Utilizing Nanomaterials in Microfluidic Devices for Disease Detection and Treatment
Abstract
:1. Introduction
2. Application of Microfluidic Technology Integrated with Nanomaterials in Disease Detection
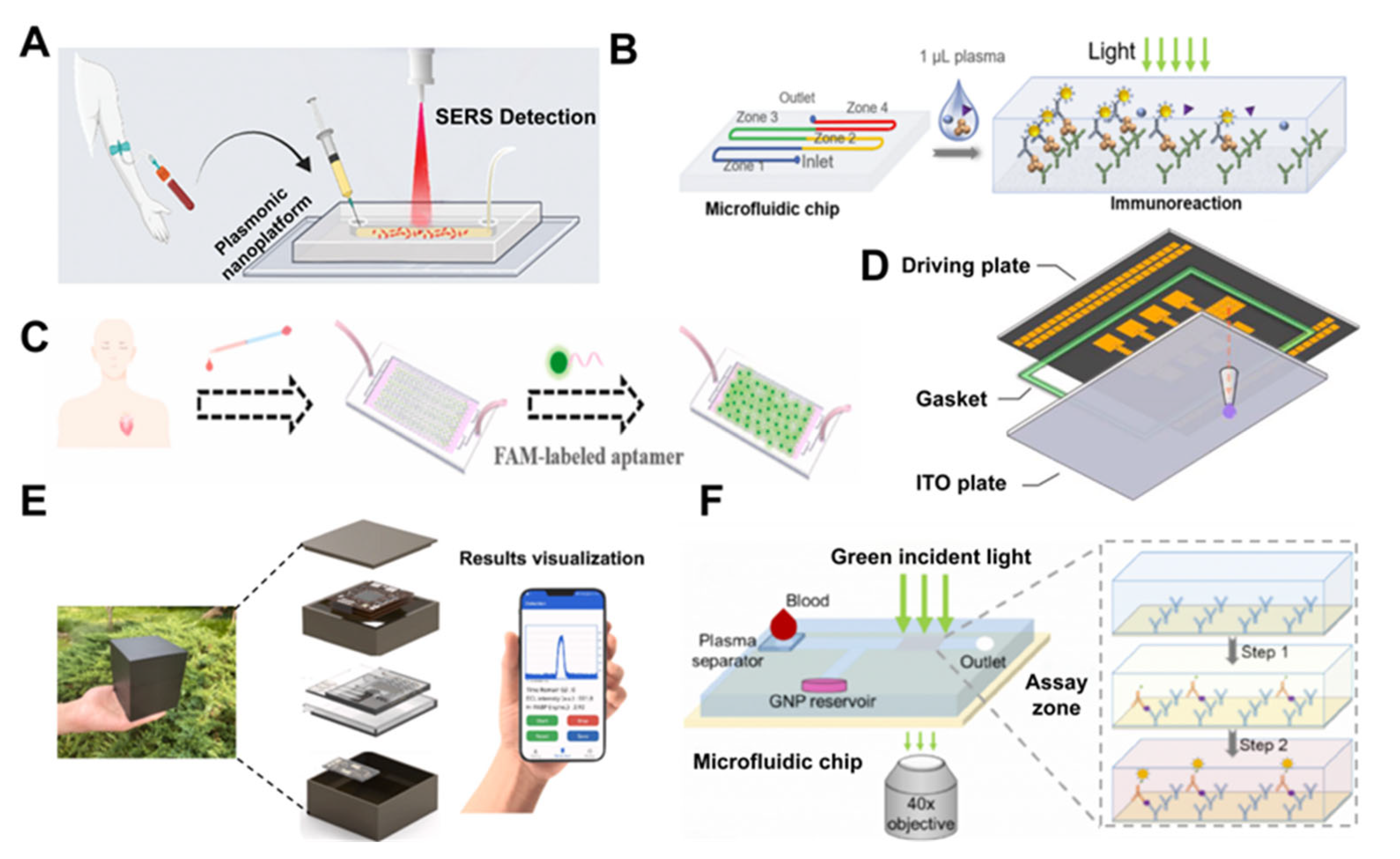
2.1. Detection of Cardiovascular Disease Biomarkers
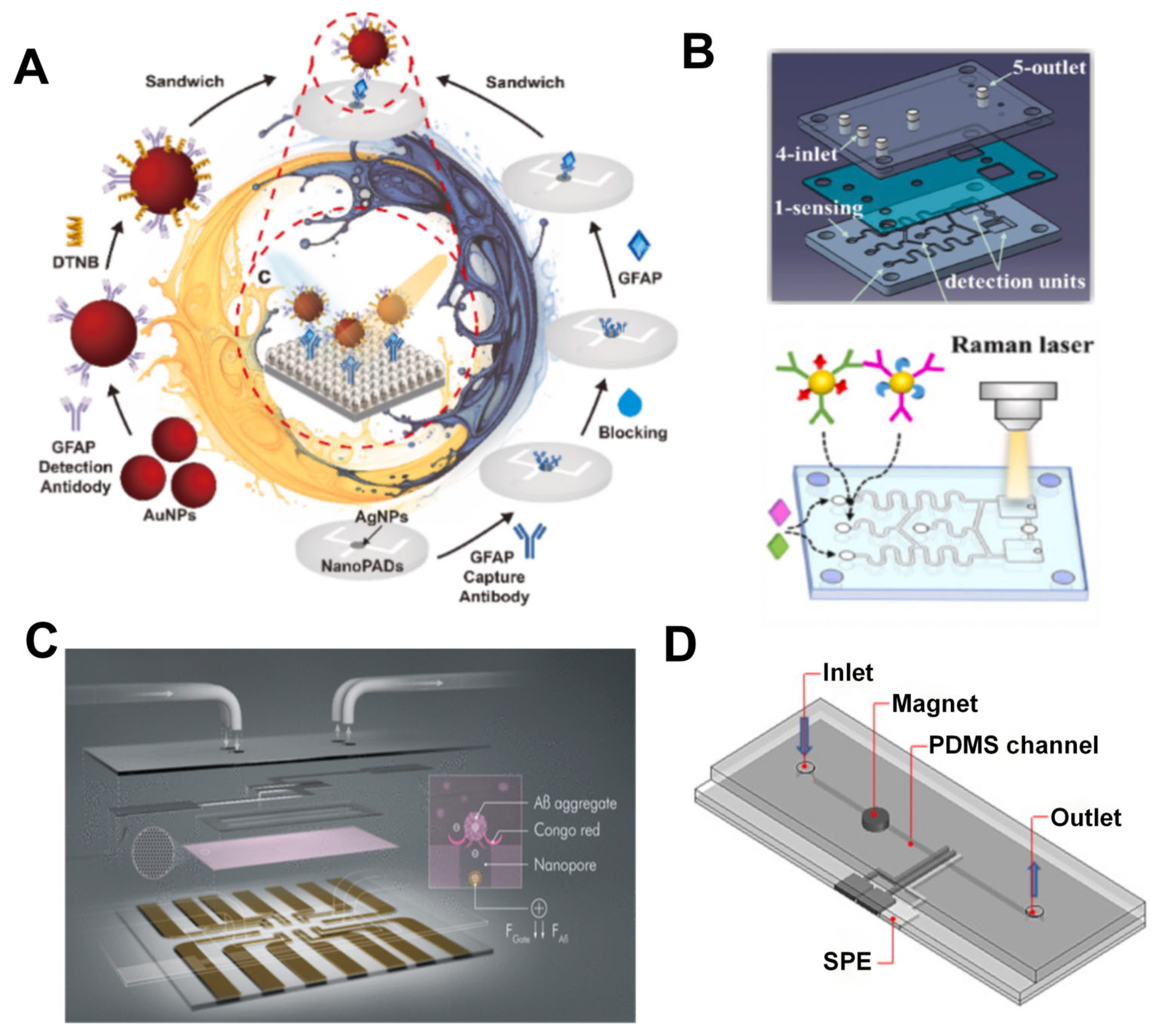
2.2. Detection of Neurological Disease Biomarkers
2.3. Detection of Cancer Biomarkers
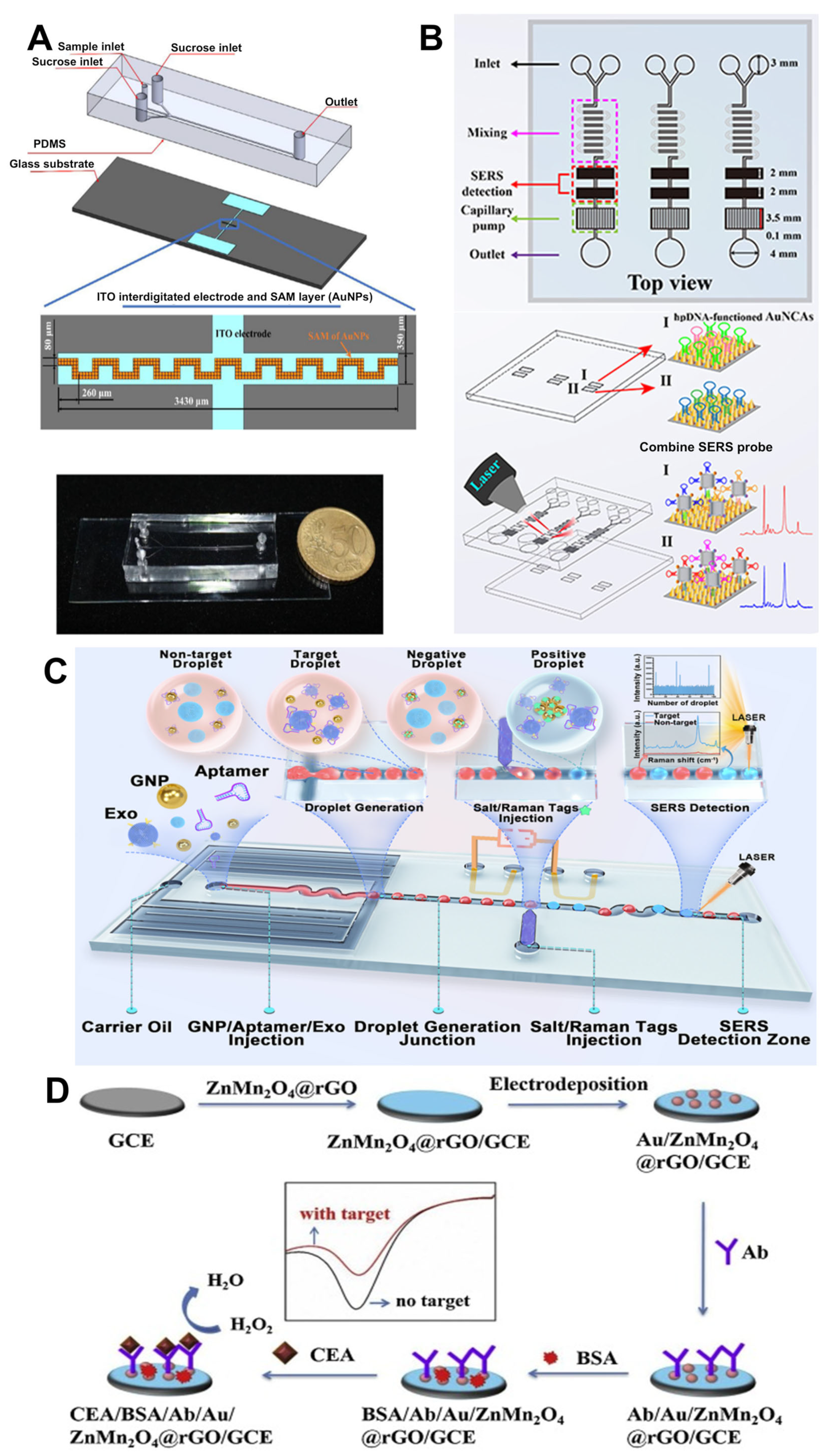
2.3.1. Detection of CTCs and ctDNA
2.3.2. Detection of Cancer-Related Extracellular Vesicles and Protein Biomarkers
3. The Integration of Nanomaterials with Microfluidic Technology for Disease Treatment
| Nanomaterial Name | Size | Shape | Function | References |
|---|---|---|---|---|
| RGN ps, CGNps | 3 nm/20 nm, 20 nm | sphere | reducing oxidative stress in endothelial cells | [95] |
| pNPs | 20 nm | sphere | researching vascular barrier permeability | [96] |
| GCPIH | 263 nm | sphere | enhancing thrombolytic effects | [97] |
| tPA-DPNs | 1000 nm of diameter, 400 nm of height | discoidal | improving thrombolytic efficiency | [98] |
| Tf@pSiNPs, BSA@pSiNPs | 182 ± 1 nm, 174 ± 1 nm | sphere | enhancing the permeability of BBB | [99] |
| angiopep-2 functionalized lipid cubosomes | 300 nm | cubic phase | encapsulating TMZ and CDDP, treating GBM | [37] |
| multiple NPs | N/D | sphere | treating GBM | [100] |
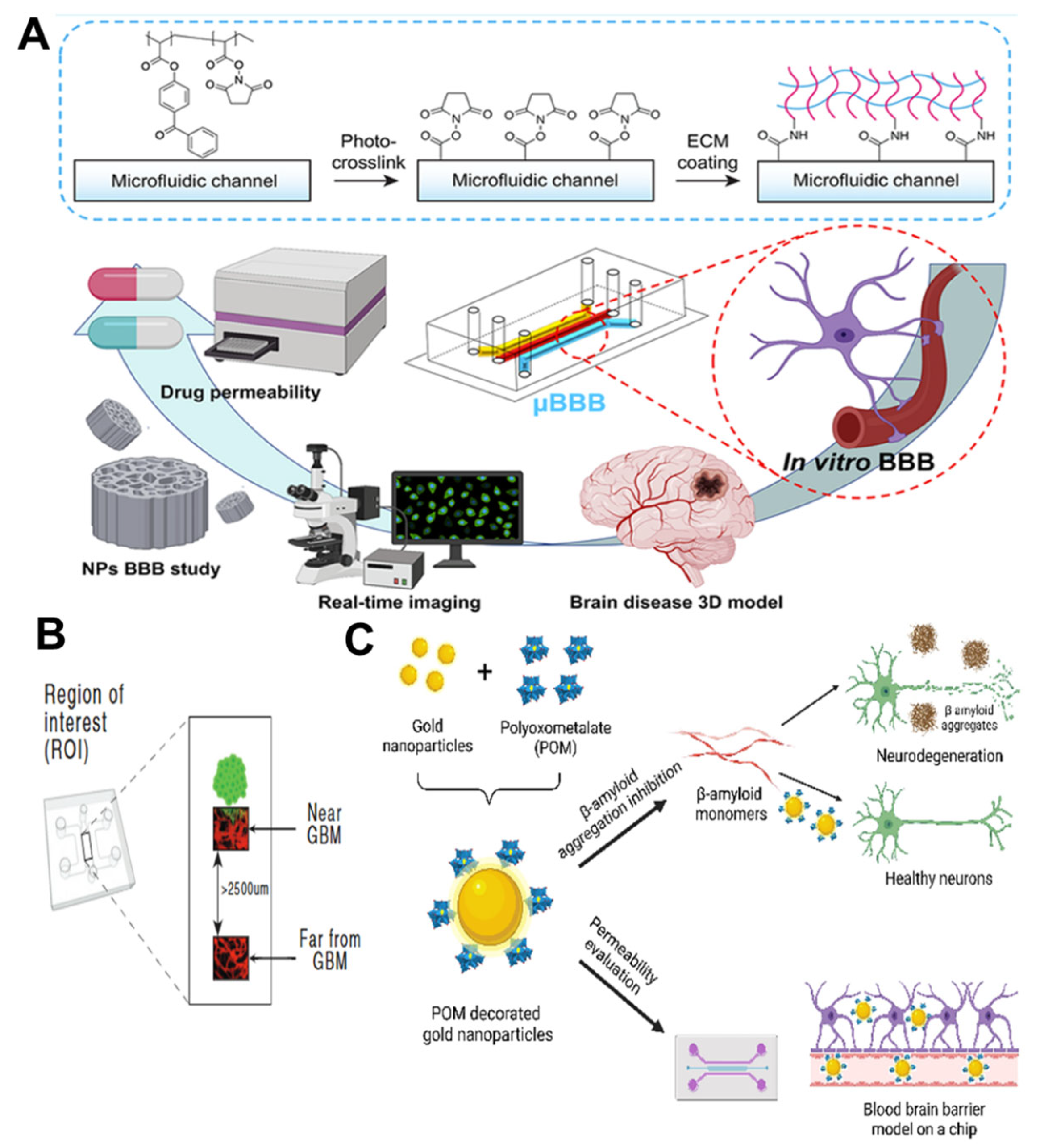
| AuNPs@POM@PEG | 17.7 ± 2.3 nm | sphere | inhibiting the aggregation of β- amyloid | [101] |
| D-T7/Tet1-lipids@PL | 68.93 ± 0.59 nm | core-shell structure | delivering LTG, treating epilepsy | [102] |
| DTXL-SPN | N/D | sphere | as a carrier of DTXL | [103] |
| IMQ-HA-GEM | 52.4 nm | sphere | delivering GEM and IMQ, enhancing therapeutic efficacy | [104] |
| HGNs@anti-MUC1 | N/D | spherical hollow structure | as a photothermal agent, photothermally treating tumors | [105] |
| PEG-liposomes, PEG-PLGA NPs | 70 nm | sphere | as a drug carrier | [106] |
| PTX-PLGA-SH NPs | 133.6 ± 2.1 nm | sphere | encapsulating and delivering PTX | [107] |
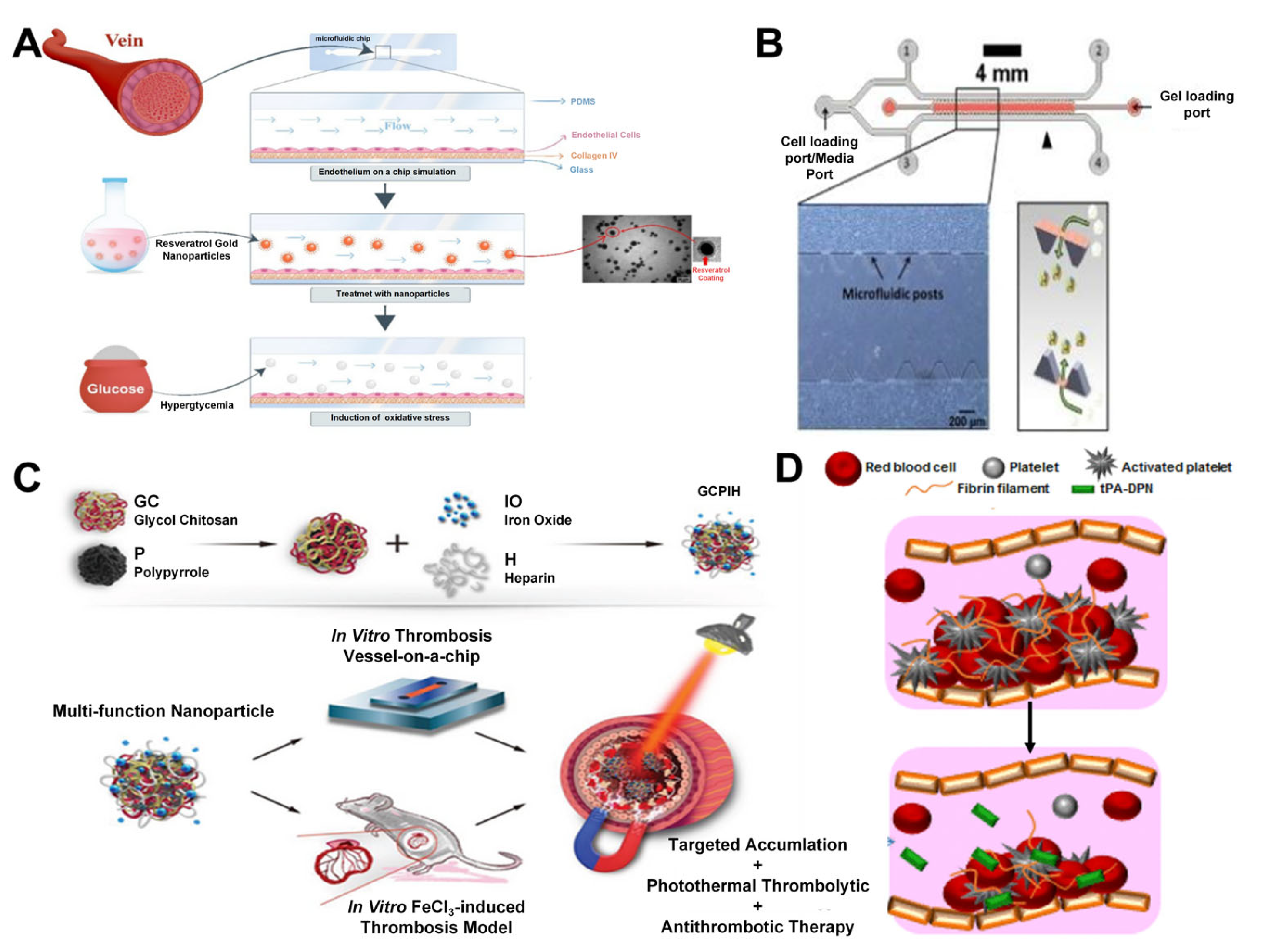
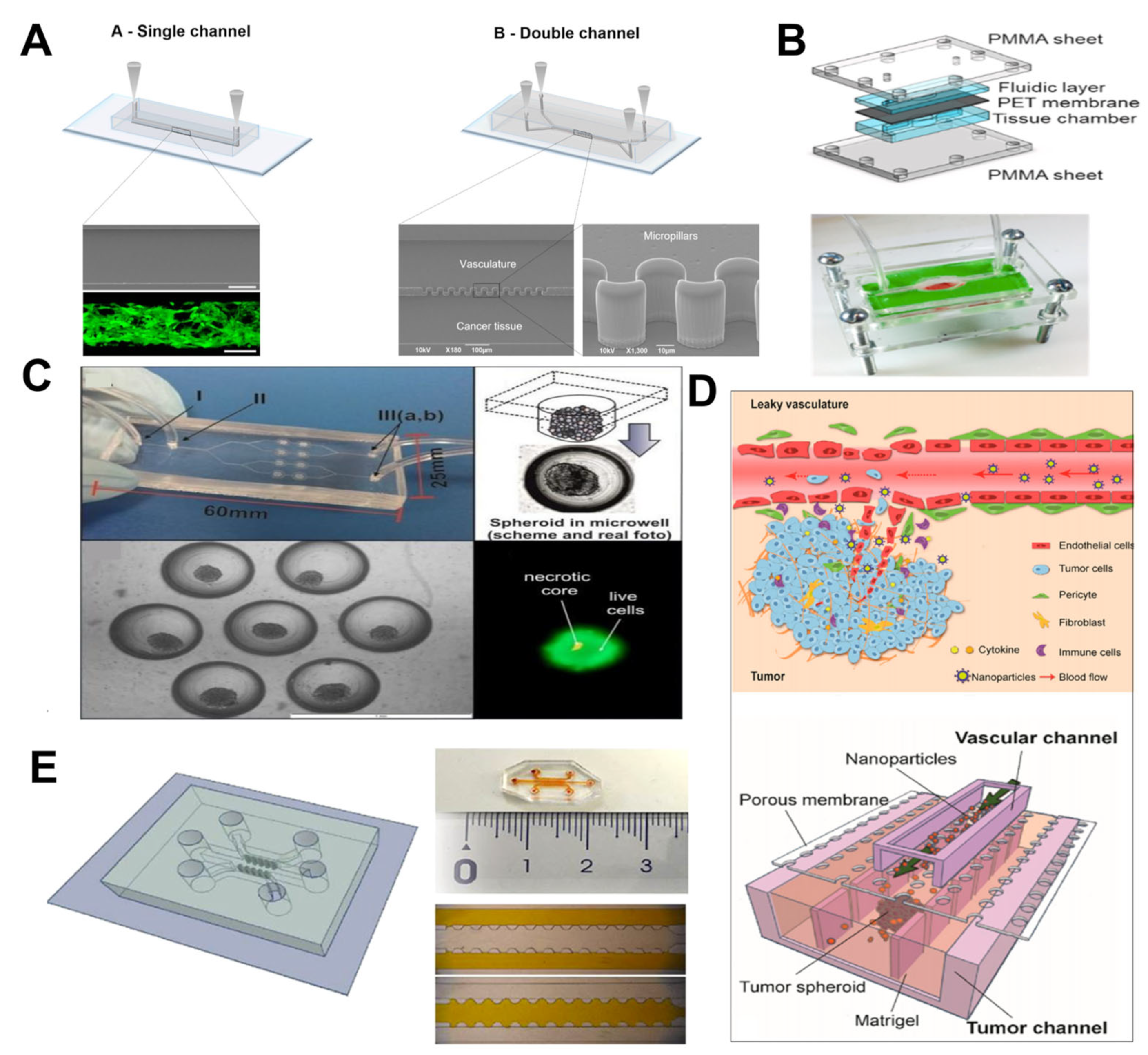
3.1. Vascular on a Chip
3.2. Blood–Brain Barrier Chip
3.3. Tumor on a Chip
4. Novel Medical Applications of Microfluidic Technology Integrated with Nanomaterials
4.1. Skin-Interfacing Devices
4.2. Medical Imaging
5. Conclusions and Perspectives
5.1. Feasibility and Clinical Transformation
5.2. Safety of Nanomaterials
5.2.1. Storage and Preparation of Nanomaterials
5.2.2. Blood Compatibility of Nanomaterials
5.2.3. Toxicity of Nanomaterials
5.3. Nanoparticle Production
5.4. Industrial Standardization of Microfluidic Technology
5.5. Novel Detection Strategies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| EDL | Electrical Double Layer |
| CT | Computed tomography |
| CVD | Cardiovascular diseases |
| WHO | World Health Organization |
| cTnI | Cardiac troponin I |
| LSPR | Localized surface plasmon resonance |
| SERS | Surface-enhanced Raman spectroscopy |
| ELISA | Enzyme-linked immunosorbent assays |
| hs-cTnT | High-sensitivity cardiac troponin T |
| PhC | Photonic crystal |
| CSWCNs | Carboxylated single-walled carbon nanotubes |
| Myo | Myoglobin |
| BNP | B-type natriuretic peptide |
| h-FABP | Heart-type fatty acid-binding protein |
| AMI | Acute myocardial infarction |
| AIENPs | Aggregation-induced emission nanoparticles |
| ECL | Electrochemiluminescence |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| POC | Point-of-care |
| GNPs | Gold nanoparticles |
| PCT | Procalcitonin |
| IL-6 | Interleukin-6 |
| SA-B-HRP | Streptavidin-biotin-horseradish peroxidase |
| MIS | Microfluidic immunoassay system |
| SA | Streptavidin |
| B | Biotin |
| HRP | Horseradish peroxidase |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MS | Multiple sclerosis |
| Aβ | Amyloid β |
| P-tau | Phosphorylated tau protein |
| GFAP | Glial fibrillary acidic protein |
| NanoPADs | Microfluidic device for SERS immunoassay based on nanocellulose paper |
| AgNPs | Silver nanoparticles |
| PS | Polystyrene |
| AuNPs | Gold nanoparticles |
| OECT | Organic electrochemical transistor |
| μf-OECT | Microfluidic integrated organic electrochemical transistor |
| ApoE | Apolipoprotein E |
| SPE | Screen-printed electrodes |
| Streptavidin-QD655 | Streptavidin-quantum dots655 |
| CTCs | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| DEP | Dielectrophoresis |
| PDMS | Polydimethylsiloxane |
| AuNCAs | Au nanocone arrays |
| SPM | Superparamagnetic |
| P-mesh | Padlock probes-conjugated nanomesh |
| EVs | Extracellular vesicles |
| CLD7 | Claudin7 |
| CRC | Colorectal cancer |
| MIL-125-NH2 | Materials Institute Lavoisier |
| hnRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 |
| S100P | S100 calcium-binding protein P |
| AuNCA | Au nanocrown array |
| MMP-9 | Matrix metalloproteinase-9 |
| Fe3O4@AuNPs | Gold-coated iron tetroxide particles |
| AuNCs | Gold nanocages |
| CEA | Carcinoembryonic antigen |
| ZnMn2O4@rGO | ZnMn2o4@reduced graphene oxide |
| MFC | Microfluidic chip |
| RGNps | Resveratrol gold nanoparticles |
| CGNps | Citrate gold nanoparticles |
| HUVECs | Human umbilical vein endothelial cells |
| ROS | Reactive oxygen species |
| pNPs | Polystyrene nanoparticles |
| GCPIH | Glycol chitosan-polypyrrole-iron oxide-heparin |
| tPA | Tissue plasminogen activator |
| tPA-DPNs | Tpa-discoidal polymeric nanoconstructs |
| BBB | Blood–brain barrier |
| μBBB | Blood–brain barrier microfluidic model |
| Tf@pSiNPs | Transferrin-functionalized porous silicon nanoparticles |
| BSA@pSiNPs | Bovine Serum Albumin-functionalized porous silicon |
| GBM | Glioblastoma multiforme |
| CDDP | Cisplatin |
| TMZ | Temozolomide |
| PEG | Polyethylene glycol |
| POM | Polyoxometalates |
| D-T7/Tet1-lipids@PL | D-T7/Tet1-lipids@PLGA-Lamotrigine nanoparticles |
| LTG | Lamotrigine |
| U87-MG | Glioblastoma multiforme |
| DTXL | Docetaxel |
| DTXL-SPN | Docetaxel-spherical polymeric nanoparticles |
