Responses of Tomato Photosystem II Photochemistry to Pegylated Zinc-Doped Ferrite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Zinc-Doped Ferrite Nanoparticles
2.2. Characterization of Zinc-Doped Ferrite Nanoparticles
2.3. Plant Material and Growth Conditions
2.4. Plant Treatment with Zinc-Doped Ferrite Nanoparticles
2.5. Chlorophyll Fluorescence Measurements
2.6. Statistical Analysis
3. Results
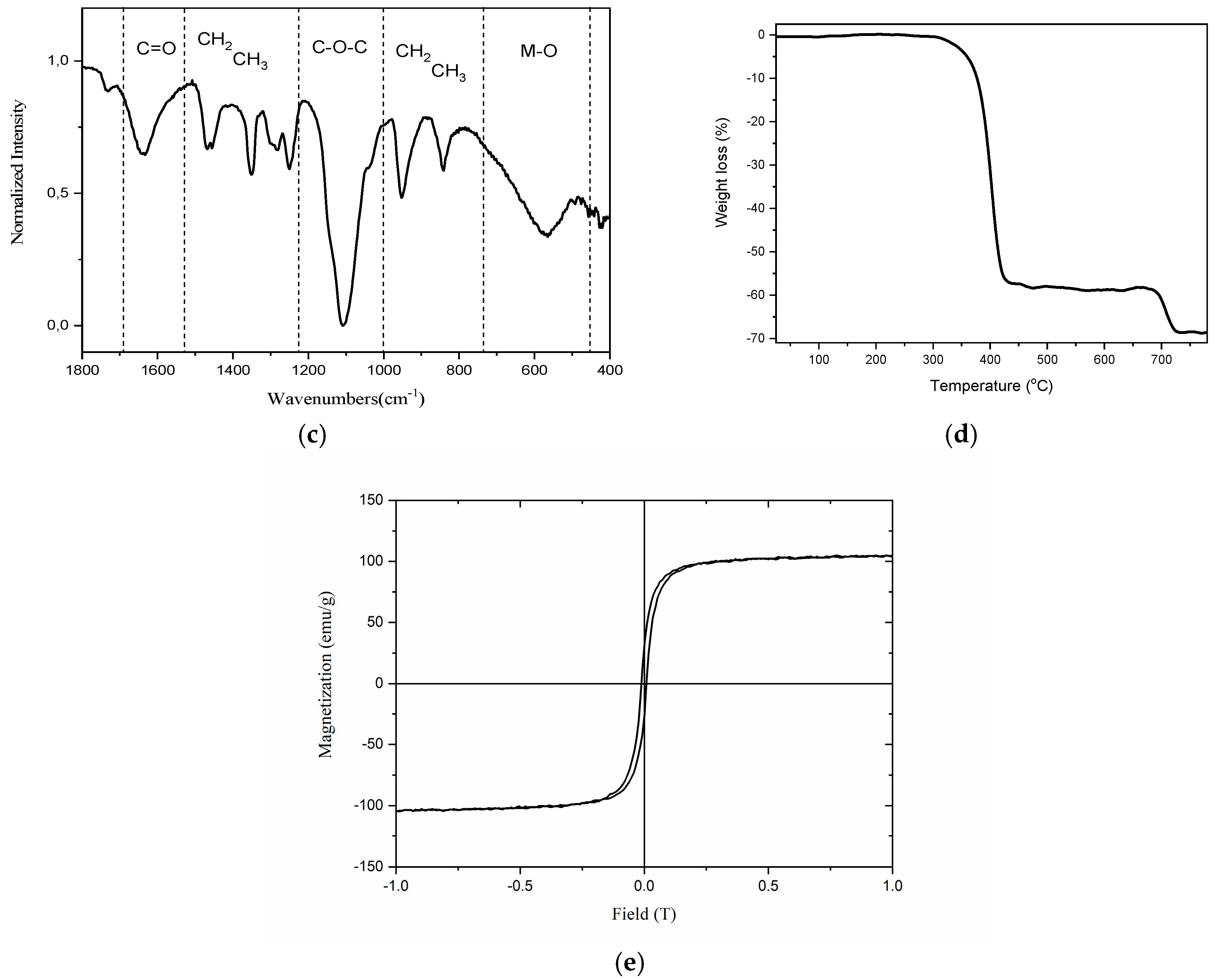
3.1. Synthesis and Characterization of Zinc-Doped Ferrite Nanoparticles
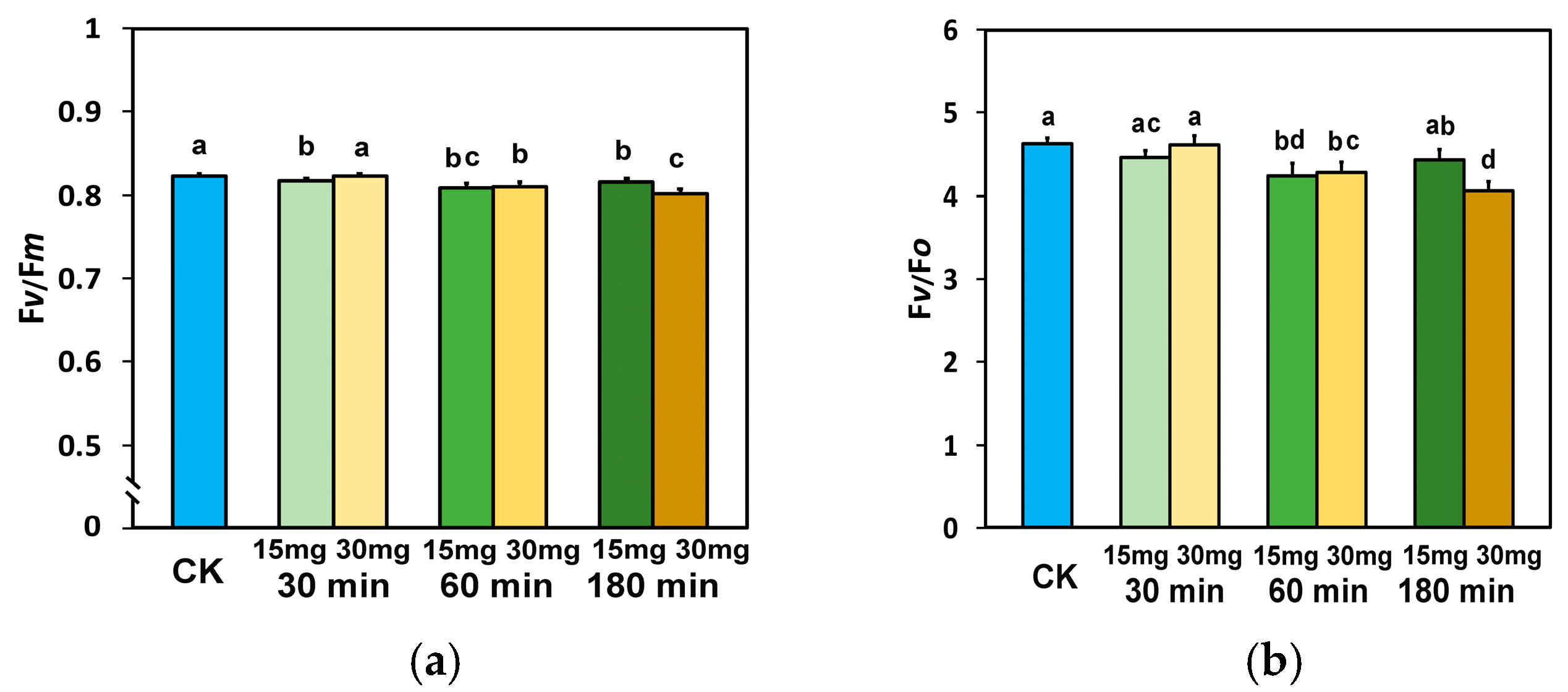
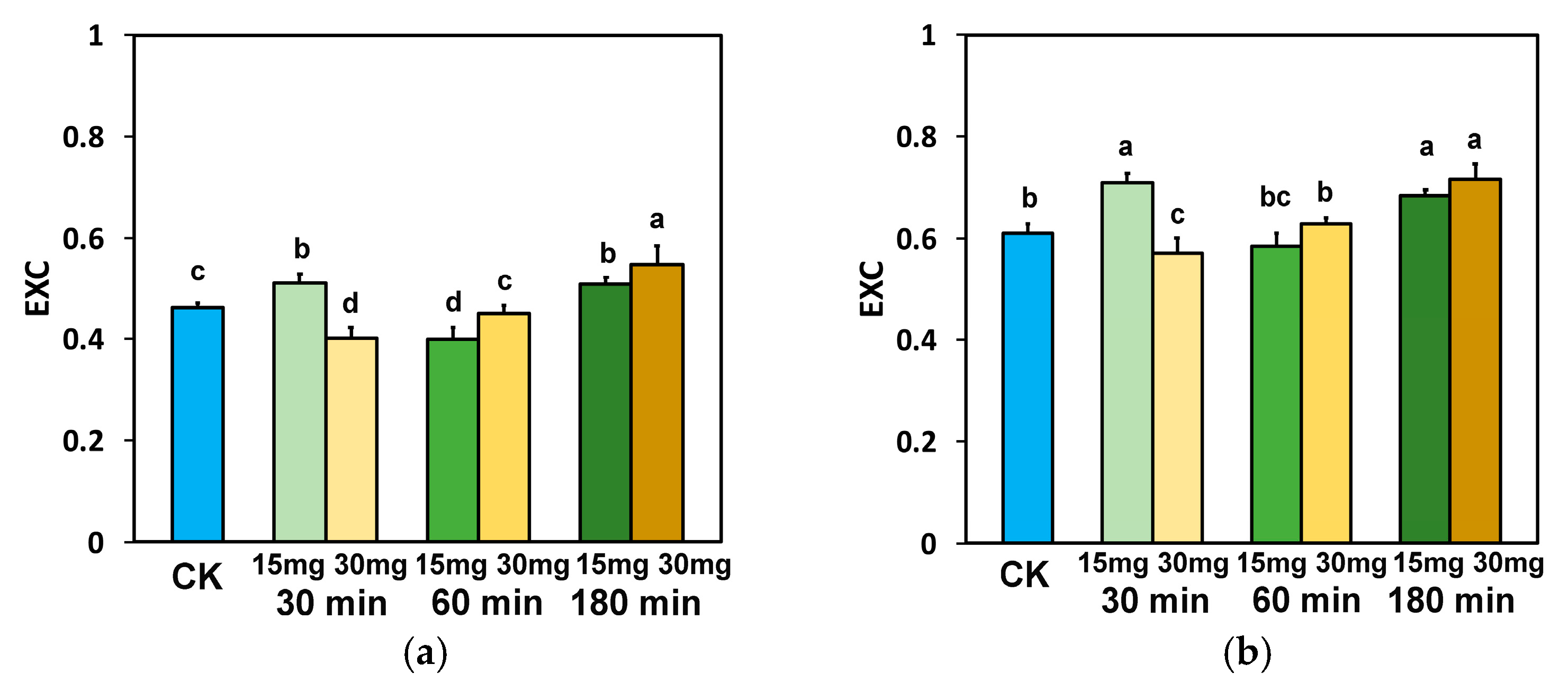
3.2. Maximum Efficiency of Photosystem II and Efficiency of the Oxygen-Evolving Complex in Tomato Leaflets Sprayed with Zinc-Doped Ferrite Nanoparticles
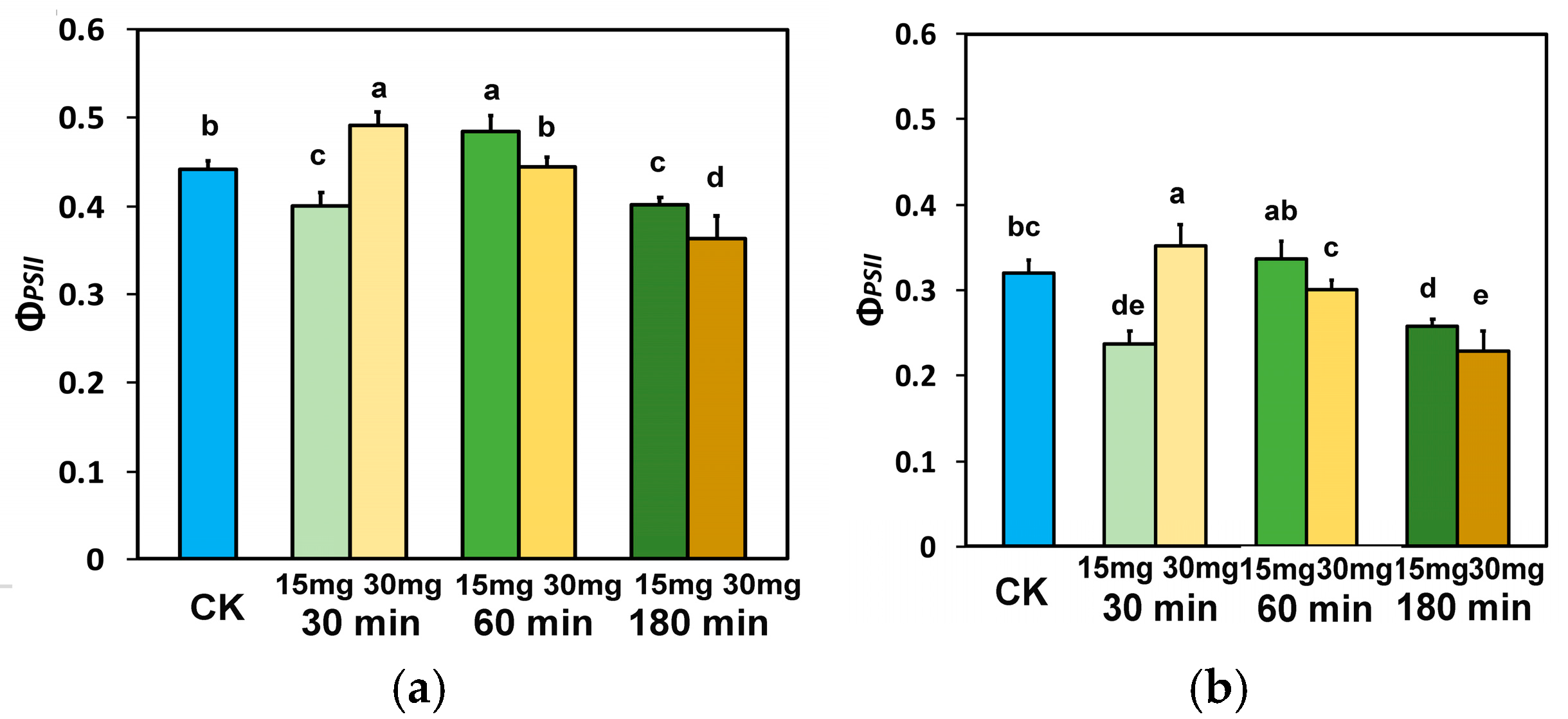
3.3. Distribution of the Absorbed Light Energy in Photosystem II in Tomato Leaflets Sprayed with Zinc-Doped Ferrite Nanoparticles
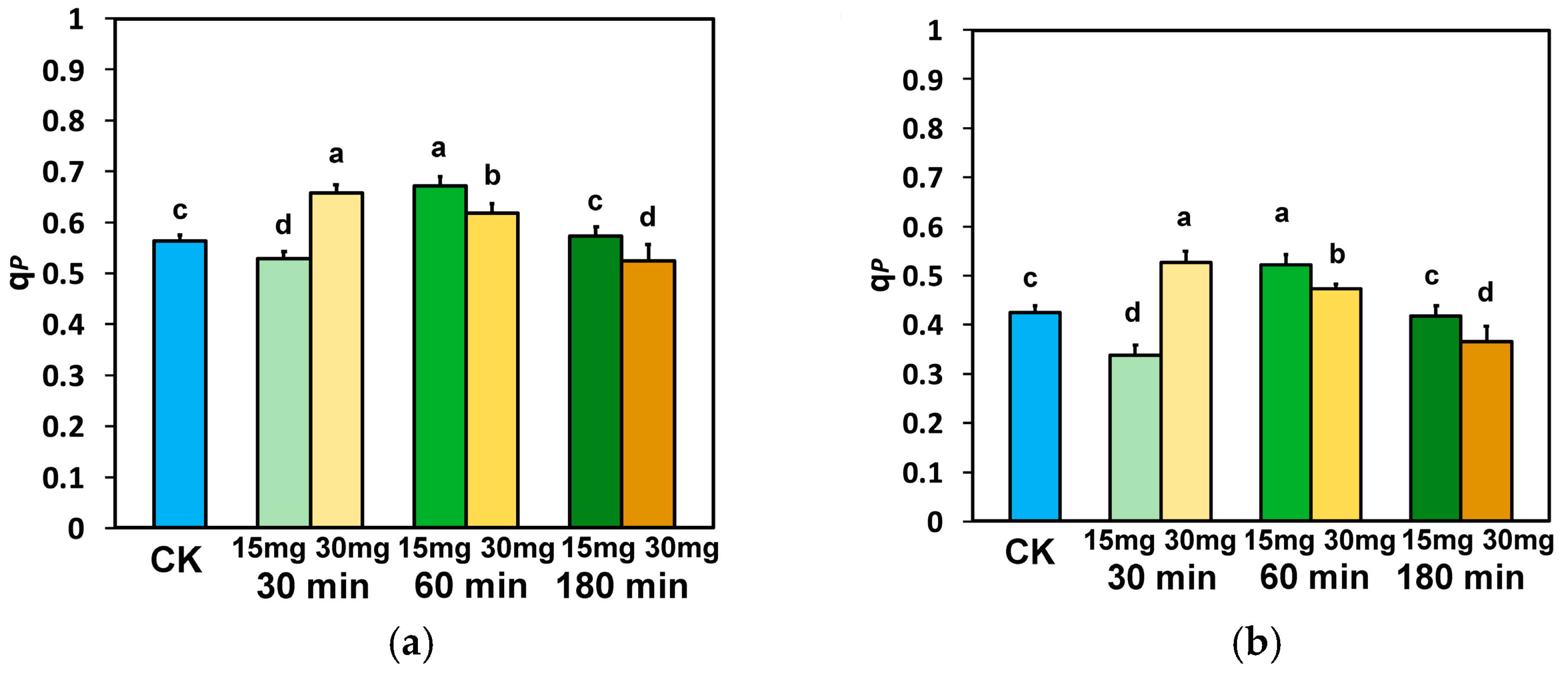
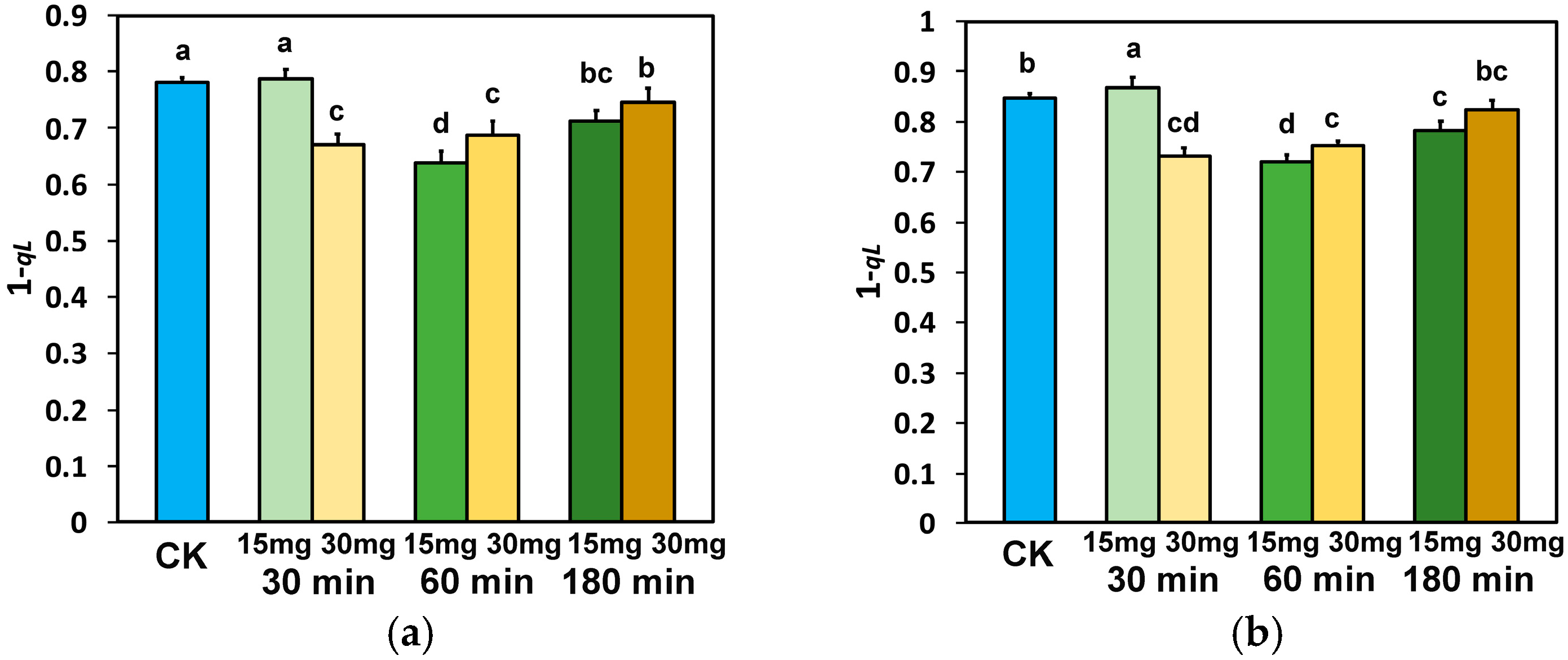
3.4. The Fraction of Open PSII Reaction Centers and the Electron Transport Efficiency in Tomato Leaflets Sprayed with Zinc-Doped Ferrite Nanoparticles
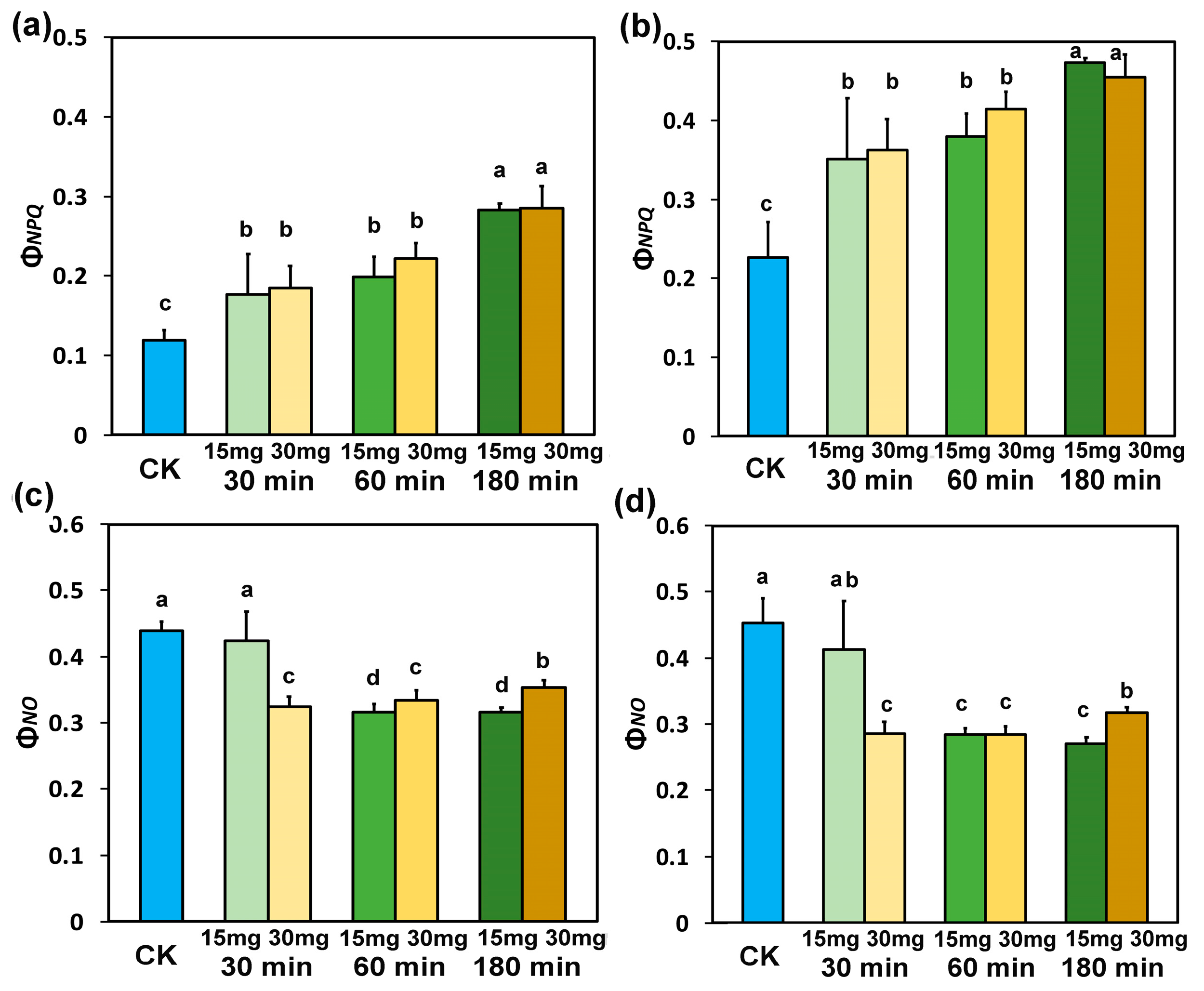
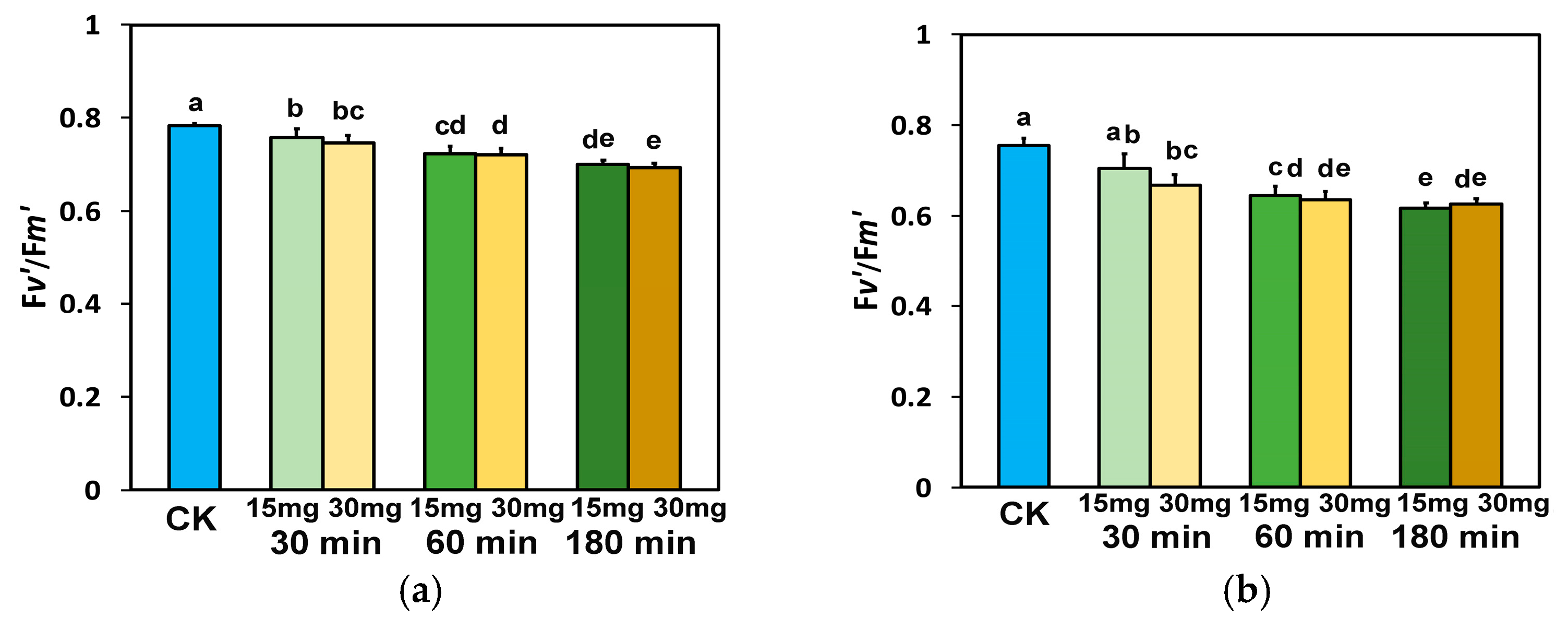
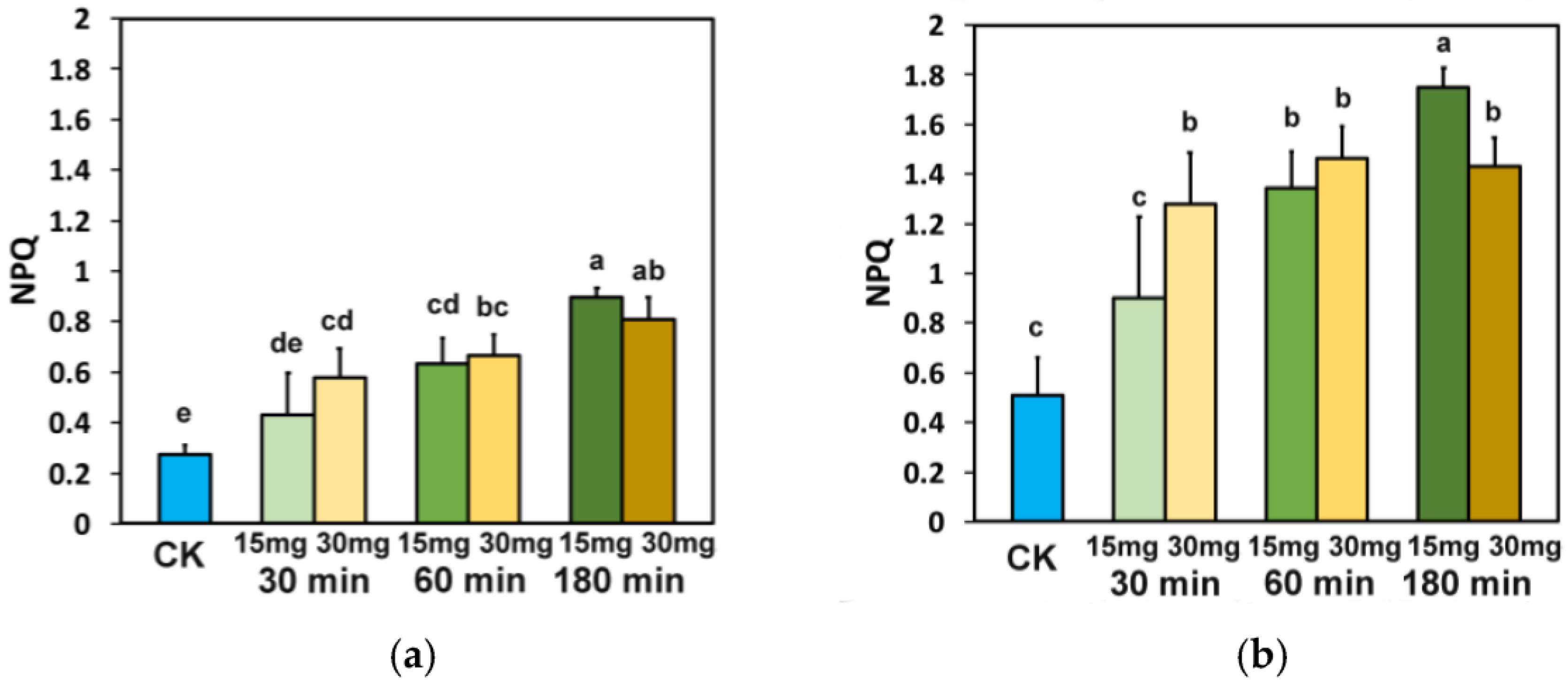
3.5. Impact of Zinc-Doped Ferrite Nanoparticles on the Efficiency of PSII Reaction Centers and the Non-Photochemical Quenching
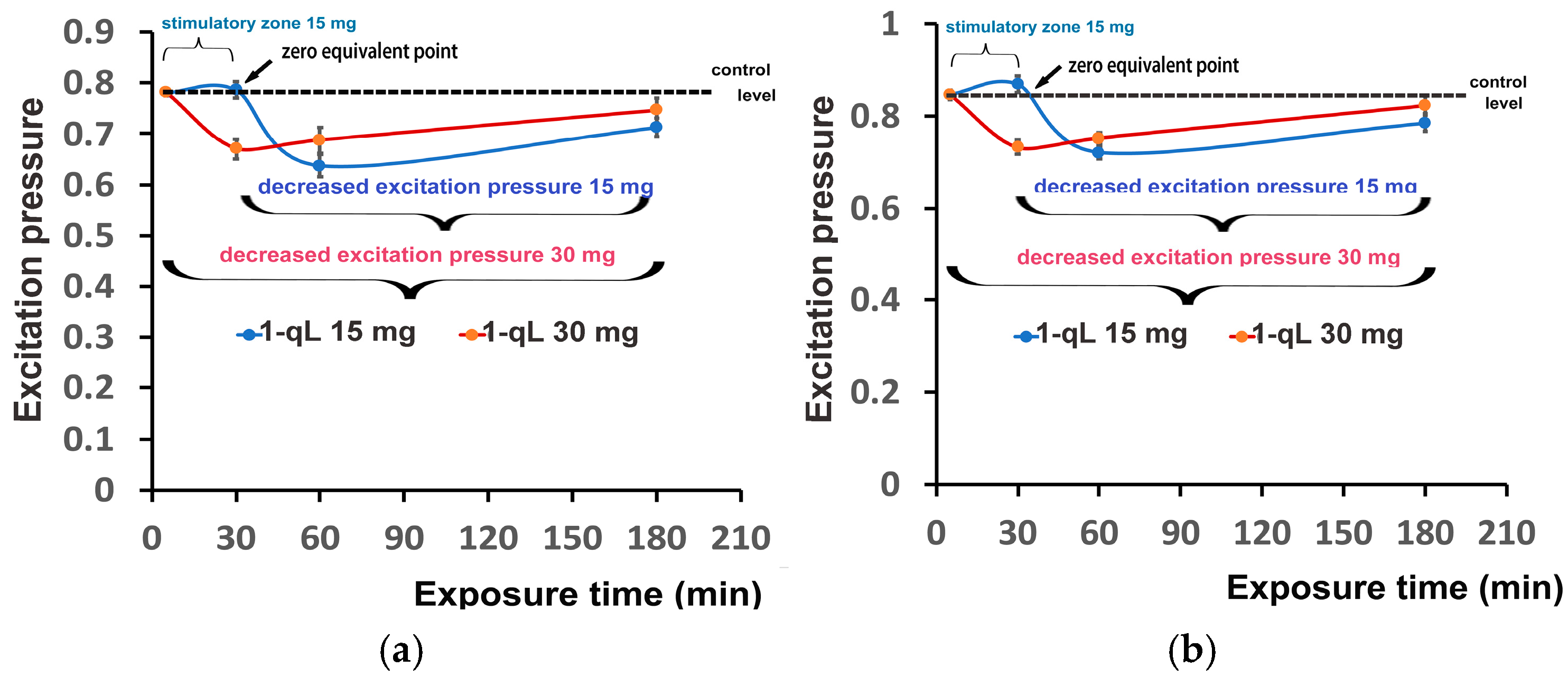
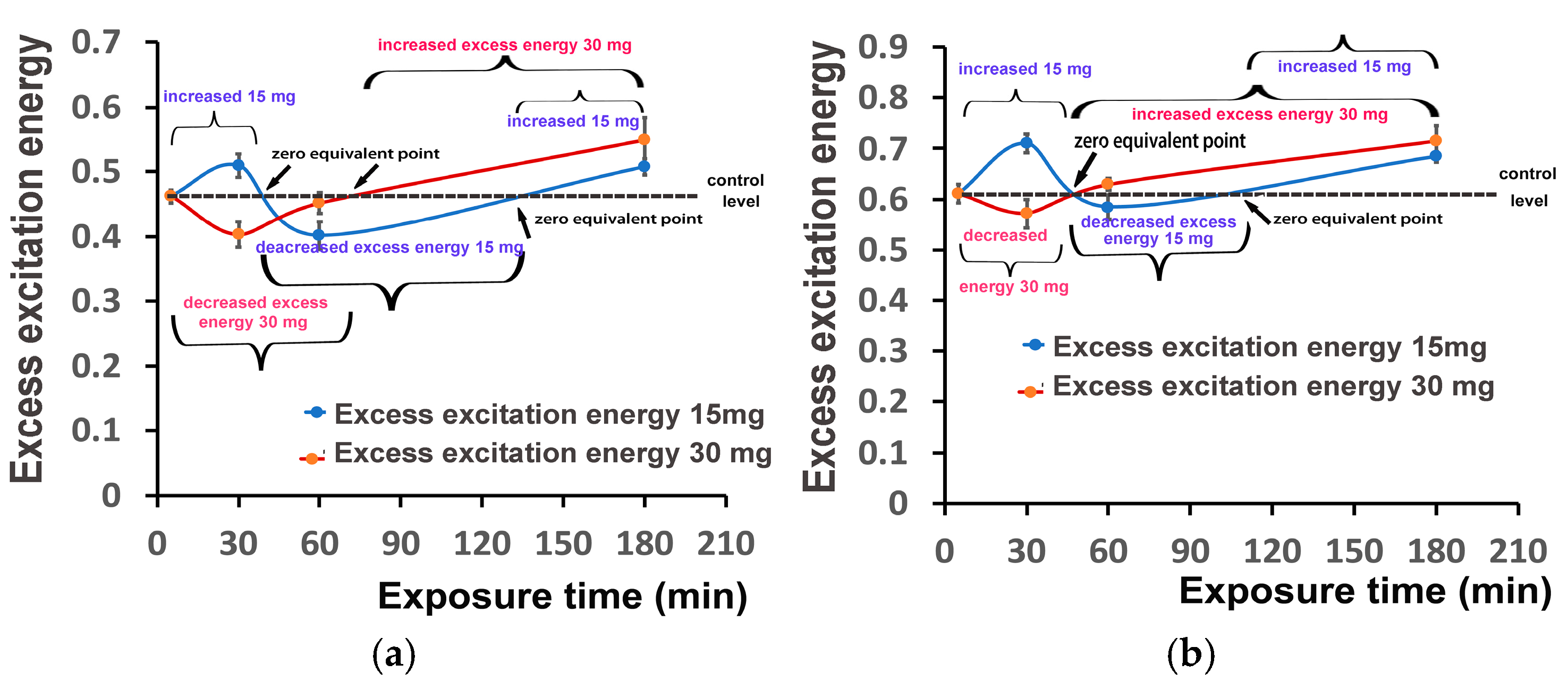
3.6. Impact of Zinc-Doped Ferrite Nanoparticles on PSII Excitation Presure and on Excess Excitation Energy at PSII
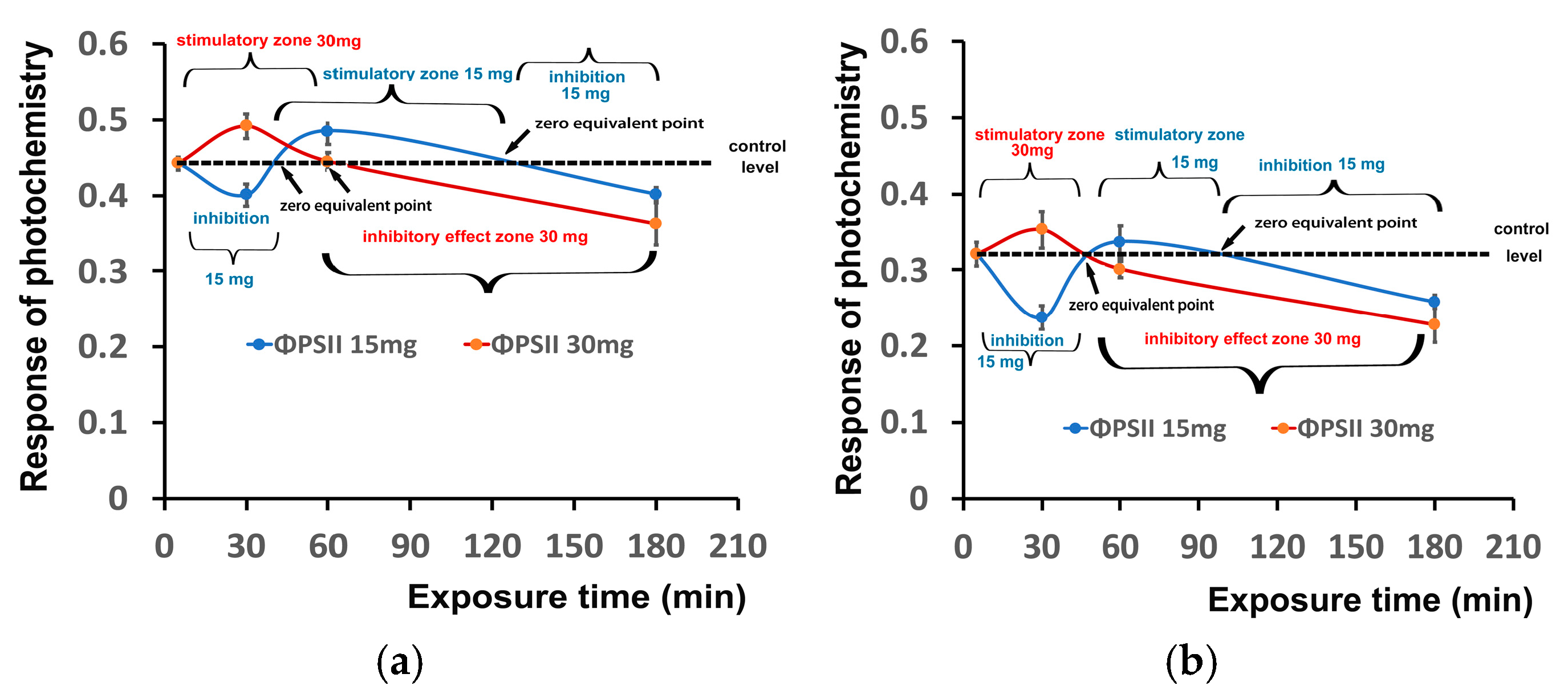
3.7. Hormetic Responses of Photosystem II in Tomato Leaflets Sprayed with Zinc-Doped Ferrite Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Metabolomics as a tool to understand nano-plant interactions: The case study of metal-based nanoparticles. Plants 2023, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Ferreira de Oliveira, J.M.P.; Dias, M.C.; Silva, A.M.S.; Santos, C. Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J. Hazard. Mater. 2019, 380, 120889. [Google Scholar] [CrossRef] [PubMed]
- Cantu, J.M.; Ye, Y.; Hernandez-Viezcas, J.A.; Zuverza-Mena, N.; White, J.C.; Gardea-Torresdey, J.L. Tomato fruit nutritional quality is altered by the foliar application of various metal oxide nanomaterials. Nanomaterials 2022, 12, 2349. [Google Scholar] [CrossRef] [PubMed]
- Tombuloglu, G.; Tombuloglu, H.; Slimani, Y.; Almessiere, M.A.; Baykal, A.; Bostancioglu, S.M.; Kirat, G.; Ercan, I. Effects of foliar iron oxide nanoparticles (Fe3O4) application on photosynthetic parameters, distribution of mineral elements, magnetic behaviour, and photosynthetic genes in tomato (Solanum lycopersicum var. cerasiforme) plants. Plant Physiol. Biochem. 2024, 210, 108616. