Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements
Abstract
:1. Introduction
2. Amino Acid Electroactivity
3. Electrochemical Analysis of Amino Acids
3.1. Sulphur Containing Amino Acids
3.1.1. Cysteine
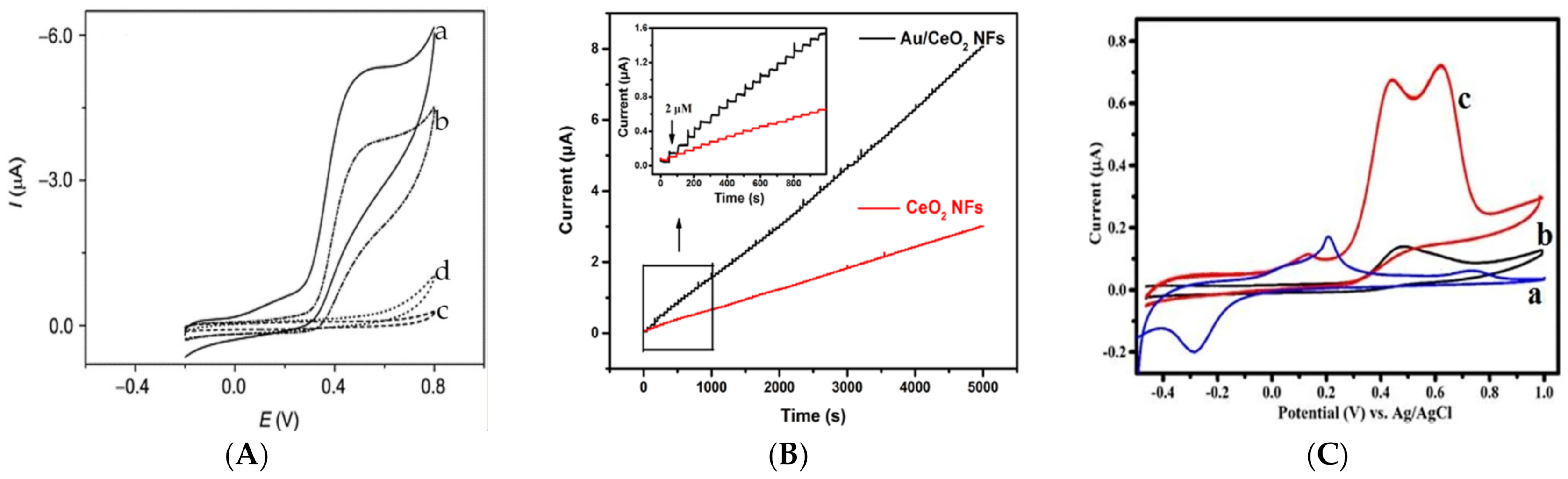
3.1.2. Methionine
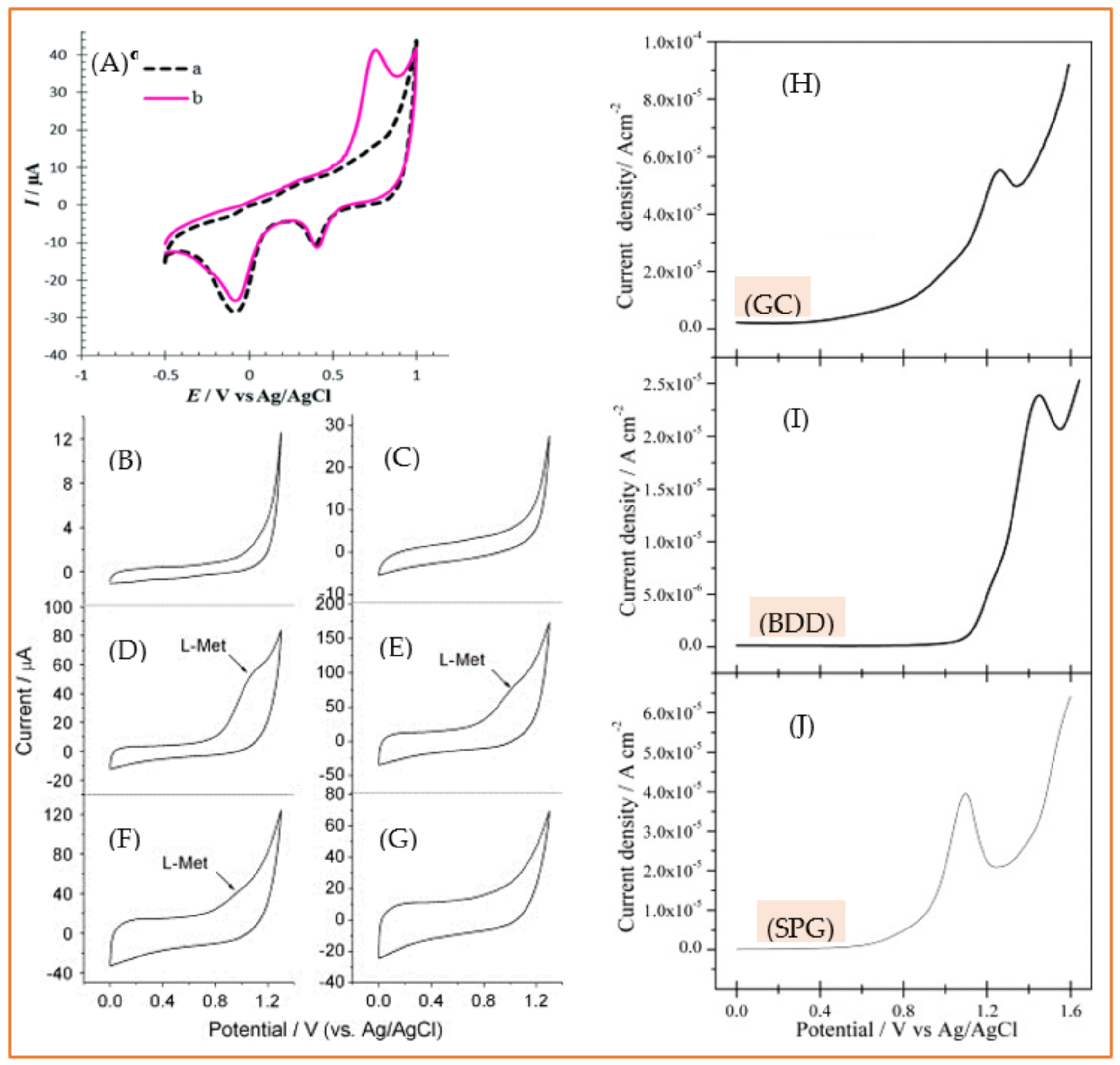
3.2. Aromatic Amino Acids
3.2.1. Tryptophan

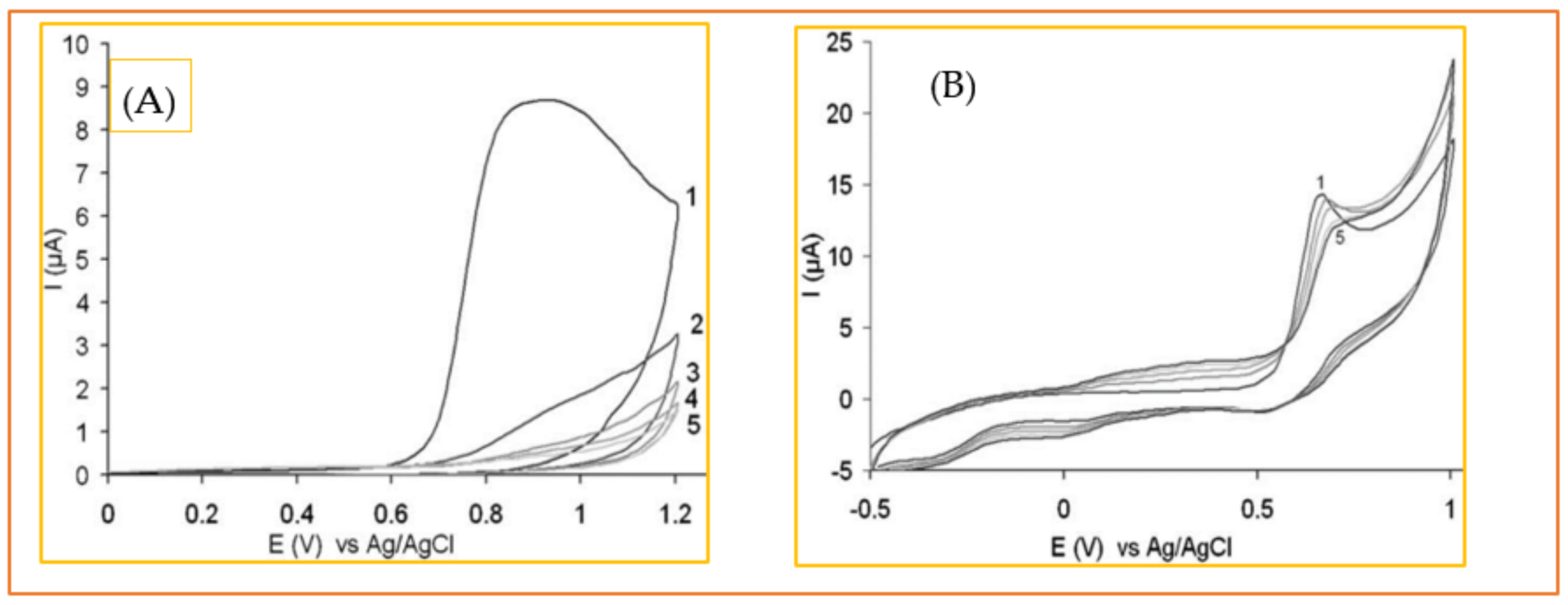
3.2.2. Tyrosine
3.3. Basic Amino Acid
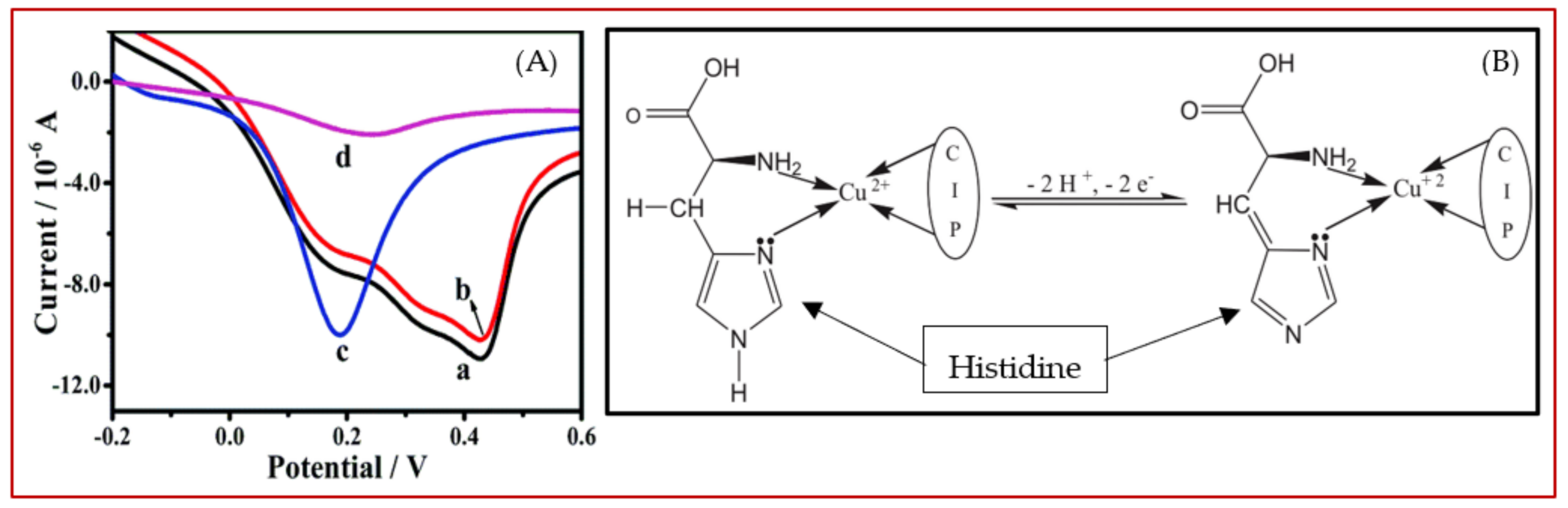
Histidine
| Sensing Part | Method | LDR | LOD | L.T. Stability | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| graphene quantum dot-scaffolded melamine and copper nanocomposites | LSV | 0.1 pM 24–70 µM | 0.025 pM | - | urine | [396] |
| tetrahedral copper metal organic framework | LSV | 0.1–200 µM | 0.025 µM | ˃6 months | human blood | [397] |
| reduced copper metal-organic framework | SWV | 0.010–100 µM | 0.002 μM | ˃12 months | red wine and urine | [398] |
| polydopamine Decorated Co3O4/rGO | AMP | 10–260 | 1.5 | - | l-His supplement | [405] |
| dl-homocysteine functionalized fullerene-C60-gold nanocomposite | SWV | 0.01 pM–100 µM | 1 fM 25 | 82% after 25 days | bovine serum albumin | [407] |
| copper germanate nanowires | CV | 5–2000 | 1.3 | - | - | [408] |
| hourglass-like nickel hydroxide nanostructure | CV | 0.1–500 | 0.08 | - | blood serum | [403] |
| nickel hydroxide nanostructures | CV | 0.1–100 | 0.013 | - | blood serum | [404] |
| complex imprinted polymers | ASDPV 26 | 9.99–323.6 ng/mL | 1.98 ng/mL | 90% after one month | bharmaceutical and blood serum | [399] |
| MIP/MWCNTs | DPV | 2–1000 | 5.8 nM | - | human blood serum | [400] |
4. Conclusions: Challenges and Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jane, B.; Reece, L.A.U.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Campbell Biology, 9th ed.; Pearson: London, UK, 2011. [Google Scholar]
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.K.; Gajra, B. Plasma and urine amino acid profiles in a healthy adult population of Singapore. Ann. Acad. Med. Singap. 2006, 35, 468–475. [Google Scholar]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, A.; Olszowy, P.; Ciborowski, P. 2—Biomolecules. In Proteomic Profiling and Analytical Chemistry, 2nd ed.; Ciborowski, P., Silberring, J., Eds.; Elsevier: Boston, MA, USA, 2016; pp. 7–24. [Google Scholar]
- Joint, F.A.; World Health Organization. Amino Acid Requirements in Human, Food, N, Agriculture Organization of the United, O. World Health, and U. United Nations. In Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Cho, W.; Yoon, Y.; Liu, S.L.; Baek, K.; Sheng, R. Chapter Two—Fluorescence-Based In Situ Quantitative Imaging for Cellular Lipids. In Methods in Enzymology; Gelb, M.H., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 19–33. [Google Scholar]
- Nur Izzah Ismail, Y.Z.H.-Y.H.; Jamal, P.; Othman, R.; Salleh, H.M. Production of Cysteine: Approaches, Challenges and Potential Solution. Int. J. Biotechnol. Wellness Ind. 2014, 3, 95–101. [Google Scholar]
- Kurpad, A.V.; Regan, M.M.; Varalakshmi, S.; Vasudevan, J.; Gnanou, J.; Raj, T.; Young, V.R. Daily methionine requirements of healthy Indian men, measured by a 24-h indicator amino acid oxidation and balance technique. Am. J. Clin. Nutr. 2003, 77, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Willke, T. Methionine production—A critical review. Appl. Microbiol. Biotechnol. 2014, 98, 9893–9914. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. l-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Sadeghiyan-Rizi, T.; Fooladi, J.; Sadrai, S. Preliminary Study on Cost-Effective l-Tryptophan Production from Indole and l-Serine by E. coli Cells. Avicenna J. Med. Biotechnol. 2016, 8, 188–192. [Google Scholar] [PubMed]
- Lütke-Eversloh, T.; Santos, C.N.S.; Stephanopoulos, G. Perspectives of biotechnological production of l-tyrosine and its applications. Appl. Microbiol. Biotechnol. 2007, 77, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Li, Y.; Xia, X.; Zhou, J.; Shi, G. Production of l-tyrosine using tyrosine phenol-lyase by whole cell biotransformation approach. Enzyme Microb. Technol. 2019, 131, 109430. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Noh, M.H.; Lim, H.G.; Kang, C.W.; Im, D.K.; Oh, M.K.; Jung, G.Y. Precise tuning of the glyoxylate cycle in Escherichia coli for efficient tyrosine production from acetate. Microb. Cell Fact. 2019, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Kulis-Horn, R.K.; Persicke, M.; Kalinowski, J. Histidine biosynthesis, its regulation and biotechnological application in Corynebacterium glutamicum. Microb. Biotechnol. 2014, 7, 5–25. [Google Scholar] [CrossRef]
- Nagashima, Y.; Kako, K.; Kim, J.D.; Fukamizu, A. Enhanced histamine production through the induction of histidine decarboxylase expression by phorbol ester in Jurkat cells. Mol. Med. Rep. 2012, 6, 944–948. [Google Scholar] [CrossRef]
- Huhtanen, P.; Vanhatalo, A.; Varvikko, T. Effects of abomasal infusions of histidine, glucose, and leucine on milk production and plasma metabolites of dairy cows fed grass silage diets. J. Dairy Sci. 2002, 85, 204–216. [Google Scholar] [CrossRef]
- Malykh, E.A.; Butov, I.A.; Ravcheeva, A.B.; Krylov, A.A.; Mashko, S.V.; Stoynova, N.V. Specific features of l-histidine production by Escherichia coli concerned with feedback control of AICAR formation and inorganic phosphate/metal transport. Microb. Cell Fact. 2018, 17, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Q.; Liu, H.; Liu, L.; Du, Y. Determining the contents of protein and amino acids in peanuts using near-infrared reflectance spectroscopy. J. Sci. Food Agric. 2013, 93, 118–124. [Google Scholar] [CrossRef]
- Faizan, M.; Ahmad, S. Experimental vibrational spectroscopy (FTIR and FT-Raman) of d-tryptophan and its anharmonic theoretical studies using density functional theory. J. Mol. Struct. 2018, 1171, 315–322. [Google Scholar] [CrossRef]
- Del Galdo, S.; Mancini, G.; Daidone, I.; Polzi, L.Z.; Amadei, A.; Barone, V. Tyrosine absorption spectroscopy: Backbone protonation effects on the side chain electronic properties. J. Comput. Chem. 2018, 39, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, D.; You, R.; Liu, J.; Huang, L.; Su, J.; Feng, S. Diazotization-coupling reaction-based determination of tyrosine in urine using ag nanocubes by surface-enhanced raman spectroscopy. Nanomaterials 2018, 8, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Ran, P.; Zhu, S.; Mo, F.; Wang, C.; Fu, Y. A highly sensitive electrochemiluminescence sensor for the detection of l-cysteine based on the rhombus-shaped rubrene microsheets and platinum nanoparticles. Sens. Actuators B Chem. 2019, 278, 97–102. [Google Scholar] [CrossRef]
- Miyamoto, T.; Sekine, M.; Ogawa, T.; Hidaka, M.; Homma, H.; Masaki, H. Generation of enantiomeric amino acids during acid hydrolysis of peptides detected by the liquid chromatography/tandem mass spectroscopy. Chem. Biodivers. 2010, 7, 1644–1650. [Google Scholar] [CrossRef]
- Mika, J.; Barek, J.; Zima, J.; Prokešová, E.; Dejmkova, H. Comparison of Glassy Carbon and Copper Microparticles as a Renewable Working Electrode Material for Amperometric Determination of Amino Acids Using Flow Through Detector. Electroanalysis 2019, 31, 357–362. [Google Scholar] [CrossRef]
- Deo, R.P.; Lawrence, N.S.; Wang, J. Electrochemical detection of amino acids at carbon nanotube and nickel-carbon nanotube modified electrodes. Analyst 2004, 129, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Citartan, M.; Tang, T.H. Recent developments of aptasensors expedient for point-of-care (POC) diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef]
- García-Carmona, L.; González, M.C.; Escarpa, A. Nanomaterial-based electrochemical (bio)-sensing: One step ahead in diagnostic and monitoring of metabolic rare diseases. Trends Anal. Chem. 2019, 118, 29–42. [Google Scholar] [CrossRef]
- Paleček, E.; Tkáč, J.; Bartošík, M.; Bertók, T.; Ostatná, V.; Paleček, J. Electrochemistry of nonconjugated proteins and glycoproteins. Toward sensors for biomedicine and glycomics. Chem. Rev. 2015, 115, 2045–2108. [Google Scholar]
- Xu, J.J.; Peng, Y.; Bao, N.; Xia, X.H.; Chen, H.Y. Simple method for the separation and detection of native amino acids and the identification of electroactive and non-electroactive analytes. J. Chromatogr. A 2005, 1095, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Peptide methionine sulfoxide reductase A (MsrA): Direct electrochemical oxidation on carbon electrodes. Bioelectrochemistry 2013, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dourado, A.H.B.; Pastrian, F.C.; Torresi, S.I.C. The long and successful journey of electrochemically active amino acids. From fundamental adsorption studies to potential surface engineering tools. An. Acad. Bras. Cienc. 2018, 90, 607–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enache, T.A.; Oliveira-Brett, A.M. Alzheimer’s disease amyloid beta peptides in vitro electrochemical oxidation. Bioelectrochemistry 2017, 114, 13–23. [Google Scholar] [CrossRef]
- Sattarahmady, N.; Heli, H. An electrocatalytic transducer for l-cysteine detection based on cobalt hexacyanoferrate nanoparticles with a core-shell structure. Anal. Biochem. 2011, 409, 74–80. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Boron doped diamond and glassy carbon electrodes comparative study of the oxidation behaviour of cysteine and methionine. Bioelectrochemistry 2011, 81, 46–52. [Google Scholar] [CrossRef]
- Prasad, B.B.; Pandey, I.; Srivastava, A.; Kumar, D.; Tiwari, M.P. Multiwalled carbon nanotubes-based pencil graphite electrode modified with an electrosynthesized molecularly imprinted nanofilm for electrochemical sensing of methionine enantiomers. Sens. Actuators B Chem. 2013, 176, 863–874. [Google Scholar] [CrossRef]
- Paz Zanini, V.I.; Giménez, R.E.; Pérez, O.E.L.; de Mishima, B.A.L.; Borsarelli, C.D. Enhancement of amperometric response to tryptophan by proton relay effect of chitosan adsorbed on glassy carbon electrode. Sens. Actuators B Chem. 2015, 209, 391–398. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wrona, M.Z.; Dryhurst, G. Electrochemical oxidation of tryptophan. J. Electroanal. Chem. Interfacial Electrochem. 1986, 199, 101–126. [Google Scholar] [CrossRef]
- Ogura, K.; Kobayashi, M.; Nakayama, M.; Miho, Y. In-situ FTIR studies on the electrochemical oxidation of histidine and tyrosine. J. Electroanal. Chem. 1999, 463, 218–223. [Google Scholar] [CrossRef]
- Paleček, E.; Bartošík, M.; Ostatná, V.; Trefulka, M. Electrocatalysis in proteins, nucleic acids and carbohydrates. Chem. Rec. 2012, 12, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.K.; Lewis, F.W.; Kutner, M.H.; Bate, D.M.; Roy, R.G.B.; Rudman, D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology 1984, 87, 770–776. [Google Scholar] [CrossRef]
- Singh, M.; Jaiswal, N.; Tiwari, I.; Foster, C.W.; Banks, C.E. A reduced graphene oxide-cyclodextrin-platinum nanocomposite modified screen printed electrode for the detection of cysteine. J. Electroanal. Chem. 2018, 829, 230–240. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; Song, J. Determination of l-cysteine in amino acid mixture and human urine by flow-injection analysis with a biamperometric detector. Anal. Biochem. 2001, 297, 170–176. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Wu, D.; Zhang, J.; Liu, X.; Gao, S.; Kong, Y. Electrochemical Chiral Recognition of Tryptophan Isomers Based on Nonionic Surfactant-Assisted Molecular Imprinting Sol-Gel Silica. ACS Appl. Mater. Interfaces 2019, 11, 2840–2848. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Habibollahi, S.; Shahidi, L. Simultaneous electrochemical sensing of cysteine, uric acid and tyrosine using a novel Au-nanoparticles/poly-Trypan Blue modified glassy carbon electrode. J. Electroanal. Chem. 2017, 789, 140–147. [Google Scholar] [CrossRef]
- Sornambikai, S.; Kadir, M.R.A.; Kumar, A.S.; Ponpandian, N.; Viswanathan, C. Selective and low potential electrocatalytic oxidation and sensing of l-cysteine using metal impurity containing carbon black modified electrode. Anal. Methods 2017, 9, 6791–6800. [Google Scholar] [CrossRef]
- Kannan, A.; Sevvel, R. Gold nanoparticles embedded electropolymerized thin film of pyrimidine derivative on glassy carbon electrode for highly sensitive detection of l-cysteine. Mater. Sci. Eng. C 2017, 78, 513–519. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Salavati, H.; Banitaba, S.H.; Kazemi, F. Simultaneous determination of cysteine, uric acid and tyrosine using Au-nanoparticles/poly(E)-4-(p-tolyldiazenyl)benzene-1,2,3-triol film modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 59, 120–128. [Google Scholar] [CrossRef]
- Gu, J.; Dai, H.; Kong, Y.; Tao, Y.; Chu, H.; Tong, Z. Chiral electrochemical recognition of cysteine enantiomers with molecularly imprinted overoxidized polypyrrole-Au nanoparticles. Synth. Met. 2016, 222, 137–143. [Google Scholar] [CrossRef]
- Wang, X.; Luo, C.; Li, L.; Duan, H. Highly selective and sensitive electrochemical sensor for l-cysteine detection based on graphene oxide/multiwalled carbon nanotube/manganese dioxide/gold nanoparticles composite. J. Electroanal. Chem. 2015, 757, 100–106. [Google Scholar] [CrossRef]
- Abbas, M.N.; Saeed, A.A.; Singh, B.; Radowan, A.A.; Dempsey, E. A cysteine sensor based on a gold nanoparticle-iron phthalocyanine modified graphite paste electrode. Anal. Methods 2015, 7, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wen, Y.; Lu, L.; Xu, J.; Zhang, L.; Yao, Y.; He, H. A Novel l-Cysteine Electrochemical Sensor Using Sulfonated Graphene-poly(3,4-Ethylenedioxythiophene) Composite Film Decorated with Gold Nanoparticles. Electroanalysis 2014, 26, 648–655. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Karuppiah, C.; Chen, S.M.; Mani, V.; Vasantha, V.S.; Ramaraj, S. Highly selective determination of cysteine using a composite prepared from multiwalled carbon nanotubes and gold nanoparticles stabilized with calcium crosslinked pectin. Microchim. Acta 2014, 182, 727–735. [Google Scholar] [CrossRef]
- Silva, F.D.A.D.S.; da Silva, M.G.A.; Lima, P.R.; Meneghetti, M.R.; Kubota, L.T.; Goulart, M.O.F. A very low potential electrochemical detection of l-cysteine based on a glassy carbon electrode modified with multi-walled carbon nanotubes/gold nanorods. Biosens. Bioelectron. 2013, 50, 202–209. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, W.S. Electrochemical oxidation of cysteine at a film gold modified carbon fiber microelectrode its application in a flow-through voltammetric sensor. Sensors 2012, 12, 3562–3577. [Google Scholar] [CrossRef] [Green Version]
- Perevezentseva, D.O.; Gorchakov, E.V. Voltammetric determination of cysteine at a graphite electrode modified with gold nanoparticles. J. Solid State Electrochem. 2012, 16, 2405–2410. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Hou, S.; Ma, H. Highly sensitive and selective electrochemical detection of l-cysteine using nanoporous gold. Microchim. Acta 2012, 177, 427–433. [Google Scholar] [CrossRef]
- Liu, X.; Luo, L.; Ding, Y.; Kang, Z.; Ye, D. Simultaneous determination of l-cysteine and l-tyrosine using Au-nanoparticles/poly-eriochrome black T film modified glassy carbon electrode. Bioelectrochemistry 2012, 86, 38–45. [Google Scholar] [CrossRef]
- Ge, S.; Yan, M.; Lu, J.; Zhang, M.; Yu, F.; Yu, J.; Song, X.; Yu, S. Electrochemical biosensor based on graphene oxide-Au nanoclusters composites for l-cysteine analysis. Biosens. Bioelectron. 2012, 31, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Miao, L.; Kan, M.; Kong, L.; Zhang, H. Oxidation and detection of l-cysteine using a modified Au/Nafion/glass carbon electrode. Sci. China Chem. 2011, 54, 521–525. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Su, W.Y.; Cheng, J.R.; Cheng, S.H. Electrochemical determination of cysteine based on conducting polymers/gold nanoparticles hybrid nanocomposites. Electrochim. Acta 2011, 56, 6887–6895. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Noor, A.M.; Huang, N.M. Amperometric determination of l-cysteine using a glassy carbon electrode modified with palladium nanoparticles grown on reduced graphene oxide in a Nafion matrix. Microchim. Acta 2018, 185, 246. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.; Ganesh, V. Simple and facile preparation of silver-polydopamine (Ag-PDA) core-shell nanoparticles for selective electrochemical detection of cysteine. RSC Adv. 2016, 6, 49578–49587. [Google Scholar] [CrossRef]
- Li, H.; Chen, D.; Wang, H.; Li, J.; Wang, W. Sub-picomole level photoelectrochemical sensing of l-cysteine based on plasmonic silver nanoparticles modified hierarchically structured zinc oxide. J. Electroanal. Chem. 2015, 759, 21–26. [Google Scholar] [CrossRef]
- Jin, G.P.; Chen, L.L.; Hang, G.P.; Yang, S.Z.; Wu, X.J. Stripping chronopotentiometric analysis of cysteine on nano-silver coat polyquercetin-MWCNT modified platinum electrode. J. Solid State Electrochem. 2010, 14, 1163–1169. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Y.; Zhang, X.; Ding, S.; Li, L.; Zha, W. Molecularly imprinted electrochemical sensor based on the synergic effect of nanoporous gold and copper nanoparticles for the determination of cysteine. J. Solid State Electrochem. 2016, 20, 2037–2044. [Google Scholar] [CrossRef]
- Zhang, L.; Ning, L.; Zhang, Z.; Li, S.; Yan, H.; Pang, H.; Ma, H. Fabrication and electrochemical determination of l-cysteine of a composite film based on V-substituted polyoxometalates and Au@2Ag core-shell nanoparticles. Sens. Actuators B Chem. 2015, 221, 28–36. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Zhang, H.M. One Step Fabrication of Au Nanoparticles-Ni-Al Layered Double Hydroxide Composite Film for the Determination of l-Cysteine. Electroanalysis 2015, 27, 1195–1201. [Google Scholar] [CrossRef]