| HA | Hyaluronic acid |
| GEM | Gemcitabine |
| IMQ | Imiquimod |
| IMQ-HA-GEM | Hyaluronic acid-gemcitabine-imiquimod |
| GelMA | Gelatin methacryloyl |
| MUC1 | Mucin1 |
| HGNs | Hollow gold nanoshells |
| HGNs@anti-MUC1 | PEG |
| PTT | Photothermal therapy |
| TVOC | Tumor vasculature-on-a-chip |
| ECM | Extracellular matrix |
| TNF-α | Tumor necrosis factor-α |
| PEG-PLGA NPs | Poly(ethylene glycol))/poly(lactide-co-glycolic acid) nanoparticles |
| FA | Folic acid |
| TMOC | Tumor microenvironment-on-a-Chip |
| PTX | Paclitaxel |
| PTX-PLGA-SH NPs | PTX-loaded nanoparticles |
| LIG | Laser-induced graphene |
| FC-ZnONRs | Flower cluster-shaped zinc oxide nanorods |
| H2O2 | Hydrogen peroxide |
| MoS2-X | Molybdenum disulfide with sulfur vacancies |
| CNTs | Carbon nanotubes |
| TiO2 | Titanium dioxide |
| CNTs/MoS2-X/TiO2 | Carbon Nanotubes/Molybdenum Disulfide with Sulfur Vacancies/Titanium Dioxide |
| MXene/MWCNT | Mxene/Multi-Walled Carbon Nanotubes |
| CRP | C-reactive protein |
| GO | Graphene oxide |
| Fe3O4-GO | Iron (III) oxide-Graphene Oxide |
| MRI | Magnetic resonance imaging |
| Ch-SPIONs | Chitosan-superparamagnetic iron oxide composite nanoparticles |
| HDL | High-density lipoprotein |
| AI | Artificial intelligence |
| IVD | In vitro diagnostic products |
| U.S. FDA | U.S. Food and Drug Administration |
| LNPs | Lipid nanoparticles |
| MNPs | Magnetic nanoparticles |
| DI | Deformation index |
| RBCs | Red blood cells |
References
- Torul, H.; Arslan, Z.; Tezcan, T.; Kayiş, E.; Çalımcı, M.; Gumustas, A.; Yildirim, E.; Külah, H.; Tamer, U. Microfluidic-based blood immunoassays. J. Pharm. Biomed. Anal. 2023, 228, 115313. [Google Scholar] [CrossRef]
- Gu, Y.; Jin, L.; Wang, L.; Ma, X.; Tian, M.; Sohail, A.; Wang, J.; Wang, D. Preparation of Baicalin Liposomes Using Microfluidic Technology and Evaluation of Their Antitumor Activity by a Zebrafish Model. ACS Omega 2024, 9, 41289–41300. [Google Scholar] [CrossRef] [PubMed]
- Abouhagger, A.; Celiešiūtė-Germanienė, R.; Bakute, N.; Stirke, A.; Melo, W.C.M.A. Electrochemical biosensors on microfluidic chips as promising tools to study microbial biofilms: A review. Front. Cell. Infect. Microbiol. 2024, 14, 1419570. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Raj, A.; Suthanthiraraj, P.P.A.; Sen, A.K. Pressure-driven flow through PDMS-based flexible microchannels and their applications in microfluidics. Microfluid. Nanofluidics 2018, 22, 128. [Google Scholar] [CrossRef]
- Lim, A.E.; Goh, S. Effect of Microchannel Diameter on Electroosmotic Flow Hysteresis. Energies 2023, 16, 2154. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Kang, J.W.; Kwon, S.B.; Hong, J. Compact Three-Dimensional Digital Microfluidic Platforms with Programmable Contact Charge Electrophoresis Actuation. Langmuir 2022, 38, 5759–5764. [Google Scholar] [CrossRef]
- Kibar, G.; Sarıarslan, B.; Doğanay, S.; Yıldız, G.; Usta, O.B.; Çetin, B. Novel 3D-Printed Microfluidic Magnetic Platform for Rapid DNA Isolation. Anal. Chem. 2024, 96, 1985–1992. [Google Scholar] [CrossRef]
- Mi, S.; Du, Z.; Xu, Y.; Sun, W. The crossing and integration between microfluidic technology and 3D printing for organ-on-chips. J. Mater. Chem. B 2018, 6, 6191–6206. [Google Scholar] [CrossRef]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. Semin. Cell Dev. Biol. 2023, 144, 41–54. [Google Scholar] [CrossRef]
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Feng, X.; Cheng, L.K.W.; Wu, A.R. Vascularized human brain organoid on-chip. Lab Chip 2023, 23, 2693–2709. [Google Scholar] [CrossRef] [PubMed]
- Quintard, C.; Tubbs, E.; Jonsson, G.; Jiao, J.; Wang, J.; Werschler, N.; Laporte, C.; Pitaval, A.; Bah, T.S.; Pomeranz, G.; et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat. Commun. 2024, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Fu, N.; Zeng, Y.; Zhang, J.; Zhang, P.; Zhang, H.; Yang, S.; Zhang, J. A Facile Strategy for PEGylated Nanoprodrug of Bortezomib with Improved Stability, Enhanced Biocompatibility, pH-Controlled Disassembly, and Release. Macromol. Biosci. 2024, 25, e2400383. [Google Scholar] [CrossRef]
- Tran, U.T.; Kitami, T. Chemical screens for particle-induced macrophage death identifies kinase inhibitors of phagocytosis as targets for toxicity. J. Nanobiotechnol. 2024, 22, 621. [Google Scholar] [CrossRef]
- Hodaei, H.; Esmaeili, Z.; Erfani, Y.; Esnaashari, S.S.; Geravand, M.; Adabi, M. Preparation of biocompatible Zein/Gelatin/Chitosan/PVA based nanofibers loaded with vitamin E-TPGS via dual-opposite electrospinning method. Sci. Rep. 2024, 14, 23796. [Google Scholar] [CrossRef]
- Sarac, B.; Soprunyuk, V.; Herwig, G.; Gümrükçü, S.; Kaplan, E.; Yüce, E.; Schranz, W.; Eckert, J.; Boesel, L.F.; Sarac, A.S. Thermomechanical properties of confined magnetic nanoparticles in electrospun polyacrylonitrile nanofiber matrix exposed to a magnetic environment: Structure, morphology, and stabilization (cyclization). Nanoscale Adv. 2024, 6, 6184–6195. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Y.; Hu, H.; Yang, X.; Xing, X.; Wu, J.; Zhu, Y.; Zhang, Y. Augment of Ferroptosis with Photothermal Enhanced Fenton Reaction and Glutathione Inhibition for Tumor Synergistic Nano-Catalytic Therapy. Int. J. Nanomed. 2024, 19, 11923–11940. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Zhang, Y.; Lv, K.; Zhu, J.; Liu, M.; Xu, H.; Jiao, G.; Yang, W.; Sun, G.; et al. Three-arm polyrotaxanes with multidirectional molecular motions as the nanocarrier for nitric oxide-enhanced photodynamic therapy against bacterial biofilms in septic arthritis. J. Nanobiotechnol. 2024, 22, 727. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Li, N.; Yang, G.; Cheng, Z.; Du, X.; Sun, L.; Wang, W.; Li, B. Advances in the application of carbon dots-based fluorescent probes in disease biomarker detection. Colloids Surf. B Biointerfaces 2024, 245, 114360. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Kanike, C.; Lu, Q.; Li, Y.; Wu, H.; Niestanak, V.D.; Maeda, N.; Atta, A.; Unsworth, L.D.; Zhang, X. Streamlined Flow Synthesis of Plasmonic Nanoparticles and SERS Detection of Uremic Toxins with Trace-Level Liquid Volumes in a Microchamber. ACS Appl. Mater. Interfaces 2024, 16, 63268–63283. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Guan, M.; Wang, Y.; Mi, F.; Zhang, S.; Rao, X. A double boronic acid affinity “sandwich” SERS biosensor based on magnetic boronic acid controllable-oriented imprinting for high-affinity biomimetic specific recognition and rapid detection of target glycoproteins. Mikrochim. Acta 2024, 191, 444. [Google Scholar] [CrossRef]
