Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease
Abstract
1. Introduction
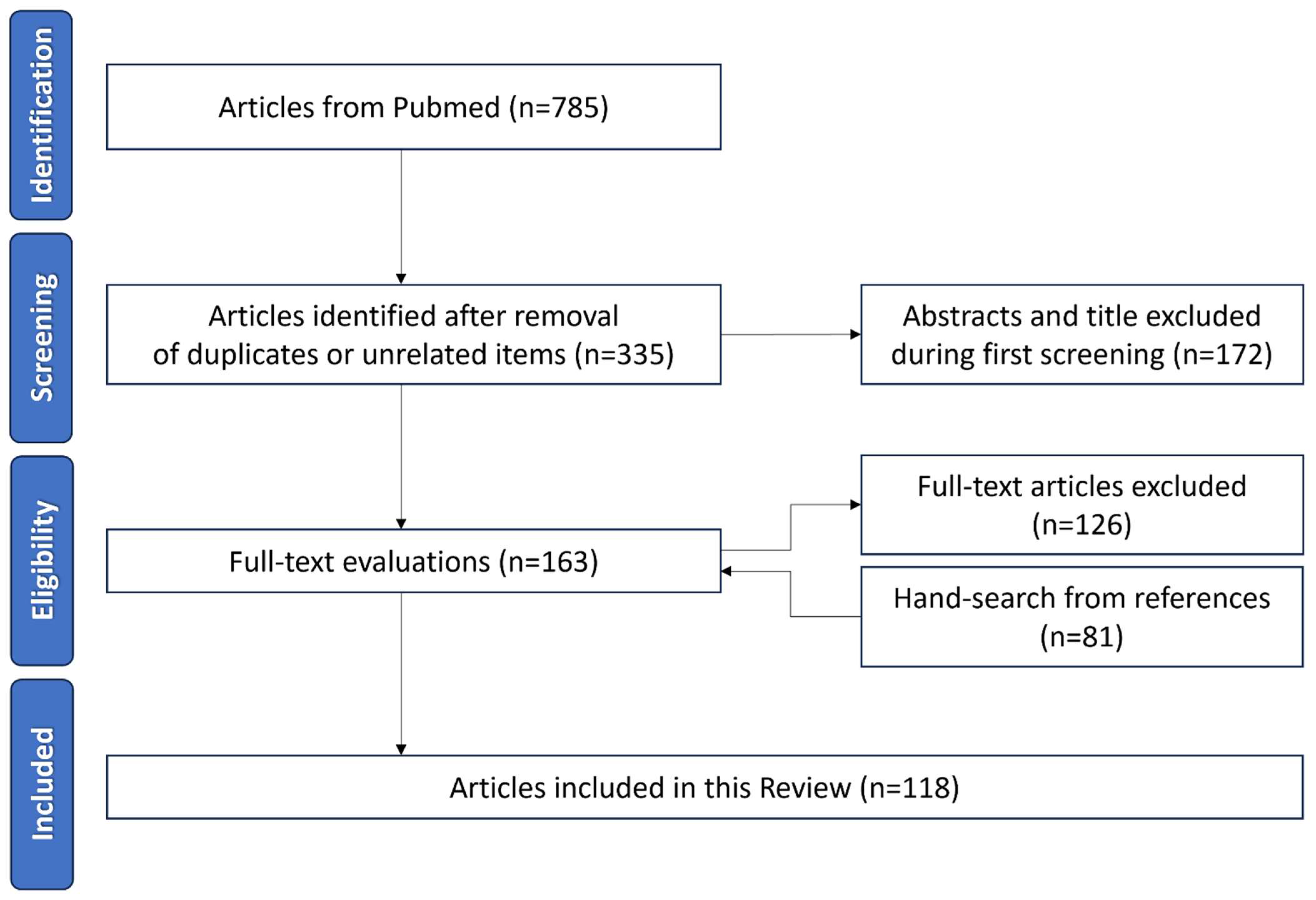
2. Methods
3. Ocular Surface Drugs Barriers
3.1. Tear Film Barrier
3.2. Corneal Barrier
4. Properties of Nanoformulations
5. Nano-Based Drug Delivery Systems in Dry Eye Disease
5.1. Nanoemulsions
5.2. Nanomicelles and Polymeric Micelles
5.3. Nanosuspensions
5.4. Liposomes, Niosomes, and Cubosomes
5.4.1. Liposomes
5.4.2. Niosomes
5.4.3. Cubosomes
5.5. Polymeric Nanoparticles, Solid Lipid Nanoparticles, and Nanostructured Lipid Carriers
5.5.1. Polymeric Nanoparticles
5.5.2. Solid Lipid Nanocapsules and Nanostructured Lipid Nanoparticles
5.6. Nanowafers
5.7. Dendrimers
5.8. In Situ Hydrogels
5.9. Drug-Eluting Contact Lenses
5.10. Nanogels
5.11. Nanozymes
6. Nanotechnologies Currently Approved for Dry Eye Disease
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Al-Saedi, Z.; Zimmerman, A.; Bachu, R.D.; Dey, S.; Shah, Z.; Baugh, R.; Boddu, S.H.S. Dry Eye Disease: Present Challenges in the Management and Future Trends. Curr. Pharm. Des. 2016, 22, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-Del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the Vicious Circle of Dry Eye Disease: A Focus on the Pathophysiology of Meibomian Gland Dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, S.; Zeng, G.; Yao, K.; Han, H. Recent Progress of Nanomedicine in Managing Dry Eye Disease. Adv. Ophthalmol. Pract. Res. 2024, 4, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Benítez-del-Castillo, J.; Labetoulle, M.; Baudouin, C.; Rolando, M.; Akova, Y.A.; Aragona, P.; Geerling, G.; Merayo-Lloves, J.; Messmer, E.M.; Boboridis, K. Visual Acuity and Quality of Life in Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.J. Assessment and Management of Dry Eye Disease. Eye 2018, 32, 200. [Google Scholar] [CrossRef]
- Dogru, M.; Nakamura, M.; Shimazaki, J.; Tsubota, K. Changing Trends in the Treatment of Dry-Eye Disease. Expert. Opin. Investig. Drugs 2013, 22, 1581–1601. [Google Scholar] [CrossRef]
- Lu, Q.; Al-Sheikh, O.; Elisseeff, J.H.; Grant, M.P. Biomaterials and Tissue Engineering Strategies for Conjunctival Reconstruction and Dry Eye Treatment. Middle East. Afr. J. Ophthalmol. 2015, 22, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Yang, C.-q.; Sun, W.; Gu, Y.-s. A Clinical Study of the Efficacy of Topical Corticosteroids on Dry Eye. J. Zhejiang Univ. Sci. B 2006, 7, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-Induced Glaucoma: A Review of the Literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous Serum Eye Drops for Dry Eye. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Coco, G.; Ambrosini, G.; Poletti, S.; Meliante, L.A.; Taloni, A.; Scorcia, V.; Giannaccare, G. Recent Advances in Drug Treatments for Dry Eye Disease. Expert. Opin. Pharmacother. 2023, 24, 2059–2079. [Google Scholar] [CrossRef] [PubMed]
- Ako-Adounvo, A.-M.; Nagarwal, R.C.; Oliveira, L.; Boddu, S.H.S.; Wang, X.S.; Dey, S.; Karla, P.K. Recent Patents on Ophthalmic Nanoformulations and Therapeutic Implications. Recent. Pat. Drug Deliv. Formul. 2014, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.; Singh, V.; Basu, S.; Singh, S. Biomaterials for Dry Eye Disease Treatment: Current Overview and Future Perspectives. Exp. Eye Res. 2023, 226, 109339. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current Trends in Pharmaceutical Treatment of Dry Eye Disease: A Review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Nanotechnology in Ophthalmology. Can. J. Ophthalmol. 2010, 45, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Maltese, A.; Drago, F. When Nanotechnology Meets the Ocular Surface. Expert. Rev. Ophthalmol. 2008, 3, 325–332. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Zhang, Y. Advance of the Application of Nano-Controlled Release System in Ophthalmic Drug Delivery. Drug Deliv. 2016, 23, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Coco, G.; Taloni, A.; Scorcia, V.; Giannaccare, G. The Vicious Cycle of Dry Eye Disease: A Look into Promising Novel Drug Therapies. Expert. Rev. Ophthalmol. 2023, 18, 235–247. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent Design to Treat Complex Disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef]
- Lakkireddy, H.R.; Bazile, D.V. Nano-Carriers for Drug Routeing—Towards a New Era. J. Drug Target. 2019, 27, 525–541. [Google Scholar] [CrossRef]
- Kamaleddin, M.A. Nano-Ophthalmology: Applications and Considerations. Nanomedicine 2017, 13, 1459–1472. [Google Scholar] [CrossRef]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Lohiya, G.; Katti, D.S. Fundamentals, Challenges, and Nanomedicine-Based Solutions for Ocular Diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1548. [Google Scholar] [CrossRef]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations. AAPS PharmSciTech 2021, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- McCulley, J.P.; Shine, W.E. Meibomian Gland Function and the Tear Lipid Layer. Ocul. Surf. 2003, 1, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Fukuoka, S.; Morishige, N. Functional Morphology of the Lipid Layer of the Tear Film. Cornea 2017, 36 (Suppl. S1), S60–S66. [Google Scholar] [CrossRef] [PubMed]