- Mo, Z.; Zhao, F.; Xiao, F.; Zeng, B. Preparation and characterization of AuPt alloy nanoparticle-multi-walled carbon nanotube-ionic liquid composite film for electrocatalytic oxidation of cysteine. J. Solid State Electrochem. 2010, 14, 1615–1620. [Google Scholar] [CrossRef]
- Murugavelu, M.; Karthikeyan, B. Study of Ag-Pd bimetallic nanoparticles modified glassy carbon electrode for detection of l-cysteine. Superlattices Microstruct. 2014, 75, 916–926. [Google Scholar] [CrossRef]
- Cao, F.; Dong, Q.; Li, C.; Kwak, D.; Huang, Y.; Song, D.; Lei, Y. Sensitive and Selective Electrochemical Determination of l-Cysteine Based on Cerium Oxide Nanofibers Modified Screen Printed Carbon Electrode. Electroanalysis 2018, 30, 1133–1139. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Z.; Yan, K.; Zhao, H.; Zhang, J. One-Step Synthesis of CuO–Cu2O Heterojunction by Flame Spray Pyrolysis for Cathodic Photoelectrochemical Sensing of l-Cysteine. ACS Appl. Mater. Interfaces 2017, 9, 40452–40460. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Wang, G.; Deng, D.; Qu, L. A novel electrochemical sensor based on Fe2O3 nanoparticles/N-doped graphene for electrocatalytic oxidation of l-cysteine. J. Solid State Electrochem. 2015, 19, 3613–3620. [Google Scholar] [CrossRef]
- Gupta, V.K.; Shamsadin-Azad, Z.; Cheraghi, S.; Agarwai, S.; Taher, M.A.; Karimi, F. Electrocatalytic determination of l-cysteine in the presence of tryptophan using carbon paste electrode modified with MgO nanoparticles and acetylferrocene. Int. J. Electrochem. Sci. 2018, 13, 4309–4318. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Wu, D.; Ma, J.; Tao, Y.; Qin, Y.; Kong, Y. Multi-templates based molecularly imprinted sodium alginate/MnO2 for simultaneous enantiorecognition of lysine, alanine and cysteine isomers. Int. J. Biol. Macromol. 2019, 129, 786–791. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Liu, L.; Wang, G.; Wang, D.; Qu, L. Preparation of nickel oxide nanoparticles on N-doped reduced graphene oxide: A two-dimensional hybrid for electrocatalytic sensing of l-cysteine. J. Alloys Compd. 2017, 691, 834–840. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, J. A nonenzymatic l-cysteine sensor based on SnO2-MWCNTs nanocomposites. J. Mol. Liq. 2014, 196, 280–284. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, S.; Chu, W.; Wei, T.; Tao, H.; Zhang, C.; Sun, Y. Enhanced photoelectrochemical detection of l-cysteine based on the ultrathin polythiophene layer sensitized anatase TiO2 on F-doped tin oxide substrates. Sens. Actuators B Chem. 2016, 232, 448–453. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, K.; Chen, L.; Guo, L.; Ai, S. A novel photoelectrochemical sensor based on PPIX-functionalized WO3-rGO nanohybrid-decorated ITO electrode for detecting cysteine. Biosens. Bioelectron. 2013, 44, 48–51. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, Y.; Wang, G.; Qu, L. Amperometric l-cysteine sensor based on a carbon paste electrode modified with Y2O3 nanoparticles supported on nitrogen-doped reduced graphene oxide. Microchim. Acta 2016, 183, 1351–1357. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Qu, C.; Wang, G.; Wang, D. Simple synthesis of ZnO nanoparticles on N-doped reduced graphene oxide for the electrocatalytic sensing of l-cysteine. RSC Adv. 2017, 7, 35004–35011. [Google Scholar] [CrossRef] [Green Version]
- Szot-Karpińska, K.; Leśniewski, A.; Jönsson-Niedziółka, M.; Marken, F.; Niedziółka-Jönsson, J. Electrodes modified with bacteriophages and carbon nanofibres for cysteine detection. Sens. Actuators B Chem. 2019, 287, 78–85. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Budnikov, H. Selective electrochemical sensor based on the electropolymerized p-coumaric acid for the direct determination of l-cysteine. Electrochim. Acta 2018, 270, 369–377. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Song, D.; Xu, X.; Zhao, Y.; Liu, X.; Su, Z. Amperometric l-cysteine Sensor Using a Gold Electrode Modified with Thiolated Catechol. Electroanalysis 2017, 29, 2410–2416. [Google Scholar] [CrossRef]
- Sundaram, S.; Kadir, M.R.A. A New Highly Conducting Carbon Black (CL-08) Modified Electrode Functionalized with Syringic Acid for Sensitive and Selective l-Cysteine Electrocatalysis at Low Potential. Electrochim. Acta 2017, 224, 475–486. [Google Scholar] [CrossRef]
- Safavi, A.; Ahmadi, R.; Mahyari, F.A. Simultaneous electrochemical determination of l-cysteine and l-cysteine disulfide at carbon ionic liquid electrode. Amino Acids 2014, 46, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. The selective electrochemical detection of homocysteine in the presence of glutathione, cysteine, and ascorbic acid using carbon electrodes. Analyst 2014, 139, 3755–3762. [Google Scholar] [CrossRef]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. The use of screen-printed electrodes in a proof of concept electrochemical estimation of homocysteine and glutathione in the presence of Cysteine using catechol. Sensors 2014, 14, 10395–10411. [Google Scholar] [CrossRef]
- Lee, P.T.; Lowinsohn, D.; Compton, R.G. Simultaneous Detection of Homocysteine and Cysteine in the Presence of Ascorbic Acid and Glutathione Using a Nanocarbon Modified Electrode. Electroanalysis 2014, 26, 1488–1496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wu, H.; Wu, Y.; Shi, H.; Feng, X.; Huang, H.; Li, J.; Song, W. Large surface area carbon material with ordered mesopores for highly selective determination of l-tyrosine in the presence of l-cysteine. Electrochim. Acta 2013, 112, 90–94. [Google Scholar] [CrossRef]
- Liu, X.; Lv, H.; Sun, Q.; Zhong, Y.; Zhao, J.; Fu, J.; Lin, M.; Wang, J. Differential Pulse Voltammetric Determination of l-Cysteine After Cyclic Voltammetry in Presence of Catechol with Glassy Carbon Electrode. Anal. Lett. 2012, 45, 2246–2256. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Taher, M.A.; Beitollahi, H.; Hosseinzadeh, R. Electrocatalytic determination of l-cysteine using a modified carbon nanotube paste electrode: Application to the analysis of some real samples. Chin. Chem. Lett. 2012, 23, 981–984. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Dadkhah-Tehrani, S.; Karimi-Maleh, H. A voltammetric sensor for the simultaneous determination of l-cysteine and tryptophan using a p-aminophenol-multiwall carbon nanotube paste electrode. Anal. Sci. 2011, 27, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Liu, Y.; Hou, H.; You, T. Electrochemical determination of l-Tryptophan, l-Tyrosine and l-Cysteine using electrospun carbon nanofibers modified electrode. Talanta 2010, 80, 2182–2186. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakania, M.; Taleata, Z.; Beitollahia, H.; Naeimib, H. Selective determination of cysteine in the presence of tryptophan by carbon paste electrode modified with quinizarine. J. Iran. Chem. Soc. 2010, 7, 251–259. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M−S Bonds (M = Au, Ag, and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef]
- Han, J.; Zhao, J.; Li, Z.; Zhang, H.; Yan, Y.; Cao, D.; Wang, G. Nanoporous carbon derived from dandelion pappus as an enhanced electrode material with low cost for amperometric detection of tryptophan. J. Electroanal. Chem. 2018, 818, 149–156. [Google Scholar] [CrossRef]
- Ye, M.L.; Xu, B.; Zhang, W.D. Sputtering deposition of Pt nanoparticles on vertically aligned multiwalled carbon nanotubes for sensing l-cysteine. Microchim. Acta 2011, 172, 439–446. [Google Scholar] [CrossRef]
- Liu, L.P.; Yin, Z.J.; Yang, Z.S. A l-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 2010, 79, 84–89. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, X.; Ran, X.; Tan, X.; Li, T.; Cao, M.; Yang, L.; Du, G. Layer-by-layer assembly of anionic-/cationic-pillar(5)arenes multilayer films as chiral interface for electrochemical recognition of tryptophan isomers. Electrochim. Acta 2018, 277, 1–8. [Google Scholar] [CrossRef]
- Vladislavić, N.; Rončević, I.Š.; Buljac, M.; Brinić, S.; Krivić, D.; Buzuk, M. Electroanalytical determination of cysteine using the electrodes based on ternary silver-copper sulfides. Sensors 2018, 18, 3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farjami, F.; Mosalman, F.K.; Ebrahimpourmoghaddam, S.; Sharghi, H. Electrocatalytic Determination of Cysteine Using a Carbon Ionic Liquid Electrode Modified with Terpyridine Copper(II) Complex. Anal. Lett. 2016, 49, 1412–1423. [Google Scholar] [CrossRef]
- Derikvand, Z.; Azadbakht, A. Copper inorganic-organic hybrid coordination compound as a novel l-cysteine electrochemical sensor: Synthesis, characterization, spectroscopy and crystal structure. J. Chem. Sci. 2015, 127, 2005–2014. [Google Scholar] [CrossRef] [Green Version]
- Pei, L.Z.; Cai, Z.Y.; Pei, Y.Q.; Xie, Y.K.; Fan, C.G.; Fu, D.G. Electrochemical determination of l-cysteine using polyaniline/CuGeO3 nanowire modified electrode. Russ. J. Electrochem. 2014, 50, 458–467. [Google Scholar] [CrossRef]
- Majidi, M.R.; Asadpour-Zeynali, K.; Hafezi, B. Sensing l-cysteine in urine using a pencil graphite electrode modified with a copper hexacyanoferrate nanostructure. Microchim. Acta 2010, 169, 283–288. [Google Scholar] [CrossRef]
- Dong, Y.; Pei, L.; Chu, X.; Zhang, W.; Zhang, Q. Electrochemical behavior of cysteine at a CuGeO3 nanowires modified glassy carbon electrode. Electrochim. Acta 2010, 55, 5135–5141. [Google Scholar] [CrossRef]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
- Zhou, H.; Ran, G.; Masson, J.F.; Wang, C.; Zhao, Y.; Song, Q. Rational Design of Magnetic Micronanoelectrodes for Recognition and Ultrasensitive Quantification of Cysteine Enantiomers. Anal. Chem. 2018, 90, 3374–3381. [Google Scholar] [CrossRef]
- Bonacin, J.A.; Santos, P.L.D.; Katic, V.; Foster, C.W.; Banks, C.E. Use of Screen-Printed Electrodes Modified by Prussian Blue and Analogues in Sensing of Cysteine. Electroanalysis 2018, 30, 170–179. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. A magnetic CoFe2O4/SiO2 nanocomposite fabricated by the sol-gel method for electrocatalytic oxidation and determination of l-cysteine. Microchim. Acta 2017, 184, 825–833. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Li, G.; Liu, Q.; Wei, T.; Li, B.; Jiang, C.; Sun, Y. Electrochemical detection of l-cysteine using a glassy carbon electrode modified with a two-dimensional composite prepared from platinum and Fe3O4 nanoparticles on reduced graphene oxide. Microchim. Acta 2016, 183, 3221–3228. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @ Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of l-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Kohilarani, K.; Ramaraj, S. Determination of l-cysteine at iron tetrasulfonated phthalocyanine decorated multiwalled carbon nanotubes film modified electrode. Int. J. Electrochem. Sci. 2015, 10, 682–690. [Google Scholar]
- Corrêa, C.C.; Jannuzzi, S.A.V.; Santhiago, M.; Timm, R.A.; Formiga, A.L.B.; Kubota, L.T. Modified electrode using multi-walled carbon nanotubes and a metallopolymer for amperometric detection of l-cysteine. Electrochim. Acta 2013, 113, 332–339. [Google Scholar] [CrossRef]
- Shaidarova, L.G.; Ziganshina, S.A.; Gedmina, A.V.; Chelnokova, I.A.; Budnikov, G.K. Electrochemical behavior and voltammetric determination of cysteine and cystine at carbon-paste electrodes modified with metal phthalocyanines. J. Anal. Chem. 2011, 66, 633–641. [Google Scholar] [CrossRef]
- Liu, S.; Dai, G. Preparation and electrochemical behaviour of silver pentacyanonitrosylferrate film modified glassy carbon electrode and its electrocatalytic oxidation to l-cysteine. J. Chin. Chem. Soc. 2011, 58, 617–622. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Mohammadpour, Z. Homogeneous electrocatalytic oxidation and voltammetric determination of l-cysteine by 1,1′-Ferrocenedicarboxylic acid at glassy carbon electrode. Anal. Bioanal. Electrochem. 2010, 2, 24–35. [Google Scholar]
- Benvidi, A.; Ansaripour, M.M.; Rajabzadeh, N.; Zare, H.R.; Mirjalili, B.B.F. Developing a nanostructure electrochemical sensor for simultaneous determination of cysteine and tryptophan. Anal. Methods 2015, 7, 3920–3928. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of l-Cysteine at a screen-printed diamond electrode. Carbon 2021, 173, 395–402. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Xia, N.; Wang, Y.; Liu, P.; Qu, L. Fabrication of hierarchical 3D prickly ball-like Co–La oxides/reduced graphene oxide composite for electrochemical sensing of l-cysteine. J. Alloys Compd. 2021, 853, 157077. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Xia, N.; Liu, P.; Wang, Y.; Qu, L. High performance electrochemical l-cysteine sensor based on hierarchical 3D straw-bundle-like Mn-La oxides/reduced graphene oxide composite. J. Electroanal. Chem. 2020, 877, 114654. [Google Scholar] [CrossRef]
- Balasubramanian, P.; He, S.-B.; Deng, H.-H.; Peng, H.-P.; Chen, W. Defects engineered 2D ultrathin cobalt hydroxide nanosheets as highly efficient electrocatalyst for non-enzymatic electrochemical sensing of glucose and l-cysteine. Sens. Actuators B Chem. 2020, 320, 128374. [Google Scholar] [CrossRef]
- Zhai, X.; Li, S.; Chen, X.; Hua, Y.; Wang, H. Coating silver metal-organic frameworks onto nitrogen-doped porous carbons for the electrochemical sensing of cysteine. Microchim. Acta 2020, 187, 493. [Google Scholar] [CrossRef]
- Jerome, R.; Keerthivasan, P.V.; Murugan, N.; Devi, N.R.; Sundramoorthy, A.K. Preparation of Stable CuO/Boron Nitride Nanocomposite Modified Electrode for Selective Electrochemical Detection of l-Cysteine. ChemistrySelect 2020, 5, 9111–9118. [Google Scholar] [CrossRef]
- Fallahi, M.; Norouzi, B. Synthesis of cobalt oxide nanoparticles using Cirsium vulgare leaves extract and evaluation of electrocatalytic effects on oxidation of l-cysteine. Ionics 2020, 26, 1951–1961. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Alizadeh, Z. A Certain Electrochemical Nanosensor Based on Functionalized Multi-Walled Carbon Nanotube for Determination of Cysteine in the Presence of Paracetamol. J. Nanostruct. 2020, 10, 258–267. [Google Scholar]
- Vladislavić, N.; Rončević, I.Š.; Buzuk, M.; Buljac, M.; Drventić, I. Electrochemical/chemical synthesis of hydroxyapatite on glassy carbon electrode for electroanalytical determination of cysteine. J. Solid State Electrochem. 2020, 25, 841. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L. 3D pothole-rich hierarchical carbon framework-encapsulated Ni nanoparticles for highly selective nonenzymatic cysteine detection. Electrochim. Acta 2019, 328, 135126. [Google Scholar] [CrossRef]
- De Souza Magossi, M.; Fernandes, D.S.; do Carmo, D.R. Synthesis of a novel hybrid nanocomposite based on copper pentacyanonitrosylferrate and octa(aminopropyl)silsesquioxane and its behavior on l-cysteine electrooxidation. Solid State Sci. 2019, 95, 105931. [Google Scholar] [CrossRef]
- Kumar, D.R.; Baynosa, M.L.; Shim, J.-J. Cu2+-1,10-phenanthroline-5,6-dione@electrochemically reduced graphene oxide modified electrode for the electrocatalytic determination of l-cysteine. Sens. Actuators B Chem. 2019, 293, 107–114. [Google Scholar] [CrossRef]
- Maheshwari, H.; Vilà, N.; Herzog, G.; Walcarius, A. Selective Detection of Cysteine at a Mesoporous Silica Film Electrode Functionalized with Ferrocene in the Presence of Glutathione. ChemElectroChem 2020, 7, 2095–2101. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, M.R.; Kumar, R.M.; Jayavel, R. CeO2-based heterostructure nanocomposite for electrochemical determination of l-cysteine biomolecule. Inorg. Chem. Commun. 2020, 113, 107793. [Google Scholar] [CrossRef]
- Le, H.T.; Tran, D.T.; Doan, T.L.L.; Kim, N.H.; Lee, J.H. Hierarchical Cu@CuxO nanowires arrays-coated gold nanodots as a highly sensitive self-supported electrocatalyst for l-cysteine oxidation. Biosens. Bioelectron. 2019, 139, 111327. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Ponraj, J.; Mahmoud, K.A. Sensitive electrochemical detection of l-cysteine based on a highly stable Pd@Ti3C2Tx (MXene) nanocomposite modified glassy carbon electrode. Anal. Methods 2019, 11, 3851–3856. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Kumar, M.R.; Kumar, R.M. Facile synthesis of CeO2-SnO2 nanocomposite for electrochemical determination of l-cysteine. J. Alloys Compd. 2019, 792, 1150–1161. [Google Scholar] [CrossRef]
- Atacan, K. CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for l-cysteine detection. J. Alloys Compd. 2019, 791, 391–401. [Google Scholar] [CrossRef]
- Heidari, M.; Ghaffarinejad, A. Electrochemical sensor for l-cysteine by using a cobalt(II)/aluminum(III) layered double hydroxide as a nanocatalyst. Microchim. Acta 2019, 186, 365. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, H.; Cai, J.; Peng, Y.; Niu, Y.; Chen, H.; Bai, L.; Zhang, S.; Jin, J.; Liu, L.; et al. A novel oxidation-reduction method for highly selective detection of cysteine over reduced glutathione based on synergistic effect of fully fluorinated cobalt phthalocyanine and ordered mesoporous carbon. Sens. Actuators B Chem. 2019, 288, 180–187. [Google Scholar] [CrossRef]
- Beitollahi, H.; Ganjali, M.R.; Norouzi, P.; Movlaee, K.; Hosseinzadeh, R.; Tajik, S. A novel electrochemical sensor based on graphene nanosheets and ethyl 2-(4-ferrocenyl-[1,2,3]triazol-1-yl) acetate for electrocatalytic oxidation of cysteine and tyrosine. Meas. J. Int. Meas. Confed. 2020, 152, 107302. [Google Scholar] [CrossRef]
