Comparison of Single- and Mixed-Sized Gold Nanoparticles on Lateral Flow Assay for Albumin Detection
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Synthesis of GNPs
2.3. Characterization of the Synthesized GNPs (20 nm & 50 nm)
2.4. Conjugation of GNPs with Anti-BSA Antibodies
2.5. Preparation of LFA Strip
2.6. Sensitivity Determination and Specificity of the Prepared LFA
2.7. Data Analysis
3. Results and Discussion
3.1. Characterization of GNPs
3.2. Bioconjugation of GNPs
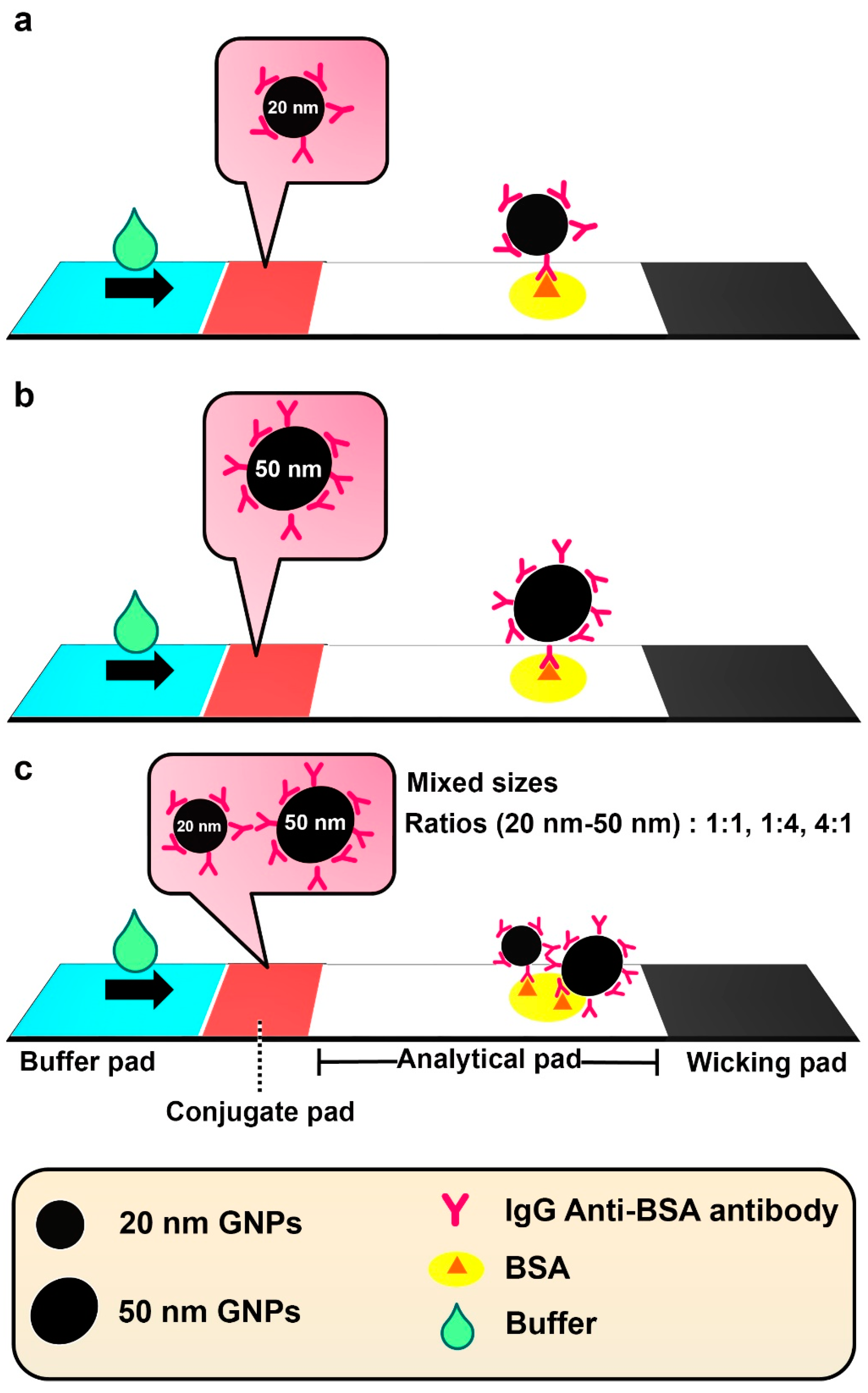
3.3. Evaluation of the Influence of Single- and Mixed-Sized GNPs@anti-BSA Conjugates on LFA Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pissuwan, D.; Gazzana, C.; Mongkolsuk, S.; Cortie, M.B. Single and Multiple Detections of Foodborne Pathogens by Gold Nanoparticle Assays. WIRES Nanomed. Nanobiotechnol. 2020, 12, e1584. [Google Scholar] [CrossRef]
- Han, G.-R.; Kim, M.-G. Highly Sensitive Chemiluminescence-Based Lateral Flow Immunoassay for Cardiac Troponin I Detection in Human Serum. Sensors 2020, 20, 2593. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, G. Signal-Enhanced Lateral Flow Immunoassay with Dual Gold Nanoparticle Conjugates for the Detection of Hepatitis B Surface Antigen. ACS Omega 2019, 4, 5083–5087. [Google Scholar] [CrossRef] [Green Version]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; Gric, T., Ed.; Intechopen: London, UK, 2018; ISBN 978-1-83881-738-1. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, T.E.P.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Lou, S.; Ye, J.Y.; Li, K.Q.; Wu, A. A Gold Nanoparticle-Based Immunochromatographic Assay: The Influence of Nanoparticulate Size. Analyst 2012, 137, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, Y.T.; Hong, S.B.; Kim, J.; Heo, N.S.; Lee, M.K.; Lee, S.J.; Kim, B.I.; Kim, I.S.; Huh, Y.S.; et al. Development of Lateral Flow Assay Based on Size-Controlled Gold Nanoparticles for Detection of Hepatitis B Surface Antigen. Sensors 2016, 16, 2154. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Guo, S.Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Huang, Y.; Wang, J.; Zhang, L.; Rong, Y.; Lai, W.; Chen, T. A Remarkable Sensitivity Enhancement in a Gold Nanoparticle-Based Lateral Flow Immunoassay for the Detection of Escherichia Coli O157:H7. RSC Adv. 2015, 5, 45092. [Google Scholar] [CrossRef]
