The Current Trends of Biosensors in Tissue Engineering
Abstract
:1. Introduction
2. Current Technology for Fabrication of Biosensors
3. The Applications of Biosensors in Tissue Engineering
3.1. Biosensors for Cellular Applications
3.1.1. Biosensors for Cell Polarity
3.1.2. Biosensors for Cell Behaviors Such as Metabolism, the Proliferation/Differentiation of Stem Cells
3.2. The Methods of Biosensors Detecting Cell-Related Analytes
3.2.1. Label-Free Biosensors
3.2.2. Surface Plasmon Resonance (SPR) Based Biosensors
3.2.3. Impedance-Based Biosensors
3.3. Biosensors in Tissue/Organ and Their Derivative Disease Models
3.3.1. Biosensors in Neural Disease Models
3.3.2. Biosensors in Cardiac Disease Models
3.3.3. Biosensors in Live/Lung and Immune Systems Disease Models
3.3.4. Biosensors in Cancer Detection
3.4. Biosensors in Bioimaging Applications
4. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [PubMed] [Green Version]
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front Bioeng. Biotech. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, X.; Zou, R.; Wu, H.; Shi, H.; Yu, S.; Liu, Y. Multifunctional glucose biosensors from Fe(3)O(4) nanoparticles modified chitosan/graphene nanocomposites. Sci. Rep. 2015, 5, 11129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Chen, C.P.; Yang, D.X.; Dong, G.X.; Jia, S.J.; Zhao, B.X.; Yan, L.; Yao, Q.Q.; Sunna, A.; Liu, Y. Optical Biosensors Based on Nitrogen-Doped Graphene Functionalized with Magnetic Nanoparticles. Adv. Mater. Interfaces 2016, 3, 1600590. [Google Scholar] [CrossRef]
- Bousse, L. Whole cell biosensors. Sensor. Actuators B-Chem. 1996, 34, 270–275. [Google Scholar] [CrossRef]
- Ma, C.; Fan, R.; Ahmad, H.; Shi, Q.; Comin-Anduix, B.; Chodon, T.; Koya, R.C.; Liu, C.-C.; Kwong, G.A.; Radu, C.G.; et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nat. Med. 2011, 17, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Stybayeva, G.; Macal, M.; Ramanculov, E.; George, M.D.; Dandekar, S.; Revzin, A. A microdevice for multiplexed detection of T-cell-secreted cytokines. Lab Chip 2008, 8, 2197–2205. [Google Scholar] [CrossRef]
- Du, H.; Strohsahl, C.M.; Camera, J.; Miller, B.L.; Krauss, T.D. Sensitivity and Specificity of Metal Surface-Immobilized “Molecular Beacon” Biosensors. J. Amn. Chem. Soc. 2005, 127, 7932–7940. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.E.; Aguilar, O. Suppressing Non-Specific Binding of Proteins onto Electrode Surfaces in the Development of Electrochemical Immunosensors. Biosensors (Basel) 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, S.K.; Park, M.K. 4-Fluoro-3-nitrophenyl grafted gold electrode based platform for label free electrochemical detection of interleukin-2 protein. Biosens. Bioelectron. 