A Comparative Study of Approaches to Improve the Sensitivity of Lateral Flow Immunoassay of the Antibiotic Lincomycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactants
2.2. Preparation of Monoclonal Anti-LIN Antibodies
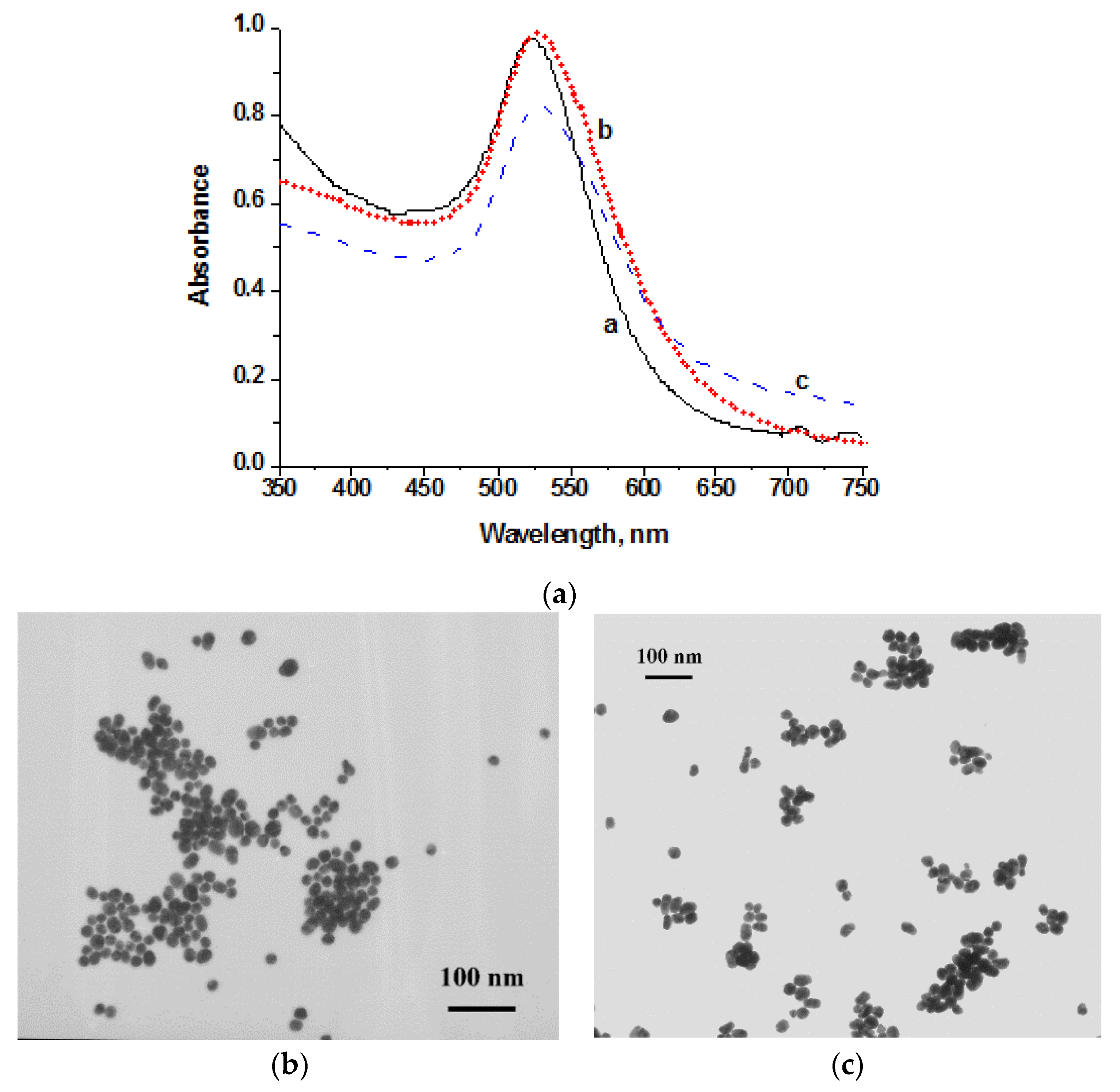
2.3. Synthesis and Characterization of AuNPs
2.4. Conjugation of Antibodies to AuNPs
2.5. Conjugation of Antibodies with QDs
2.6. Synthesis of the Raman Reporter Bioprobe
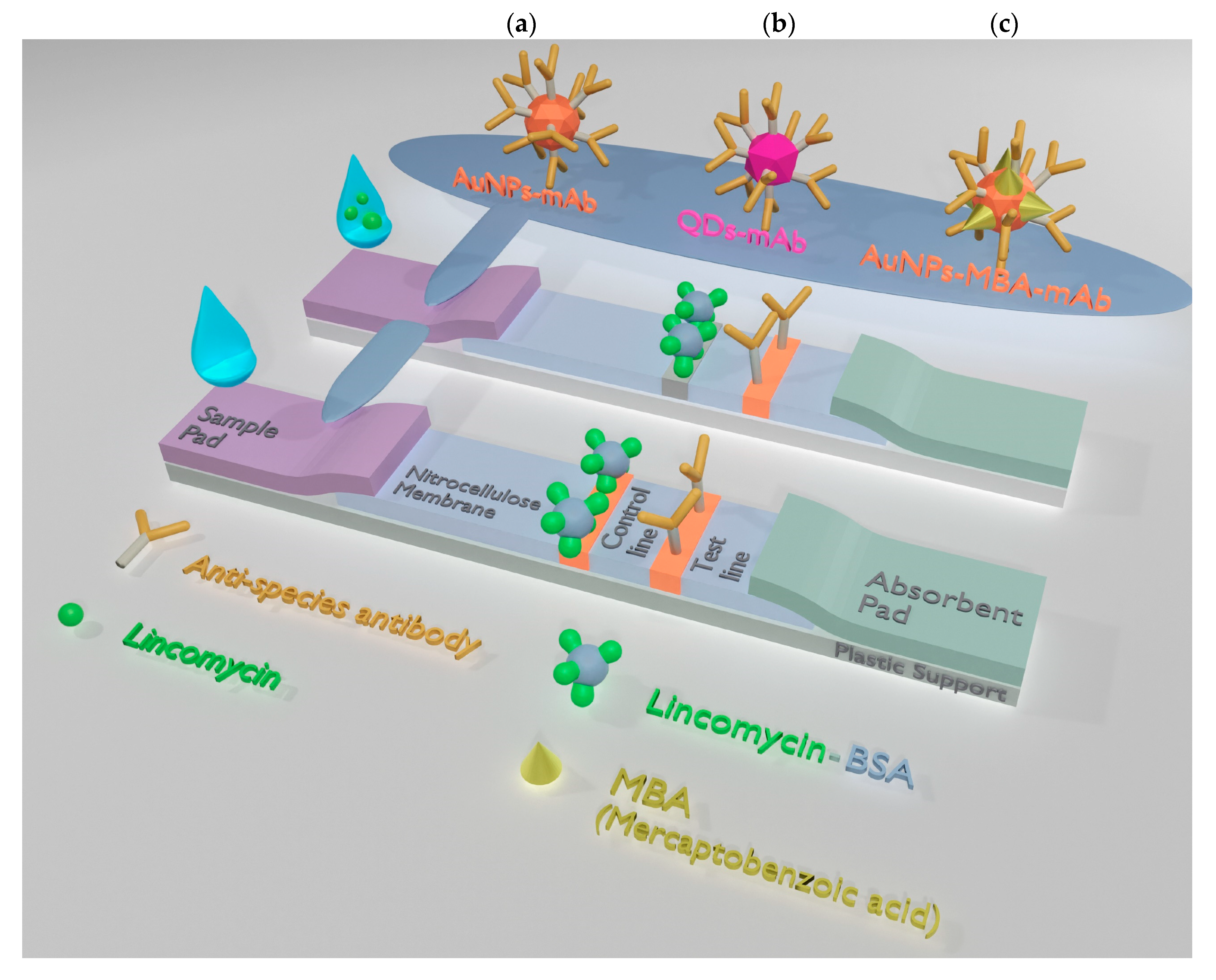
2.7. Preparation of Test Strips
2.8. LFIA Procedures
2.8.1. Colorimetric and Fluorescent LFIAs
2.8.2. SERS-Based LFIA
3. Results and Discussion
3.1. Synthesis and Characterization of Signal Markers
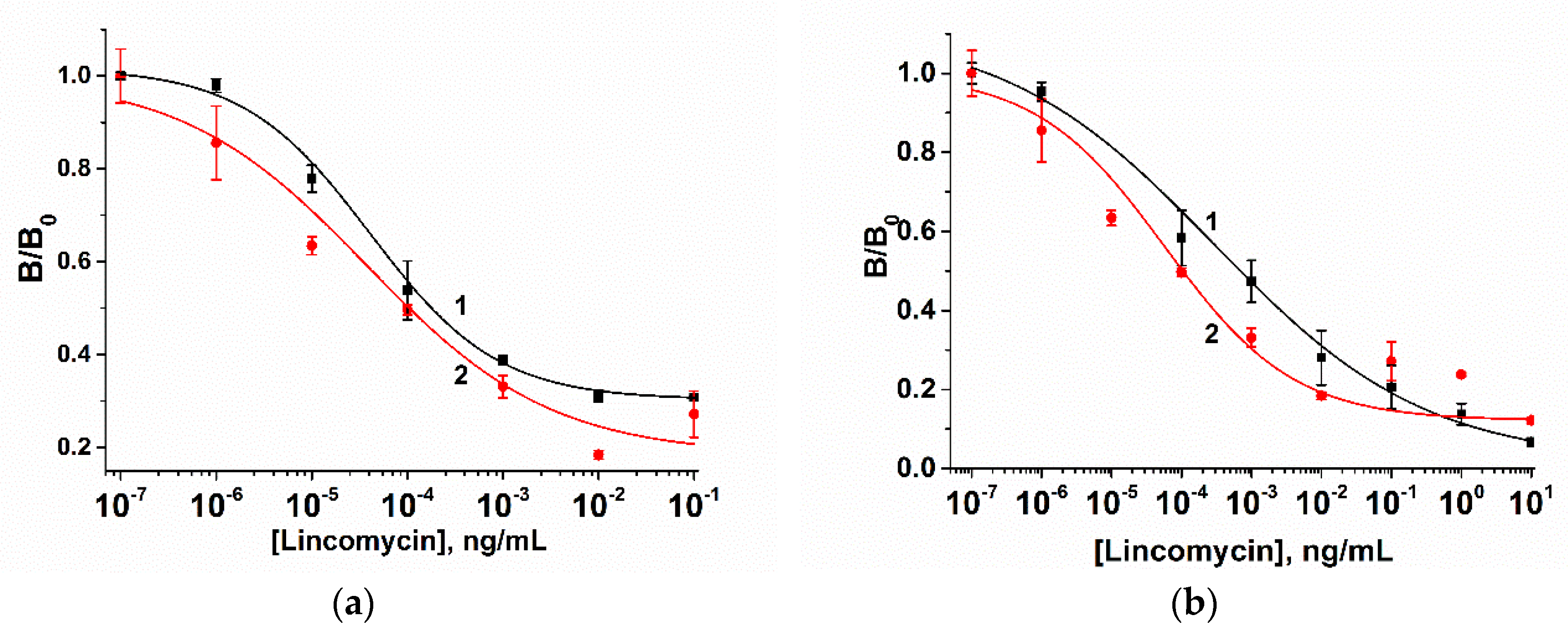
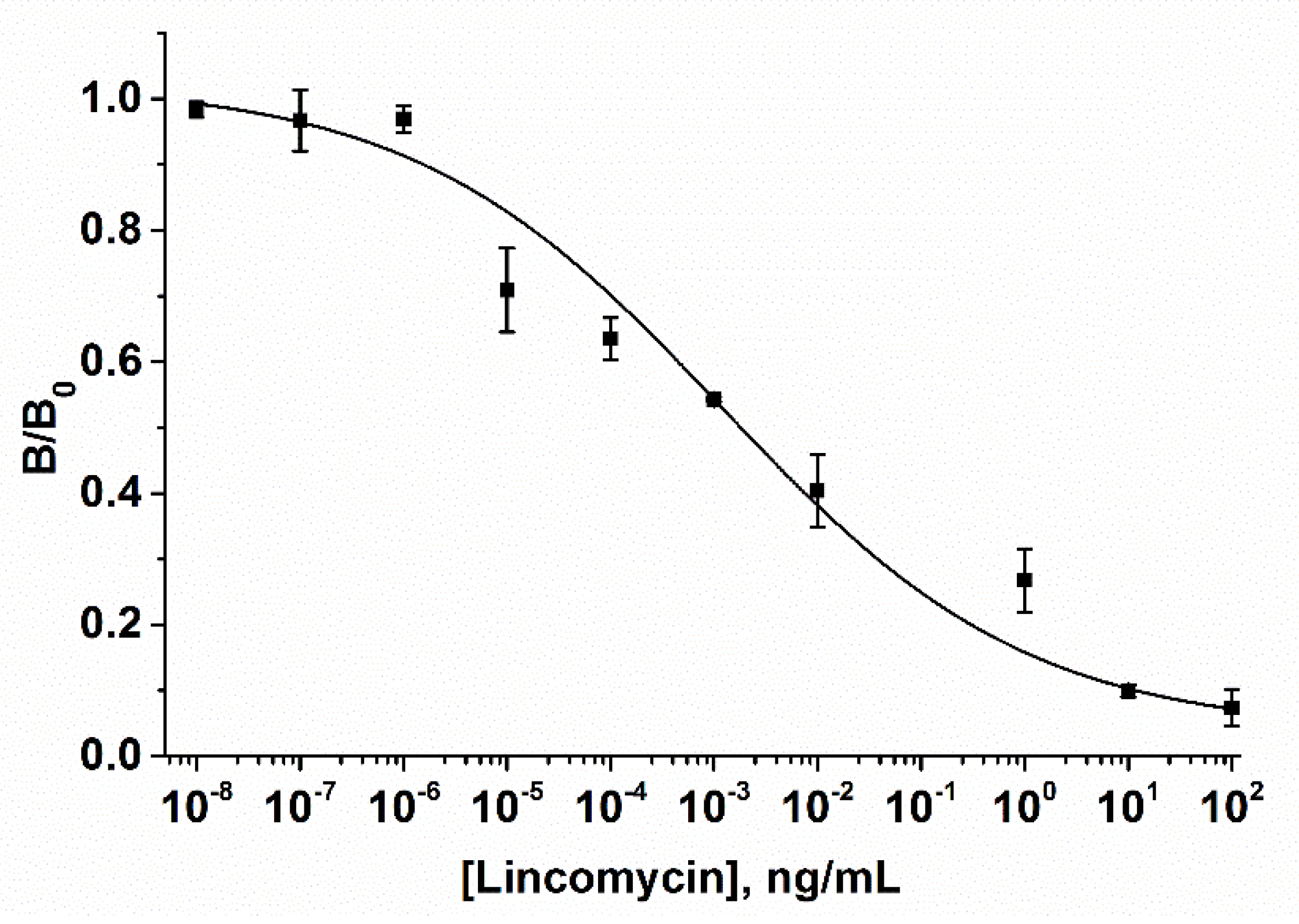
3.2. AuNP-Based LFIA
3.3. QD-Based LFIA
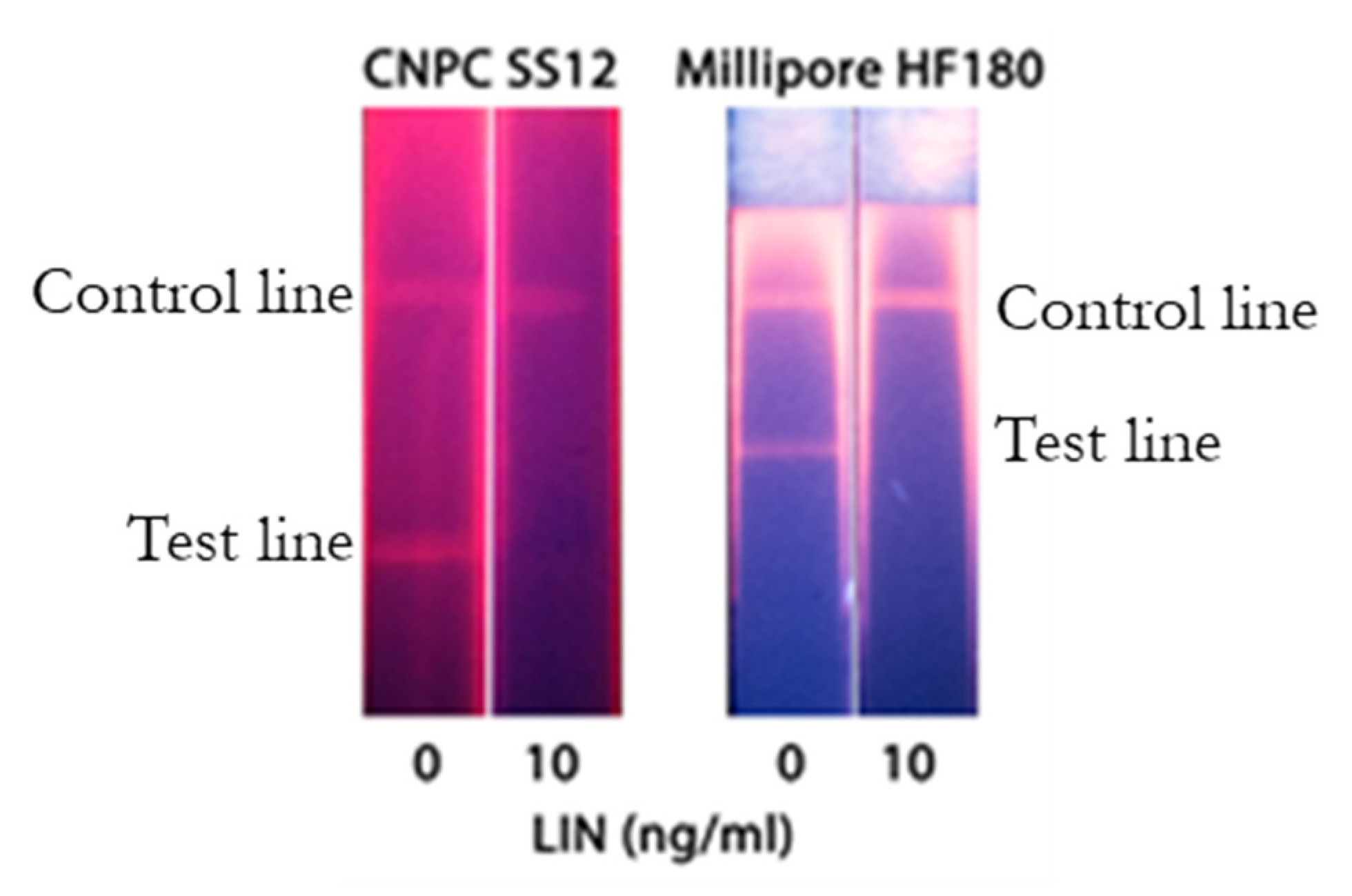
3.4. SERS-Based LFIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards Lateral flow quantitative assays: Detection approaches. Biosensors 2019, 9, 89. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, K.; Alfranca, G.; de la Fuente, J.M.; Cui, D. A plasmonic thermal sensing based portable device for lateral flow assay detection and quantification. Nanoscale Res. Lett. 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, S.; Choo, J. Application of a SERS-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale 2016, 8, 11418–11425. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Bratashov, D.N.; Byzova, N.A.; Dzantiev, B.B.; Khlebtsov, N.G. SERS-based lateral flow immunoassay of troponin I by using gap-enhanced Raman tags. Nano Res. 