- Hussain, M.; Khaliq, N.; Nisar, A.; Khan, M.; Karim, S.; Khan, A.A.; Yi, X.; Maqbool, M.; Ali, G. TiO2 nanotube array-modified electrodes for l-cysteine biosensing: Experimental and density-functional theory study. Nanotechnology 2020, 31, 505501. [Google Scholar] [CrossRef]
- Norouzi, B.; Gorji, A. Preparation of cobalt-poly (naphthylamine)/sodium dodecylsulfate-modified carbon paste electrode as a sensitive sensor for l-cysteine. Ionics 2019, 25, 797–807. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kurniawan, F.; Gunawan, F.; Chou, S.H.; Wang, M.J. Disposable electrochemical sensor based on copper-electrodeposited screen-printed gold electrode and its application in sensing l-Cysteine. Electrochim. Acta 2019, 293, 318–327. [Google Scholar] [CrossRef]
- Keshavananda Prabhu, C.P.; Manjunatha, N.; Shambhulinga, A.; Imadadulla, M.; Shivaprasad, K.H.; Amshumali, M.K.; Lokesh, K.S. Synthesis and characterization of novel imine substituted phthalocyanine for sensing of l-cysteine. J. Electroanal. Chem. 2019, 834, 130–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Wei, T.; Lin, F.F.; Qiu, F.L.; Pei, L.Z. Polyaniline/zinc bismuthate nanocomposites for the enhanced electrochemical performance of the determination of l-Cysteine. Meas. J. Int. Meas. Confed. 2018, 128, 55–62. [Google Scholar] [CrossRef]
- Tajyani, S.; Babaei, A. A new sensing platform based on magnetic Fe3O4@NiO core/shell nanoparticles modified carbon paste electrode for simultaneous voltammetric determination of Quercetin and Tryptophan. J. Electroanal. Chem. 2018, 808, 50–58. [Google Scholar] [CrossRef]
- Premlatha, S.; Selvarani, K.; Bapu, G.N.K.R. Facile Electrodeposition of Hierarchical Co-Gd2O3 Nanocomposites for Highly Selective and Sensitive Electrochemical Sensing of l-Cysteine. ChemistrySelect 2018, 3, 2665–2674. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wei, T.; Lin, N.; Zhang, H.; Fan, C.G. Bismuth Tellurate Nanospheres and Electrochemical Behaviors of l-Cysteine at the Nanospheres Modified Electrode. Russ. J. Electrochem. 2018, 54, 84–91. [Google Scholar] [CrossRef]
- Bananezhad, A.; Karimi-Maleh, H.; Ganjali, M.R.; Norouzi, P. MnO2-TiO2 Nanocomposite and 2-(3,4-Dihydroxyphenethyl) Isoindoline-1,3-Dione as an Electrochemical Platform for the Concurrent Determination of Cysteine, Tryptophan and Uric Acid. Electroanalysis 2018, 30, 1759–1765. [Google Scholar] [CrossRef]
- Wen, Y.; Pei, L.Z.; Wei, T. Growth of Li doped bismuth oxide nanorods and its electrochemical performance for the determination of l-cysteine. Mater. Res. 2017, 20, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.F.; Wei, T.; Pei, L.Z.; Lin, N. Sensitive electrochemical determination of l-cysteine using bismuth nickelate nanorods modified electrode. J. Bionanosci. 2017, 11, 177–182. [Google Scholar] [CrossRef]
- Hooshmand, S.; Es’haghi, Z. Simultaneous quantification of arginine, alanine, methionine and cysteine amino acids in supplements using a novel bioelectro-nanosensor based on CdSe quantum dot/modified carbon nanotube hollow fiber pencil graphite electrode via Taguchi method. J. Pharm. Biomed. Anal. 2017, 146, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, M.; Sivakumar, M.; Chen, S.M.; Lun-Cheng, W. Thulium (III) hexacyanoferrate star fruit-like structure modified electrode for l-cysteine electrochemical sensor. Int. J. Electrochem. Sci. 2016, 11, 8016–8026. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wei, T.; Lin, N.; Cai, Z.Y.; Fan, C.G.; Yang, Z. Synthesis of Zinc bismuthate nanorods and electrochemical performance for sensitive determination of l-Cysteine. J. Electrochem. Soc. 2016, 163, H1–H8. [Google Scholar] [CrossRef]
- Pazalja, M.; Kahrović, E.; Zahirović, A.; Turkušić, E. Electrochemical sensor for determination of l-cysteine based on carbon electrodes modified with Ru(III) Schiff base complex, carbon nanotubes and nafion. Int. J. Electrochem. Sci. 2016, 11, 10939–10952. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; Sanjuán, I.; Montiel, M.Á.; Foster, C.W.; Banks, C.E.; Iniesta, J. l-Cysteine determination in embryo cell culture media using Co(II)-phthalocyanine modified disposable screen-printed electrodes. J. Electroanal. Chem. 2016, 780, 303–310. [Google Scholar] [CrossRef]
- Hashemi, H.S.; Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. A novel cysteine sensor based on modification of carbon paste electrode by Fe(II)-exchanged zeolite X nanoparticles. Mater. Sci. Eng. C 2016, 58, 286–293. [Google Scholar] [CrossRef]
- Geng, D.; Li, M.; Bo, X.; Guo, L. Molybdenum nitride/nitrogen-doped multi-walled carbon nanotubes hybrid nanocomposites as novel electrochemical sensor for detection l-cysteine. Sens. Actuators B Chem. 2016, 237, 581–590. [Google Scholar] [CrossRef]
- Da Silveira, T.S.; Fernandes, D.S.; Magossi, M.S.; Barbosa, P.P.; Souza, T.R.; Magossi, M.S.; Do Carmo, D.R. A novel composite obtained through of chemical interaction of zirconium (IV) phosphated with silver hexacyanoferrate (III) for voltammetric detection of l-cysteine. Int. J. Electrochem. Sci. 2016, 11, 7527–7539. [Google Scholar] [CrossRef]
- Areias, M.C.C.; Shimizu, K.; Compton, R.G. Cysteine determination: Via adsorptive stripping voltammetry using a bare glassy carbon electrode. Analyst 2016, 141, 5563–5570. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Abbaspour, A.; Ajamian, M. Determination of Cysteine at Bismuth Nanostructure—Carbon Ionic Liquid Electrode by Square Wave Voltammetry. Electroanalysis 2015, 27, 2335–2340. [Google Scholar] [CrossRef]
- Lee, P.T.; Thomson, J.E.; Karina, A.; Salter, C.; Johnston, C.; Davies, S.G.; Compton, R.G. Selective electrochemical determination of cysteine with a cyclotricatechylene modified carbon electrode. Analyst 2015, 140, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, M.A.; Karimi-Maleh, H.; Gupta, V.K. A Nanostructure Based Electrochemical Sensor for Square Wave Voltammetric Determination of l-Cysteine in the Presence of High Concentration of Folic Acid. Electroanalysis 2015, 27, 1766–1773. [Google Scholar] [CrossRef]
- Vladislavic’, N.; Brinic’, S.; Grubac, Z.; Buzuk, M. Study of bi film formation on different carbon based electrodes for possible applicability in electroanalytical determination of cysteine. Int. J. Electrochem. Sci. 2014, 9, 6020–6032. [Google Scholar]
- Aswini, K.K.; Mohan, A.M.V.; Biju, V.M. Molecularly imprinted polymer based electrochemical detection of l-cysteine at carbon paste electrode. Mater. Sci. Eng. C 2014, 37, 321–326. [Google Scholar] [CrossRef]
- Majd, S.M.; Teymourian, H.; Salimi, A. Fabrication of an electrochemical l-cysteine sensor based on graphene nanosheets decorated manganese oxide nanocomposite modified glassy carbon electrode. Electroanalysis 2013, 25, 2201–2210. [Google Scholar] [CrossRef]
- Liu, S.; Luo, T.; Li, L. Sensitive l-cysteine amperometric sensor based on a glassy carbon electrode modified by MnO2 nanoparticles. Instrum. Sci. Technol. 2013, 41, 382–393. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, W.; Liu, L.; Gao, F.; Li, M. The electrochemical determination of l-cysteine at a Ce-doped Mg-Al layered double hydroxide modified glassy carbon electrode. Electrochim. Acta 2012, 70, 193–198. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Hashemi, H.S. Voltammetric determination of cysteine using carbon paste electrode modified with Co(II)-Y zeolite. Talanta 2012, 88, 201–208. [Google Scholar] [CrossRef]
- Cumba, L.R.; Bicalho, U.O.; Carmo, D.R. Preparation and voltammetric studies of titanium (IV) Phosphate modified with silver hexacyanoferrate to a voltammetric determination of l-cysteine. Int. J. Electrochem. Sci. 2012, 7, 4465–4478. [Google Scholar]
- Xiao, C.; Chen, J.; Liu, B.; Chu, X.; Wu, L.; Yao, S. Sensitive and selective electrochemical sensing of l-cysteine based on a caterpillar-like manganese dioxide–carbon nanocomposite. Phys. Chem. Chem. Phys. 2011, 13, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yang, S.; Li, G.; Yang, R.; Li, J.; Yu, L. Preparation of yttrium hexacyanoferrate/carbon nanotube/Nafion nanocomposite film-modified electrode: Application to the electrocatalytic oxidation of l-cysteine. Electrochim. Acta 2011, 56, 2934–2940. [Google Scholar] [CrossRef]
- Long, Y.T.; Kong, C.; Li, D.W.; Li, Y.; Chowdhury, S.; Tian, H. Ultrasensitive determination of cysteine based on the photocurrent of nafion-functionalized CdS-MV quantum dots on an ITO electrode. Small 2011, 7, 1624–1628. [Google Scholar] [CrossRef]
- Kannan, P.; John, S.A. Ultrasensitive detection of l-cysteine using gold-5-amino-2-mercapto-1,3,4-thiadiazole core-shell nanoparticles film modified electrode. Biosens. Bioelectron. 2011, 30, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Ganguly, A.; Chen, L.C.; Chen, K.H. Direct voltammetric sensing of l-Cysteine at pristine GaN nanowires electrode. Biosens. Bioelectron. 2010, 26, 1688–1691. [Google Scholar] [CrossRef]
- Stabler, S.P.; Marcell, P.D.; Podell, E.R.; Allen, R.H. Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 1987, 162, 185–196. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Kawde, A.N.; Ibrahim, M. In-situ single-step electrochemical AgO modified graphite pencil electrode for trace determination of dl-methionine in human serum sample. Sens. Actuators B Chem. 2019, 281, 765–773. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Khorshidi, E. Preparation of Ru-Pt bimetallic monolayer on nanoporous gold film electrode and its application as an ultrasensitive sensor for determination of methionine. RSC Adv. 2017, 7, 21827–21836. [Google Scholar] [CrossRef] [Green Version]
- Murugavelu, M.; Karthikeyan, B. Synthesis, characterization of Ag-Au core-shell bimetal nanoparticles and its application for electrocatalytic oxidation/sensing of l-methionine. Mater. Sci. Eng. C 2017, 70, 656–664. [Google Scholar] [CrossRef]
- Perevezentseva, D.O.; Skirdin, K.V.; Gorchakov, E.V.; Bimatov, V.I. Electrochemical activity of methionine at graphite electrode modified with gold nanoparticles. Key Eng. Mater. 2016, 685, 563–568. [Google Scholar] [CrossRef]
- Li, Y.; Mei, S.; Liu, S.; Hun, X. A photoelectrochemical sensing strategy based on single-layer MoS2 modified electrode for methionine detection. J. Pharm. Biomed. Anal. 2019, 165, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H. Simultaneous electrochemical determination of dopamine, melatonin, methionine and caffeine. Sens. Actuators B Chem. 2015, 208, 195–203. [Google Scholar] [CrossRef]
- Sasikumar, R.; Chen, T.W.; Chen, S.M.; Rwei, S.P.; Yu, M.C. Facile synthesis of Mn2O3 for highly active catalytic oxidation and reduction of organic substances and electrochemical determination of l-Methionine. Int. J. Electrochem. Sci. 2018, 13, 4561–4574. [Google Scholar] [CrossRef]
- Pourbahman, F.; Sattarahmady, N.; Heli, H. Electrodeposition of Nickel Hydroxide Nanoparticles on Glassy Carbon Electrode-Applied to Electroanalysis of l-Methionine. Sens. Lett. 2016, 14, 65–71. [Google Scholar] [CrossRef]
- Chekin, F.; Bagheri, S.; Hamid, S.B.A. Synthesis of Pt doped TiO2 nanoparticles: Characterization and application for electrocatalytic oxidation of l-methionine. Sens. Actuators B Chem. 2013, 177, 898–903. [Google Scholar] [CrossRef]
- Jeevagan, A.J.; John, S.A. Electrochemical determination of l-methionine using the electropolymerized film of non-peripheral amine substituted Cu(II) phthalocyanine on glassy carbon electrode. Bioelectrochemistry 2012, 85, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tabeshnia, M.; Rashvandavei, M.; Amini, R.; Pashaee, F. Electrocatalytic oxidation of some amino acids on a cobalt hydroxide nanoparticles modified glassy carbon electrode. J. Electroanal. Chem. 2010, 647, 181–186. [Google Scholar] [CrossRef]
- Zor, E.; Saglam, M.E.; Alpaydin, S.; Bingol, H. A reduced graphene oxide/α-cyclodextrin hybrid for the detection of methionine: Electrochemical, fluorometric and computational studies. Anal. Methods 2014, 6, 6522–6530. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, C.; Li, S.; Zhang, R.; Yan, H.; Miao, H.; Fan, Y.; Yuan, B. Electrochemically controlling oxygen functional groups in graphene oxide for the optimization in the electro-catalytic oxidation of dihydroxybenzene isomers and l-methionine. J. Electroanal. Chem. 2014, 717, 219–224. [Google Scholar] [CrossRef]
- Gómez-Mingot, M.; Iniesta, J.; Montiel, V.; Kadara, R.O.; Banks, C.E. Direct oxidation of methionine at screen printed graphite macroelectrodes: Towards rapid sensing platforms. Sens. Actuators B Chem. 2011, 155, 831–836. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Grigor’Eva, L.; Morozov, M.; Gilmutdinov, A.; Budnikov, H. Electrochemical oxidation of sulfur-containing amino acids on an electrode modified with multi-walled carbon nanotubes. Microchim. Acta 2009, 165, 353–359. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; de Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A. Sensing of l-methionine in biological samples through fully 3D-printed electrodes. Anal. Chim. Acta 2021, 1142, 135–142. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H.; Hosseinzadeh, R.; Ranjbar, M. Preparation, Characterization and Electrochemical Application of ZnS/ZnAl2S4 Nanocomposite for Voltammetric Determination of Methionine and Tryptophan Using Modified Carbon Paste Electrode. Electroanalysis 2016, 28, 656–662. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohadesi, A.; Ghorbani, F.; Maleh, H.K.; Baghayeri, M.; Hosseinzadeh, R. Electrocatalytic measurement of methionine concentration with a carbon nanotube paste electrode modified with benzoylferrocene. Chin. J. Catal. 2013, 34, 1333–1338. [Google Scholar] [CrossRef]
- Revin, S.B.; John, S.A. Selective and Sensitive Electrochemical Sensor for l-Methionine at Physiological pH Using Functionalized Triazole Polymer Film Modified Electrode. Electroanalysis 2012, 24, 1277–1283. [Google Scholar] [CrossRef]
- Tan, W.T.; Goh, J.K. Electrochemical oxidation of methionine mediated by a fullerene-C 60 modified gold electrode. Electroanalysis 2008, 20, 2447–2453. [Google Scholar] [CrossRef]
- Idili, A.; Gerson, J.; Parolo, C.; Kippin, T.; Plaxco, K.W. An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan. Anal. Bioanal. Chem. 2019, 411, 4629. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F. Effects of age on serum tryptophan and urine indican in adults given a tryptophan load test. Eur. J. Drug Metab. Pharmacokinet. 1982, 7, 55–58. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Ghoreishi, F.S.; Mousavi, S. Voltammetric determination of tryptophan in the presence of uric acid and dopamine using carbon paste electrode modified with multi-walled carbon nanotubes. Arab. J. Chem. 2017, 10, S1546–S1552. [Google Scholar] [CrossRef] [Green Version]
- Roushani, M.; Sarabaegi, M. Novel electrochemical sensor based on carbon nanodots/chitosan nanocomposite for the detection of tryptophan. J. Iran. Chem. Soc. 2015, 12, 1875–1882. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Li, J.; Qu, L.; Harrington, P.B. A highly selective and sensitive electrochemical sensor for tryptophan based on the excellent surface adsorption and electrochemical properties of PSS functionalized graphene. Talanta 2019, 196, 309–316. [Google Scholar] [CrossRef]
- Sakthivel, R.; Mutharani, B.; Chen, S.M.; Kubendhiran, S.; Chen, T.W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. A simple and rapid electrochemical determination of l-tryptophan based on functionalized carbon black/poly-l-histidine nanocomposite. J. Electrochem. Soc. 2018, 165, B422–B430. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Lu, S.; Huang, C.; Su, W.; Zhou, S.; Sheng, J.; Huang, S. An electrochemical chiral sensor based on amino-functionalized graphene quantum dots/β-cyclodextrin modified glassy carbon electrode for enantioselective detection of tryptophan isomers. J. Iran. Chem. Soc. 2017, 14, 1957–1970. [Google Scholar] [CrossRef]
- Yu, L.Y.; Liu, Q.; Wu, X.W.; Jiang, X.Y.; Yu, J.G.; Chen, X.Q. Chiral electrochemical recognition of tryptophan enantiomers at a multi-walled carbon nanotube-chitosan composite modified glassy carbon electrode. RSC Adv. 2015, 5, 98020–98025. [Google Scholar] [CrossRef]
- Zou, J.; Huang, L.; Jiang, X.; Jiao, F.; Yu, J. Enhanced chiral electrochemical recognition of tryptophan enantiomers using a novel triple-layered GO/BSA/CS modified glassy carbon electrode. Nanosci. Nanotechnol. Lett. 2017, 9, 1700–1707. [Google Scholar] [CrossRef]
- Ou, J.; Tao, Y.; Xue, J.; Kong, Y.; Dai, J.; Deng, L. Electrochemical enantiorecognition of tryptophan enantiomers based on graphene quantum dots-chitosan composite film. Electrochem. Commun. 2015, 57, 5–9. [Google Scholar] [CrossRef]
- Ba, X.; Luo, L.; Ding, Y.; Liu, X. Determination of l-tryptophan in the presence of ascorbic acid and dopamine using poly(sulfosalicylic acid) modified glassy carbon electrode. Sens. Actuators B Chem. 2013, 187, 27–32. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.M.; Marikkani, S.; Elangovan, A.; Muthuraj, V. Green synthesis of a novel flower-like cerium vanadate microstructure for electrochemical detection of tryptophan in food and biological samples. J. Colloid Interface Sci. 2017, 496, 78–86. [Google Scholar] [CrossRef]
- Yang, X.; Kirsch, J.; Fergus, J.; Simonian, A. Modeling analysis of electrode fouling during electrolysis of phenolic compounds. Electrochim. Acta 2013, 94, 259–268. [Google Scholar] [CrossRef]
- Singh, V.V.; Nigam, A.K.; Batra, A.; Boopathi, M.; Singh, B.; Vijayaraghavan, R. Applications of Ionic Liquids in Electrochemical Sensors and Biosensors. Int. J. Electrochem. 2012, 2012, 165683. [Google Scholar] [CrossRef] [Green Version]