- Cwiklik, L. Tear Film Lipid Layer: A Molecular Level View. Biochim. Biophys. Acta 2016, 1858, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Dey, M.; Dixit, H.N.; Feng, J.J. Tear-Film Breakup: The Role of Membrane-Associated Mucin Polymers. Phys. Rev. E 2021, 103, 013108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. Tear Analysis in Ocular Surface Diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target Strategies for Drug Delivery Bypassing Ocular Barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Ruponen, M.; Urtti, A. Undefined Role of Mucus as a Barrier in Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 96, 442–446. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical Biochemistry of Tears. Surv. Ophthalmol. 1981, 26, 84–96. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological Functions of Tear Film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, L.C.; Pillay, V.; Choonara, Y.E.; Govender, T.; Carmichael, T. Ocular Drug Delivery—A Look towards Nanobioadhesives. Expert. Opin. Drug Deliv. 2011, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Van Santvliet, L.; Ludwig, A. Determinants of Eye Drop Size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Chow, J.; Liu, J. Focus: Sensory Biology and Pain: Corneal Innervation and Sensation: The Eye and Beyond. Yale J. Biol. Med. 2018, 91, 13. [Google Scholar]
- Ramos, T.; Scott, D.; Ahmad, S. An Update on Ocular Surface Epithelial Stem Cells: Cornea and Conjunctiva. Stem Cells Int. 2015, 2015, 601731. [Google Scholar] [CrossRef]
- Ramsay, E.; Ruponen, M.; Picardat, T.; Tengvall, U.; Tuomainen, M.; Auriola, S.; Toropainen, E.; Urtti, A.; del Amo, E.M. Impact of Chemical Structure on Conjunctival Drug Permeability: Adopting Porcine Conjunctiva and Cassette Dosing for Construction of In Silico Model. J. Pharm. Sci. 2017, 106, 2463–2471. [Google Scholar] [CrossRef]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Del Pozo Rodriguez, A.; Solinis, M.A. Application of Lipid Nanoparticles to Ocular Drug Delivery. Expert. Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Ünlü, N. Development and Characterization of Cyclosporine A Loaded Nanoparticles for Ocular Drug Delivery: Cellular Toxicity, Uptake, and Kinetic Studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-Penetrating Nanoparticles for Drug and Gene Delivery to Mucosal Tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, H.; Hong, J.; Chang, M.; Kim, M.; Chuck, R.S.; Lee, J.K.; Park, C.Y. The Effect of Silica Nanoparticles on Human Corneal Epithelial Cells. Sci. Rep. 2016, 6, 37762. [Google Scholar] [CrossRef] [PubMed]
- Toropainen, E.; Fraser-Miller, S.J.; Novakovic, D.; Del Amo, E.M.; Vellonen, K.S.; Ruponen, M.; Viitala, T.; Korhonen, O.; Auriola, S.; Hellinen, L.; et al. Biopharmaceutics of Topical Ophthalmic Suspensions: Importance of Viscosity and Particle Size in Ocular Absorption of Indomethacin. Pharmaceutics 2021, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Rojanasakul, Y.; Robinson, J.R. Transport Mechanisms of the Cornea: Characterization of Barrier Permselectivity. Int. J. Pharm. 1989, 55, 237–246. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and Mucoadhesive in Situ Ocular Gel for Effective Local Delivery and Antifungal Activity of Itraconazole Nanocrystal in the Treatment of Fungal Keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Han, H.; Gao, Y.; Chai, M.; Zhang, X.; Liu, S.; Huang, Y.; Jin, Q.; Grzybowski, A.; Ji, J.; Yao, K. Biofilm Microenvironment Activated Supramolecular Nanoparticles for Enhanced Photodynamic Therapy of Bacterial Keratitis. J. Control. Release 2020, 327, 676–687. [Google Scholar] [CrossRef]
- Jindal, A.B. The Effect of Particle Shape on Cellular Interaction and Drug Delivery Applications of Micro- and Nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Sun, Y.N.; Wang, C.D.; Zhang, X.M.; Ren, L.; Tian, X.H. Shape Dependence of Gold Nanoparticles on in Vivo Acute Toxicological Effects and Biodistribution. J. Nanosci. Nanotechnol. 2011, 11, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kröger, M.; Liu, W.K. Shape Effect in Cellular Uptake of PEGylated Nanoparticles: Comparison between Sphere, Rod, Cube and Disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef]
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle Drug Loading as a Design Parameter to Improve Docetaxel Pharmacokinetics and Efficacy. Biomaterials 2013, 34, 8424–8429. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-Responsive Liposomes for Biomedical Applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yin, L.; Zhang, Y.; Yu, W.; Wang, Q.; Zhan, Z. Preparation and Study of Two Kinds of Ophthalmic Nano-Preparations of Everolimus. Drug Deliv. 2019, 26, 1235. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and Chemical Stability of Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for Drug Delivery to the Anterior Segment of the Eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yasueda, S.I.; Isowaki, A.; Yamamoto, M.; Kimura, M.; Inada, K.; Ohtori, A. Formulation of an Ophthalmic Lipid Emulsion Containing an Anti-Inflammatory Steroidal Drug, Difluprednate. Int. J. Pharm. 2005, 301, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Alany, R.G.; Rades, T.; Nicoll, J.; Tucker, I.G.; Davies, N.M. W/O Microemulsions for Ocular Delivery: Evaluation of Ocular Irritation and Precorneal Retention. J. Control. Release 2006, 111, 145–152. [Google Scholar] [CrossRef] [PubMed]