- Wilar, G.; Suhandi, C.; Wathoni, N.; Fukunaga, K.; Kawahata, I. Nanoparticle-Based Drug Delivery Systems Enhance Treatment of Cognitive Defects. Int. J. Nanomed. 2024, 19, 11357–11378. [Google Scholar] [CrossRef]
- Elsayed, N. Selective imaging, gene, and therapeutic delivery using PEGylated and pH-Sensitive nanoparticles for enhanced lung disorder treatment. Int. J. Pharm. 2024, 666, 124819. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Irfan, S.; Anjum, N.; Althobaiti, T.; Alotaibi, A.A.; Siddiqui, A.B.; Ramzan, N. Heartbeat Classification and Arrhythmia Detection Using a Multi-Model Deep-Learning Technique. Sensors 2022, 22, 5606. [Google Scholar] [CrossRef]
- Sacco, S.; Caverzasi, E.; Papinutto, N.; Cordano, C.; Bischof, A.; Gundel, T.; Cheng, S.; Asteggiano, C.; Kirkish, G.; Mallott, J.; et al. Neurite Orientation Dispersion and Density Imaging for Assessing Acute Inflammation and Lesion Evolution in MS. AJNR Am. J. Neuroradiol. 2020, 41, 2219–2226. [Google Scholar] [CrossRef]
- Probst, D.; Batchu, K.; Younce, J.R.; Sode, K. Levodopa: From Biological Significance to Continuous Monitoring. ACS Sens. 2024, 9, 3828–3839. [Google Scholar] [CrossRef] [PubMed]
- Campu, A.; Muresan, I.; Potara, M.; Lazar, D.R.; Lazar, F.L.; Cainap, S.; Olinic, D.M.; Maniu, D.; Astilean, S.; Focsan, M. Portable microfluidic plasmonic chip for fast real-time cardiac troponin I biomarker thermoplasmonic detection. J. Mater. Chem. B 2024, 12, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.R.S.; Gil, J.F.; Guillot, A.J.; Li, J.; Pinto, R.J.B.; Santos, H.A.; Gonçalves, G. Advances in Microfluidic-Based Core@Shell Nanoparticles Fabrication for Cancer Applications. Adv. Healthc. Mater. 2024, 13, e2400946. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Nie, Y.; Shen, T.; Zhang, J.X. Microfluidics-enabled rational design of immunomagnetic nanomaterials and their shape effect on liquid biopsy. Lab Chip 2018, 18, 1997–2002. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Z.; Zhang, W.; Yu, J.; Li, H.; Di, W.; Duan, Y. Substrate-induced growth of micro/nanostructured Zn (OH) F arrays for highly sensitive microfluidic fluorescence assays. ACS Appl. Mater. Interfaces 2021, 13, 28462–28471. [Google Scholar] [CrossRef]
- Li, Y.; Huang, R.; Duan, Y.; Deng, D.; Chen, H.; Xia, T.; Duan, Y.; Lei, H.; Luo, L. Ultrasensitive lab-on-paper electrochemical device via heterostructure copper/cuprous sulfide@N-doped C@Au hollow nanoboxes as signal amplifier for alpha-fetoprotein detection. Biosens. Bioelectron. 2025, 267, 116827. [Google Scholar] [CrossRef]
- Cai, X.; Refaat, A.; Gan, P.-Y.; Fan, B.; Yu, H.; Thang, S.H.; Drummond, C.J.; Voelcker, N.H.; Tran, N.; Zhai, J. Angiopep-2-Functionalized Lipid Cubosomes for Blood–Brain Barrier Crossing and Glioblastoma Treatment. ACS Appl. Mater. Interfaces 2024, 16, 12161–12174. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Shin, S.R.; Bañobre-López, M. Brain-on-a-chip: An emerging platform for studying the nanotechnology-biology interface for neurodegenerative disorders. J. Nanobiotechnol. 2024, 22, 573. [Google Scholar] [CrossRef]
- Dhauria, M.; Mondal, R.; Deb, S.; Shome, G.; Chowdhury, D.; Sarkar, S.; Benito-León, J. Advancing Non-Invasive Diagnostics and Prognostics. Int. J. Mol. Sci. 2024, 25, 10911. [Google Scholar] [CrossRef]
- Virga, A.; Gianni, C.; Palleschi, M.; Angeli, D.; Merloni, F.; Maltoni, R.; Ulivi, P.; Martinelli, G.; De Giorgi, U.; Bravaccini, S. A Novel AKT1, ERBB2, ESR1, KRAS, PIK3CA, and TP53 NGS Assay: A Non-Invasive Tool to Monitor Resistance Mechanisms to Hormonal Therapy and CDK4/6 Inhibitors. Biomedicines 2024, 12, 2183. [Google Scholar] [CrossRef]
- Hasanabadi, S.; Aghamiri, S.M.R.; Abin, A.A.; Abdollahi, H.; Arabi, H.; Zaidi, H. Enhancing Lymphoma Diagnosis, Treatment, and Follow-Up Using 18F-FDG PET/CT Imaging: Contribution of Artificial Intelligence and Radiomics Analysis. Cancers 2024, 16, 3511. [Google Scholar] [CrossRef]
- Jing, W.; Wang, Y.; Chen, C.; Zhang, F.; Yang, Y.; Ma, G.; Yang, E.H.; Snozek, C.L.N.; Tao, N.; Wang, S. Gradient-Based Rapid Digital Immunoassay for High-Sensitivity Cardiac Troponin T (hs-cTnT) Detection in 1 μL Plasma. ACS Sens. 2021, 6, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Zhang, N.; Zhao, J.; Zhou, Y.; Zhou, Q.; Gu, Z.; Zhang, D. Carbon nanotubes integrated photonic barcodes in Herringbone Microfluidics for Multiplex Biomarker Quantification. Biosens. Bioelectron. 2024, 258, 116350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Cao, Y.; Wang, Y.; Zhao, M.; Wu, Y.; Xu, B.; Lin, G. Rapid and sensitive detection of heart-type fatty acid binding protein using aggregation-induced emission nanoparticles on digital microfluidics workstation. Biosens. Bioelectron. 2024, 262, 116563. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fu, W.; Zhu, B.; Feng, Q.; Ying, X.; Li, S.; Chen, J.; Xie, X.; Pan, C.; Liu, J.; et al. An integrated microfluidic electrochemiluminescence device for point-of-care testing of acute myocardial infarction. Talanta 2023, 262, 124626. [Google Scholar] [CrossRef]