- Safavi, A.; Momeni, S. Electrocatalytic oxidation of tryptophan at gold nanoparticle-modified carbon ionic liquid electrode. Electroanalysis 2010, 22, 2848–2855. [Google Scholar] [CrossRef]
- Thomas, T.; Mascarenhas, R.J.; D’Souza, O.J.; Martis, P.; Dalhalle, J.; Swamy, B.E.K. Multi-walled carbon nanotube modified carbon paste electrode as a sensor for the amperometric detection of l-tryptophan in biological samples. J. Colloid Interface Sci. 2013, 402, 223–229. [Google Scholar] [CrossRef]
- Fiorucci, A.R.; Cervini, P.; Filho, O.F.; Cavalheiro, É.T.G. Tryptophan determination at carbon fiber ultramicroelectrodes by fast-scan cyclic voltammetry. J. Braz. Chem. Soc. 2013, 24, 1228–1236. [Google Scholar] [CrossRef]
- Rejithamol, R.; Krishnan, R.G.; Beena, S. Disposable pencil graphite electrode decorated with a thin film of electro-polymerized 2, 3, 4, 6, 7, 8, 9, 10-octahydropyrimido [1, 2-a] azepine for simultaneous voltammetric analysis of dopamine, serotonin and tryptophan. Mater. Chem. Phys. 2021, 258, 123857. [Google Scholar] [CrossRef]
- Deng, P.; Nie, X.; Wu, Y.; Tian, Y.; Li, J.; He, Q. A cost-saving preparation of nickel nanoparticles/nitrogen-carbon nanohybrid as effective advanced electrode materials for highly sensitive tryptophan sensor. Microchem. J. 2021, 160, 105744. [Google Scholar] [CrossRef]
- Zagitova, L.R.; Maistrenko, V.N.; Yarkaeva, Y.A.; Zagitov, V.V.; Zilberg, R.A.; Kovyazin, P.V.; Parfenova, L.V. Novel chiral voltammetric sensor for tryptophan enantiomers based on 3-neomenthylindene as recognition element. J. Electroanal. Chem. 2021, 880, 114939. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Tang, Z.; Wang, H.; Deng, P.; Tang, Y. Sensitive and selective determination of tryptophan using a glassy carbon electrode modified with nano-CeO2/reduced graphene oxide composite. Microchem. J. 2020, 159, 105367. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Zhang, X. Electrode material fabricated by loading cerium oxide nanoparticles on reduced graphene oxide and its application in electrochemical sensor for tryptophan. J. Alloys Compd. 2020, 842, 155934. [Google Scholar] [CrossRef]
- Mousavi, S.-F.; Alimoradi, M.; Shirmardi, A.; Zare-Shahabadi, V. Preparation, characterization and electrochemical application of an Ag/zeolite nanocomposite: Application to sub-micromolar quantitation of tryptophan. J. Porous Mater. 2020, 27, 1505–1514. [Google Scholar] [CrossRef]
- Yıldız, C.; Bayraktepe, D.E.; Yazan, Z. Electrochemical low-level detection of l-tryptophan in human urine samples: Use of pencil graphite leads as electrodes for a fast and cost-effective voltammetric method. Chem. Mon. 2020, 151, 871–879. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Torabi, F.; Baharifar, H.; Sajjadi-Jazi, S.M.; Afjeh, M.S.; Faridbod, F.; Larijani, B.; Khorramizadeh, M.R. Determination of the biomarker l-tryptophan level in diabetic and normal human serum based on an electrochemical sensing method using reduced graphene oxide/gold nanoparticles/18-crown-6. Anal. Bioanal. Chem. 2020, 412, 3615–3627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jamal, R.; Ge, Y.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Functionalized PProDOT@nitrogen-doped carbon hollow spheres composites for electrochemical sensing of tryptophan. Carbon 2020, 161, 842–855. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- GunaVathana, S.D.; Thivya, P.; Wilson, J.; Peter, A.C. Sensitive voltammetric sensor based on silver dendrites decorated polythiophene nanocomposite: Selective determination of l-Tryptophan. J. Mol. Struct. 2020, 1205, 127649. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Feng, J.; Wu, Y.; Tian, Y.; Li, G.; Chen, D. Sensitive Voltammetric Sensor for Tryptophan Detection by Using Polyvinylpyrrolidone Functionalized Graphene/GCE. Nanomaterials 2020, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Sangili, A.; Vinothkumar, V.; Chen, S.-M.; Veerakumar, P.; Chang, C.-W.; Muthuselvam, I.P.; Lin, K.-C. Highly Selective Voltammetric Sensor for l-Tryptophan Using Composite-Modified Electrode Composed of CuSn(OH)6 Microsphere Decorated on Reduced Graphene Oxide. J. Phys. Chem. C 2020, 124, 25821–25834. [Google Scholar] [CrossRef]
- Pandey, P.C.; Shukla, S.; Pandey, G.; Narayan, R.J. Organotrialkoxysilane-mediated synthesis of functional noble metal nanoparticles and their bimetallic for electrochemical recognition of l-tryptophan. MRS Adv. 2020, 5, 2429–2444. [Google Scholar] [CrossRef]
- Gao, J.; Li, H.; Li, M.; Wang, G.; Long, Y.; Li, P.; Li, C.; Yang, B. Polydopamine/graphene/MnO2 composite-based electrochemical sensor for in situ determination of free tryptophan in plants. Anal. Chim. Acta 2020, 1145, 103. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, F.; Zeng, B. A molecularly imprinted copolymer based electrochemical sensor for the highly sensitive detection of l-Tryptophan. Talanta 2020, 206, 120245. [Google Scholar] [CrossRef]
- Zhang, Y.; Waterhouse, G.I.N.; Xiang, Z.-p.; Che, J.; Chen, C.; Sun, W. A highly sensitive electrochemical sensor containing nitrogen-doped ordered mesoporous carbon (NOMC) for voltammetric determination of l-tryptophan. Food Chem. 2020, 326, 126976. [Google Scholar] [CrossRef]
- Erbilen, N.; Zor, E.; Saf, A.O.; Akgemci, E.G.; Bingol, H. An electrochemical chiral sensor based on electrochemically modified electrode for the enantioselective discrimination of d-/l-tryptophan. J. Solid State Electrochem. 2019, 23, 2695–2705. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.-F.; Pan, W.C.; Subramanian, B.; Ramalingam, R.J.; Al-lohedan, H. Facile sonochemical synthesis of perovskite-type SrTiO3 nanocubes with reduced graphene oxide nanocatalyst for an enhanced electrochemical detection of α-amino acid (tryptophan). Ultrason. Sonochem. 2019, 56, 193–199. [Google Scholar] [CrossRef]
- Prongmanee, W.; Alam, I.; Asanithi, P. Hydroxyapatite/Graphene oxide composite for electrochemical detection of l-Tryptophan. J. Taiwan Inst. Chem. Eng. 2019, 102, 415–423. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsakova, V.; Lete, C.; Marin, M.; Lupu, S. Poly(3,4-ethylenedioxythiophene)-modified electrodes for tryptophan voltammetric sensing. J. Electroanal. Chem. 2019, 848, 113309. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Mo, Z.; Liu, N.; Guo, R.; Pan, Z.; Liu, Z. Electrochemical chiral sensing of tryptophan enantiomers by using 3D nitrogen-doped reduced graphene oxide and self-assembled polysaccharides. Microchim. Acta 2019, 186, 557. [Google Scholar] [CrossRef] [PubMed]
- Kokulnathan, T.; Chen, T.-W.; Chen, S.-M.; Kumar, J.V.; Sakthinathan, S.; Nagarajan, E.R. Hydrothermal synthesis of silver molybdate/reduced graphene oxide hybrid composite: An efficient electrode material for the electrochemical detection of tryptophan in food and biological samples. Compos. Part B Eng. 2019, 169, 249–257. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Ding, Z.; Li, G.; Liu, J.; He, Q. A Simple and Efficient Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Tryptophan. Biomolecules 2019, 9, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Niu, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z. Perylene-functionalized graphene sheets modified with chitosan for voltammetric discrimination of tryptophan enantiomers. Microchim. Acta 2019, 186, 333. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials 2019, 9, 811. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Yi, Y.; Zhang, D.; Zhu, G. Electrochemical recognition of tryptophan enantiomers using a multi-walled carbon nanotube@polydopamine composite loaded with copper(II). Microchim. Acta 2019, 186, 358. [Google Scholar] [CrossRef]
- Duan, S.; Wang, W.; Yu, C.; Liu, M.; Yu, L. Development of Electrochemical Sensor for Detection of l-Tryptophan Based on Exfoliated Graphene/PEDOT:PSS. Nano 2019, 14, 1950058. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; Deng, P.; Chen, D. Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Rebekah, A.; Kokulnathan, T.; Wang, T.-J.; Viswanathan, C.; Ponpandian, N. MnCo2O4-rGO Hybrid Magnetic Nanocomposite Modified Glassy Carbon Electrode for Sensitive Detection of l-Tryptophan. J. Electrochem. Soc. 2019, 166, B845–B852. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; He, Q.; Yang, S.; Xie, M.; Deng, P.; Xia, Y.; Li, G. Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan. Biomolecules 2019, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, I.A.; Baccarin, M.; Cervini, P.; Cavalheiro, É.T.G. Electrochemical investigation of a graphite-polyurethane composite electrode modified with electrodeposited gold nanoparticles in the voltammetric determination of tryptophan. J. Electroanal. Chem. 2019, 835, 212–219. [Google Scholar] [CrossRef]
- Taleb, M.; Ivanov, R.; Bereznev, S.; Kazemi, S.H.; Hussainova, I. Alumina/graphene/Cu hybrids as highly selective sensor for simultaneous determination of epinephrine, acetaminophen and tryptophan in human urine. J. Electroanal. Chem. 2018, 823, 184–192. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Li, M.; Li, C.; Dai, H.; Sun, D.; Yang, B. Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sens. Actuators B Chem. 2018, 259, 433–442. [Google Scholar] [CrossRef]
- Song, J.; Yang, C.; Ma, J.; Han, Q.; Ran, P.; Fu, Y. Voltammetric chiral discrimination of tryptophan using a multilayer nanocomposite with implemented amino-modified β-cyclodextrin as recognition element. Microchim. Acta 2018, 185, 230. [Google Scholar] [CrossRef]
- Shamsipur, M.; Taherpour, A.; Sharghi, H.; Pashabadi, A. Transduction of interaction between trace tryptophan and surface-confined chromium salen using impedance spectroscopy. A sensing device that works based on highly selective inhibition of mediator’s Faradaic process. Anal. Chim. Acta 2018, 1030, 70–76. [Google Scholar] [CrossRef]
- Saini, A.K.; Saraf, M.; Kumari, P.; Mobin, S.M. A highly selective and sensitive chemosensor for l-tryptophan by employing a Schiff based Cu(II) complex. New J. Chem. 2018, 42, 3509–3518. [Google Scholar] [CrossRef]
- Rezaee, E.; Honarasa, F. Determination of Tryptophan by Using of Activated Multi-Walled Carbon Nanotube Ionic Liquid Electrode. Russ. J. Electrochem. 2018, 54, 1073–1080. [Google Scholar] [CrossRef]
- Porifreva, A.V.; Gorbatchuk, V.V.; Evtugyn, V.G.; Stoikov, I.I.; Evtugyn, G.A. Glassy Carbon Electrode Modified with Silver Nanodendrites Implemented in Polylactide-Thiacalix[4]arene Copolymer for the Electrochemical Determination of Tryptophan. Electroanalysis 2018, 30, 641–649. [Google Scholar] [CrossRef]
- Naganathan, D.; Thangamani, P.; Selvam, T.; Narayanasamy, T. Ce doped ZnO/f-MWCNT moss ball like nanocomposite: A strategy for high responsive current detection of l-tryptophan. Microchim. Acta 2018, 185, 96. [Google Scholar] [CrossRef]
- Mukdasai, S.; Poosittisak, S.; Ngeontae, W.; Srijaranai, S. A highly sensitive electrochemical determination of l-tryptophan in the presence of ascorbic acid and uric acid using in situ addition of tetrabutylammonium bromide on the ß-cyclodextrin incorporated multi-walled carbon nanotubes modified electrode. Sens. Actuators B Chem. 2018, 272, 518–525. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Hassanzadeh, M. Voltammetric determination of tryptophan using a carbon paste electrode modified with magnesium core shell nanocomposite and ionic liquids. Anal. Bioanal. Chem. Res. 2018, 5, 55–65. [Google Scholar]
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Pd–Cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim. Acta 2018, 260, 526–535. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Karikalan, N.; Chen, S.M.; Sundaresan, P.; Karthik, R. Synergistic activity of single crystalline bismuth sulfide and sulfur doped graphene towards the electrocatalysis of tryptophan. J. Catal. 2018, 367, 252–263. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Liu, X.; Tang, Y.; Zhu, J.; Hu, W.; Liu, X. A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(l-arginine) for simultaneous voltammetric determination of dopamine, serotonin and l-tryptophan. Microchim. Acta 2018, 185, 439. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Sarkary, A.; Khorablou, Z.; Dorraji, P.S. Electrochemical analysis of tryptophan using a nanostructuring electrode with multi-walled carbon nanotubes and cetyltrimethylammonium bromide nanocomposite. J. Nanostruct. 2018, 8, 266–275. [Google Scholar]
- Karimi, S.; Heydari, M. Voltammetric mixture analysis of tyrosine and tryptophan using carbon paste electrode modified by newly synthesized mesoporous silica nanoparticles and clustering of variable-partial least square: Efficient strategy for template extraction in mesoporous silica nanoparticle synthesis. Sens. Actuators B Chem. 2018, 257, 1134–1142. [Google Scholar]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, N. Magnetic graphene quantum dots as a functional nanomaterial towards voltammetric detection of l-tryptophan at physiological pH. J. Nanostruct. 2018, 8, 21–30. [Google Scholar]
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on reduced graphene oxide decorated with polypyrrole nanofibers and zinc oxide-copper oxide p-n junction heterostructures for the simultaneous voltammetric determination of ascorbic acid, dopamine, paracetamol, and tryptophan. New J. Chem. 2018, 42, 8512–8523. [Google Scholar]
- Bahmanzadeh, S.; Noroozifar, M. Fabrication of modified carbon paste electrodes with Ni-doped Lewatit FO36 nano ion exchange resin for simultaneous determination of epinephrine, paracetamol and tryptophan. J. Electroanal. Chem. 2018, 809, 153–162. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, Y.; Ding, Y.; Fu, R. An amperometric l-tryptophan sensor platform based on electrospun tricobalt tetroxide nanoparticles decorated carbon nanofibers. Sens. Actuators B Chem. 2017, 241, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, H.; Bagheri, H.; Monsef-Khoshhesab, Z.; Khoshsafar, H.; Hajian, A. Nanomolar simultaneous determination of tryptophan and melatonin by a new ionic liquid carbon paste electrode modified with SnO2-Co3O4@rGO nanocomposite. Mater. Sci. Eng. C 2017, 71, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, Z.; Sun, P.; Wang, R.; Zhao, X.; Wang, Q. Facile longitudinal unzipped multiwalled carbon nanotubes incorporated overoxidized poly(p-aminophenol) modified electrode for sensitive simultaneous determination of dopamine, uric acid and tryptophan. J. Electroanal. Chem. 2017, 801, 395–402. [Google Scholar] [CrossRef]
- Tığ, G.A. Development of electrochemical sensor for detection of ascorbic acid, dopamine, uric acid and l-tryptophan based on Ag nanoparticles and poly(l-arginine)-graphene oxide composite. J. Electroanal. Chem. 2017, 807, 19–28. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M.; Karimi-Maleh, H. Incorporation of graphene oxide–NiO nanocomposite and n-hexyl-3-methylimidazolium hexafluoro phosphate into carbon paste electrode: Application as an electrochemical sensor for simultaneous determination of benserazide, levodopa and tryptophan. J. Iran. Chem. Soc. 2017, 14, 955–961. [Google Scholar] [CrossRef]
- Nunes da Silva, D.; Tarley, C.R.T.; Pereira, A.C. Development of a Sensor Based on Modified Carbon Paste with Com Iron(III) Protoporphyrin Immobilized on SiNbZn Silica Matrix for l-tryptophan Determination. Electroanalysis 2017, 29, 2793–2802. [Google Scholar] [CrossRef]
- Narouei, F.H.; Tammandani, H.K.; Ghalandarzehi, Y.; Sabbaghi, N.; Noroozifar, M. An electrochemical sensor based on conductive polymers/graphite paste electrode for simultaneous determination of dopamine, uric acid and tryptophan in biological samples. Int. J. Electrochem. Sci. 2017, 12, 7739–7753. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Di, J.; Long, Y.; Li, W.; Tu, Y. Graphene-like carbon nitride nanosheet as a novel sensing platform for electrochemical determination of tryptophan. J. Colloid Interface Sci. 2017, 505, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Yeon, S.H.; Huh, Y.S.; Han, Y.K. Electrochemical determination of tryptophan using a glassy carbon electrode modified with flower-like structured nanocomposite consisting of reduced graphene oxide and SnO2. Sens. Actuators B Chem. 2017, 239, 1221–1230. [Google Scholar] [CrossRef]
- Chen, J.; He, P.; Bai, H.; He, S.; Zhang, T.; Zhang, X.; Dong, F. Poly(β-cyclodextrin)/carbon quantum dots modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of dopamine, uric acid and tryptophan. Sens. Actuators B Chem. 2017, 252, 9–16. [Google Scholar] [CrossRef]
- Habibi, B.; Ayazi, Z.; Dadkhah, M. Multi-walled carbon nanotubes/ionic liquid nanocomposite modified carbonceramic electrode: Electrochemistry and measurement of tryptophan in the presence of uric acid. Anal. Bioanal. Chem. Res. 2017, 4, 155–169. [Google Scholar]
- Ghoreishi, S.M.; Malekian, M. Curve resolution on overlapped voltammograms for simultaneous determination of tryptophan and tyrosine at carbon paste electrode modified with ZnFe2O4 nanoparticles. J. Electroanal. Chem. 2017, 805, 1–10. [Google Scholar] [CrossRef]
- Alizadeh, T.; Amjadi, S. A tryptophan assay based on the glassy carbon electrode modified with a nano-sized tryptophan-imprinted polymer and multi-walled carbon nanotubes. New J. Chem. 2017, 41, 4493–4502. [Google Scholar] [CrossRef]
- Yokuş, Ö.A.; Kardaş, F.; Akyildirim, O.; Eren, T.; Atar, N.; Yola, M.L. Sensitive voltammetric sensor based on polyoxometalate/reduced graphene oxide nanomaterial: Application to the simultaneous determination of l-tyrosine and l-tryptophan. Sens. Actuators B Chem. 2016, 233, 47–54. [Google Scholar] [CrossRef]