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Moustakas, M.; Dendrinou-Samara, C. Impact of coated zinc oxide nanoparticles on photosystem II of tomato plants. Materials 2023, 16, 5846. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Dendrinou-Samara, C.; Moustakas, M. Modification of tomato photosystem II photochemistry with engineered zinc oxide nanorods. Plants 2023, 12, 3502. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.-S. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Abu-Elsaad, N.I.; Abdel Latef, A.A.H.; Metwally, R.A. Tracking of zinc ferrite nanoparticle effects on pea (Pisum sativum L.) plant growth, pigments, mineral content and arbuscular mycorrhizal colonization. Plants 2021, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic materials and systems: Domain structure visualization and other characterizationtechniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Di, S.; Ning, T.; Yu, J.; Chen, P.; Yu, H.; Wang, J.; Yang, H.; Zhu, S. Recent advances and applications of magnetic nanomaterials in environmental sample analysis. Trends Anal. Chem. 2020, 126, 115864. [Google Scholar] [CrossRef]
- Targuma, S.; Njobeh, P.B.; Ndungu, P.G. Current applications of magnetic nanomaterials for extraction of mycotoxins, pesticides, and pharmaceuticals in food commodities. Molecules 2021, 26, 4284. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Płóciennik, A.; Machnicka, B. Functionalized magnetic Fe3O4 nanoparticles for targeted methotrexate delivery in ovarian cancer therapy. Int. J. Mol. Sci. 2024, 25, 9098. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A review on photocatalysis used for wastewater treatment: Dye degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R. Fabrication of highly visible-light-responsive ZnFe2O4/TiO2 heterostructures for the enhanced photocatalytic degradation of organic dyes. RSC Adv. 2016, 6, 103428–103437. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Moustaka, J.; Adamakis, I.-D.S.; Giannousi, K.; Dendrinou-Samara, C.; Moustakas, M. Hormetic response of photosystem II function induced by nontoxic calcium hydroxide nanoparticles. Int. J. Mol. Sci. 2024, 25, 8350. [Google Scholar] [CrossRef]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health 2018, 6, 77–83. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; ALmoshadak, A.S.; Shafi, M.E.; Albaqami, N.M.; Saad, A.M.; El-Tahan, A.M.; Desoky, E.M.; Elnahal, A.S.M.; Almakas, A.; Abd El-Mageed, T.A.; et al. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J. Biol. Sci. 2021, 28, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Calatayud, A. Non-invasive tools to estimate stress-induced changes in photosynthetic performance in plants inhabiting Mediterranean areas. Environ. Exp. Bot. 2014, 103, 42–52. [Google Scholar] [CrossRef]