2014, 61, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, M.; Luo, F.; Xu, K.; Lin, Z.; Zhang, L. Stimulus-response click chemistry based aptamer-functionalized mesoporous silica nanoparticles for fluorescence detection of thrombin. Talanta 2018, 178, 563–568. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Y.; Wang, Y.; Hu, L.; Ma, H.; Wang, G.; Wie, Q. Label-free Electrochemiluminescent Immunosensor for Detection of Prostate Specific Antigen based on Aminated Graphene Quantum Dots and Carboxyl Graphene Quantum Dots. Sci. Rep. 2016, 6, 20511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors (Basel) 2017, 17, 1623. [Google Scholar] [CrossRef] [Green Version]
- Raut, N.; O’Connor, G.; Pasini, P.; Daunert, S. Engineered cells as biosensing systems in biomedical analysis. Anal. Bioanal. Chem. 2012, 402, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ferrie, A.M.; Fontaine, N.H.; Mauro, J.; Balakrishnan, J. Resonant Waveguide Grating Biosensor for Living Cell Sensing. Biophys. J. 2006, 91, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Bavli, D.; Prill, S.; Ezra, E.; Levy, G.; Cohen, M.; Vinken, M.; Vanfleteren, J.; Jaeger, M.; Nahmias, Y. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E2231–E2240. [Google Scholar] [CrossRef] [Green Version]
- Hammarback, J.A.; Palm, S.L.; Furcht, L.T.; Letourneau, P.C. Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J. Neurosci. Res. 1985, 13, 213–220. [Google Scholar] [CrossRef]
- Derkus, B. Applying the miniaturization technologies for biosensor design. Biosens. Bioelectron. 2016, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Dondapati, S.K.; Duarte, M.V.; Chatzichristidi, M.; Misiakos, K.; Petrou, P.; Kakabakos, S.E.; Argitis, P.; Katakis, I. Electrochemical biosensor microarray functionalized by means of biomolecule friendly photolithography. Biosens. Bioelectron. 2010, 25, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Dong, T.; Yang, Z. A fluorimetric nitrite biosensor with polythienothiophene-fullerene thin film detectors for on-site water monitoring. Analyst 2019, 144, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Diakoumakos, C.D.; Douvas, A.; Raptis, I.; Kakabakos, S.; Dimotikalli, D.; Terzoudi, G.; Argitis, P. Dilute aqueous base developable resists for environmentally friendly and biocompatible processes. Microelectron. Eng. 2002, 61–62, 819–827. [Google Scholar] [CrossRef]
- Petrou, P.S.; Chatzichristidi, M.; Douvas, A.M.; Argitis, P.; Misiakos, K.; Kakabakos, S.E. A biomolecule friendly photolithographic process for fabrication of protein microarrays on polymeric films coated on silicon chips. Biosens. Bioelectron. 2007, 22, 1994–2002. [Google Scholar] [CrossRef]