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Qian, J.; Xing, C.; Ge, Y.; Rui, L.; Aitong, L.; Yan, W. Gold nanostars-enhanced Raman fingerprint strip for rapid detection of trace tetracycline in water samples. Spectrochim. Acta A 2020, 232, 118146. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Hou, Y.; Zhang, C.; Li, D.; Li, H.; Yang, M.; Fan, C.; Sun, B. A SERS-based lateral flow assay biosensor for quantitative and ultrasensitive detection of interleukin-6 in unprocessed whole blood. Biosens. Bioelectron. 2019, 141, 111432. [Google Scholar] [CrossRef]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef]
- Tripathi, P.; Upadhyay, N.; Nara, S. Recent advancements in lateral flow immunoassays: A journey for toxin detection in food. Crit. Rev. Food Sci. Nutr. 2018, 58, 1715–1734. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Anfossi, L.; Giovannoli, C.; Baggiani, C.; Roda, A.; Mirasoli, M. A multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1 quantitative detection in maize flour. Analyst 2014, 140, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Naghdi, T.; Zor, E.; Merkoçi, A. Photoluminescent lateral-flow immunoassay revealed by graphene oxide: Highly sensitive paper-based pathogen detection. Anal. Chem. 2015, 87, 8573–8577. [Google Scholar] [CrossRef] [PubMed]