- Oláh, V.; Hepp, A.; Irfan, M.; Mészáros, I. Chlorophyll fluorescence imaging-based duckweed phenotyping to assess acute phytotoxic effects. Plants 2021, 10, 2763. [Google Scholar] [CrossRef] [PubMed]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll fluorescence imaging for early detection of drought and heat stress in strawberry plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Wolosiuk, R.A.; Malkin, R. Photosynthesis. In Biochemistry & Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 508–566. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Wrzaczek, M.; Brosché, M.; Kangasjärvi, J. ROS signaling loops-production, perception, regulation. Curr. Opin. Plant Biol. 2013, 16, 575–582. [Google Scholar] [CrossRef]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kogoshi, M.; Nishio, D.; Kitahata, N.; Ohwada, H.; Kuchitsu, K.; Mizuno, H.; Kurusu, T. Novel in silico screening system for plant defense activators using deep learning-based prediction of reactive oxygen species accumulation. Plant Methods 2023, 19, 142. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Root-associated entomopathogenic fungi modulate their host plant’s photosystem II photochemistry and response to herbivorous insects. Molecules 2022, 27, 207. [Google Scholar] [CrossRef]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Harayama, H. On the non-monotonic, hormetic photoprotective response of plants to stress. Dose Response 2019, 17, 1559325819838420. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st Century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Ż.; Pogrzeba, M.; Kalaji, H.M.; Zieleźnik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Wei, C.; Jiao, Q.; Agathokleous, E.; Liu, H.; Li, G.; Zhang, J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef]
- Agathokleous, E.; Sonne, C.; Benelli, G.; Calabrese, E.J.; Guedes, R.N.C. Low-dose chemical stimulation and pest resistance threaten global crop production. Sci. Total Environ. 2023, 878, 162989. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Sperdouli, I.; Hanć, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid hormetic responses of photosystem II photochemistry of clary sage to cadmium exposure. Int. J. Mol. Sci. 2021, 22, 41. [Google Scholar] [CrossRef]
- Bayçu, G.; Moustaka, J.; Gevrek-Kürüm, N.; Moustakas, M. Chlorophyll fluorescence imaging analysis for elucidating the mechanism of photosystem II acclimation to cadmium exposure in the hyperaccumulating plant Noccaea caerulescens. Materials 2018, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Adamakis, I.S.; Moustaka, J.; Apostolova, E. A hormetic spatiotemporal photosystem II response mechanism of salvia to excess zinc exposure. Int. J. Mol. Sci. 2022, 23, 11232. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Şaş, B.; İşgören, S.; Moustaka, J.; Morales, F. Mechanistic approach on melatonin-induced hormesis of photosystem II function in the medicinal plant Mentha spicata. Plants 2023, 12, 4025. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.-L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.-D.S.; Moustakas, M. Hormetic responses of photosystem II in tomato to Botrytis cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Cheah, B.H.; Chen, Y.L.; Lo, J.C.; Tang, I.C.; Yeh, K.C.; Lin, Y.F. Divalent nutrient cations: Friend and foe during zinc stress in rice. Plant Cell Environ. 2021, 44, 3358–3375. [Google Scholar] [CrossRef]
- Sperdouli, I.; Adamakis, I.D.S.; Dobrikova, A.; Apostolova, E.; Hanć, A.; Moustakas, M. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef]
- Doncheva, S.; Stoyanova, Z.; Velikova, V. The influence of succinate on zinc toxicity of pea plant. J. Plant Nutr. 2001, 24, 789–804. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakas, M. Tolerance mechanisms of the aromatic and medicinal plant Salvia sclarea L. to excess zinc. Plants 2021, 10, 194. [Google Scholar] [CrossRef]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smith, S. Mineral nutrition. In Plant Physiology and Development; Taiz, L., Zeiger, E., Møller, I.M., Murphy, A., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; pp. 119–142. [Google Scholar]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J. Agron. Crop Sci. 2022, 208, 314–334. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Ma, Y.; Liu, L.; Wu, D.; Shu, X.; Pan, L.; Lai, Q. Combination of high Zn density and low phytic acid for improving Zn bioavailability in rice (Oryza sativa L.) grain. Rice 2021, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.G.; Liu, Y.M.; Chen, X.X.; Cao, W.Q.; Ding, T.B.; Zou, C.Q. Foliar zinc application to wheat may lessen the zinc deficiency burden in rural Quzhou, China. Front. Nutr. 2021, 8, 697817. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Huertas, R.; Karpinska, B.; Ngala, S.; Mkandawire, B.; Malingá, J.; Wajenkeche, E.; Kimani, P.M.; Boesch, C.; Stewart, D.; Hancock, R.D.; et al. Biofortification of common bean (Phaseolus vulgaris L.) with iron and zinc: Achievements and challenges. Food Energy Secur. 2023, 12, e406. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. Hormesis can enhance agricultural sustainability in a changing world. Glob. Food Secur. 2019, 20, 150–155. [Google Scholar] [CrossRef]
- Wang, K.; Miao, Y.; Wang, X.; Li, Y.; Li, F.; Song, H. Research on the construction of a knowledge graph for tomato leaf pests and diseases based on the named entity recognition model. Front. Plant Sci. 2024, 15, 1482275. [Google Scholar] [CrossRef]
- Bora, U.; Kumar, K.D.; Kumar, S.; Sharma, P.; Nahar, P. Photochemical activation of polyethylene glycol and its application in PEGylation of protein. Process Biochem. 2011, 46, 1380–1383. [Google Scholar] [CrossRef]
