Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors
Abstract
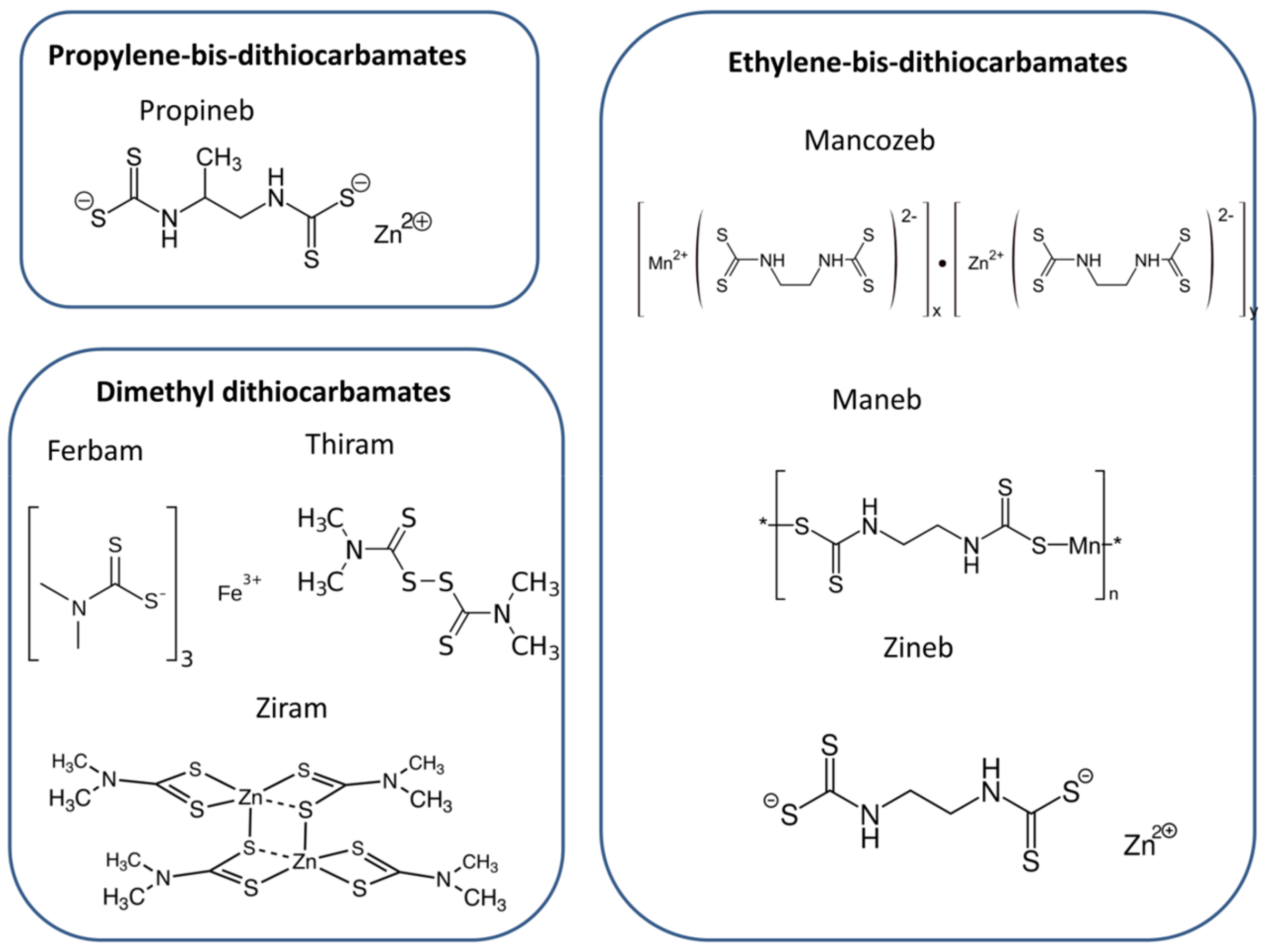
:1. Introduction
2. Advances in DTFs Detection
2.1. Standard Chromatographic Methods for DTFs Detection
2.2. Spectroscopy-Based Analysis Methods
2.3. Optical and Electrochemical Assays
2.3.1. Electrochemical Sensors

2.3.2. Optical Assays
2.4. Biosensors Based on Enzyme Inhibition
2.4.1. Examples of Biosensors for the Determination of DTFs
2.4.2. Extremozymes as Potential Biorecognition Elements in Biosensors for DTFs
2.4.3. Challenges in the Application of Biosensors for DTFs Determination in Real Samples
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefton, J.B.; Pekar, K.B.; Runčevski, T. The Crystal Structure of Zineb, Seventy-Five Years Later. Cryst. Growth Des. 2020, 20, 851–857. [Google Scholar] [CrossRef]
- Fungicides Market Report—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017−2025. Available online: https://www.prnewswire.com/news-releases/fungicides-market-mancozeb-chlorothalonil-metalaxyl-strobilurin-and-others-for-cereals--grains-oilseeds--pulses-fruits--vegetables-and-other-crops---global-industry-analysis-size-share-growth-trends-and-forecast-20-300243678.html (accessed on 29 November 2020).