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Dev. 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Ganter, P.; Lotsch, B.V. Photocatalytic Nanosheet Lithography: Photolithography based on Organically Modified Photoactive 2D Nanosheets. Angew. Chem. Int. 2017, 56, 8389–8392. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E.G. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435. [Google Scholar] [CrossRef] [Green Version]
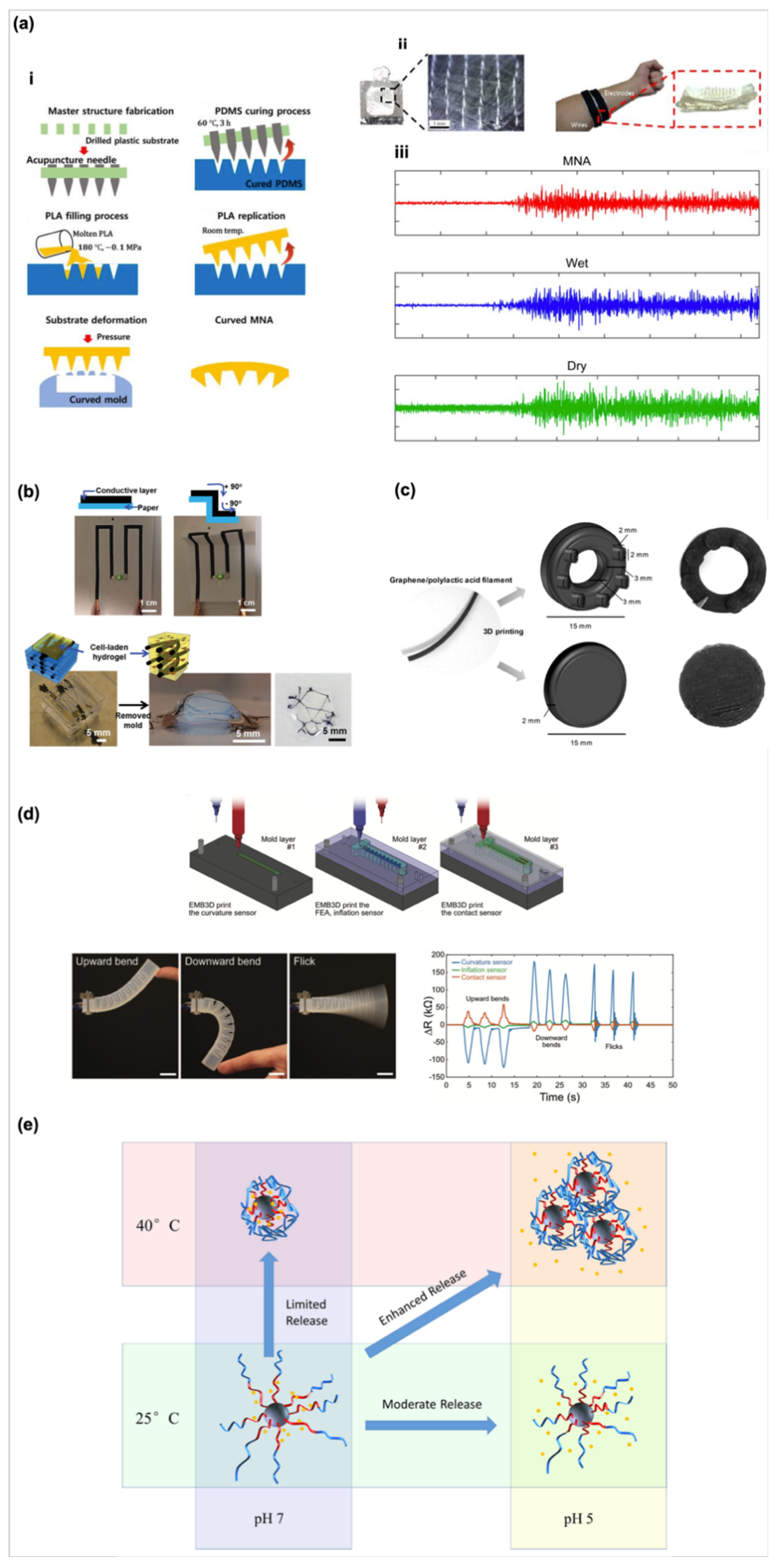
- Kim, M.; Kim, T.; Kim, D.S.; Chung, W.K. Curved Microneedle Array-Based sEMG Electrode for Robust Long-Term Measurements and High Selectivity. Sensors (Basel) 2015, 15, 16265–16280. [Google Scholar] [CrossRef]
- Shin, S.R.; Farzad, R.; Tamayol, A.; Manoharan, V.; Mostafalu, P.; Zhang, Y.S.; Akbari, M.; Jung, S.M.; Kim, D.; Comotto, M.; et al. A Bioactive Carbon Nanotube-Based Ink for Printing 2D and 3D Flexible Electronics. Adv. Mater. 2016, 28, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Manzanares Palenzuela, C.L.; Novotny, F.; Krupicka, P.; Sofer, Z.; Pumera, M. 3D-Printed Graphene/Polylactic Acid Electrodes Promise High Sensitivity in Electroanalysis. Anal. Chem. 2018, 90, 5753–5757. [Google Scholar] [CrossRef] [PubMed]
- Truby, R.L.; Wehner, M.; Grosskopf, A.K.; Vogt, D.M.; Uzel, S.G.M.; Wood, R.J.; Lewis, J.A. Soft Somatosensitive Actuators via Embedded 3D Printing. Adv. Mater. 2018, 30, e1706383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. pH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Qin, W.; Yu, Q.; Cheng, H.; Yu, X.; Wu, H. Transfer Printing and its Applications in Flexible Electronic Devices. Nanomaterials (Basel) 2019, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Shyu, T.C.; Kotov, N.A. Origami and Kirigami Nanocomposites. ACS Nano 2017, 11, 7587–7599. [Google Scholar] [CrossRef]
- Sim, K.; Chen, S.; Li, Y.; Kammoun, M.; Peng, Y.; Xu, M.; Gao, Y.; Song, J.; Zhang, Y.; Ardebili, H.; et al. High Fidelity Tape Transfer Printing Based On Chemically Induced Adhesive Strength Modulation. Sci. Rep. 2015, 5, 16133. [Google Scholar] [CrossRef] [Green Version]
- Rafsanjani, A.; Zhang, Y.; Liu, B.; Rubinstein, S.M.; Bertoldi, K. Kirigami skins make a simple soft actuator crawl. Sci. Robot. 2018, 3, eaar7555. [Google Scholar] [CrossRef] [Green Version]
- Evke, E.E.; Meli, D.; Shtein, M. Developable Rotationally Symmetric Kirigami-Based Structures as Sensor Platforms. Adv. Mater. Technol. 2019, 4, 1900563. [Google Scholar] [CrossRef]