- Chen, C.; Porter, R.; Zhou, X.; Snozek, C.L.; Yang, E.H.; Wang, S. Microfluidic Digital Immunoassay for Point-of-Care Detection of NT-proBNP from Whole Blood. Anal. Chem. 2024, 96, 10569–10576. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Yang, M.; Wang, Y.; Zhang, C.; Yang, M.; Sun, J.; Xie, M.; Jiang, X. Streptavidin-biotin-peroxidase nanocomplex-amplified microfluidics immunoassays for simultaneous detection of inflammatory biomarkers. Anal. Chim. Acta 2017, 982, 138–147. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, H.; Li, R.; Yong, R.; Mitrovic, I.; Lim, E.G.; Duan, S.; Song, P. A SERS nanocellulose-paper-based analytical device for ultrasensitive detection of Alzheimer’s disease. Anal. Chim. Acta 2024, 1301, 342447. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Wang, L.; Zhang, X.; Luo, C.; Hua, J.; Feng, M.; Chen, Z.; Wang, M.; Xu, C. Construction of a microcavity-based microfluidic chip with simultaneous SERS quantification of dual biomarkers for early diagnosis of Alzheimer’s disease. Talanta 2023, 261, 124677. [Google Scholar] [CrossRef]
- Koklu, A.; Wustoni, S.; Musteata, V.E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic Integrated Organic Electrochemical Transistor with a Nanoporous Membrane for Amyloid-β Detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Morales-Narváez, E.; Merkoçi, A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014, 54, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Su, T.C.; Huang, S.J.; Jen, C.P. Enhancing the efficiency of lung cancer cell capture using microfluidic dielectrophoresis and aptamer-based surface modification. Electrophoresis 2024, 45, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, V.A.; Chen, K.; George, T.J.; Fan, Z.H. Gold Nanoparticle-Based Microfluidic Chips for Capture and Detection of Circulating Tumor Cells. Biosensors 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gu, Y.; Deng, J.; Cai, Z.; Wang, Y.; Zhou, R.; Zhu, D.; Lu, H.; Wang, Z. Combined SERS Microfluidic Chip with Gold Nanocone Array for Effective Early Lung Cancer Prognosis in Mice Model. Int. J. Nanomed. 2023, 18, 3429–3442. [Google Scholar] [CrossRef]
- Balakrishnan, S.G.; Ahmad, M.R.; Koloor, S.S.R.; Petrů, M. Separation of ctDNA by superparamagnetic bead particles in microfluidic platform for early cancer detection. J. Adv. Res. 2021, 33, 109–116. [Google Scholar] [CrossRef]
- Huang, N.; Chen, M.; Chen, S.; Dang, K.; Guo, H.; Wang, X.; Yan, S.; Tian, J.; Liu, Y.; Ye, Q. A Specific Nucleic Acid Microfluidic Capture Device Based on Stable DNA Nanostructure. ACS Appl. Mater. Interfaces 2021, 13, 24487–24492. [Google Scholar] [CrossRef]
- Ho, K.H.W.; Lai, H.; Zhang, R.; Chen, H.; Yin, W.; Yan, X.; Xiao, S.; Lam, C.Y.K.; Gu, Y.; Yan, J.; et al. SERS-Based Droplet Microfluidic Platform for Sensitive and High-Throughput Detection of Cancer Exosomes. ACS Sens. 2024, 9, 4860–4869. [Google Scholar] [CrossRef]
- Ortega, F.G.; Gomez, G.E.; Boni, C.; García, I.C.; Navas, C.G.; D’Vries, R.F.; Molina Vallejos, M.P.; Serrano, M.J.; Messina, G.A.; Hernández, J.E.; et al. Microfluidic amperometric immunosensor based on porous nanomaterial towards claudin7 determination for colorectal cancer diagnosis. Talanta 2023, 251, 123766. [Google Scholar] [CrossRef]
- Cao, X.; Liu, Z.; Qin, X.; Gu, Y.; Huang, Y.; Qian, Y.; Wang, Z.; Li, H.; Zhu, Q.; Wei, W. LoC-SERS platform for rapid and sensitive detection of colorectal cancer protein biomarkers. Talanta 2024, 270, 125563. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, M.; Jiang, F.; Lu, C.; Zhu, Q.; Yang, Y.; Fu, L.; Li, L.; Liu, J.; Wang, Z.; et al. Microfluidic-SERS sensing system based on dual signal amplification and aptamer for gastric cancer detection. Mikrochim. Acta 2024, 191, 668. [Google Scholar] [CrossRef]
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Huan, K.; Luo, L. A sensitive amperometric immunosensor for the detection of carcinoembryonic antigen using ZnMn2O4@reduced graphene oxide composites as signal amplifier. Sens. Actuators B Chem. 2021, 339, 129852. [Google Scholar] [CrossRef]
- Ritngam, A.; Kalampakorn, S.; Lagampan, S.; Jirapongsuwan, A. Effectiveness of a Nurse-Led Workplace Intervention in Reducing Cardiovascular Risks Among Thai Workers: A Randomized Controlled Trial. J. Prim. Care Community Health 2024, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Zhang, T.; Zhu, T.; Yao, S.; Wang, R.; Wang, W.; Dilimulati, H.; Ge, J.; An, S. Exploring shared biomarkers and shared pathways in insomnia and atherosclerosis using integrated bioinformatics analysis. Front. Mol. Neurosci. 2024, 17, 1477903. [Google Scholar] [CrossRef]
- Bagheri, B.; Jalalian, R.; Mousavi, F.S.; Azizi, S.; Alipour, A.; Mousavi, F.; Ghadirzadeh, E. The role of hemoglobin A1c as a predictor of major adverse cardiovascular events in patients with type 2 diabetes mellitus after percutaneous coronary intervention: A case-cohort study. BMC Cardiovasc. Disord. 2024, 24, 583. [Google Scholar] [CrossRef]
- Gao, R.; Mao, Y.; Ma, C.; Wang, Y.; Jia, H.; Chen, X.; Lu, Y.; Zhang, D.; Yu, L. SERS-Based Immunoassay of Myocardial Infarction Biomarkers on a Microfluidic Chip with Plasmonic Nanostripe Microcones. ACS Appl. Mater. Interfaces 2022, 14, 55414–55422. [Google Scholar] [CrossRef]
- Beck, F.; Horn, C.; Baeumner, A.J. Dry-reagent microfluidic biosensor for simple detection of NT-proBNP via Ag nanoparticles. Anal. Chim. Acta 2022, 1191, 339375. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsystems Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef]
- Wu, S.; Zou, S.; Wang, S.; Li, Z.; Ma, D.L.; Miao, X. CTnI diagnosis in myocardial infarction using G-quadruplex selective Ir(Ⅲ) complex as effective electrochemiluminescence probe. Talanta 2022, 248, 123622. [Google Scholar] [CrossRef]
- Alhadi, H.A.; Fox, K.A. Do we need additional markers of myocyte necrosis: The potential value of heart fatty-acid-binding protein. QJM Mon. J. Assoc. Physicians 2004, 97, 187–198. [Google Scholar] [CrossRef]