- European Commission Regulation 2016/1 of 3 December 2015 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Bifenazate, Boscalid, Cyazofamid, Cyromazine, Dazomet, Dithiocarbamates, Fluazifop-P, Mepanipyrim, Metrafenone, Picloram, Propamocarb, Pyridaben, Pyriofenone, Sulfoxaflor, Tebuconazole, Tebufenpyrad and Thiram in or on Certain Products. Available online: https://eur-lex.europa.eu/eli/reg/2016/1/oj (accessed on 29 November 2020).
- Atuhaire, A.; Kaye, E.; Mutambuze, I.L.; Matthews, G.; Friedrich, T.; Jørs, E. Assessment of Dithiocarbamate Residues on Tomatoes Conventionally Grown in Uganda and the Effect of Simple Washing to Reduce Exposure Risk to Consumers. Environ. Health Insights 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Agency. The 2017 European Union report on pesticide residues in food. EFSA J. 2019, 17, e05743. [Google Scholar]
- Nakamura, M.; Noda, S.; Kosugi, M.; Ishiduka, N.; Mizukoshi, K.; Taniguchi, M.; Nemoto, S. Determination of dithiocarbamates and milneb residues in foods by gas chromatography-mass spectrometry. J. Food Hyg. Soc. Jpn. 2010, 51, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Crnogorac, G.; Schmauder, S.; Schwack, W. Trace analysis of dithiocarbamate fungicide residues on fruits and vegetables by hydrophilic interaction liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2539–2546. [Google Scholar] [CrossRef]
- Kakitani, A.; Yoshioka, T.; Nagatomi, Y.; Harayama, K. A rapid and sensitive analysis of dithiocarbamate fungicides using modified QuEChERS method and liquid chromatography-tandem mass spectrometry. J. Pestic. Sci. 2017, 42, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Dong, C.; Yang, Q.; An, W.; Zheng, Z.; Jiao, B. Simultaneous Determination of Ethylenebisdithiocarbamate (EBDC) and Propylenebisdithiocarbamate (PBDC) Fungicides in Vegetables, Fruits, and Mushrooms by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2019, 12, 2045–2055. [Google Scholar] [CrossRef]
- Van Lishaut, H.; Schwack, W. Selective trace determination of dithiocarbamate fungicides in fruits and vegetables by reversed-phase ion-pair liquid chromatography with ultraviolet and electrochemical detection. J. AOAC Int. 2000, 83, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Al-Alam, J.; Bom, L.; Chbani, A.; Fajloun, Z.; Millet, M. Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J. Chromatogr. Sci. 2017, 55, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Ubeda, M.R.; Escribano, M.T.S.; Hernandez, L.H. Determination of Thiram by high-performance liquid chromatography with amperometric detection in river water and fungicide formulations. Microchem. J. 1990, 41, 22–28. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Yaroslavtseva, L.N.; Merkulova, N.L.; Yashin, A.Y.; Shpigun, O.A. Separation of pesticides by high-performance liquid chromatography with amperometric detection. J. Anal. Chem. 2009, 64, 164–170. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Chailapakul, O.; Siangproh, W. Development of gold nanoparticles modified screen-printed carbon electrode for the analysis of thiram, disulfiram and their derivative in food using ultra-high performance liquid chromatography. Talanta 2015, 132, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Chatzimichail, S.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. A Review of Portable High-Performance Liquid Chromatography: The Future of the Field? Chromatographia 2020, 83, 1165–1195. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Pérez-Junquera, A.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Towards single-molecule in situ electrochemical SERS detection with disposable substrates. Chem. Commun. 2018, 54, 5748–5751. [Google Scholar] [CrossRef] [Green Version]
- Mylrajan, M. SERS, FT-Raman and FT-IR studies of dithiocarbamates. J. Mol. Struct. 1995, 348, 257–260. [Google Scholar] [CrossRef]
- Tse Yuen, K. SERS of dithiocarbamates and xanthates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 2177–2192. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, R.; Wang, S.; Han, G.; Han, M.-Y.; Jiang, C.; Zhang, Z. Single clusters of self-assembled silver nanoparticles for surface-enhanced Raman scattering sensing of a dithiocarbamate fungicide. J. Mat. Chem. 2011, 21, 16264–16270. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Shi, X.; Yang, F.; Meng, G.; Xiong, Q.; Ke, Y.; Wang, H.; Lu, Y.; Wu, N. Detection of Dithiocarbamate Pesticides with a Spongelike Surface-Enhanced Raman Scattering Substrate Made of Reduced Graphene Oxide-Wrapped Silver Nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 39618–39625. [Google Scholar] [CrossRef]