- Han, G.R.; Kim, M.G. Highly Sensitive chemiluminescence-based lateral flow immunoassay for cardiac troponin i detection in human serum. Sensors 2020, 20, 2593. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Dou, L.; Bu, T. Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chem. 2018, 261, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Noguera, P.; Posthuma-Trumpie, G.A.; van Tuil, M.; van der Wal, F.J.; de Boer, A.; Moers, A.P.H.A.; van Amerongen, A. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Anal. Bioanal. Chem. 2011, 399, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wiriyachaiporn, N.; Sirikett, H.; Maneeprakorn, W.; Dharakul, T. Carbon nanotag based visual detection of influenza A virus by a lateral flow immunoassay. Microchim. Acta 2017, 184, 1827–1835. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Monette, C.E.; Bracero, S.; Saha, M.S. Methods for field measurement of antibiotic concentrations: Limitations and outlook. FEMS Microbiol. Ecol. 2018, 94, fiy 105. [Google Scholar] [CrossRef]
- Benetti, C.; Piro, R.; Binato, G.; Angeletti, R.; Biancotto, G. Simultaneous determination of lincomycin and five macrolide antibiotic residues in honey by liquid chromatography coupled to electrospray ionisation mass spectrometry. Food Addit. Contam. 2006, 23, 1099–1108. [Google Scholar] [CrossRef]
- Jank, L.; Martins, M.; Bazzan, J.; Magalhães, T.; Motta, C.; Hoff, R.; Barreto, F.; Pizzolato, T. High-throughput method for macrolides and lincosamides antibiotics residues analysis in milk and muscle using a simple liquid–liquid ex- traction technique and liquid chromatography–electrospray–tandem mass spectrometry analysis (LC–MS/MS). Talanta 2015, 144, 686–695. [Google Scholar] [CrossRef]
