Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing
Abstract
:1. Introduction
- Reveal the efficiency of ATO as a reinforcement agent in the PETG polymer.
- Investigate the ability of ATO to modify the electrical properties of the PETG polymer.
- Propose PETG/ATO nanocomposites for the MEX 3D printing process with enhanced performance. To improve the efficiency of applications, the PETG polymer is used overall, further expanding its use.
2. Materials and Methods
2.1. Materials
2.2. Preparation of the PETG and PETG/ATO Filaments and 3D Printing of the Specimens
2.3. SEM and EDS Analysis of ATO and the Nanocomposites’ Specimens
2.4. Micro-Computed Tomography (μ-CT)
2.5. Mechanical Characterization
2.6. Raman Spectroscopy Analysis and Fourier Transform Infrared (FTIR) Spectroscopy
- Acquisition spectral range from 50 up to 3900 cm−1;
- Spectrometer grating 600 grooves/mm, resulting in 2 cm−1 spectral resolution;
- Sample exposure time is 10 s at each measurement point;
- We acquired a total of five accumulations at each point for statistical purposes and to improve the signal-to-noise ratio, which in turn would yield high-quality Raman spectra;
- Imaging resolution was 1.7 μm lateral and 2 μm axial.
2.7. Rheometric Performance Examination
2.8. Thermal Properties Examination
2.9. Broadband Dielectric Spectroscopy (BDS) Measurements
3. Results
3.1. Raman Spectroscopy Characterization
3.2. Thermal Properties Characterization via TGA and DSC
3.3. Viscosity and Melt Flow Rate Results
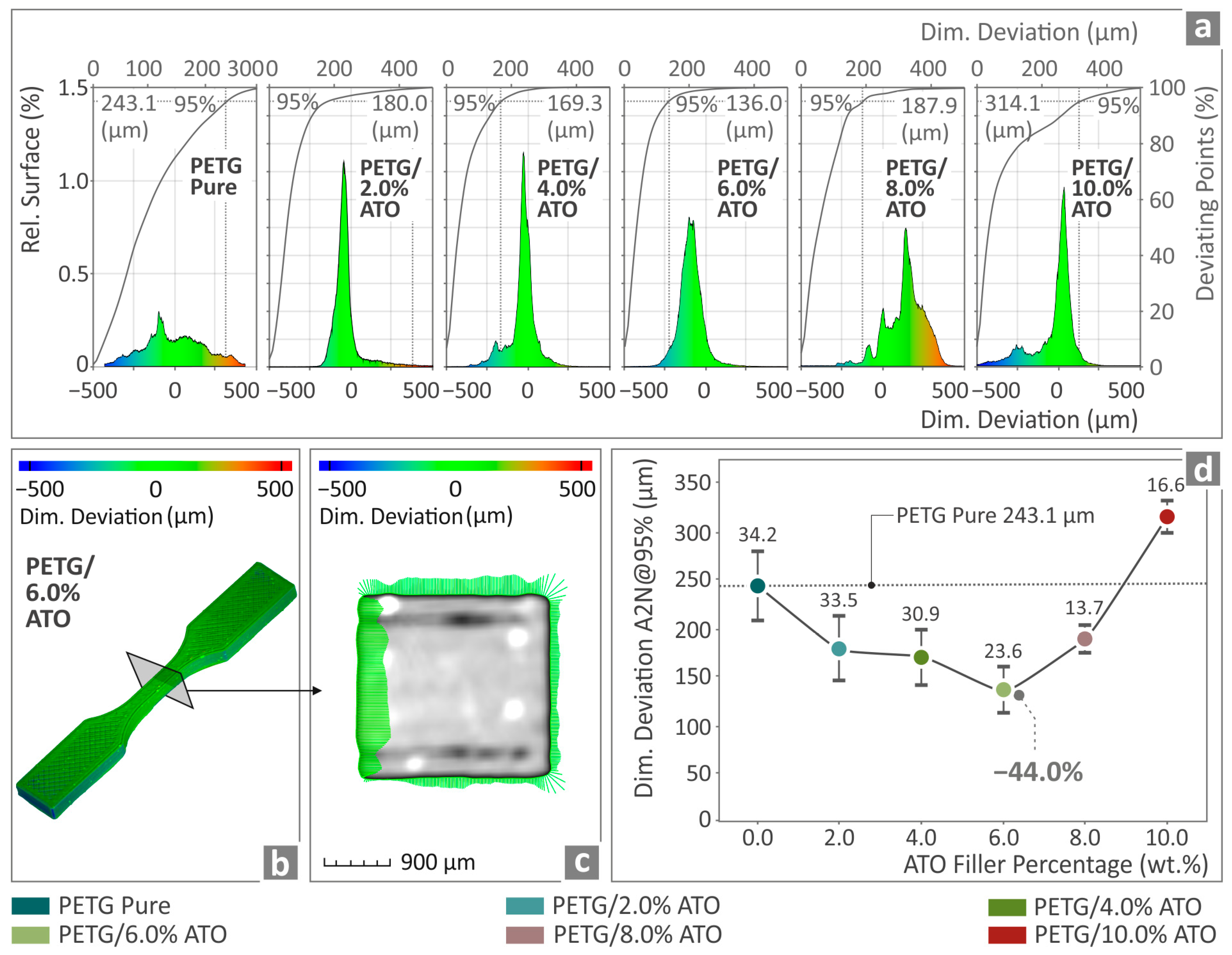
3.4. Filament Inspection and Quality Control
3.5. Characterization of Specimens’ Mechanical Performance
3.6. Electrical/Dielectric Characterization of PETG/ATO Composites’ Samples
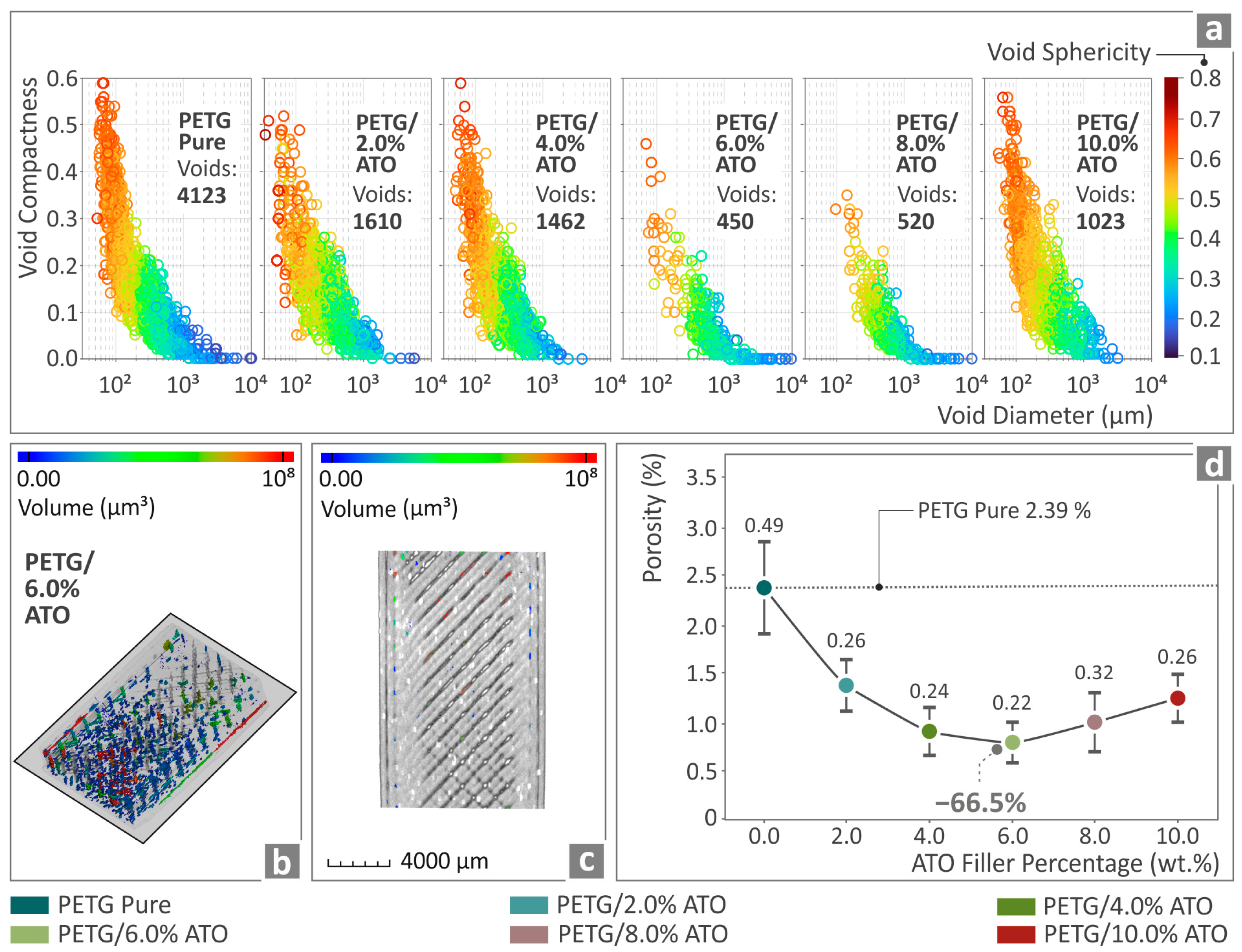
3.7. Micro-Computed Tomography of the Specimens
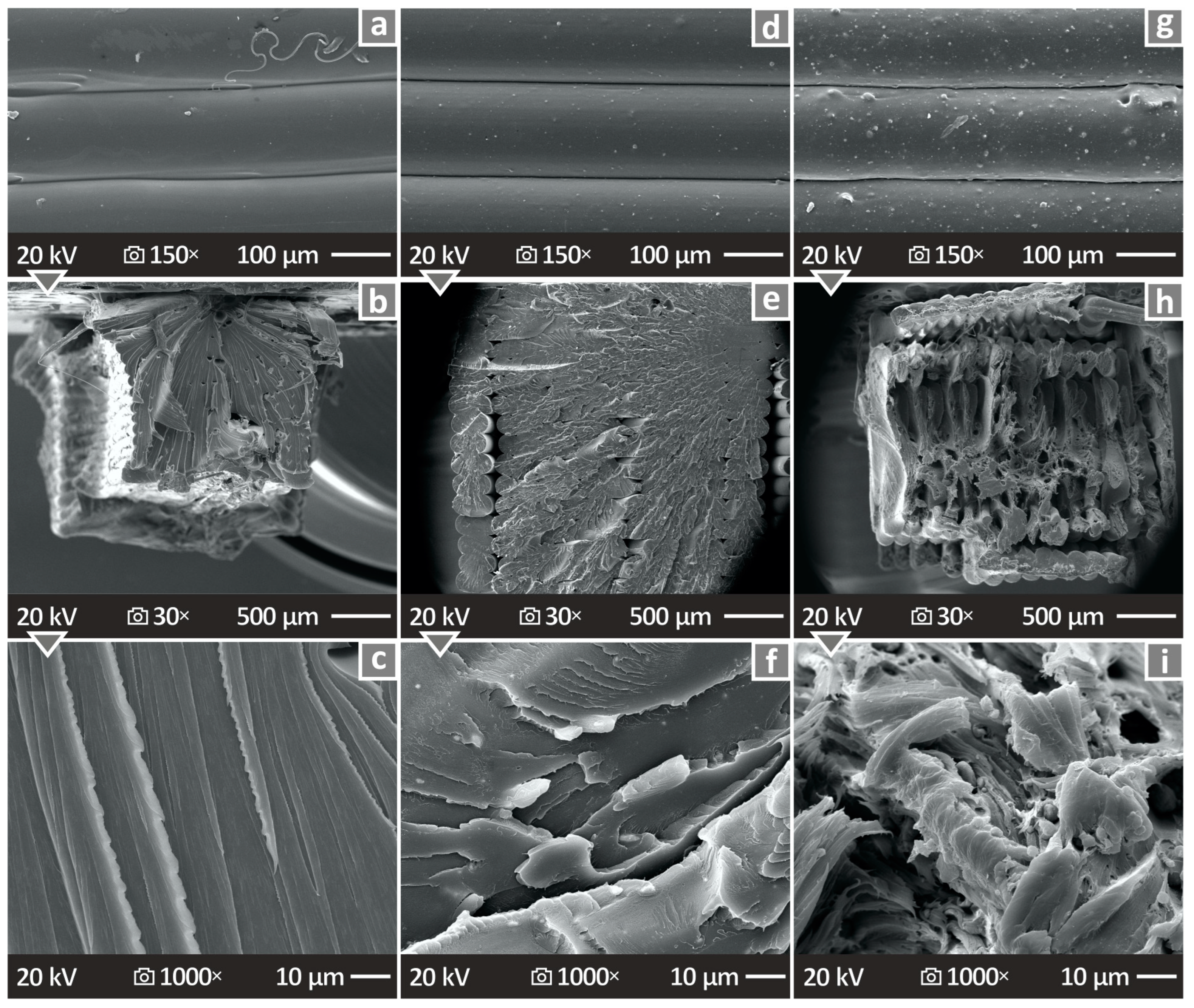
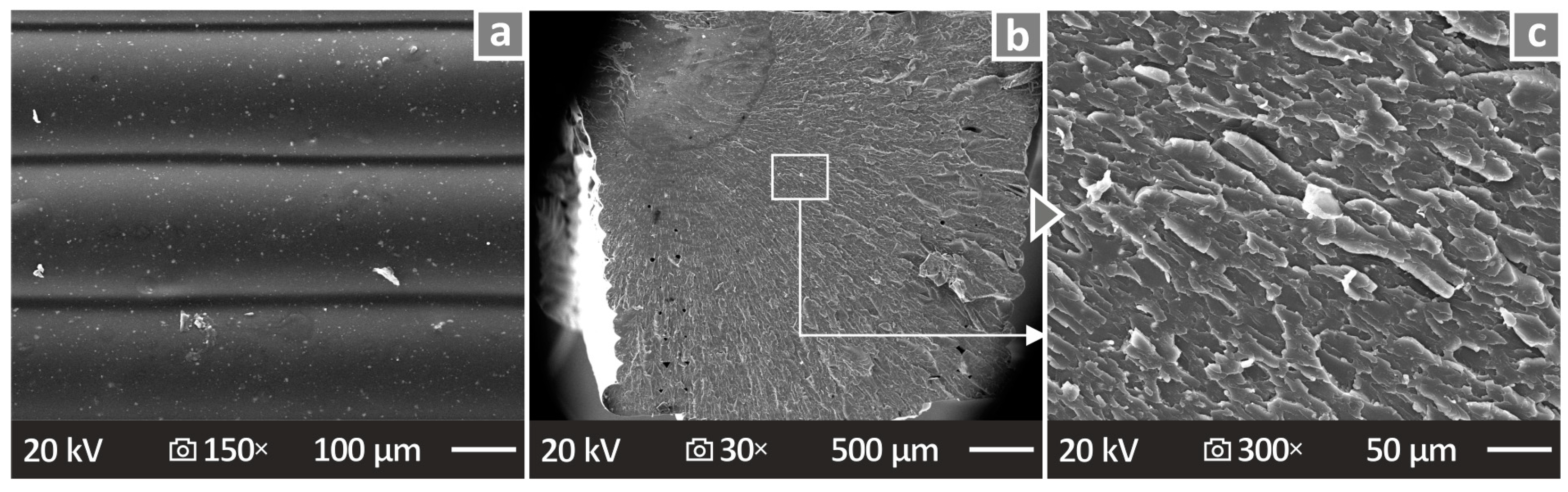
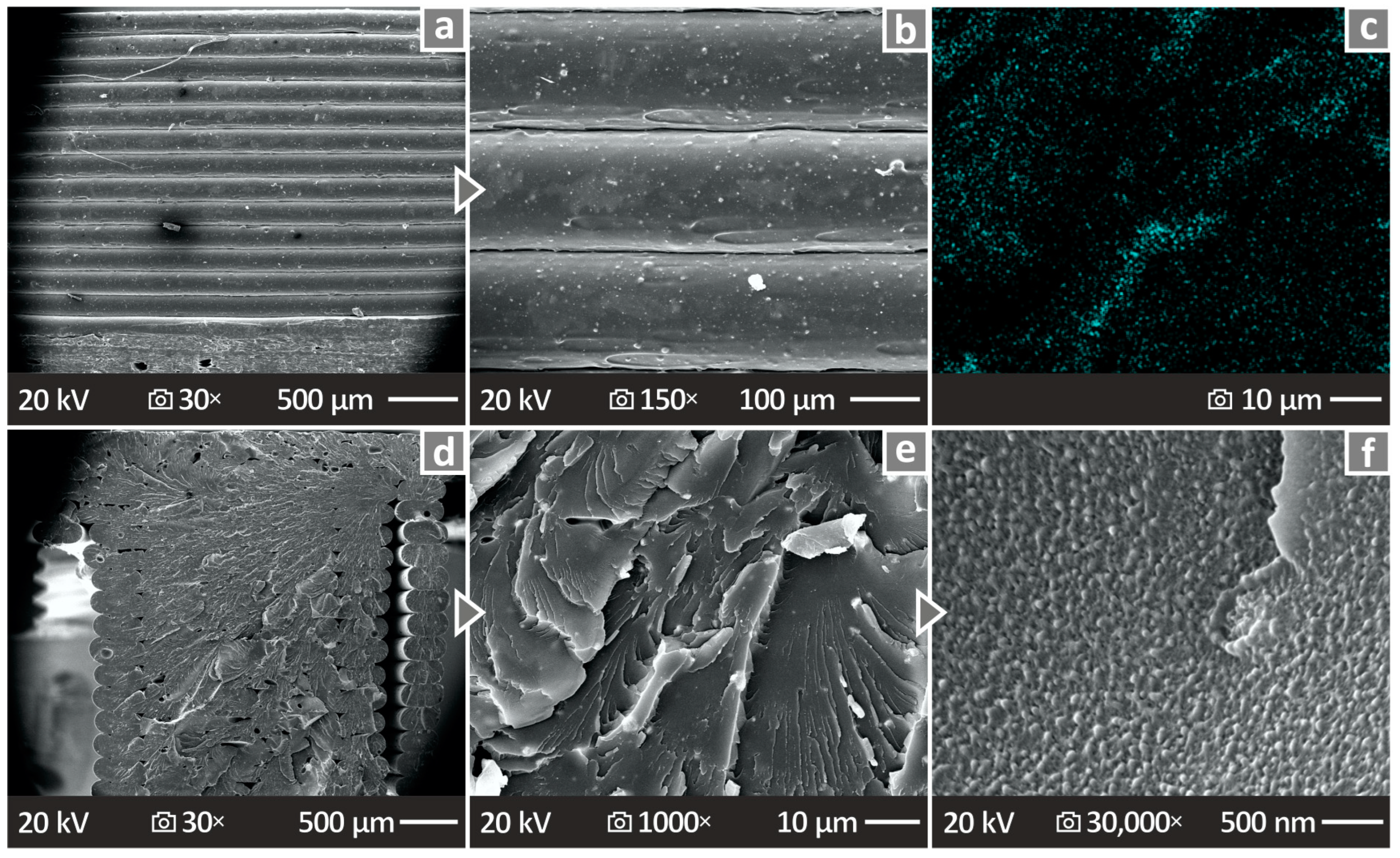
3.8. SEM and EDS Mapping of the 3D-P Specimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durgashyam, K.; Indra Reddy, M.; Balakrishna, A.; Satyanarayana, K. Experimental Investigation on Mechanical Properties of PETG Material Processed by Fused Deposition Modeling Method. Mater. Today Proc. 2019, 18, 2052–2059. [Google Scholar] [CrossRef]