- Nowicka, A.B.; Czaplicka, M.; Kowalska, A.A.; Szymborski, T.; Kamińska, A. Flexible PET/ITO/Ag SERS Platform for Label-Free Detection of Pesticides. Biosensors 2019, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Soni, R.K. Silver Nanocube- and Nanowire-Based SERS Substrates for Ultra-low Detection of PATP and Thiram Molecules. Plasmonics 2020, 15, 1577–1589. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C. Highly Sensitive and Rapid Surface Enhanced Raman Spectroscopic (SERS) Determination of Thiram on the Epidermis of Fruits and Vegetables Using A Silver Nanoparticle-Modified Fibrous Swab. Anal. Lett. 2020, 53, 973–983. [Google Scholar] [CrossRef]
- Saute, B.; Premasiri, R.; Ziegler, L.; Narayanan, R. Gold nanorods as surface enhanced Raman spectroscopy substrates for sensitive and selective detection of ultra-low levels of dithiocarbamate pesticides. Analyst 2012, 137, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Saute, B.; Narayanan, R. Solution-based SERS method to detect dithiocarbamate fungicides in different real-world matrices. J. Raman Spectrosc. 2013, 44, 1518–1522. [Google Scholar] [CrossRef]
- Zhao, Y.; Pérez-Segarra, W.; Shi, Q.; Wei, A. Dithiocarbamate Assembly on Gold. JACS 2005, 127, 7328–7329. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Luo, W.; Liu, Q.; Hao, N.; Zhu, Y.; Liu, M.; Wang, L.; Yang, H.; Chen, X. Simultaneous In Situ Extraction and Fabrication of Surface-Enhanced Raman Scattering Substrate for Reliable Detection of Thiram Residue. Anal. Chem. 2018, 90, 13647–13654. [Google Scholar] [CrossRef]
- Ibáñez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Detection of dithiocarbamate, chloronicotinyl and organophosphate pesticides by electrochemical activation of SERS features of screen-printed electrodes. Spectrochim. Acta A 2020, 119174. [Google Scholar] [CrossRef]
- Cassella, A.R.; Cassella, R.J.; Garrigues, S.; Santelli, R.E.; de Campos, R.C.; de la Guardia, M. Flow injection-FTIR determination of dithiocarbamate pesticides. Analyst 2000, 125, 1829–1833. [Google Scholar]
- Cassella, A.R.; Garrigues, S.; de Campos, R.C.; de la Guardia, M. Fourier transform infrared spectrometric determination of Ziram. Talanta 2001, 54, 1087–1094. [Google Scholar] [CrossRef]
- Halls, D.J. The properties of dithiocarbamates A Review. Microchim Acta 1969, 57, 62–77. [Google Scholar] [CrossRef]
- Bond, A.M.; Martin, R.L. Electrochemistry and redox behaviour of transition metal dithiocarbamates. Coord. Chem. Rev. 1984, 54, 23–98. [Google Scholar] [CrossRef]
- Giannakopoulos, E.; Deligiannakis, Y. Thermodynamics of Adsorption of Dithiocarbamates at the Hanging Mercury Drop. Langmuir 2007, 23, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.; Mocák, J.; Bustin, D.I. Electrochemical study of the diethyldithiocarbamate anion and of its oxidation products. Chem. Pap. 1981, 36, 633–664. [Google Scholar]
- Silva, R.A.G.; Silva, L.A.J.; Munoz, R.A.A.; Richter, E.M.; Oliveira, A.C. Fast and direct determination of mancozeb through batch injection analysis with amperometric detection on boron-doped diamond electrodes. J. Electroanal. Chem. 2014, 733, 85–90. [Google Scholar] [CrossRef]
- Qiu, P.; Ni, Y.N. Determination of ziram in vegetable samples by square wave voltammetry. Chin. Chem. Lett. 2008, 19, 1337–1340. [Google Scholar] [CrossRef]
- Silva, L.M.; De Souza, D. Ziram herbicide determination using a polished silver solid amalgam electrode. Electrochim. Acta 2017, 224, 541–550. [Google Scholar] [CrossRef]
- Procopio, J.R.; Sevilla Escribano, M.T.; Hernandez, L.H. Determination of thiram in water and soils by cathodic stripping voltammetry based on adsorptive accumulation. Fresenius Z. Anal. Chem. 1988, 331, 27–29. [Google Scholar] [CrossRef]
- Sevilla, M.T.; Procopio, J.R.; Pinilla, J.M.; Hernandez, L. Voltammetric determination of thiram following adsorptive accumulation on a rotating gold disk electrode. Electroanalysis 1990, 2, 475–479. [Google Scholar] [CrossRef]
- Fernández, C.; Reviejo, A.J.; Pingarrón, J.M. Development of graphite-poly(tetrafluoroethylene) composite electrodes Voltammetric determination of the herbicides thiram and disulfiram. Anal. Chim. Acta 1995, 305, 192–199. [Google Scholar] [CrossRef]