- Sheikhi, S.; Aliannezhadi, M.; Tehrani, F.S. The effect of PEGylation on optical and structural properties of ZnO nanostructures for photocatalyst and photodynamic applications. Mater. Today Commun. 2023, 34, 105103. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Khan, M.; Saleem, S.; Ali, S.; Hussain, F.; Muhammad, R.; Khesro, A.; Abdullah; Ling, Y. Non-stoichiometric zinc ferrite nanostructures: Dielectric, magnetic, optical and photoelectrochemical properties. Mater. Today Commun. 2021, 28, 102662. [Google Scholar]
- Zhang, X.; Fu, J.; Hiromasa, Y.; Pan, H.; Bai, G. Differentially expressed proteins associated with Fusarium head blight resistance in wheat. PLoS ONE 2013, 8, e82079. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Moustaka, J.; Sperdouli, I.; Moustakas, M. Light energy use efficiency in photosystem II of tomato is related to leaf age and light intensity. Crops 2024, 4, 623–635. [Google Scholar] [CrossRef]
- Retta, M.A.; Van Doorselaer, L.; Driever, S.M.; Yin, X.; de Ruijter, N.C.A.; Verboven, P.; Nicolaï, B.M.; Struik, P.C. High photosynthesis rates in Brassiceae species are mediated by leaf anatomy enabling high biochemical capacity, rapid CO2 diffusion and efficient light use. New Phytol. 2024, 244, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Long, S.P. One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proc. R. Soc. B. 2016, 283, 20152578. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.J.; Masclaux-Daubresse, C.; Strittmatter, G.; Weber, A.P.M.; Taylor, S.H.; Harbinson, J.; Yin, X.; Long, S.; Paul, M.J.; Westhoff, P.; et al. Improving crop yield potential: Underlying biological processes and future prospects. Food Energy Secur. 2023, 12, e435. [Google Scholar] [CrossRef]
- Garcia, A.; Gaju, O.; Bowerman, A.F.; Buck, S.A.; Evans, J.R.; Furbank, R.T.; Gilliham, M.; Millar, A.H.; Pogson, B.J.; Reynolds, M.P.; et al. Enhancing crop yields through improvements in the efficiency of photosynthesis and respiration. New Phytol. 2023, 237, 60–77. [Google Scholar] [CrossRef]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on improving photosynthesis to increase crop yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Struik, P.C. Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: From leaf biochemistry to canopy physiology and crop ecology. J. Exp. Bot. 2015, 66, 6535–6549. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nosberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J. Improving photosynthetic metabolism for crop yields: What is going to work? Front. Plant Sci. 2021, 12, 743862. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kasajima, I.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H. Estimation of the relative sizes of rate constants for chlorophyll de-excitation processes through comparison of inverse fluorescence intensities. Plant Cell Physiol. 2009, 50, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams III, W.W.; Baker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocation to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- De Bianchi, S.; Betterle, N.; Kouril, R.; Cazzaniga, S.; Boekema, E.; Bassi, R.; Dall’Osto, L. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 2011, 23, 2659–2679. [Google Scholar] [CrossRef]