- Hardin, J.O.; Ober, T.J.; Valentine, A.D.; Lewis, J.A. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv. Mater. 2015, 27, 3279–3284. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. B 2011, 98, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Patents Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioen. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Whatley, B.R.; Li, X.; Zhang, N.; Wen, X. Magnetic-directed patterning of cell spheroids. J. Biomed. Mater. Res. A 2014, 102, 1537–1547. [Google Scholar] [CrossRef]
- Koch, L.; Gruene, M.; Unger, C.; Chichkov, B. Laser assisted cell printing. Curr. Pharm. Biotechnol. 2013, 14, 91–97. [Google Scholar]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-Printed Biosensor Arrays for Medical Diagnostics. Micromachines (Basel) 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Sakamoto, S.; Hatakeyama, M.; Yamaguchi, Y.; Suematsu, M.; Itonaga, M.; Handa, H. Application of high-performance magnetic nanobeads to biological sensing devices. Anal. Bioanal. Chem. 2019, 411, 1825–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.H.; Woo, M.K.; Yoon, H.Y.; Jang, J.W.; Wu, J.H.; Lim, C.S.; Kim, Y.K. Isolation of DNA using magnetic nanoparticles coated with dimercaptosuccinic acid. Anal. Biochem. 2014, 447, 114–118. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.-K.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. BioMed. Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.E.; Chen, J.; Chan, J.R.; Langen, R. Engineering a polarity-sensitive biosensor for time-lapse imaging of apoptotic processes and degeneration. Nat. Methods 2010, 7, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.A.; Tzortzis, K.N.; Dupont, E.; Selvadurai, S.; Perbellini, F.; Cantwell, C.D.; Ng, F.S.; Simon, A.R.; Terracciano, C.M.; Peters, N.S. Concurrent micro- to macro-cardiac electrophysiology in myocyte cultures and human heart slices. Sci. Rep. 2018, 8, 6947. [Google Scholar] [CrossRef] [Green Version]
- Knöll, R.; Hoshijima, M.; Hoffman, H.M.; Person, V.; Lorenzen-Schmidt, I.; Bang, M.-L.; Hayashi, T.; Shiga, N.; Yasukawa, H.; Schaper, W.; et al. The Cardiac Mechanical Stretch Sensor Machinery Involves a Z Disc Complex that Is Defective in a Subset of Human Dilated Cardiomyopathy. Cell 2002, 111, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Caluori, G.; Pribyl, J.; Pesl, M.; Jelinkova, S.; Rotrekl, V.; Skladal, P.; Raiteri, R. Non-invasive electromechanical cell-based biosensors for improved investigation of 3D cardiac models. Biosens. Bioelectron. 2019, 124–125, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Son, K.J.; Gheibi, P.; Stybayeva, G.; Rahimian, A.; Revzin, A. Detecting cell-secreted growth factors in microfluidic devices using bead-based biosensors. Microsyst. Nanoeng. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