- Fernández, C.; Reviejo, A.J.; Pingarrón, J.M. Graphite-poly(tetrafluoroethylene) electrodes as electrochemical detectors in flowing systems. Anal. Chim. Acta 1995, 314, 13–22. [Google Scholar] [CrossRef]
- Mathew, L.; Reddy, M.L.P.; Rao, T.P.; Iyer, C.S.P.; Damodaran, A.D. Differential pulse anodic stripping voltammetric determination of ziram (a dithiocarbamate fungicide). Talanta 1996, 43, 73–76. [Google Scholar] [CrossRef]
- Shan Lin, M.; Iuan Jan, B.; Leu, H.-J.; Shing Lin, J. Trace measurement of dithiocarbamate based pesticide by adsorptive stripping voltammetry. Anal. Chim. Acta 1999, 388, 111–117. [Google Scholar] [CrossRef]
- Shaidarova, L.G.; Budnikov, G.K.; Zaripova, S.A. Electrocatalytic Determination of Dithiocarbamate-Based Pesticides Using Electrodes Modified with Metal Phthalocyanines. J. Anal. Chem. 2001, 56, 748–753. [Google Scholar] [CrossRef]
- Lin, M.S.; Wang, J.S. Determination of an Ethylene Bisdithiocarbamate Based Pesticide (Nabam) by Cobalt Phthalocyanine Modified Carbon Ink Electrode. Electroanalysis 2004, 16, 904–909. [Google Scholar] [CrossRef]
- Zhao, Y.-G.; Zheng, X.-W.; Huang, Z.-Y.; Yang, M.-M. Voltammetric study on the complex of thiram–copper(II) and its application. Anal. Chim. Acta 2003, 482, 29–36. [Google Scholar] [CrossRef]
- Barroso, M.F.; Paíga, P.; Vaz, M.C.V.F.; Delerue-Matos, C. Study of the voltammetric behaviour of metam and its application to an amperometric flow system. Anal. Bioanal. Chem. 2005, 383, 880–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nováková, K.; Navrátil, T.; Dytrtová, J.J.; Chýlková, J. The use of copper solid amalgam electrodes for determination of the pesticide thiram. J. Solid State Electrochem. 2013, 17, 1517–1528. [Google Scholar] [CrossRef]
- Abbaci, A.; Azzouz, N.; Bouznit, Y. A new copper doped montmorillonite modified carbon paste electrode for propineb detection. Appl. Clay Sci. 2014, 90, 130–134. [Google Scholar] [CrossRef]
- López-Fernández, O.; Barroso, M.F.; Fernandes, D.M.; Rial-Otero, R.; Simal-Gándara, J.; Morais, S.; Nouws, H.P.A.; Freire, C.; Delerue-Matos, C. Voltammetric analysis of mancozeb and its degradation product ethylenethiourea. J. Electroanal. Chem. 2015, 758, 54–58. [Google Scholar] [CrossRef]
- Stanković, D.M.; Kalcher, K. Amperometric quantification of the pesticide ziram at boron doped diamond electrodes using flow injection analysis. Sens. Actuators B Chem. 2016, 233, 144–147. [Google Scholar] [CrossRef]
- Stanković, D.M. Electroanalytical Approach for Quantification of Pesticide Maneb. Electroanalysis 2017, 29, 352–357. [Google Scholar] [CrossRef]
- Nishiyama, K.; Sawada, N.; Kataoka, M.; Tsuruta, K.; Yamamura, K.; Origuchi, S.; Yoshimoto, S. Electrochemical detection of manzeb using reductive desorption from Au(111) and Au(100). Electrochemistry 2018, 86, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Sequeira, R.; Alvarado-Hidalgo, F.; Robles-Chaves, D.; Sáenz-Arce, G.; Avendano-Soto, E.D.; Sánchez-Kopper, A.; Starbird-Perez, R. Electrochemical Characterization of Mancozeb Degradation for Wastewater Treatment Using a Sensor Based on Poly (3,4-ethylenedioxythiophene) (PEDOT) Modified with Carbon Nanotubes and Gold Nanoparticles. Polymers 2019, 11, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maximiano, E.M.; Cardoso, C.A.L.; Arruda, G.J. Simultaneous Electroanalytical Determination of Thiram and Carbendazim in Samples of Fresh Fruit Juices in the Presence of Surfactants. Food Anal. Methods 2020, 13, 119–130. [Google Scholar] [CrossRef]
- Ragam, P.N.; Mathew, B. Unmodified silver nanoparticles for dual detection of dithiocarbamate fungicide and rapid degradation of water pollutants. Int. J. Environ. Sci. Technol. 2020, 17, 1739–1752. [Google Scholar] [CrossRef]
- Alves Sá da Silva, V.; da Silva Santos, A.; Ferreira, T.L.; Codognoto, L.; Agostini Valle, E.M. Electrochemical Evaluation of Pollutants in the Environment: Interaction Between the Metal Ions Zn(II) and Cu(II) with the Fungicide Thiram in Billings Dam. Electroanalysis 2020, 32, 1582–1589. [Google Scholar] [CrossRef]
- Da Silva, M.d.P.; Procopio, J.R.; Hernández, L. Electrochemical detection in the determination of several dithiocarbamates by reverse-phase liquid chromatography. J. Liq. Chromatogr. Rel. Technol. 1999, 22, 463–475. [Google Scholar] [CrossRef]