- Michielsen, E.C.; Diris, J.H.; Kleijnen, V.W.; Wodzig, W.K.; Van Dieijen-Visser, M.P. Interpretation of cardiac troponin T behaviour in size-exclusion chromatography. Clin. Chem. Lab. Med. 2006, 44, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Myhre, P.L. NT-proBNP Response to Heart Failure Therapies: An Imperfect Surrogate. J. Am. Coll. Cardiol. 2021, 78, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. JAD 2019, 67, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Olek, M.J. Multiple Sclerosis. Ann. Intern. Med. 2021, 174, Itc81–itc96. [Google Scholar] [CrossRef]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Qiu, Y.; Sun, D.; Zhou, H. Peripheral GFAP and NfL as early biomarkers for dementia: Longitudinal insights from the UK Biobank. BMC Med. 2024, 22, 192. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Fang, J.; Jia, D.; Tian, T.; Du, Y.; Wei, Q.; Li, F. Analysis of okadaic acid using electrochemiluminescence imaging on microfluidic biosensing chip. Biosens. Bioelectron. 2024, 264, 116690. [Google Scholar] [CrossRef]
- Sahil; Jaryal, V.B.; Sharma, R.; Thakur, K.K.; Ataya, F.S.; Gupta, N.; Singh, D. Facile One-Pot Synthesis of Fe3O4─MoS2@MXene Nanocomposite as an Electrochemical Sensor for the Detection of Levofloxacin. ChemistrySelect 2025, 10, e202405959. [Google Scholar] [CrossRef]
- Saputra, H.A.; Jannath, K.A.; Kim, K.B.; Park, D.S.; Shim, Y.B. Conducting polymer composite-based biosensing materials for the diagnosis of lung cancer: A review. Int. J. Biol. Macromol. 2023, 252, 126149. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Amn Zia, M.; Shoukat, M.; Kaleem, I.; Bashir, S. Nanoparticles as a novel key driver for the isolation and detection of circulating tumour cells. Sci. Rep. 2024, 14, 22580. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Kavgaci, G.; Sahin, T.K.; Muderrisoglu, T.; Ileri, S.; Guven, D.C.; Aksoy, S. Post-operative serum CEA predicts prognosis in HR-positive/HER2-negative early breast cancer. Expert Rev. Anticancer Ther. 2024, 24, 1319–1326. [Google Scholar] [CrossRef]
- Novikova, S.E.; Soloveva, N.A.; Farafonova, T.E.; Tikhonova, O.V.; Liao, P.-C.; Zgoda, V.G. Proteomic Signature of Extracellular Vesicles for Lung Cancer Recognition. Molecules 2021, 26, 6145. [Google Scholar] [CrossRef]
- Astiawati, T.; Rohman, M.S.; Wihastuti, T.; Sujuti, H.; Endharti, A.T.; Sargowo, D.; Oceandy, D.; Lestari, B.; Triastuti, E.; Nugraha, R.A. Efficacy of Colchicine in Reducing NT-proBNP, Caspase-1, TGF-β, and Galectin-3 Expression and Improving Echocardiography Parameters in Acute Myocardial Infarction: A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. J. Clin. Med. 2025, 14, 1347. [Google Scholar] [CrossRef]
- Jawaid, S.; Joshi, Y.; Neelofar, N.; Khursheed, K.; Shams, S.; Chaudhary, M.; Arora, M.; Mahajan, K.; Anwar, F. A Cross-talk between Nanomedicines and Cardiac Complications: Comprehensive View. Curr. Pharm. Des. 2024, 31, 741–752. [Google Scholar] [CrossRef]
- Peng, F.; Wang, Z.; Qiu, Z.; Zhang, W.; Zhao, Y.; Li, C.; Shi, B. Nanomedicine in cardiology: Precision drug delivery for enhanced patient outcomes. Life Sci. 2024, 358, 123199. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.; Luo, L.; Pan, Y.; Han, L.; Yu, Y. Efficient bionic nanozyme based on AuPt NPs@ZIF-90 used for cyclic catalysis multimodal tumor therapy. J. Mater. Chem. B 2024, 12, 12597–12607. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.S.; Zhang, X.; Liu, C. Organ-on-a-chip platforms for accelerating the evaluation of nanomedicine. Bioact. Mater. 2021, 6, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, M.; Phung, N.; Grimm, J.; Andreou, C. Organ-on-chip systems as a model for nanomedicine. Nanoscale 2023, 15, 9927–9940. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Fayazbakhsh, F.; Hataminia, F.; Eslam, H.M.; Ajoudanian, M.; Kharrazi, S.; Sharifi, K.; Ghanbari, H. Evaluating the antioxidant potential of resveratrol-gold nanoparticles in preventing oxidative stress in endothelium on a chip. Sci. Rep. 2023, 13, 21344. [Google Scholar] [CrossRef]
- Ho, Y.T.; Adriani, G.; Beyer, S.; Nhan, P.-T.; Kamm, R.D.; Kah, J.C.Y. A Facile Method to Probe the Vascular Permeability of Nanoparticles in Nanomedicine Applications. Sci. Rep. 2017, 7, 707. [Google Scholar] [CrossRef]
- Liu, K.T.; Quiñones, E.D.; Liu, M.H.; Lin, C.W.; Chen, Y.T.; Chiang, C.C.; Wu, K.C.W.; Fan, Y.J.; Chuang, E.Y.; Yu, J.S. A Biomimicking and Multiarm Self-Indicating Nanoassembly for Site-Specific Photothermal-Potentiated Thrombolysis Assessed in Microfluidic and In Vivo Models. Adv. Healthc. Mater. 2023, 12, e2300682. [Google Scholar] [CrossRef]
- Colasuonno, M.; Palange, A.L.; Aid, R.; Ferreira, M.; Mollica, H.; Palomba, R.; Emdin, M.; Del Sette, M.; Chauvierre, C.; Letourneur, D.; et al. Erythrocyte-Inspired Discoidal Polymeric Nanoconstructs Carrying Tissue Plasminogen Activator for the Enhanced Lysis of Blood Clots. ACS Nano 2018, 12, 12224–12237. [Google Scholar] [CrossRef]
- Peng, B.; Tong, Z.; Tong, W.Y.; Pasic, P.J.; Oddo, A.; Dai, Y.; Luo, M.; Frescene, J.; Welch, N.G.; Easton, C.D.; et al. In Situ Surface Modification of Microfluidic Blood–Brain-Barriers for Improved Screening of Small Molecules and Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 56753–56766. [Google Scholar] [CrossRef]
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119. [Google Scholar] [CrossRef]
- Perxés Perich, M.; Palma-Florez, S.; Solé, C.; Goberna-Ferrón, S.; Samitier, J.; Gómez-Romero, P.; Mir, M.; Lagunas, A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood-Brain Barrier in a µphysiological Model. Nanomaterials 2023, 13, 2697. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Wang, L.; Xiao, F.; Wang, L.; Liu, X.; Zhu, L.; Lu, Y.; Zheng, W.; Jiang, X. Dual Targeting Nanoparticles for Epilepsy Therapy. Chem. Sci. 2022, 13, 12913–12920. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Brito, A.; Barbato, M.G.; Felici, A.; Reis, R.L.; Pires, R.A.; Pashkuleva, I.; Decuzzi, P. Efficacy of molecular and nano-therapies on brain tumor models in microfluidic devices. Biomater. Adv. 2023, 144, 213227. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Pan, D.C.; Zhao, Z.; Gao, Y.; Zhang, Y.S.; Mitragotri, S. Imiquimod-gemcitabine nanoparticles harness immune cells to suppress breast cancer. Biomaterials 2022, 280, 121302. [Google Scholar] [CrossRef]