- El Tayar, N.; Mark, A.E.; Vallat, P.; Brunne, R.M.; Testa, B.; van Gunsteren, W.F. Solvent-Dependent Conformation and Hydrogen-Bonding Capacity of Cyclosporin A: Evidence from Partition Coefficients and Molecular Dynamics Simulations. J. Med. Chem. 1993, 36, 3757–3764. [Google Scholar] [CrossRef]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D. Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef]
- Bose, A.; Burman, D.R.; Sikdar, B.; Patra, P. Nanomicelles: Types, Properties and Applications in Drug Delivery. IET Nanobiotechnol. 2021, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, A.; Dutt Vadlapudi, A.; Mitra, K.A. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent. Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Nagai, N.; Otake, H. Novel Drug Delivery Systems for the Management of Dry Eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Li, Y.; Yang, W.; Tu, J.; Shen, Y. Improving the Topical Ocular Pharmacokinetics of Lyophilized Cyclosporine A-Loaded Micelles: Formulation, in Vitro and in Vivo Studies. Drug Deliv. 2018, 25, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Pınar, S.G.; Oktay, A.N.; Karaküçük, A.E.; Çelebi, N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics 2023, 15, 1520. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K. Eudragit, a Nifty Polymer for Anticancer Preparations: A Patent Review. Recent. Pat. Anticancer. Drug Discov. 2022, 17, 92–101. [Google Scholar] [CrossRef]
- Wu, X.G.; Xin, M.; Yang, L.N.; Shi, W.Y. The Biological Characteristics and Pharmacodynamics of a Mycophenolate Mofetil Nanosuspension Ophthalmic Delivery System in Rabbits. J. Pharm. Sci. 2011, 100, 1350–1361. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as Vehicles for Topical Ophthalmic Drug Delivery and Ocular Surface Protection. Expert. Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.P.; Singh, S.; Thacker, M.; Pati, F.; Vemuganti, G.K.; Basu, S.; Singh, V. Newer Approaches to Dry Eye Therapy: Nanotechnology, Regenerative Medicine, and Tissue Engineering. Indian J. Ophthalmol. 2023, 71, 1292. [Google Scholar] [CrossRef] [PubMed]
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Garg, A.; Singla, A.K.; Aggarwal, D. Vesicular Systems in Ocular Drug Delivery: An Overview. Int. J. Pharm. 2004, 269, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, B.K.; Holzchuh, R.; Chiacchio, B.B.; Santo, R.M.; Shimazaki, J.; Hida, R.Y. Clinical Treatment of Dry Eye Using 0.03% Tacrolimus Eye Drops. Cornea 2012, 31, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y.; et al. Hyaluronic Acid-Coated Niosomes Facilitate Tacrolimus Ocular Delivery: Mucoadhesion, Precorneal Retention, Aqueous Humor Pharmacokinetics, and Transcorneal Permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef]
- Başaran, E.; Yenilmez, E.; Berkman, M.S.; Büyükköroǧlu, G.; Yazan, Y. Chitosan Nanoparticles for Ocular Delivery of Cyclosporine A. J. Microencapsul. 2014, 31, 49–57. [Google Scholar] [CrossRef]
- Modi, D.; Mohammad; Warsi, M.H.; Garg, V.; Bhatia, M.; Kesharwani, P.; Jain, G.K. Formulation Development, Optimization, and in Vitro Assessment of Thermoresponsive Ophthalmic Pluronic F127-Chitosan in Situ Tacrolimus Gel. J. Biomater. Sci. Polym. Ed. 2021, 32, 1678–1702. [Google Scholar] [CrossRef]
- Modi, D.; Nirmal, J.; Warsi, M.H.; Bhatia, M.; Hasan, N.; Kesharwani, P.; Jain, G.K. Formulation and Development of Tacrolimus-Gellan Gum Nanoformulation for Treatment of Dry Eye Disease. Colloids Surf. B Biointerfaces 2022, 211, 112255. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L. Feasibility of Lipid Nanoparticles for Ocular Delivery of Anti-Inflammatory Drugs. Curr. Eye Res. 2010, 35, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Carbone, C.; Puglia, C.; Offerta, A.; Bonina, F.P.; Puglisi, G. Ophthalmic Applications of Lipid-Based Drug Nanocarriers: An Update of Research and Patenting Activity. Ther. Deliv. 2015, 6, 1297–1318. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of Drug Safety by the Use of Lipid-Based Nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Deng, Y.; Jin, X.; Ping, Q.; Su, Z.; Li, L. Thiolated Nanostructured Lipid Carriers as a Potential Ocular Drug Delivery System for Cyclosporine A: Improving in Vivo Ocular Distribution. Int. J. Pharm. 2010, 402, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Niamprem, P.; Teapavarapruk, P.; Srinivas, S.P.; Tiyaboonchai, W. Impact of Nanostructured Lipid Carriers as an Artificial Tear Film in a Rabbit Evaporative Dry Eye Model. Cornea 2019, 38, 485–491. [Google Scholar] [CrossRef]