- Yang, Y.J.; Yu, X. Cetyltrimethylammonium bromide assisted self-assembly of phosphotungstic acid on graphene oxide nanosheets for selective determination of tryptophan. J. Solid State Electrochem. 2016, 20, 1697–1704. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q.; Xuan, C.; Xia, Q.; Lin, X.; Fu, Y. Chiral Recognition of Tryptophan Enantiomers Based on β-Cyclodextrin-platinum Nanoparticles/Graphene Nanohybrids Modified Electrode. Electroanalysis 2016, 28, 868–873. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, S.; Huang, C.; Su, W.; Huang, S. Novel N-doped carbon dots/β-cyclodextrin nanocomposites for enantioselective recognition of tryptophan enantiomers. Sensors 2016, 16, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ouyang, X.; Ding, Y.; Liu, B.; Xu, D.; Liao, L. An electrochemical sensor for determination of tryptophan in the presence of da based on poly(l-methionine)/graphene modified electrode. RSC Adv. 2016, 6, 10662–10669. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Liu, Z.; Guo, Y.; Li, C.; Dong, C.; Shuang, S. An ultra-sensitive sensor based on β-cyclodextrin modified magnetic graphene oxide for detection of tryptophan. J. Electroanal. Chem. 2016, 781, 363–370. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, R.; Zhang, H.; Zheng, J. A l-tryptophan sensor based on tellurium nanorods modified glassy carbon electrode. J. Iran. Chem. Soc. 2016, 13, 1189–1195. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.M.; Goyal, R.N. A facile method to anchor reduced graphene oxide polymer nanocomposite on the glassy carbon surface and its application in the voltammetric estimation of tryptophan in presence of 5-hydroxytryptamine. Sens. Actuators B Chem. 2016, 233, 445–453. [Google Scholar]
- Tashkhourian, J.; Daneshi, M.; Nami-Ana, S.F. Simultaneous determination of tyrosine and tryptophan by mesoporous silica nanoparticles modified carbon paste electrode using H-point standard addition method. Anal. Chim. Acta 2016, 902, 89–96. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Coupling of NiO nanoparticles and room temperature ionic liquid for fabrication of highly sensitive voltammetric sensor in tryptophan analysis. Anal. Bioanal. Electrochem. 2016, 8, 578–588. [Google Scholar]
- Peng, Z.; Jiang, Z.; Huang, X.; Li, Y. A novel electrochemical sensor of tryptophan based on silver nanoparticles/metal-organic framework composite modified glassy carbon electrode. RSC Adv. 2016, 6, 13742–13748. [Google Scholar] [CrossRef]
- Khaleghi, F.; Irai, A.E.; Gupta, V.K.; Agarwal, S.; Bijad, M.; Abbasghorbani, M. Highly sensitive nanostructure voltammetric sensor employing Pt/CNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate for determination of tryptophan in food and pharmaceutical samples. J. Mol. Liq. 2016, 223, 431–435. [Google Scholar] [CrossRef]
- Jarošová, R.; Rutherford, J.; Swain, G.M. Evaluation of a nitrogen-incorporated tetrahedral amorphous carbon thin film for the detection of tryptophan and tyrosine using flow injection analysis with amperometric detection. Analyst 2016, 141, 6031–6041. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Su, W.; Wang, T.; Hu, J. Electrochemical sensor of tryptophan based on an Ag/CP electrode prepared by the filtered cathodic vacuum arc technique. J. Electrochem. Soc. 2016, 163, B107–B112. [Google Scholar] [CrossRef]
- Yang, F.; Xie, Q.; Zhang, H.; Yu, S.; Zhang, X.; Shen, Y. Simultaneous determination of ascorbic acid, uric acid, tryptophan and adenine using carbon-supported NiCoO2 nanoparticles. Sens. Actuators B Chem. 2015, 210, 232–240. [Google Scholar] [CrossRef]
- Wang, C.; Zou, X.; Zhao, X.; Wang, Q.; Tan, J.; Yuan, R. Cu-nanoparticles incorporated overoxidized-poly(3-amino-5-mercapto-1,2,4-triazole) film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. J. Electroanal. Chem. 2015, 741, 36–41. [Google Scholar] [CrossRef]
- Taei, M.; Shavakhi, M.; Hadadzadeh, H.; Movahedi, M.; Rahimi, M.; Habibollahi, S. Simultaneous determination of epinephrine, acetaminophen, and tryptophan using Fe2O3(0.5)/SnO2(0.5) nanocomposite sensor. J. Appl. Electrochem. 2015, 45, 185–195. [Google Scholar] [CrossRef]
- Tadayon, F.; Sepehri, Z. A new electrochemical sensor based on a nitrogen-doped graphene/CuCo2O4 nanocomposite for simultaneous determination of dopamine, melatonin and tryptophan. RSC Adv. 2015, 5, 65560–65568. [Google Scholar] [CrossRef]
- Kim, S.; Sun, S.; Li, Y.; He, X. One-pot synthesis of graphene oxide coated with sol-gel for electrochemical determination of tryptophan. Anal. Methods 2015, 7, 6352–6359. [Google Scholar] [CrossRef]
- Baytak, A.K.; Aslanoglu, M. Voltammetric quantification of tryptophan using a MWCNT modified GCE decorated with electrochemically produced nanoparticles of nickel. Sens. Actuators B Chem. 2015, 220, 1161–1168. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Zhao, X.E.; Wang, H.; Xu, G.; You, J. Electrochemical behavior and voltammetric determination of l-tryptophan and l-tyrosine using a glassy carbon electrode modified with single-walled carbon nanohorns. Microchim. Acta 2014, 181, 445–451. [Google Scholar] [CrossRef]
- Xia, X.; Zheng, Z.; Zhang, Y.; Zhao, X.; Wang, C. Synthesis of Ag-MoS2/chitosan nanocomposite and its application for catalytic oxidation of tryptophan. Sens. Actuators B Chem. 2014, 192, 42–50. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Xu, W.; Zhang, W.; Ying, X. An Electrochemical Sensor for l-Tryptophan Using a Molecularly Imprinted Polymer Film Produced by Copolymerization of o-Phenylenediamine and Hydroquinone. Anal. Lett. 2014, 47, 1712–1725. [Google Scholar] [CrossRef]
- Rajalakshmi, K.; John, S.A. Sensitive and selective determination of l-tryptophan at physiological pH using functionalized multiwalled carbon nanotubes-nanostructured conducting polymer composite modified electrode. J. Electroanal. Chem. 2014, 734, 31–37. [Google Scholar] [CrossRef]
- Pang, T.T.; Cai, Z.F.; Liu, H.L.; Du, L.M.; Guo, M.D.; Fu, Y.L. Determination of Tryptophan using a p-Sulfonated Calix [4,6,8]arene Modified Gold Electrode. Anal. Lett. 2014, 47, 1808–1820. [Google Scholar] [CrossRef]
- Divya, P.; Sudarvizhi, A.; Pandian, K. Single step synthesis of gold nanoparticles decorated graphene oxide using aniline as reducing agent and study its application on elecrocatalytic detection of tryptophan. Adv. Mater. Res. 2014, 938, 182–191. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Feng, Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater. Sci. Eng. C 2014, 35, 54–60. [Google Scholar] [CrossRef]
- Boonchiangma, S.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. A highly selective electrochemical sensor for l-tryptophan based on a screen-printed carbon electrode modified with poly-p-phenylenediamine and CdS quantum dots. J. Appl. Polym. Sci. 2014, 131, 40356. [Google Scholar] [CrossRef]
- Wu, F.H.; Chen, L.; Chu, X.F.; Wei, X.W. Synthesis of ruthenium xanthate complex and its electrocatalytic activity for tryptophan oxidation. Chem. Res. Chin. Univ. 2013, 29, 574–578. [Google Scholar] [CrossRef]
- Majidi, M.R.; Salimi, A.; Alipour, E. Development of voltammetric sensor for determination of tryptophan using MWCNTs-modified sol-gel electrode. J. Chin. Chem. Soc. 2013, 60, 1473–1478. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Liu, Y.; Yang, Z. A sensitive sensor for determination of l-tryptophan based on gold nanoparticles/poly(alizarin red S)-modified glassy carbon electrode. J. Solid State Electrochem. 2013, 17, 2623–2631. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Thomas, T.; Namboothiri, I.N.N.; Rajamathi, M.; Martis, P.; Dalhalle, J. Electrochemical determination of l-Tryptophan based on a multiwall carbon nanotube/Mg-Al layered double hydroxide modified carbon paste electrode as a sensor. J. Electroanal. Chem. 2013, 704, 220–226. [Google Scholar] [CrossRef]
- Deng, K.-Q.; Zhou, J.-H.; Li, X.-F. Direct electrochemical reduction of graphene oxide and its application to determination of l-tryptophan and l-tyrosine. Colloids Surf. B Biointerfaces 2013, 101, 183–188. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, H.; Bo, X.; Guo, L. Electrochemical sensor for amino acids based on gold nanoparticles/macroporous carbon composites modified glassy carbon electrode. J. Electroanal. Chem. 2012, 687, 117–122. [Google Scholar] [CrossRef]
- Ye, D.; Luo, L.; Ding, Y.; Liu, B.; Liu, X. Fabrication of Co3O4 nanoparticles-decorated graphene composite for determination of l-tryptophan. Analyst 2012, 137, 2840–2845. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ma, M.; Ma, Y. Electrochemical determination of tryptophan based on silicon dioxide nanopartilces modified carbon paste electrode. Russ. J. Electrochem. 2012, 48, 489–494. [Google Scholar] [CrossRef]
- Xu, C.-X.; Huang, K.-J.; Fan, Y.; Wu, Z.-W.; Li, J.; Gan, T. Simultaneous electrochemical determination of dopamine and tryptophan using a TiO2-graphene/poly(4-aminobenzenesulfonic acid) composite film based platform. Mater. Sci. Eng. C 2012, 32, 969–974. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Guo, Y.; Liu, W.; Dong, C.; Wu, Y.; Li, S.; Shuang, S. β-Cyclodextrin/Fe3O4 hybrid magnetic nano-composite modified glassy carbon electrode for tryptophan sensing. Sens. Actuators B Chem. 2012, 163, 171–178. [Google Scholar] [CrossRef]
- Wan, M.; Li, W.; Long, Y.; Tu, Y. Electrochemical determination of tryptophan based on Si-doped nano-TiO2 modified glassy carbon electrode. Anal. Methods 2012, 4, 2860–2865. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y. A novel approach for the selective determination of tryptophan in blood serum in the presence of tyrosine based on the electrochemical reduction of oxidation product of tryptophan formed in situ on graphite electrode. Biosens. Bioelectron. 2012, 31, 26–31. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Kuang, Y.; Deng, P.; Zhang, S.; Xu, J. A carbon paste electrode modified with a cobalt(II) coordination polymer for the direct voltammetric determination of tryptophan. Microchim. Acta 2012, 176, 455–461. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, Y.; Li, W.; Deng, P.; Deng, J. Carbon paste electrode modified with a binuclear manganese complex as a sensitive voltammetric sensor for tryptophan. Microchim. Acta 2011, 174, 239–245. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Bayat, M. Pyrolytic graphite electrode modified with a thin film of a graphite/diamond nano-mixture for highly sensitive voltammetric determination of tryptophan and 5-hydroxytryptophan. Microchim. Acta 2011, 174, 361–366. [Google Scholar] [CrossRef]
- Prabhu, P.; Babu, R.S.; Narayanan, S.S. Electrocatalytic oxidation of l-tryptophan using copper hexacyanoferrate film modified gold nanoparticle graphite-wax electrode. Colloids Surf. B Biointerfaces 2011, 87, 103–108. [Google Scholar] [CrossRef]
- Kia, M.; Islamnezhad, A.; Shariati, S.; Biparva, P. Preparation of voltammetric biosensor for tryptophan using multi-walled carbon nanotubes. Korean J. Chem. Eng. 2011, 28, 2064–2068. [Google Scholar] [CrossRef]
- Güney, S.; Yildiz, G. Determination of tryptophan using electrode modified with poly(9-aminoacridine) functionalized multi-walled carbon nanotubes. Electrochim. Acta 2011, 57, 290–296. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Pashabadi, A.; Azadbakht, A.; Menati, S. A nano-structured Ni(II)-ACDA modified gold nanoparticle self-assembled electrode for electrocatalytic oxidation and determination of tryptophan. Electrochim. Acta 2011, 56, 4022–4030. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.H.; Lu, H.T.; Zhang, Q. Electrochemistry and voltammetric determination of l-tryptophan and l-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Microchim. Acta 2011, 173, 241–247. [Google Scholar] [CrossRef]
- Szunerits, S.; Coffinier, Y.; Galopin, E.; Brenner, J.; Boukherroub, R. Preparation of boron-doped diamond nanowires and their application for sensitive electrochemical detection of tryptophan. Electrochem. Commun. 2010, 12, 438–441. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Gaichore, R.R. Macrocyclic compounds based chemically modified electrodes for voltammetric determination of l-tryptophan using electrocatalytic oxidation. Anal. Lett. 2010, 43, 1933–1950. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, W.; Jiao, K. Electrochemical behavior and determination of l-tryptophan on carbon ionic liquid electrode. J. Anal. Chem. 2010, 65, 648–651. [Google Scholar] [CrossRef]
- Huang, K.J.; Xu, C.X.; Sun, J.Y.; Xie, W.Z.; Peng, L. Electrochemical oxidation of tryptophan and its analysis in pharmaceutical formulations at a poly(methyl red) film-modified electrode. Anal. Lett. 2010, 43, 176–185. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Fang, Y.; Dong, S. Gold nanoparticle/carbon nanotube hybrids as an enhanced material for sensitive amperometric determination of tryptophan. Electrochim. Acta 2010, 55, 3927–3931. [Google Scholar] [CrossRef]
- Shumyantseva, V.; Bulko, T.; Kuzikov, A.; Masamrekh, R.; Archakov, A. Analysis of l-tyrosine based on electrocatalytic oxidative reactions via screen-printed electrodes modified with multi-walled carbon nanotubes and nanosized titanium oxide (TiO2). Amino Acids 2018, 50, 823–829. [Google Scholar] [CrossRef]
- Maes, M.; Verkerk, R.; Vandoolaeghe, E.; van Hunsel, F.; Neels, H.; Wauters, A.; Demedts, P.; Scharpé, S. Serotonin-immune interactions in major depression: Lower serum tryptophan as a marker of an immune-inflammatory response. Eur. Arch. Psychiatry Clin. Neurosci. 1997, 247, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Mayersbach, P.; Becker, K.; Schennach, H.; Fuchs, D.; Gostner, J.M. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines 2015, 26, 31. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health. Clinical Review Report: Nitisinone (Nitisinone Tablets): (Cycle Pharmaceuticals Ltd.): Indication: For the Treatment of Patients with Hereditary Tyrosinemia Type 1 in Combination with Dietary Restriction of Tyrosine and Phenylalanine [Internet]; Appendix 4, Validity of Outcome Measures; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Tallan, H.H.; Bella, S.T.; Stein, W.H.; Moore, S. Tyrosine-O-sulfate as a constituent of normal human urine. J. Biol. Chem. 1955, 217, 703–708. [Google Scholar] [CrossRef]
- Holme, E.; Lindstedt, S. Nontransplant treatment of tyrosinemia. Clin. Liver Dis. 2000, 4, 805–814. [Google Scholar] [CrossRef]
- Baig, N.; Kawde, A.N. A novel, fast and cost effective graphene-modified graphite pencil electrode for trace quantification of l-tyrosine. Anal. Methods 2015, 7, 9535–9541. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Satpati, A.K.; Aiman, L.V.; Mekhalif, Z. Electrocatalytic oxidation of l-tyrosine at carboxylic acid functionalized multi-walled carbon nanotubes modified carbon paste electrode. Ionics 2016, 22, 405–414. [Google Scholar] [CrossRef]
- Yu, X.; Mai, Z.; Xiao, Y.; Zou, X. Electrochemical behavior and determination of l-tyrosine at single-walled carbon nanotubes modified glassy carbon electrode. Electroanalysis 2008, 20, 1246–1251. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Talebi, F. Graphene Quantum Dots Incorporated into β-cyclodextrin: A Novel Polymeric Nanocomposite for Non-Enzymatic Sensing of l-Tyrosine at Physiological pH. J. Anal. Chem. 2018, 73, 602–612. [Google Scholar] [CrossRef]
- Wang, Q.; Vasilescu, A.; Subramanian, P.; Vezeanu, A.; Andrei, V.; Coffinier, Y.; Li, M.; Boukherroub, R.; Szunerits, S. Simultaneous electrochemical detection of tryptophan and tyrosine using boron-doped diamond and diamond nanowire electrodes. Electrochem. Commun. 2013, 35, 84–87. [Google Scholar] [CrossRef]
- Habibi, E.; Heidari, H. Renewable Surface Carbon-Composite Electrode Bulk Modified with GQD-RuCl3 Nano-Composite for High Sensitive Detection of l-Tyrosine. Electroanalysis 2016, 28, 2559–2564. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, C.; Zhou, L.; Wu, L.; Bian, K.; Zeng, J.; Wang, J.; Feng, Z.; Yin, Y.; Cao, Z. Highly sensitive determination of l-tyrosine in pig serum based on ultrathin CuS nanosheets composite electrode. Biosens. Bioelectron. 2019, 140, 111356. [Google Scholar] [CrossRef]
- Gu, W.; Wang, M.; Mao, X.; Wang, Y.; Li, L.; Xia, W. A facile sensitive l-tyrosine electrochemical sensor based on a coupled CuO/Cu2O nanoparticles and multi-walled carbon nanotubes nanocomposite film. Anal. Methods 2015, 7, 1313–1320. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Satpati, A.K.; Namboothiri, I.N.N.; Detriche, S.; Mekhalif, Z.; Delhalle, J. A multi-walled carbon nanotube/poly-2,6-dichlorophenolindophenol film modified carbon paste electrode for the amperometric determination of l-tyrosine. RSC Adv. 2015, 5, 91472–91481. [Google Scholar] [CrossRef]
- Quintana, C.; Suárez, S.; Hernández, L. Nanostructures on gold electrodes for the development of an l-tyrosine electrochemical sensor based on host-guest supramolecular interactions. Sens. Actuators B Chem. 2010, 149, 129–135. [Google Scholar] [CrossRef]
- Ma, Q.; Ai, S.; Yin, H.; Chen, Q.; Tang, T. Towards the conception of an amperometric sensor of l-tyrosine based on Hemin/PAMAM/MWCNT modified glassy carbon electrode. Electrochim. Acta 2010, 55, 6687–6694. [Google Scholar] [CrossRef]
- Chekin, F.; Bagheri, S. Tyrosine sensing on phthalic anhydride functionalized chitosan and carbon nanotube film coated glassy carbon electrode. Russ. J. Electrochem. 2016, 52, 174–180. [Google Scholar] [CrossRef]