- Ruban, A.V. Quantifying the efficiency of photoprotection. Philos. Trans. R. Soc. B 2017, 372, 20160393. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Moustakas, M.; Panteris, E.; Moustaka, J.; Aydın, T.; Bayçu, G.; Sperdouli, I. Modulation of photosystem ΙΙ function in celery via foliar-applied salicylic acid during gradual water deficit stress. Int. J. Mol. Sci. 2024, 25, 6721. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustaka, J.; Ouzounidou, G.; Moustakas, M. Leaf age-dependent photosystem II photochemistry and oxidative stress responses to drought stress in Arabidopsis thaliana are modulated by flavonoid accumulation. Molecules 2021, 26, 4157. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef]
- Fallah, S.; Yusefi-Tanhaa, E.; Peralta-Videa, J.R. Interaction of nanoparticles and reactive oxygen species and their impact on macromolecules and plant production. Plant Nano Biol. 2024, 10, 100105. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant andanimal cells. Free Radic. Biol. Med. 2018, 22, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyeva, L.I. ROS as signaling molecules to initiate the process of plant acclimatization to abiotic stress. Int. J. Mol. Sci. 2024, 25, 11820. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Vinyard, D.J.; Ananyev, G.M.; Dismukes, G.C. Photosystem II: The reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 2013, 82, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M.; Ghobadi, M.Z.; Haghighi, B.; Eaton-Rye, J.J.; Tomo, T.; Shen, J.R.; Allakhverdiev, S.I. Nano-sized manganese-calcium cluster in photosystem II. Biochemistry 2014, 79, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Noguchi, T. Intermediate formation via proton release during the photoassembly of the water-oxidizing Mn4CaO5 cluster in photosystem II. J. Phys. Chem. B 2024, 128, 8145–8161. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Yachandra, V. Mn4Ca cluster in photosynthesis: Where and how water is oxidized to dioxygen. Chem. Rev. 2014, 114, 4175–4205. [Google Scholar] [CrossRef]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol. Plant. 2004, 121, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Carucci, M.G.; Campanella, A.; Lorenzini, G.; Nali, C. Ozone stress in Melissa officinalis plants assessed by photosynthetic function. Environ. Exp. Bot. 2011, 73, 94–101. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Hayat, S. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol. Biochem. 