- Makhsin, S.R.; Razak, K.A.; Noordin, R.; Zakaria, N.D.; Chun, T.S. The Effects of Size and Synthesis Methods of Gold Nanoparticle-Conjugated MαHIgG4 for Use in an Immunochromatographic Strip Test to Detect Brugian Filariasis. Nanotechnology 2012, 23, 495719. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.-B.; Kim, M.-G. A Dual Gold Nanoparticle Conjugate-Based Lateral Flow Assay (LFA) Method for the Analysis of Troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Z.; Liu, D.; Peng, T.; Liu, K.; Wang, S.; Xiong, Y.; Wei, H.; Xu, H.; Lai, W. Dual Gold Nanoparticle Lateral Flow Immunoassay for Sensitive Detection of Escherichia Coli O157:H7. Anal. Chim. Acta. 2015, 876, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Masutani, K.; Torisu, K.; Katafuchi, R.; Tanaka, S.; Tsuchimoto, A.; Mitsuiki, K.; Tsuruya, K.; Kitazono, T. Association between Serum Albumin Level and Incidence of End-Stage Renal Disease in Patients with Immunoglobulin a Nephropathy: A Possible Role of Albumin as an Antioxidant Agent. PLoS ONE 2018, 13, e0196655. [Google Scholar] [CrossRef] [PubMed]
- Kittanamongkolchai, W.; Thongprayoon, C.; Cheungpasitporn, W.; Nasr, S. Serum Albumin as a Quantitative Definition of Nephrotic Syndrome. Am. J. Kidney Dis. 2015, 65, A50. [Google Scholar]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A.J. Turkevich Method for Gold Nanoparticle Synthesis Revisited. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, M.J.; Trabuco, J.R.C.; Vu, B.V.; Garvey, G.; Khodadady, M.; Azevedo, A.M.; Aires-Barros, M.R.; Chang, L.; Kourentzi, K.; Litvinov, D.; et al. Enhancement of Lateral Flow Assay Performance by Electromagnetic Relocation of Reporter Particles. PLoS ONE 2018, 13, e0186782. [Google Scholar]
- Kim, J.; Cao, X.E.; Finkelstein, J.L.; Cárdenas, W.B.; Erickson, D.; Mehta, S. A Two-Colour Multiplexed Lateral Flow Immunoassay System to Differentially Detect Human Malaria Species on a Single Test Line. Malar. J. 2019, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors Determining the Stability, Size Distribution, and Cellular Accumulation of Small, Monodisperse Chitosan Nanoparticles as Candidate Vectors for Anticancer Drug Delivery: Application to the Passive Encapsulation of [14C]-Doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pissuwan, D.; Cortie, C.B.; Valenzuela, S.M.; Cortie, M.B. Gold Nanosphere-Antibody Conjugates for Hyperthermal Therapeutic Applications. Gold Bull. 2007, 40, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing BSA Binding to Citrate-Coated Gold Nanoparticles and Surfaces. Langmuir 2005, 21, 9303–9307. [Google Scholar] [CrossRef]
- Pissuwan, D.; Valenzuela, S.M.; Miller, C.M.; Killingworth, M.C.; Cortie, M.B. Destruction and Control of Toxoplasma Gondii Tachyzoites Using Gold Nanoparticle/Antibody Conjugates. Small 2009, 5, 1030–1034. [Google Scholar] [CrossRef] [Green Version]