- Zou, J.; Mao, D.; Wee, A.T.S.; Jiang, J. Micro/nano-structured ultrathin g-C3N4/Ag nanoparticle hybrids as efficient electrochemical biosensors for l-tyrosine. Appl. Surf. Sci. 2019, 467, 608–618. [Google Scholar] [CrossRef]
- Zhao, G.; Qi, Y.; Tian, Y. Simultaneous and direct determination of tryptophan and tyrosine at boron-doped diamond electrode. Electroanalysis 2006, 18, 830–834. [Google Scholar] [CrossRef]
- Zribi, R.; Maalej, R.; Gillibert, R.; Donato, M.G.; Gucciardi, P.G.; Leonardi, S.G.; Neri, G. Simultaneous and selective determination of dopamine and tyrosine in the presence of uric acid with 2D-MoS2 nanosheets modified screen-printed carbon electrodes. FlatChem 2020, 24, 100187. [Google Scholar] [CrossRef]
- Zhao, J.; Cong, L.; Ding, Z.; Zhu, X.; Zhang, Y.; Li, S.; Liu, J.; Chen, X.; Hou, H.; Fan, Z.; et al. Enantioselective electrochemical sensor of tyrosine isomers based on macroporous carbon embedded with sulfato-β-Cyclodextrin. Microchem. J. 2020, 159, 105469. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Feng, P.-Q.; Guo, Z.; Wei, X. Water-STable 1D Double-Chain Cu Metal–Organic Framework-Based Electrochemical Biosensor for Detecting l-Tyrosine. Langmuir 2020, 36, 14123–14129. [Google Scholar] [CrossRef]
- Zou, J.; Lan, X.-W.; Zhao, G.-Q.; Huang, Z.-N.; Liu, Y.-P.; Yu, J.-G. Immobilization of 6-O-α-maltosyl-β-cyclodextrin on the surface of black phosphorus nanosheets for selective chiral recognition of tyrosine enantiomers. Microchim. Acta 2020, 187, 636. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Uddin, M.T.; Asiri, A.M.; Rahman, M.M.; Islam, M.A. Detection of l-Tyrosine by electrochemical method based on binary mixed CdO/SnO2 nanoparticles. Measurement 2020, 163, 107990. [Google Scholar] [CrossRef]
- Zou, H.-Y.; Lu, X.-Y.; Kong, F.-Y.; Wang, Z.-X.; Li, H.-Y.; Fang, H.-L.; Wang, W. A voltammetric sensor based on reduced graphene oxide-hemin-Ag nanocomposites for sensitive determination of tyrosine. RSC Adv. 2020, 10, 28026–28031. [Google Scholar] [CrossRef]
- Cioates Negut, C.; Stefanov, C.; van Staden, J.F. Graphite Based Microsensors Developed for the Electrochemical Determination of l-Tyrosine from Pharmaceutical Samples. Electroanalysis 2020, 32, 1488–1497. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Y.; Lin, L.; Chen, H.; Zhao, M. Au nanoparticles @metal organic framework/polythionine loaded with molecularly imprinted polymer sensor: Preparation, characterization, and electrochemical detection of tyrosine. J. Electroanal. Chem. 2020, 863, 114052. [Google Scholar] [CrossRef]
- Khoobi, A.; Shahdost-fard, F.; Arbabi, M.; Akbari, M.; Mirzaei, H.; Nejati, M.; Lotfinia, M.; Sobhani-Nasab, A.; Banafshe, H.R. Sonochemical synthesis of ErVO4/MnWO4 heterostructures: Application as a novel nanostructured surface for electrochemical determination of tyrosine in biological samples. Polyhedron 2020, 177, 114302. [Google Scholar] [CrossRef]
- Zribi, R.; Maalej, R.; Messina, E.; Gillibert, R.; Donato, M.G.; Maragò, O.M.; Gucciardi, P.G.; Leonardi, S.G.; Neri, G. Exfoliated 2D-MoS2 nanosheets on carbon and gold screen printed electrodes for enzyme-free electrochemical sensing of tyrosine. Sens. Actuators B Chem. 2020, 303, 127229. [Google Scholar] [CrossRef]
- Karthika, A.; Rosaline, D.R.; Inbanathan, S.S.R.; Suganthi, A.; Rajarajan, M. Fabrication of Cupric oxide decorated β-cyclodextrin nanocomposite solubilized Nafion as a high performance electrochemical sensor for l-tyrosine detection. J. Phys. Chem. Solids 2020, 136, 109145. [Google Scholar] [CrossRef]
- Feng, J.; Deng, P.; Xiao, J.; Li, J.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. New voltammetric method for determination of tyrosine in foodstuffs using an oxygen-functionalized multi-walled carbon nanotubes modified acetylene black paste electrode. J. Food Compos. Anal. 2021, 96, 103708. [Google Scholar] [CrossRef]
- Parsa, A.; Akbarzadeh-Torbati, N.; Beitollahi, H. Rapid and sensitive electrochemical monitoring of tyrosine using NiO nanoparticles modified graphite screen printed electrode. Int. J. Electrochem. Sci. 2019, 14, 1556–1565. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F. Simultaneous determination of l-DOPA, l-tyrosine and uric acid by cysteic acid—Modified glassy carbon electrode. Mater. Sci. Eng. C 2019, 98, 496–502. [Google Scholar] [CrossRef]
- Arani, N.H.; Ghoreishi, S.M.; Khoobi, A. Increasing the electrochemical system performance using a magnetic nanostructured sensor for simultaneous determination of l-tyrosine and epinephrine. Anal. Methods 2019, 11, 1159–1167. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, M.; Liu, W.; Yu, S.; Niu, L.; Li, G.; Li, H.; Liu, W. Electrochemical sensor based on molecularly imprinted polymer/reduced graphene oxide composite for simultaneous determination of uric acid and tyrosine. J. Electroanal. Chem. 2018, 813, 75–82. [Google Scholar] [CrossRef]
- Heidari, H.; Habibi, E. Lead-doped carbon ceramic electrode as a renewable surface composite electrode for the preparation of lead dioxide film and detection of l-tyrosine. J. Iran. Chem. Soc. 2018, 15, 885–892. [Google Scholar] [CrossRef]
- García-Carmona, L.; Moreno-Guzmán, M.; Sierra, T.; González, M.C.; Escarpa, A. Filtered carbon nanotubes-based electrodes for rapid sensing and monitoring of l-tyrosine in plasma and whole blood samples. Sens. Actuators B Chem. 2018, 259, 762–767. [Google Scholar] [CrossRef]
- Ermiş, N.; Tinkiliç, N. Preparation of molecularly imprinted polypyrrole modified gold electrode for determination of tyrosine in biological samples. Int. J. Electrochem. Sci. 2018, 13, 2286–2298. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, L.; Tan, D.; Chen, S.; Gan, Y. Sonochemical synthesis of “sea-island” structure silver/polyaniline nanocomposites for the detection of l-tyrosine. J. Thermoplast. Compos. Mater. 2017, 30, 1033–1044. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of Tyrosine Based on a Novel Magnetic Electrochemical Chiral Sensor. Electrochim. Acta 2017, 241, 386–394. [Google Scholar] [CrossRef]
- Tian, F.; Li, H.; Li, M.; Li, C.; Lei, Y.; Yang, B. A tantalum electrode coated with graphene nanowalls for simultaneous voltammetric determination of dopamine, uric acid, l-tyrosine, and hydrochlorothiazide. Microchim. Acta 2017, 184, 1611–1619. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ganjali, M.R.; Norouzi, P.; Bananezhad, A. Amplified nanostructure electrochemical sensor for simultaneous determination of captopril, acetaminophen, tyrosine and hydrochlorothiazide. Mater. Sci. Eng. C 2017, 73, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Sheikhshoaie, I. rGO/ZnO nanocomposite modified carbon paste electrode as sensor for tyrosine analysis. Anal. Bioanal. Electrochem. 2017, 9, 834–840. [Google Scholar]
- Narayanan, K.R.; Sadanandhan, N.K.; Devaki, S.J. Silver Patterned Supramolecular Liquid Crystalline Gels as Electrochemical Sensor of Tyrosine. ChemistrySelect 2017, 2, 320–328. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, M.; Li, X.; Zhang, C. Voltammetric Separation and Determination of Glutathione and l-tyrosine with Chlorogenic Acid as an Electrocatalytic Mediator. Electroanalysis 2017, 29, 1141–1146. [Google Scholar] [CrossRef]
- Foroughi, M.M.; Tajik, S. SiO2@Fe3O4 nanocomposite decorated graphene modified carbon ionic liquid electrode as an electrochemical sensor for the determination of tyrosine. Anal. Bioanal. Electrochem. 2017, 9, 495–505. [Google Scholar]
- Dong, S.; Bi, Q.; Qiao, C.; Sun, Y.; Zhang, X.; Lu, X.; Zhao, L. Electrochemical sensor for discrimination tyrosine enantiomers using graphene quantum dots and β-cyclodextrins composites. Talanta 2017, 173, 94–100. [Google Scholar] [CrossRef]
- Shrestha, S.; Mascarenhas, R.J.; D’Souza, O.J.; Satpati, A.K.; Mekhalif, Z.; Dhason, A.; Martis, P. Amperometric sensor based on multi-walled carbon nanotube and poly (Bromocresol purple) modified carbon paste electrode for the sensitive determination of l-tyrosine in food and biological samples. J. Electroanal. Chem. 2016, 778, 32–40. [Google Scholar] [CrossRef]
- Yola, M.L.; Eren, T.; Atar, N. A sensitive molecular imprinted electrochemical sensor based on gold nanoparticles decorated graphene oxide: Application to selective determination of tyrosine in milk. Sens. Actuators B Chem. 2015, 210, 149–157. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lopa, N.S.; Kim, K.; Lee, J.J. Selective detection of l-tyrosine in the presence of ascorbic acid, dopamine, and uric acid at poly(thionine)-modified glassy carbon electrode. J. Electroanal. Chem. 2015, 754, 87–93. [Google Scholar] [CrossRef]
- Razavian, A.S.; Ghoreishi, S.M.; Esmaeily, A.S.; Behpour, M.; Monzon, L.M.A.; Coey, J.M.D. Simultaneous sensing of l-tyrosine and epinephrine using a glassy carbon electrode modified with nafion and CeO2 nanoparticles. Microchim. Acta 2014, 181, 1947–1955. [Google Scholar] [CrossRef]
- Kanchana, P.; Lavanya, N.; Sekar, C. Development of amperometric l-tyrosine sensor based on Fe-doped hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2014, 35, 85–91. [Google Scholar] [CrossRef]
- Cipri, A.; Del Valle, M. Pd nanoparticles/multiwalled carbon nanotubes electrode system for voltammetric sensing of tyrosine. J. Nanosci. Nanotechnol. 2014, 14, 6692–6698. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Zhong, Y.; Yu, J. Construction of europium hexacyanoferrate film and its electrocatalytic activity to tyrosine determination. Appl. Surf. Sci. 2010, 256, 3148–3154. [Google Scholar] [CrossRef]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Teloh, J.K.; Dohle, D.-S.; Petersen, M.; Verhaegh, R.; Waack, I.N.; Roehrborn, F.; Jakob, H.; de Groot, H. Histidine and other amino acids in blood and urine after administration of Bretschneider solution (HTK) for cardioplegic arrest in patients: Effects on N-metabolism. Amino Acids 2016, 48, 1423–1432. [Google Scholar] [CrossRef] [Green Version]
- Alevridis, A.; Tsiasioti, A.; Zacharis, C.K.; Tzanavaras, P.D. Fluorimetric Method for the Determination of Histidine in Random Human Urine Based on Zone Fluidics. Molecules 2020, 25, 1665. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Li, Y.; Gu, W.; Liu, X. Spiropyran-modified upconversion nanocomposite as a fluorescent sensor for diagnosis of histidinemia. RSC Adv. 2020, 10, 26664–26670. [Google Scholar] [CrossRef]
- Guo Nan, C.; Ping, W.X.; Ping, D.J.; Qing, C.H. A study on electrochemistry of histidine and its metabolites based on the diazo coupling reaction. Talanta 1999, 49, 319–330. [Google Scholar] [CrossRef]
- Hua, Y.; Li, S.; Cai, Y.; Liu, H.; Wan, Y.; Yin, M.; Wang, F.; Wang, H. A sensitive and selective electroanalysis strategy for histidine using the wettable well electrodes modified with graphene quantum dot-scaffolded melamine and copper nanocomposites. Nanoscale 2019, 11, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Lv, X.; Cai, Y.; Liu, H.; Li, S.; Wan, Y.; Wang, H. Highly selective and reproducible electroanalysis for histidine in blood with turn-on responses at a potential approaching zero using tetrahedral copper metal organic frameworks. Chem. Commun. 2019, 55, 1271–1274. [Google Scholar] [CrossRef]
- Hua, Y.; Cai, Y.; Liu, H.; Wan, Y.; Ding, X.; Li, S.; Wang, H. A highly selective “turn-on” electroanalysis strategy with reduced copper metal–organic frameworks for sensing histamine and histidine. Nanoscale 2019, 11, 17401–17406. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Kumar, D.; Madhuri, R.; Tiwari, M.P. Metal ion mediated imprinting for electrochemical enantioselective sensing of l-histidine at trace level. Biosens. Bioelectron. 2011, 28, 117–126. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Zhang, H.; Luo, L.; Yao, S. Layer-by-layer assembly sensitive electrochemical sensor for selectively probing l-histidine based on molecular imprinting sol-gel at functionalized indium tin oxide electrode. Biosens. Bioelectron. 2010, 26, 696–702. [Google Scholar] [CrossRef]
- Stefan-Van Staden, R.I. Enantioanalysis of d-histidine based on its interaction with [5,6]fullerene-C70 and diethyl (1,2-methanofullerene-C 70)-71,71-dicarboxylate. New J. Chem. 2010, 34, 1141–1147. [Google Scholar] [CrossRef]
- Stefan-van Staden, R.I.; Holo, L. Enantioanalysis of l-histidine using enantioselective, potentiometric membrane electrodes based on maltodextrins. Anal. Lett. 2011, 44, 968–975. [Google Scholar] [CrossRef]
- Chen, Z.; Nai, J.; Ma, H.; Li, Z. Nickel hydroxide nanocrystals-modified glassy carbon electrodes for sensitive l-histidine detection. Electrochim. Acta 2014, 116, 258–262. [Google Scholar] [CrossRef]
- Nai, J.; Chen, Z.; Li, H.; Li, F.; Bai, Y.; Li, L.; Guo, L. Structure-dependent electrocatalysis of Ni(OH)2 hourglass-like nanostructures towards l-histidine. Chem. Eur. J. 2013, 19, 501–508. [Google Scholar] [CrossRef]
- Mers, S.V.S.; Muthurasu, A.; Ganesh, V. Polydopamine Decorated Co3O4/Reduced Graphene Oxide Composite for Efficient and Selective Sensing of Histidine: Restructuring α-Cobalt Hydroxide to Highly Crystalline Co3O4 Sheets. J. Electrochem. Soc. 2018, 165, B753–B761. [Google Scholar] [CrossRef]
- Chen, L.-C.; Chang, C.-C.; Chang, H.-C. Electrochemical oxidation of histidine at an anodic oxidized boron-doped diamond electrode in neutral solution. Electrochim. Acta 2008, 53, 2883–2889. [Google Scholar] [CrossRef]
- Sutradhar, S.; Jacob, G.V.; Patnaik, A. Structure and dynamics of a dl-homocysteine functionalized fullerene-C60-gold nanocomposite: A femtomolar l-histidine sensor. J. Mater. Chem. B 2017, 5, 5835–5844. [Google Scholar] [CrossRef]
- Pei, L.Z.; Wei, T.; Lin, N.; Cai, Z.Y. Electrochemical sensing of histidine based on the copper germanate nanowires modified electrode. J. Bionanosci. 2015, 9, 161–165. [Google Scholar] [CrossRef]
- Suprun, E.V.; Radko, S.P.; Kozin, S.A.; Mitkevich, V.A.; Makarov, A.A. Electrochemical detection of Zn(II)-induced amyloid-β aggregation: Insights into aggregation mechanisms. J. Electroanal. Chem. 2018, 830, 34–42. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; Oliveira-Brett, A.M. Electrochemistry of alzheimer disease amyloid beta peptides. Curr. Med. Chem. 2018, 25, 4066–4083. [Google Scholar] [CrossRef]
- Suprun, E.V.; Radko, S.P.; Farafonova, T.E.; Kozin, S.A.; Makarov, A.A.; Archakov, A.I.; Shumyantseva, V.V. Electrochemical detection of protein post-translational modifications: Phosphorylation and nitration of amyloid-beta (1–16). Electrochim. Acta 2017, 258, 1182–1190. [Google Scholar] [CrossRef]
- Suprun, E.V.; Khmeleva, S.A.; Radko, S.P.; Kozin, S.A.; Archakov, A.I.; Shumyantseva, V.V. Direct electrochemical oxidation of amyloid-β peptides via tyrosine, histidine, and methionine residues. Electrochem. Commun. 2016, 65, 53–56. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z.; Ouyang, M. Voltammetric enantiomeric differentiation of tryptophan by using multiwalled carbon nanotubes functionalized with ferrocene and β-cyclodextrin. Electrochim. Acta 2019, 297, 650–659. [Google Scholar] [CrossRef]
- Fedorowski, J.; LaCourse, W.R. A review of pulsed electrochemical detection following liquid chromatography and capillary electrophoresis. Anal. Chim. Acta 2015, 861, 1–11. [Google Scholar] [CrossRef]
- Johll, M.E.; Williams, D.G.; Johnson, D.C. Activated pulsed amperometric detection of cysteine at platinum electrodes in acidic media. Electroanalysis 1997, 9, 1397–1402. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Nardiello, D. A pulsed potential waveform displaying enhanced detection capabilities towards sulfur-containing compounds at a gold working electrode. J. Chromatogr. A 2005, 1066, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jandik, P.; Avdalovic, N. Use of disposable gold working electrodes for cation chromatography-integrated pulsed amperometric detection of sulfur-containing amino acids. J. Chromatogr. A 2003, 997, 73–78. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]

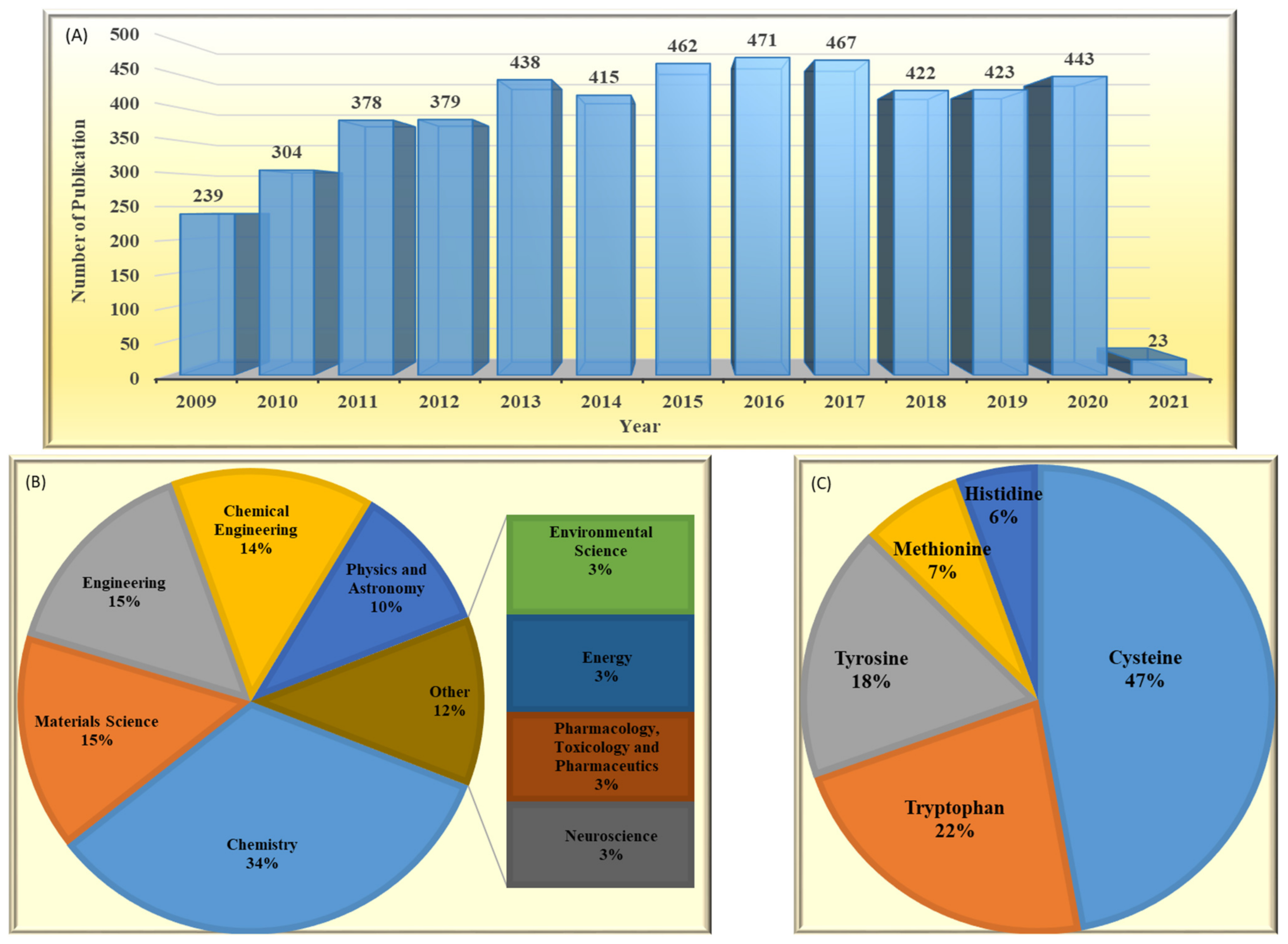

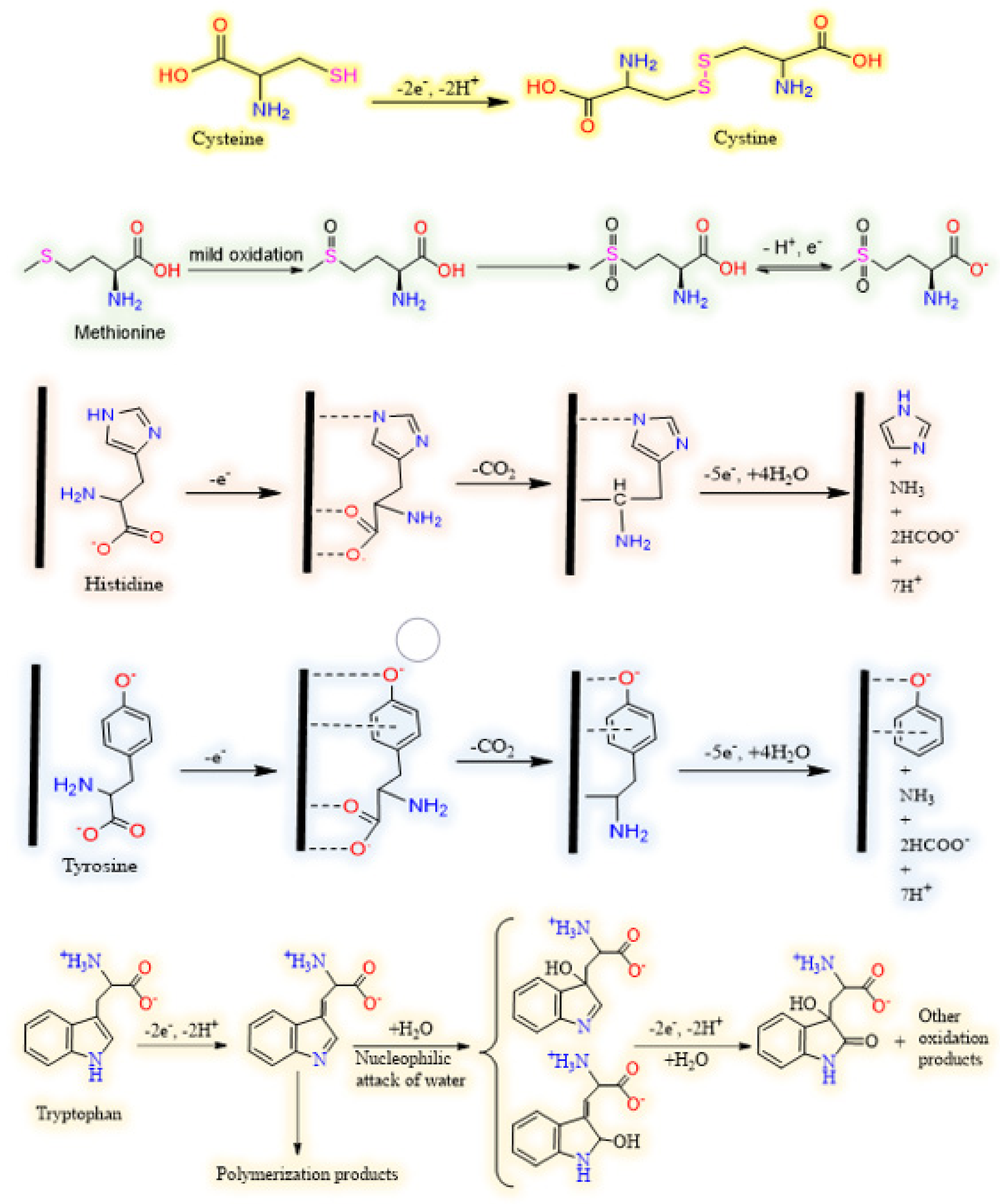
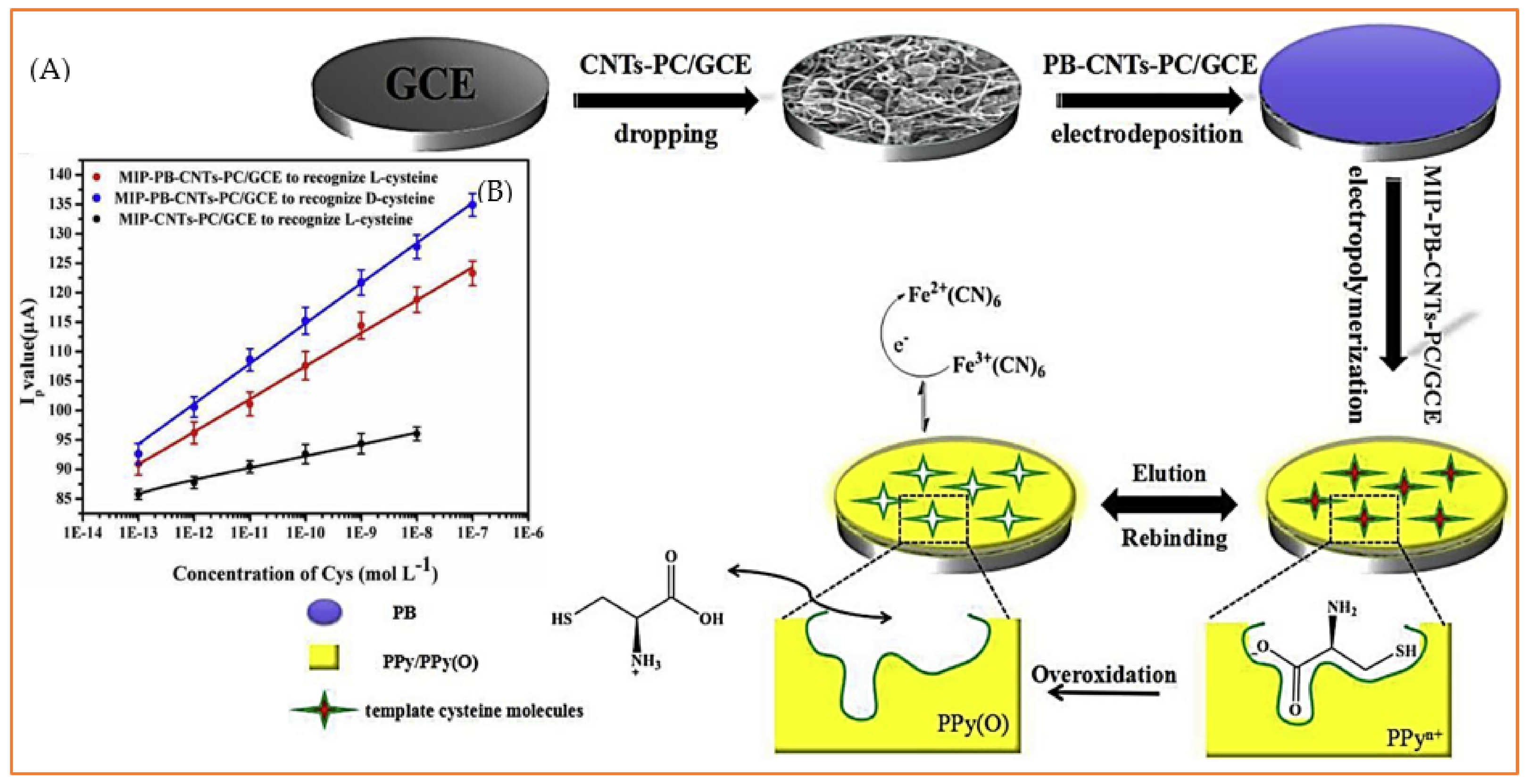
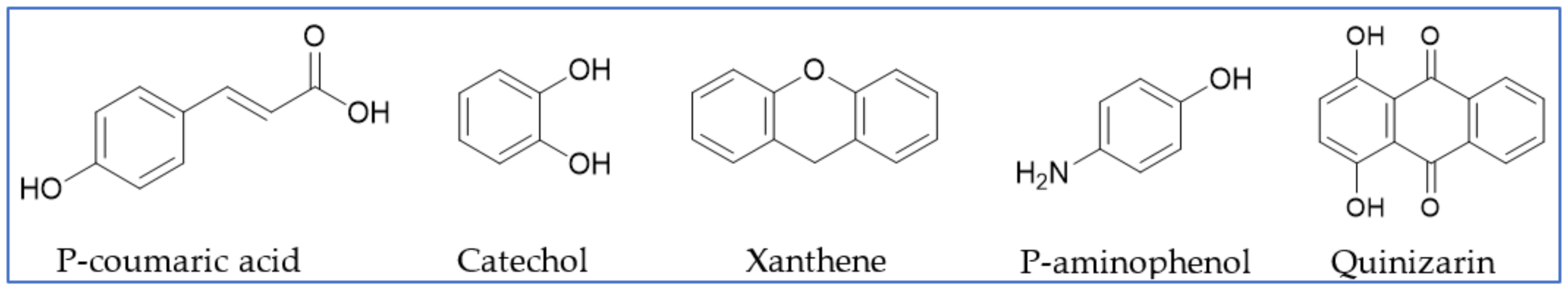
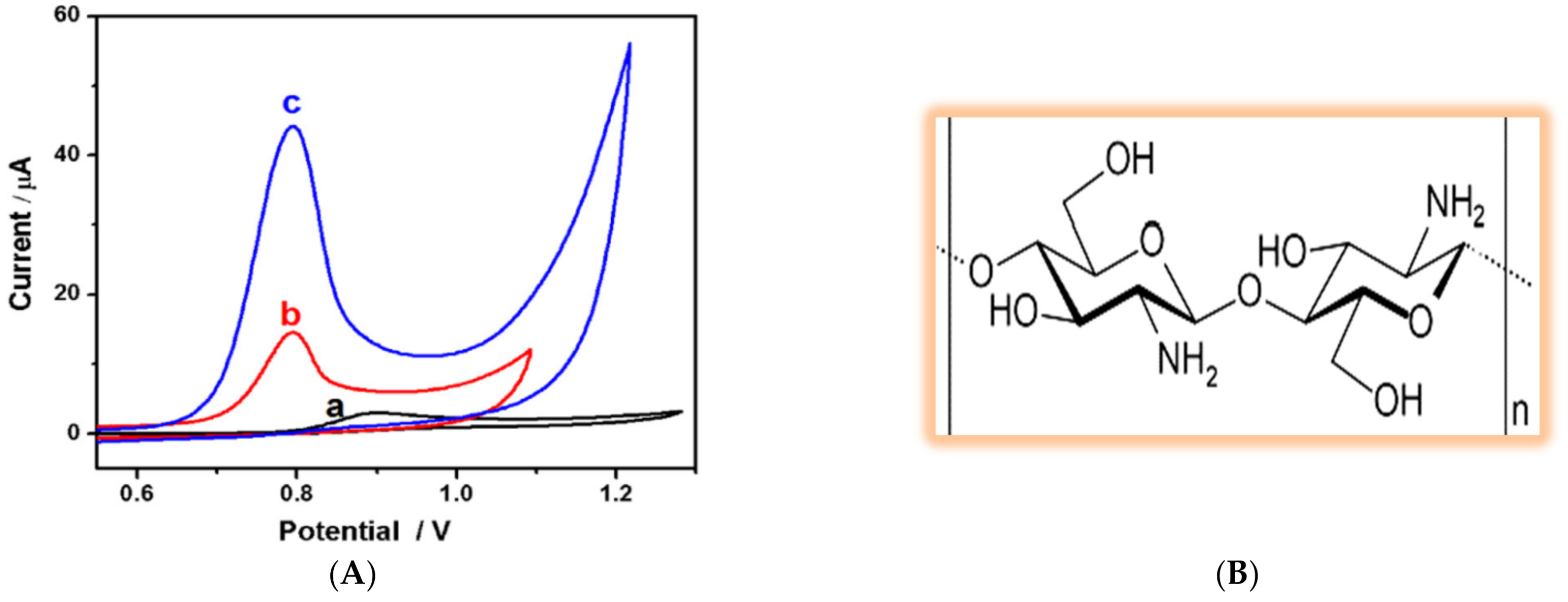
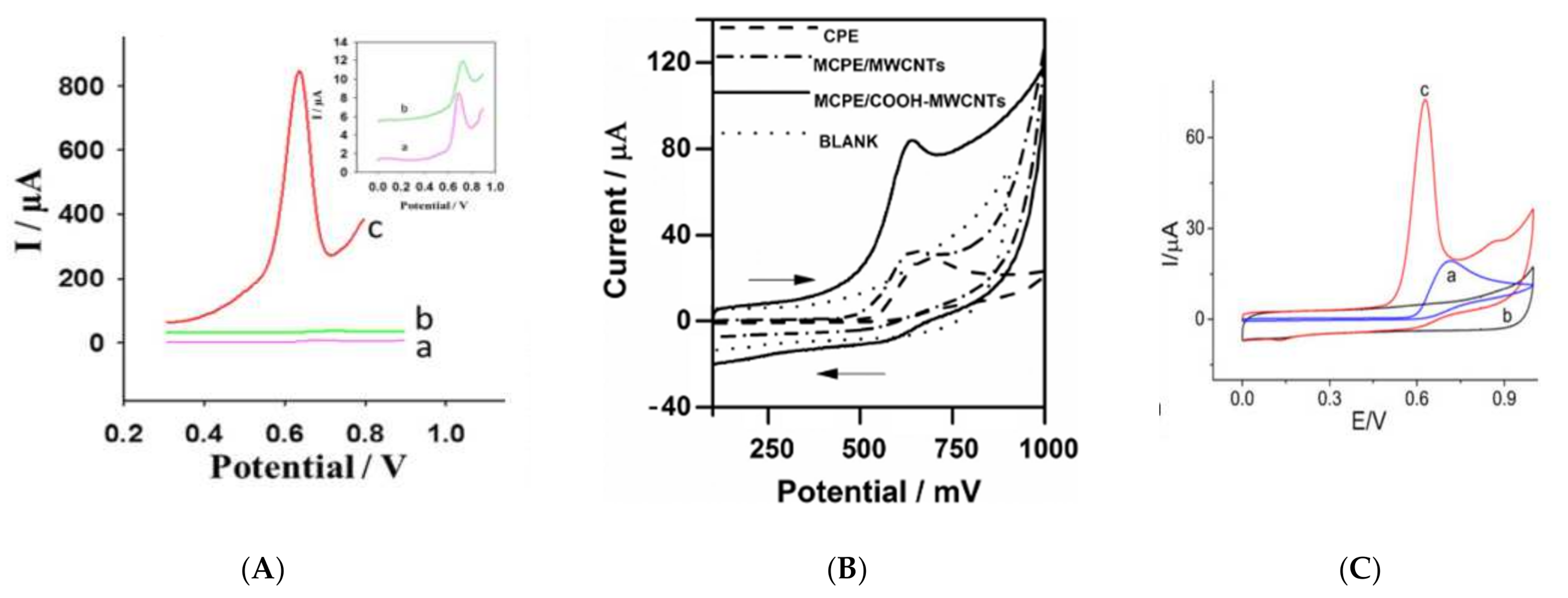
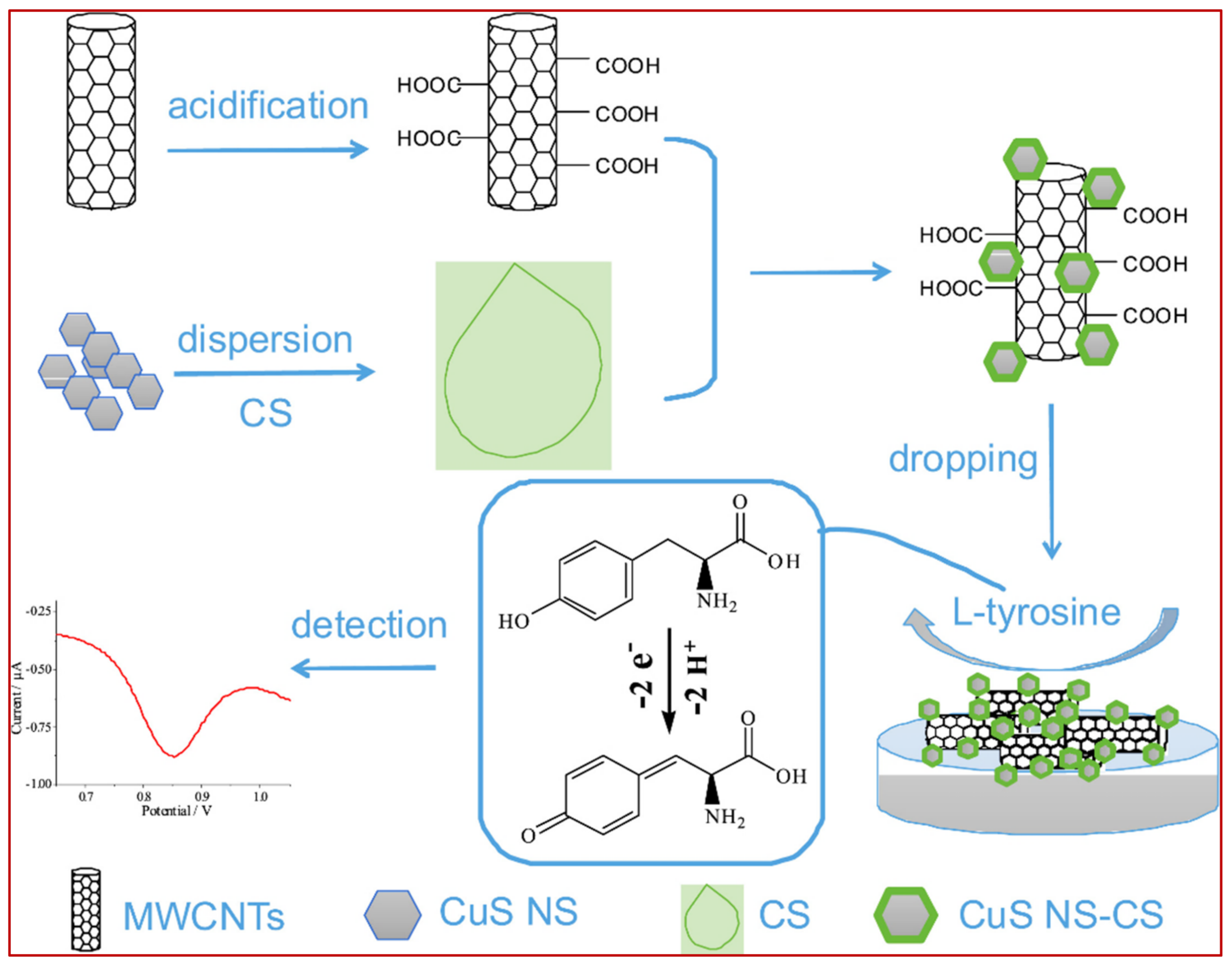

| Amino Acid | Body Function Importance | Industrial Importance |
|---|---|---|
Cysteine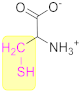 |
|
|
Methionine |
|
|
Tryptophan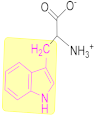 |
|
|
Tyrosine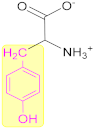 |
|
|
Histidine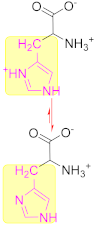 |
|
|
| Sensing Part | Method | LDR 1 (µM) | LOD 2 (µM) | L.T. Stability 3 | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| screen-printed diamond electrode | CV | 1–194 | 0.62 | - | bovine plasma | [124] |
| Co–La oxides/rGO composite | AMP | 1–888 | 0.1 | - | serum and commercial syrup | [125] |
| Mn-La oxides/reduced graphene oxide composite | AMP | 0.5–832.5 | 0.1 | 93.2% after one week | commercial serum and syrup samples | [126] |
| cobalt hydroxide nanosheets | AMP | 0.2–1940 | 0.0765 | - | human blood serum | [127] |
| silver metal-organic frameworks coated onto nitrogen-doped porous carbon | LSV | 0.1–1300 | 0.05 | ˃6 months | milk sample | [128] |
| CuO/Boron Nitride Nanocomposite | AMP | 1–10 | 0.58 | 98% after 25 days | blood serum | [129] |
| Co3O4 nanoparticles | AMP | 0.2–75 | 0.07 | - | urine sample | [130] |
| functionalized MWCNT | DPV | 0.7nM-200µM | 0.16 nM | ˃one week | blood serum | [131] |
| graphite-polyurethane composite | AMP | 30–130 | 4.24 | - | food supplements | [132] |