- Yu, F.; Zheng, M.; Zhang, A.Y.; Han, Z. A Cerium Oxide Loaded Glycol Chitosan Nano-System for the Treatment of Dry Eye Disease. J. Control. Release 2019, 315, 40–54. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, R.; Ran, M.; Deng, Y.; Ge, Y.; Zhu, Y.; Tao, X.; Shang, L.; Gou, J.; He, H.; et al. A Novel Eyes Topical Drug Delivery System: CsA-LNC for the Treatment of DED. Pharm. Res. 2020, 37, 146. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; de Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.; Yu, C.; Wilmoth, A.H.; Chen, M.; Sharma, A.; Yiu, S. Dendrimers in Corneal Drug Delivery: Recent Developments and Translational Opportunities. Pharmaceutics 2023, 15, 1591. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(Amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Kaga, S.; Arslan, M.; Sanyal, R.; Sanyal, A. Dendrimers and Dendrons as Versatile Building Blocks for the Fabrication of Functional Hydrogels. Molecules 2016, 21, 497. [Google Scholar] [CrossRef] [PubMed]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival Injectable Dendrimer-Dexamethasone Gel for the Treatment of Corneal Inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, L.; Shi, H.; Xu, C.; Liu, M.; Li, Q.; Zheng, L.; Chi, H.; Wang, M.; Liu, Z.; et al. Effectiveness of an Ocular Adhesive Polyhedral Oligomeric Silsesquioxane Hybrid Thermo-Responsive FK506 Hydrogel in a Murine Model of Dry Eye. Bioact. Mater. 2022, 9, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chow, D.W.Y.; Lau, C.M.L.; Zhou, G.; Back, W.; Xu, J.; Carim, S.; Chau, Y. A Bioinspired Synthetic Soft Hydrogel for the Treatment of Dry Eye. Bioeng. Transl. Med. 2021, 6, e10227. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact Lenses: Promising Devices for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended Release of Hyaluronic Acid from Hydrogel Contact Lenses for Dry Eye Syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Li, Y.; Jin, R.; Shrestha, T.; Choi, J.S.; Lee, W.J.; Moon, M.J.; Ju, H.T.; Choi, W.; Yoon, K.C. The Efficiency of Cyclosporine A-Eluting Contact Lenses for the Treatment of Dry Eye. Curr. Eye Res. 2019, 44, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Fonseca, B.; Carracedo, G.; Martin-Gil, A.; Martinez-Aguila, A.; Pintor, J. Dry Eye Treatment Based on Contact Lens Drug Delivery: A Review. Eye Contact Lens 2016, 42, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tao, Q.; Xie, J.; Lu, L.; Xie, X.; Zhang, Y.; Jin, Y. Advances in Nanogels for Topical Drug Delivery in Ocular Diseases. Gels 2023, 9, 292. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Lin, P.H.; Jian, H.J.; Li, Y.J.; Huang, Y.F.; Anand, A.; Huang, C.C.; Lin, H.J.; Lai, J.Y. Alleviation of Dry Eye Syndrome with One Dose of Antioxidant, Anti-Inflammatory, and Mucoadhesive Lysine-Carbonized Nanogels. Acta Biomater. 2022, 141, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Zhao, M.; Rong, S.; Jhe, W.; Cai, X.; Xiao, Y.; Zhang, W.; Geng, X.; Li, Z.; Zhang, X.; et al. Dual-Atom Nanozyme Eye Drops Attenuate Inflammation and Break the Vicious Cycle in Dry Eye Disease. Nanomicro Lett. 2024, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, H.; Xu, B.; Liang, L.; Shen, L.; Lin, Q. Regenerative Cerium Oxide Nanozymes Alleviate Oxidative Stress for Efficient Dry Eye Disease Treatment. Regen. Biomater. 2022, 9, rbac070. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and Nanotechnology in Drug Development: Focus on Ocular Targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, N.; Yalavarthi, P.; Vadlamudi, H.; Thanniru, J.; Yaga, G.; Haritha, K. Cubosomes as Targeted Drug Delivery Systems—A Biopharmaceutical Approach. Curr. Drug Discov. Technol. 2014, 11, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone Nanowafer as an Effective Therapy for Dry Eye Disease. J. Control. Release 2015, 213, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G.; Yang, H. Dendrimers for Ocular Drug Delivery. Can. J. Chem. 2017, 95, 897. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Wang, P. Recent Advances in Hydrogels for the Diagnosis and Treatment of Dry Eye Disease. Gels 2022, 8, 816. [Google Scholar] [CrossRef]
- Ames, P.; Galor, A. Cyclosporine Ophthalmic Emulsions for the Treatment of Dry Eye: A Review of the Clinical Evidence. Clin. Investig. 2015, 5, 267–285. [Google Scholar] [CrossRef]
- Silva-Cunha, A.; Da Silva, G.R.; De Castro, W.V.; Fialho, S.L. Evaluation of the Pharmacokinetics and Ocular Tolerance of a Microemulsion Containing Tacrolimus. J. Ocul. Pharmacol. Ther. 2014, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Dixon, P.; Sekar, P.; Chauhan, A. Incorporation of Drug Particles for Extended Release of Cyclosporine A from Poly-Hydroxyethyl Methacrylate Hydrogels. Eur. J. Pharm. Biopharm. 2017, 120, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two Multicenter Randomized Studies of the Efficacy and Safety of Cyclosporine Ophthalmic Emulsion in Moderate to Severe Dry Eye Disease. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Kunert, K.S.; Tisdale, A.S.; Gipson, I.K. Goblet Cell Numbers and Epithelial Proliferation in the Conjunctiva of Patients with Dry Eye Syndrome Treated with Cyclosporine. Arch. Ophthalmol. 2002, 120, 330–337. [Google Scholar] [CrossRef]
- Daull, P.; Lallemand, F.; Garrigue, J.S. Benefits of Cetalkonium Chloride Cationic Oil-in-Water Nanoemulsions for Topical Ophthalmic Drug Delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef]
- Baudouin, C.; De La Maza, M.S.; Amrane, M.; Garrigue, J.S.; Ismail, D.; Figueiredo, F.C.; Leonardi, A. One-Year Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease. Eur. J. Ophthalmol. 2017, 27, 678–685. [Google Scholar] [CrossRef]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef] [PubMed]