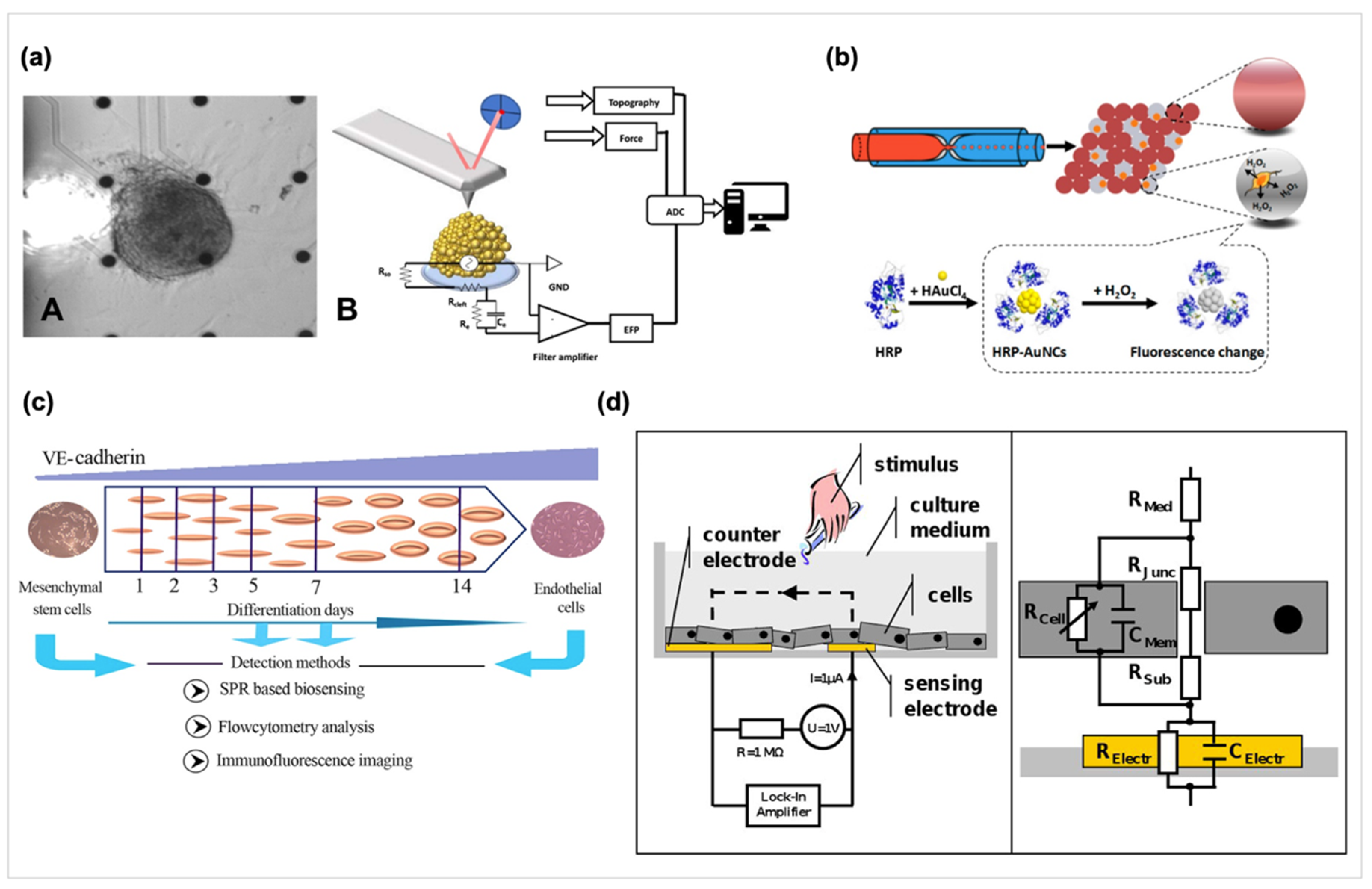
- Shen, R.; Liu, P.; Zhang, Y.; Yu, Z.; Chen, X.; Zhou, L.; Nie, B.; Żaczek, A.; Chen, J.; Liu, J. Sensitive Detection of Single-Cell Secreted H2O2 by Integrating a Microfluidic Droplet Sensor and Au Nanoclusters. Anal. Chem. 2018, 90, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, S. A novel nitric oxide biosensor based on electropolymerization poly(toluidine blue) film electrode and its application to nitric oxide released in liver homogenate. Biosens. Bioelectron. 2006, 22, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Visser, C.W.; Kamperman, T.; Karbaat, L.P.; Lohse, D.; Karperien, M. In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Sci. Adv. 2018, 4, eaao1175. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Ishimuro, T.; Arima, Y.; Hirata, I.; Iwata, H. High-Throughput Immunophenotyping by Surface Plasmon Resonance Imaging. Anal. Chem. 2007, 79, 8616–8623. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Ho, J.H.; Yen, T.-J.; Chen, H.-F.; Lee, O.K.-S. Development of a surface plasmon resonance biosensor for real-time detection of osteogenic differentiation in live mesenchymal stem cells. PLoS ONE 2011, 6, e22382. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; Steel, D.; Asp, J.; Dahlenborg, K.; Jonsson, M.; Jeppsson, A.; Lindahl, A.; Kågedal, B.; Sartipy, P.; Mandenius, C.-F. Assaying cardiac biomarkers for toxicity testing using biosensing and cardiomyocytes derived from human embryonic stem cells. J. Biotech. 2010, 150, 175–181. [Google Scholar] [CrossRef]
- Fathi, F.; Rezabakhsh, A.; Rahbarghazi, R.; Rashidi, M.-R. Early-stage detection of VE-cadherin during endothelial differentiation of human mesenchymal stem cells using SPR biosensor. Biosens. Bioelectron. 2017, 96, 358–366. [Google Scholar] [CrossRef]
- Rutten, M.J.; Laraway, B.; Gregory, C.R.; Xie, H.; Renken, C.; Keese, C.; Gregory, K.W. Rapid assay of stem cell functionality and potency using electric cell-substrate impedance sensing. Stem Cell Res. Ther. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, D.T.; Rahman, A.R.; Bhansali, S. Design rule for optimization of microelectrodes used in electric cell-substrate impedance sensing (ECIS). Biosens. Bioelectron. 2009, 24, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 51300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Luong, J.H.T. On-Line Monitoring of Cell Growth and Cytotoxicity Using Electric Cell-Substrate Impedance Sensing (ECIS). Biotech. Prog. 2003, 19, 1000–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C 2016, 69, 554–560. [Google Scholar] [CrossRef]