2018, 129, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Mensuali Sodi, A. UV-B physiological changes under conditions of distress and eustress in sweet basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, D.; Soni, V. Performance of chlorophyll a fluorescence parameters in Lemna minor under heavy metal stress induced by various concentration of copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M.; et al. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2023, 224, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, A.; Kornaś, A.; Skoczowski, A.; Oliwa, J.; Jurkow, R.; Gil, J.; Sękara, A.; Sałata, A.; Caruso, G. Leaf chlorophyll fluorescence and reflectance of oakleaf lettuce exposed to metal and metal(oid) oxide nanoparticles. BMC Plant Biol. 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farrag, E.S.; Mahmoud, S.Y. Dieback of royal poinciana (Delonix regia) trees induced by Alternaria tenuissima and its impact on photochemical efficiency of photosystem II. Physiol. Mol. Plant Pathol. 2024, 133, 102357. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Nagy, V.; Puthur, J.T.; Kovács, L.; Garab, G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef]
- Széles, E.; Kuntam, S.; Vidal-Meireles, A.; Nagy, V.; Nagy, K.; Ábrahám, Á.; Kovács, L.; Tóth, S.Z. Single-cell microfluidics in combination with chlorophyll a fluorescence measurements to assess the lifetime of the Chlamydomonas PSBO protein. Photosynthetica 2023, 61, 417–424. [Google Scholar] [CrossRef]
- Gao, S.; Han, H.; Feng, H.L.; Zhao, S.J.; Meng, Q.W. Overexpression and suppression of violaxanthin de-epoxidase affects the sensitivity of photosystem II photoinhibition to high light and chilling stress in transgenic tobacco. J. Integr. Plant Biol. 2010, 52, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.C.; Hikosaka, K.; Hirotsu, N.; Makino, A.; Hirose, T. The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol. 2003, 44, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim. Biophys. Acta 2005, 1706, 68–80. [Google Scholar] [CrossRef]
- Ohnishi, N.; Allakhverdiev, S.I.; Takahashi, S.; Higashi, S.; Watanabe, M.; Nishiyama, Y.; Murata, N. Two-step mechanism of photodamage to photosystem II: Step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 2005, 44, 8494–8499. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Oguchi, R.; Terashima, I.; Chow, W.S. The involvement of dual mechanisms of photoinactivation of photosystem II in Capsicum annuum L. plants. Plant Cell Physiol. 2009, 50, 1815–1825. [Google Scholar] [CrossRef]
- Campbell, D.A.; Tyystjärvi, E. Parameterization of photosystem II photoinactivation and repair. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zavafer, A.; Koinuma, W.; Chow, W.S.; Cheah, M.H.; Mino, H. Mechanism of photodamage of the oxygen evolving Mn cluster of photosystem II by excessive light energy. Sci. Rep. 2017, 7, 7604. [Google Scholar] [CrossRef] [PubMed]