- Fernández, C.; Reviejo, A.J.; Polo, L.M.; Pingarrón, J. HPLC-Electrochemical detection with graphite-poly (tetrafluoroethylene) electrode: Determination of the fungicides thiram and disulfiram. Talanta 1996, 4, 1341–1348. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M. Applications of copper nanoparticles for colorimetric detection of dithiocarbamate pesticides. J. Nanostructure Chem. 2019, 9, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M. Silver Nanoparticles with Sodium Dodecyl Sulfate as a Colorimetric Probe for the Detection of Dithiocarbamate Pesticides in Environmental Samples. Anal. Sci. 2019, 35, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannoulis, K.M.; Giokas, D.L.; Tsogas, G.Z.; Vlessidis, A.G. Ligand-free gold nanoparticles as colorimetric probes for the non-destructive determination of total dithiocarbamate pesticides after solid phase extraction. Talanta 2014, 119, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, D.; Duan, H.; Xu, Y.; Zhou, Z.; Wang, P. Catechol Dyes–Tyrosinase System for Colorimetric Determination and Discrimination of Dithiocarbamate Pesticides. J. Agric. Food Chem. 2020, 68, 9252–9259. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Wang, J.; Sun, H.; Jiang, L. Double-decrease of the fluorescence of CdSe/ZnS quantum dots for the detection of zinc(II) dimethyldithiocarbamate (ziram) based on its interaction with gold nanoparticles. Microchim. Acta 2018, 185, 472. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Zhao, T.; Liu, B.; Wang, Z.; Zhang, Z. Selective phosphorescence sensing of pesticide based on the inhibition of silver(I) quenched ZnS:Mn2+ quantum dots. Sens. Actuators B Chem. 2017, 252, 1083–1088. [Google Scholar] [CrossRef]
- Waseem, A.; Yaqoob, M.; Nabi, A. Determination of thiram in natural waters using flow-injection with cerium(IV)–quinine chemiluminescence system. Luminescence 2010, 25, 71–75. [Google Scholar] [CrossRef]
- Asghar, M.; Yaqoob, M.; Haque, N.; Nabi, A. Determination of Thiram and Aminocarb Pesticides in Natural Water Samples Using Flow Injection with Tris(2,2′-bipyridyl)ruthenium(II)-diperiodatoargentate(III) Chemiluminescence Detection. Anal. Sci. 2013, 29, 1061–1066. [Google Scholar] [CrossRef] [Green Version]
- Girotti, S.; Maiolini, E.; Ghini, S.; Ferri, E.; Fini, F.; Nodet, P.; Eremin, S. Quantification of Thiram in Honeybees: Development of a Chemiluminescent ELISA. Anal. Lett. 2008, 41, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Queffelec, A.-L.; Boisdé, F.; Larue, J.-P.; Haelters, J.-P.; Corbel, B.; Thouvenot, D.; Nodet, P. Development of an Immunoassay (ELISA) for the Quantification of Thiram in Lettuce. J. Agric. Food Chem. 2001, 49, 1675–1680. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 2011, 82, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Amine, A. Biosensors based on enzyme inhibition. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 140, pp. 299–326. [Google Scholar]
- Oliveira, T.M.B.F.; Fátima Barroso, M.; Morais, S.; Araújo, M.; Freire, C.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Laccase-Prussian blue film-graphene doped carbon paste modified electrode for carbamate pesticides quantification. Biosens. Bioelectron. 2013, 47, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Noguer, T.; Balasoiu, A.-M.; Vasilescu, A.; Marty, J. Development of a disposable biosensor for the detection of metam-sodium and its metabolite MITC. Anal. Lett. 2001, 34, 513–528. [Google Scholar] [CrossRef]
- Oliveira, T.; Barroso, M.F.; Morais, S.; Araújo, M.; Freire, C.; Lima-Neto, P.; Correia, A.; Oliveira, M.; Delerue-Matos, C. Sensitive bi-enzymatic biosensor based on polyphenoloxidases-gold nanoparticles-chitosan hybrid film-graphene doped carbon paste electrode for carbamates detection. Bioelectrochemistry 2014, 98C, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.S.; Nunes, G.S.; Noguer, T.; Marty, J.-L. Biossensor enzimático para detecção de fungicidas ditiocarbamatos: Estudo cinético da enzima aldeído desidrogenase e otimização do biossensor. Quim. Nova 2007, 30, 9–17. [Google Scholar] [CrossRef]
- Noguer, T.; Marty, J.-L. High sensitive bienzymic sensor for the detection of dithiocarbamate fungicides. Anal. Chim. Acta 1997, 347, 63–70. [Google Scholar] [CrossRef]
