Rapid Detection of Gut Microbial Metabolite Trimethylamine N-Oxide for Chronic Kidney Disease Prevention
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
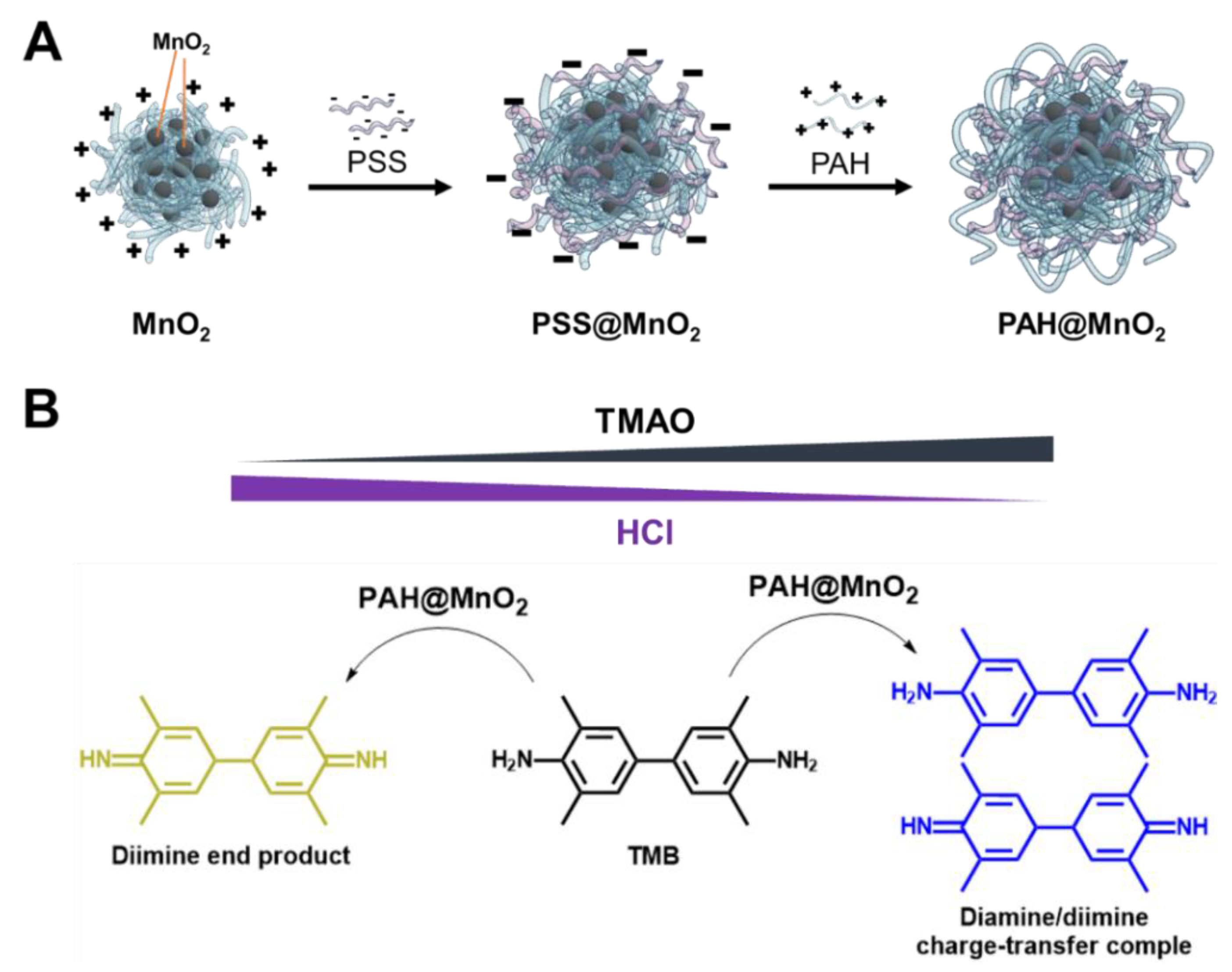
2.2. Preparation of PAH-Capped MnO2 NPs (PAH@MnO2 NPs)
2.3. Stability Tests
2.4. pH Effect on the Peroxidation Activity
2.5. Colourimetric Detection of TMAO
2.6. Preparation of an Artificial Urine Solution
2.7. TMAO Rapid Detection in Plasma from CKD Rats
2.8. Statistics
3. Results and Discussion
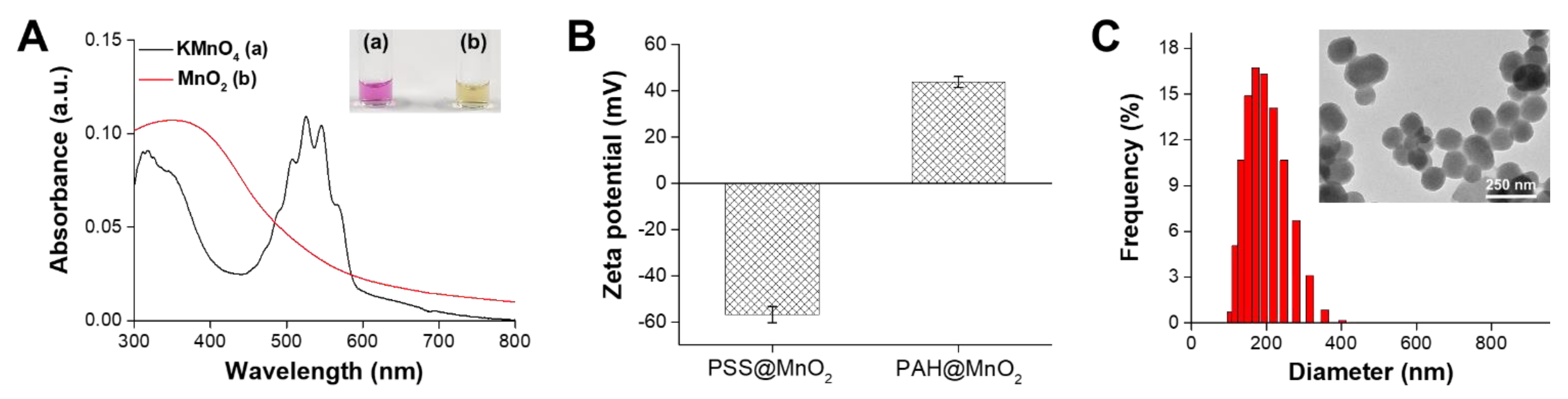
3.1. Characterisation of the PAH@MnO2 NPs
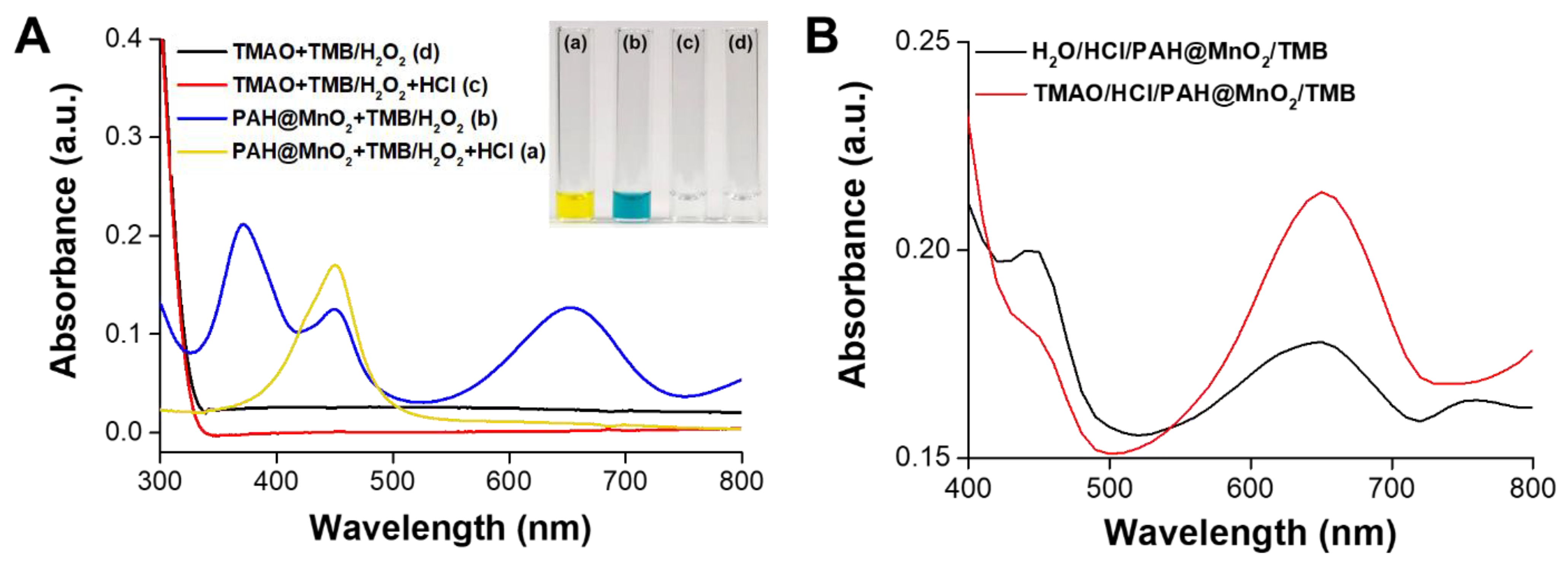
3.2. Mechanism of TMAO Detection Using PAH@MnO2 NPs
3.3. Comparison of PAH@MnO2 and HRP in Terms of Peroxidation Activity and Stability
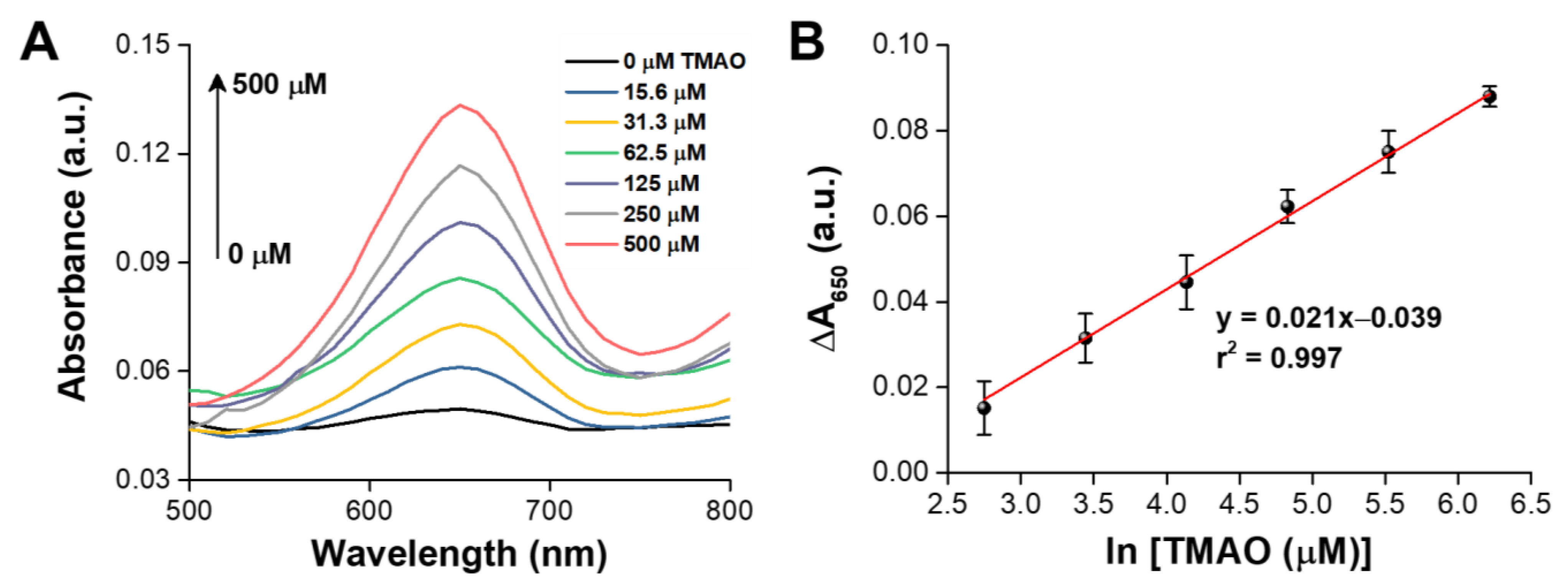
3.4. Colourimetric Analysis of TMAO
3.5. Recovery of TMAO in Spiked Urine Samples
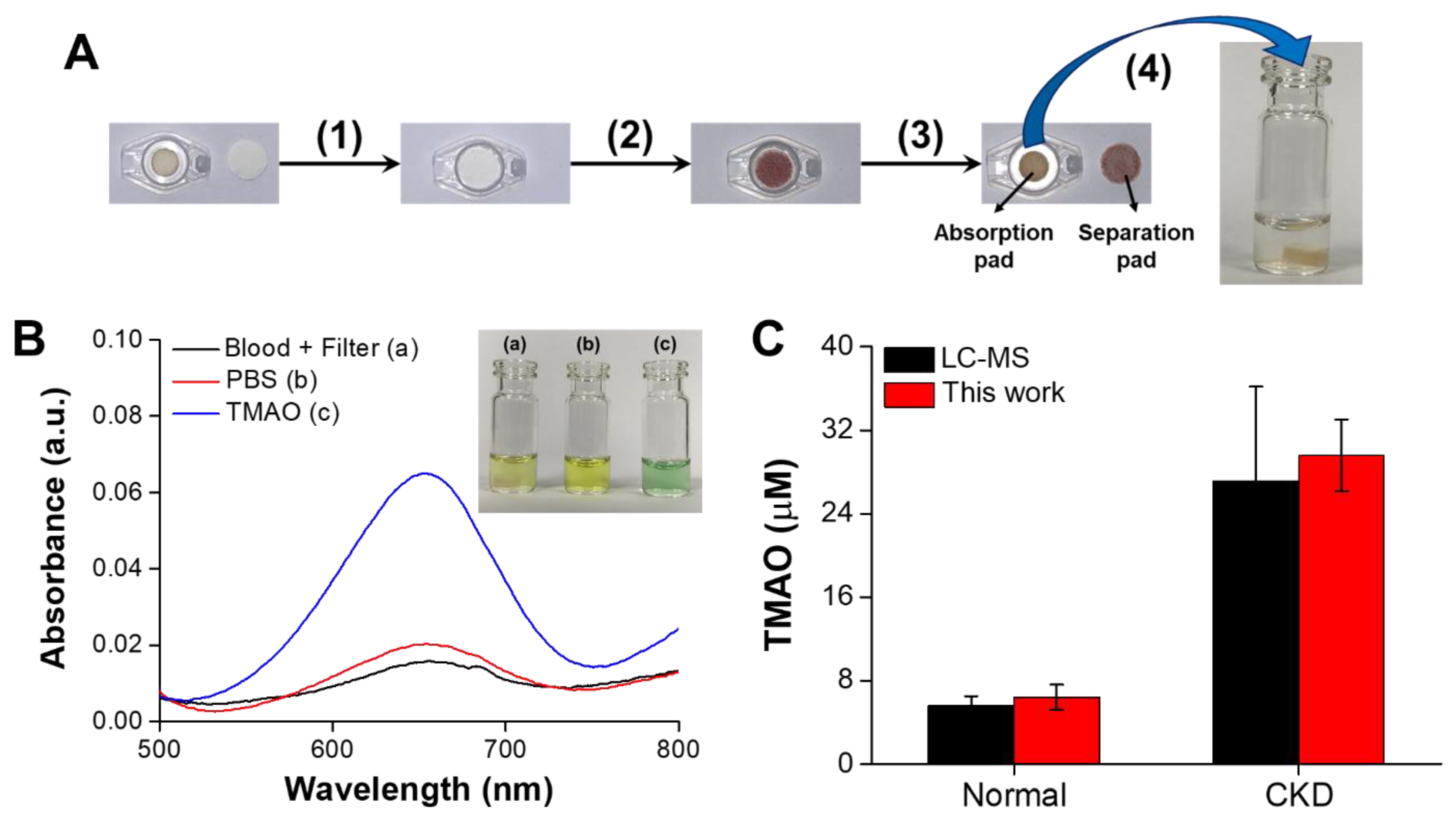
3.6. TMAO Rapid Detection in Plasma from CKD Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.; Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Yuan, C.; Zhang, Y.; Wang, W.; Hu, S.; Liu, L.; Wang, Y. Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark. Med. 2017, 11, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Wishnok, J.S.; Deen, W.M. Metabolism and excretion of methylamines in rats. Toxicol. Appl. Pharmacol. 1994, 125, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Lee, J.A.; Lee, H.A.; Sadler, P.J.; Wilkie, D.R.; Woodham, R.H. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: Identification of trimethylamine-N-oxide. Biochim. Biophys. Acta Mol. Basis Dis. 1991, 1096, 101–107. [Google Scholar] [CrossRef]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.; Nolin, T.D.; et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zeisel, S.H.; Zhang, S. Rapid Lc-MRM-Ms assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N-oxide, and creatinine in human plasma and urine. Electrophoresis 2015, 36, 2207–2214. [Google Scholar] [CrossRef]
- Wang, Z.; Levison, B.S.; Hazen, J.E.; Donahue, L.; Li, X.M.; Hazen, S.L. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem Mass spectrometry. Anal. Biochem. 2014, 455, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Wolak-Dinsmore, J.; Wang, Z.; Li, X.S.; Bennett, D.W.; Connelly, M.A.; Otvos, J.D.; Hazen, S.L.; Jeyarajah, E.J. NMR quantification of trimethylamine-N-oxide in human serum and plasma in the clinical laboratory setting. Clin. Biochem. 2017, 50, 947–955. [Google Scholar] [CrossRef]