- Buggage, R.R.; Amrane, M.; Ismail, D.; Deniaud, M.; Lemp, M.A.; Baudouin, C. The Effect of Cyclokat® (Preservative-Free Cyclosporine 0.1% Cationic Emulsion) on Dry Eye Disease Signs and Symptoms in Sjogren and Non-Sjogren Patients with Moderate to Severe DED in a Phase III Randomized Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2012, 53, 576. [Google Scholar]
- Hoy, S.M. Ciclosporin Ophthalmic Emulsion 0.1%: A Review in Severe Dry Eye Disease. Drugs 2017, 77, 1909–1916. [Google Scholar] [CrossRef]
- Luschmann, C.; Herrmann, W.; Strauß, O.; Luschmann, K.; Goepferich, A. Ocular Delivery Systems for Poorly Soluble Drugs: An in-Vivo Evaluation. Int. J. Pharm. 2013, 455, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.; Kannarr, S.; Luchs, J.; Malhotra, R.; Justice, A.; Ogundele, A.; Darby, C.; Bacharach, J. Efficacy and Safety of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, for the Treatment of Keratoconjunctivitis Sicca: Pooled Analysis of a Phase 2b/3 and Phase 3 Study. Eye Contact Lens 2020, 46 (Suppl. 1), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Kramer, W.G. Ocular Distribution of Cyclosporine Following Topical Administration of OTX-101 in New Zealand White Rabbits. J. Ocul. Pharmacol. Ther. 2019, 35, 395–402. [Google Scholar] [CrossRef]
- Tauber, J.; Schechter, B.A.; Bacharach, J.; Toyos, M.M.; Smyth-Medina, R.; Weiss, S.L.; Luchs, J.I. A Phase II/III, Randomized, Double-Masked, Vehicle-Controlled, Dose-Ranging Study of the Safety and Efficacy of OTX-101 in the Treatment of Dry Eye Disease. Clin. Ophthalmol. 2018, 12, 1921–1929. [Google Scholar] [CrossRef]
- Craig, J.P.; Purslow, C.; Murphy, P.J.; Wolffsohn, J.S.W. Effect of a Liposomal Spray on the Pre-Ocular Tear Film. Cont. Lens Anterior Eye 2010, 33, 83–87. [Google Scholar] [CrossRef]
- Pult, H.; Gill, F.; Riede-Pult, B.H. Effect of Three Different Liposomal Eye Sprays on Ocular Comfort and Tear Film. Cont. Lens Anterior Eye 2012, 35, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, Y.P.; Shin, Y.J. Vitamin D Enhances the Efficacy of Topical Artificial Tears in Patients with Dry Eye Disease. Cornea 2019, 38, 304–310. [Google Scholar] [CrossRef]
- Fogagnolo, P.; De Cilla, S.; Alkabes, M.; Sabella, P.; Rossetti, L. A Review of Topical and Systemic Vitamin Supplementation in Ocular Surface Diseases. Nutrients 2021, 13, 1998. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Bian, Y.; Gupta, P.K. Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease. Clin. Ophthalmol. 2022, 16, 349–355. [Google Scholar] [CrossRef]
- Korenfeld, M.; Nichols, K.K.; Goldberg, D.; Evans, D.; Sall, K.; Foulks, G.; Coultas, S.; Brazzell, K. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients with Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea 2021, 40, 564–570. [Google Scholar] [CrossRef]
- Akpek, E.K.; Wirta, D.L.; Downing, J.E.; Tauber, J.; Sheppard, J.D.; Ciolino, J.B.; Meides, A.S.; Krösser, S. Efficacy and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.; Tauber, J.; Reis, B.L. Efficacy and Safety of Cyclosporin A Ophthalmic Emulsion in the Treatment of Moderate-to-Servere Dry Eye Disease: A Dose-Ranging, Randomized Trial. Ophthalmology 2000, 107, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; Quisisana, C.; Caretti, A.; Marchina, D.; Dei Cas, M.; Melardi, E.; Rossetti, L. Efficacy and Safety of VisuEvo® and Cationorm® for the Treatment of Evaporative and Non-Evaporative Dry Eye Disease: A Multicenter, Double-Blind, Cross-Over, Randomized Clinical Trial. Clin. Ophthalmol. 2020, 14, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Irkeç, M.; Messmer, E.M.; Benítez-del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical Impact of Inflammation in Dry Eye Disease: Proceedings of the ODISSEY Group Meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A Delivery to the Eye: A Pharmaceutical Challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318. [Google Scholar] [CrossRef]
- Goldberg, D.F.; Malhotra, R.P.; Schechter, B.A.; Justice, A.; Weiss, S.L.; Sheppard, J.D. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology 2019, 126, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Profazio, V.; Giannaccare, G.; Fresina, M.; Campos, E.C. Discomfort Symptoms Reduction and Ocular Surface Parameters Recovery with Artelac Rebalance Treatment in Mild-Moderate Dry Eye. Eur. J. Ophthalmol. 2013, 23, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R. [Treatment of Dry Eye with a New Gel in Eyedrop Form]. Klin. Monbl. Augenheilkd. 1986, 189, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Pattmöller, M.; Szentmáry, N.; Eppig, T.; Gro, D.; Seitz, B. Safety of Hyaluronic Acid in Postoperative Treatment after Penetrating Keratoplasty. Klin. Monbl. Augenheilkd. 2018, 235, 64–72. [Google Scholar] [CrossRef]
- Wilson, C.G.; Zhu, Y.P.; Frier, M.; Rao, L.S.; Gilchrist, P.; Perkins, A.C. Ocular Contact Time of a Carbomer Gel (GelTears) in Humans. Br. J. Ophthalmol. 1998, 82, 1131–1134. [Google Scholar] [CrossRef]