- Callahan, F.E.; Becker, D.W.; Cheniae, G.M. Studies on the photo-inactivation of the water-oxidizing enzyme. II. Characterization of weak light photoinhibition of PSII and its light-induced recovery. Plant Physiol. 1986, 82, 261–269. [Google Scholar] [CrossRef]
- Chen, G.X.; Kazimir, J.; Cheniae, G.M. Photoinhibition of hydroxylamine-extracted photosystem II membranes: Studies of the mechanism. Biochemistry 1992, 31, 11072–11083. [Google Scholar] [CrossRef]
- Anderson, J.M.; Park, Y.I.; Chow, W.S. Unifying model for the photoinactivation of photosystem II in vivo: A hypothesis. Photosynth. Res. 1998, 56, 1–13. [Google Scholar] [CrossRef]
- Sarvikas, P.; Hakala, M.; Pätsikkä, E.; Tyystjärvi, T.; Tyystjärvi, E. Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of beta-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta 2015, 1847, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Dabagh, S.; Haris, S.A.; Ertas, Y.N. Engineered Polyethylene Glycol-Coated Zinc Ferrite Nanoparticles as a Novel Magnetic Resonance Imaging Contrast Agent. ACS Biomater. Sci. Eng. 2023, 9, 4138–4148. [Google Scholar]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol. Plant. 2015, 37, 222. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, J.; Xie, J.M.; Yu, J.H.; Dawuda, M.M.; Lyv, J.; Tang, Z.Q.; Zhang, J.; Zhang, X.D.; Tang, C.N. Changes in photosynthesis and carotenoid composition of pepper (Capsicum annuum L.) in response to low-light stress and low temperature combined with low-light stress. Photosynthetica 2020, 58, 125–136. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Xie, J.; Li, J.; Zhang, X.; Wang, C. Exogenous strigolactones alleviate low-temperature stress in peppers seedlings by reducing the degree of photoinhibition. BMC Plant Biol. 2024, 24, 907. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperdouli, I.; Giannousi, K.; Moustaka, J.; Antonoglou, O.; Dendrinou-Samara, C.; Moustakas, M. Responses of Tomato Photosystem II Photochemistry to Pegylated Zinc-Doped Ferrite Nanoparticles. Nanomaterials 2025, 15, 288. https://doi.org/10.3390/nano15040288
Sperdouli I, Giannousi K, Moustaka J, Antonoglou O, Dendrinou-Samara C, Moustakas M. Responses of Tomato Photosystem II Photochemistry to Pegylated Zinc-Doped Ferrite Nanoparticles. Nanomaterials. 2025; 15(4):288. https://doi.org/10.3390/nano15040288
Chicago/Turabian StyleSperdouli, Ilektra, Kleoniki Giannousi, Julietta Moustaka, Orestis Antonoglou, Catherine Dendrinou-Samara, and Michael Moustakas. 2025. "Responses of Tomato Photosystem II Photochemistry to Pegylated Zinc-Doped Ferrite Nanoparticles" Nanomaterials 15, no. 4: 288. https://doi.org/10.3390/nano15040288
APA StyleSperdouli, I., Giannousi, K., Moustaka, J., Antonoglou, O., Dendrinou-Samara, C., & Moustakas, M. (2025). Responses of Tomato Photosystem II Photochemistry to Pegylated Zinc-Doped Ferrite Nanoparticles. Nanomaterials, 15(4), 288. https://doi.org/10.3390/nano15040288






