Defect-Engineered Z-Scheme Heterojunction of Fe-MOFs/Bi2WO6 for Solar-Driven CO2 Conversion: Synergistic Surface Catalysis and Interfacial Charge Dynamics
Abstract
1. Introduction
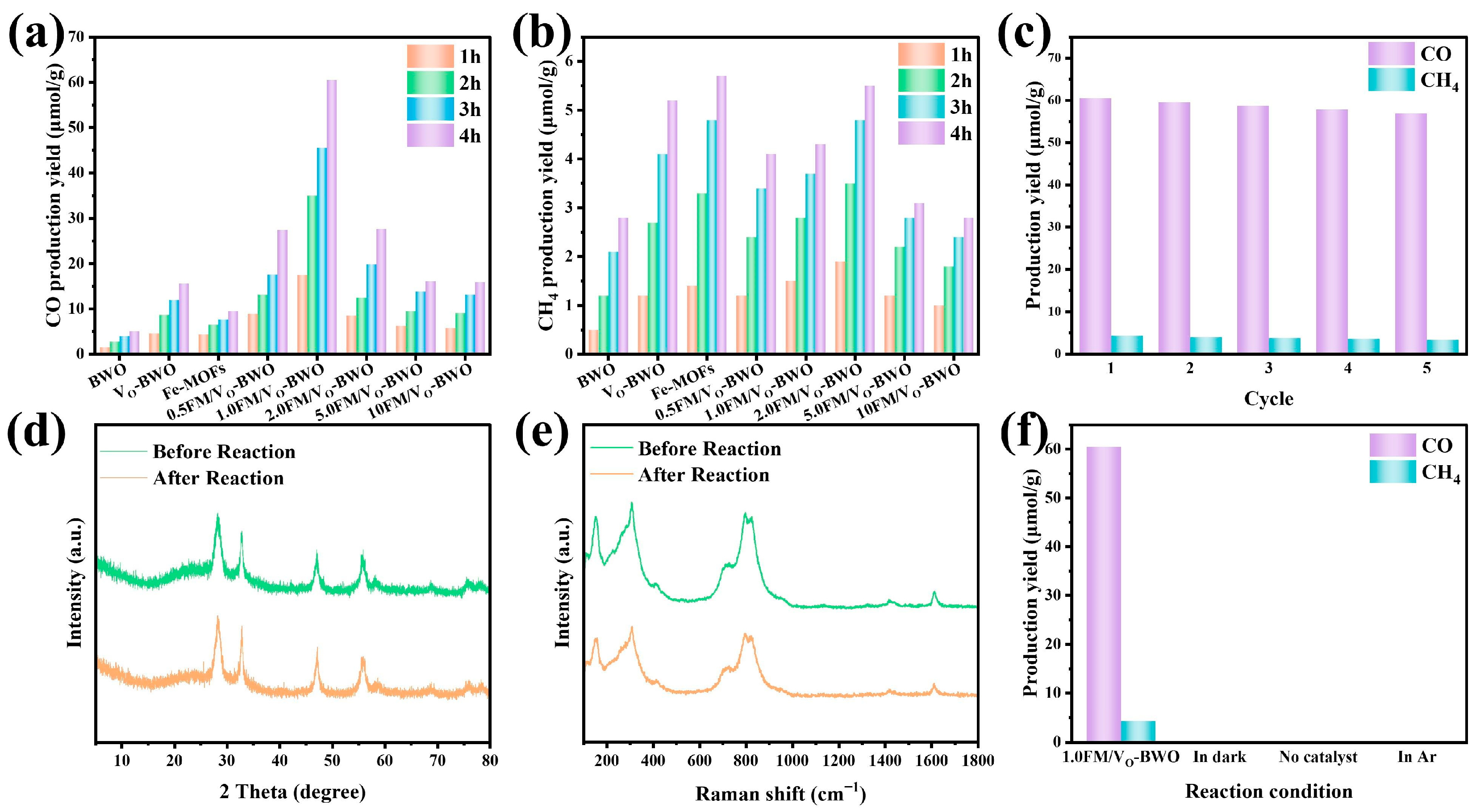
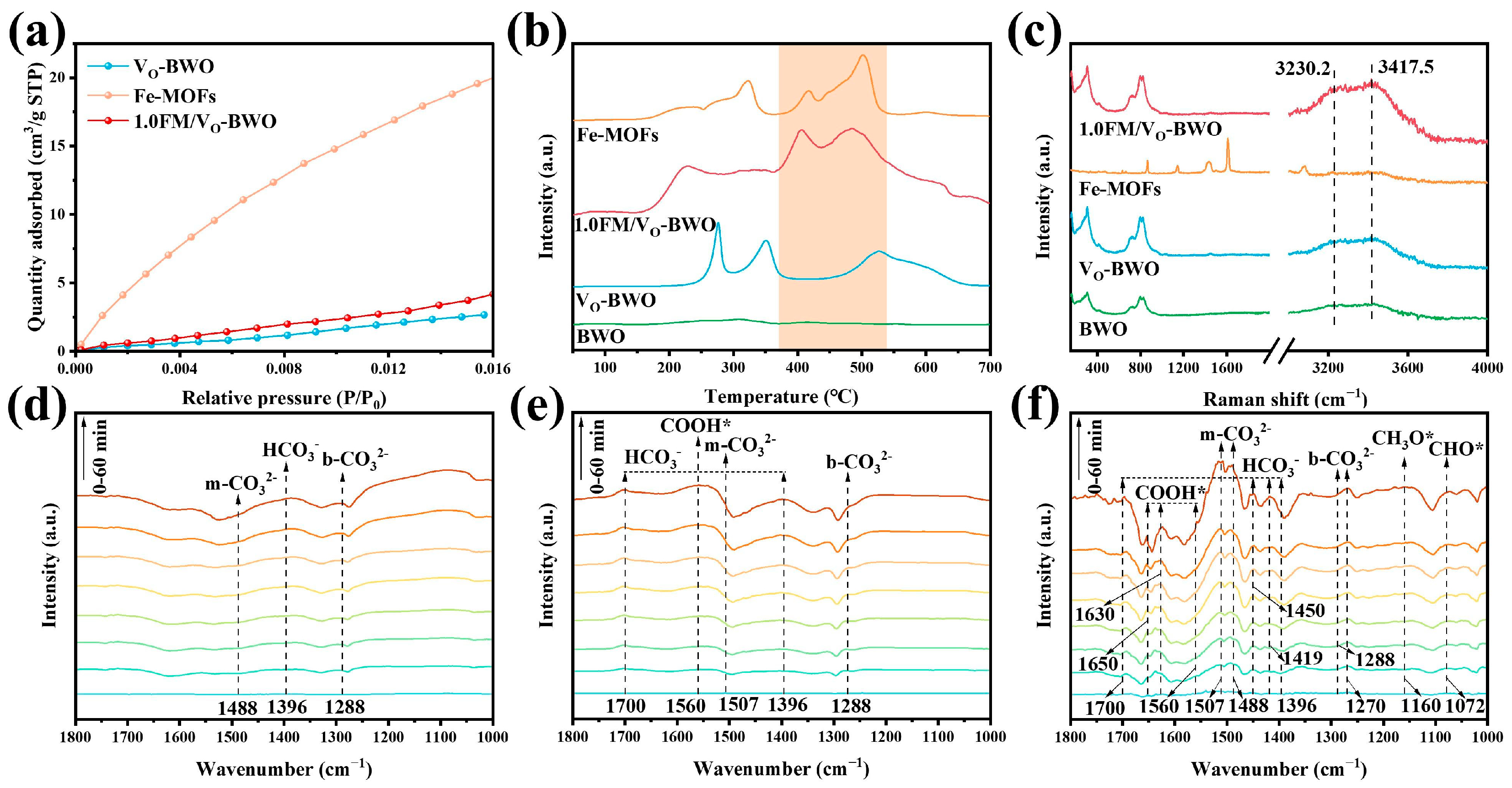
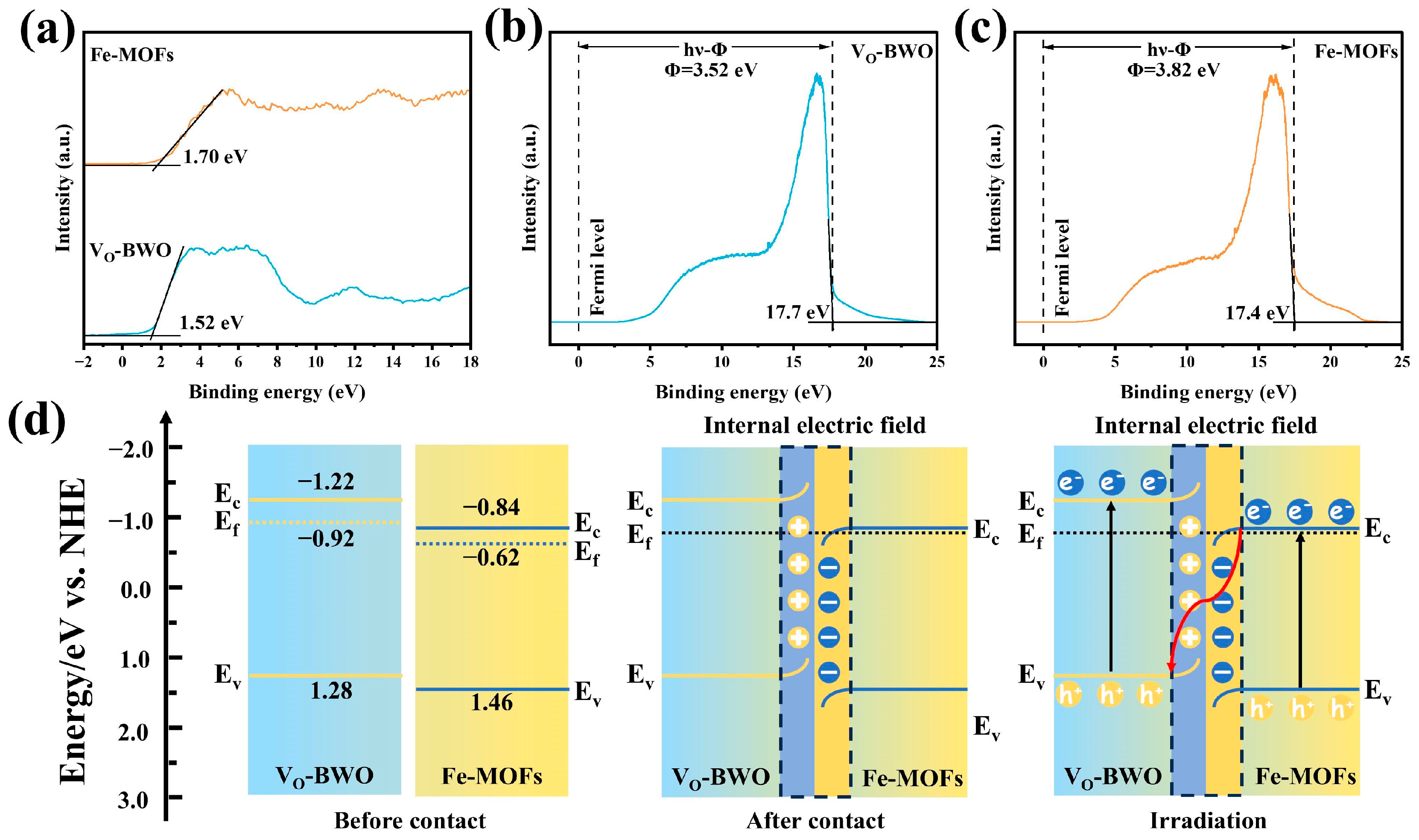
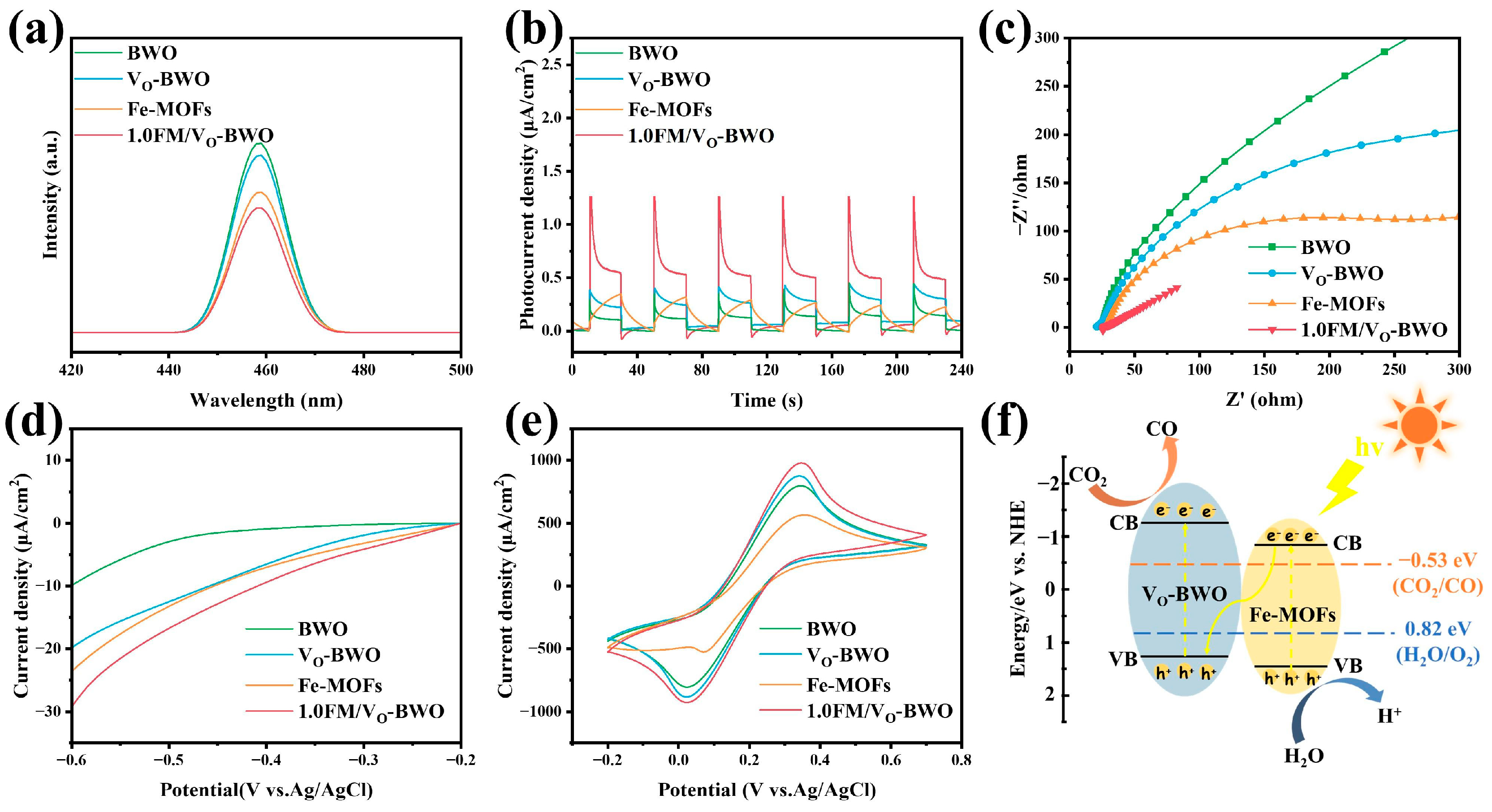
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis of Layered Bi2WO6
4.3. Synthesis of VO-Bi2WO6 Nanosheets
4.4. Synthesis of Fe-MOFs
4.5. Synthesis of Fe-MOFs/VO-Bi2WO6 Composites
4.6. General Characterization
4.7. In Situ Raman Spectroscopy
4.8. In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy (In Situ DRIFTS)
4.9. Evaluation of Photocatalytic CO2 Reduction Performance
4.10. Photoelectrochemical Measurements
4.11. Synchrotron-Radiation X-Ray Absorption Fine Structure (XAFS) Spectroscopy
4.12. Positron Annihilation Lifetime Spectral (PALS)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Mohamed, A.R.; Ong, W.-J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem. Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring Global Carbon Emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Meys, R.; Kätelhön, A.; Bachmann, M.; Winter, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving Net-Zero Greenhouse Gas Emission Plastics by a Circular Carbon Economy. Science 2021, 374, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, S.; He, H.; Li, X.; Cheng, Z.; Yang, Y.; Wang, J.; Shen, Q.; Wang, X.; Xiong, Y.; et al. Vacancy-Defect Modulated Pathway of Photoreduction of CO2 on Single Atomically Thin AgInP2S6 Sheets into Olefiant Gas. Nat. Commun. 2021, 12, 4747. [Google Scholar] [CrossRef]
- Cheng, C.; Xu, H.; Ni, M.; Guo, C.; Zhao, Y.; Hu, Y. Interfacial Electron Interactions Governed Photoactivity and Selectivity Evolution of Carbon Dioxide Photoreduction with Spinel Cobalt Oxide Based Hollow Hetero-Nanocubes. Appl. Catal. B Environ. 2024, 345, 123705. [Google Scholar] [CrossRef]
- Wang, Y.; Godin, R.; Durrant, J.R.; Tang, J. Efficient Hole Trapping in Carbon Dot/Oxygen-Modified Carbon Nitride Heterojunction Photocatalysts for Enhanced Methanol Production from CO2 under Neutral Conditions. Angew. Chem. Int. Ed. 2021, 60, 20811–20816. [Google Scholar] [CrossRef]