- Anchan, A.; Kalogirou-Baldwin, P.; Johnson, R.; Kho, D.T.; Joseph, W.; Hucklesby, J.; Finlay, G.J.; O’Carroll, S.J.; Angel, C.E.; Graham, E.S. Real-Time Measurement of Melanoma Cell-Mediated Human Brain Endothelial Barrier Disruption Using Electric Cell-Substrate Impedance Sensing Technology. Biosensors 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc.Natl. Acad.Sci. USA 2011, 108, 6462. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, S.; Jahnke, H.-G.; Fritsche, E.; Girard, M.; Robitzki, A.A. Comprehensive human stem cell differentiation in a 2D and 3D mode to cardiomyocytes for long-term cultivation and multiparametric monitoring on a multimodal microelectrode array setup. Biosens. Bioelectron. 2019, 126, 624–631. [Google Scholar] [CrossRef]
- Kalmykov, A.; Huang, C.; Bliley, J.; Shiwarski, D.; Tashman, J.; Abdullah, A.; Rastogi, S.K.; Shukla, S.; Mataev, E.; Feinberg, A.W.; et al. Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Raknim, P.; Lan, K.C. Gait Monitoring for Early Neurological Disorder Detection Using Sensors in a Smartphone: Validation and a Case Study of Parkinsonism. Telemed. J. e-Health 2016, 22, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, J.P.; Wu, F.; Wise, K.D.; Yoon, E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017, 3, 16066. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Vato, A.; Tedesco, M.; Marcoli, M.; Davide, F.; Martinoia, S. Networks of neurons coupled to microelectrode arrays: A neuronal sensory system for pharmacological applications. Biosens. Bioelectron. 2003, 18, 627–634. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Gerhardt, G.A.; Laranjinha, J.; Barbosa, R.M. Neurometabolic and electrophysiological changes during cortical spreading depolarization: Multimodal approach based on a lactate-glucose dual microbiosensor arrays. Sci. Rep. 2017, 7, 6764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-C.; Lee, I.C.; Lei, K.F. Toward the Development of an Artificial Brain on a Micropatterned and Material-Regulated Biochip by Guiding and Promoting the Differentiation and Neurite Outgrowth of Neural Stem/Progenitor Cells. ACS Appl. Mater. Inter. 2018, 10, 5269–5277. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, X.; Lei, K.F.; Huang, C.-H. Quantitative Impedimetric Monitoring of Cell Migration Under the Stimulation of Cytokine or Anti-Cancer Drug in a Microfluidic Chip. Biomicrofluidics 2015, 9, 034109. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.F.; Tseng, H.-P.; Lee, C.-Y.; Tsang, N.-M. Quantitative Study of Cell Invasion Process under Extracellular Stimulation of Cytokine in a Microfluidic Device. Sci. Rep. 2016, 6, 25557. [Google Scholar] [CrossRef] [Green Version]