- Noguer, T.; Gradinaru, A.; Ciucu, A.; Marty, J. A New Disposable Biosensor for the Accurate and Sensitive Detection of Ethylenebis(Dithiocarbamate) Fungicides. Anal. Lett. 1999, 32, 1723–1738. [Google Scholar] [CrossRef]
- Flampouri, K.; Mavrikou, S.; Kintzios, S.; Miliadis, G.; Aplada-Sarlis, P. Development and validation of a cellular biosensor detecting pesticide residues in tomatoes. Talanta 2010, 80, 1799–1804. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Herrera, M.C. Enzyme inhibition-based biosensors and biosensing systems: Questionable analytical devices. Biosens. Bioelectron. 2003, 18, 279–294. [Google Scholar] [CrossRef]
- Zapp, E.; Brondani, D.; Vieira, I.C.; Scheeren, C.W.; Dupont, J.; Barbosa, A.M.J.; Ferreira, V.S. Biomonitoring of methomyl pesticide by laccase inhibition on sensor containing platinum nanoparticles in ionic liquid phase supported in montmorillonite. Sens. Actuators B Chem. 2011, 155, 331–339. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Fátima Barroso, M.; Morais, S.; de Lima-Neto, P.; Correia, A.N.; Oliveira, M.B.P.P.; Delerue-Matos, C. Biosensor based on multi-walled carbon nanotubes paste electrode modified with laccase for pirimicarb pesticide quantification. Talanta 2013, 106, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Milton, R.D.; Abdellaoui, S.; Hickey, D.P.; Minteer, S.D. Laccase Inhibition by Arsenite/Arsenate: Determination of Inhibition Mechanism and Preliminary Application to a Self-Powered Biosensor. Anal. Chem. 2016, 88, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titoiu, A.M.; Lapauw, M.; Necula-Petrareanu, G.; Purcarea, C.; Fanjul-Bolado, P.; Marty, J.-L.; Vasilescu, A. Carbon Nanofiber and Meldola Blue Based Electrochemical Sensor for NADH: Application to the Detection of Benzaldehyde. Electroanalysis 2018, 30, 2676–2688. [Google Scholar] [CrossRef]
- Noguer, T.; Leca, B.; Jeanty, G.; Marty, J.-L. Biosensors based on enzyme inhibition: Detection of organophosphorus and carbamate insecticides and dithiocarbamate fungicides. Field Anal. Chem. Technol. 1999, 3, 171–178. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Llopis, X.; Pumera, M.; Alegret, S.; Merkoçi, A. Lab-on-a-chip for ultrasensitive detection of carbofuran by enzymatic inhibition with replacement of enzyme using magnetic beads. Lab Chip 2009, 9, 213–218. [Google Scholar] [CrossRef]
- Vasudevan, N.; Jayshree, A. Extremozymes and Extremoproteins in Biosensor Applications. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; pp. 1711–1736. [Google Scholar]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Xu, F. Applications of oxidoreductases: Recent progress. Ind. Biotechnol. 2005, 1, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Liao, L.; Xu, X.W.; Oren, A.; Wu, M. Aldehyde dehydrogenase of the haloalkaliphilic archaeon Natronomonas pharaonis and its function in ethanol metabolism. Extremophiles 2008, 12, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Kazuoka, T.; Yoshida, M.; Yamanaka, K.; Oikawa, T.; Soda, K. Thermostable aldehyde dehydrogenase from psychrophile, Cytophaga sp. KUC-1: Enzymological characteristics and functional properties. Biochem. Biophys. Res. Commun. 2002, 298, 632–637. [Google Scholar] [CrossRef]
- Kim, H.-j.; Joo, W.-A.; Cho, C.-W.; Kim, C.-W. Halophile Aldehyde Dehydrogenase from Halobacterium salinarum. J. Proteome Res. 2006, 5, 192–195. [Google Scholar] [CrossRef]
- Keller, M.W.; Lipscomb, G.L.; Nguyen, D.M.; Crowley, A.T.; Schut, G.J.; Scott, I.; Kelly, R.M.; Adams, M.W.W. Ethanol production by the hyperthermophilic archaeon Pyrococcus furiosus by expression of bacterial bifunctional alcohol dehydrogenases. Microb. Biotechnol. 2017, 10, 1535–1545. [Google Scholar] [CrossRef]
- Esser, D.; Kouril, T.; Talfournier, F.; Polkowska, J.; Schrader, T.; Bräsen, C.; Siebers, B. Unraveling the function of paralogs of the aldehyde dehydrogenase super family from Sulfolobus solfataricus. Extremophiles 2013, 17, 205–216. [Google Scholar] [CrossRef]
- Tamaki, N.; Hama, T. Aldehyde dehydrogenase from bakers’ yeast. Methods Enzymol. 1982, 89, 469–473. [Google Scholar]
- Titoiu, A.M.; Necula-Petrareanu, G.; Visinescu, D.; Dinca, V.; Bonciu, A.; Mihailescu, C.N.; Purcarea, C.; Boukherroub, R.; Szunerits, S.; Vasilescu, A. Flow injection enzymatic biosensor for aldehydes based on a Meldola Blue-Ni complex electrochemical mediator. Microchim. Acta 2020, 187, 550. [Google Scholar] [CrossRef]