- Yu, F.; Jing, X.; Wang, Y.; Sun, M.; Duan, C. Cover Picture: Hierarchically Porous Metal–Organic Framework/MoS2 Interface for Selective Photocatalytic Conversion of CO2 with H2O into CH3COOH (Angew. Chem. Int. Ed. 47/2021). Angew. Chem. Int. Ed. 2021, 60, 24729. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.-J.; Lin, Z. Nickel Metal–Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, W.; Li, X.; Jiao, X.; Zhu, J.; Sun, Y.; Xie, Y. Asymmetric Triple-Atom Sites Confined in Ternary Oxide Enabling Selective CO2 Photothermal Reduction to Acetate. J. Am. Chem. Soc. 2021, 143, 18233–18241. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Rao, Z.; Yu, F.; Bao, W.; Tang, Y.; Zhao, H.; Zhang, J.; Wang, Z.; Li, J.; et al. Experimental and Theoretical Insights into an Enhanced CO2 Methanation Mechanism over a Ru-Based Catalyst. Appl. Catal. B Environ. 2022, 319, 121903. [Google Scholar] [CrossRef]
- Zhang, T.; Han, X.; Nguyen, N.T.; Yang, L.; Zhou, X. TiO2-Based Photocatalysts for CO2 Reduction and Solar Fuel Generation. Chin. J. Catal. 2022, 43, 2500–2529. [Google Scholar] [CrossRef]
- Patial, S.; Kumar, R.; Raizada, P.; Singh, P.; Van Le, Q.; Lichtfouse, E.; Le Tri Nguyen, D.; Nguyen, V.-H. Boosting Light-Driven CO2 Reduction into Solar Fuels: Mainstream Avenues for Engineering ZnO-Based Photocatalysts. Environ. Res. 2021, 197, 111134. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, C.; Ning, J.; Zhong, Y.; Chen, D.; Hu, Y. Oxygen-Vacancy-Assisted Construction of FeOOH/CdS Heterostructure as an Efficient Bifunctional Photocatalyst for CO2 Conversion and Water Oxidation. Appl. Catal. B Environ. 2021, 293, 120203. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galvan, M.C.; de la Mano, J.A.V.; Al-Zahrani, S.M.; Fierro, J.L.G. A Framework for Visible-Light Water Splitting. Energy Environ. Sci. 2010, 3, 1865–1882. [Google Scholar] [CrossRef]
- Navarro, R.M.; del Valle, F.; Villoria de la Mano, J.A.; Álvarez-Galván, M.C.; Fierro, J.L.G. Photocatalytic Water Splitting Under Visible Light: Concept and Catalysts Development. In Advances in Chemical Engineering; de Lasa, H.I., Serrano Rosales, B., Eds.; Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2009; Volume 36, pp. 111–143. [Google Scholar]