- Lei, K.F.; Lin, B.-Y.; Tsang, N.-M. Real-time and Label-Free Impedimetric Analysis of the Formation and Drug Testing of Tumor Spheroids Formed via the Liquid Overlay Technique. RSC Adv. 2017, 7, 13939–13946. [Google Scholar] [CrossRef] [Green Version]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly Stable Glassy Carbon Interfaces for Long-Term Neural Stimulation and Low-Noise Recording of Brain Activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Liu, J.; Fu, T.M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Soler, M.; Özdemir, C.I.; Belushkin, A.; Yesilköy, F.; Altug, H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip 2017, 17, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Lin, C.W.; Wei, K.C.; Huang, C.Y.; Hsu, P.H.; Liu, H.L.; Lu, Y.J.; Lin, S.C.; Yang, H.W.; Ma, C.C. Non-invasive screening for early Alzheimer’s disease diagnosis by a sensitively immunomagnetic biosensor. Sci. Rep. 2016, 6, 25155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441–5444. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Ha, G.; Wright, D.E.; Ma, Y.; Sen-Gupta, E.; Haubrich, N.R.; Branche, P.C.; Li, W.; Huppert, G.L.; Johnson, M.; et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. NPJ Digit. Med. 2018, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Bolonduro, O.A.; Hu, N.; Ju, J.; Rao, A.A.; Duffy, B.M.; Huang, Z.; Black, L.D.; Timko, B.P. Heart-on-a-Chip Model with Integrated Extra- and Intracellular Bioelectronics for Monitoring Cardiac Electrophysiology under Acute Hypoxia. Nano Lett. 2020, 20, 2585–2593. [Google Scholar] [CrossRef]
- Pan, L.H.; Pang, S.T.; Fang, P.Y.; Chuang, C.K.; Yang, H.W. Label-Free Biochips for Accurate Detection of Prostate Cancer in the Clinic: Dual Biomarkers and Circulating Tumor Cells. Theranostics 2017, 7, 4289–4300. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Sheng, Y.; Kwak, K.J.; Shi, J.; Yu, B.; Lee, L.J. A signal-amplifiable biochip quantifies extracellular vesicle-associated RNAs for early cancer detection. Nat. Commun. 2017, 8, 1683. [Google Scholar] [CrossRef]
- Duranteau, J.; Chandel, N.S.; Kulisz, A.; Shao, Z.; Schumacker, P.T. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 1998, 273, 11619–11624. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Minchole, A.; Quinn, T.A.; Rodriguez, B. Electrophysiological properties of computational human ventricular cell action potential models under acute ischemic conditions. Prog. Biophys. Mol. Biol. 2017, 129, 40–52. [Google Scholar] [CrossRef]
- Feiner, R.; Fleischer, S.; Shapira, A.; Kalish, O.; Dvir, T. Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J. Control Release 2018, 281, 189–195. [Google Scholar] [CrossRef]