- Vasilescu, A.; Titoiu, A.M.; Purcarea, C.; Necula-Petrareanu, G. Method for Determining the Fungicide Thiram Based on Enzymatic Inhibition and Electrochemical Sensor. Romanian OSIM Patent Application No. A/00587, 13 August 2018. [Google Scholar]
- Nagy, I.; Schoofs, G.; Compernolle, F.; Proost, P.; Vanderleyden, J.; de Mot, R. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J. Bacteriol. 1995, 177, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.S.; Philp, J.C.; Aw, D.W.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol. 1998, 85, 195–210. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R.; da Fonseca, M.M.R. The remarkable Rhodococcus erythropolis. Appl. Microbiol. Biotechnol. 2005, 67, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Van der Geize, R.; Dijkhuizen, L. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr. Opin. Microbiol. 2004, 7, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from prokaryotes: A new source for an old enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326. [Google Scholar] [CrossRef]
- Prakash, O.; Mahabare, K.; Yadav, K.K.; Sharma, R. Fungi from Extreme Environments: A Potential Source of Laccases Group of Extremozymes. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S.M., Grube, M., Eds.; Springer International Publishing: Cham, Switzerland; Amsterdam, The Netherlands, 2019; pp. 441–462. [Google Scholar]
- Bains, J.; Capalash, N.; Sharma, P. Laccase from a non-melanogenic, alkalotolerant gamma-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol. Lett. 2003, 25, 1155–1159. [Google Scholar] [CrossRef]
- Suzuki, T.; Endo, K.; Ito, M.; Tsujibo, H.; Miyamoto, K.; Inamori, Y. A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Biosci. Biotechnol. Biochem. 2003, 67, 2167–2175. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Sousa, C.P.; Salazar-Banda, G.R.; de Lima-Neto, P.; Correia, A.N.; Morais, S. Current overview and perspectives on carbon-based (bio)sensors for carbamate pesticides electroanalysis. TrAC Trends Anal. Chem. 2020, 124, 115779. [Google Scholar] [CrossRef]
- Pichon, V.; Delaunay, N.; Combès, A. Sample Preparation Using Molecularly Imprinted Polymers. Anal. Chem. 2020, 92, 16–33. [Google Scholar] [CrossRef]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2016, 76, 180–194. [Google Scholar] [CrossRef]
- Del Valle, M. Bioelectronic Tongues Employing Electrochemical Biosensors. In Trends in Bioelectroanalysis; Matysik, F.-M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 143–202. [Google Scholar]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable Flexible and Stretchable Glove Biosensor for On-Site Detection of Organophosphorus Chemical Threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef]






| Dithiocarbamate Pesticides Investigated | Electrode Surface (Catalyst/Modifier) a | Real Samples Investigated | Signal Basis b | L.O.D. (Analytical Ranges Reported) | Ref. |
|---|---|---|---|---|---|
| Ziram | Polished silver solid amalgam electrode | Spiked river waters | SWV | 0.24 µM | [38] |
| Thiram | Hg | CS-DPV peak at −0.55 V vs. Ag/AgCl | 0.12 μM | [39] | |
| Thiram | Rotating gold disk electrode | Commercial formulations; spiked water samples | Ads-LSV, peak at +1.4 to +1.5 V vs. Ag/AgCl | 16 nM | [40] |
| Thiram Disulfiram | Graphite-PTFE composite electrode | Extracts of spiked strawberry samples | Ads-LSV, peaks at +0.85 V vs. SCE | Thiram: 54 nM (0.2 to 1 µM) Disulfiram: 20 nM (0.2 to 1 µM) | [41] |
| Thiram Disulfiram | Graphite-PTFE composite electrode | Spiked tap and well water samples | FIA-CA at +1V vs. Ag/AgCl | Thiram: 43 nM (0.1 to 1 µM) Disulfiram: 20 nM (0.1 to 1 µM) | [42] |
| Ziram | Hg | Extracts of spiked rice samples | CS-DPV | 32 nM i.e., 10 ppb | [43] |
| Zineb | Hg | AdSV, cathodic peak at −0.455 V vs. Ag/AgCl | 1 nM | [44] | |
| Carbathion, Ferbam, Nabam, Thiram, Thiuram, Zineb, Ziram | Carbon paste electrode -Fe(II)metallophthalocyanine composite | Ads-LSV | Ranged from 10 nM (carbathion) to 200 nM (Thiuram) | [45] | |
| Nabam | GCE, modified with Co(II) phthalocyanine and carbon ink | LSV, peak at −0.2V vs. Ag/AgCL | 28.8 nM | [46] | |
| Thiram | GCE | Commercial formulations; plant sample extracts exposed to thiram | SWV at +0.34 V vs. Ag/AgCl | n.r. | [47] |
| Carbathion | GCE | CV, peak forming at +1.46 vs. Ag/AgCl | 9.3 μM (132 μM to 224 μM) | [48] | |