- MNavarro, R.M.; Sanchez-Sanchez, M.C.; Alvarez-Galvan, M.C.; Del Valle, F.; Fierro, J.L.G. Hydrogen Production from Renewable Sources: Biomass and Photocatalytic Opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Tomita, O.; Otsubo, T.; Higashi, M.; Ohtani, B.; Abe, R. Partial Oxidation of Alcohols on Visible-Light-Responsive WO3 Photocatalysts Loaded with Palladium Oxide Cocatalyst. ACS Catal. 2016, 6, 1134–1144. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Sun, C.; Liu, L.; Cui, Y.; Liang, B. Performance Enhancement Strategies of Bi-Based Photocatalysts: A Review on Recent Progress. Chem. Eng. J. 2020, 389, 124402. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, X.; Hu, C.; Wang, L.; Shi, B.; Cao, X. Enhanced Photocatalytic Destruction of Pollutants by Surface W Vacancies in VW-Bi2WO6 under Visible Light. J. Colloid Interface Sci. 2020, 576, 385–393. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Gao, C.; Hu, Q.; Li, M.; Ding, Y.; Di, J.; Xia, J.; Li, H. In-Situ Preparation of MIL-125(Ti)/Bi2WO6 Photocatalyst with Accelerating Charge Carriers for the Photodegradation of Tetracycline Hydrochloride. J. Photochem. Photobiol. Chem. 2020, 387, 112149. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.; Zhang, Y.; Ma, T. Oxygen Vacant Semiconductor Photocatalysts. Adv. Funct. Mater. 2021, 31, 2100919. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-Based Metal Organic Frameworks (Fe-MOFs) for Organic Pollutants Removal via Photo-Fenton: A Review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Cheng, H.; Zang, C.; Bian, F.; Jiang, Y.; Yang, L.; Dong, F.; Jiang, H. Boosting Free Radical Type Photocatalysis over Pd/Fe-MOFs by Coordination Structure Engineering. Catal. Sci. Technol. 2021, 11, 5543–5552. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, K.; Peng, W.; Huang, J. G-C3N4/Uio-66-NH2 Nanocomposites with Enhanced Visible Light Photocatalytic Activity for Hydrogen Evolution and Oxidation of Amines to Imines. New J. Chem. 2020, 44, 3052–3061. [Google Scholar] [CrossRef]