- Bron, A.J.; Daubas, P.; Siou-Mermet, R.; Trinquand, C. Comparison of the Efficacy and Safety of Two Eye Gels in the Treatment of Dry Eyes: Lacrinorm and Viscotears. Eye 1998, 12 Pt 5, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wirta, D.L.; Torkildsen, G.L.; Moreira, H.R.; Lonsdale, J.D.; Ciolino, J.B.; Jentsch, G.; Beckert, M.; Ousler, G.W.; Steven, P.; Krösser, S. A Clinical Phase II Study to Assess Efficacy, Safety, and Tolerability of Waterfree Cyclosporine Formulation for Treatment of Dry Eye Disease. Ophthalmology 2019, 126, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent Advances in Topical Ophthalmic Drug Delivery with Lipid-Based Nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J. Polyoxyethylated Nonionic Surfactants and Their Applications in Topical Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- Oualikene-Gonin, W.; Sautou, V.; Ezan, E.; Bastos, H.; Bellissant, E.; Belgodère, L.; Maison, P.; Ankri, J. Regulatory Assessment of Nano-Enabled Health Products in Public Health Interest. Position of the Scientific Advisory Board of the French National Agency for the Safety of Medicines and Health Products. Front. Public Health 2023, 11, 1125577. [Google Scholar] [CrossRef]
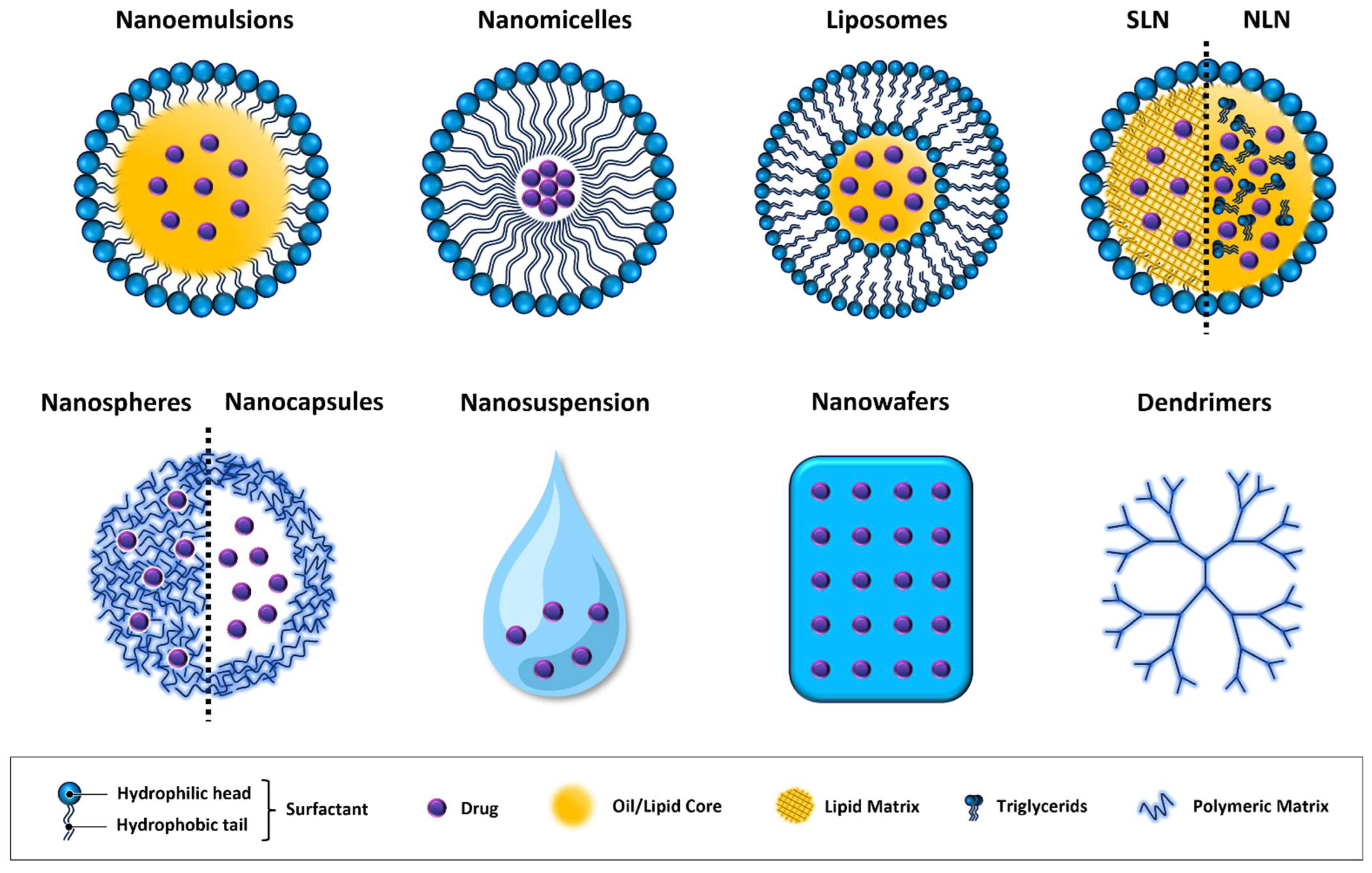
| Nanotechnology | Composition | Advantages | Disadvantages |
|---|---|---|---|
| Nanoemulsions [67,68] | Two-phase system of water, oil, and amphiphilic surfactants. The oil phase integrates with the lipid layer of the tear film, while the water phase integrates with the aqueous layer and emulsifiers with the mucous layer. | Long residence time, can be used as reservoir for slow drug release. | Thermodynamic instability. Potential intolerance due to high surfactant concentration. |
| Nanomicelles [71] | Hydrophobic core and hydrophilic shell. Employed to encapsulate, solubilize, and deliver hydrophobic drugs. | High water solubility, forming clear aqueous solutions which do not cause vision blurring. | Potential stability issues. |
| Nanosuspensions [78] | Colloidal dispersions where drug particles are reduced to the nanometer scale and dispersed in a liquid medium. | Enhances bioavailability of poorly soluble drugs. | Physical instability issues (sedimentation) and potential toxicity due to the use of surfactants. |
| Liposomes [81,114,115] | One or more concentric phospholipid bilayers separated by an aqueous buffer, which allow the encapsulation of both hydrophobic (in lipid bilayer) and hydrophilic (in aqueous compartment) drug molecules. | High biocompatibility. Protects drug molecules from enzymatic degradation. Encapsulate both hydrophobic and hydrophilic drugs. | Potential risk of aggregation and fusion. Limited stability in storage. |
| Niosomes [86] | Structurally different from liposomes due to the absence of phospholipids. Composed of non-ionic surfactants and cholesterol. | Encapsulate both hydrophilic and hydrophobic drugs. | Structurally different from natural membranes for the absence of phospholipids. Stability concerns in aqueous environments. |
| Cubosomes [116] | Composed of specific amphiphilic lipids in the presence of an appropriate stabilizer to form cubic liquid crystalline phase | Suitable for hydrophobic, hydrophilic, and amphiphilic compounds. | Complex manufacturing process. Stability issues related to the crystalline phase. |
| Polymeric Nanoparticles [117] | Depending on the preparation method can form nanospheres (drug is uniformly dispersed throughout the polymer matrix) or nanocapsules (drug is encapsulated within the polymer shell). | Potential for targeted delivery to specific tissues. | Complex manufacturing process. Potential toxicity due to polymers. |
| Solid Lipid Nanoparticles (SLNs) [87] | Structures made of a lipid core, in a solid state at room temperature, that provides a stable matrix for drug encapsulation and a surrounding phospholipid layer that contributes to stability and biocompatibility. | Controlled drug release. Protects active molecules from degradation. Good biocompatibility and safety profile. | Poor drug-loading capacity and drug expulsion after polymeric transition during storage and relatively high water content of the dispersions. |
| Nanostructured Lipid Carriers (NLCs) [94,95] | Similar to SLNs. They include at least 30% triglycerides in a liquid state at room temperature. | Controlled drug release. Protects active ingredients from degradation. Enhanced physical stability. Higher drug-loading capacity. | Complex manufacturing process. Potential drug leakage. |
| Nanowafers [118] | Biodegradable polymers loaded with drugs, with a thin and flat design. | Extended drug contact time. Protect corneal surface. | Potential discomfort upon application. |
| Dendrimers [119] | Tree-like or dendritic structure, featuring highly branched repeating units radiating from a central core. Star-shaped multi-branched structure enables it to encapsulate a large number of lipophilic or hydrophilic drugs. | High drug encapsulation efficiency. Controlled drug release. | Complex manufacturing process. Potential cytotoxicity. |
| In Situ Hydrogels [120] | Three-dimensional structure of hydrophilic polymers (hyaluronic acid, chitosan, and methylcellulose) that can absorb a significant amount of water without dissolving. | Responsive to environmental stimuli. Prolonged drug retention. Customizable in various shapes and thicknesses. | Variable sol–gel transition rates. Potentially inconsistent drug release. |