- Szykiedans, K.; Credo, W.; Osiński, D. Selected Mechanical Properties of PETG 3-D Prints. Procedia Eng. 2017, 177, 455–461. [Google Scholar] [CrossRef]
- Szykiedans, K.; Credo, W. Mechanical Properties of FDM and SLA Low-Cost 3-D Prints. Procedia Eng. 2016, 136, 257–262. [Google Scholar] [CrossRef]
- Valvez, S.; Silva, A.P.; Reis, P.N.B. Optimization of Printing Parameters to Maximize the Mechanical Properties of 3D-Printed PETG-Based Parts. Polymers 2022, 14, 2564. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prathap, P.; Raj, A.; Aswinth Kannan, S.; Deepak, V. Influence of Fused Deposition Modeling Process Parameters on the Mechanical Properties of PETG Parts. Mater. Today Proc. 2020, 27, 1877–1883. [Google Scholar] [CrossRef]
- Thomas, C.L.; Gaffney, T.M.; Kaza, S.; Lee, C.H. Rapid Prototyping of Large Scale Aerospace Structures. In Proceedings of the 1996 IEEE Aerospace Applications Conference, Aspen, CO, USA, 10 February 1996; pp. 219–230. [Google Scholar]
- Bacciaglia, A.; Ceruti, A.; Liverani, A. Towards Large Parts Manufacturing in Additive Technologies for Aerospace and Automotive Applications. Procedia Comput. Sci. 2022, 200, 1113–1124. [Google Scholar] [CrossRef]
- Mohanavel, V.; Ashraff Ali, K.S.; Ranganathan, K.; Allen Jeffrey, J.; Ravikumar, M.M.; Rajkumar, S. The Roles and Applications of Additive Manufacturing in the Aerospace and Automobile Sector. Mater. Today Proc. 2021, 47, 405–409. [Google Scholar] [CrossRef]
- Najmon, J.C.; Raeisi, S.; Tovar, A. Review of Additive Manufacturing Technologies and Applications in the Aerospace Industry. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–31. [Google Scholar]
- Tepylo, N.; Huang, X.; Patnaik, P.C. Laser-Based Additive Manufacturing Technologies for Aerospace Applications. Adv. Eng. Mater. 2019, 21, 1900617. [Google Scholar] [CrossRef]
- de Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High Performance Polymer Nanocomposites for Additive Manufacturing Applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Joshi, S.C.; Sheikh, A.A. 3D Printing in Aerospace and Its Long-Term Sustainability. Virtual Phys. Prototyp. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Sepasgozar, S.M.E.; Shi, A.; Yang, L.; Shirowzhan, S.; Edwards, D.J. Additive Manufacturing Applications for Industry 4.0: A Systematic Critical Review. Buildings 2020, 10, 231. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; McMurtrey, M.D.; Jerred, N.D.; Liou, F.; Li, J. Additive Manufacturing for Energy: A Review. Appl. Energy 2021, 282, 116041. [Google Scholar] [CrossRef]