- Kalinowska, D.; Grabowska-Jadach, I.; Liwinska, M.; Drozd, M.; Pietrzak, M.; Dybko, A.; Brzozka, Z. Studies on effectiveness of PTT on 3D tumor model under microfluidic conditions using aptamer-modified nanoshells. Biosens. Bioelectron. 2019, 126, 214–221. [Google Scholar] [CrossRef]
- Wang, H.-F.; Ran, R.; Liu, Y.; Hui, Y.; Zeng, B.; Chen, D.; Weitz, D.A.; Zhao, C.-X. Tumor-Vasculature-on-a-Chip for Investigating Nanoparticle Extravasation and Tumor Accumulation. ACS Nano 2018, 12, 11600–11609. [Google Scholar] [CrossRef]
- Wang, H.-F.; Liu, Y.; Wang, T.; Yang, G.; Zeng, B.; Zhao, C.-X. Tumor-Microenvironment-on-a-Chip for Evaluating Nanoparticle-Loaded Macrophages for Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 5040–5050. [Google Scholar] [CrossRef]
- Li, J.; Xie, F.; Ma, X. Advances in nanomedicines: A promising therapeutic strategy for ischemic cerebral stroke treatment. Nanomedicine 2024, 19, 811–835. [Google Scholar] [CrossRef]
- Yang, Q.; Li, R.; Hong, Y.; Liu, H.; Jian, C.; Zhao, S. Curcumin-Loaded Gelatin Nanoparticles Cross the Blood-Brain Barrier to Treat Ischemic Stroke by Attenuating Oxidative Stress and Neuroinflammation. Int. J. Nanomed. 2024, 19, 11633–11649. [Google Scholar] [CrossRef]
- Lin, J.; Chen, S.; Zhang, C.; Liao, J.; Chen, Y.; Deng, S.; Mao, Z.; Zhang, T.; Tian, N.; Song, Y.; et al. Recent advances in microfluidic technology of arterial thrombosis investigations. Platelets 2024, 35, 2316743. [Google Scholar] [CrossRef]
- Illath, K.; Kar, S.; Gupta, P.; Shinde, A.; Wankhar, S.; Tseng, F.G.; Lim, K.T.; Nagai, M.; Santra, T.S. Microfluidic nanomaterials: From synthesis to biomedical applications. Biomaterials 2022, 280, 121247. [Google Scholar] [CrossRef] [PubMed]
- Herland, A.; Maoz, B.M.; Das, D.; Somayaji, M.R.; Prantil-Baun, R.; Novak, R.; Cronce, M.; Huffstater, T.; Jeanty, S.S.F.; Ingram, M.; et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 2020, 4, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Anwarkhan, S.; Koilpillai, J.; Narayanasamy, D. Utilizing Multifaceted Approaches to Target Drug Delivery in the Brain: From Nanoparticles to Biological Therapies. Cureus 2024, 16, e68419. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patel, K.; Patel, A.; Patel, S.; Desai, J.; Patel, M.; Shah, U.; Patel, A.; Solanki, N. Nanomaterials as drug delivery agents for overcoming the blood-brain barrier: A comprehensive review. ADMET DMPK 2024, 12, 63–105. [Google Scholar] [CrossRef]
- Xu, K.; Duan, S.; Wang, W.; Ouyang, Q.; Qin, F.; Guo, P.; Hou, J.; He, Z.; Wei, W.; Qin, M. Nose-to-brain delivery of nanotherapeutics: Transport mechanisms and applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1956. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Navigating Neurotoxicity and Safety Assessment of Nanocarriers for Brain Delivery: Strategies and Insights. Acta Biomater 2024, 189, 25–56. [Google Scholar] [CrossRef]
- Fithroni, A.B.; Inoue, H.; Zhou, S.; Hakim, T.F.N.; Tada, T.; Suzuki, M.; Sakurai, Y.; Ishimoto, M.; Yamada, N.; Sauriasari, R.; et al. Novel Drug Delivery Particles Can Provide Dual Effects on Cancer “Theranostics” in Boron Neutron Capture Therapy. Cells 2025, 14, 60. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Wang, Y.; Lu, S. Cellulose Based Nano-Scaffolds for Targeted Cancer Therapies: Current Status and Future Perspective. Int. J. Nanomed. 2025, 20, 199–213. [Google Scholar] [CrossRef]
- Lee, A.; Di Mascolo, D.; Francardi, M.; Piccardi, F.; Bandiera, T.; Decuzzi, P. Spherical polymeric nanoconstructs for combined chemotherapeutic and anti-inflammatory therapies. Nanomedicine 2016, 12, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Ni, Z. Innovations in Microfluidics to Enable Novel Biomedical Applications. Biosensors 2024, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Maxim, F.; Vitaly, M.; Anna, L.; Elizaveta, D.; Evgenii, P.; Konstantin, B.; Shuang, L.; Lang, M.; Chong, C.; Ekaterina, P.; et al. Smart Graphene Textiles for Biopotential Monitoring: Laser-Tailored Electrochemical Property Enhancement. ACS Sens. 2024, 9, 1809–1819. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, S.; Zhan, X.; Meng, W.; Wang, H.; Liu, C.; Zhang, T.; Zhang, K.; Su, S. Smartphone-based Wearable Microfluidic Electrochemical Sensor for On-Site Monitoring of Copper Ions in Sweat Without External Driving. Talanta 2023, 266, 125015. [Google Scholar] [CrossRef]
- Ying, Z.; Qiao, L.; Liu, B.; Gao, L.; Zhang, P. Development of a microfluidic wearable electrochemical sensor for the non-invasive monitoring of oxidative stress biomarkers in human sweat. Biosens. Bioelectron. 2024, 261, 116502. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Xu, T.; Zhang, X. Autonomous Sweating Wearable Platform for Bilirubin Sensing Based on Thermal Stimulation. Anal. Chem. 2024, 96, 20247–20254. [Google Scholar] [CrossRef]
- Ye, C.; Wang, M.; Min, J.; Tay, R.Y.; Lukas, H.; Sempionatto, J.R.; Li, J.; Xu, C.; Gao, W. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 2024, 19, 330–337. [Google Scholar] [CrossRef]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.-Y.; et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar] [CrossRef]
- Keerthanaa, M.R.; Panicker, L.R.; Narayan, R.; Kotagiri, Y.G. Biopolymer-protected graphene-Fe3O4 nanocomposite based wearable microneedle sensor: Toward real-time continuous monitoring of dopamine. RSC Adv. 2024, 14, 7131–7141. [Google Scholar] [CrossRef]
- Kafali, M.; Şahinoğlu, O.B.; Tufan, Y.; Orsel, Z.C.; Aygun, E.; Alyuz, B.; Saritas, E.U.; Erdem, E.Y.; Ercan, B. Antibacterial properties and osteoblast interactions of microfluidically synthesized chitosan—SPION composite nanoparticles. J. Biomed. Mater. Res. Part A 2023, 111, 1662–1677. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; van Leent, M.M.T.; Lameijer, M.; Fisher, E.A.; Fayad, Z.A.; Pérez-Medina, C. High-Density Lipoprotein Nanobiologics for Precision Medicine. Acc. Chem. Res. 2017, 51, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Z.; Jia, Q.; Xiong, J.; Wang, H. Bio-Skin-Inspired Flexible Pressure Sensor Based on Carbonized Cotton Fabric for Human Activity Monitoring. Sensors 2024, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, X.; Yan, X.; Ni, W.; Wu, M.; Liu, J. Nanoengineering Ultrathin Flexible Pressure Sensors with Superior Sensitivity and Wide Range via Nanocomposite Structures. ACS Sens. 2024, 9, 4176–4185. [Google Scholar] [CrossRef]