- Leng, Y.; Hu, F.; Ma, C.; Du, C.; Ma, L.; Xu, J.; Lin, Q.; Sang, Z.; Luand, Z. Simple, rapid, sensitive, selective and label-free lincomycin detection by using HAuCl4 and NaOH. RSC Adv. 2019, 9, 28248–28252. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Yin, G.; Zhang, Q.; Luo, J.; Wu, N. Aptamer-molecularly imprinted sensor base on 2916 electrogenerated chemiluminescence energy transfer for detection of lincomycin. Biosens. Bioelectron. 2017, 91, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Zherdev, A.V.; Godjevargova, T.; Xu, C.; Dzantiev, B.B. Development of a double immunochromatographic test system for simultaneous determination of lincomycin and tylosin antibiotics in foodstuffs. Food Chem. 2020, 318, 126510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, K.; Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Ding, S. Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine. J. Agric. Food Chem. 2014, 62, 12061–12066. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Popravko, D.S.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. An immunochromatographic test system for the determination of lincomycin in foodstuffs of animal origin. J. Chromatogr. B 2020, 1141, 122014. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Song, S.; Liu, L.; Kong, N.; Kuang, H.; Xu, C. Comparison of an enzyme-linked immunosorbent assay with an immunochromatographic assay for detection of lincomycin in milk and honey. Immunol. Investig. 2015, 44, 438–450. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Shanin, I.A.; Zherdev, A.V.; Tarannum, N.; Dzantiev, B.B. Highly sensitive immunochromatographic detection of antibiotic ciprofloxacin in milk. Appl. Biochem. Microbiol. 2018, 54, 670–676. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Song, Y.; Zhu, J.; Wang, D.; Li, W. Surface-enhanced Raman spectroscopy (SERS)-based immunochromatographic assay (ICA) for the simultaneous detection of two pyrethroid pesticides. Sens. Actuators B 2019, 283, 230–238. [Google Scholar] [CrossRef]
- Serebrennikova, K.; Samsonova, J.; Osipov, A.; Senapati, D.; Kuznetsov, D. Gold nanoflowers and gold nanospheres as labels in lateral flow immunoassay of procalcitonin. Nano Hybrids Compos. 2017, 13, 47–53. [Google Scholar] [CrossRef]
- Fu, X.; Chu, Y.; Zhao, K.; Li, J.; Deng, A. Ultrasensitive detection of the β-adrenergic agonist brombuterol by a SERS-based lateral flow immunochromatographic assay using flower-like gold-silver core-shell nanoparticles. Microchim. Acta 2017, 184, 1711–1719. [Google Scholar] [CrossRef]
- Alasel, M.; Keusgen, M. Two-protein modified gold nanoparticles for one-step serological diagnosis. Phys. Status Solidi A 2018, 215, 1700700. [Google Scholar] [CrossRef]
- Karnorachai, K.; Sakamoto, K.; Laocharoensuk, R.; Suwussa, B.; Dharakul, T.; Miki, K. SERS-based immunoassay on 2D-arrays of Au@Ag core–shell nanoparticles: Influence of the sizes of the SERS probe and sandwich immunocomplex on the sensitivity. RSC Adv. 2017, 7, 14099–14106. [Google Scholar] [CrossRef]