- Goh, G.L.; Zhang, H.; Chong, T.H.; Yeong, W.Y. 3D Printing of Multilayered and Multimaterial Electronics: A Review. Adv. Electron. Mater. 2021, 7, 2100445. [Google Scholar] [CrossRef]
- Giannatsis, J.; Dedoussis, V. Additive Fabrication Technologies Applied to Medicine and Health Care: A Review. Int. J. Adv. Manuf. Technol. 2009, 40, 116–127. [Google Scholar] [CrossRef]
- Ovsianikov, A. Additive Manufacturing Technologies for Biomedical Applications. Ph.D. Thesis, Technische Universität Wien, Vienna, Austria, 2017. [Google Scholar]
- Chauhan, I.; Chattopadhyay, S.; Mohanty, P. Fabrication of Titania Nanowires Incorporated Paper Sheets and Study of Their Optical Properties. Mater. Express 2013, 3, 343–349. [Google Scholar] [CrossRef]
- Hwa, L.C.; Uday, M.B.; Ahmad, N.; Noor, A.M.; Rajoo, S.; Zakaria, K. Bin Integration and Fabrication of the Cheap Ceramic Membrane through 3D Printing Technology. Mater. Today Commun. 2018, 15, 134–142. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. Open-Source-Based 3D Printing of Thin Silica Gel Layers in Planar Chromatography. Anal. Chem. 2017, 89, 2116–2122. [Google Scholar] [CrossRef]
- Ahmed, W.; Siraj, S.; Al-Marzouqi, A.H. Embracing Additive Manufacturing Technology through Fused Filament Fabrication for Antimicrobial with Enhanced Formulated Materials. Polymers 2021, 13, 1523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Song, Y.; Yan, Y.; Zhang, R.; Xu, D.; Wang, F. Manufacture of the Die of an Automobile Deck Part Based on Rapid Prototyping and Rapid Tooling Technology. J. Mater. Process Technol. 2002, 120, 237–242. [Google Scholar] [CrossRef]
- Vasco, J.C. Additive Manufacturing for the Automotive Industry. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 505–530. [Google Scholar]
- ISO/ASTM 52900:2015; Standard Terminology for Additive Manufacturing—General Principles—Terminology. ASTM (American Society for Testing and Materials) International: West Conshohocken, PA, USA, 2015.