| 3D pothole-rich hierarchical carbon framework-encapsulated Ni nanoparticles | CV | 0.8–85 | 0.15 | 90% after one week | Blood serum and urine | [133] |
| copper pentacyanonitrosylferrate and octa(aminopropyl)silsesquioxane | AMP | 200–2000 | 125 | - | - | [134] |
| Cu2 +-phen-dione@rGO | AMP | 10–32,344 | 2 | ˃15 days | urine sample | [135] |
| ferrocene-functionalized mesoporous silica | CV | 3–20 | 3 | - | - | [136] |
| CeO2-CuO nanocomposite | AMP | 10–5000 | 0.16 | 93.7% after 5 days | pond water | [137] |
| Au–Cu@CuxO | AMP | 1.25–1940 | 1.25 | ˃5 weeks | human blood serum | [138] |
| Pd@Ti3C2Tx nanocomposite | AMP | 0.5–10 | 0.14 | ˃one week | urine sample | [139] |
| CeO2-SnO2 nanocomposite | AMP | 10–2000 | 0.016 | ˃4 days | pond water | [140] |
| CuFe2O4/rGO–Au composite | CV | 50–200 | 0.383 | 93% after 4 week | urine sample | [141] |
| Co(II)–Al(III) layered double hydroxide | DPV | 10−4–1 | 10−4 | - | pharmaceutical samples | [142] |
| fluorinated cobalt phthalocyanine and ordered mesoporous carbon | DPV | 20–20,000 | 1 | 90% after 2 weeks | cell lysate, human serum and urine | [143] |
| ethyl 2-(4 ferrocenyl [1-3]triazol-1-yl) acetate/graphene | SWV | 4.0–2300.0 | 0.9 | - | blood serum and urine | [144] |
| free-standing TiO2 nanotube | AMP | 100–10,000 | 100 | 93% after 80 days | human serum sample | [145] |
| bacteriophage particles-carbon nanofiber | CV | 20–1000 | 20 | - | - | [87] |
| cobalt-poly (naphthylamine)/sodium dodecyl sulphate | AMP | 1–100 | 0.8 | ˃5 weeks | human urine | [146] |
| Au nanoparticles/ anthraquinone-2-carboxylic acid | DPV | 15–500 | 1.873 | 92.5% after 2 weeks | blood serum | [49] |
| electrodeposited copper/SPE | AMP | 1–1800 | 0.21 | - | human and rabbit blood serum | [147] |
| imine substituted cobalt(II) phthalocyanine | CV | 0.01–0.10 | 0.003 | - | human urine and cysteine tablet samples | [148] |
| molecularly imprinted Prussian blue-porous carbon-CNTs/polypyrrole | DPV | 1.0×10–z7–0.1 | 6.01 pM | 94.42% after 15 days | blood serum | [112] |
| poly(p-coumaric acid)/MWNT | DPV | 7.5–1000 | 1.1 | - | human urine | [88] |
| Cu2+ modified Fe3O4@polydopamine | SWV | 0.010–500 | 83.0 pM | - | blood serum | [113] |
| polyaniline/zinc bismuthate | AMP | 50–2000 | 0.19 | - | - | [149] |
| 4 RGO/Nafion film decorated Pd nanoparticles | AMP | 0.5–10 | 0.15 | 92.27% after 5 days | human urine | [67] |
| silver-copper sulphide | AMP | 1–100 | 0.24 | Should be kept in dark condition | dietary supplement | [106] |
| Fe3O4@NiO magnetic nanoparticles | DPV | 0.1–120 | 0.014 | 89% after one month | human breast milk, cow milk and honey | [150] |
| Co-Gd2O3 nanocomposite | AMP | 1–100 | 0.23 | 92.3% after one month | milk, cysteine capsule | [151] |
| bismuth tellurate nanospheres | CV | 0.1–2000 | 0.46 | ˃2 weeks | - | [152] |
| hollow cubic Cu2O particles/Nafion | CV | 5–200 | 0.4 | 96% after 10 days | amino acid injections | [105] |
| MgO nanoparticle/acetylferrocene | DPV | 0.1–7000 | 0.03 | 95% after 25 days | urine, pharmaceutical serum | [79] |
| Au/CeO2 nanofibers composite | AMP | 2–200 | 0.01 | 90% after one week | blood serum | [102] |
| CeO2 nanofibers | AMP | 2–200 | 0.02 | - | blood serum | [76] |
| Prussian blue | AMP | 100–600 | 67.4 | - | - | [114] |
| MnO2-TiO2 nanocomposite/2-(3,4 dihydroxyphenethyl) isoindoline-1,3-dione | SWV | 0.025–200 | 0.013 | 94% after one month | blood serum, urine, cysteine capsule | [153] |
| CuO–Cu2O heterojunction | 5 PEC | 0.2–10 | 0.05 | ˃6 weeks | urine sample | [77] |
| ZnO nanoparticle/N-doped RGO | AMP | 0.1–705.0 | 0.1 | - | cysteine capsule | [86] |
| nickel oxide NPs/N-doped RGO | AMP | 0.3–1620.8 | 0.1 | 93.1% after 7 days | syrup sample | [81] |
| thiolated catechol | AMP | 0.12–34.6 | 0.0605 | - | urine sample | [89] |
| Li-doped bismuth oxide nanorods | CV | 0.1–2000 | 0.17 | - | blood serum | [154] |
| Au-nanoparticles/poly-Trypan Blue | DPV | 5–270 | 0.006 | - | blood serum and urine | [50] |
| carbon black functionalized with syringic acid | CV | 20–1000 | 0.639 | - | chicken flesh and blood serum | [90] |
| Impurity-containing carbon black | AMP | 50–700 | 0.0459 | - | blood serum | [51] |
| bismuth nickelate nanorods | CV | 0.5–2000 | 0.087 | - | - | [155] |
| gold nanoparticles incorporated polypyrimidine derivative | DPV | 2–500 | 0.02 | 94.9% after one month | blood serum and urine | [52] |
| CdSe quantum dot-modified/MWCNT 6 hollow fiber | DPV | 0.287–33,670 | 0.116 | - | bodybuilding supplements | [156] |
| magnetic CoFe2O4/SiO2 spinel-type | DPV | 0.02–425 | 0.2 | 70% after one week | milk sample | [115] |
| MIP 7/CuNPs/nonporous gold | DPV | 0.5–10,000 nM | 70 nM | 95.4% after one week | bovine serum and sauce of instant noodle samples | [71] |
| Y2O3 nanoparticles/nitrogen-doped RGO | AMP | 1.3–720 | 0.8 | - | syrup sample | [85] |
| polythiophene layer sensitized anatase TiO2 | PEC | 100–800 | 12.6 | 93.9% after 2 weeks | - | [83] |
| Pt-Fe3O4/RGO | AMP | 100–1000 | 10 | 94.2% after 2 weeks | - | [116] |
| polypyrrole/graphene quantum dots@Prussian Blue | AMP | 0.2–1000 | 0.15 | - | cysteine tablets | [117] |
| polydopamine-capped silver nanoparticles | LSV 8 | 0.05–300 | 0.023 | ˃2 months | blood serum | [68] |
| Au nanoparticles/poly(E)-4-(p-tolyldiazenyl)benzene-1,2,3-triol | DPV | 2–540 | 0.04 | - | human urine | [53] |
| thulium hexacyanoferrate | DPV | 0.5–8.92 | 0.016 | 91% after one week | human urine, river water | [157] |
| zinc bismuthate nanorods | CV | 0.1–2000 | 0.074 | ˃2 weeks | - | [158] |
| Ru(III) Schiff base complex, multi-walled carbon nanotubes and Nafion | AMP | 50–500 mg/L | 0.11 mg/L | - | pharmaceutical products | [159] |
| Co(II)-phthalocyanine | SWV | 2.6–200 | 4 | - | embryo cell culture | [160] |
| Fe(II)-exchanged zeolite | SWV | 0.005–300 | 0.00015 | ˃8 months | human serum, urine and N-acetyl cysteine tablets | [161] |
| molybdenum nitride nanosheets/N-doped MWCNT | CV | 5–12,600 | 3.64 | 92.9% after 2 weeks | blood serum | [162] |
| carbon ionic liquid electrode with terpyridine copper(II) complex | CV | 0.1–40 | 0.01 | 93.4% after 15 days | blood serum | [107] |
| zirconium (IV) phosphate/Ag hexacyanoferrate (III) | AMP | 10–80 | 10.2 | - | - | [163] |
| bare glassy carbon electrode | CV | 1–10 | 0.03 | - | - | [164] |
| V-substituted polyoxometalates/Au@2Ag core–shell nanoparticles | AMP | 0.025–7.625 | 0.0276 | 90% after 2 weeks | milk sample | [72] |
| Fe2O3 nanoparticles supported on N-doped graphene | AMP | 0.2–400 | 0.1 | 90.6% after one week | syrup sample | [78] |
| Au NPs-Ni-Al layered double hydroxide composite | DPV | 10–1000 | 6.0 | 79.3% after 4 days | - | [73] |
| GO/CCNTs/AuNPs@MnO2 9 | DPV | 0.01–7.0 | 0.0034 | - | human urine | [55] |
| bismuth nanostructure incorporated into ionic liquid | SWV | 1–2000 | 0.5 | - | blood serum | [165] |
| silver nanoparticles modified hierarchically structured ZnO | PEC | 0.67–34.77 pM 10 | 0.21 pM | 94.1% after one month | human urine | [69] |
| cyclotricatechylene | CV | 0–20 | 0.9 | - | cell tissue media | [166] |
| (DMBQ) 11/ZnO nanoparticles | SWV | 0.09–340.0 | 0.05 | ˃one month | urine, water and pharmaceutical serum | [167] |
| iron tetrasulphonated phthalocyanine decorated MWCNT | AMP | 10–200 | 1 | 95.16% after one month | blood serum | [118] |
| copper inorganic-organic hybrid coordination compound | AMP | 10–80 | 2.1 | - | - | [108] |
| 14-(4-hydroxyphenyl)-14-H-dibenzo[a,j]-xanthene/MWCNT | DPV | 4–1000 | 1 | - | human serum, acetyl cysteine tablets | [123] |
| gold nanoparticle (AuNP)-iron(iii) phthalocyanine | DPV | 50–1000 | 0.27 | - | pharmaceutical sachets, dietary supplement | [56] |
| Sulphonated Graphene-poly(3,4-Ethylenedioxythiophene)/Au NPs | AMP | 0.1–382 | 0.02 | 95.25% after 10 days | human urine | [57] |
| bismuth film | SWV | 1–10 | 0.028 | - | dietary supplement | [168] |
| carbon ionic liquid | LSV | 1–450 | 0.298 | - | artificial urine and nutrient broth | [91] |
| polyaniline/CuGeO3 nanowire | CV | 1–2000 | 0.44 | - | - | [109] |
| nanocarbon (carbon black) | SWV | 0–100 | 5 | - | - | [94] |
| SnO2–MWCNTs | AMP | 0.1–554.5 | 0.03 | 92% after one month | - | [82] |
| MWCNTs/gold NPs stabilized with calcium cross-linked pectin | AMP | 0.1–1000 | 0.019 | - | blood serum | [58] |
| methacrylic acid based MIP | DPV | 0.02–0.18 | 0.0096 | - | blood plasma, tapwater samples | [169] |
| protoporphyrin/WO3/RGO | PEC | 0.1–100 | 0.025 | 93.74% after 4 weeks | [84] | |
| MWCNTs/ gold nanorods | AMP | 5.0–200 | 0.0085 | ˃3 months | blood serum | [59] |
| graphene nanosheets/manganese oxide nanoparticles | AMP | 1–24 | 0.075 | - | - | [170] |
| MnO2 nanoparticles | AMP | 10–640 | 0.8 | - | blood serum | [171] |
| dispersion of MWCNTs in metallopolymer | AMP | 0.025–0.151 | 0.006 | ˃one month | - | [119] |
| Ce-doped Mg–Al layered double hydroxide | AMP | 10–5400 | 4.2 | 92.7% after 2 weeks | syrup sample | [172] |
| gold nanoparticles | CV | 1–14 pM | 0.6 pM | - | - | [61] |
| Co(II)-exchanged zeolite Y | CV | 1 nM–1 mM | 0.24 nM | ˃9 months | blood serum, urine, N-acetylcysteine tablet and powdered poultry feed samples | [173] |
| nanoporous gold | AMP | 1–400 | 0.05 | - | human urine | [62] |
| Au-NPs/poly-eriochrome black T | AMP | 0.05–100 | 0.008 | 95% after 10 days | - | [63] |
| graphene oxide/Au nanocluster | CV | 0.05–20 | 0.02 | 87.3% after 12 weeks | human urine | [64] |
| titanium (IV) phosphate composite | CV | 200–9000 | 334 | - | - | [174] |
| benzoylferrocene-modified MWCNTs | SWV | 0.7–350 | 0.1 | - | human hair, N-acetylcysteine tablet | [97] |
| vertically aligned MWCNTs modified with Pt nanoparticles | AMP | 1–500 | 0.5 | - | human urine | [103] |
| caterpillar-like manganese dioxide–carbon nanocomposite | AMP | 0.5–680 | 0.022 | - | ˃one month | [175] |
| Au NPs dispersed in Nafion | AMP | 4.0–80.0 | 1.0 | - | - | [65] |
| cobalt hexacyanoferrate NPs with a core-shell structure | AMP | 3–37 | 0.04 | - | blood serum and urine samples | [39] |
| yttrium hexacyanoferrate nanoparticle /MWCNT/Nafion | AMP | 0.2–11.4 | 0.16 | - | - | [176] |
| CdS quantum dot-methyl viologen complex | PEC | 0.2–2.8 | 0.1 | - | - | [177] |
| silver pentacyanonitrosylferrate | CV | 0.1–20 | 0.035 | - | ˃three months | [121] |
| gold-aminomercaptothiadiazole core-shell NPs | AMP | 0.01–0.14 | 3 pM | 96.5% after 10 days | blood serum and urine samples | [178] |
| conducting polymer/Au NPs | AMP | 0.5–200 | 0.05 | - | l-cysteine capsule | [66] |
| p-aminophenol/MWCNTs | DPV | 0.5–100 | 0.3 | - | urine, river water, blood plasma and serum samples | [98] |
| electrospun carbon nanofibers | AMP | 0.15–64 | 0.1 | - | - | [99] |
| 1,1′-Ferrocenedicarboxylic acid | DPV | 20–500 | 9.8 | - | water samples | [122] |
| AuPt alloy/MWCNTs-ionic liquid | AMP | 0.5–40 | - | 95% after 15 days | - | [74] |
| quinizarine | DPV | 1–1000 | 0.22 | - | blood serum, acetylcysteine tablet | [100] |
| copper hexacyanoferrate | AMP | 1–13 | 0.13 | - | human urine | [110] |
| Pt nanoparticles/poly(o-aminophenol) film | AMP | 0.4–630 | 0.08 | 93% after one month | syrup sample | [104] |
| gallium nitride nanowires | CV | 0.5–75 | 0.5 | 86% after 5 days | - | [179] |
| silver nanoparticles coated polyquercetin | CPM 12 | 0.1–90 nM | 0.03 nM | - | - | [70] |
| CuGeO3 nanowire | CV | 1–1000 | 0.9 | ˃2 weeks | tapwater | [111] |
| Sensing Part | Method | LDR 13 | LOD 14 | L.T. Stability 15 | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| 3D-printed electrodes | SWV | 5–230 | 1.39 | - | human serum | [196] |
| silver oxide | AMP | 60–500 | 0.42 | - | blood serum | [181] |
| single layer MoS2 | PEC | 0.1–1000 nM | 0.03 nM | ˃28 days | blood serum | [185] |
| Mn2O3 | DPV | 1–610 | 0.001 | seafood sample | [187] | |
| ruthenium/platinum bimetallic monolayer coated on a nanoporous gold film | DPV | 0.006–102 | 0.002 | 97.3% after 3 weeks | human urine | [182] |
| Ag–Au core-shell bimetal nanoparticles | AMP | 50–1000 | 30 | 96% after a week | - | [183] |
| ZnS/ZnAl2S4 nanocomposite | SWV | 0.05–800 | 0.01 | - | blood serum and urine | [197] |
| RGO/α-cyclodextrin | AMP | 170–1200 | 40 | ˃7 days | - | [192] |
| graphene oxide | DPV | 450–4950 | 100 | - | - | [193] |
| imprinted polybenzidine/MWCNTs functionalized –COOH | DPV | 11.7–206.3 ng/L | 3 ng/L | - | pharmaceutical and blood serum samples | [41] |
| Pt doped TiO2 NPs/CNT | AMP | 0.5–100 | 0.1 | 88% after 2 weeks | blood serum | [189] |
| benzoylferrocene modified MWCNTs | SWV | 0.1–200 | 0.058 | - | urine sample | [198] |
| electropolymerized functionalized triazole polymer | AMP | 0.1–100 | 4.1 × 10−4 | 97.65% after 2 weeks | urine sample | [199] |
| electropolymerized film of non-peripheral amine substituted Cu(II) phthalocyanine | DPV | 50–500 | 0.027 | ˃one month | blood serum | [190] |
| bare screen-printed graphite electrodes | DPV | 50–5000 | 95 | - | pharmaceutical products | [194] |
| cobalt hydroxide nanoparticles | AMP | 245–1210 | 160 | - | - | [191] |
| MWCNTs | AMP | 360–6900 | 270 | - | pharmaceutical product | [195] |
| fullerene-C60 modified gold electrode | CV | Up to 100 | 8.2 | - | root beer syrup and methionine pill | [200] |
| Sensing Part | Method | LDR (µM) | LOD (µM) | L.T. Stability | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| octahydropyrimido[1, 2-a] azepine | DPV | 1.5–750 | 0.05 | ˃one month | blood and urine samples | [218] |
| nickel nanoparticle/Nitrogen-carbon nanohybrid | SDLSV 16 | 0.01–80 | 0.006 | 92.77% after 2 weeks | human serum and pharmaceutical samples | [219] |
| 3-neomenthylindene | DPV | 2.5–300 | 1.71 | 95.4% after 5 days | urine and blood plasma | [220] |
| CeO2/rGO composite | SDLSV | 0.01–10 | 0.006 | 88.7% after 2 weeks | amino acid injection, human serum and urine | [221] |
| CeO2/rGO composite | DPV | 0.2–25 | 0.08 | ˃one month | milk and bovine serum samples | [222] |
| silver zeolite nanocomposite | DPV | 0.01–1.2 | 0.0063 | - | wheat flour, goat and cow milk | [223] |
| pencil graphite electrode | ASDPV 17 | 0.154–200 | 0.046 | - | urine sample | [224] |
| rGO decorated with 18-crown-6 and gold nanoparticles | SWV | 0.1–2.5 | 0.05 | 77% after 50 days | human serum | [225] |
| poly (3,4-proplenedioxy thiophene)@nitrogen-doped carbon hollow spheres composites | DPV | 0.1–100 | 0.0092 | - | - | [226] |
| rGO/gold nanoparticles | DPV | 0.5–500 | 0.39 | - | saliva, serum and plasma | [227] |
| polythiophene/silver dendrites composite | SWV | 0.2–400 | 0.02 | - | soybeans extract | [228] |
| MWCNT and molecularly imprinted polymer | SDLSV | 0.002–100 | 0.001 | 91% after 2 weeks | human serum | [229] |
| polyvinylpyrrolidone functionalized graphene | SDLSV | 0.06–100 | 0.01 | 88% after 20 days | urine, serum and injection samples | [230] |
| CuSn(OH)6 microsphere decorated on rGO | DPV | 0.05–175.8 | 0.002 | 95% after 15 days | urine sample | [231] |
| Pd-Ag nanoparticles | DPV | 0.1–1000 | 0.1 (at pH 4) | - | - | [232] |
| polydopamine/rGO/MnO2 composite | AMP | 1.23–303.26 | 0.24 | 94% after one month | tomato fruit and juice | [233] |
| molecularly imprinted copolymer/ MWCNT | DPV | 0.008–26 | 0.006 | 92.7% after 30 days | amino acid oral liquid and human serum samples | [234] |
| nitrogen-doped ordered mesoporous carbon | CV | 0.5–200 | 0.035 | 95.4% after one month | amino acid cocktails | [235] |
| rGO, gold nanoparticles, poly-l-cysteine, and poly-l-phenylalanine methyl ester | AMP | 100–800 | 44 | 87.7% after 5 weeks | - | [236] |
| perovskite-type SrTiO3 nanocubes/rGO | AMP | 0.03–917.3 | 0.0071 | 96.2% after 2 weeks | urine and blood serum | [237] |
| hydroxyapatite/graphene oxide | LSV | 7–1000 | 5.5 | - | sunflower and pumpkin seeds | [238] |
| poly(3,4-ethylenedioxythiophene) | CV | 10–400 | 7.2 | - | urine and serum samples | [239] |
| 3D nitrogen-doped reduced graphene oxide and self-assembled polysaccharides | DPV | 10–5000 | 0.0035 | 96.9% after 22 days | human urine and serum | [240] |
| silver molybdate/rGO | AMP | 0.002–146.9 | 0.0057 | - | milk and oat samples | [241] |
| molecularly imprinted chitosan film | SDLSV | 0.01–100 | 0.008 | 91% after 20 days | human serum, amino acid injections | [242] |
| graphene functionalized with 3,4,9,10-perylene tetracarboxylic acid and chitosan | DPV | 1–10 mM | 1.2 | 96.7% after 2 weeks | human urine and serum | [243] |
| Ta2O5-rGO | SDLSV | 1–800 | 0.84 | - | human serum | [244] |
| MWCNT@polydopamine composite loaded with copper(II) | DPV | 1–100 | 0.15 | 93.4% after 30 days | - | [245] |
| exfoliated graphene and poly (3,4-ethylenedioxythiophene):poly (styrene sulphonate) | DPV | 0.1–1000 | 0.015 | 98.9% after 2 weeks | - | [246] |
| cuprous oxide and electrochemically rGO | SWV | 0.02–20 | 0.01 | 94.28% after 2 weeks | human serum and commercial amino acid injections | [247] |
| poly(sodium 4-styrenesulphonate) functionalized graphene | LSV | 0.04–10 | 0.02 | 89.2% after 7 days | human serum sample | [205] |
| manganese cobaltite entrapped rGO | AMP | 0.004–112.9 | 0.001 | - | milk sample | [248] |
| Fe3O4/C composite | SDLSV | 1–800 | 0.26 | 90.4% after one week | human blood serum | [249] |
| gold nanoparticles electrodeposited onto graphite-polyurethane | DPV | 0.6–2.0 | 0.053 | - | synthetic urine and commercial poly-amino acids supplement | [250] |
| anionic/cationic-pillar [5]arenes multilayer film | DPV | 1–300 | 0.3 | - | blood serum | [105] |
| alumina/graphene/Cu hybrid | DPV | 1–1000 | 0.009 | ˃10 days | urine sample | [251] |
| flowerlike Fe3O4@NiO magnetic nanoparticles | DPV | 0.1–120 | 0.014 | 89% after one month | human breast milk, cow milk and honey | [150] |
| NiO/carbon nanotube/PEDOT 18 composite | DPV | 1–41 | 0.21 | 100.5% after 37 days | blood serum | [252] |