- Leersnyder, I.D.; Gelder, L.D.; Driessche, I.V.; Vermeir, P. Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials 2019, 9, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, J.R.G.; Werts, M.H.V. Resonant light scattering spectroscopy of gold, silver and gold-silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Xiong, B.; Peng, L.; Li, H.; He, Y.; Yeung, E.S. Recent Advances in Optical Imaging with Anisotropic Plasmonic Nanoparticles. Anal. Chem. 2015, 87, 200–215. [Google Scholar] [CrossRef]
- Pan, R.; Jiang, Y.; Sun, L.; Wang, R.; Zhuang, K.; Zhao, Y.; Wang, H.; Ali, A.; Xu, H.; Man, C.J. Gold Nanoparticle-Based Enhanced Lateral Flow Immunoassay for Detection of Cronobacter Sakazakii in Powdered Infant Formula. J. Dairy Sci. 2018, 101, 3835–3843. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, B.; Khlebtsov, N. Enhanced Solid-Phase Immunoassay Using Gold Nanoshells: Effect of Nanoparticle Optical Properties. Nanotechnology 2008, 19, 435703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Pan, S.; Zhang, Y.; Chang, J.; Huang, Z.; Li, T.; Zhang, C.; Fuentea, J.M.; Zhang, Q.; Cui, D. Carbon-Gold Hybrid Nanoprobes for Real-Time Imaging, Photothermal/Photodynamic and Nanozyme Oxidative Therapy. Theranostics 2019, 9, 3443–3458. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. A Network Composed of Gold Nanoparticles and a Poly(vinyl alcohol) Hydrogel for Colorimetric Determination of Ceftriaxone. Microchim. Acta 2020, 187, 133. [Google Scholar] [CrossRef]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.B.; Ashrafizadeh, M.; Zare, E.N.; Agarwal, T.; Padil, V.V.T.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117–139. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Hydrogel Based Aptasensor for Thrombin Sensing by Resonance Rayleigh Scattering. Anal. Chim. Acta 2019, 1079, 180–191. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotithammakul, S.; Cortie, M.B.; Pissuwan, D. Comparison of Single- and Mixed-Sized Gold Nanoparticles on Lateral Flow Assay for Albumin Detection. Biosensors 2021, 11, 209. https://doi.org/10.3390/bios11070209
Chotithammakul S, Cortie MB, Pissuwan D. Comparison of Single- and Mixed-Sized Gold Nanoparticles on Lateral Flow Assay for Albumin Detection. Biosensors. 2021; 11(7):209. https://doi.org/10.3390/bios11070209
Chicago/Turabian StyleChotithammakul, Sasima, Michael B. Cortie, and Dakrong Pissuwan. 2021. "Comparison of Single- and Mixed-Sized Gold Nanoparticles on Lateral Flow Assay for Albumin Detection" Biosensors 11, no. 7: 209. https://doi.org/10.3390/bios11070209
APA StyleChotithammakul, S., Cortie, M. B., & Pissuwan, D. (2021). Comparison of Single- and Mixed-Sized Gold Nanoparticles on Lateral Flow Assay for Albumin Detection. Biosensors, 11(7), 209. https://doi.org/10.3390/bios11070209