| SWV, peak forming at +1.46 vs. Ag/AgCl | 85 nM (2 μM to 7.7 μM)) | ||||
| FIA-CA potential of +1.3 V vs. Ag/AgCl | 10 nM (1.2 μM to 6 μM) | ||||
| Ziram | Hg | Extracts of spiked vegetable samples | SWV, −1.1V vs. Ag/AgCl. | 23 nM (33 to 328 nM) | [37] |
| Thiram | Copper-mercury amalgam electrode | Spiked river water samples | CS-SPV, peak between −0.59 and −0.8 V vs. Ag/AgCl | 16 nM | [49] |
| Propineb | Carbon-paste electrode (Cu2+-enriched montmorillonite) | Commercial formulation | Ads-SWV, peak at ~−0.1V vs. SCE | 1 μM | [50] |
| Mancozeb | BDD | PAD at +0.3V vs. Ag/AgCl) | 0.514 µM (40 to 650 µM) | [36] | |
| Mancozeb | GCE | Commercial formulation | Ads-SWV, peaks forming at −0.7V vs. Ag/AgCl | 7 µM | [51] |
| Ziram | BDD | Spiked river water samples | FIA-CA at +0.55 V | 2.7 nM | [52] |
| Maneb | BDD | River water | DPV peak at +0.9V vs. Ag/AgCl | 24 nM (80 nM to 3 µM) | [53] |
| Mancozeb | Single-crystal (Au(111) and Au(110) | Ads-LSV, peaks at −0.6 to −0.96V vs. Ag/AgCl | Au(110): 100 nM Au(111): 500 nM | [54] | |
| Mancozeb | Gold electrode modified with Poly (3,4-ethylene dioxythiophene), multi-walled carbon nanotubes, and gold nanoparticles | Water | CV, anodic peak +0.65 V vs. Ag/AgCl | 5 μM | [55] |
| Thiram | Carbon paste electrode modified with zeolite | Aqueous extracts of fruit juices | DPV, anodic wave at +0.70V vs. Ag/AgCl; | 4 nM (14 nM to 4.2 μM) | [56] |
| Thiram | Platinum, modified with silver nanoparticles | Tap, canal, and river water | DPV and CV | 0.731 μM or 0.18 ppm | [57] |
| Thiram | GCE (dissolved Zn2+ and Cu2+ cations) | River water | CS-LSV: −1.330 V vs. Ag/AgCl for Zn-Thiram; +0.020V for Cu-Thiram complexes. | n.r. (5 to 50 μM) | [58] |
| DTF Investigated | Electrode Surface (Catalyst/Modifier) | Sample | Applied Potential | L.O.D. | Ref. |
|---|---|---|---|---|---|
| Thiram | CPE | Spiked river water | +1.1V vs. Ag/AgCl | 2.07 µM, | [12] |
| Thiram Disulfiram | Composite PTFE-graphite paste electrodes | Spiked apple samples | +1V vs. Ag/AgCl | Thiram: 1.66 µM Disulfiram: 3.37 μM | [60] |
| Carbathion Thiram Zineb | GCE | Spiked fruit pulp samples | +1.1 V vs. Pd | 0.7 μM Thiram: 1.5 μM Carbathion: 0.7 μM | [59] |
| Carbathion Mancozeb Propineb Ziram | not reported | +0.6V vs. Pd. | Carbathion: 31 nM Mancozeb: 7 nM Propineb: 26 nM Ziram: 26 nM | [10] | |
| Thiram | GCE | Spiked tap water and beetroot juice | +1.4V vs. Ag/AgCl. | 13.4 nM | [13] |
| Thiram, disulfiram | AuNP-SPCE | Spiked apple, grape and lettuce samples | +1.2 V vs. Ag/AgCl | Thiram: 91 nM Disulfiram: 0.56 µM | [14] |
| Fungicide | Detection Method | Enzyme | Limit of Detection | Incubation Time | Reference |
|---|---|---|---|---|---|
| Ziram | Square wave voltammetry/GPE | LACC 1, adsorption on electrodeposited Prussian Blue film | 0.002 ppm | 15 min | [77] |
| Ziram | Square wave voltammetry/GPE | LACC-TYR-AuNPs -CS electrodeposited film | 1 ppb | 20 min | [79] |
| Maneb | Amperometry/Pt electrode | ALDH+DP, entrapment in PVA/SbQ | 1.48 ppb | 15 min | [81] |
| Zineb | Amperometry/Pt-sputtered SPCE | ALDH and NADH oxidase/entrapment in PVA/SbQ | 8 ppm 8–80 ppb | 5 min | [82] |
| MITC | Amperometry/MBRS SPCE | ALDH/entrapment in PVA/SbQ | 100 ppm | 10 min | [78] |
| Maneb and zineb | Chronoamperometry/MBRS-SPCE | ALDH/ entrapment in PVA/SbQ or cross-linking with glutaraldehyde | 31.5 ppb–maneb 35 ppb-zineb | 10 min | [80] |
| Propineb (and organophosphates) | Potentiometry/Ag coated with AgCl | Working electrode inserted into Calcium-alginate beads containing 5 × 104 cultured N2a or Vero mammalian cells. | 0.33 μM (Vero cells) to 1.65 μM (N2a) | 2.5 min | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors 2021, 11, 12. https://doi.org/10.3390/bios11010012
Fanjul-Bolado P, Fogel R, Limson J, Purcarea C, Vasilescu A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors. 2021; 11(1):12. https://doi.org/10.3390/bios11010012
Chicago/Turabian StyleFanjul-Bolado, Pablo, Ronen Fogel, Janice Limson, Cristina Purcarea, and Alina Vasilescu. 2021. "Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors" Biosensors 11, no. 1: 12. https://doi.org/10.3390/bios11010012
APA StyleFanjul-Bolado, P., Fogel, R., Limson, J., Purcarea, C., & Vasilescu, A. (2021). Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors, 11(1), 12. https://doi.org/10.3390/bios11010012