| Drug-eluting Contact Lenses [105] | Contact lenses engineered using polymeric materials, such as hydrogels, which encapsulate drug molecules. | Extended contact with the ocular surface. Increased drug absorption. Reduced drug loss via tear ducts. | Need for lens compatibility. Risk of lens-related complications. |
| Nanogels [109] | Three-dimensional crosslinked polymeric network. Categorized based on the type of bonds in the polymer network (non-covalent, covalent). | Easy manufacturing process. High drug-loading capacity. Smart nanogels are thermosensitive. | Fragility of physically crosslinked nanogels. Potential toxicity in chemically crosslinked varieties. |
| Nanozymes [112] | Nanozymes mimic natural enzymes’ activity. The active sites for the catalysis of the reaction usually consist of single or multiple metal atoms. | Mimic a naturally occurring process. High selectivity. | More studies are necessary to assess tolerability. |
| Category | Drug | Nanosystem | Study Model | Outcomes | References |
|---|---|---|---|---|---|
| Emulsions | Cyclosporine A | Emulsion of glycerin, castor oil, polysorbate 80, carbomer copolymer A | In vivo (animal and humans) | -Improved dry eye symptoms and signs | Ames P. et al. [121] |
| Tacrolimus | Microemulsion prepared by titration with propylene glycol and polysorbate 80 | In vitro and in vivo (rabbit model) | -Increased drug penetration -No toxicity to corneal and conjunctival cells | Silva-Cunha A. et al. [122] | |
| Micelles | Cyclosporine A | Methoxy poly (ethylene glycol)-poly (lactide) polymer (mPEG-PLA) micelles | In vitro and in vivo | -Stability for at least 3 months and sustained release -Enhanced retention time with a longer effect toward DED symptoms | Yu Y. et al. [76] |
| Cyclosporine A | HCO-40/OC-40 based non-ionic nanomicelles | Preclinical and clinical trials | -Highly effective and safe -Rapid onset of action | Mandal A. et al. [77] | |
| Nanosuspensions | Mycophenolate Mofetil | Chitosan-modified nanosuspensions | In vivo (rabbit model) | -Increase corneal mucoadhesion and drug absorption -Prolonged survival time of high-risk allografts -Inhibition of corneal immune rejection in the rabbit models of penetrating keratoplasty | Wu XG et al. [80] |
| Liposomes | Lactoferrin | Hyaluronic acid-coated liposomes | In vitro and in vivo | -Physical stability -Prolonged release of the drug -Biocompatible without any sign of ocular irritation or cytotoxicity | López-Machado A et al. [83] |
| Niosomes | Tacrolimus | Hyaluronic acid-coated niosomes | In vivo (rabbit model) | -Prolonged residence time of the drug -Enhancement in transcorneal permeability | Zeng W. et al. [86] |
| Nanoparticles | Tacrolimus | Gellan gum nanoparticles | In vitro and in vivo (rabbit model) | -Prolonged drug release throughout 12 h and higher precorneal retention compared to tacrolimus solution -Amelioration in DED symptoms in rabbits | Modi D et al. [90] |
| Cerium oxide | Water-soluble glycol chitosan nanoparticle | In vitro and in vivo (murine model) | -No cytotoxic effects -Improvement in dry eye disease models by stabilizing the tear film, promoting and maintaining corneal and conjunctival cell growth and integrity | Yu F. et al. [96] | |
| Cyclosporine A | Lipid nanocapsule | In vitro and in vivo (rabbit model) | -Improvement in bioavailability and permeability -Amelioration in BUT, fluorescein staining, tear production, and histopathology tests | Zhang A. et al. [97] | |
| Nanostructured lipid carriers | Cyclosporine A | Thiolated nanostructured lipid carrier | In vitro and in vivo (rabbit model) | -Higher concentration of CsA in aqueous, humor, tear, and eye tissues | Shen J. et al. [94] |
| Nanowafers | Dexamethasone | Polydimethylsiloxane nanowafers | In vivo (mice model) | -Preservation of corneal clarity -Decreasing expression of metalloproteinases and inflammatory cytokines | Bian F. et al. [98] |
| Dendrimers | Dexamethasone | Subconjunctival injectable gel based on G4-PAMAM dendrimer and hyaluronic acid | In vivo (rat model) | -Reduction in corneal inflammation more effective than with free-dexamethasone -Enhanced corneal clarity without causing an increase in intraocular pressure levels | Soiberman et al. [102] |
| Hydrogels | Cyclosporine A | Nanostructured poly (2-hydroxyethyl methacrylate) (p-HEMA) hydrogels containing microemulsions or micelles of Brij 97 | In vitro | -Sustained and controlled release (20 days) of drugs. -Resistance after exposure to all the relevant processing conditions | Kapoor Y. et al. [123] |
| Hyaluronic acid | Soft hydrogels | In vivo (canine model) | -Biocompatibility -In combination with CsA, improved clinical signs in more than 65% of dog patients previously unresponsive to Cyclosporine treatment | Yu Y. et al. [104] | |
| Drug-eluting contact lenses | Hyaluronic acid | Contact lenses prepared by soaking method or direct entrapment method | In vivo (rabbit model) | -Safe profile -Increased residence time of hyaluronic acid with lenses compared to conventional eye drop treatments | Maulvi FA et al. [106] |