- Madhusudan, P.; Shi, R.; Xiang, S.; Jin, M.; Chandrashekar, B.N.; Wang, J.; Wang, W.; Peng, O.; Amini, A.; Cheng, C. Construction of Highly Efficient Z-Scheme ZnxCd1-xS/Au@g-C3N4 Ternary Heterojunction Composite for Visible-Light-Driven Photocatalytic Reduction of CO2 to Solar Fuel. Appl. Catal. B Environ. 2021, 282, 119600. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, L.; Li, M.; Lu, H.; Wei, G.; Qu, Y.; Wang, G. Z-Scheme Heterojunction Photocatalyst Based on Lanthanum Single-Atom Anchored on Black Phosphorus for Regulating Surface Active Sites, Therefore Enhancing Photocatalytic CO2 Reduction with ≈100% CO Selectivity. Adv. Funct. Mater. 2022, 32, 2207330. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Zhang, Q.; Lin, Y.; Peng, F. Enhanced Photocatalytic CO2 Reduction in H2O Vapor by Atomically Thin Bi2WO6 Nanosheets with Hydrophobic and Nonpolar Surface. Appl. Catal. B Environ. 2021, 283, 119630. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-Based MOFs for Photocatalytic CO2 Reduction: Role of Coordination Unsaturated Sites and Dual Excitation Pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Ji, M.; Xia, J.; Yan, C.; Hao, W.; Li, S.; Li, H.; Liu, Z. Bismuth Vacancy Mediated Single Unit Cell Bi2WO6 Nanosheets for Boosting Photocatalytic Oxygen Evolution. Appl. Catal. B Environ. 2018, 238, 119–125. [Google Scholar] [CrossRef]
- Su, Y.; Tan, G.; Liu, T.; Lv, L.; Wang, Y.; Zhang, X.; Yue, Z.; Ren, H.; Xia, A. Photocatalytic Properties of Bi2WO6/BiPO4 Z-Scheme Photocatalysts Induced by Double Internal Electric Fields. Appl. Surf. Sci. 2018, 457, 104–114. [Google Scholar] [CrossRef]
- Jakhade, A.P.; Biware, M.V.; Chikate, R.C. Two-Dimensional Bi2 WO6 Nanosheets as a Robust Catalyst toward Photocyclization. ACS Omega 2017, 2, 7219–7229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, H.-Y.; Li, J.-Y.; Liao, J.-F.; Zhang, H.-H.; Wang, X.-D.; Kuang, D.-B. Z-Scheme 2D/2D Heterojunction of CsPbBr3/Bi2WO6 for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2020, 30, 2004293. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, K.; Wan, J.; Fu, F.; Fan, J. Novel Phosphorus-Doped Bi2WO6 Monolayer with Oxygen Vacancies for Superior Photocatalytic Water Detoxication and Nitrogen Fixation Performance. Chem. Eng. J. 2021, 411, 128629. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Hu, P.; Liu, T.; Weng, B.; Ye, K.; Luo, Y.; Ji, H. Vacancy Pair Induced Surface Chemistry Reconstruction of Cs2AgBiBr6/Bi2WO6 Heterojunction to Enhance Photocatalytic CO2 Reduction. Appl. Catal. B Environ. Energy 2024, 351, 123956. [Google Scholar] [CrossRef]