- Buc, J.; Santini, C.L.; Giordani, R.; Czjzek, M.; Wu, L.F.; Giordano, G. Enzymatic and physiological properties of the tungsten-substituted molybdenum TMAO reductase from Escherichia coli. Mol. Microbiol. 1999, 32, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mitrova, B.; Waffo, A.F.T.; Kaufmann, P.; Iobbi-Nivol, C.; Leimkühler, S.; Wollenberger, U. Trimethylamine N-oxide electrochemical biosensor with a chimeric enzyme. ChemElectroChem 2019, 6, 1732–1737. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Geng, W.C.; Zheng, Z.; Gao, J.; Guo, D.S.; Wang, Y. Facile fluorescence monitoring of gut microbial metabolite trimethylamine N-oxide via molecular recognition of guanidinium-modified calixarene. Theranostics 2019, 9, 4624–4632. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Suslick, K.S. Ultrasonic preparation of porous silica-dye microspheres: Sensors for quantification of urinary trimethylamine N-oxide. ACS Appl. Mater. Interfaces 2018, 10, 15820–15828. [Google Scholar] [CrossRef]
- Shmaefsky, B.R. Artificial urine for laboratory testing. Am. Biol. Teach. 1990, 52, 170–172. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chan, J.Y.H.; Wu, K.L.H.; Yu, H.R.; Lee, W.C.; Hou, C.Y.; Tain, Y.L. Altered gut microbiota and its metabolites in hypertension of developmental origins: Exploring differences between fructose and antibiotics exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef]
- Song, M.; Liu, T.; Shi, C.; Zhang, X.; Chen, X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano 2016, 10, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Li, N.S.; Hsu, Y.P.; Peng, C.; Yang, H.W. Direct glucose detection in whole blood by colorimetric assay based on glucose oxidase-conjugated graphene oxide/MnO2 nanozymes. Analyst 2019, 144, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Osborn, L.J.; Wang, Z. Simultaneous measurement of urinary trimethylamine (TMA) and trimethylamine N-oxide (TMAO) by liquid chromatography–mass spectrometry. Molecules 2020, 25, 1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Spiked Concentration (μM) | Detected (μM) | Recovery (%) |
|---|---|---|---|
| Artificial urine | 100 | 101.7 ± 7.2 | 101.7 ± 7.2 |