- Guedes, G.; Uribe, K.B.; Martínez-Parra, L.; Aires, A.; Beraza, M.; Ruiz-Cabello, J.; Cortajarena, A.L. Engineering Protein-Nanoparticle Hybrids as Targeted Contrast Agents. ACS Appl. Mater. Interfaces 2024, 16, 59849–59861. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Ribeiro, E.; Fernandes, S.; da Silva, J.E.; Vale, N.; Esteves da Silva, L. Safety Evaluation of Carbon Dots in UM-UC-5 and A549 Cells for Biomedical Applications. Cancers 2024, 16, 3332. [Google Scholar] [CrossRef]
- Singh, N.; Kaushik, A.; Ghori, I.; Rai, P.; Dong, L.; Sharma, A.; Malhotra, B.D.; John, R. Electrochemical and Plasmonic Detection of Myocardial Infarction Using Microfluidic Biochip Incorporated with Mesoporous Nanoscaffolds. ACS Appl Mater Interfaces 2024, 16, 32794–32811. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Z.; Ge, S.; Mao, Y.; Gu, Y.; Cao, X.; Lu, D. A microfluidic chip using Au@SiO2 array–based highly SERS-active substrates for ultrasensitive detection of dual cervical cancer–related biomarkers. Anal. Bioanal. Chem. 2022, 414, 7659–7673. [Google Scholar] [CrossRef]
- Zeng, Y.; Gan, X.; Xu, Z.; Hu, X.; Hu, C.; Ma, H.; Tu, H.; Chai, B.; Yang, C.; Hu, S.; et al. AIEgens-enhanced rapid sensitive immunofluorescent assay for SARS-CoV-2 with digital microfluidics. Anal. Chim. Acta 2024, 1298, 342398. [Google Scholar] [CrossRef]
- Liu, J.; Du, H.; Huang, L.; Xie, W.; Liu, K.; Zhang, X.; Chen, S.; Zhang, Y.; Li, D.; Pan, H. AI-powered microfluidics: Shaping the future of phenotypic drug discovery. ACS Appl. Mater. Interfaces 2024, 16, 38832–38851. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Y.; Feng, Y.Z.; Niu, X.; Zhao, Y.; Zhao, J.; Dong, Y.; Tan, M.; Xianyu, Y.; Chen, Y. Computer Vision-Based Artificial Intelligence-Mediated Encoding-Decoding for Multiplexed Microfluidic Digital Immunoassay. ACS Nano 2023, 17, 13700–13714. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.G.; Mansur, H.S.; Mansur, A.A.P.; Caires, A.; Carvalho, S.M.; Krambrock, K.; Outon, L.E.F.; Ardisson, J.D. Synthesis and characterization of iron oxide nanoparticles/carboxymethyl cellulose core-shell nanohybrids for killing cancer cells in vitro. Int. J. Biol. Macromol. 2019, 132, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Satarzadeh, R.; Motallebi, A.A.; Hosseini, H.; Ahari, H. The Impact of Chitosan Nanoparticles Coating with Sodium Lactate on Beef Hamburger Quality during Storage at 4 °C: Oxidative Stability, Microbial and Sensorial Characteristics. Arch. Razi Inst. 2024, 79, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Sathiensathaporn, S.; Solé-Porta, A.; Baowan, D.; Pissuwan, D.; Wongtrakoongate, P.; Roig, A.; Katewongsa, K.P. Nanoencapsulation of vitamin B(2) using chitosan-modified poly(lactic-co-glycolic acid) nanoparticles: Synthesis, characterization, and in vitro studies on simulated gastrointestinal stability and delivery. J. Food Sci. 2025, 90, e17631. [Google Scholar] [CrossRef]
- Tkachenko, A. Hemocompatibility studies in nanotoxicology: Hemolysis or eryptosis? (A review). Toxicol Vitr. 2024, 98, 105814. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanoparticle Res. 2016, 18, 194. [Google Scholar] [CrossRef]
- Lage, T.; Faustino, V.; Rodrigues, R.O.; Lima, R.A.; Minas, G. Haemocompatibility test of simple Magnetic Nanoparticles using the distribution of deformed RBCs. In Proceedings of the 2019 IEEE 6th Portuguese Meeting on Bioengineering (ENBENG), Lisbon, Portugal, 22–23 February 2019; pp. 1–4. [Google Scholar]
- Naskar, S.; Panda, A.K.; Jana, A.; Kanagaraj, S.; Basu, B. UHMWPE-MWCNT-nHA based hybrid trilayer nanobiocomposite: Processing approach, physical properties, stem/bone cell functionality, and blood compatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2320–2343. [Google Scholar] [CrossRef]
- Oddo, A.; Morozesk, M.; Lombi, E.; Schmidt, T.B.; Tong, Z.; Voelcker, N.H. Risk assessment on-a-chip: A cell-based microfluidic device for immunotoxicity screening. Nanoscale Adv. 2021, 3, 682–691. [Google Scholar] [CrossRef]
- Li, F.; Zhou, X.; Zhu, J.; Ma, J.; Huang, X.; Wong, S.T. High content image analysis for human H4 neuroglioma cells exposed to CuO nanoparticles. BMC Biotechnol. 2007, 7, 66. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wang, Y. Patterned Fibers Embedded Microfluidic Chips Based on PLA and PDMS for Ag Nanoparticle Safety Testing. Polymers 2016, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Mahler, G.J.; Stokol, T.; Shuler, M.L. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014, 14, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ruan, F.; Jiang, S.; Zeng, J.; Yin, H.; Liu, R.; Zhang, Y.; Huang, L.; Wang, C.; Ma, S.; et al. Black Phosphorus Quantum Dots Cause Nephrotoxicity in Organoids, Mice, and Human Cells. Small 2020, 16, e2001371. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.A.; Shah, N.; Parekh, K.; Dhas, N.; Patel, J.K. Surface-Modified PLGA Nanoparticles for Targeted Drug Delivery to Neurons. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 33–71. [Google Scholar]
- Dong, R.; Li, L.; Chang, H.; Song, G.; Liu, S. Study on the mechanisms of defective spermatogenesis induced by TiO2 NPs based on 3D blood-testis barrier microfluidic chip. Toxicology 2024, 507, 153888. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-Small Iron Nanoparticles Target Mitochondria Inducing Autophagy, Acting on Mitochondrial DNA and Reducing Respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef]