- Li, R.; Lv, H.; Zhang, X.; Liu, P.; Chen, L.; Cheng, J.; Zhao, B. Vibrational spectroscopy and density functional theory study of 4-mercaptobenzoic acid. Spectrochim. Acta Part. A 2015, 148, 369–374. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, B. Evolution in lateral flow–based immunoassay systems. In Lateral Flow Immunoassay; Wong, R.C., Tse, H.Y., Eds.; Humana Press: New York, NY, USA, 2009; Chapter 1; p. 236. [Google Scholar]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced raman spectroscopy of self-assembled monolayers: sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Smith, G.; Girardon, J.S.; Paul, J.F.; Berrier, E. Dynamics of a plasmon-activated p-mercaptobenzoic acid layer deposited over Au nanoparticles using time-resolved SERS. Phys. Chem. Chem. Phys. 2016, 18, 19567–19573. [Google Scholar] [CrossRef]
- Bartosh, A.V.; Sotnikov, D.V.; Hendrickson, O.D.; Zherdev, A.V.; Dzantiev, B.B. Design of multiplex lateral flow tests: A case study for simultaneous detection of three antibiotics. Biosensors 2020, 10, 17. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Immunochromatographic assay for rapid on site detection of lincosamide residues in milk, egg, beef, and honey samples. Biotechnol. J. 2019, 15, 1900174. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef]
- Shu, L.; Zhou, J.; Yuan, X.; Petti, L.; Chen, J.; Jia, Z.; Mormile, P. Highly sensitive immunoassay based on SERS using nano-Au immune probes and a nano-Ag immune substrate. Talanta 2014, 123, 161–168. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Wei, C.; Xu, M.; Yao, J.; Li, Y.; Deng, A.; Gu, R. A femtogram level competitive immunoassay of mercury (II) based on surface-enhanced Raman spectroscopy. Chem. Commun. (Camb. Engl.) 2014, 50, 9112–9114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Lu, L.; Rong, Z.; Wang, C.; Peng, Y.; Wang, F.; Wang, J.; Sun, M.; Dong, J.; Wang, D.; et al. Portable and multiplexed lateral flow immunoassay reader based on SERS for highly sensitive point-of-care testing. Biosens. Bioelectron. 2020, 168, 112524. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Walkenfort, B.; König, M.; Salehi, M.; Schlücker, S. Rapid, quantitative, and ultrasensitive point-of-care testing: A portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. (Int. Ed. Engl.) 2019, 58, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, K.; Xiao, K.; Qin, W.; Lu, W.; Tao, W.; Cui, D. Smartphone-based dual-modality imaging system for quantitative detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Res. Lett. 2017, 12, 291. [Google Scholar] [CrossRef]
- Ruppert, C.; Phogat, N.; Laufer, S.; Kohl, M.; Deigner, H.-P. A smartphone readout system for gold nanoparticle-based lateral flow assays: Application to monitoring of digoxigenin. Microchim. Acta 2019, 186, 119. [Google Scholar] [CrossRef]



| Target Analyte | LFIA Formats | Signal Marker | Limit of Detection | References |
|---|---|---|---|---|
| Lincomycin (and chloramphenicol, tetracycline) | Multiplex LFIA | 30 nm AuNPs | 0.4 ng/mL | [37] |
| Lincomycin (and gentamicin, kanamycin, streptomycin, neomycin) | Multiplex LFIA | 15 nm AuNPs | 2.5 ng/mL | [38] |