| 300 | 303.4 ± 8.9 | 101.1 ± 3.0 | |
| 500 | 487.4 ± 8.7 | 97.5 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chu, Y.-H.; Wang, C.-C.; Wang, C.-H.; Tain, Y.-L.; Yang, H.-W. Rapid Detection of Gut Microbial Metabolite Trimethylamine N-Oxide for Chronic Kidney Disease Prevention. Biosensors 2021, 11, 339. https://doi.org/10.3390/bios11090339
Chang Y-C, Chu Y-H, Wang C-C, Wang C-H, Tain Y-L, Yang H-W. Rapid Detection of Gut Microbial Metabolite Trimethylamine N-Oxide for Chronic Kidney Disease Prevention. Biosensors. 2021; 11(9):339. https://doi.org/10.3390/bios11090339
Chicago/Turabian StyleChang, Yu-Chun, Yi-Hsuan Chu, Chien-Cheng Wang, Chih-Hsuan Wang, You-Lin Tain, and Hung-Wei Yang. 2021. "Rapid Detection of Gut Microbial Metabolite Trimethylamine N-Oxide for Chronic Kidney Disease Prevention" Biosensors 11, no. 9: 339. https://doi.org/10.3390/bios11090339
APA StyleChang, Y.-C., Chu, Y.-H., Wang, C.-C., Wang, C.-H., Tain, Y.-L., & Yang, H.-W. (2021). Rapid Detection of Gut Microbial Metabolite Trimethylamine N-Oxide for Chronic Kidney Disease Prevention. Biosensors, 11(9), 339. https://doi.org/10.3390/bios11090339