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Velidakis, E.; Mountakis, N.; Grammatikos, S.A. Polyamide 12/Multiwalled Carbon Nanotube and Carbon Black Nanocomposites Manufactured by 3D Printing Fused Filament Fabrication: A Comparison of the Electrical, Thermoelectric, and Mechanical Properties. C 2021, 7, 38. [Google Scholar] [CrossRef]
- Suteja, T.J.; Soesanti, A. Mechanical Properties of 3D Printed Polylactic Acid Product for Various Infill Design Parameters: A Review. J. Phys. Conf. Ser. 2020, 1569, 42010. [Google Scholar] [CrossRef]
- Jaisingh Sheoran, A.; Kumar, H. Fused Deposition Modeling Process Parameters Optimization and Effect on Mechanical Properties and Part Quality: Review and Reflection on Present Research. Mater. Today Proc. 2020, 21, 1659–1672. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of Fused Deposition Modeling Process Parameters: A Review of Current Research and Future Prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive Manufacturing of Carbon Fiber Reinforced Thermoplastic Composites Using Fused Deposition Modeling. Compos. B Eng. 2015, 80, 369–378. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; You, H. Photodegradation Behavior and Mechanism of Poly(Ethylene Glycol-Co-1,4-Cyclohexanedimethanol Terephthalate) (PETG) Random Copolymers: Correlation with Copolymer Composition. RSC Adv. 2016, 6, 102778–102790. [Google Scholar] [CrossRef]
- Latko-Durałek, P.; Dydek, K.; Boczkowska, A. Thermal, Rheological and Mechanical Properties of PETG/RPETG Blends. J. Polym. Environ. 2019, 27, 2600–2606. [Google Scholar] [CrossRef]
- Petrov, P.; Agzamova, D.; Pustovalov, V.; Zhikhareva, E.; Saprykin, B.; Chmutin, I.; Shmakova, N. Research into the Effect of the 3D-Printing Mode on Changing the Properties of PETG Transparent Plastic. In Proceedings of the 24th International Conference on Material Forming—ESAFORM, Liège, Belgium, 14–16 April 2021. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The Role of Crystalline, Mobile Amorphous and Rigid Amorphous Fractions in the Performance of Recycled Poly (Ethylene Terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Zhang, Q.; Chen, F.; Fu, Q. Synergistic Enhancement in Tensile Strength and Ductility of ABS by Using Recycled PETG Plastic. J. Appl. Polym. Sci. 2009, 113, 1207–1215. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in Orthodontic Clear Aligner Materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef]
- Yan, C.; Kleiner, C.; Tabigue, A.; Shah, V.; Sacks, G.; Shah, D.; DeStefano, V. PETG: Applications in Modern Medicine. Eng. Regen. 2024, 5, 45–55. [Google Scholar] [CrossRef]
- Devsingh, D.; Dev, A.D.; Avala, B.; Reddy, R.; Arjula, S. Characterization of Additive Manufactured PETG and Carbon Fiber-PETG. Int. J. Res. Eng. Appl. Manag. 2018, 4, 2. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Grammatikos, S.A.; Porfyrakis, E.; Maniadi, A.; Mountakis, N. Sustainable Additive Manufacturing: Mechanical Response of Polyethylene Terephthalate Glycol over Multiple Recycling Processes. Materials 2021, 14, 1162. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Mechtcherine, V.; Tzounis, L. On the Strain Rate Sensitivity of Fused Filament Fabrication (Fff) Processed Pla, Abs, Petg, Pa6, and Pp Thermoplastic Polymers. Polymers 2020, 12, 2924. [Google Scholar] [CrossRef] [PubMed]
- Petousis, M.; Ntintakis, I.; David, C.; Sagris, D.; Nasikas, N.K.; Korlos, A.; Moutsopoulou, A.; Vidakis, N. A Coherent Assessment of the Compressive Strain Rate Response of PC, PETG, PMMA, and TPU Thermoplastics in MEX Additive Manufacturing. Polymers 2023, 15, 3926. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, C.V.; Srinivasa Reddy, Y.; Inturi, V.; Indra Reddy, M. Dynamic Mechanical Analysis of 3D Printed PETG Material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1057, 012031. [Google Scholar] [CrossRef]
- Kopar, M.; Yildiz, A.R. Experimental Investigation of Mechanical Properties of PLA, ABS, and PETG 3-d Printing Materials Using Fused Deposition Modeling Technique. Mater. Test. 2023, 65, 1795–1804. [Google Scholar] [CrossRef]
- Kasmi, S.; Ginoux, G.; Allaoui, S.; Alix, S. Investigation of 3D Printing Strategy on the Mechanical Performance of Coextruded Continuous Carbon Fiber Reinforced PETG. J. Appl. Polym. Sci. 2021, 138, 50955. [Google Scholar] [CrossRef]
- Kannan, S.; Ramamoorthy, M.; Sudhagar, E.; Gunji, B. Mechanical Characterization and Vibrational Analysis of 3D Printed PETG and PETG Reinforced with Short Carbon Fiber. AIP Conf. Proc. 2020, 2270, 030004. [Google Scholar] [CrossRef]
- Bhandari, S.; Lopez-Anido, R.A.; Gardner, D.J. Enhancing the Interlayer Tensile Strength of 3D Printed Short Carbon Fiber Reinforced PETG and PLA Composites via Annealing. Addit. Manuf. 2019, 30, 100922. [Google Scholar] [CrossRef]
- Alarifi, I.M. PETG/Carbon Fiber Composites with Different Structures Produced by 3D Printing. Polym. Test. 2023, 120, 107949. [Google Scholar] [CrossRef]