- Giana, G.; Romano, E.; Porfirio, M.C.; D’Ambrosio, R.; Giovinazzo, S.; Troianiello, M.; Barlocci, E.; Travaglini, D.; Granstrem, O.; Pascale, E.; et al. Detection of auto-antibodies to DAT in the serum: Interactions with DAT genotype and psycho-stimulant therapy for ADHD. J. Neuroimmunol. 2015, 278, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Sanchez-Tirado, E.; Martinez-Garcia, G.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J.M. Electrochemical biosensor for the simultaneous determination of rheumatoid factor and anti-cyclic citrullinated peptide antibodies in human serum. Analyst 2020. [Google Scholar] [CrossRef] [PubMed]
- Fayanju, O.M.; Jeffe, D.B.; Elmore, L.; Ksiazek, D.N.; Margenthaler, J.A. Patient and process factors associated with late-stage breast cancer diagnosis in Safety-Net patients: A pilot prospective study. Ann. Surg. Oncol. 2013, 20, 723–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, K.-i.; Yoshiki, K.; Hirai, Y.; Ito, S.; Satoh, J.; Oka, A.; Tsuchiya, T.; Chenad, Y.; Tabatab, O. Integrated heart/cancer on a chip to reproduce the side effects of anti-cancer drugs in vitro. RSC Adv. 2017, 7, 36777–36786. [Google Scholar] [CrossRef] [Green Version]
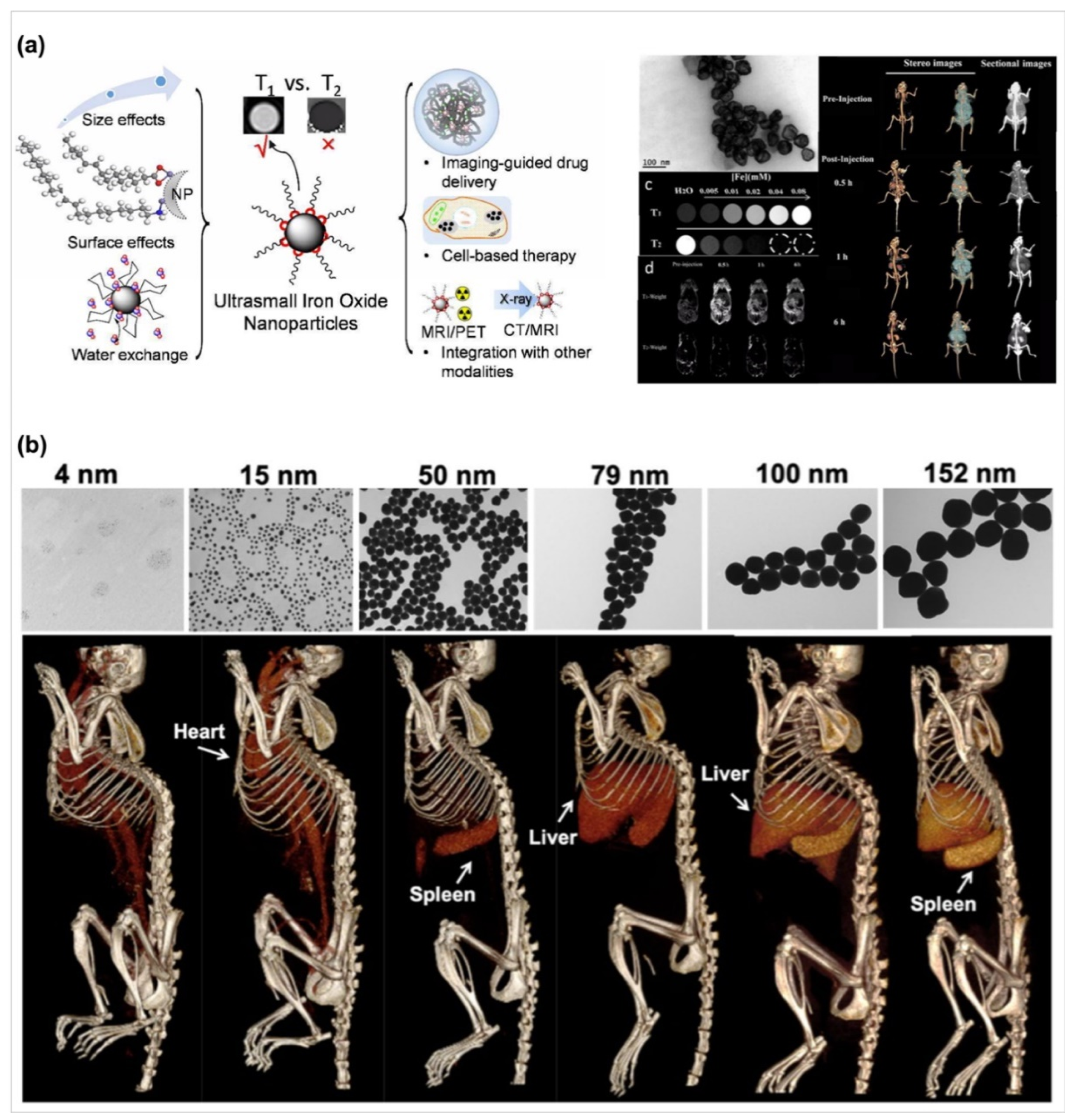
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Palantavida, S.; Tang, R.; Sudlow, G.P.; Akers, W.J.; Achilefu, S.; Sokolov, I. Ultrabright NIR fluorescent mesoporous silica nanoparticles. J. Mater. Chem. B 2014, 2, 3107–3114. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Hola, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots-highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2020, 2020 6, 1280–1290. [Google Scholar]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lu, X.; Chen, J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014, 2, 25–32. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chen, C.C.; Hsu, S.M.; Chuang, H.S. Contact-Lens Biosensors. Sensors (Basel) 2018, 18, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.E.; Lee, I.-C. The Current Trends of Biosensors in Tissue Engineering. Biosensors 2020, 10, 88. https://doi.org/10.3390/bios10080088
Li Y-CE, Lee I-C. The Current Trends of Biosensors in Tissue Engineering. Biosensors. 2020; 10(8):88. https://doi.org/10.3390/bios10080088
Chicago/Turabian StyleLi, Yi-Chen Ethan, and I-Chi Lee. 2020. "The Current Trends of Biosensors in Tissue Engineering" Biosensors 10, no. 8: 88. https://doi.org/10.3390/bios10080088
APA StyleLi, Y.-C. E., & Lee, I.-C. (2020). The Current Trends of Biosensors in Tissue Engineering. Biosensors, 10(8), 88. https://doi.org/10.3390/bios10080088