- Liu, Y.; Yoo, E.; Mahler, G.J.; Doiron, A.L. Endothelial barrier dysfunction induced by nanoparticle exposure through actin remodeling via caveolae/raft-regulated calcium signalling. NanoImpact 2018, 11, 82–91. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, W.; Chen, X.; Zou, M.; Guo, W.; Feng, X. Evaluating the safety and efficiency of nanomaterials: A focus on mitochondrial health. Biomed. Pharmacother. 2024, 180, 117484. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Kang, X.; Zhang, D.; Dou, X.; Wang, X.; Guo, G. A scalable synthesis of ternary nanocatalysts for a high-efficiency electrooxidation catalysis by microfluidics. Nanoscale 2020, 12, 12647–12654. [Google Scholar] [CrossRef]
- Yu, X.; Dutta, S.; Andreo, J.; Wuttke, S. Identifying synthetic variables influencing the reproducible microfluidic synthesis of ZIF nano- and micro-particles. Commun. Mater. 2024, 5, 210. [Google Scholar] [CrossRef]
- Wang, L.; Karadaghi, L.R.; Brutchey, R.L.; Malmstadt, N. Self-optimizing parallel millifluidic reactor for scaling nanoparticle synthesis. Chem. Commun. 2020, 56, 3745–3748. [Google Scholar] [CrossRef]


| Nanomaterial Name | Size | Shape | Function | References |
|---|---|---|---|---|
| gold nanobipyramids | 85 ± 2 nm in length, 26 ± 4 nm in width | bicone | converting light energy into thermal energy, promoting the detection of cTnI | [32] |
| gold NPs | 150 nm | sphere | binding with antibodies, enhancing the detection signal of hs-cTnT | [42] |
| CSWCNs, silicon NPs | N/D | tubular, sphere | constructing PHC barcodes | [43] |
| AIENPs, magnetic NPs | 300 nm, N/D | sphere | as fluorescent probes and capture particles, detecting h-FABP | [44] |
| Ru(bpy)32+ loaded silica NPs | 250 nm | porous sphere | as ECL probes, enhancing the sensitivity of h-FABP detection | [45] |
| GNPs | 40 nm | sphere | marking NT-proBNP immune complexes | [46] |
| SA-B-HRP | 220 nm | sphere | enhancing the luminous signal, improving the detection sensitivity of PCT and IL-6 | [47] |
| AgNPs, AuNPs | 55.37 ± 6.7 nm, 40 ± 4.78 nm | sphere | detecting GFAP, enhancing signal | [48] |
| PS microspheres, AuNPs | 5 μm, 40 ± 4.78 nm | sphere | enhancing signal | [49] |
| nanoporous membrane, AuNPs | 41 ± 2 nm, N/D | nanopore structure, sphere | detecting Aβ, enhancing signal | [50] |
| Streptavidin-QD655 | N/D | sphere | detecting ApoE | [51] |
| AuNPs | 12–14 nm | sphere | detecting CTCs | [52] |
| AuNPs | 5 nm | sphere | detecting CTCs | [53] |
| AuNCAs | N/D | tapered array | detecting ctDNA | [54] |
| SPM | 1 μm | sphere | detecting ctDNA | [55] |
| P-mesh | N/D | network topology | detecting ctDNA | [56] |
| GNPs | 15 nm | sphere | detecting HER2-positive exosomes | [57] |
| MIL-125-NH2 NPs | N/D | plate crystal | detecting CLD7 | [58] |
| AuNCA | N/D | coronal structure | detecting hnRNPA1 and S100P | [59] |
| Fe3O4@AuNPs, AuNCs | N/D, 40 nm | sphere, hollow cube | enhancing signal | [60] |
| ZnMn2O4@rGO | N/D | sphere | improving the detection sensitivity of CEA | [61] |
| Type | Methods | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Traditional | ELISA | simple operation, high sensitivity | false positive results, cross-reactivity, prolonged duration | [88] |
| Novel | optical detection (SERS, LSPR and others) | high sensitivity, high specificity, multiple detection | complex equipment, signal interference | [42,46,49,54,57,59,60] |
| electrochemical detection | high sensitivity, fast response | limited selectivity, changes in the stability of the electrode | [45,50,51,52,58,61] | |
| hot plasma detection | high sensitivity, rapid response, multiple detection | high equipment cost, variation in thermal stability of nanomaterials | [32] | |
| fluorescence detection | high sensitivity, high specificity, multiple detection | prolonged photobleaching, background signal interference | [44,53,56] | |
| magnetic separation technology | efficient separation, simple operation | sample requires pretreatment, changes in the stability of magnetic beads | [55] | |
| chemiluminescence immune assay | high sensitivity, high specificity | poor stability of reagents and substrates, complicated operation | [47] |
| Nanomaterial Name | Size | Shape | Function | References |
|---|---|---|---|---|
| FC-ZnONRs, LIG | N/D | flower cluster structure, 3D porous structure | improving the electrochemical detection sensitivity of copper ions, increasing electrical conductivity | [124] |
| CNTs/MoS2-X/TiO2 | N/D | core-shell structure | detecting H2O2 and phosphorylated proteins | [125] |
| MXene/MWCNT | N/D | core-shell structure | enhancing the signal of bilirubin | [126] |
| AuNPs, MXene | 22 nm, N/D | sphere, stratified structure | enhancing the signal of estradiol | [127] |
| AuNPs | 20 nm | sphere | detecting CRP | [128] |
| Fe3O4, GO | N/D | sphere, stratified structure | improving the electrochemical detection sensitivity of dopamine | [129] |
| Ch-SPIONs | 8.8 ± 1.2 nm | sphere | as MRI contrast agent, enhancing image contrast and clarity | [130] |
| HDL nanobiologics | 8–9 nm, 20–30 nm, 40–400 nm | disc, sphere | integrating multiple imaging labels, suiting various imaging technologies | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Fu, Y.; Dang, Z.; Guo, T.; Li, W.; Zhang, J. Utilizing Nanomaterials in Microfluidic Devices for Disease Detection and Treatment. Nanomaterials 2025, 15, 434. https://doi.org/10.3390/nano15060434
Tian Z, Fu Y, Dang Z, Guo T, Li W, Zhang J. Utilizing Nanomaterials in Microfluidic Devices for Disease Detection and Treatment. Nanomaterials. 2025; 15(6):434. https://doi.org/10.3390/nano15060434
Chicago/Turabian StyleTian, Zhibiao, Yatian Fu, Zhiyong Dang, Tao Guo, Wenjuan Li, and Jing Zhang. 2025. "Utilizing Nanomaterials in Microfluidic Devices for Disease Detection and Treatment" Nanomaterials 15, no. 6: 434. https://doi.org/10.3390/nano15060434
APA StyleTian, Z., Fu, Y., Dang, Z., Guo, T., Li, W., & Zhang, J. (2025). Utilizing Nanomaterials in Microfluidic Devices for Disease Detection and Treatment. Nanomaterials, 15(6), 434. https://doi.org/10.3390/nano15060434