- Bex, G.J.P.; Ingenhut, B.L.J.; ten Cate, T.; Sezen, M.; Ozkoc, G. Sustainable Approach to Produce 3D-Printed Continuous Carbon Fiber Composites: “A Comparison of Virgin and Recycled PETG”. Polym. Compos. 2021, 42, 4253–4264. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, H.; Saran, C.; Tripathy, T.S.; Sangwan, K.S.; Herrmann, C. A Comparative Study on the Life Cycle Assessment of a 3D Printed Product with PLA, ABS & PETG Materials. Procedia CIRP 2022, 107, 15–20. [Google Scholar] [CrossRef]
- Wachtman, J.B.; Cannon, W.R.; Matthewson, M.J. Mechanical Properties of Ceramics; John Wiley& Sons: Hoboken, NJ, USA, 2009; ISBN 9780471735816. [Google Scholar]
- Wei, Z.-Y.; Meng, G.-H.; Chen, L.; Li, G.-R.; Liu, M.-J.; Zhang, W.-X.; Zhao, L.-N.; Zhang, Q.; Zhang, X.-D.; Wan, C.-L.; et al. Progress in Ceramic Materials and Structure Design toward Advanced Thermal Barrier Coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.-X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in Ultra-High Temperature Ceramics, Composites, and Coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Antoniadis, A.; Vidakis, N.; Bilalis, N. Fatigue Fracture Investigation of Cemented Carbide Tools in Gear Hobbing, Part 2: The Effect of Cutting Parameters on the Level of Tool Stresses—A Quantitative Parametric Analysis. J. Manuf. Sci. Eng. 2002, 124, 792–798. [Google Scholar] [CrossRef]
- Bouzakis, K.-D.; Vidakis, N.; Kallinikidis, D.; Leyendecker, T.; Erkens, G.; Wenke, R.; Fuss, H.-G. Fatigue Failure Mechanisms of Multi- and Monolayer Physically Vapour-Deposited Coatings in Interrupted Cutting Processes. Surf. Coat. Technol. 1998, 108–109, 526–534. [Google Scholar] [CrossRef]
- Dresch, A.B.; Venturini, J.; Arcaro, S.; Montedo, O.R.K.; Bergmann, C.P. Ballistic Ceramics and Analysis of Their Mechanical Properties for Armour Applications: A Review. Ceram. Int. 2021, 47, 8743–8761. [Google Scholar] [CrossRef]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive Manufacturing of Bio-Inspired Ceramic Bone Scaffolds: Structural Design, Mechanical Properties and Biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Mark, J.E. Ceramic-Reinforced Polymers and Polymer-Modified Ceramics. Polym. Eng. Sci. 1996, 36, 2905–2920. [Google Scholar] [CrossRef]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef] [PubMed]
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of Polymer–Ceramic Composites for Improved Fire Resistance. J. Mater. Process Technol. 2004, 153–154, 401–407. [Google Scholar] [CrossRef]
- Bhargava, A.K. Engineering Materials: Polymers, Ceramics and Composites; PHI Learning Pvt. Ltd.: Delhi, India, 2012; ISBN 9788120346215. [Google Scholar]
- Vidakis, N.; Michailidis, N.; Argyros, A.; Mountakis, N.; Papadakis, V.; Spiridaki, M.; Moutsopoulou, A.; Sfakiotakis, E.; Petousis, M. A Coherent Optimization Course of the Silicon Nitride Nanofiller Load in Medical Grade Isotactic Polypropylene for Material Extrusion Additive Manufacturing: Rheology, Engineering Response, and Cost-Effectiveness. Colloid Polym. Sci. 2024. [Google Scholar] [CrossRef]
- Petousis, M.; Michailidis, N.; Papadakis, V.M.; Korlos, A.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Charou, C.; Moutsopoulou, A.; Vidakis, N. Optimizing the Rheological and Thermomechanical Response of Acrylonitrile Butadiene Styrene/Silicon Nitride Nanocomposites in Material Extrusion Additive Manufacturing. Nanomaterials 2023, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Argyros, A.; Papadakis, V.; Moutsopoulou, A.; Rogdakis, K.; Kymakis, E. Optimization Course of Hexagonal Boron Carbide Ceramic Nanofiller Content in Polypropylene for Material Extrusion Additive Manufacturing: Engineering Response, Nanostructure, and Rheology Insights. Next Nanotechnol. 2024, 5, 100054. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Nasikas, N.; Papadakis, V.; Argyros, A.; Mountakis, N.; Charou, C.; Moutsopoulou, A. Optimizing Titanium Carbide (TiC) Ceramic Nanofiller Loading in Isotactic Polypropylene for MEX Additive Manufacturing: Mechano-Thermal and Rheology Aspects. Mater. Today Commun. 2023, 37, 107368. [Google Scholar] [CrossRef]
- Petousis, M.; Vidakis, N.; Mountakis, N.; Moutsopoulou, A.; Papadakis, V.M.; Maravelakis, E. Emmanouel Maravelakis On the Substantial Mechanical Reinforcement of Polylactic Acid with Titanium Nitride Ceramic Nanofillers in Material Extrusion 3D Printing. Ceram. Int. 2023, 49, 16397–16411. [Google Scholar] [CrossRef]
- Moutsopoulou, A.; Petousis, M.; Michailidis, N.; Mountakis, N.; Argyros, A.; Papadakis, V.; Spiridaki, M.; Charou, C.; Ntintakis, I.; Vidakis, N. Optimization of Isotactic Polypropylene Nanocomposite Content of Tungsten Carbide for Material Extrusion 3D Printing. J. Compos. Sci. 2023, 7, 393. [Google Scholar] [CrossRef]