- Ou, G.; Xu, Y.; Wen, B.; Lin, R.; Ge, B.; Tang, Y.; Liang, Y.; Yang, C.; Huang, K.; Zu, D.; et al. Tuning Defects in Oxides at Room Temperature by Lithium Reduction. Nat. Commun. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Z.; Wang, J.; Zhang, D.; Zhou, F.; Li, J.; Huang, J. Scheme-II Heterojunction of Bi2WO6@Br-COFs Hybrid Materials for CO2 Photocatalytic Reduction. Chem. Eng. J. 2023, 471, 144559. [Google Scholar] [CrossRef]
- Zhu, K.; Qin, W.; Gan, Y.; Huang, Y.; Jiang, Z.; Chen, Y.; Li, X.; Yan, K. Acceleration of Fe3+/Fe2+ Cycle in Garland-like MIL-101(Fe)/MoS2 Nanosheets to Promote Peroxymonosulfate Activation for Sulfamethoxazole Degradation. Chem. Eng. J. 2023, 470, 144190. [Google Scholar] [CrossRef]
- Ren, X.; Yao, J.; Cai, L.; Li, J.; Cao, X.; Zhang, Y.; Wang, B.; Wei, Y. Band Gap Engineering of BiOI via Oxygen Vacancies Induced by Graphene for Improved Photocatalysis. New J. Chem. 2019, 43, 1523–1530. [Google Scholar] [CrossRef]
- Achary, L.S.K.; Kumar, A.; Rout, L.; Kunapuli, S.V.S.; Dhaka, R.S.; Dash, P. Phosphate Functionalized Graphene Oxide with Enhanced Catalytic Activity for Biginelli Type Reaction under Microwave Condition. Chem. Eng. J. 2018, 331, 300–310. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zheng, S.; Yang, W.-M.; Zhou, R.-Y.; He, Q.-F.; Radjenovic, P.; Dong, J.-C.; Li, S.; Zheng, J.; Yang, Z.-L.; et al. In Situ Raman Spectroscopy Reveals the Structure and Dissociation of Interfacial Water. Nature 2021, 600, 81–85. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Liu, Q.; Chen, Z.; Chen, Z.; Feng, X.; Chen, H. Interfacial Engineering of Bi19 Br3 S27 Nanowires Promotes Metallic Photocatalytic CO2 Reduction Activity under Near-Infrared Light Irradiation. J. Am. Chem. Soc. 2021, 143, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Chen, M.; Wang, J.; Wang, L. In Situ Growth Strategy Synthesis of Single-Atom Nickel/Sulfur Co-Doped g-C3N4 for Efficient Photocatalytic Tetracycline Degradation and CO2 Reduction. Chem. Eng. J. 2022, 442, 136208. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, M.; Li, Z.; Zhu, Z.; Chen, Y.; Thummavichai, K.; Ola, O.; Wang, N.; Zhu, Y. Interfacial Construction of P25/Bi2 WO6 Composites for Selective CO2 Photoreduction to CO in Gas–Solid Reactions. RSC Adv. 2023, 13, 8564–8576. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Z.; Yu, P.; Balu, A.M.; Li, K.; Li, L.; Zeng, L.; Zhang, C.; Luque, R.; Yan, K.; et al. Understanding Bridging Sites and Accelerating Quantum Efficiency for Photocatalytic CO2 Reduction. Nano-Micro Lett. 2024, 16, 5. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Deng, F.; Huang, J.; Han, L.; He, C.; Chen, Z.; Luo, Y.; Zhu, Y. Double-Defect-Induced Polarization Enhanced OV-BiOBr/Cu2−xS High-Low Junction for Boosted Photoelectrochemical Hydrogen Evolution. Appl. Catal. B Environ. 2022, 314, 121502. [Google Scholar] [CrossRef]
- Tang, C.; Bao, T.; Li, S.; Li, X.; Rao, H.; She, P.; Qin, J. Rapid Charge Transfer Endowed by Hollow-structured Z-Scheme Heterojunction for Coupling Benzylamine Oxidation with CO2 Reduction. Adv. Funct. Mater. 2024, 35, 2415280. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Shi, Z.; Liu, Z.; Zhao, Z.; Li, J.; Li, N.; Huang, H. Enabling Interfacial Lattice Matching by Selective Epitaxial Growth of CuS Crystals on Bi2WO6 Nanosheets for Efficient CO2 Photoreduction into Solar Fuels. Angew. Chem. Int. Ed. 2025, 64, e202418496. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.; Zhang, Q.; Xie, P.; Deng, L.; Wang, S. CuInS2 Quantum Dots Embedded in Bi2WO6 Nanoflowers for Enhanced Visible Light Photocatalytic Removal of Contaminants. Appl. Catal. B Environ. 2018, 221, 215–222. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, F.; Li, Y.; Wang, F.; Li, C.; Li, H.; Jiang, Y. Bi2WO6@Cu2O-GOx Bio-Heterojunctionp-n Spray for Accelerating Chronic Diabetic Wound Repairment with Bilaterally Enhanced Sono-Catalysis and Glycolytic Inhibition Antisepsis. Biomaterials 2025, 317, 123046. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yu, J.; Wu, Z.; Zhou, Q. Preparation of Flower-like Co3O4 QDs/Bi2WO6 p-n Heterojunction Photocatalyst and Its Degradation Mechanism of Efficient Visible-Light-Driven Photocatalytic Tetracycline Antibiotics. Appl. Surf. Sci. 2022, 585, 152547. [Google Scholar] [CrossRef]