| amino-modified β-cyclodextrin (NH2-β-CD), gold-platinum core-shell microspheres | DPV | 10–5000 | 4.3 | 91.7% after 15 days | milk samples | [253] |
| surface-confined chromium-salen complex | EIS 19 | 4–60 nM | 0.78 nM | - | blood serum | [254] |
| functionalized carbon black/poly-l-histidine nanocomposite | AMP | 0.025–125.0 | 0.008 | 92% after 2 weeks | milk and human urine | [206] |
| Schiff-based Cu(II) complex | CV | 7–48 | 0.185 | ˃3 weeks | milk sample | [255] |
| activated MWCNTs Ionic Liquid | CV | 5–1000 | 2.3 | - | commercial amino acid injection and blood serum | [256] |
| silver nanodendrites implemented in polylactide-thiacalix[4]arene copolymer | DPV | 0.1–100 | 0.03 | 90% after 6 weeks | tryptophan sedative medication | [257] |
| cerium-doped ZnO and functionalized MWCNTs | DPV | 0.01–0.1 | 0.001 | 97% after one week | blood serum and milk samples | [258] |
| tetrabutylammonium bromide on the ß-cyclodextrin incorporated MWCNTs | DPV | 1.5–30.5 | 0.07 | 90% after 20 days | blood serum | [259] |
| magnetic coreshell manganese ferrite nanoparticles/ionic liquid | SWV | 5–400 | 1.1 | - | urine sample | [260] |
| Pd−Cu@Cu2O/N-rGO | DPV | 0.01–40.0 | 1.9 nM | - | urine and milk samples | [261] |
| bismuth sulphide/sulphur doped graphene nanocomposite | DPV | 0.01–120 | 0.004 | - | - | [262] |
| poly(l-arginine)/rGO and gold nanoparticles | DPV | 0.01–100 | 0.1 | 95% after 2 weeks | urine sample | [263] |
| MWCNTs-CTAB 20 nanocomposite | DPV | 4.9–64.1 | 1.6 | - | blood serum | [264] |
| mesoporous silica nanoparticles | DPV | 0.05–600 | 0.011 | - | artificial urine | [265] |
| Fe3O4 magnetic nanoparticles/graphene quantum dots | DPV | 0.08–150 | 0.08 | - | - | [266] |
| nanoporous carbon | AMP | 1–103 | 0.03 | 93.7% after 3 weeks | amino acid injection, fetal calf serum samples | [102] |
| rGO decorated with polypyrrole nanofibers and zinc oxide-copper oxide | DPV | 0.053–480 | 0.01 | 97.86% after 2 weeks | blood serum | [267] |
| Ni-doped Lewatit FO36 nano ion exchange resin | DPV | 4–560 | 0.38 | 89.7% after 2 months | water, urine, serum and pharmaceutical samples | [268] |
| tricobalt tetroxide nanoparticles decorated carbon nanofibers | AMP | 0.005–40 | 0.002 | 96% after 20 days | pharmaceutical samples | [269] |
| rGO decorated with SnO2–Co3O4 nanoparticles. | DPV | 0.02–6.0 | 0.0032 | 95.7% after 14 days | blood serum, urine and pharmaceutical samples | [270] |
| amino-functionalized graphene quantum dots/β-cyclodextrin | DPV | 1–30 | 0.65 | 94%after 2 months | - | [207] |
| unzipped MWCNT incorporated overoxidized poly(p-aminophenol) | DPV | 5–1265 | 0.47 | 94.7% after one month | blood serum and urine samples | [271] |
| AgNPs/graphene oxide-poly(l-arginine) | DPV | 1–150 | 0.122 | - | urine sample | [272] |
| graphene oxide/NiO nanocomposite and n-hexyl-3-methylimidazolium hexafluoro phosphate | SWV | 5–700 | 1 | - | urine and pharmaceutical samples | [273] |
| mixed oxide SiO2/Nb2O5/ZnO metallization with iron(III) and inserted into the porphyrin ring | SWV | 10–70 | 3.28 | - | pharmaceutical samples | [274] |
| polythiophene nanostructures | LSV | 6–180 | 0.61 | - | blood serum and urine | [275] |
| graphite-like carbon nitride nanosheets | LSV | 0.1–110 | 0.024 | 91.8% after 30 days | amino acid injection and rat blood serum | [276] |
| flower-like cerium vanadate | DPV | 0.1–94 | 0.024 | 98.4% after one week | milk and urine samples | [212] |
| rGO) decorated with SnO2 | DPV | 1–100 | 0.04 | ˃2 weeks | milk and amino acid injection samples | [277] |
| poly(β-cyclodextrin)/carbon quantum dots composite | DPV | 5–270 | 0.16 | 94.7% after 2 weeks | urine sample | [278] |
| MWCNT/ ionic liquid nanocomposite | DPV | 0.5–70 | 0.32 | - | dough sample | [279] |
| ZnFe2O4 nanoparticles | DPV | 0.1–200 | 0.04 | - | blood serum and urine samples | [280] |
| MWCNTs | DPV | 0.6–100 | 0.065 | - | blood serum | [203] |
| MWVNTs/1-(allyloxy)-4-nitrobenzene | DPV | 0.06–40 | 0.007 | - | blood serum, milk and pharmaceutical samples | [281] |
| polyoxometalate functionalized rGO | SWV | 0.001–1 nM | 0.002 nM | 97.75% after 45 days | blood serum | [282] |
| CTAB/phosphotungstic acid/rGO | DPV | 0.1–300 | 0.02 | 99.73%after one month | amino acids injection sample | [283] |
| β-cyclodextrin-platinum nanoparticles/graphene nanohybrids | DPV | 50–5000 | 17 | 93.7% after one week | Trp enantiomers mixture | [284] |
| N-doped carbon dots/β-cyclodextrin | DPV | 5–70 | 1.7 | - | Trp enantiomers in riboflavin sample | [285] |
| poly(l-methionine) and graphene composite film | DPV | 0.2–150 | 0.017 | 89.7% after one month | milk and blood serum samples | [286] |
| β-cyclodextrin modified magnetic graphene oxide | DPV | 0.5–750 | 0.3 | 97.4% after 15 days | commercial amino acid preparations | [287] |
| tellurium nanorods | AMP | 0.02–11.48 | 0.01 | 90% after 20 days | commercial amino acid injection | [288] |
| nickel and copper oxides-decorated graphene | SWV | 0.3–40 | 0.1 | 95% after one month | blood serum and pharmaceutical samples | [289] |
| 4-amino-3-hydroxy-1-naphthalenesulphonic acid/rGO based polymer | SWV | 0.5–200 | 0.31 | 93.33% after one month | pharmaceutical formulations, human urine and plasma samples | [290] |
| SiO2 | DPV | 0.05–400 | 0.034 | ˃2 months | artificial urine sample | [291] |
| NiO nanoparticle coupled ionic liquid | SWV | 0.08–350 | 0.04 | - | urine and water samples | [292] |
| metal-organic framework/silver nanoparticles composite | DPV | 1–150 | 0.14 | - | urine sample | [293] |
| Pt/CNTs nanocomposite/ionic liquid | SWV | 0.1–400 | 0.04 | ˃40 days | meat and pharmaceutical samples | [294] |
| nitrogen-incorporated tetrahedral amorphous carbon thin film | AMP | 0.1–100 | 0.089 | - | - | [295] |
| silver film loaded on carbon paper | LSV | 0.1–330 | 0.04 | 93% after 2 weeks | milk sample | [296] |
| carbon-supported NiCoO2 nanoparticles | DPV | 50–943.4 | 5.7 | 90.31% after 20 days | blood serum, urine and pharmaceutical samples | [297] |
| Cu NPs/overoxidized poly(3-amino-5-mercapto-1,2,4-triazole) film | DPV | 4–144 | 0.16 | blood serum and urine samples | [298] | |
| Fe2O3/SnO2 composite | DPV | 0.6–70 | 0.1 | - | blood serum and pharmaceutical samples | [299] |
| nitrogen-doped graphene nanosheets/CuCo2O4 nanoparticles | DPV | 0.01–3.0 | 0.0041 | - | urine, serum and pharmaceutical samples | [300] |
| carbon nanodots/chitosan | DPV | Up to 90 | 0.09 | 98.4% after 3 days | blood serum | [204] |
| chitosan film | DPV | 0.1–130 | 0.04 | ˃one week | pharmaceutical samples | [42] |
| graphene/silicon oxide | DPV | 0.5–200 | 0.495 | 96.5% after 2 months | - | [301] |
| MWCNTs decorated with Nickel NPs | SWV | 0.02–1.0 | 0.0066 | 95.1% after 3 weeks | milk and pharmaceutical samples | [302] |
| SWCNTs | LSV | 0.5–50 | 0.05 | - | blood serum | [303] |
| Ag-MoS2/chitosan | DPV | 0.5–120 | 0.05 | - | urine sample | [304] |
| MIP from co-electropolymerization of o-phenylenediamine and hydroquinone | DPV | 0.01–1.0 | 0.005 | - | Trp enantiomers mixture | [305] |
| MWCNT functionalized with 5-amino-2-mercapto-1,3,4-thiadiazole | AMP | 25–300 nM | 0.54 nM | - | blood serum | [306] |
| p-sulphonated calix[4]arene complex | CV | 0.1–10 | 0.03 | 90% after one week | blood serum and amino acid injection samples | [307] |
| gold nanoparticles decorated graphene oxide nanocomposite | AMP | 5–25 | 0.29 | - | - | [308] |
| acetylene black/graphene | DLSV 21 | 1–100 | 0.06 | 92% after 2 weeks | compound amino acid injections and humanserum samples | [309] |
| p-phenylenediamine covalently linked with cysteamine capped cadmium sulphide quantum dots | SWV | 100–500 | 14.74 | - | beverage sample | [310] |
| ruthenium xanthate complex | DPV | 0.25–50 | 0.083 | ˃3 days | pharmaceutical samples | [311] |
| boron-doped diamond (BDD) electrodes and wires | DPV | Up to 250 | 0.5 | - | blood serum | [216] |
| MWCNTs | AMP | 0.6–100 | 0.033 | 94.6% after one week | milk and blood serum samples | [216] |
| MWCNT modified sol-gel | DPV | 0.2–15 | 0.139 | - | milk sample | [312] |
| Au NPs/poly(alizarin red S) film | AMP | 0.02–20 | 0.0067 | - | - | [313] |
| carbon fiber ultramicroelectrodes | CV | 50–200 | 16.7 | - | pharmaceutical samples | [217] |
| MWCNT/Mg-Al-CO3 layered double hydroxide | LSV | 3–1000 | 0.0068 | 93.7% after one week | milk and blood serum samples | [314] |
| poly-sulphosalicylic acid | DPV | 0.05–10 | 0.0068 | 95.24% after 4 weeks | blood serum and amino acid injection samples | [211] |
| electrochemically reduced graphene oxide | DPV | 0.2–40 | 0.1 | 91.6% after 2 weeks | - | [315] |
| gold nanoparticles/macroporous carbon composites | DPV | 10–1000 | 0.024 | 91% after 3 weeks | blood serum | [316] |
| Co3O4 nanoparticles-decorated graphene | AMP | 0.05–10 | 0.01 | 94% after 4 weeks | liquid Dulbecco’s modified Eagle medium and amino acid injection samples | [317] |
| SiO2 nanoparticles | LSV | 0.1–50 | 0.036 | 95.4% after one week | blood serum and pharmaceutical samples | [318] |
| TiO2-graphene/4-aminobenzenesulphonic acid composite | DPV | 1–400 | 0.3 | 96.08% after 20 days | human blood serum | [319] |
| β-cyclodextrin functionalized Fe3O4 magnetic nanoparticles | DPV | 0.8–300 | 0.5 | - | amino acid injection sample | [320] |
| Si-doped nano-TiO2 | CV | 1–400 | 0.5 | 90.3% after one month | amino acid injection sample | [321] |
| oxidation product of TRP | DPV | 0.5–50 | 0.05 | ˃30 days | blood serum | [322] |
| cobalt(II) coordination polymer | DLSV | 0.2–80 | 0.1 | ˃30 days | amino acid injection sample | [323] |
| binuclear manganese(II) complex | DLSV | 0.1–80 | 0.08 | ˃30 days | amino acid injection sample | [324] |
| nano-mixture of graphite/diamond | LSV | 0.1–80 | 0.03 | - | human synthetic serum | [325] |
| copper hexacyanoferrate film on cysteamine-gold nanoparticle graphite-wax composite | CV | 0.085–120 | 0.0185 | ˃25 days | milk sample | [326] |
| chemical vapor deposited MWCNTs | CV | 0.001–100 | 0.22 nM | ˃2 months | pharmaceutical samples | [327] |
| poly(9-aminoacridine) functionalized MWCNT | DPV | 1–500 | 0.81 | ˃2 months | pharmaceutical samples | [328] |
| nano-structured Ni (II)/(2-amino-1-cyclopentene-1-dithiocarboxylic acid) film | AMP | 0.085–43.0 | 0.023 | - | blood serum | [329] |
| nafion and TiO2-graphene nanocomposite, | DPV | 5–140 | 0.7 | 92% after 2 weeks | - | [330] |
| p-aminophenol/MWCNTs | DPV | 10–300 | 5.7 | - | urine, river water, blood plasma and serum samples | [98] |
| boron-doped diamond nanowires | DPV | 0.5–50 | 0.5 | - | - | [331] |
| dibenzo-18-crown-6 and Ni2+ ion | DPV | 1.96–1010 | 0.0979 | - | apple, guava, red grape juice, milk and pharmaceutical samples | [332] |
| gold nanoparticles | SWV | 5–900 | 4 | - | amino acid injection sample | [215] |
| carbon ionic liquid | CV | 8–1000 | 4.8 | 96.87% after 4 weeks | synthetic amino acid mixtures | [333] |
| poly(methyl red) film | LSV | 0.1–100 | 0.04 | - | amino acid injection sample | [334] |
| gold nanoparticles (AuNPs) onto carbon nanotube (CNT) films | AMP | 0.03–2.5 | 0.01 | - | pharmaceutical samples | [335] |
| Sensing Part | Method | LDR | LOD | L.T. Stability | Real Sample | Ref. |
|---|---|---|---|---|---|---|
| 2D-MoS2 | LSV | 1–500 | 0.5 | 70% after 4 months | - | [356] |
| macroporous carbon embedded β-cyclodextrin | DPV | 1–500 | 0.2 | - | tapwater | [357] |
| double-chain Cu metal organic framework | DPV | 10–90 | 5.8 | - | - | [358] |
| black phosphorus nanosheets/β-cyclodextrin | SWV | 10–100 | 4.81 | - | - | [359] |
| CdO/SnO2 nanoparticle | AMP | 0.1nM-10 µM | 0.098 nM | - | human, rabbit and mouse blood serum samples | [360] |
| rGO-hemin-Ag | DPV | 0.1–1000 | 0.03 | 92.1% after 15 days | urine sample | [361] |
| plain graphite | DPV | 0.01–100 | 0.002 | - | pharmaceutical capsule | [362] |
| Au nanoparticles @metal organic framework/polythionine loaded with molecularly imprinted polymer | DPV | 0.01–4 | 0.79 nM | 96.8% after 2 weeks | blood serum | [363] |
| ErVO4/MnWO4 heterostructure | DPV | 0.08–400 | 0.0077 | - | blood serum | [364] |
| 2D-MoS2 nanosheets | CV | 0–100 | 0.5 | - | commercial food integrator | [365] |
| cupric oxide decorated β-cyclodextrin | AMP | 0.01–100 | 0.0082 | 97% after 15 days | food sample, urine and serum samples | [366] |
| acetylene black paste electrode modified by oxygen-functionalized MWCNTs | SDLSV | 0.04–600 | 0.02 | 92.5% after 2 weeks | milk, yogurt, beer and cheese samples | [367] |
| ultrathin g-C3N4/Ag layers | DPV | 1–150 | 0.14 | 85.8% after 4 weeks | pharmaceutical samples | [354] |
| copper sulphide nanosheets modified with chitosan and acidified MWCNTs | DPV | 0.08–1.0 | 4.9 nM | 93.54% after 10 days | pig serum samples | [348] |
| NiO nanoparticles | DPV | 0.15–450 | 0.1 | - | urine and pharmaceutical samples | [368] |
| electrodeposited Cysteic acid | DPV | 3.5–96 | 1.1 | ˃one month | blood serum | [369] |
| iron oxide nanoparticles | DPV | 0.4–270 | 0.05 | ˃3 weeks | blood serum | [370] |
| molecularly imprinted polymer/rGO | DPV | 0.1–400 | 0.046 | 90.6% after 20 days | blood serum and urine samples | [371] |
| MWCNT/TiO2 | DPV | 0.001–100 | 0.001 | - | human serum albumin and bovine serum albumin samples | [336] |
| graphene quantum dot-β-cyclodextrin | DPV | 0.1–1.5 | 0.03 | ˃6 days | - | [345] |
| mesoporous silica nanoparticles | DPV | 0.3–600 | 0.049 | - | artificial urine sample | [265] |
| lead-doped carbon ceramic | AMP | 5–1458 | 0.77 | - | pharmaceutical samples | [372] |
| filtered MWCNTs | DPV | 25–750 | 8 | - | plasma and whole blood samples | [373] |
| molecularly imprinted polypyrrole film | SWV | 0.005–0.025 | 0.0025 | 94.4% after 10 days | plasma sample | [374] |
| poly-(diallyldimethylammonium chloride)/gold nanoparticles | CCR 22 | 0.3–10 | 0.01 | - | - | [375] |
| graphene oxide/MnO2 microspheres/chitosan | DPSV 23 | 0.02–20 | 0.0083 | 98.2% after one month | milk and dried blood spots samples | [376] |
| graphene nanowalls deposited on a tantalum | DPV | 8–100 | 0.8 | ˃94 days | blood serum and pharmaceutical samples | [377] |
| Au-nanoparticles/poly-Trypan blue | DPV | 0.5–880 | 0.008 | 97.1% after 4 weeks | blood serum and urine samples | [50] |
| graphene-zinc oxide (ZnO/GR) nanocomposite film | DPV | 1–800 | 0.5 | - | urine sample | [378] |
| graphene oxide/ZnO nanocomposite | SWV | 0.1–400 | 0.07 | - | pharmaceutical serum and water samples | [379] |
| silver nanoparticle patterned functional liquid crystalline gel | DPV | 0.2–500 | 0.01 | 95% after one month | blood serum | [380] |
| acetylene black and chitosan | DPV | 2.5–430 | 0.92 | 90.4% after 28 days | urine sample | [381] |
| ZnFe2O4 nanoparticles | DPV | 0.4–175 | 0.1 | - | blood serum and urine samples | [280] |
| SiO2@Fe3O4/GR nanocomposite decorated graphene/carbon ionic liquid | DPV | 1–800 | 0.5 | - | urine sample | [382] |
| graphene quantum dots (GQDs) and β-cyclodextrins | CV | 6–1500 | 0.0067 | 98.31% after 10 days | blood serum | [383] |
| polyoxometalate (H3PW12O40) functionalized rGO | SWV | 0.01–1 nM | 0.002 nM | 99.03% after 45 days | blood serum | [282] |
| SiO2 | DPV | 0.5–600 | 0.15 | ˃2 months | artificial urine sample | [291] |
| MWCNT/poly (Bromocresol purple) | AMP | 2–100 | 0.191 | 95.8% after 7 days | milk and blood serum samples | [384] |
| graphene quantum dot/RuCl3 nanocomposite | AMP | 1–937 | 0.23 | 94% after 6 weeks | - | [347] |
| carboxylic acid functionalized MWCNT | AMP | 0.8–100 | 0.014 | 93.1% after one week | milk and blood serum samples | [343] |
| phthalic anhydride functionalized chitosan/carbon nanotube film | AMP | 1–800 | 0.3 | 85% after 2 weeks | blood erum | [353] |
| gold nanoparticles involved in 2-aminoethanethiol functionalized graphene oxide | DPV | 1–20 nM | 0.15 nM | 98.13% after 60 days | milk sample | [385] |
| poly(thionine) | DPV | 1–250 | 0.57 | 95% after one week | blood serum | [386] |
| copper oxide/cuprous oxide nanoparticles/MWCNT nanocomposite | AMP | 0.2–200 | 0.0096 | 90% after 3 weeks | urine sample | [349] |
| MWCNT/poly-2,6-dichlorophenolindophenol film | AMP | 0.3–110 | 0.075 | 93.8% after 2 weeks | blood serum and soya sauce | [350] |
| rGO | SWV | 0.8–60 | 0.07 | - | urine sample | [342] |
| SWCNTs | LSV | 2–30 | 0.4 | - | blood serum | [303] |
| Nafion and cerium dioxide nanoparticles | DPV | 2–160 | 0.09 | 94% after 2 weeks | blood serum | [387] |
| Fe-doped hydroxyapatite nanoparticles | AMP | 0.1–10 | 0.245 | 80% after 2 weeks | - | [388] |
| palladium decorated MWCNT | LSV | 0.1–10 nM | 0.146 nM | - | - | [389] |
| ordered mesoporous carbon | DPV | 15–900 | 10 | 95.4% after 2 weeks | - | [95] |
| old nanoparticles/macroporous carbon (GNPs–MPC) composites | DPV | 5–1000 | 0.074 | 87% after 3 weeks | blood serum | [316] |
| thiolated β-cyclodextrins | DPV | 36–240 | 12 | - | pharmaceutical samples | [351] |
| hemin immobilized onto the poly (amidoamine)/MWCNT | AMP | 0.1–28.8 | 0.01 | - | - | [352] |
| europium hexacyanoferrate film | AMP | 10–600 | 8 | - | - | [390] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulaee, K.; Neri, G. Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements. Biosensors 2021, 11, 502. https://doi.org/10.3390/bios11120502
Moulaee K, Neri G. Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements. Biosensors. 2021; 11(12):502. https://doi.org/10.3390/bios11120502
Chicago/Turabian StyleMoulaee, Kaveh, and Giovanni Neri. 2021. "Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements" Biosensors 11, no. 12: 502. https://doi.org/10.3390/bios11120502
APA StyleMoulaee, K., & Neri, G. (2021). Electrochemical Amino Acid Sensing: A Review on Challenges and Achievements. Biosensors, 11(12), 502. https://doi.org/10.3390/bios11120502






