68] |
| IR-780 | C36H44ClIN2 | 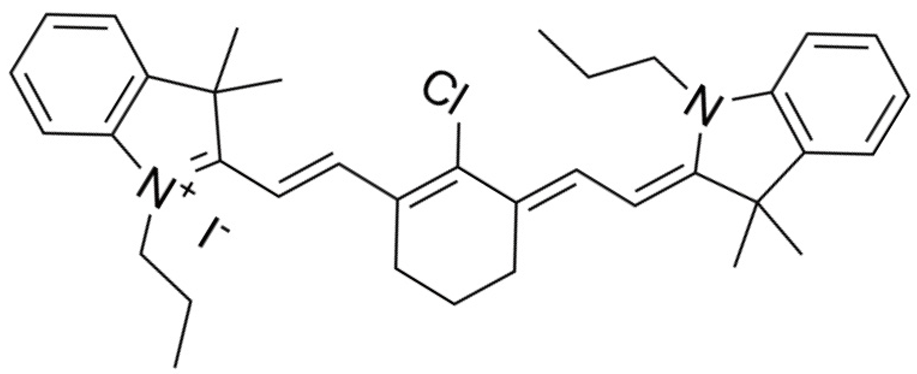 | [32] |
| Sorafenib | C21H16ClF3N4O3 | 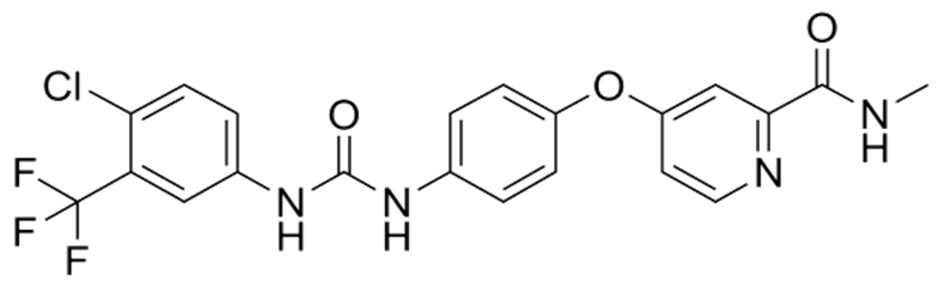 | [68] |
| DOX | C27H30ClNO11 | 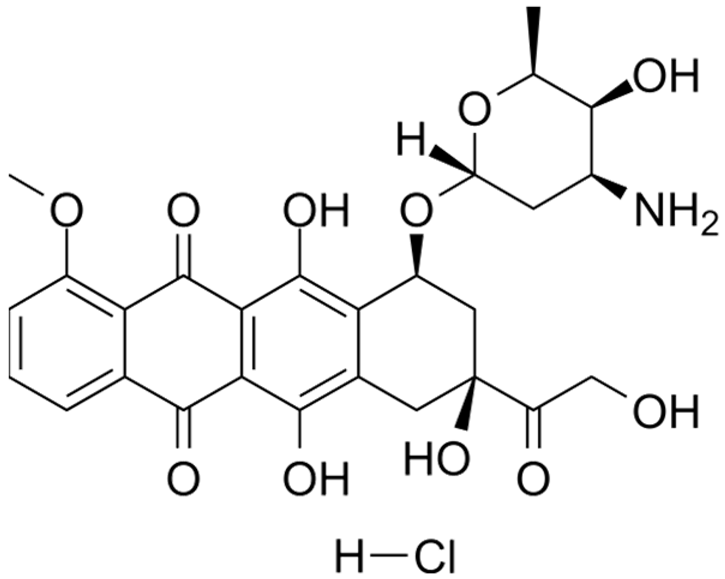 | [71] |
| AIPH | C12H24Cl2N6 |  | [41] |
| Verteporfin | C82H84N8O16 | 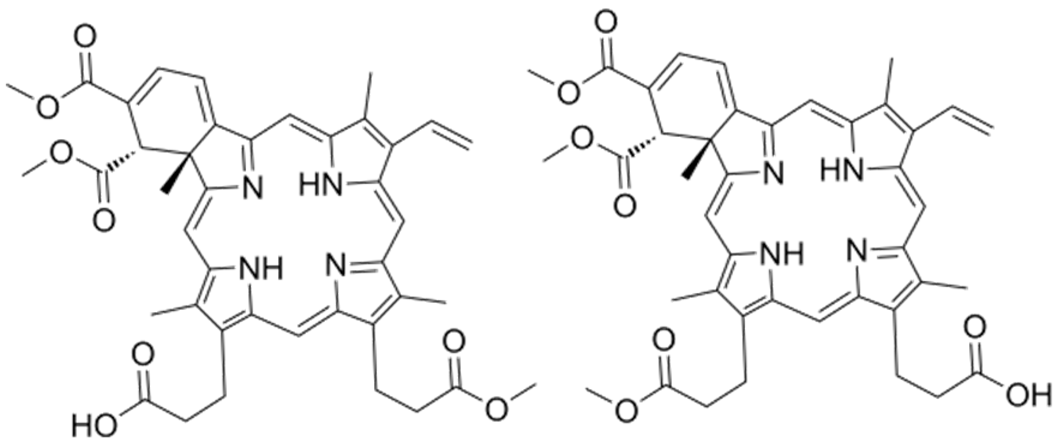 | [100] |
| Tetrakis(4-carboxyphenyl) porphyrin | C48H30N4O8 | 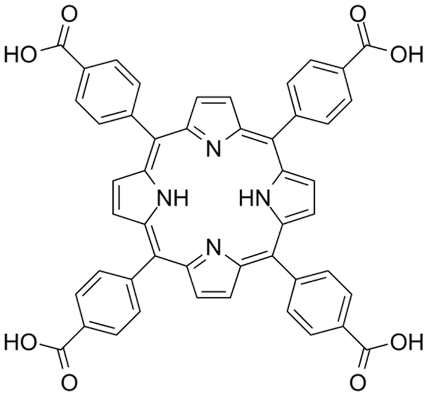 | [44] |
| DON | C6H9N3O3 | 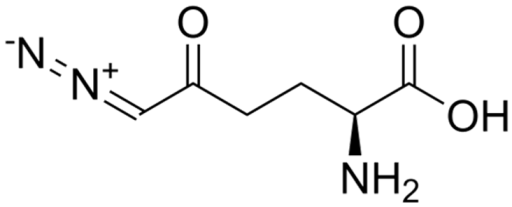 | [35] |
| Cisplatin | Cl2H6N2Pt | 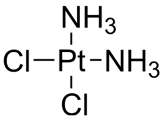 | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Huang, Y.; Huang, Y. Advances in Nanodynamic Therapy for Cancer Treatment. Nanomaterials 2024, 14, 648. https://doi.org/10.3390/nano14070648
Zhang B, Huang Y, Huang Y. Advances in Nanodynamic Therapy for Cancer Treatment. Nanomaterials. 2024; 14(7):648. https://doi.org/10.3390/nano14070648
Chicago/Turabian StyleZhang, Bingchang, Yan Huang, and Yong Huang. 2024. "Advances in Nanodynamic Therapy for Cancer Treatment" Nanomaterials 14, no. 7: 648. https://doi.org/10.3390/nano14070648
APA StyleZhang, B., Huang, Y., & Huang, Y. (2024). Advances in Nanodynamic Therapy for Cancer Treatment. Nanomaterials, 14(7), 648. https://doi.org/10.3390/nano14070648