- Wu, W.; Guan, X.; Zhang, X.; Zhang, C.; Guo, X.; Liu, J.; Li, R.; Fan, C. In-Situ Construction of Bi2WO6/BiOCl p-n Heterojunction Film for Photocatalytic CO2 Reduction. Inorg. Chem. Commun. 2023, 158, 111454. [Google Scholar] [CrossRef]
- Pan, Y.-X.; You, Y.; Xin, S.; Li, Y.; Fu, G.; Cui, Z.; Men, Y.-L.; Cao, F.-F.; Yu, S.-H.; Goodenough, J.B. Photocatalytic CO2 Reduction by Carbon-Coated Indium-Oxide Nanobelts. J. Am. Chem. Soc. 2017, 139, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, Y.; Yang, X.; Jia, X.; Xu, J.; Song, H. Photocatalytic H2O2 Generation Reaction with a Benchmark Rate at Air-Liquid-Solid Joint Interfaces. Adv. Mater. 2024, 36, 2307967. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.; Ji, H.; Zhang, L.; Ni, J.; Li, N.; Qian, T.; Yan, C.; Lu, J. Solvent-in-Gas System for Promoted Photocatalytic Ammonia Synthesis on Porous Framework Materials. Adv. Mater. 2023, 35, 2211730. [Google Scholar] [CrossRef]
- Feng, C.; Hu, M.; Zuo, S.; Luo, J.; Castaño, P.; Ren, Y.; Rueping, M.; Zhang, H. Ru-OV Site-Mediated Product Selectivity Switch for Overall Photocatalytic CO2 Reduction. Adv. Mater. 2025, 37, 2411813. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The Enhancement of CdS Photocatalytic Activity for Water Splitting via Anti-Photocorrosion by Coating Ni2P Shell and Removing Nascent Formed Oxygen with Artificial Gill. Appl. Catal. B Environ. 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Wei, D.; Li, Z. Ultrasonic-Assisted Fabrication of Cs2AgBiBr6/Bi2WO6 S-Scheme Heterojunction for Pho-tocatalytic CO2 Reduction under Visible Light. Chin. J. Catal. 2022, 43, 2606–2614. [Google Scholar] [CrossRef]
- Hao, M.; Wei, D.; Li, Z. Rational Design of an Efficient S-Scheme Heterojunction of CdS/Bi2WO6–S Nanocomposites for Pho-tocatalytic CO2 Reduction. Energy Fuels 2022, 36, 11524–11531. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Liu, R.-R.; Mu, Y.-F.; Feng, Y.-X.; Dong, G.-X.; Zhang, M.; Lu, T.-B. In Situ Construction of Lead-Free Perovskite Direct Z-Scheme Heterojunction Cs3Bi2I9/Bi2WO6 for Efficient Photocatalysis of CO2 Reduction. Sol. RRL 2021, 5, 2000691. [Google Scholar] [CrossRef]