- Fan, G.; Wang, Z.; Sun, K.; Liu, Y.; Fan, R. Doping-Dependent Negative Dielectric Permittivity Realized in Mono-Phase Antimony Tin Oxide Ceramics. J. Mater. Chem. C 2020, 8, 11610–11617. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Chen, F.; Li, X.; Schoenung, J.M.; Shen, Q. Spark Plasma Sintering of Antimony-Doped Tin Oxide (ATO) Nanoceramics with High Density and Enhanced Electrical Conductivity. J. Asian Ceram. Soc. 2013, 1, 114–119. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Wu, J.; Shen, Q.; Schoenung, J.M.; Zhang, L. Effect of Post-Annealing on the Electrical Conductivity of Spark Plasma Sintered Antimony-Doped Tin Oxide (ATO) Ceramics. Scr. Mater. 2013, 68, 297–300. [Google Scholar] [CrossRef]
- Pan, W.; Zou, H. Characterization of PAN/ATO Nanocomposites Prepared by Solution Blending. Bull. Mater. Sci. 2008, 31, 807–811. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(Ε-caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Wei, P.; Xiaowei, H.; Yan, C. Preparation and Characterization of Poly (Vinyl Alcohol)/Antimony-Doped Tin Oxide Nanocomposites. Int. J. Polym. Mater. 2010, 60, 223–232. [Google Scholar] [CrossRef]
- Egdell, R.G.; Flavell, W.R.; Tavener, P. Antimony-Doped Tin(IV) Oxide: Surface Composition and Electronic Structure. J. Solid State Chem. 1984, 51, 345–354. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Cox, D.F.; Woodson, G.L.; Laitinen, H.A. Surface Characteristics of Antimony-Doped Tin Oxide Films. Thin Solid Films 1981, 78, 357–363. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Liebscher, M.; Tzounis, L. Mechanical Properties of 3D-Printed Acrylonitrile–Butadiene–Styrene TiO2 and ATO Nanocomposites. Polymers 2020, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.R.S.; Oliveira, P.W.; Schmidt, H.K. Enhanced Mechanical and Electrical Properties of Antimony-Doped Tin Oxide Coatings. Semicond. Sci. Technol. 2008, 23, 035013. [Google Scholar] [CrossRef]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent Conductors—A Status Review. Thin Solid Films 1983, 102, 1–46. [Google Scholar] [CrossRef]
- Texter, J.; Lelental, M. Network Formation in Nanoparticulate Tin Oxide−Gelatin Thin Films. Langmuir 1999, 15, 654–661. [Google Scholar] [CrossRef]
- Grunlan, J.C.; Gerberich, W.W.; Francis, L.F. Lowering the Percolation Threshold of Conductive Composites Using Particulate Polymer Microstructure. J. Appl. Polym. Sci. 2001, 80, 692–705. [Google Scholar] [CrossRef]
- Geblinger, N.; Thiruvengadathan, R.; Regev, O. Preparation and Characterization of a Double Filler Polymeric Nanocomposite. Compos. Sci. Technol. 2007, 67, 895–899. [Google Scholar] [CrossRef]
- Ginley, D.S.; Bright, C. Transparent Conducting Oxides. MRS Bull. 2000, 25, 15–18. [Google Scholar] [CrossRef]
- Gordon, R.G. Criteria for Choosing Transparent Conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef]
- Lewis, B.G.; Paine, D.C. Applications and Processing of Transparent Conducting Oxides. MRS Bull. 2000, 25, 22–27. [Google Scholar] [CrossRef]
- Shanthi, E.; Dutta, V.; Banerjee, A.; Chopra, K.L. Electrical and Optical Properties of Undoped and Antimony-doped Tin Oxide Films. J. Appl. Phys. 1980, 51, 6243–6251. [Google Scholar] [CrossRef]
- Jain, G.; Kumar, R. Electrical and Optical Properties of Tin Oxide and Antimony Doped Tin Oxide Films. Opt. Mater. 2004, 26, 27–31. [Google Scholar] [CrossRef]
- Valvez, S.; Silva, A.P.; Reis, P.N.B. Compressive Behaviour of 3D-Printed PETG Composites. Aerospace 2022, 9, 124. [Google Scholar] [CrossRef]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive Characterization of the Polycarbonate—Octadecylamine Interface by Surface Enhanced Raman Spectroscopy. Polym. Test. 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence Spectroscopy of Polycarbonate Matrices Irradiated with Different Energy 28Si+ Ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Bichara, L.C.; Alvarez, P.E.; Fiori Bimbi, M.V.; Vaca, H.; Gervasi, C.; Brandán, S.A. Structural and Spectroscopic Study of a Pectin Isolated from Citrus Peel by Using FTIR and FT-Raman Spectra and DFT Calculations. Infrared Phys. Technol. 2016, 76, 315–327. [Google Scholar] [CrossRef]
- Synytsya, A. Fourier Transform Raman and Infrared Spectroscopy of Pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature Studies of Polycarbonate Using Fourier Transform Raman Spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; He, Z.; Liang, X.; Wang, M.; Jin, G. Thermal Degradation Kinetics Study of Molten Polylactide Based on Raman Spectroscopy. Polym. Eng. Sci. 2021, 61, 201–210. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Li, W.; Cao, G.; Chen, W. Kinetics of Thermo-Oxidative and Thermal Degradation of Poly(d,l-Lactide) (PDLLA) at Processing Temperature. Polym. Degrad. Stab. 2006, 91, 3259–3265. [Google Scholar] [CrossRef]
- Jaggy, F.; Zinelis, S.; Polychronis, G.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. ATR-FTIR Analysis and One-Week Stress Relaxation of Four Orthodontic Aligner Materials. Materials 2020, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Nafee, S.S.; Hamdalla, T.A.; Shaheen, S.A. FTIR and Optical Properties for Irradiated PVA–GdCl3 and Its Possible Use in Dosimetry. Phase Transit. 2017, 90, 439–448. [Google Scholar] [CrossRef]