| Nanogels | Lysine hydrochloride | Carbonized nanogels | In vitro and in vivo (rabbit model) | -High biocompatibility -Reduction in the therapeutic dose and extended dosing interval | Lin PH et al. [111] |
| Nanozymes | Cerium oxide | Cerium oxide nanozyme combined with branched poly(ethylene imine)-graft-poly(ethylene glycol) | In vitro and in vivo (murine model) | -Biocompatibility -Antioxidant activity -In vivo reduction in corneal epithelial defects and increased goblet cells | Zou et al. [113] |
| Dual-atom (Fe-Mn) | Fe and Mn atoms embedded in N-doped carbon material and modified with hydrophilic polymer | In vitro and in vivo (murine model) | -Inhibition of NLPR3 inflammasome activation -Antioxidant activity -Reduced corneal opacity -Reduced fluorescein staining | Chu et al. [112] |
| Trade Name | Therapeutic Agent | Nanosystem | Outcomes | References |
|---|---|---|---|---|
| Restasis® | Cyclosporine A | 0.05% oil-in-water anionic nanoemulsion. Polysorbate 80 as surfactant and castor oil as solubilizer | -Increase in conjunctival goblet cell density -Reduction in punctate fluorescein staining -Amelioration in symptoms of blurred vision -Safe profile of action | Sall K. et al. [124] Stevenson D. et al. [142] |
| Lacrinmune® | Cyclosporine A | Oil-in-water emulsion | -The composition is similar to Restasis® but with the addition of sodium hyaluronate, which allows an increased viscosity and a prolonged retention time on the ocular surface | Lv Z. et al. [6] |
| Cationorm® | Lipids, glycerol | Nanoemulsion | -Effective in evaporative and non-evaporative DED -Excellent safety profile -Transient blurred vision observed in some patients | Fogagnolo P. et al. [143] |
| Ikervis® | Cyclosporine A | Cationic emulsion 0.1% | -Improvement in global symptom and corneal staining scores at 6 months -Greater bioavailability of CsA to the ocular surface compared to anionic emulsion | Baudouin C. et al. [144] Lallemand F. et al. [145] |
| Cyclokat® | Cyclosporine A | Cationic emulsion 0.1% | -Improvement in signs and symptoms in patients suffering from moderate-to-severe dry eye syndrome | Buggage RR et al. [129] |
| Cequa® (OTX-101 0.09%) | Cyclosporine A | Aqueous nanomicellar solution | -Improved corneal and conjunctival staining -Good tolerability -Rapid onset of action | Goldberg DF et al. [146] Mandal A. et al. [77] |
| Vyseo® | Vitamin A and vitamin E | Phospholipid liposomal spray | -Useful for the treatment of patients with mild-to-moderate evaporative DED | Nagai N. et al. [74] |
| Clarimist® | Vitamin A palmitate and vitamin E | Liposomal spray | ||
| Tears Again® | Hyaluronic acid | Phospholipid liposomal spray | -Improvement in tear film stability, symptoms, and visual acuity | Craig JP et al. [135] |
| Lacrisek® | Vitamin A palmitate and vitamin E | Liposomal spray | -Local vitamin A supplementation is useful in improving goblet cell density and epithelial health | Fogagnolo P. et al. [138] |
| Artelac Rebalance® | Vitamin B12 | Liposomal spray | -2-months application in mild-to-moderate dry eye cases resulted in a reduction in ocular inflammation parameters, ocular surface damage, and subjective discomfort symptoms -High tolerability and satisfaction -No adverse events reported | Versura P. et al. [147] |
| Vidisc®gel | Polymerizate acrylic acid | Hydrogel | -Longer viability compared to other tear substitutes -Well tolerated and effective | Marquardt R. [148] |
| Hylo®gel | Hyaluronic acid 0.2% | Hydrogel | -Significant improvements in objective findings and subjective symptoms when used as a lubricant after penetrating keratoplasty | Pattmöller M. et al. [149] |
| GelTears® | Carbomer 980 | Hydrogel | -Extended contact of solutes or suspended solids with the corneal surface | Wilson CG et al. [150] |
| Viscotears® | Carbomer 980 | Polyacrylic acid 0.2% hydrogel | -Local tolerability upon instillation -Improvement in subjective symptoms and objective test results after 30 days of treatment | Bron AJ et al. [151] |
| Eysuvis® (KPI-121 0.25%) | Loteprednol etabonate | Nanoparticles coated with Poloxamer 407 | -Good tolerability -No significant increase in intraocular pressure after 2-week treatment | Korenfeld M. et al. [140] Venkateswaran N et al. [139] |
| Cyclasol® | Cyclosporine A | Nonaqueous solution without water, oil, surfactants, or preservatives | -Enhanced bioavailability and efficacy -Early therapeutic effects on the ocular surface -Safety, tolerability, and comfort profile | Akpek EK et al. [141] Wirta DL et al. [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coco, G.; Buffon, G.; Taloni, A.; Giannaccare, G. Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease. Nanomaterials 2024, 14, 669. https://doi.org/10.3390/nano14080669
Coco G, Buffon G, Taloni A, Giannaccare G. Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease. Nanomaterials. 2024; 14(8):669. https://doi.org/10.3390/nano14080669
Chicago/Turabian StyleCoco, Giulia, Giacinta Buffon, Andrea Taloni, and Giuseppe Giannaccare. 2024. "Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease" Nanomaterials 14, no. 8: 669. https://doi.org/10.3390/nano14080669
APA StyleCoco, G., Buffon, G., Taloni, A., & Giannaccare, G. (2024). Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease. Nanomaterials, 14(8), 669. https://doi.org/10.3390/nano14080669