- Dao, X.-Y.; Guo, J.-H.; Wei, Y.-P.; Guo, F.; Liu, Y.; Sun, W.-Y. Solvent-Free Photoreduction of CO2 to CO Catalyzed by Fe-MOFs with Superior Selectivity. Inorg. Chem. 2019, 58, 8517–8524. [Google Scholar] [CrossRef]
- Wu, L.; Mu, Y.; Guo, X.; Zhang, W.; Zhang, Z.; Zhang, M.; Lu, T. Encapsulating Perovskite Quantum Dots in Iron-Based Metal–Organic Frameworks (MOFs) for Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 9491–9495. [Google Scholar] [CrossRef] [PubMed]
- Xuan, R.; Chen, J.; Dou, Y.; Jiang, Z.; Li, X.; Ding, D.; Wang, C.; Yan, J.; Sun, L. Novel Bimetallic MIL-53(Fe/Mn) as Dual Co-catalysts Promote Charge Separation Efficiency of CdS for Photocatalytic Reduction of CO2. J. Environ. Chem. Eng. 2025, 13, 115293. [Google Scholar] [CrossRef]
- Liu, N.; Tang, K.; Wang, D.; Fei, F.; Cui, H.; Li, F.; Lei, J.; Crawshaw, D.; Zhang, X.; Tang, L. Enhanced Photocatalytic CO2 Reduction by Integrating an Iron Based Metal-Organic Framework and a Photosensitizer. Sep. Purif. Technol. 2024, 332, 125873. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Wu, Y.; Wang, H.; Lu, J.; Luo, Y. Defect-Engineered Z-Scheme Heterojunction of Fe-MOFs/Bi2WO6 for Solar-Driven CO2 Conversion: Synergistic Surface Catalysis and Interfacial Charge Dynamics. Nanomaterials 2025, 15, 618. https://doi.org/10.3390/nano15080618
Liu T, Wu Y, Wang H, Lu J, Luo Y. Defect-Engineered Z-Scheme Heterojunction of Fe-MOFs/Bi2WO6 for Solar-Driven CO2 Conversion: Synergistic Surface Catalysis and Interfacial Charge Dynamics. Nanomaterials. 2025; 15(8):618. https://doi.org/10.3390/nano15080618
Chicago/Turabian StyleLiu, Ting, Yun Wu, Hao Wang, Jichang Lu, and Yongming Luo. 2025. "Defect-Engineered Z-Scheme Heterojunction of Fe-MOFs/Bi2WO6 for Solar-Driven CO2 Conversion: Synergistic Surface Catalysis and Interfacial Charge Dynamics" Nanomaterials 15, no. 8: 618. https://doi.org/10.3390/nano15080618
APA StyleLiu, T., Wu, Y., Wang, H., Lu, J., & Luo, Y. (2025). Defect-Engineered Z-Scheme Heterojunction of Fe-MOFs/Bi2WO6 for Solar-Driven CO2 Conversion: Synergistic Surface Catalysis and Interfacial Charge Dynamics. Nanomaterials, 15(8), 618. https://doi.org/10.3390/nano15080618