- Schoonover, J.R.; Zhang, S.L.; Bridgewater, J.S.; Havrilla, G.J.; Fletcher, M.A.; Lightfoot, J.M. Polymer Degradation Study by Factor Analysis of GPC-FT-IR Data. Appl. Spectrosc. 2001, 55, 927–934. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Cheng, W.-T.; Wei, Y.-S.; Lin, H.-L. DSC-FTIR Microspectroscopy Used to Investigate the Heat-Induced Intramolecular Cyclic Anhydride Formation between Eudragit E and PVA Copolymer. Polym. J. 2011, 43, 577–580. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and Ac Impedance Spectroscopic Study on PVA Based Polymer Electrolyte Doped with NH4X (X=Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Ma, N.; Liu, W.; Ma, L.; He, S.; Liu, H.; Zhang, Z.; Sun, A.; Huang, M.; Zhu, C. Crystal Transition and Thermal Behavior of Nylon 12. e-Polymers 2020, 20, 346–352. [Google Scholar] [CrossRef]
- Rihawy, M.S.; Alzier, A.; Allaf, A.W. Investigation of Chloramphenicol Release from PVA/CMC/HEA Hydrogel Using Ion Beam Analysis, UV and FTIR Techniques. Appl. Radiat. Isot. 2019, 153, 108806. [Google Scholar] [CrossRef] [PubMed]
- Veselý, P.; Froš, D.; Hudec, T.; Sedláček, J.; Ctibor, P.; Dušek, K. Dielectric Spectroscopy of PETG/TiO2 Composite Intended for 3D Printing. Virtual Phys. Prototyp. 2023, 18, e2170253. [Google Scholar] [CrossRef]
- Dhotel, A.; Rijal, B.; Delbreilh, L.; Dargent, E.; Saiter, A. Combining Flash DSC, DSC and Broadband Dielectric Spectroscopy to Determine Fragility. J. Therm. Anal. Calorim. 2015, 121, 453–461. [Google Scholar] [CrossRef]
- Knauert, S.T.; Douglas, J.F.; Starr, F.W. The Effect of Nanoparticle Shape on Polymer-Nanocomposite Rheology and Tensile Strength. J. Polym. Sci. B Polym. Phys. 2007, 45, 1882–1897. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel Nanoparticle-Reinforced Metal Matrix Composites with Enhanced Mechanical Properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Concepts and Conflicts in Nanoparticles Reinforcement to Polymers beyond Hydrodynamics. Prog. Mater. Sci. 2016, 84, 1–58. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, T.H.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of Nanoparticles on the Thermal and Mechanical Properties of Epoxy Coatings. J. Nanosci. Nanotechnol. 2016, 16, 9874–9881. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Xu, C.; Li, Y.; Liu, Q.; Wang, S.; Yan, S. Effect of Nanoparticle Size on the Mechanical Properties of Polymer Nanocomposites. Polymer 2022, 252, 124944. [Google Scholar] [CrossRef]
- Chang, A.; Babhadiashar, N.; Barrett-Catton, E.; Asuri, P. Role of Nanoparticle–Polymer Interactions on the Development of Double-Network Hydrogel Nanocomposites with High Mechanical Strength. Polymers 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Navarro Oliva, F.S.; Sahihi, M.; Lenglet, L.; Ospina, A.; Guenin, E.; Jaramillo-Botero, A.; Goddard, W.A.; Bedoui, F. Nanoparticle Size and Surface Chemistry Effects on Mechanical and Physical Properties of Nano-Reinforced Polymers: The Case of PVDF-Fe3O4 Nano-Composites. Polym. Test. 2023, 117, 107851. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of Nanoparticles Aggregation/Agglomeration on the Interfacial/Interphase and Tensile Properties of Nanocomposites. Compos. B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Zare, Y. Study of Nanoparticles Aggregation/Agglomeration in Polymer Particulate Nanocomposites by Mechanical Properties. Compos. Part. A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Jin, Y.; Gerhardt, R.A. Prediction of the Percolation Threshold and Electrical Conductivity of Self-Assembled Antimony-Doped Tin Oxide Nanoparticles into Ordered Structures in PMMA/ATO Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 22264–22271. [Google Scholar] [CrossRef]
- Vidakis, N.; Michailidis, N.; Petousis, M.; Nasikas, N.K.; Saltas, V.; Papadakis, V.; Mountakis, N.; Argyros, A.; Spiridaki, M.; Valsamos, I. Multifunctional HDPE/Cu Biocidal Nanocomposites for MEX Additive Manufactured Parts: Perspectives for the Defense Industry. Def. Technol. 2024. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Pakuła, D.; Frydrych, M.; Špitalský, Z.; Przekop, R.E. Natural and Synthetic Polymer Fillers for Applications in 3D Printing—FDM Technology Area. Solids 2022, 3, 508–548. [Google Scholar] [CrossRef]
- Gama, N.; Magina, S.; Barros-Timmons, A.; Ferreira, A. Enhanced Compatibility between Coconut Fibers/PP via Chemical Modification for 3D Printing. Prog. Addit. Manuf. 2022, 7, 213–223. [Google Scholar] [CrossRef]
- Coleman, E.A. 21—Plastics Additives. In Applied Plastics Engineering Handbook, 2nd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 489–500. ISBN 978-0-323-39040-8. [Google Scholar]
- Abdelkareem, M.A.; Maghrabie, H.M.; Abo-Khalil, A.G.; Adhari, O.H.K.; Sayed, E.T.; Radwan, A.; Elsaid, K.; Wilberforce, T.; Olabi, A.G. Battery Thermal Management Systems Based on Nanofluids for Electric Vehicles. J. Energy Storage 2022, 50, 104385. [Google Scholar] [CrossRef]