| Lincomycin | Fluorescence LFIA | Fluorescent microspheres | 0.69 ng/mL | [23] |
| Lincomycin (and clindamycin, pirlimycin) | Conventional LFIA | 20 nm AuNPs | 10 ng/mL | [39] |
| Lincomycin | Indirect LFIA | 30 nm AuNPs | 8 pg/mL | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serebrennikova, K.V.; Hendrickson, O.D.; Zvereva, E.A.; Popravko, D.S.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. A Comparative Study of Approaches to Improve the Sensitivity of Lateral Flow Immunoassay of the Antibiotic Lincomycin. Biosensors 2020, 10, 198. https://doi.org/10.3390/bios10120198
Serebrennikova KV, Hendrickson OD, Zvereva EA, Popravko DS, Zherdev AV, Xu C, Dzantiev BB. A Comparative Study of Approaches to Improve the Sensitivity of Lateral Flow Immunoassay of the Antibiotic Lincomycin. Biosensors. 2020; 10(12):198. https://doi.org/10.3390/bios10120198
Chicago/Turabian StyleSerebrennikova, Kseniya V., Olga D. Hendrickson, Elena A. Zvereva, Demid S. Popravko, Anatoly V. Zherdev, Chuanlai Xu, and Boris B. Dzantiev. 2020. "A Comparative Study of Approaches to Improve the Sensitivity of Lateral Flow Immunoassay of the Antibiotic Lincomycin" Biosensors 10, no. 12: 198. https://doi.org/10.3390/bios10120198
APA StyleSerebrennikova, K. V., Hendrickson, O. D., Zvereva, E. A., Popravko, D. S., Zherdev, A. V., Xu, C., & Dzantiev, B. B. (2020). A Comparative Study of Approaches to Improve the Sensitivity of Lateral Flow Immunoassay of the Antibiotic Lincomycin. Biosensors, 10(12), 198. https://doi.org/10.3390/bios10120198