- Aruguete, D.M.; Hochella, M.F. Bacteriananoparticle Interactions and Their Environmental Implications. Environ. Chem. 2010, 7, 3–9. [Google Scholar] [CrossRef]
- Mo, Y.; Gu, A.; Tollerud, D.J.; Zhang, Q. Chapter Five—Nanoparticle Toxicity and Environmental Impact. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 117–143. ISBN 978-0-12-803269-5. [Google Scholar]
- Elsaid, K.; Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A.; Sayed, E.T. Environmental Impacts of Nanofluids: A Review. Sci. Total Environ. 2021, 763, 144202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qiu, J.; Shi, L.; Liu, Y.; Pan, B.; Xing, B. The Mechanisms and Environmental Implications of Engineered Nanoparticles Dispersion. Sci. Total Environ. 2020, 722, 137781. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.A.; Tzounis, L. Mechanical Reinforcement Course of 3D Printed Polypropylene–Antimony Doped Tin Oxide Nanocomposites versus Filler Loading. Adv. Compos. Mater. 2022, 31, 235–256. [Google Scholar] [CrossRef]


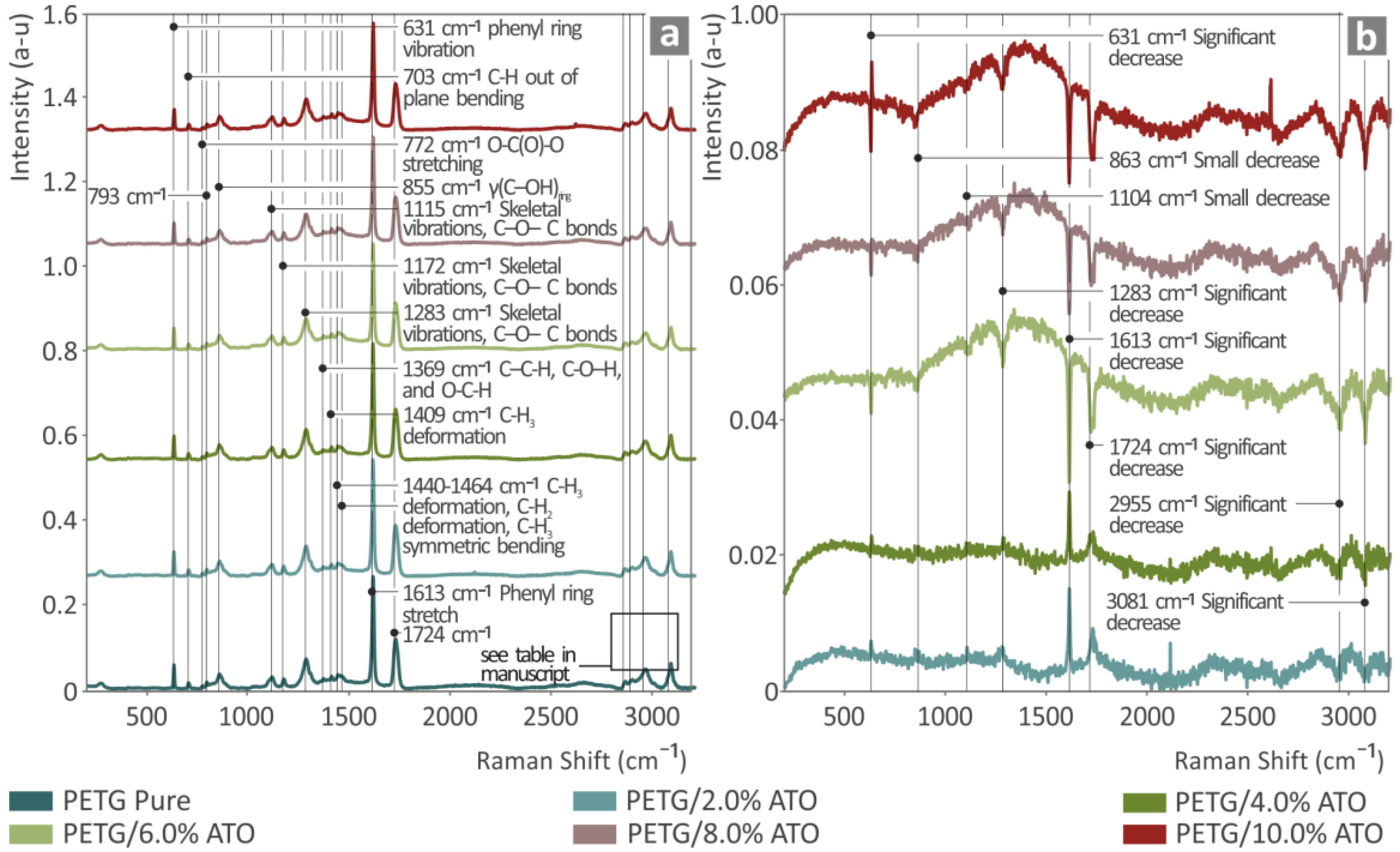


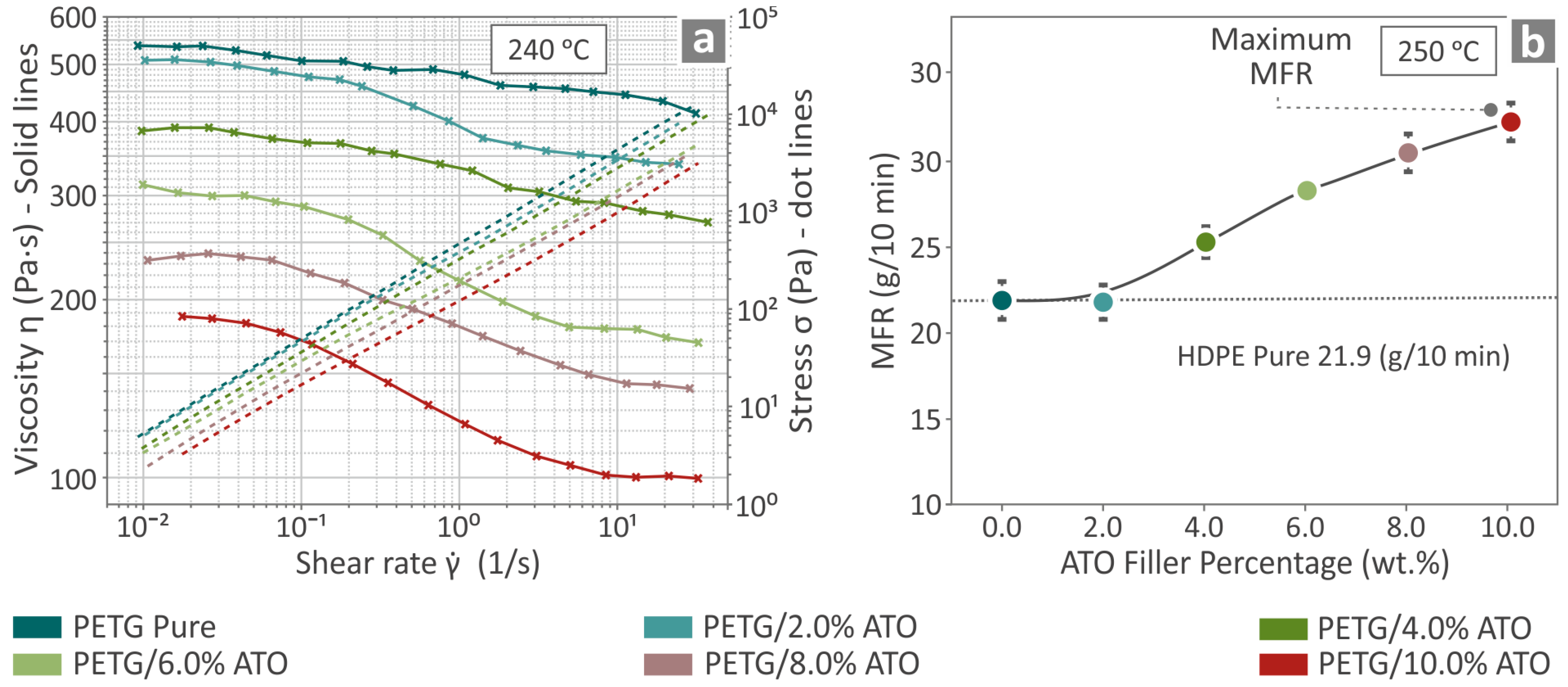


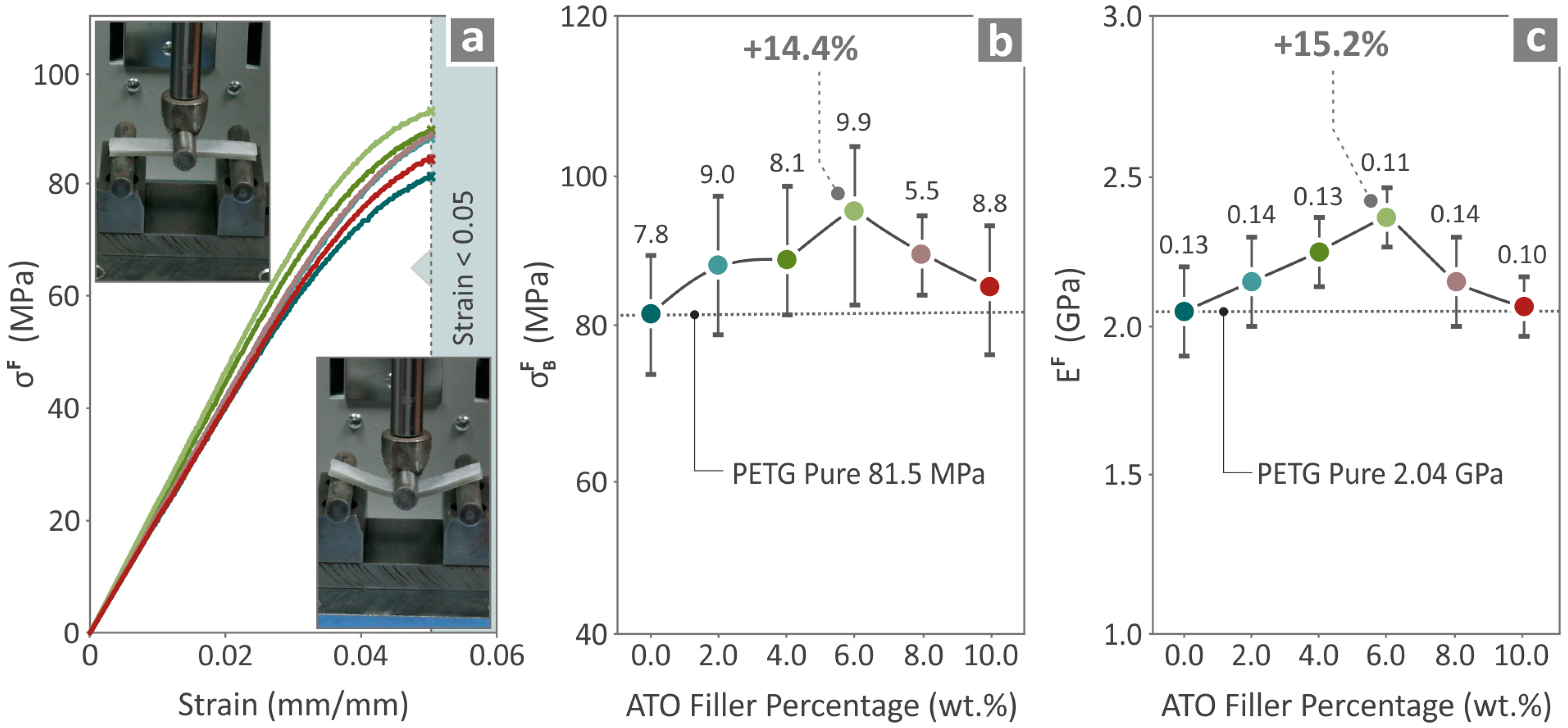

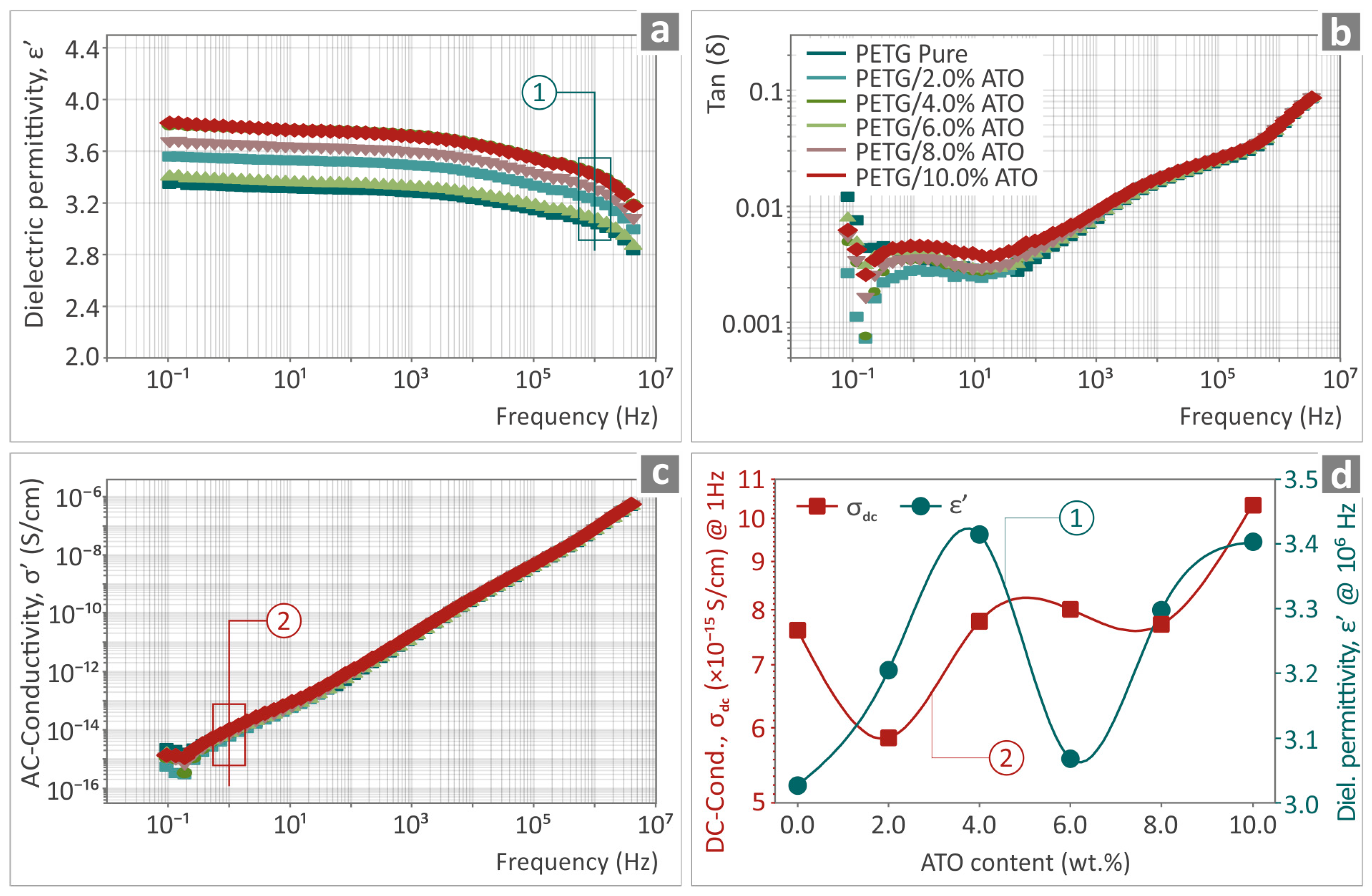

| Wavenumber (cm−1) | Intensity | Raman Peak Assignment |
|---|---|---|
| 631 | Strong | phenyl ring vibration [88,89] |
| 703 | Medium | C–H out-of-plane bending [88] |
| 772 | Small | O–C(O)–O stretching [89] |
| 793 | Medium | |
| 855 | Strong | γ(C–OH)ring [90,91] |
| 1115 | Strong | Skeletal vibrations, C–O–C bonds [92] |
| 1172 | Strong | Skeletal vibrations, C–O–C bonds [88,92] |
| 1283 | Strong | Skeletal vibrations, C–O–C bonds [88,92] |
| 1369 | Small | C–C–H, C–O–H, and O–C–H [92] |
| 1409 | Medium | C-H3 deformation [93] |
| 1440–1464 | Medium | C–H3 deformation [88,93]; C–H2 deformation [88,93]; C–H3 symmetric bending [89,93,94]; |
| 1613 | Very Strong | Phenyl ring stretch [89] |
| 1724 | Very Strong | |
| 2857 | Medium | C–H2 symmetric stretching [92] |
| 2890 | Medium | CH2 symmetric stretching [92,95] |
| 2955 | Strong | CH2 asymmetric stretching [92] |
| 3081 | Strong | C-H stretching [93] |
| 631 | Gradual drop | Significant decrease |
| 863 | Gradual drop | Small decrease |
| 1104 | Gradual drop | Small decrease |
| 1283 | Gradual drop | Significant decrease |
| 1613 | Gradual drop | Significant decrease |
| 1724 | Gradual drop | Significant decrease |
| 2955 | Gradual drop | Significant decrease |
| 3081 | Gradual drop | Significant decrease |
| Wavenumber (cm−1) | Intensity | ATR/FTIR Peak Assignment |
|---|---|---|
| 725 | Strong | CH vibration [96] |
| 792 | Weak | Unidentified |
| 872 | Medium | CH vibration [96] |
| 956 | Medium | C–C stretching [97] |
| 1016 | Strong | CH3 rocking [98]; C–O–C asymmetric stretching [99] |
| 1043 | Weak | C–O stretching [97,99] |
| 1093 | Strong | C–O stretching [97] |
| 1114 | Strong | C–O–C–O–C symmetric vibration [98] |
| 1173 | Weak | CH2 and CH3 rocking [98] |
| 1240 | Strong | CH3 rocking [98]; C–O stretching [96,99] |
| 1340 | Weak | CH2 wagging vibration [97] |
| 1371 | Weak | CH2 wagging vibration [100] |
| 1408 | Medium | C–H bending [100] |
| 1450 | Medium | CH2 bending [97,100] |
| 1504 | Weak | CH vibration [96] |
| 1578 | Weak | C–N stretching [101] |
| 1713 | Strong | C=O vibration [96,98,99,100,102] |
| 2850 | Medium | CH symmetric stretching [97] |
| 2923 | Medium | CH asymmetric stretching [97] |
| Wavenumber (cm−1) | Change | Importance |
|---|---|---|
| 668 (592–720) | Gradual increase | Significant |
| 733 | Gradual drop | Significant |
| 1066 | Gradual increase | Significant |
| 1213 (1137–1301) | Gradual increase | Significant |
| 1699 (1648–1738) | Gradual increase | Significant |
| 2850 | Gradual drop | Medium |
| 2923 | Gradual drop | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petousis, M.; Michailidis, N.; Saltas, V.; Papadakis, V.; Spiridaki, M.; Mountakis, N.; Argyros, A.; Valsamos, J.; Nasikas, N.K.; Vidakis, N. Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing. Nanomaterials 2024, 14, 761. https://doi.org/10.3390/nano14090761
Petousis M, Michailidis N, Saltas V, Papadakis V, Spiridaki M, Mountakis N, Argyros A, Valsamos J, Nasikas NK, Vidakis N. Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing. Nanomaterials. 2024; 14(9):761. https://doi.org/10.3390/nano14090761
Chicago/Turabian StylePetousis, Markos, Nikolaos Michailidis, Vassilis Saltas, Vassilis Papadakis, Mariza Spiridaki, Nikolaos Mountakis, Apostolos Argyros, John Valsamos, Nektarios K. Nasikas, and Nectarios Vidakis. 2024. "Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing" Nanomaterials 14, no. 9: 761. https://doi.org/10.3390/nano14090761
APA StylePetousis, M., Michailidis, N., Saltas, V., Papadakis, V., Spiridaki, M., Mountakis, N., Argyros, A., Valsamos, J., Nasikas, N. K., & Vidakis, N. (2024). Mechanical and Electrical Properties of Polyethylene Terephthalate Glycol/Antimony Tin Oxide Nanocomposites in Material Extrusion 3D Printing. Nanomaterials, 14(9), 761. https://doi.org/10.3390/nano14090761











